Abstract
The control of pollen fertility is central to the production of F1-hybrid seed in self-pollinating crops, and is potentially applicable to the containment of transgenes deployed in crop plants. Pollen sterility can be achieved through cytoplasmic male sterility (CMS) encoded by the plant mitochondrial genome, or through genic male sterility encoded by the nuclear genome. Both routes have been exploited in schemes for hybrid seed production. Recently, pollen sterility has been achieved through novel strategies involving nuclear or plastid transgenes. Here we review the applications of pollen sterility, and the genetic systems used for the control of pollen fertility.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
The control of pollen fertility is central to the production of F1-hybrid seed in self-pollinating crops, and is potentially applicable to the containment of transgenes deployed in crop plants. Pollen sterility can be achieved through cytoplasmic male sterility (CMS) encoded by the plant mitochondrial genome, or through genic male sterility encoded by the nuclear genome. Both routes have been exploited in schemes for hybrid seed production. Recently, pollen sterility has been achieved through novel strategies involving nuclear or plastid transgenes. Here we review the applications of pollen sterility, and the genetic systems used for the control of pollen fertility.
2 Applications of Pollen Sterility
While F1-hybrid seed production has been the primary driving force behind strategies to achieve pollen sterility, the control of pollen function also plays a role in other valued phenotypes. Furthermore, the expanding plantings of transgenic crops have created interest in the use of pollen sterility to limit the flow of transgenes to nearby native plants and non-transgenic crops.
2.1 Hybrid Seed Production
Hybrid vigor (or heterosis), uniformity, broad adaptation, and variety protection are desired features of crop plants that can be achieved through the use of F1-hybrid varieties (Duvick 1959, 2001; Perez-Prat and van Lookeren Campagne 2002; Virmani et al. 2003; Troyer 2006; Springer and Stupar 2007; Cheng et al. 2007). In the case of self-pollinating crops, uniform populations of pollen-sterile plants must be created, to then be cross pollinated for the production of F1-hybrid seed.
2.1.1 Hybrid Seeds via Cytoplasmic Male Sterility
In hybrid seed production, pollen sterility is often achieved through the use of CMS (Duvick 1959; Budar and Pelletier 2001; Havey 2004; Pelletier and Budar 2007; Cheng et al. 2007). Maternally inherited CMS results from genes in the plant mitochondrial genome (reviewed in Budar and Pelletier 2001; Hanson and Bentolila 2004; Chase 2007). These genes do not segregate, but expression of the CMS trait can be conveniently controlled by the presence or absence of nuclear restorer-of-fertility (restorer) alleles (reviewed in Schnable and Wise 1998; Wise and Pring 2002; Hanson and Bentolila 2004; Chase 2007; Pelletier and Budar 2007). Uniform populations of pollen-sterile plants are developed by crossing CMS plants with normal-cytoplasm, pollen-fertile plants that do not carry nuclear restorer alleles. The resulting pollen-sterile progeny produce F1-hybrid seed via cross fertilization with pollen-fertile plants grown nearby. Pollen fertility may or may not be restored in the F1 generation. If the F1 seed is grown to produce vegetative structures such as roots, tubers, or leaves, pollen fertility need not be restored in the F1 generation (Duvick 1959; Havey 2004). In the case of hybrid crops grown for a seed product, pollen fertility is achieved in the F1 generation via nuclear restorer alleles introduced in the previous generation (Duvick 1959; Havey 2004). Alternatively, pollen-sterile F1 hybrids can be grown along with male-fertile pollinators. In crops such as maize, F1-hybrid seed can be produced through the use of CMS or through hand emasculation (Duvick 1959). CMS (pollen-sterile) and normal-cytoplasm (pollen-fertile) versions of the same hybrid can be produced and blended for planting (Stamp et al. 2000; Weingartner et al. 2002a, b; Havey 2004).
In some cases, CMS confers a yield advantage independent of heterosis. Pollen-sterile CMS maize hybrids, fertilized with isonuclear pollen-fertile hybrids, yield more grain than do isonuclear pollen-fertile hybrids (Stamp et al. 2000). The Plus-Hybrid system combines CMS and a xenia effect to create a further yield advantage when pollen-sterile CMS hybrids are crossed with unrelated pollinators (Weingartner et al. 2002a, b). CMS lines of wheat out-yield isonuclear lines produced through use of chemical sterilizing agents (Adugna et al. 2004). Work in sorghum (Moran and Rooney 2003) and millet (Chandra-Shekara et al. 2007) demonstrates genetic variation of male sterility-inducing cytoplasms with respect to yield.
CMS is used for hybrid seed production in many crops, including alfalfa, onion, sugar and table beets, oilseed and vegetable brassicas, maize, millet, rice, pepper, rye, sorghum, sunflower, and wheat (Duvick 1959; Havey 2004; Adugna et al. 2004; Cheng et al. 2007). T-cytoplasm maize carries an unusual CMS gene that also conditions susceptibility to the fungal pathogen Bipolaris maydis (reviewed in Pring and Lonsdale 1989; Wise et al. 1999). Although this caused concerns regarding the use of CMS for crop production, CMS genes are diverse and work by different mechanisms (Schnable and Wise 1998; Hanson and Bentolila 2004; Linke and Borner 2005; Chase 2007; Carlsson et al. 2008). Hence, undesirable phenotypes are not necessarily expected to result from every application of CMS in hybrid crop production.
2.1.2 Hybrid Seeds via Nuclear Male Sterility
In contrast to CMS, pollen sterility conditioned by dominant or recessive nuclear mutations is not true-breeding. The male-sterile plants must be propagated through pollination with wild-type, male-fertile plants. In some cases, this problem can be circumvented. Genic male-sterile leek plants are propagated asexually, and then cross pollinated to produce hybrid seed (Smith and Crowther 1995; Havey 2004). In China, considerable hybrid rice seed is developed through environmental genic male sterility systems. In photo-thermo genic male sterility, the seed parent line is amplified under environmental conditions that cause this line to be pollen-fertile (cool temperatures and shorter photoperiod). For the production of F1-hybrid seed, the seed parent is grown and cross-fertilized under environmental conditions that cause pollen sterility (warmer temperatures and longer day-length; Virmani et al. 2003; Cheng et al. 2007). Female parent propagation has been described as the key limiting factor for the production of hybrids in many crops (Perez-Prat and van Lookeren Campagne 2002). Asexual propagation is labor-intensive, and environmental factors are not always uniform enough to ensure complete pollen sterility. Novel strategies to address these challenges via the deployment of nuclear transgenes are described below.
2.2 Value-Added Traits
The control of pollen fertility has implications for plant improvement beyond hybrid seed production. Pollen sterility, combined with parthenocarpy, results in the production of desirable seedless fruits (Possingham 1998; Schijlen et al. 2007). Pollen is an allergen of significant importance (Goldberg et al. 1998; Mahillon et al. 2006), and pollination induces the rapid senescence of floral organs in many species (Rubinstein 2000; Rogers 2006). Hence, ornamental species might be improved through control of pollen fertility (Daniell 2002).
2.3 Transgene Containment
The expansion of transgenic crops has raised concerns about the flow of transgenes to nearby cross-compatible crops and native plants (Daniell 2002; Chapman and Burke 2006; Lee and Natesan 2006; Brunner et al. 2007). Many factors influence transgene flow, including dispersal via seeds, pollen, or vegetative propagules (Brunner et al. 2007), and the fitness of the plants resulting from genetic exchange with the transgenic crop (Chapman and Burke 2006; Lee and Natesan 2006). No single strategy of containment will be 100% effective in any situation, and the best combined approaches must be determined on a case-by-case basis (Chapman and Burke 2006; Lee and Natesan 2006). Pollen sterility can make a significant contribution toward transgene containment (Brunner et al. 2007). This strategy is suited to species amenable to vegetative propagation, and has been applied to creeping bentgrass (Luo et al. 2005). The maize Plus-Hybrid system can also be adapted to limit transgene flow, through use of a pollen-sterile transgenic hybrid and a non-transgenic pollinator (Feil et al. 2003). Transgene containment in F1 hybrids of seed crops is also possible by crossing a homozygous male-sterile tobacco line, produced by metabolic engineering of glutamine, with a homozygous male-fertile line. The resulting heterozygous F1 plants produce 50% fertile pollen, and all of the fertile pollen is non-transgenic (Ribarits et al. 2007; see below).
3 Cytoplasmic Male Sterility Systems
CMS is observed in a wide array of plant species that exhibit diversity with respect to the causal gene and its phenotypic effects. Although the maternally inherited mitochondrial genome encodes CMS, nuclear restorer alleles regulate the expression of this trait (Schnable and Wise 1998; Wise and Pring 2002; Hanson and Bentolila 2004; Chase 2007).
3.1 CMS Genes
Most CMS genes are mosaic open reading frames (orfs) that include segments from normal mitochondrial gene coding and flanking sequences, and segments of unknown origin (Schnable and Wise 1998; Hanson and Bentolila 2004; Linke and Borner 2005; Kubo and Newton 2008). These orfs, when fused to plant mitochondrial promoters or co-transcribed courtesy of upstream mitochondrial genes (Hanson and Bentolila 2004), become gain-of-function mutations. Recombination in plant mitochondrial genomes likely favors the creation of CMS genes, and places these in contexts that support gene expression (Sandhu et al. 2007; Kubo and Newton 2008). Functional copies of all essential mitochondrial genes are almost always retained in CMS mutants. Two examples of CMS involving essential mitochondrial respiratory genes, deletion of the NADH dehydrogenase subunit 7 gene in Nicotiana sylvestris (Pla et al. 2005), and truncation of the cytochrome oxidase subunit 2 gene in wild beet (Ducos et al. 2001), seem not to condition deleterious vegetative phenotypes. Perhaps alternative pathways of electron flow in plant mitochondria (Rasmusson et al. 2004; Rhoades and Subbaiah 2007; Noctor et al. 2007) compensate for the effects of these mutations.
3.2 CMS Phenotypes
CMS genes condition a wide array of reproductive abnormalities (Laser and Lersten 1972; Schnable and Wise 1998; Zubko 2004; Hanson and Bentolila 2004; Linke and Borner 2005; Chase 2007; Carlsson et al. 2008). In some cases, male reproductive organs (stamens) are transformed into petals or into female reproductive organs (carpels). Other CMS mutations condition degeneration of anthers and/or developing pollen.
3.2.1 Homeotic CMS
CMS involving the transformation of stamens to petals (petaloid) or stamens to carpels (carpeloid; Zubko 2004; Linke and Borner 2005; Carlsson et al. 2008) copies the phenotypes conditioned by mutations in nuclear floral organ identity genes (Coen and Meyerowitz 1991; Krizek and Fletcher 2005). In dicotyledonous plants, four whorls of plant floral organs (sepals, petals, stamens, and carpels) develop in response to spatial patterns established by three major classes of floral organ identity genes (A, B, and C). These encode MADS box transcription factors having distinct domains of expression that overlap, in part, to specify sepals (A alone), petals (A and B), stamens (B and C), and carpels (C alone). Decreased accumulation of B-class MADS box transcription factors occurs in several carpeloid CMS systems (Murai et al. 2002; Linke et al. 2003; Zubko 2004; Teixeira et al. 2005). Unusually, late expression of B-class genes is associated with abnormal anther and pollen development of CMS B. napus of the Napus (nap) type (Geddy et al. 2005). Nuclear MADs box genes are therefore targets of mitochondrial influence. Further study of homeotic CMS will enhance our understanding of retrograde (mitochondria-to-nucleus) signaling pathways in plants.
3.2.2 Degenerative CMS
In CMS sunflower, anther tissues exhibit features of apoptotic programmed cell death (PCD) observed in vertebrates, namely, the release of cytochrome c from the mitochondria, cleavage of nuclear DNA, and condensation of cytoplasm containing intact organelles (Balk and Leaver 2001). Declining ATP levels promote PCD in animal cells (Bras et al. 2005), and perhaps in sunflower, where the CMS gene encodes a protein related to mitochondrial ATP synthase subunit 8. Abundance of the ATP synthase complex is reduced in CMS compared to male-fertile sunflower (Sabar et al. 2003). The identity and targets of mitochondrial cell death signals in plants are not yet clear, as the caspase proteases, primary downstream target of mitochondria-signaled PCD in animals, are not conserved in plants (Jones 2000; Lam 2004; Logan 2006).
When ATP levels are insufficient to support PCD, cell death can occur by necrosis (Bras et al. 2005). In CMS-T maize plants, the tapetal cells lining the anther exhibit features of necrotic cell death, including the swelling and lysis of organelles and cells (Warmke and Lee 1977). In vegetative tissues, the CMS-T gene product (URF13) forms a mitochondrial pore upon exposure to a toxin produced by Bipolaris maydis (reviewed in Wise et al. 1999). In tapetal cells, this pore might form in the absence of toxin, leading to mitochondrial dysfunction and necrosis (Flavell 1974).
3.2.3 Male Specificity
In many cases, CMS gene products accumulate throughout the plant, but condition a phenotype only in male reproductive organs (Dewey et al. 1987; Wise et al. 1987; Nivison and Hanson 1989; Laver et al. 1991; Krishnasamy and Makaroff 1994). Warmke and Lee (1978) observed that a 20- to 40-fold increase in the number of mitochondria accompanies the normal development of tapetal cells and pollen within the anther. They proposed that CMS mutations compromise the energy status of tapetum and/or developing pollen. Energy considerations might explain both homeotic and degenerative CMS phenotypes, if the proper regulation of nuclear floral organ identity genes requires ATP-dependent proteolysis (Teixeira et al. 2005), and if declining levels of ATP trigger mitochondria-signaled PCD in anther tissues (Sabar et al. 2003). Alternatively, positive regulators of cell death produced by anthers or developing pollen (Flavell 1974) might induce or act synergistically with mitochondrial cell death signals, or negative regulators might repress these pathways in all but male reproductive organs. The large number of mitochondria in male reproductive organs (Warmke and Lee 1978) could amplify such mitochondrial signaling pathways.
3.3 Fertility Restoration
Expression of CMS can be suppressed by system-specific nuclear restorer alleles. The molecular cloning of several restorer genes identified pentatricopeptide repeat (PPR) protein coding genes in all but one case (reviewed in Schanble and Wise 1998; Wise and Pring 2002; Hanson and Bentolila 2004; Chase 2007).
3.3.1 Fertility Restoration Genetics
Nuclear fertility restoration systems can be sporophytic, acting in the diploid plant, or gametophytic, acting in the haploid pollen. In sporophytic restoration, all pollen produced by a CMS plant heterozygous for a dominant restoring allele (CMS Rf/rf) will function regardless of pollen genotype. In gametophytic restoration, only pollen carrying the restoring allele will function. Some fertility restoration systems require the complementary action of restoring alleles at two loci (Pring et al. 1999; Wise et al. 1999), and some CMS systems can be reversed through the action of two or more independent restoration systems (Zabala et al. 1997; Sarria et al. 1999; Wang et al. 2006).
3.3.2 Fertility Restoration Mechanisms
In many cases, mitochondria-encoded CMS gene products fail to accumulate in the presence of a nuclear restorer allele (Dewey et al. 1987; Nivison and Hanson 1989; Krishnasamy and Makaroff 1994; Moneger et al. 1994; Abad et al. 1995; Wang et al. 2006). The decreased abundance or internal cleavage of CMS transcripts accompanies this protein loss in some (Dewey et al. 1987; Pruitt and Hanson 1991; Moneger et al. 1994; Wang et al. 2006) but not all cases (Chase 1994; Krishnasamy and Makaroff 1994). It is not known whether these transcript effects are the basis for the protein phenotype, or a secondary consequence of failure to translate a CMS transcript.
3.3.3 Fertility Restoration Genes
Rf2 of CMS-T maize encodes a mitochondrial aldehyde dehydrogenase (Cui et al. 1996; Liu et al. 2001), and restores pollen fertility through a mechanism of metabolic compensation. All other restorer genes cloned to date encode PPR proteins (Bentolila et al. 2002; Kazama and Toriyama 2003; Brown et al. 2003; Desloire et al. 2003; Koizuka et al. 2003; Akagi et al. 2004; Komori et al. 2004; Klein et al. 2005; Wang et al. 2006), members of a highly expanded plant protein family (Lurin et al. 2004). PPR proteins are comprised largely of degenerate 35-amino acid repeats, which might confer sequence-specific RNA binding capability to mediate interactions between enzymes and RNA substrates (Lurin et al. 2004; Stern et al. 2004; Shikanai 2006; Delannoy et al. 2007). In Arabidopsis, 54% of the PPR proteins are predicted to be located in the mitochondria, and 19% are predicted to locate in the plastids (Lurin et al. 2004). PPR proteins function in the translation (Schmitz-Linneweber et al. 2005), editing (Kotera et al. 2005; Okuda et al. 2007), processing and splicing (Schmitz-Linneweber et al. 2006; Hattori et al. 2007) of plastid RNAs, and likely perform similar roles in the expression of normal plant mitochondrial genes. Restorer genes encoding PPR proteins might have evolved through duplication of genes encoding PPR proteins essential to normal mitochondrial gene expression, followed by divergence of mitochondrial targets (Touzet and Budar 2004; Geddy and Brown 2007).
3.4 Transgenic Approaches to CMS
Novel transgenic strategies have recently been applied to induce CMS. One strategy exploits genetic transformation of maternally inherited plastid genomes. Another modifies mitochondrial genome organization via nuclear genome transformation.
3.4.1 CMS via Plastid Transgenes
There is currently no technology for the genetic transformation of plant mitochondrial genomes (Pelletier and Budar 2007), but the genetic transformation of plastid genomes provides a route to engineering CMS. Ruiz and Daniell (2005) transformed the tobacco plastid genome with a bacterial gene (phaA) encoding the enzyme β-ketothiolase. In plants expressing a plastid phaA gene, tapetal cells undergo premature degeneration, anthers are misshapen, and pollen is collapsed. These plants are female-fertile, producing uniform pollen-sterile progenies after fertilization with wild-type pollen. β-ketothiolase might disrupt fatty acid metabolism critical to tapetal cell function and pollen development. Tapetal cell abnormalities often appear to be the basis of male sterility in mitochondrial CMS systems (reviewed in Hanson and Bentolila 2004; Chase 2007). Full restoration of male fertility has not been achieved for CMS conditioned by plastid-expressed phaA, but this example lays the groundwork for plastid-based CMS. While plastid transformation is not routine in all crops, advances have been made in some plants (Daniell et al. 2005; Okumura et al. 2006; C.W. Liu et al. 2007; Wurbs et al. 2007). It must be remembered, however, that plastid inheritance is paternal or bi-parental in a number of plant species (Hagemann 2004).
3.4.2 CMS via Nuclear Transgenes
The plant mitochondrial genome has a complex, multipartite structure that is under the control of nuclear genes (reviewed in Kubo and Newton 2008). The Arabidopsis nuclear gene MSH1 encodes an organelle-targeted homolog of the Escherichia coli mismatch repair protein MutS. Mutations in MSH1 condition mitochondrial genome rearrangements (Abdelnoor et al. 2003). The down-regulation of tobacco and tomato MSH1 genes by an RNA interference (RNAi) transgene conditions mitochondrial genome rearrangements associated with male sterility. Male-sterile phenotypes are maternally inherited, and genetically stable following removal of the inducing RNAi construct via Mendelian segregation (Sandhu et al. 2007). This strategy potentially allows for the development of stable non-transgenic CMS systems in any plant amenable to genetic transformation. It remains to be seen whether conventional genetic screens will identify fertility restoration genes for these novel CMS systems.
4 Nuclear-Encoded Male Sterility Systems
4.1 Nuclear Male Sterility Genes
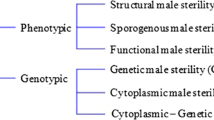
Natural nuclear mutations that impair pollen and anther development, but do not disturb female reproductive development, have been described in more than 175 plant species (Kaul 1988). Male-sterile mutants can be defined as structural (physical barriers preventing successful self-fertilization), sporogenous (pollen abortion), and functional (failure of anther dehiscence). In addition, various induced mutant approaches have been exploited to disrupt the complex network of developmental pathways involved in pollen development, anther dehiscence, and pollen germination (Chaudhury 1993; Williams 1995; Horner and Palmer 1995; McCormick 2004; X. Liu et al. 2007). Many such mutants have been evaluated for their adaptability with respect to F1-hybrid breeding, and are or have been used in breeding programs (Horner and Palmer 1995; Budar and Pelletier 2001; Perez-Prat and van Lookeren Campagne 2002; Atanassova 2007). They are referred to as genic male sterility (GMS) or nuclear male sterility (NMS) systems. Of practical importance is the environmental GMS found in rice and other crops (Ku et al. 2001; S. Li et al. 2007) that depends on stimuli like temperature or photoperiod, and has been used to develop hybrid rice varieties in the past.
Although nuclear male-sterile plants are valuable in elucidating pollen development, their application in breeding programs is limited, as no homozygous male-sterile populations can be produced (Williams 1995). In most systems, male-sterile plants must be propagated through pollination with male-fertile plants, which leads to segregation in the progeny and hampers the maintenance of the female (i.e., male-sterile) parent (Perez-Prat and van Lookeren Campagne 2002). In a few cases, both a male sterility gene and a restorer gene are available, and three-line systems of F1-hybrid seed production have been applied to create 100% male-sterile populations (Lu et al. 2004), but the complexity of genotypes involved requires tremendous efforts. In contrast, transgenes offer virtually unlimited options to create male sterility traits, and several transgenic nuclear male sterility systems targeting pollen have been developed.
As many crop species are now amenable to nuclear transformation, genetically engineering nuclear male sterility is an important alternative to CMS and non-GMO NMS. To overcome the limitations of NMS, in particular the problem of segregation of the male sterility trait, numerous strategies have been devised to deploy and regulate nuclear genes for the purposes of creating and propagating uniformly pollen-sterile plant populations. Reversible male sterility (also called conditional male fertility) systems avoid the elimination of segregating male-fertile plants. Moreover, a novel technology adds the creation of homozygous male-sterile plants to reversible male sterility.
4.2 Genetically Engineered Nuclear Male Sterility
Due to the remarkable complexity of reproductive development (McCormick 2004; Scott et al. 2004), numerous approaches have been tried to induce nuclear male sterility. Male sterility phenotypes, i.e., abortion of pollen, can occur at different stages during pollen development, ranging from meiosis until dehiscence or pollen germination (Chaudhury 1993; Williams 1995; Perez-Prat and van Lookeren-Campagne 2002; Gleba et al. 2004). Both engineering natural plant genes and introducing genes from other sources, e.g., fungi and bacteria, have led to male sterility (Williams 1995; Budar and Pelletier 2001; Perez-Prat and van Lookeren-Campagne 2002; Gleba et al. 2004).
4.2.1 Restoration and Elimination of Segregating Male-Fertile Plants
The tapetum surrounds the developing pollen until first pollen mitosis, and plays a crucial role in the formation of microspores (Wilson and Yang 2004). Premature degeneration of the tapetal cells inevitably leads to pollen abortion. Severe defects in pollen development have been observed when toxic genes like barnase or diphtheria toxin A were transferred into plants under control of cell type-specific promoters (Mariani et al. 1990; Guerineau et al. 2003; Liu and Liu 2008). Similarly, when the tobacco-derived TA29 promoter (Koltunow et al. 1990) was silenced in transgenic tobacco by RNAi, the tapetal cells degenerated and pollen aborted (Nawaz-ul-Rehman et al. 2007). Targeting the tapetum allowed for the development of the only commercialized transgenic male sterility, SeedLink™, used in canola and based on the work of Mariani et al. (1990). Pollen sterility resulting from the selective destruction of tapetal cells in transgenic tobacco and canola plants was engineered by the tapetum-specific expression of a natural cytotoxic ribonuclease, the barnase gene of Bacillus amyloliquefaciens under control of the TA29 promoter.
SeedLink™ sterility was reversed when the male-sterile plants were crossed with transgenic male-fertile plants expressing the barnase inhibitor barstar, which protected the tapetal cells from degradation via barnase-barstar protein complex formation (Mariani et al. 1992). To enhance the expression of the restorer (barstar) gene in male-sterile TA29-barnase lines of Brassica juncea, two promoters (TA29 from tobacco, and A9 from Arabidopsis thaliana) were used to express, and thereby extend, the temporal expression of two independent wild-type and codon-modified transcriptional units of barstar (Bisht et al. 2004). The two different versions of the barstar gene presumably also minimized co-suppression effects.
The barnase/barstar F1-hybrid technology was faced with the general problem inherent in NMS, i.e., segregation of male-sterile and male-fertile plants in maintenance breeding of the male-sterile line. Linking the barnase gene to the bialaphos resistance (bar; Reynaerts et al. 1993) or mutated acetolactate synthase (ALS; Ray et al. 2007) herbicide resistance genes allowed to eliminate male-fertile plants by herbicide treatments. Still, excess seeds needed to be sown to avoid yield penalties.
Another strategy to remove unwanted fertile plants in barnase-mediated NMS was a two-component system based on independent transgenic tomato lines carrying inactive partial barnase peptides (Burgess et al. 2002). Expression of partial peptides alone did not affect pollen viability, allowing for the maintenance of inbred parents in a homozygous state by selfing. When the inbreds were crossed, barnase activity was reconstituted by complementation, and destroyed pollen in the progeny. A recent variation on this theme was a system that combined the barnase gene with mutated ALS genes (Gils et al. 2008). Male sterility and linked herbicide resistance resulted from the functional complementation of split barnase and ALS fragments (respectively) fused to trans-splicing inteins, and located on two homologous chromosomes. This system acts, by design, only in heterozygous plants that combine all elements. Plants lacking one of the elements, e.g., after hybridization with a wild-type inbred line, are pollen-fertile.
Inactivation of transcription factors has frequently led to male sterility (e.g., Preston et al. 2004), but practical application has been discussed in only in a few cases. The transcription factor AtMYB103 is essential to tapetum development in Arabidopsis. An insertion mutant disrupting the AtMYB103 gene led to premature vacuolation and degeneration of the tapetum, and complete disruption of pollen development (S.F. Li et al. 2007). Expression of the MYB103 gene by a strong anther-specific promoter successfully restored pollen fertility. Based on the occurrence of MYB103 homologs in important crop plants like rice, barley, and canola, the authors suggested the use of the system for F1-hybrid seed production. Over-expression of another transcription factor, MYB26, caused ectopic lignification that included the anther wall, causing failure of the anthers to dehisce (functional male sterility; Yang et al. 2007). Pollen was viable in the over-expression lines, and could be released mechanically from the anthers. Consequently, restoration was not required for the reversal of MYB26-mediated pollen sterility (Steiner-Lange et al. 2003; Yang et al. 2007).
4.2.2 Reversible Male Sterility
Pollen requires for its development nutrients and signals that are provided by the tapetum and other tissues of the anther. The nutritional requirements of developing pollen became evident when isolated microspores were able to develop into mature, fertile pollen under appropriate culture conditions (Benito Moreno et al. 1988; Tupý et al. 1991), and when pollen tube growth was enhanced upon addition of flavonols to in vitro matured pollen (Ylstra et al. 1992). Down-regulation or over-expression of enzymes involved in the synthesis of metabolites that are essential for pollen development offer numerous possibilities to engineer male sterility. Application of the missing nutrient should lead to restoration of pollen fertility (reversible male sterility).
Metabolic engineering of primary metabolites has targeted glutamine, carbohydrates, and pyruvate. The amino acid glutamine is essential for pollen development (Benito Moreno et al. 1988). Transferring into tobacco plants a gene driven by the tapetum-specific promoter TA29 and encoding a dominant-negative version of glutamine synthetase (dnGS) resulted in male sterility (Ribarits et al. 2007). Spraying plants with glutamine restored male fertility. Anthers of the male-sterile lines contained viable microspores, and restoration was also achieved by isolating the microspores, maturing these in vitro in the presence of glutamine, and using the in vitro matured pollen for pollination of emasculated flowers in situ (Touraev and Heberle-Bors 1999). A similar strategy has been tried in tobacco plants. Here, knocking down an extracellular invertase gene by an anti-sense construct resulted in carbohydrate deficiency of pollen (Goetz et al. 2001), but no seeds were obtained after pollination with pollen matured in vitro in a sugar-containing medium. Down-regulation of pyruvate dehydrogenase was used to mimic CMS, and led to hypertrophy and vacuolation of the tapetal cells (Yui et al. 2003). However, no restoration strategies were devised in this case.
Male sterility resulting from disruption of flavonoid biosynthesis by anti-sense suppression, co-suppression, or mutations (Taylor and Jorgensen 1992; van der Meer et al. 1992; Napoli et al. 1999) was restored when flavonols were applied manually during pollination (Mo et al. 1992; Ylstra et al. 1994), or by expressing the key enzyme in flavonoid biosynthesis, chalcone synthase (Napoli et al. 1999). Exploiting the metabolic similarities of stilbene synthase and chalcone synthetase, tapetum-specific expression of grapevine stilbene synthase was shown to cause male sterility in tobacco, which could partially be reversed by application of 4-coumarate, resveratrol, and flavonols (Fischer et al. 1997). Jasmonic acid biosynthesis is also essential for pollen development and anther dehiscence, and suppression of several enzymes involved in this pathway has been shown to lead to male sterility that can be reversed by the application of linolenic or jasmonic acid (McConn and Browse 1996; Stintzi and Browse 2000; Ishiguro et al. 2001; Park et al. 2002).
Impaired reception and signal transduction of hormones in pollen, and reversal by exogenous application of growth regulators such as kinetin, gibberellic, or jasmonic acid have also been employed to engineer male sterility (Huang et al. 2003; Al-Ahmad and Gressel 2005). No restoration, however, has been reported for tobacco lines carrying an over-expressed ethylene receptor (Ishimaru et al. 2006).
4.2.3 Induced Male Sterility
An alternative to reversible male sterility is induced male sterility. The strategy described by Kriete et al. (1996) involved transgenic tobacco plants expressing the bacterial argE gene specifically in the tapetum. When these plants were treated with the non-toxic compound N-acetyl-phosphinothricin, it was converted into the herbicide phosphinotricin, thereby destroying the tapetal cells. F1-hybrid breeding based on induced male sterility employs two male-fertile inbred lines, one of which is sprayed with N-acetyl-phosphinothricin for F1-hybrid seed production.
4.2.4 Homozygous Nuclear Male-Sterile Lines
An F1-hybrid seed production system that combines reversible male sterility with a general strategy to produce homozygous male-sterile plants is based on the metabolic engineering of glutamine, as described above (Ribarits et al. 2007). To overcome segregation of the nuclear TA29-dnGS gene in maintenance breeding of the male-sterile inbred line, viable microspores contained in its anthers were recovered and used to produce doubled haploid plants through in vitro microspore culture in the presence of glutamine (Touraev and Heberle-Bors 1999). Being 100% homozygous, these doubled haploid plants did not segregate into male-sterile and male-fertile plants in the offspring, but produced only male-sterile plants. Doubled haploids are widely used in plant breeding, for example, in barley, wheat, and canola, not only in selection and combination breeding, but also as inbred lines in F1-hybrid breeding (Forster et al. 2007).
F1 hybrids produced by this method were male-sterile, and could be used for crops in which the vegetative parts of the plants are used. To make this technology applicable also for F1-hybrid breeding in seed crops, the microspore-specific NTM19 promoter was employed to drive the dnGS gene. Transgenic plants containing one NTM19-dnGS locus were male-fertile, but 50% of the pollen (the pollen carrying NTM19-dnGS) was not viable. To achieve complete male sterility, viable microspores produced by these lines were isolated and cultured in vitro, in the presence of glutamine, to produce homozygous doubled haploid plants. Doubled haploid lines homozygous for the NTM19-dnGS gene were indeed male-sterile, and did not segregate into male-sterile and male-fertile plants in the offspring. To maintain the male-sterile line, plants were sprayed with glutamine, an innocuous chemical that restored pollen fertility. Crossing these lines with male-fertile wild-type lines produced heterozygous F1 lines that were male-fertile, due to the presence of 50% wild-type pollen.
In conclusion, this technology combined essential features of F1-hybrid breeding, i.e., a method to create inbred lines (doubled haploidy), a method to produce F1-hybrid seeds (male sterility), and a method to maintain nuclear male-sterile lines as homozygotes (fertility restoration by reversible sterility and doubled haploids).
5 Summary and Future Prospects
The past 15 years have seen a virtual explosion in attempts to engineer male sterility for the breeding of F1 hybrids. Breeders are today faced with a variety of options. Not all, however, apply to any given crop species, and some of the new published methods lack a complete strategy for F1-hybrid seed production. Given the choice between CMS and NMS, CMS provides the benefit that male-sterile lines can easily be propagated due to the maternal inheritance of mitochondrial and plastid genomes. CMS is, however, difficult and time-consuming to introduce in a new species, and even more so the corresponding restorer genes. NMS can more easily be introduced, particularly since today many crop species are amenable to nuclear transformation. In addition, the large number of genes expressed in pollen, and the availability of different molecular and genetic tools to engineer gene expression offer numerous possibilities to block pollen development, often in a conditional manner. However, NMS faces the problem of segregation during maintenance breeding. Yet, also for this problem recent developments have offered solutions. Given that most breeders today are familiar with GMOs and doubled haploids, F1-hybrid seed technologies that combine methods to create inbred lines by doubled haploidy, to produce F1-hybrid seeds by NMS, and to maintain nuclear male-sterile lines as homozygotes by fertility restoration through reversible sterility and doubled haploids seem to be a promising path to the future. Another is induced male sterility.
References
Abad AR, Mehrtens BJ, Mackenzie SA (1995) Specific expression in reproductive tissues and fate of a mitochondrial sterility-associated protein in cytoplasmic male-sterile bean. Plant Cell 7:271–285
Abdelnoor RV, Yule R, Elo A, Christensen AC, Meyer-Gauen G, Mackenzie SA (2003) Substoichiometric shifting in the plant mitochondrial genome is influenced by a gene homologous to MutS. Proc Natl Acad Sci USA 13:5968–5973
Adugna A, Nanda GS, Singh K, Bains NS (2004) A comparison of cytoplasmic and chemically-induced male sterility systems for hybrid seed production in wheat (Triticum aestivum L.). Euphytica 135:297–304
Akagi H, Nakamura A, Yokozeki-Misono Y, Inagaki A, Takahashi H, Mori K, Fujimura T (2004) Positional cloning of the rice Rf-1 gene, a restorer of BT-type cytoplasmic male sterility that encodes a mitochondria-targeting PPR protein. Theor Appl Genet 108:1449–1457
Al-Ahmad H, Gressel J (2005) Transgene containment using cytokinin-reversible male sterility in constitutive, gibberellic acid-insensitive (Δgai) transgenic tobacco. J Plant Growth Regul 24:19–27
Atanassova B (2007) Genic male sterility and its application in tomato (Lycopersicon esculentum Mill.) hybrid breeding and hybrid seed production. Acta Hort 729:45–51
Balk J, Leaver CJ (2001) The PET1-CMS mitochondrial mutation in sunflower is associated with premature programmed cell death and cytochrome c release. Plant Cell 13:1803–1818
Benito Moreno R, Macke F, Hauser M, Alwen A, Heberle-Bors E (1988) Sporophytes and male gametophytes from in vitro cultured, immature tobacco pollen. In: Cresti M, Jori P, Pacini E (eds) Sexual reproduction in higher plants. Springer, Berlin Heidelberg New York, pp 137–142
Bentolila S, Alfonso AA, Hanson MR (2002) A pentatricopeptide repeat-containing gene restores fertility to cytoplasmic male-sterile plants. Proc Natl Acad Sci USA 99:10887–10892
Bisht NC, Jagannath A, Gupta V, Burma PK, Pental D (2004) A two gene-two promoter system for enhanced expression of a restorer gene (barstar) and development of improved fertility restorer lines for hybrid seed production in crop plants. Mol Breed 14:120–144
Bras M, Queenan B, Susin SA (2005) Programmed cell death via mitochondria: different modes of dying. Biochemistry (Mosc) 70:231–239
Brown GG, Formanova N, Jin H, Warzachuk R, Dandy C, Pati P, Leforest M, Zhang J, Cheung W, Landry BS (2003) The radish Rfo restorer gene of Ogura cytoplasmic male sterility encodes a protein with multiple pentatricopeptide repeats. Plant J 35:262–272
Brunner AM, Li J, DiFazio SP, Shevchenko O, Montgomery BE, Mohamed R, Wei H, Ma C, Elias AA, VanWormer K, Strauss SH (2007) Genetic containment of forest plantations. Tree Genet Genomics 3:75–100
Budar F, Pelletier G (2001) Male sterility in plants: occurrence, determinism, significance and use. C R Acad Sci Paris 324:543–550
Burgess DG, Ralston EJ, Hanson WG, Heckert M, Ho M, Jenq T, Palys JM, Tang K, Gutterson N (2002) A novel, two-component system for cell lethality and its use in engineering nuclear male-sterility in plants. Plant J 31:113–125
Carlsson J, Leino M, Sohlberg J, Sundström JF, Glimelius K (2008) Mitochondrial regulation of flower development. Mitochondrion 8:74–86
Chandra-Shekara AC, Prasanna BM, Singh BB, Unnikrishnan KV, Seetharam A (2007) Effect of cytoplasm and cytoplasm-nuclear interaction on combining ability and heterosis for agronomic traits in pearl millet [Pennisetum glaucum (L) Br. R]. Euphytica 153:15–26
Chapman MA, Burke JM (2006) Letting the gene out of the bottle: the population genetics of genetically modified crops. New Phytol 170:429–443
Chase CD (1994) Expression of CMS-unique and flanking mitochondrial DNA sequences in Phaseolus vulgaris L. Curr Genet 25:245–251
Chase CD (2007) Cytoplasmic male sterility: a window to the world of plant mitochondrial-nuclear interactions. Trends Genet 23:81–90
Chaudhury AM (1993) Nuclear genes controlling male fertility. Plant Cell 5:1277–1283
Cheng S-H, Zhuang J-Y, Fan Y-Y, Du J-H, Cao L-Y (2007) Progress in research and development on hybrid rice: a super-domesticate in China. Ann Bot 100:959–966
Coen ES, Meyerowitz EM (1991) The war of the whorls: genetic interactions controlling flower development. Nature 353:31–37
Cui X, Wise RP, Schnable PS (1996) The rf2 nuclear restorer gene of male-sterile T-cytoplasm maize. Science 272:1334–1336
Daniell H (2002) Molecular strategies for gene containment in transgenic crops. Nature Biotechnol 20:581–586
Daniell H, Kumar S, Dufourmantel N (2005) Breakthrough in chloroplast genetic engineering of agronomically important crops. Trends Biotechnol 5:238–245
Delannoy E, Stanley WA, Bond CS, Small ID (2007) Pentatricopeptide repeat (PPR) proteins as sequence-specificity factors in post-transcriptional processes in organelles. Biochem Soc Trans 35:1643–1647
Desloire S, Gherbi H, Laloui W, Marhadour S, Clouet V, Cattolico L, Falentin C, Giancola S, Renard M, Budar F, Small I, Caboche M, Delourme R, Bendahmane A (2003) Identification of the fertility restoration locus, Rfo, in radish, as a member of the pentatricopeptide-repeat protein family. EMBO Rep 4:588–594
Dewey RE, Timothy DH, Levings III CS (1987) A mitochondrial protein associated with cytoplasmic male sterility in the T cytoplasm of maize. Proc Natl Acad Sci USA 84:5374–5378
Ducos E, Touzet P, Boutry M (2001) The male sterile G cytoplasm of wild beet displays modified mitochondrial respiratory complexes. Plant J 26:171–180
Duvick DN (1959) The use of cytoplasmic male-sterility in hybrid seed production. Econ Bot 13:167–195
Duvick DN (2001) Biotechnology in the 1930s: the development of hybrid maize. Nature Rev Genet 2:69–74
Feil B, Weingartner U, Stamp P (2003) Controlling release of pollen from genetically modified maize and increasing its grain yield by growing mixtures of male-sterile and male-fertile plants. Euphytica 130:163–165
Fischer R, Budde I, Hain R (1997) Stilbene synthase gene expression causes changes in flower colour and male sterility in tobacco. Plant J 11:489–498
Flavell RB (1974) A model for the mechanism of cytoplasmic male sterility with special reference to maize. Plant Sci Lett 3:259–263
Forster BP, Heberle-Bors E, Kasha KJ, Touraev A (2007) The resurgence of haploids in higher plants. Trends Plant Sci 12:368–375
Geddy R, Brown GG (2007) Genes encoding pentatricopeptide repeat (PPR) proteins are not conserved in location in plant genomes and may be subject to diversifying selection. BMC Genomics 8:130–142
Geddy R, Mahé L, Brown GG (2005) Cell-specific regulation of a Brassica napus CMS-associated gene by a nuclear restorer with related effects on a floral homeotic gene promoter. Plant J 41:333–345
Gils M, Marillonnet S, Werner S, Grutzner R, Giritch A, Engler C, Schachschneider R, Klimyuk V, Gleba Y (2008) A novel hybrid seed system for plants. Plant Biotechnol J 6:226–235
Gleba Y, Marillonnet S, Klimyuk V (2004) Design of safe and biologically contained transgenic plants: tools and technologies for controlled transgene flow and expression. Biotechnol Genet Eng Rev 21:325–367
Goetz M, Godt DE, Guivarc'h A, Kahmann U, Chriqui D, Roitsch T (2001) Induction of male sterility in plants by metabolic engineering of the carbohydrate supply. Proc Natl Acad Sci USA 98:6522–6527
Goldberg A, Confino-Cohen R, Waisel Y (1998) Allergic responses to pollen of ornamental plants: high incidence in the general atopic population and especially among flower growers. J Allergy Clin Immunol 102:210–214
Guerineau F, Sorensen AM, Fenby N, Scott RJ (2003) Temperature sensitive diphtheria toxin confers conditional male-sterility in Arabidopsis thaliana. Plant Biotechnol J 1:33–42
Hagemann R (2004) The sexual inheritance of plant organelles. In: Daniell H, Chase CD (eds) Molecular biology and biotechnology of plant organelles. Springer, Berlin Heidelberg New York, pp 93–114
Hanson MR, Bentolila S (2004) Interactions of mitochondrial and nuclear genes that affect male gametophyte development. Plant Cell 16:S154–S169
Hattori M, Miyake H, Sugita M (2007) A Pentatricopeptide repeat protein is required for RNA processing of clpP Pre-mRNA in moss chloroplasts. J Biol Chem 282:10773–10782
Havey M(2004) The use of cytoplasmic male sterility for hybrid seed production. In: Daniell H, Chase CD (eds) Molecular biology and biotechnology of plant organelles. Springer, Berlin Heidelberg New York, pp 623–634
Horner H, Palmer R (1995) Mechanisms of genic male sterility. Crop Sci 35:1527–1535
Huang S, Cerny RE, Qi Y, Bhat D, Aydt CM, Hanson DD, Malloy KP, Ness LA (2003) Transgenic studies on the involvement of cytokinin and gibberellin in male development. Plant Physiol 131:1270–1282
Ishiguro S, Kawai-Oda A, Ueda J, Nishida I, Okada K (2001) The DEFECTIVE IN ANTHER DEHISCIENCE1 gene encodes a novel phospholipase A1 catalyzing the initial step of jasmonic acid biosynthesis, which synchronizes pollen maturation, anther dehiscence, and flower opening in Arabidopsis. Plant Cell 13:2191–2209
Ishimaru K, Takada K, Watanabe S, Kamada H, Ezura H (2006) Stable male sterility induced by the expression of mutated melon ethylene receptor genes in Nicotiana tabacum. Plant Sci 171:355–359
Jones A (2000) Does the plant mitochondrion integrate cellular stress and regulate programmed cell death? Trends Plant Sci 5:225–230
Kaul MLH (1988) Male sterility in higher plants. Springer, Berlin Heidelberg New York
Kazama T, Toriyama K (2003) A pentatricopeptide repeat-containing gene that promotes the processing of aberrant atp6 RNA of cytoplasmic male-sterile rice. FEBS Lett 544:99–102
Klein RR, Klein PE, Mullet JE, Minx P, Rooney WL, Schertz KF (2005) Fertility restorer locus Rf1 of sorghum (Sorghum bicolor L.) encodes a pentatricopeptide repeat protein not present in the colinear region of rice chromosome 12. Theor Appl Genet 111:994–1012
Koizuka N, Imai R, Fujimoto H, Hayakawa T, Kimura Y, Kohno-Murase J, Sakai T, Kawasaki S, Imamura J (2003) Genetic characterization of a pentatricopeptide repeat protein gene, orf687, that restores fertility in the cytoplasmic male-sterile Kosena radish. Plant J 34:407–415
Koltunow AM, Truettner J, Cox KH, Wallroth M, Goldberg RB (1990) Different temporal and spatial gene expression patterns occur during anther development. Plant Cell 2:201–1224
Komori T, Ohta S, Murai N, Takakura, Y, Kuraya Y, Suzuki S, Hiei Y, Imaseki H, Nitta N (2004) Map-based cloning of a fertility restorer gene, Rf-1 in rice (Oryza sativa L.). Plant J 37:315–325
Kotera E, Tasaka M, Shikanai T (2005) A pentatricopeptide repeat protein is essential for RNA editing in chloroplasts. Nature 433:326–330
Kriete G, Niehaus K, Perlick AM, Puhler A, Broer I (1996) Male sterility in transgenic tobacco plants induced by tapetum-specific deacetylation of the externally applied non-toxic compound N-acetyl-L-phosphinothricin. Plant J 9:809–818
Krishnasamy S, Makaroff CA (1994) Organ-specific reduction in the abundance of a mitochondrial protein accompanies fertility restoration in cytoplasmic male-sterile radish. Plant Mol Biol 26:935–946
Krizek BA, Fletcher JC (2005) Molecular mechanisms of flower development: an armchair guide. Nature Rev Genet 6:688–698
Ku SJ, Cho KH, Choi YJ, Baek WK, Kim S, Su HS, Chung YY (2001) Cytological observation of two environmental genic male-sterile lines of rice. Mol Cells 12:403–406
Kubo T, Newton KJ (2008) Angiosperm mitochondrial genomes and mutations. Mitochondrion 8:5–14
Lam E (2004) Controlled cell death, plant survival and development. Nature Rev Mol Cell Biol 5:305–315
Laser KD, Lersten NR (1972) Anatomy and cytology of microsporogenesis in cytoplasmic male sterile angiosperms. Bot Rev 38:425–454
Laver H, Reynolds SJ, Monéger F, Leaver CJ (1991) Mitochondrial genome organization and expression associated with cytoplasmic male sterility in sunflower (Helianthus annuus). Plant J 1:185–193
Lee D, Natesan E (2006) Evaluating genetic containment strategies for transgenic plants. Trends Biotechnol 24:109–114
Li S, Yang D, Zhu Y (2007) Characterization and use of male sterility in hybrid rice breeding. J Integr Biol 49:791–804
Li SF, Iacuone S, Parish RW (2007) Suppression and restoration of male fertility using a transcription factor. Plant Biotechnol J 5:297–312
Linke B, Borner T (2005) Mitochondrial effects on flower and pollen development. Mitochondrion 5:389–402
Linke B, Nothnagel T, Börner T (2003) Flower development in carrot CMS plants: mitochondria affect the expression of MADS box genes homologous to GLOBOSA and DEFICIENS. Plant J 34:27–37
Liu Z, Liu Z (2008) The second intron of AGAMOUS drives carpel- and stamen-specific expression sufficient to induce complete sterility in Arabidopsis. Plant Cell Rep 27(5):855–863
Liu F, Cui X, Horner HT, Weiner H, Schnable PS (2001) Mitochondrial aldehyde dehydrogenase activity is required for male fertility in maize. Plant Cell 13:1063–1078
Liu CW, Lin CC, Chen JJ, Tseng MJ (2007) Stable chloroplast transformation in cabbage (Brassica oleracea L. var. capitata L.) by particle bombardment. Plant Cell Rep 26:1733–1744
Liu X, Wang S, Wang Y, Wei S (2007) Genetic analysis and molecular mapping of a nuclear recessive male sterility gene, ms91(t), in rice. Genome 50:796–801
Logan DC (2006) Plant mitochondrial dynamics. Biochim Biophys Acta 1763:430–441
Lu G, Yang G, Fu T (2004) Molecular mapping of a dominant genic male sterility gene Ms in rapeseed (Brassica napus). Plant Breed 123:262–265
Luo H, Kausch AP, Hu Q, Nelson K, Wipff JK, Fricker CCR, Owen TP, Moreno MA, Lee JY, Hodges TK (2005) Controlling transgene escape in GM creeping bentgrass. Mol Breed 16:185–188
Lurin C, Andrés C, Aubourg S, Bellaoui M, Bitton F, Bruyère C, Caboche M, Debast C, Gualberto J, Hoffmann B, Lecharny A, Le Ret M, Martin-Magniette ML, Mireau H, Peeters N, Renou JP, Szurek B, Taconnat L, Small IC (2004) Genome-wide analysis of Arabidopsis pentatricopeptide repeat proteins reveals their essential role in organelle biogenesis. Plant Cell 16:2089–2103
Mahillon V, Saussez S, Michel O (2006) High incidence of sensitization to ornamental plants in allergic rhinitis. Allergy 61:1138–1140
Mariani C, de Beuckeleer M, Truttner J, Leemans J, Goldberg RB (1990) Induction of male sterility in plants by a chimaeric ribonuclease gene. Nature 347:737–741
Mariani C, Gossele V, de Beuckeleer M, de Block M, Goldberg RB, de Greef W, Leemans J (1992) A chimaeric ribonuclease inhibitor gene restores fertility to male-sterile plants. Nature 257:384–387
McConn M, Browse J (1996) The critical requirement for linolenic acid is pollen development, not photosynthesis, in an Arabidopsis mutant. Plant Cell 8:403–416
McCormick S (2004) Control of male gametophyte development. Plant Cell 16 Suppl:S142–S153
Mo Y, Nagel C, Taylor LP (1992) Biochemical complementation of chalcone synthase mutants defines a role for flavonols in functional pollen. Proc Natl Acad Sci USA 89:7213–7217
Moneger F, Smart SJ, Leaver CJ (1994) Nuclear restoration of cytoplasmic male sterility in sunflower is associated with the tissue-specific regulation of a novel mitochondrial gene. EMBO J 13:8–17
Moran JL, Rooney WL (2003) Effect of cytoplasm on the agronomic performance of grain sorghum hybrids. Crop Sci 43:777–781
Murai K, Takumi S, Koga H, Ogihara Y (2002) Pistilloidy, homeotic transformation of stamens into pistil-like structures, caused by nuclear-cytoplasm interaction in wheat. Plant J 29:169–181
Napoli CA, Fahy D, Wang HY, Taylor LP (1999) white anther: a petunia mutant that abolishes pollen flavonol accumulation, induces male sterility, and is complemented by a chalcone synthase transgene. Plant Physiol 120:615–622
Nawaz-ul-Rehman MS, Mansoor S, Khan AA, Zafar Y, Briddon RW (2007) RNAi-mediated male sterility of tobacco by silencing TA29. Mol Biotechnol 36:159–165
Nivison HT, Hanson MR (1989) Identification of a mitochondrial protein associated with cytoplasmic male sterility in petunia. Plant Cell 1:1121–1130
Noctor G, De Paepe R, Foyer CH (2007) Mitochondrial redox biology and homeostasis in plants. Trends Plant Sci 12:125–134
Okuda K, Myouga F, Motohashi R, Shinozaki K, Shikanai T (2007) Conserved domain structure of pentatricopeptide repeat proteins involved in chloroplast RNA editing. Proc Natl Acad Sci USA 104:8178–8183
Okumura S, Sawada M, Park YW, Hayashi T, Shimamura M, Takase H, Tomizawa K (2006) Transformation of poplar (Populus alba) plastids and expression of foreign proteins in tree chloroplasts. Transgenic Res 15:637–646
Park JH, Halitschke R, Kim HB, Baldwin IT, Feldmann KA, Feyereisen R (2002) A knock-out mutation in allene oxide synthase results in male sterility and defective wound signal transduction in Arabidopsis due to a block in jasmonic acid biosynthesis. Plant J 31:1–12
Pelletier G, Budar F (2007) The molecular biology of cytoplasmically inherited male sterility and prospects for its engineering. Curr Opin Biotechnol 18:121–125
Perez-Prat E, van Lookeren Campagne MM (2002) Hybrid seed production and the challenge of propagating male-sterile plants. Trends Plant Sci 7:199–203
Pla M, Mathieu C, De Paepe R, Chetrit P, Vedel F (1995) Deletion of the last two exons of the mitochondrial nad7 gene results in lack of the NAD7 polypeptide in a Nicotiana sylvestris CMS mutant. Mol Gen Genet 248:79–88
Possingham JV (1998) Fruit and vegetable quality in the 21st century - the influence of Japan. J Japan Soc Hort Sci 67:1250–1254
Preston J, Wheeler J, Heazlewood J, Li SF, Parish RW (2004) AtMYB32 is required for normal pollen development in Arabidopsis thaliana. Plant J 40:979–995
Pring DR, Lonsdale DM (1989) Cytoplasmic male sterility and maternal inheritance of disease susceptibility in maize. Annu Rev Phytopathol 27:483–502
Pring DR, Tang HV, Howad W, Kempken F (1999) A unique two-gene gametophytic male sterility system in sorghum involving a possible role of RNA editing in fertility restoration. J Hered 90:386–393
Pruitt KD, Hanson MR (1991) Transcription of the Petunia mitochondria CMS-associated PCF locus in male sterile and fertility-restored lines. Mol Gen Genet 227:348–355
Rasmusson, AG, Soole KL, Elthon TE (2004) Alternative NAD(P)H dehydrogenases of plant mitochondria. Annu Rev Plant Biol 55:23–39
Ray K, Bisht N, Pental D, Burma PK (2007) Development of barnase/barstar transgenics for hybrid seed production in Indian oilseed mustard (Brassica juncea L. Czern & Coss) using a mutant acetolactate synthase gene conferring resistance to imidazolinone-based herbicide ‘Pursuit’. Curr Sci 93:1390–1396
Reynaerts A, Van de Wiele H, De Sutter G, Janssens J (1993) Engineered genes for fertility control and their application in hybrid seed production. Sci Hort 55:125–139
Rhoades DM, Subbaiah CC (2007) Mitochondrial retrograde regulation in plants. Mitochondrion 7:177–194
Ribarits A, Mamun AN, Li S, Resch T, Fiers M, Heberle-Bors E, Liu CM, Touraev A (2007) Combination of reversible male sterility and doubled haploid production by targeted inactivation of cytoplasmic glutamine synthetase in developing anthers and pollen. Plant Biotechnol J 5:483–494
Rogers HJ (2006) Programmed cell death in floral organs: how and why do flowers die? Ann Bot 97:309–315
Rubinstein B (2000) Regulation of cell death in flower petals. Plant Mol Biol 44:303–318
Ruiz O, Daniell H (2005) Cytoplasmic male sterility engineered via the plastid genome. Plant Physiol 138:1232–1246
Sabar M, Gagliardi D, Balk J, Leaver CJ (2003) ORFB is a subunit of F1F0-ATP synthase: insight into the basis of cytoplasmic male sterility in sunflower. EMBO Rep 4:381–386
Sandhu AP, Abdelnoor RV, Mackenzie SA (2007) Transgenic induction of mitochondrial rearrangements for cytoplasmic male sterility in crop plants. Proc Natl Acad Sci USA 104:1766–1770
Sarria R, Janska H, Arrieta-Montiel M, Lyznik A, Mackenzie SA (1999) Two nuclear-directed means of suppressing a dominant mitochondrial mutation in common bean. J Hered 90:357–361
Schijlen EGWM, de Vos CHR, Martens S, Jonker HH, Rosin FM, Molthoff JW, Tikunov YM, Angenent GC, van Tunen AJ, Bovy AG (2007) RNA interference silencing of chalcone synthase, the first step in the flavonoid biosynthesis pathway, leads to parthenocarpic tomato fruits. Plant Physiol 144:1520–1530
Schmitz-Linneweber C, Williams-Carrier R, Barkan A (2005) RNA immunoprecipitation and microarray analysis show a chloroplast pentatricopeptide repeat protein to be associated with the 5' region of mRNAs whose translation it activates. Plant Cell 17:2791–2804
Schmitz-Linneweber C, Williams-Carrier RE, Williams-Voelker PM, Kroeger TS, Vichas A, Barkan A (2006) A pentatricopeptide repeat protein facilitates the trans-splicing of the maize chloroplast rps12 pre-mRNA. Plant Cell 18:2650–2663
Schnable P, Wise R (1998) The molecular basis of cytoplasmic male sterility and fertility restoration. Trends Plant Sci 3:175–180
Scott RJ, Spielman M, Dickinson HG (2004) Stamen structure and function. Plant Cell 16 Suppl:S46–S60
Shikanai T (2006) RNA editing in plant organelles: machinery, physiological function and evolution. Cell Mol Life Sci 63:698–708
Smith B, Crowther T (1995) Inbreeding depression and single cross hybrids in leeks (Allium ampeloprasum ssp. porrum). Euphytica 86:87–94
Springer NM, Stupar RM (2007) Allelic variation and heterosis in maize: how do two halves make more than a whole? Genome Res 17:264–275
Stamp P, Chowchond S, Menzi M, Weingartner U, Kaeser O (2000) Increase in the yield of cytoplasmic male sterile maize revisited. Crop Sci 40:1586–1587
Steiner-Lange S, Unte US, Eckstein L, Yang C, Wilson ZA, Schmelzer E, Dekker K, Saedler H (2003) Disruption of Arabidopsis thaliana MYB26 results in male sterility due to non-dehiscent anthers. Plant J 34:519–528
Stern DB, Hanson MR, Barkan A (2004) Genetics and genomics of chloroplast biogenesis: maize as a model system. Trends Plant Sci 9:293–301
Stintzi A, Browse J (2000) The Arabidopsis male-sterile mutant, opr3, lacks the 12-oxophytodienoic acid reductase required for jasmonate synthesis. Proc Natl Acad Sci USA 97:10625–10630
Taylor L, Jorgensen R (1992) Conditional male fertility in chalcone synthase-deficient petunia. J Hered 83:11–17
Teixeira RT, Farbos I, Glimelius K (2005) Expression levels of meristem identity and homeotic genes are modified by nuclear-mitochondrial interactions in alloplasmic male-sterile lines of Brassica napus. Plant J 42:731–742
Touraev A, Heberle-Bors E (1999) Microspore embryogenesis and in vitro pollen maturation in tobacco. Methods Mol Biol 111:281–291
Touzet P, Budar F(2004) Unveiling the molecular arms race between two conflicting genomes in cytoplasmic male sterility. Trends Plant Sci 19:568–570
Troyer AF (2006) Adaptedness and heterosis in corn and mule hybrids. Crop Sci 46:528–543
Tupý J, Rcaronihová L, Zcaronárský V (1991) Production of fertile tobacco pollen from microspores in suspension culture and its storage for in situ pollination. Sex Plant Reprod 4:284–287
van der Meer IM, Stam ME, van Tunen AJ, Mol JN, Stuitje AR (1992) Antisense inhibition of flavonoid biosynthesis in petunia anthers results in male sterility. Plant Cell 4:253–262
Virmani SS, Sun ZX, Mou TM, Jauhar Ali A, Mao CX (2003) Two-line hybrid rice breeding manual. International Rice Research Institute, Los Banos, Philippines
Wang Z, Zou Y, Li X, Zhang Q, Chen L, Wu H, Su D, Chen Y, Guo J, Luo D, Long Y, Zhong Y, Liu YG (2006) Cytoplasmic male sterility of rice with boro II cytoplasm is caused by a cytotoxic peptide and is restored by two related PPR motif genes via distinct modes of mRNA silencing. Plant Cell 18:676–687
Warmke HE, Lee S-LJ (1977) Mitochondrial degeneration in Texas cytoplasmic male-sterile corn anthers. J Hered 68:213–222
Warmke HE, Lee S-LJ (1978) Pollen abortion in T cytoplasmic male-sterile corn (Zea mays): a suggested mechanism. Science 200:561–562
Weingartner U, Prest TJ, Camp KH, Stamp P (2002a) The plus-hybrid system: a method to increase grain yield by combined cytoplasmic male sterility and xenia. Maydica 47:127–134
Weingartner U, Kaeser O, Long M, Stamp P (2002b) Combining cytoplasmic male sterility and xenix increases grain yield of maize hybrids. Crop Sci 42:1848–1856
Williams M (1995) Genetic engineering for pollination control. Trends Biotechnol 13:344–349
Wilson ZA, Yang C (2004) Plant gametogenesis: conservation and contrasts in development. Reproduction 128:483–492
Wise RP, Pring DR (2002) Nuclear-mediated mitochondrial gene regulation and male fertility in higher plants: light at the end of the tunnel? Proc Natl Acad Sci USA 99:10240–10242
Wise RP, Fliss AE, Pring DR, Gengenbach BG (1987) Urf13T of T cytoplasm maize mitochondria encodes a 13kD polypeptide. Plant Mol Biol 9:121–126
Wise RP, Bronson CR, Schnable PS, Horner HT (1999) The genetics, pathology, and molecular biology of T-cytoplasm male sterility in maize. Adv Agron 65:79–130
Wurbs D, Ruf S, Bock R (2007) Contained metabolic engineering in tomatoes by expression of carotenoid biosynthesis genes from the plastid genome. Plant J 49:276–288
Yang XY, Li JG, Pei M, Gu H, Chen ZL, Qu LJ (2007) Over-expression of a flower-specific transcription factor gene AtMYB24 causes aberrant anther development. Plant Cell Rep 26:219–228
Ylstra B, Touraev A, Benito Moreno RM, Stöger E, Van Tunen AA, Vicente O, Mol JNM, Heberle-Bors E (1992) Flavonols stimulate development, germination and tube growth of tobacco pollen. Plant Physiol 100:902–907
Ylstra B, Busscher J, Franken J, Hollman P, Mol J, van Tunen A (1994) Flavonols and fertilization in Petunia hybrida: localization and mode of action during pollen tube growth. Plant J 6:201–212
Yui R, Iketani S, Mikami T, Kubo T (2003) Antisense inhibition of mitochondrial pyruvate dehydrogenase E1 alpha subunit in anther tapetum causes male sterility. Plant J 34:57–66
Zabala G, Gabay-Laughnan S, Laughnan JR (1997) The nuclear gene Rf3 affects the expression of the mitochondrial chimeric sequence R implicated in S-type male sterility in maize. Genetics 147:847–850
Zubko MK (2004) Mitochondrial tuning fork in nuclear homeotic functions. Trends Plant Sci 9:61–64
Acknowledgements
The Chase laboratory research on CMS and fertility restoration is supported by the Florida Agricultural Experiment Station and by USDA Cooperative State Research, Education and Extension Service NRI grant numbers 2001-0534-10888 and 2005-35301-1570. Alexandra Ribarits and Erwin Heberle-Bors are at the Department of Plant Molecular Biology, Max F. Perutz Laboratories, Vienna, Austria. Their work on male sterility has been supported by the HYBTECH project No. QKL5-CT-1999-30902 of the European Commission.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2010 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Chase, C.D., Ribarits, A., Heberle-Bors, E. (2010). Male Sterility. In: Pua, E., Davey, M. (eds) Plant Developmental Biology - Biotechnological Perspectives. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-02301-9_21
Download citation
DOI: https://doi.org/10.1007/978-3-642-02301-9_21
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-02300-2
Online ISBN: 978-3-642-02301-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)