Abstract
Crizotinib is an ATP-competitive small-molecule inhibitor of the receptor tyrosine kinases (RTK) C-Met, ALK and ROS1. There is a robust effectiveness in non-small-cell lung cancer (NSCLC) harbouring EML4-ALK-rearrangements resulting in constitutional activation of the ALK-RTK. The drug is approved for this entity, which represents no more than 3–5% of all NSCLC. However, in this population, impressive response rates are generated. The same is true for ROS-1 rearrangements; however, these only occur in approximately 1% of all NSCLC. In small series, efficacy is also reported in patients, whose tumours harbour a MET Exon 4 skipping mutation (approx. 3% of all NSCLC). Toxicities include visual impairment, nausea, peripheral edema, QT-prolongation and liver-enzyme elevation. Also, the occurrence of renal cysts is reported. The detection of ALK-protein by immunohistochemistry is a predictor of efficacy for crizotinib. In cases of doubt, fluorescence in situ hybridisation (FISH) detecting the ALK-rearrangement has to be performed on tumour tissue. FISH is also the method of choice to detect ROS1-rearrangement, whereas MET-mutations are detected by sequencing methods. The high efficacy of crizotinib in ALK- and ROS-rearranged as well as MET mutated lung cancer as new molecular targets beside the epidermal growth factor receptor (EGFR) underscores the importance of molecular typing in NSCLC.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Structure and Mechanism of Action
Crizotinib, (R)-3-[1-(2,6-dichloro-3-flourophenyl)ethoxyl]-5-(1-piperidin-4-yl-1H-pyrazol-4-yl)pyridine-2-ylamine (Fig. 1) was initially developed as a second generation, selective C-Met (Mesenchymal to epidermal transition)—inhibitor developed from a compound named PHA-665752 by Pfizer. This first-generation compound was modulated to become a potent small-molecule inhibitor of c-Met (Cui et al. 2011). It is an adenosine triphosphate (ATP) inhibitor of receptor tyrosine kinases. Besides c-met, it inhibits ALK (anaplastic lymphoma kinase), ROS-1 and possibly other targets (Table 1) (Curran 2012).
Crizotinib in complex with its target, i.e. ALK, creates an inactive conformation of this oncogenic protein by inhibiting its phosphorylation as shown by crystalline structure analysis (Sasaki et al. 2010).
2 Preclinical Data
Much preclinical data was obtained on its property of inhibiting c-Met. The IC50 of inhibiting the phosphorylation of wild-type c-Met in vitro in several human tumour cell lines has a mean of 4–8 nM. It inhibited cell growth and induced apoptosis in human GTL16 gastric carcinoma cell lines and was capable in suppression and migration of tumour cells in vitro (Rodig and Shapiro 2010).
In ALK-translocated cell lines, crizotinib inhibited downstream effector functions and induced apoptosis (Christensen et al. 2007). Moreover, the compound has antiangiogenic properties in preclinical studies (Zou et al. 2007).
3 Clinical Data
3.1 NSCLC
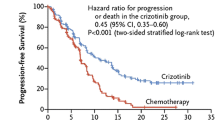
In a phase 1 trial, patients with any solid tumour and no further approved treatment option were treated with increasing doses of crizotinib. 250 mg bid was the maximum tolerated dose. In this cohort, two patients with NSCLC had improvements in tumour symptoms. Thus, an expansion cohort was created consisting of patients with NSCLC harbouring an EML4/ALK-rearrangement. They received the maximum tolerated dose (250 mg bid) in continuous 28-day cycles (Kwak et al. 2010). Median progression-free survival was 9.7 months (95% CI 7.7–12.8). Estimated overall survival (OS) at 6 and 12 months was 87.9% (95% CI 81.3–92.3) and 74.8% (66.4–81.5), respectively; however, the median was not reached by time of the publication. 39 patients continued to receive crizotinib for more than 2 weeks after progression because of perceived ongoing clinical benefit from the drug (12 for at least 6 months from the time of their initial investigator-defined disease progression) (Camidge et al. 2012). PROFILE 1007 compared crizotinib to either pemetrexed or docetaxel (by investigators’ decision) in ALK-positive patients as second-line therapy. 318 patients were randomized to either crizotinib or chemotherapy. Primary endpoint was PFS. OS was not feasible, because crossover of chemotherapy patients into a single-arm crizotinib trial (PROFILE 1005) was pre-planned. A median of 11 cycles of crizotinib and 4 cycles of chemotherapy were administered, respectively. Chemotherapy consisted of docetaxel in 41% of patients and pemetrexed in 57% of patients, respectively. Median PFS was 7.7 versus 3.0 months favouring crizotinib (HR 0.49; Confidence Interval [CI] 0.37–0.64; p < 0.0001). Interestingly, there was also a different PFS regarding to chemotherapy: patients receiving pemetrexed had a median of 4.3 months compared to docetaxel with 2.6 months (p < 0.0001) with the difference between pemetrexed and crizotinib remaining significant (p = 0.0004, Table 2). Response rates to crizotinib, pemetrexed and docetaxel were 65.7, 29.3 and 6.9% respectively. The preliminary data on OS showed no significant difference between crizotinib (20.3 months) and chemotherapy (22.8 months, p = 0.5394) because 111 out of 174 patients in the chemotherapy arm subsequently received crizotinib (Shaw et al. 2013) and thus benefitted from the drug as well.
First-line crizotinib was tested against standard chemotherapy with either cis- or carboplatin plus pemetrexed in the PROFILE 1014-trial in patients with ALK-rearranged tumours. 343 patients were randomized. Primary endpoint was again PFS, which was significantly longer in the crizotinib arm with 10.9 months versus 7.0 months under chemotherapy (HR 0.45; p < 0.001). Moreover, crizotinib was associated with better symptom control and greater improvement in quality of life (Solomon et al. 2014). Meanwhile, the OS-data are updated, showing that the median is still not reached in the crizotinib arm (CI 45.8—not reached), whereas the median is 47.5 months (32.2—not reached) with chemotherapy. Four-year OS is 56.6% versus 49.1% with crizotinib and chemotherapy, respectively. The crossover rate to crizotinib after chemotherapy was 84% (Mok et al. 2017).
There is further indirect evidence that crizotinib prolongs OS in patients harbouring an ALK-rearrangement: In a retrospective comparison of 82 patients with ALK-rearrangement receiving crizotinib, 36 patients with ALK-rearrangement not receiving crizotinib, 67 patients with an activating EGFR-mutation and 253 patients with wild-type EGFR and ALK survival were compared. ALK-positive patients treated second- or third-line with crizotinib had a 1-year survival of 70% (95%-CI 50–83%). ALK-positive patients treated with any other second- or third-line therapy had a 1-year survival of 44% (95-CI 23–64%; HR 0.36; 95% CI 0.17–0.75; p = 0.004). Survival of ALK-positive patients receiving crizotinib was comparable to those who harbour an activating EGFR-mutation receiving an EGFR-TKI (1-year survival% [95% CI 58–81] vs. 74% [61–83]). ALK-positive patients not treated with crizotinib had similar survival as ‘double-wild-type’ patients (median OS 20 months [95% CI 13–26] vs. 15 months (Costa et al. 2011; Tang et al. 2014; Peters et al. 2017; Choi et al. 2010; Gainor et al. 2016); p = 0.244) (Shaw et al. 2011).
A frequent site of treatment failure is the central nervous system, due to the fact, that crizotinib concentration in cerebrospinal fluid is much lower than in blood plasma (cerebrospinal fluid to plasma ratio 0.0026 (Costa et al. 2011)) and many patients develop brain metastases in their relatively long course of crizotinib treatment. Crizotinib is a substrate to the p-glycoprotein transporter (ABCB1), a transmembrane protein delivering the drug to the extracellular space (Tang et al. 2014). Newer ALK-inhibitors like alectinib, which are not a substrate to the p-glycoprotein transporter, show better disease control in the central nervous system (Peters et al. 2017).
Some of the molecular resistance mechanisms leading to crizotinib failure are already discovered. In fact, the first report on crizotinib resistance was published ‘back to back’ with the first clinical efficacy results described above (Choi et al. 2010). Meanwhile, a number of resistance mutations, most of them point mutations, have been identified. Drugs developed as ALK-inhibitors like ceritinib, alectinib, lorlatinib and brigatinib show effectiveness against several of these resistance mutations (Gainor et al. 2016).
In a direct comparison of crizotinib with the newer compound alectinib, it could be shown that the latter substance is superior in terms of the 12-month-PFS (68.4% vs. 48.7%, p < 0.001), a trend in the immature OS (HR 0.76, p = 0.24) and a slightly better toxicity profile (41% vs. 50% severe adverse events). The rate of CNS progression was significantly lower under alectinib compared to crizotinib (9.4% vs. 41.4%) (Peters et al. 2017).
ROS-1-rearrangements occur in about 1% of patients with NSCLC. ROS1 and ALK are ‘kissing cousins’, i.e. closely akin within the human kinome. In vitro essays with crizotinib showed that the drug was capable of inhibiting growth of ROS-1 positive NSCLC cell lines (Yasuda et al. 2012). So, it was straightforward to test crizotinib in patients with a ROS-1-rearrangement. Patients were recruited in the dose escalation trial mentioned above (Kwak et al. 2010) as an own cohort. 50 patients harbouring such a rearrangement could be identified. Overall response rate was 72% with 3 complete and 33 partial responses. Duration of response was 17.6 months and median PFS was 19.2 months (Shaw et al. 2014). Crizotinib is approved for the treatment of ROS1-translocated NSCLC in the United States and Europe.
The driving event beyond crizotinib efficacy as a MET-inhibitor is the MET Exon 14 skipping mutation, which occurs in approximately 3% of patients with NSCLC and is also described in gastric and colon carcinomas as well as in gliomas and solid cancers of unknown primary site (Lee et al. 2015; Frampton et al. 2015). In a small retrospective series of 61 patients with metastatic NSCLC, 27 were treated with a MET-inhibitor (20 of these with crizotinib) and 34 were not. Median OS was 24.6 months for patients treated with a MET-inhibitor compared to 8.1 months in those not receiving such a drug (Awad et al. 2017). These data underscore, that crizotinib could be an effective drug in patients with this driver mutation.
3.2 Other Entities
In gastric carcinomas, ROS-1 rearrangements and c-MET amplifications are described (Lee et al. 2013; Okamoto et al. 2012) suggesting a possible benefit of crizotinib in these subsets.
Targets drugable with crizotinib are also found in Ewing Sarcomas (Fleuren et al. 2013), anaplastic large cell lymphoma (Ordemann et al. 2013; Mosse et al. 2017), inflammatory myofibroblastic tumours (Mosse et al. 2017; Tothova and Wagner 2012), chronic myelomonocytic leukaemia (Cilloni et al. 2013) and neuroblastoma (Matthay et al. 2012) (Table 1). However, the clinical evidence is still sparse.
4 Toxicity
Crizotinib is comparably well tolerable. According to an analysis of phase I/II trials, most adverse events were mild to moderate with only 3–6% of treatment interruption due to adverse events. In order of frequency, the adverse events are
-
Visual disturbances (in 62% of patients) including light flashes or perception of overlying shadows or after images. However, the disturbances were all short in duration and had minimal influence on the quality of life or activities of daily living.
-
Nausea occurred in approximately 50% of patients, diarrhoea and vomiting occurred too. Again, these disturbances were mild and of short duration (Inc. P. Xalkori 2012).
-
Further frequent side effects were peripheral edema, constipation, fatigue, decreased appetite and dizziness (Inc. P. Xalkori (2012).
-
In 1.3% of cases, administration of crizotinib can result in prolongation of the QT-interval in the electrocardiogram (ECG) (Tothova and Wagner 2012). Patients already showing a prolonged QT-interval or taking drugs known to prolong it should be monitored periodically with an ECG (Curran 2012).
5 Drug Interactions
Crizotinib is primarily metabolized in the liver by CYP3A, which can result in drug interactions with CYP3A-inducers like rifampicin, resulting in decreased plasma levels of crizotinib. CYP3A-inhibitors like ketoconazole can lead to increased plasma levels of crizotinib. Finally, crizotinib itself may act as a CYP3A inhibitor, raising plasma levels of other substrates like midazolam (Curran 2012).
6 Biomarkers
The efficacy of crizotinib was tested in small subsets of patients with NSCLC, who are clearly defined on a molecular basis.
By now, three targets for crizotinib are identified: Rearrangements in ALK and ROS as well as Met Exon 14 skipping mutations. The gold-standard for detection of ALK- or ROS-rearrangements is fluorescence in situ hybridization (FISH). For ALK, 15% of cells must show this rearrangement for being classified as ‘ALK-positive’. This number is not chosen arbitrary but represents the double standard deviation from the number of ALK-rearrangements found in normal tissue. FISH is a time-consuming procedure and can only be done by specially trained personnel. It can partially be replaced by immunohistochemistry (IHC). Overexpression of ALK measured by IHC is a sufficient predictor of crizotinib efficacy, whereas negativity on ALK-IHC correlates strongly with a negative FISH test and less effectivity of the compound. Only in samples with low or moderate expression of ALK, FISH is needed as a confirmatory test (von Laffert et al. 2016).
ROS1 rearrangements have still to be determined by FISH, whereas MET-mutations are detected by sequencing techniques.
7 Summary and Perspectives
Crizotinib was the first-in-class drug to treat patients, whose tumours harbour the above-mentioned genetic alterations. Developed as a MET-inhibitor, it reveals to be prone to several pharmacodynamic and genetic resistance mechanisms in ALK-positive tumours which can be overcome by second and third generation ALK-tyrosinkinase-inhibitors. Especially because of the frequent failure in the central nervous system, its use might be challenged by these drugs in the treatment of ALK-rearranged lung cancer. However, it still has its place in the treatment of ROS1-rearranged tumours and probably in the future in those with MET Exon 14 skipping mutations.
References
Awad MM, Leonardi GC, Kravets S et al (2017) Impact of MET inhibitors on survival among patients (pts) with MET exon 14 mutant (METdel14) non-small cell lung cancer (NSCLC). J Clin Oncol: Off J Am Soc Clin Oncol 35
Camidge DR, Bang YJ, Kwak EL et al (2012) Activity and safety of crizotinib in patients with ALK-positive non-small-cell lung cancer: updated results from a phase 1 study. Lancet Oncol 13:1011–1019
Choi YL, Soda M, Yamashita Y et al (2010) EML4-ALK mutations in lung cancer that confer resistance to ALK inhibitors. N Engl J Med 363:1734–1739
Christensen JG, Zou HY, Arango ME et al (2007) Cytoreductive antitumor activity of PF-2341066, a novel inhibitor of anaplastic lymphoma kinase and c-Met, in experimental models of anaplastic large-cell lymphoma. Mol Cancer Ther 6:3314–3322
Cilloni D, Carturan S, Bracco E et al (2013) Aberrant activation of ROS1 represents a new molecular defect in chronic myelomonocytic leukemia. Leuk Res 37:520–530
Costa DB, Kobayashi S, Pandya SS et al (2011) CSF concentration of the anaplastic lymphoma kinase inhibitor crizotinib. J Clin Oncol: Off J Am Soc Clin Oncol 29:e443–e445
Cui JJ, Tran-Dube M, Shen H et al (2011) Structure based drug design of crizotinib (PF-02341066), a potent and selective dual inhibitor of mesenchymal-epithelial transition factor (c-MET) kinase and anaplastic lymphoma kinase (ALK). J Med Chem 54:6342–6363
Curran MP (2012) Crizotinib: in locally advanced or metastatic non-small cell lung cancer. Drugs 72:99–107
Fleuren ED, Roeffen MH, Leenders WP et al (2013) Expression and clinical relevance of MET and ALK in Ewing sarcomas. Int J Cancer 133:427–436
Frampton GM, Ali SM, Rosenzweig M et al (2015) Activation of MET via diverse exon 14 splicing alterations occurs in multiple tumor types and confers clinical sensitivity to MET inhibitors. Cancer Discov 5:850–859
Gainor JF, Dardaei L, Yoda S et al (2016) Molecular mechanisms of resistance to first- and second-generation ALK inhibitors in ALK-rearranged lung cancer. Cancer Discov 6:1118–1133
Inc. P. Xalkori (Crizotinib) Prescribing Information. 2012
Kwak EL, Bang YJ, Camidge DR et al (2010) Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med 363:1693–1703
Lee J, Lee SE, Kang SY et al (2013) Identification of ROS1 rearrangement in gastric adenocarcinoma. Cancer 119:1627–1635
Lee J, Ou SH, Lee JM et al (2015) Gastrointestinal malignancies harbor actionable MET exon 14 deletions. Oncotarget 6:28211–28222
Matthay KK, George RE, Yu AL (2012) Promising therapeutic targets in neuroblastoma. Clin Cancer Res 18:2740–2753
Mok T, Kim D, Wu Y et al (2017) Overall survival (OS) for first-line crizotinib versus chemotherapy in ALK+ lung cancer: updated results from PROFILE 1014. Ann Oncol 28:v605–v649
Mosse YP, Voss SD, Lim MS et al (2017) Targeting ALK with crizotinib in pediatric anaplastic large cell lymphoma and inflammatory myofibroblastic tumor: a children’s oncology group study. J Clin Oncol: Off J Am Soc Clin Oncol 35:3215–3221
Okamoto W, Okamoto I, Arao T et al (2012) Antitumor action of the MET tyrosine kinase inhibitor crizotinib (PF-02341066) in gastric cancer positive for MET amplification. Mol Cancer Ther 11:1557–1564
Ordemann R, Stohlmacher J, Beuthien-Baumann B et al (2013) Use of targeted therapy for refractory ALK-positive anaplastic large cell lymphoma as a bridging strategy prior to allogeneic transplantation. Ann Hematol 92:125–127
Peters S, Camidge DR, Shaw AT et al (2017) Alectinib versus crizotinib in untreated ALK-positive non-small-cell lung cancer. N Engl J Med 377:829–838
Rodig SJ, Shapiro GI (2010) Crizotinib, a small-molecule dual inhibitor of the c-Met and ALK receptor tyrosine kinases. Curr Opin Invest Drugs 11:1477–1490
Sasaki T, Okuda K, Zheng W et al (2010) The neuroblastoma-associated F1174L ALK mutation causes resistance to an ALK kinase inhibitor in ALK-translocated cancers. Cancer Res 70:10038–10043
Shaw AT, Yeap BY, Solomon BJ et al (2011) Effect of crizotinib on overall survival in patients with advanced non-small-cell lung cancer harbouring ALK gene rearrangement: a retrospective analysis. Lancet Oncol 12:1004–1012
Shaw AT, Kim DW, Nakagawa K et al (2013) Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med
Shaw AT, Ou SH, Bang YJ et al (2014) Crizotinib in ROS1-rearranged non-small-cell lung cancer. N Engl J Med 371:1963–1971
Solomon BJ, Mok T, Kim DW et al (2014) First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med 371:2167–2177
Tang SC, Nguyen LN, Sparidans RW, Wagenaar E, Beijnen JH, Schinkel AH (2014) Increased oral availability and brain accumulation of the ALK inhibitor crizotinib by coadministration of the P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2) inhibitor elacridar. Int J Cancer 134:1484–1494
Tothova Z, Wagner AJ (2012) Anaplastic lymphoma kinase-directed therapy in inflammatory myofibroblastic tumors. Curr Opin Oncol 24:409–413
von Laffert M, Schirmacher P, Warth A et al (2016) Statement of the German Society for Pathology and the working group thoracic oncology of the working group oncology/German Cancer Society on ALK testing in NSCLC: immunohistochemistry and/or FISH? Pathologe 37:187–192
Yasuda H, de Figueiredo-Pontes LL, Kobayashi S, Costa DB (2012) Preclinical rationale for use of the clinically available multitargeted tyrosine kinase inhibitor crizotinib in ROS1-translocated lung cancer. J Thorac Oncol 7:1086–1090
Zou HY, Li Q, Lee JH et al (2007) An orally available small-molecule inhibitor of c-Met, PF-2341066, exhibits cytoreductive antitumor efficacy through antiproliferative and antiangiogenic mechanisms. Cancer Res 67:4408–4417
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Heigener, D.F., Reck, M. (2018). Crizotinib. In: Martens, U. (eds) Small Molecules in Oncology. Recent Results in Cancer Research, vol 211. Springer, Cham. https://doi.org/10.1007/978-3-319-91442-8_4
Download citation
DOI: https://doi.org/10.1007/978-3-319-91442-8_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-91441-1
Online ISBN: 978-3-319-91442-8
eBook Packages: MedicineMedicine (R0)