Abstract
The date palm - one of the oldest domesticated fruit crops - is the tree most adapted to growing in desert areas. It has always been looked upon as a key source of stability, survival and evolution of the oasis agro-system as it constitutes the basic features of the ecological pyramid in desert regions. Tunisian date palm germplasm is characterised by high genetic diversity, with more than 250 varieties identified. However, this patrimony is seriously menaced by severe genetic erosion due to different biotic and abiotic factors. In Tunisia, as well as in North African countries, dates are cultivated for fruit production, and all parts of the tree are used for many other artisanal and/or industrial purposes. Recent efforts have focussed on the development of phenotypic, biochemical and DNA-based markers useful in characterising the genetic diversity of date palm populations and to establish the relationships between different cultivars. This chapter reviews current efforts made towards developing such selection markers for Tunisian date palm cultivars for use in breeding programmes.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
1.1 History
The date palm is recorded in ancient history extending over an area from the Indus Valley (now Pakistan) to Mesopotamia (now Iraq), the Nile Valley, Southern Persia, the Eastern Mediterranean and the Horn of Africa. References to the date in the Nile Valley and Tigris/Euphrates valleys suggest that it has been under cultivation for at least the last 5,000 years. Such a wide distribution implies that Phoenix dactylifera (2n = 36) is well adapted to quite extensive geographic, soil and climatic conditions. Another species, Phoenix sylvestris (sugar date palm or toddy palm) still occurs in the wild throughout northern India; its sap is used to produce a crude sugar.
Phoenix dactilyfera most likely grew wild as a natural hybrid of P. sylvestris in the Indus Valley, where it was appreciated as a wild fruit and probably cultivated as early as the sixth millennium BC; there have been finds of date palm seeds in association with human settlement from 5000 BC onwards. The oldest radiocarbon dated discovery of date seeds was on Dalma island, part of the Abu Dhabi Island group. Two seeds were found in 1998, the oldest was 5110 BC and the other 4670 BC. As there was no evidence of cultivation of date palms in the region at that time, it is probable that these seeds came from traders (Zohary and Spiegel-Roy 1975; Wrigley 1995).
1.2 Botanical Profile
Belonging to the Angiosperms-Monocotyledones, Palmaceae is a family of about 200 genera and 1,500 species (Dowson 1982). The genus Phoenix (Coryphoideae Phoeniceae) contains a dozen species, all native to the tropical or subtropical regions of Africa or Southern Asia, including Phoenix dactylifera L. (Munier 1973). According to Dransfield and Uhl (1986), the date palm is classified as follows:
-
Group: Spadiciflora
-
Order: Palmea
-
Family: Palmaceae
-
Sub-family: Coryphyoideae
-
Tribe: Phoeniceae
-
Genus: Phoenix
-
Species: dacttylifera L.
Twelve species of the genus Phoenix, along with their geographical distribution, were first listed (Table 17.1) by Chevalier (1952). Besides date palm, five of these species bear edible fruit (P. atlantica Chev., P. reclinata Jacq., P. farinifera Roxb., P. humilis Royle., and P. acaulis Roxb.).
The species Phoenix dactilyfera has about 19 known relatives, including Phoenix canariensis (Canary Island palm), P. reclinata (Senegal date palm) and P. sylvestris (Indian sugar date palm). All are members of the plant family, Arecaceae. The scientific name was derived from ‘Phoenix’, the legendary bird of ancient Greece. The Phoenicians dyed cloth a purple colour using dye from the Murex shellfish; this colour was also called Phoenix, possibly because it had such great appeal and value. The same colour was noted on the fruit of the date, hence the date palm genus became Phoenix. The specific name dactylifera came from the shape of the fruit, ‘dactylos’ being the ancient Greek word for ‘finger’. Date palms are dioecious; i.e. the male and female parts are on separate plants. The date palm is the tallest of the Phoenix species, growing to 30 m in some places. The trunk, in cultivation, is surrounded from the ground upward in a spiral pattern of leaf bases. The leaves are large, 4–5 m, alternate, sheathing in dense terminal rosettes, and pinnately lobed. The ends of leaf fronds are needle sharp to help protect the growth tip from grazing animals.
The fruit is also the largest of the species, with a few varieties reaching up to 100 mm x 40 mm in size. P. dactylifera is now found in tropical and sub-tropical regions all over the world as well as in temperate and arid regions of the United States, Australia, southern Spain and the Mediterranean coast of Africa and West Asia. The fruit is a ‘drupe’, with a single seed in each date. Fruit is borne on clusters often weighing 10 kg or more. A fully productive palm can support up to ten clusters carrying as much as 100 kg fruit. From the time of pollination, the fruit takes 200 days to reach the fully ripened stage.
1.3 Production Levels and Locations
The FAO estimate that worldwide production of dates peaked in 1996 at 4,492,000 tons. The world largest producer is Iran at 765,000 Mt followed by Egypt (680,000 Mt), Saudi Arabia (597,000 Mt), Iraq (550,000 Mt), Pakistan (533,000 Mt), Algeria (361,000 Mt) then the United Arab Emirates (UAE) at 240,000 Mt. Other significant producers are Libya, Morocco, Sudan, Tunisia, China, Oman, Yemen, Qatar, Bahrain, the United States and Jordan. In UAE, the Abu Dhabi Emirate is by far the largest producer of dates. There is said to be approximately 16 million date palms in the Emirate, with approximately 4 million in the remaining six Emirates. Turkey and Spain produce small quantities; Spain is of particular interest because, after its introduction by the Moors, the Spanish took the date subsequently to Mexico from where it migrated to California. Major commercial plantings in California were however, imported some time later direct from Saudi Arabia (Hodel and Johnson 2007).
1.4 Propagation
While dates grow readily from seed, the quality of the resultant plants and reliability of the crop is too ‘hit and miss’; therefore, the most common method of reproduction is the planting of suckers. There have been further refinements in propagation methods including, over the last 20 years, production of ‘tissue culture’ dates in laboratories in various parts of the world.
1.5 Date Varieties
There are more than 600 varieties, including cultivars, grown worldwide, and different countries have their favourites. In UAE the preferred fruit is Khalas but others such as Zaghloul, Khuneizi, Hilali, Howaiz, Naghal and Jaberi Fardh have their followings. These dates have different colours, flavours, sweetness, acidity and textures. A popular imported variety, mainly from Morocco, is Majool, which is a very large fruit. All major date-producing countries have their own cultivars and favoured varieties, such as Amir Hajj and Ashrashi from Iraq; Saidy and Hayany from Egypt, Deglet Nour and Thoory from Algeria, and Ruzeiz, Bukeira, Nebut, Seif and Barhi from Oman.
2 Date Palms in Tunisia
In Tunisia (Fig. 17.1), as well as in North African countries and several other tropical countries located in the Middle East and the Arabic peninsula, oasis cultures consist of date-palm groves and constitute one of the main factors determining social, environmental and economic stability in these areas. Date palm groves also most important factor in the establishment of favourable conditions in the oasian agro-system. On a commercial scale, Tunisia is one of the main date palm producing countries in the world. In addition, date palms constitute the principal financial resources and food sources of oasis cultivators, and contribute to the development of adjacent culture of fruit trees, saffron, forage plants and vegetables. Indeed, more than 10% of Tunisians depend on date palm culture for their livelihood (Haddouch 1996).
Bioclimatic map of Tunisia showing the main Tunisian date palm oases (from Zehdi-Azouzi 2005)
Well-adapted ecotypes empirically selected by farmers for their attractive fruit qualities are cultivated. As a consequence, the local germplasm is composed of a variable scale of selected ecotypes of locally named cultivars, and exhibits a great diversity. For instance, more than 220 and 800 cultivars have been reported in Morocco and in Algeria, respectively (Benkhalifa 1996), and 250 have been identified in Tunisia (Rhouma 2005). It should be mentioned here that the date palm is propagated clonally via offshoots produced by the mother tree. However, this method of propagation is relatively slow since the mother tree produces a very limited number of offshoots - too low a number to establish new plantations. To overcome this difficulty, plantlets are generated on a large-scale via in vitro culture methods, which has allowed the expansion of modern date palm plantations. Characterisation of genetic variation in this crop has become imperative to check fidelity of date palm trees produced from offshoots and/or the in vitro-derived plantlets.
3 Genetic Variation in Date Palm
In Tunisia, this important subtropical fruit crop is currently in danger due to severe genetic erosion as a consequence of the predominance of the elite cultivar Deglet Nour in modern cultures (Rhouma 1994). This tendency has led to the disappearance of many cultivars with medium and low fruit qualities. It is therefore imperative to elaborate a strategy aimed at the evaluation of the genetic diversity and the preservation of Tunisian date palm germplasm. Many studies have addressed this issue, and describe the use of either morphological traits or isozyme markers to identify Tunisian date palm varieties (Reynes et al. 1994; Rhouma 1994; Bouabidi et al. 1996; Ould Mohamed Salem et al. 2001). Among these methods, those based on morphological traits are of some benefit in the evaluation of date palm genetic resources (Mohamed et al. 1983; Reynes et al. 1994; Bouabidi et al. 1996; Belguedj 2002; El Houmaizi et al. 2006; Rhouma 2005; Ould Mohamed Salem et al. 2007). Therefore it has been assumed that criteria related to either vegetative or fruit parameters are useful for cultivar characterisation, phenotypic diversity analysis and the exploration phylogenic relationships among date-palm ecotypes.
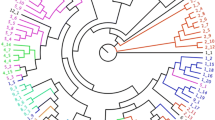
Evaluation of phenotypic diversity is a logical first step in the elaboration of a program to improve germplasm management and utilisation of any crop. However, most morphological traits are highly influenced by environmental conditions or vary with the developmental stage of the plant, and isozymes are limiting due to low levels of polymorphism (Fig. 17.2). Consequently, DNA-based techniques have been developed, and have proved effective in assessing genetic diversity because they access an almost unlimited source of potential markers to uncover differences at the molecular level.
Diagram illustrating the discrimination of 29 Tunisian date-palm ecotypes based on isozyme markers (from Ould Mohamed Salem 2001)
Microsatellites or simple sequence repeats (SSRs) consist of variable numbers of tandemly repeated units, each of 1–6 bp, and represent a class of repetitive DNA commonly found in eukaryotic genomes (Tautz and Renz 1984). They are characterised by their great abundance (Roder et al. 1995), high variability, and extensive distribution throughout different genomes (Roder et al. 1998). Microsatellites are typically multi-allelic loci since more than five alleles per locus are commonly observed in plant populations (Senior and Heun 1998). In addition, automated polymerase chain reaction (PCR)-based techniques, which enable high throughput data collection and good analytical resolution at a low cost, have been developed for microsatellites (Kresovich et al. 1995). Data based on molecular markers such as restriction fragment length polymorphisms (RFLPs), rapid amplification of polymorphic DNA (RAPDs), inter simple sequence repeats (ISSRs), amplified fragment length polymorphisms (AFLPs), random amplified microsatellite polymorphisms (RAMPOs) and SSRs have been used to characterise (Figs. 17.2–17.4) date palm genotypes (Cornicquel and Mercier 1994, 1997; Sedra et al. 1998; Ben Abdallah et al. 2000; Trifi et al. 2000; Trifi 2001; Zehdi et al. 2002; Sakka et al. 2004; Al Khalifah and Askari 2003; El Assar et al. 2005; Hussein et al. 2005; Rhouma et al. 2008). It is evident from these data that microsatellite DNA (Fig. 17.4), ISSRs, RAMPO and cleaved amplified polymorphic sequences (CAPS; Fig. 17.3) provided clear polymorphism as compared to isoenzyme patterns (Fig. 17.2). A large number of SSR alleles have been revealed, with a mean 7.14 per locus, which has permitted detection of a high degree of genetic variability in date palm. The scored values of diversity are higher at the intra-group level than at the inter-group level (Zehdi et al. 2004). In comparison to vegetative and fruiting characters for distinguishing cultivars, SSR alleles were used successfully to discriminate molecularly between a nearly unlimited number of date palms cultivars. Compared to studies reported in date palms, the scored percentage of resolution is higher than that observed using isoenzymes (Booij et al. 1995; Ould Mohamed Salem et al. 2001) and plastid DNA haplotypes (Sakka 2003). These studies have permitted the identification of markers suitable for identification of date palm varieties. However, the search for other markers continues in order to obtain a deeper comprehension of the genetic organisation in Tunisian date palm germplasm.
Diagram illustrating the discrimination of 38 Tunisian date-palm ecotypes based on cleaved amplified polymorphic sequences (CAPS) chloroplast (ct)DNA (from Sakka et al. 2004)
Diagram illustrating the discrimination of 49 Tunisian date-palm ecotypes based on three microsatellite loci. Alleles significance (size in base pairs): a1 138, a2 142, a3 144, a4 148, a5 153, a6 157, a7 159, a8 165, a9 171, a10 173, b1 175, b2 181, b3 183, b4 185, b5 187, b6 189, b7 195, b8 197, c1 219, c2 231, c3 233, c4 236, c5 246, c6 248, c7 250 (from Zehdi et al. 2004)
4 Molecular Diversity and Development of an Identification Key
The survey of genetic variation at the DNA level and establishment of precise fingerprints has become an important task in plant breeding programs and in germplasm management. For this purpose, studies reported on Tunisian date palms describe the use of isozymes (Ould Mohamed Salem 2001; Bennaceur et al, 1991; Booij et al. 1995), plastid DNA (Sakka et al. 2004) and nuclear DNA markers (Zehdi et al. 2004). In fact, all these latter authors, working independently, have clustered cultivars from different oases based on geographical origin and/or the sex of the trees. They concluded that these plants had a common genetic basis at the DNA level in spite of their phenotypic divergence. Importantly, this is in agreement with the unique domestication origin of this crop. When other molecular markers such as SSRs were used, the data showed large genetic variation in different defined groups. This is well exemplified by SSRs in the Tozeur oasis, which showed a significant deficiency in heterozygocity. However, the remaining two groups (Gabès and Kebili) showed no deviation from Hardy-Weinberg equilibrium (HWE). This result can be explained by the stronger selection pressure operating in the Tozeur oasis compared to in the Kebili and Gabès oases (Zehdi et al. 2004). In fact, scored values of diversity are higher at the intra-group level than at the inter-group level. Similar results have been reported in Moroccan, Algerian and Tunisian datepalm cultivars using isozyme markers (Torres and Tisserat 1980; Ould Mohamed Salem et al. 2001). These results are also comparable to those reported in other long-lived cultivated species such as olive (Ouazzani et al. 1995) and fig (Salhi-Hannachi et al. 2005). Taken together, our present data and the available prior isozyme information (Ould Mohamed Salem et al. 2001) suggest that genetic diversity in Tunisian date palms is high. This could be attributed to the dioecious nature of this crop. Date palms in the Tozeur oasis and male groups showed a significant deficit of heterozygocity. Using RAPD and ISSR markers, similar results have been reported in date palms (Sedra et al. 1998; Trifi et al. 2000; Zehdi et al. 2002). These authors have suggested a common genetic basis among date palm genotypes in spite of the distinctiveness of their morphometric parameters, particularly those related to fruit traits. Hence, our data suggest the existence of one ancestral date palm population, and are in agreement with the unique Mesopotamian domestication origin of this crop (Wrigley 1995).
5 Biotechnology in Tunisian Date Palms
As noted above, the date palm is propagated clonally via offshoots produced by the mother plant. This conventional propagation mode has several drawbacks: it is time consuming and produces only a low number of shoots. For instance, several genotypes do not produce offshoots and rooting percentage is low in such shoots. Moreover, a period of 5–7 years is required to verify the fidelity of the sucker-derived plants (Nixon and Carpenter 1978). To overcome these drawbacks, in vitro methods have been developed to provide an alternative strategy aimed at the mass propagation of date palm plants (El Hadrami 1995; Sharma et al. 1986). The in vitro propagation of endangered Tunisian date palm elite and/or fruity cultivars (i.e. Deglet nour, Deglet bey, Boufaggous, Gondi) through organogenesis and somatic embryogenesis has been successfully achieved (Drira and Benbadis 1985; Othmani et al. 2009a, b; Fig. 17.5). Assessment of certification of the plant tissue culture-derived progeny has, however, never been reported. Among the available molecular biology methods, we investigated RAPD (Williams et al. 1990) and AFLP (Vos et al. 1995) methods, and found them to be reliable, fast and inexpensive procedures with which to identify clones and to assess somaclonal variation in this crop. The RAPD and AFLP banding profiles (op cit.) of the derived progenies were very similar to those of the mother plant (Rhouma 2008), suggesting that no variation had cropped in. In fact, the number as well the sizes of the generated bands are similar in these profiles. This result strongly supports the true-to-type nature of the in vitro-derived date palm plantlets. Moreover, 2,4-D did not induce somaclonal variation in this crop. Similar results have been reported in plantlets regenerated via embryogenic suspension cultures, in comparison to the mother tree of the date palm cv. Deglet nour (Fki et al. 2003). In fact, using flow cytophotometry analysis, these authors examined the ploidy level in these plantlets and revealed the levels to be identical in the mother tree and its in vitro progeny. Moreover, among 100 microsatellite alleles, a difference of only one allele size was registered in only one plantlet over 150 samples studied (Zehdi-Azouzi 2005). Similar results from somatic embryogenesis have also been reported in date palm (Cohen et al. 2004; Sharma et al. 1986) and in other crops such as Norway spruce (Heinze and Schmidt 1995), conifers (Tautorus et al. 1991), Hevea brasiliensis (Michaux-Ferrière et al. 1992), and cereals (Vasil 1995).
Induction of somatic embryogenesis and plant regeneration from leaf explants of date palm cv. Deglet Nour. a Embryogenic callus within proembryogenic globular structures obtained after 6-month culture period on Murashige-Skoog (MS) medium including 1 mg l−1 2,4-D (M2). b Direct embryogenesis at the basic part of a juvenile leaf cultured on MS medium including 1 mg l−1 2,4-D for 6 months of culture. c Initiation of differentiation of embryogenic callus 1 month after transfer to MS medium supplemented with 0.1 mg l−1 2,4-D. d Matured somatic embryos obtained after 10 weeks of transfer of differentiated embryogenic callus on MS medium lacking 2,4-D. e Hardened plantlets with full radicle and shoot obtained after 3 months of transfer to half-strength MS liquid medium supplemented with 1 mg l−1 indole-3-butyric acid (IBA). f Potted plantlets 3 months after transfer to a greenhouse. g Two-year-old plants after transfer to free-living conditions. Bars a, c 10 mm; b 5 mm; d 20 mm; e 15 mm; f 100 mm; g 300 mm (from Othmani et al. 2009a)
6 Conclusions
Date palm - one of the oldest domesticated fruit crops - is the tree most adapted to growing in desert areas. It has always been looked upon as a key source of stability, survival and evolution in oasis agro-systems as it constitutes the basic features of the ecological pyramid in desert regions. Ecotypes are well suited to various usages, as locally known cultivars. All over the world, date palm germplasms are characterised by the presence of a large number of cultivars (Djerbi 1985; Rizvi and Davis 1983; Ben Khalifa 1996; Sedra 1996). In early periods, germplasm was characterised using classical morphometric and vegetative criteria. Despite their usefulness of the latter methods in the establishment of phenotypic divergence in the cultivars studied, in order to set up a catalogue of the most important date palm cultivars, both in North African and other producing countries, analytic fruit parameters and isozyme markers were used. However, taking advantage of the large panel of DNA-based markers developed in the last two decades, investigations have focussed on identifying DNA markers suitable for fingerprinting of date palm cultivars and/or for varietal identification, as well as for the survey of the genetic organisation of this crop. Data have clearly shown that among the procedures currently available, PCR-based methods are the best suited to surveying genetic variation and to precisely characterise cultivars in this crop. Moreover, among these markers, microsatellites (SSRs) are ideal for the unambiguous differentiation between cultivars via multilocus genotyping.
In addition, taking into account the mode of propagation, preservation of date palm biodiversity has been made possible through in vitro multiplication methods by providing attractive strategies for mass propagation of fruity and/or endangered cultivars. As a result, the in vitro mass propagation of several Tunisian date palm elite and/or fruity endangered cultivars has been successfully achieved (e.g. Deglet nour, Deglet bey, Boufaggous, Gondi). Furthermore, PCR-based methods have demonstrated that somaclonal variation did not occur even after cultivation of date palm cells on medium containing 2,4-D, which is known to induce such variations by chromosome aberrations.
It is obviously necessary to extend the use of in vitro methods to many other local and/or introduced cultivars in order to enhance date palm cultivation and to improve biodiversity in local germplasms. In addition, based on SSR evidence, it will be easily possible to fingerprint any cultivar from anywhere in the world.
References
Al Khalifah NS, Askari E (2003) Molecular phylogeny of date palm (Phoenix dactylifera L.) cultivars from Saudi Arabia. Theor Appl Genet 107:1266–1270
Belguedj M (2002) Les ressources génétiques du palmier dattier, caractéristiques des cultivars de dattiers dans les palmeraies du Sud-Est Algérien. Institut National de la Recherche Agronomique d'Algérie, pp 289
Ben Abdallah A, Stiti K, Lepoivre P, Du Jardin P (2000) Identification de cultivars de palmier dattier (Phoenix dactylifera L.) par l'amplification aléatoire d'ADN (RAPD). Cah Agric 9:103–10
Ben Khalifa A (1996) Diversity of date palm (Phoenix dactylifera L.). Options Méditerranéennes 28:160
Bennaceur M, Lanaud C, Chevalier MH, Bounaga N (1991) Genetic diversity of the date palm (Phoenix dactylifera L.) from Algeria revealed by enzyme markers. Plant Breed 107:56–69
Booij L, Montfort S, Ferry M (1995) Characterization of thirteen date palm (Phoenix dactylifera L.) cultivars by enzyme electrophoresis using the Phast-System. J Plant Physiol 145:62–66
Bouabidi H, Reynes M, Rouissi MB (1996) Critères de caractérisation des fruits de quelques cultivars de palmier dattier (Phoenix dactylifera L.) du sud tunisien. Ann Inst Rech Agric Tunisie 69:73–87
Chevalier A (1952) Recherches sur les Phoenix africains. Rev Int Bot Appl Agric Trop 1952:355–356
Cohen R, Korchinsky R, Tripler E (2004) Flower abnormalities cause abnormal fruit setting in tissue culture propagated date palm (Phoenix dactylifera L.). J Hortic Sci Biotechnol 79:1007–1013
Cornicquel B, Mercier L (1994) Date palm (Phoenix dactylifera L.) cultivar identification by RFLP and RAPD. J Plant Sci 101:163–172
Cornicquel B, Mercier L (1997) Identification of date palm (Phoenix dactylifera L.) cultivars by RFLP: partial characterization of a cDNA probe that contains a sequence encoding a zinc finger motif. Int J Plant Sci 158:152–156
Djerbi M (1985) Précis de Phoéniciculture. FAO, Rome, Italy
Dowson VHW (1982) Date production and protection with special reference to North Africa and the Near East. FAO Technical Bulletin 35:294
Dransfield J, Uhl NW (1986) An outline of a classification of palms. Principes 30:3–11
Drira N, Benbadis A (1985) Multiplication végétative du palmier dattier (Phoenix dactylifera L.) par réversion, en culture in vitro d'ébauches florales de pieds femelles adultes. J Plant Physiol 119:227–235
El Assar A, Krueger RR, Devanand PS, Chao CCT (2005) Genetic analysis of Egyptian date (Phoenix dactylifera L.) accessions using AFLP markers. Genet Resour Crop Evol 52:601–607
El Hadrami I (1995) L'embryogenèse somatique chez Phoenix dactylifera L.: quelques facteurs limitants et marqueurs biochimiques. PhD Thesis, Université Cadi Ayyad, Marakech, Morocco
El Houmaizi MA, Devanand PS, Fang J, Chao CT (2006) Confirmation of ‘Medjool’ date as a landrace variety through genetic analysis of ‘Medjool’ accessions in Morocco. J Am Soc Hortic Sci 131:403–407
Fki L, Masmoudi R, Drira N, Rival A (2003) An optimised protocol for plant regeneration from embryogenic suspension cultures of date palm, Phoenix dactylifera L., cv. Deglet Nour. Plant Cell Rep 21:517–524
Haddouch M (1996) Situation actuelle et perspectives de développement du palmier dattier au Maroc. Cah Options Méditerranéennes 28:63–79
Heinze B, Schmidt J (1995) Monitoring genetic fidelity vs. somaclonal variation in Norway spruce (Picea abies) somatic embryogenesis by RAPD analysis. Euphytica 85:341–345
Hodel DR, Johnson DV (2007) Imported and American varieties of dates in the United States. University of California. Agriculture and Natural Resources, pp 112
Hussein A, Adawy S, Ismail EME, El-Itriby A (2005) Molecular characterization of some Egyptian date palm germplasm using RAPD and ISSR markers. Arab J Biotechnol 8:83–98
Kresovich S, Szewc-McFadden AK, Bliek SM, McFerson JR (1995) Abundance and characterization of simple sequence repeats (SSRs) isolated from a size-fractionated genomic library of Brassica napus L. (rapeseed). Theor Appl Genet 91:206–211
Michaux-Ferrière N, Grout H, Carron MP (1992) Origin and ontogenesis of somatic embryos in Hevea brasiliensis (Euphorbiaceae). Am J Bot 79:174–180
Mohamed S, Shabana HR, Mawlod BA (1983) evaluation and identification of Iraqi date: fruit characteristics of fifty cultivars. Date Palm J 2:27–56
Munier P (1973) Le palmier dattier. Maisonneuve et Larose, Paris
Nixon RW, Carpenter B (1978) Growing date in the United States. US Dep Bull 207, pp 63
Othmani A, Bayoudh C, Drira N, Marrakchi M, Trifi M (2009a) Somatic embryogenesis and plant regeneration in date palm Phœnix dactylifera L., cv. Boufeggous is significantly improved by fine chopping and partial desiccation of embryogenic callus. Plant Cell Tissue Organ Cult 97:71–79
Othmani A, Bayoudh C, Drira N, Marrakchi M, Trifi M (2009b) Regeneration and molecular analysis of date palm (Phœnix dactylifera L.) plantlets using RAPD markers. Afr J Biotechnol 8:813–820
Ouazzani N, Lumaret R, Villemar P (1995) Apport du polymorphisme alloenzymatique à l'identification variétale de l'olivier (Oleae europeae L.). Agronomie 15:31–37
Ould Mohamed Salem A (2001) Contribution à l'évaluation des resources phytogénétiques du palmier dattier (Phoenix dactylifera L.) par analyse du polymorphisme isoenzymatique. PhD Thesis, Faculté des Sciences de Tunis, Université Tunis-El Manar
Ould Mohamed Salem A, Trifi M, Rhouma A, Marrakchi M (2001) Genetic inheritance of four enzymes in date-palm (Phoenix dactylifera L.). Genet Resour Crop Evol 48:361–368
Ould Mohamed Salem A, Rhouma S, Zehdi S, Marrakchi M, Trifi M (2007) Morphological variability of Mauritanian date-palm (Phoenix dactylifera L.) cultivars as revealed by vegetative traits. Acta Bot Croatica 67:81–90
Reynes M, Bouabidi H, Piombo G, Risterrucci AM (1994) Charactérisation des principales variétés des dattes cultivées dans la région de Tozeur. Fruits 49:289–298
Rhouma A (1994) Le palmier dattier en Tunisie. Le patrimoine génétique Vol I. Arabesques Editions et Créations, Tunis, Tunisie
Rhouma A (2005) Le palmier dattier en Tunisie. Le patrimoine génétique Vol II. IPGRI, Rome, Italy
Rhouma S (2008) Analyse de la diversité génétique chez le palmier dattier (Phoenix dactylifera L.) étude transcriptomique de la maladie des feuilles cassantes. PhD Thesis, Faculté des Sciences de Tunis, Université Tunis-El Manar
Rhouma S, Zehdi-Azouzi S, Ould Mohamed Salem A, Rhouma A, Marrakchi M, Trifi M (2008) Genetic diversity analysis in Tunisian date palm (Phoenix dactylifera L.) accessions by means of Random Amplified Microsatellite Polymorphisms (RAMPO). Sci Hortic 117:53–57
Rizvi M, Davis J (1983) Structural features of date market in Sind Pakistan. Date Palm J 2:103–122
Roder MS, Plaschke J, Konig SU, Börner A, Sorrells ME, Tanksley SD, Ganal MW (1995) Abundance, variability and chromosomal location of microsatellites in wheat. Mol Gen Genet 246:327–333
Roder MS, Korzun V, Wendehake K, Plaschke J, Tixier MH, Leroy P, Ganal MW (1998) A microsatellite map of wheat. Genetics 149:2007–2023
Sakka H (2003) Analyse de la diversité génétique chez le palmier dattier (Phoenix dactylifera L.): polymorphisme de l'AND chloroplastique. PhD Thesis, Universite?Tunis, El Manar, Tunis, Tunisie
Sakka H, Zehdi S, Ould Mohamed Salem A, Rhouma A, Marrakchi M, Trifi M (2004) Tunisian date-palm (Phoenix dactylifera L.) genotypes identification mediated by plastid PCR/RFLP based DNA. J Genet Breeding 57:259–264
Salhi-Hannachi A, Chatti K, Mars M, Marrakchi M, Trifi M (2005) Comparative analysis of genetic diversity in two collections figs cultivars based on random amplified polymorphic DNA and Inter Simple Sequence Repeats fingerprints. Genet Res Crop Evol 52:563–573
Sedra My H (1996) La palmeraie marocaine, caractéristique et potentialités. Options Méditerranéennes A 28:163
Sedra My H, Lashermes P, Trouslot P, Combes MC, Hamon S (1998) Identification and genetic diversity analysis of date palm (Phoenix dactylifera L.) varieties from Morocco using RAPD markers. Euphytica 103:75–82
Senior ML, Heun M (1998) Mapping maize microsatellites and polymerase chain reaction confirmation of the targeted repeats using a CT primer. Genome 36:884–889
Sharma DR, Deepak S, Chowdury JB (1986) Regeneration of plantlets from somatic tissues of the date palm. Indian J Exp Bot 24:763–766
Tautorus TE, Fowke LC, Dunstan DI (1991) Somatic embryogenesis in conifers. Can J For Res 69:1873–1899
Tautz D, Renz M. (1984) Simple sequences are ubiquitous repetitive components of eukaryotic genomes. Nucleic Acids Res 12: 4127–4138
Torres AM, Tisserat B (1980) Leaf isozymes as genetic markers in date palms. Am J Bot 67:162–167
Trifi M (2001) Polymorphisme moléculaire de variétés Tunisiennes de palmier dattier (Phoenix dactylifera L.: relation avec la résistance au bayoud. PhD Thesis, Faculté des Sciences de Tunis, Université Tunis-El Manar
Trifi M, Rhouma A, Marrakchi M (2000) Phylogenetic relationships in Tunisian date palm (Phoenix dactylifera L.) germplasm collection using DNA amplification fingerprinting. Agronomie 20:1–7
Vasil IK (1995) Cellular molecular genetic improvement of cereals. In: Terzi M, Cell R, Falavigna A (eds) Current issues in plant molecular and cellular biology. Kluwer, Dordrecht, pp 5–18
Vos P, Hogers R, Bleeker M, Reijans M, van de Lee T, Hornes M, Frijters A, Pot J, Peleman J, Kuiper M, Zabeau M (1995) AFLP: a new technique for DNA fingerprinting. Nucleic Acids Res 23:4407–4414
Williams JGK, Kubelik AR, Livak KJ, Rafalski JA (1990) DNA polymorphisms amplified by arbitrary primers are useful as genetic markers. Nucleic Acids Res 18:6531–6535
Wrigley G (1995) Date-palm (Phoenix dactylifera L.). In: Smartt J, Simmonds NW (eds) The evolution of crop plants, 2nd edn. Longman, Essex, pp 399–403
Zehdi S, Trifi M, Ould Mohamed Salem A, Rhouma A, Marrakchi M (2002) Survey of inter simple sequence repeat (ISSR) in Tunisian date-palms (Phoenix dactylifera L.). J Genet Breed 56:77–83
Zehdi S, Trifi M, Billotte N, Marrakchi M, Pintaud JC (2004) Genetic diversity of Tunisian date palms (Phoenix dactylifera L.) revealed by nuclear microsatellite polymorphism. Hereditas 141:278–287
Zehdi-Azouzi S (2005) Analyse moléculaire de la diversité génétique chez le palmier dattier (Phoenix dactylifera L.). PhD Thesis, Faculté des Sciences de Tunis, Université Tunis-El Manar
Zohary D, Spiegel-Roy P (1975) Beginning of fruit growing in the Old World. Science 187:319–327
Acknowledgements
This work was partially supported by grants from the IPGRI “CRRAO Degache, and the Tunisian ‘Ministère de l’Enseignement Supérieur, de la Recherche Scientifique et de la technologie' “project Lab B02” This paper is dedicated to Professor Mohamed Marrakchi, Director of the Lab B02 and promoter of this work, who succumbed in April 2008 during the investigation and evaluation process of his laboratory research activities.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2010 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Rhouma, S. et al. (2010). Genetic Variation in the Tunisian Date Palm (Phoenix dactylifera L.). In: Ramawat, K. (eds) Desert Plants. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-02550-1_17
Download citation
DOI: https://doi.org/10.1007/978-3-642-02550-1_17
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-02549-5
Online ISBN: 978-3-642-02550-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)