Abstract
Crizotinib is an ATP-competitive small-molecule inhibitor of the receptor tyrosine kinases (RTK) c-Met, anaplastic lymphoma kinase (ALK), and ROS1. There is convincing clinical evidence for the effectiveness in non-small-cell lung cancer (NSCLC) harboring EML4-ALK rearrangements resulting in constitutional activation of the ALK-RTK. The drug is approved for this entity, which represents no more than 3–5 % of all NSCLC. However, in this population, impressive response rates are generated. The same seems to be true for ROS-1 rearrangements; however, these only occur in approximately 1 % of all NSCLC. The role in c-Met altered cancers needs to be determined. Toxicities include visual impairment, nausea, peripheral edema, QT-prolongation, and liver enzyme elevation. Also, the occurrence of renal cysts is reported. Fluorescence in situ hybridization (FISH) detecting the ALK rearrangement has to be performed on tumor tissue to predict crizotinib efficacy. The role of immunohistochemistry in this setting needs to be determined. It has high concordance with FISH results when strongly positive or completely negative. The high efficacy of crizotinib in ALK- and ROS-positive lung cancer as new molecular targets beside the epidermal growth factor receptor (EGFR) underscores the importance of molecular typing in NSCLC.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Overall Survival
- Epidermal Growth Factor Receptor
- Epidermal Growth Factor Receptor Mutation
- Anaplastic Lymphoma Kinase
- Anaplastic Lymphoma Kinase Inhibitor
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Structure and Mechanism of Action
Crizotinib (R)-3-[1-(2,6-dichloro-3-flourophenyl)ethoxyl]-5-(1-piperidin-4-yl-1H-pyrazol-4-yl)pyridine-2-ylamine (Fig. 1) was initially developed as a second-generation, selective c-Met (mesenchymal to epidermal transition) inhibitor developed from a compound named PHA-665752 by Pfizer. This first-generation compound was modulated to become a potent small-molecule inhibitor of c-Met (Cui et al. 2011). It is an adenosine triphosphate (ATP) inhibitor of receptor tyrosine kinases. Besides c-Met, it inhibits anaplastic lymphoma kinase (ALK), ROS-1, and possibly other targets (Table 1) (Curran 2012).
Crizotinib in combination with its target, i.e., ALK, creates an inactive conformation of this oncogenic protein by inhibiting its phosphorylation as shown by crystalline structure analysis (Sasaki et al. 2010).
2 Preclinical Data
Much preclinical data were obtained on its property of inhibiting c-Met. The IC50 inhibiting the phosphorylation of wild-type c-Met in vitro in several human tumor cell lines has a mean of 4–8 nM. It inhibited cell growth and induced apoptosis in human GTL16 gastric carcinoma cell lines and supressed migration of tumor cells in vitro (Rodig and Shapiro 2010).
In ALK-translocated cell lines, crizotinib inhibited downstream effector functions and induced apoptosis (Christensen et al. 2007). Moreover, the compound has antiangiogenic properties in preclinical studies (Zou et al. 2007).
3 Clinical Data
3.1 NSCLC
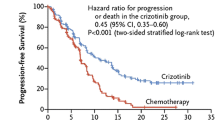
In a phase 1 trial, patients with any solid tumor and no further approved treatment option were treated with increasing doses of crizotinib. Two hundred and fifty milligrams bid was the maximum tolerated dose. In this cohort, two patients with non-small-cell lung cancer had improvements in tumor-symptoms. Thus, an expansion cohort was created consisting of patients with NSCLC harboring an EML4/ALK rearrangement. They received the maximum tolerated dose (250 mg bid) in continuous 28-day cycles (2010). Median progression-free survival was 9.7 months (95 % CI 7.7–12.8). Estimated overall survival (OS) at 6 and 12 months was 87.9 % (95 % CI 81.3–92.3) and 74.8 % (66.4–81.5), respectively; however, the median was not reached by time of the publication. Thirty-nine patients continued to receive crizotinib for more than 2 weeks after progression because of perceived ongoing clinical benefit from the drug (12 for at least 6 months from the time of their initial investigator-defined disease progression) (Camidge et al. 2012). These data were considered sufficient for accelerated approval of crizotinib in the United States. However, the European Medical Agency (EMA) demanded further trials to prove superiority of crizotinib compared to standard chemotherapy. This trial compared crizotinib to either pemetrexed or docetaxel (by investigators’ decision) in ALK-positive patients as second-line therapy (PROFILE 1007).Three hundred and eighteen patients were randomized to either crizotinib or chemotherapy. Primary endpoint was PFS. OS was not feasible, because crossover of chemotherapy patients into a single-arm crizotinib trial (PROFILE 1005) was pre-planned. A median of eleven cycles of crizotinib and four cycles of chemotherapy were administered, respectively. Chemotherapy consisted of docetaxel in 41 % of patients and pemetrexed in 57 % of patients, respectively. Median PFS was 7.7 versus 3.0 months favoring crizotinib [HR 0.49; confidence interval (CI) 0.37–0.64; p < 0.0001]. Interestingly, there was also a different PFS regarding to chemotherapy: Patients receiving pemetrexed had a median of 4.3 months compared to docetaxel with 2.6 months (p < 0.0001) with the difference between pemetrexed and crizotinib remaining significant (p = 0.0004, Table 2). Response rates to crizotinib, pemetrexed, and docetaxel were 65.7, 29.3, and 6.9 %, respectively. The preliminary data on OS showed no significant difference between crizotinib (20.3 months) and chemotherapy (22.8 months, p = 0.5394) because 111 out of 174 patients in the chemotherapy arm subsequently received crizotinib (Shaw et al. 2013a) and thus benefitted from the drug as well. These results are in line with all phase III trials of epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors in patients harboring EGFR-activating mutations: Despite impressive advantages in PFS compared to chemotherapy, no OS difference could be detected due to crossover to EGFR-TKI in subsequent lines of therapy (Hirsch et al. 2013).
There is indirect evidence that crizotinib prolongs OS in patients harboring an ALK rearrangement: In a retrospective comparison of 82 patients with ALK rearrangement receiving crizotinib, 36 patients with ALK rearrangement not receiving crizotinib, 67 patients with an activating EGFR mutation, and 253 patients with wild-type EGFR and ALK survival was compared. ALK-positive patients treated second or third line with crizotinib had a one-year survival of 70 % (95 % CI 50–83 %). ALK-positive patients treated with any other second- or third-line therapy had a one-year survival of 44 % (95 CI 23–64 %; HR 0.36; 95 % CI 0.17–0.75; p = 0.004). Survival of ALK-positive patients receiving crizotinib was comparable to those who harbor an activating EGFR mutation receiving an EGFR-TKI [one-year survival % (95 % CI 58–81) vs. 74 % (61–83)]. ALK-positive patients not treated with crizotinib had similar survival as “double-wild-type” patients [median OS 20 months (95 % CI 13–26) vs. 15 months (13–17)]; p = 0.244 (Shaw et al. 2011).
A frequent site of treatment failure is the central nervous system, possibly due to the fact that crizotinib concentration in cerebrospinal fluid is much lower than in blood plasma [cerebrospinal fluid-to-plasma ratio 0.0026 (Costa et al. 2011)] and many patients develop brain metastases in their relatively long course of crizotinib treatment. As suspected by the results of the PROFILE 1007 trial, continuation of crizotinib paired with local treatment (i.e., radiation) seems to be beneficial. In 21 ALK-positive patients who developed central nervous system progression and radiation to these lesions, seven continued crizotinib without further progression for more than 4 months (Takeda et al. 2013).
Some of the resistance mechanisms leading to crizotinib failure are already discovered. In fact, the first report on crizotinib resistance was published “back to back” with the first clinical efficacy results described above (Choi et al. 2010). Drugs which might overcome this resistance are in clinical development.
ROS-1 rearrangements occur in about 1 % of patients with NSCLC. ROS1 and ALK are “kissing cousins,” i.e., closely akin within the human kinome. In vitro essays with crizotinib showed that the drug was capable of inhibiting growth of ROS-1-positive NSCLC cell lines (Yasuda et al. 2012). So it was straightforward to test crizotinib in patients with a ROS-1 rearrangement. Patients were recruited in the dose escalation trial mentioned above (Kwak et al. 2010) as an own cohort. Forty patients harboring such a rearrangement could be identified. Thirty-five could be evaluated for response, and 32 were still receiving the drug at the time of data analysis. Overall response rate was 60 % with two complete and 19 partial responses. Disease control rate was 66 % at 16 weeks of treatment, and thus, median PFS is not reached at the time of first publication (Ou et al. 2013).
Data reporting the activity of crizotinib in c-MET-positive NSCLC cell lines suggest that its efficacy seems to be restricted to c-MET amplification rather than mutations in the corresponding gene (Tanizaki et al. 2011). A case report by Ou and colleagues reports on a patient with NSCLC harboring a c-MET amplification but no ALK rearrangement. Crizotinib resulted in a durable response in this patient (Ou et al. 2011). Because c-MET amplification is a known mechanism of EGFR resistance, a current trial investigates the safety and feasibility of combining crizotinib with a potent EGFR and HER-2 inhibitor [PF-00299804 (dacomitinib); NCT01121575].
3.2 Other Entities
In gastric carcinomas, ROS-1 rearrangements and c-MET amplifications are described (Lee et al. 2013; Okamoto et al. 2012) suggesting a possible benefit of crizotinib in these subsets.
Targets drugable with crizotinib are also found in Ewing sarcomas (Fleuren et al. 2013), anaplastic large-cell lymphoma (Ordemann et al. 2013), inflammatory myofibroblastic tumors (Tothova and Wagner 2012), chronic myelomonocytic leukemia (Cilloni et al. 2013), and neuroblastoma (Matthay et al. 2012) (Table 1). However, the clinical evidence is preliminary at best.
4 Toxicity
Crizotinib is comparably well tolerable. According to an analysis of phase I/II trials, most adverse events were mild to moderate with only 3–6 % of treatment interruption due to adverse events. In the order of frequency, the adverse events are the following:
-
Visual disturbances (in 62 % of patients) including light flashes or perception of overlying shadows or after images (Salgia et al. 2011). However, the disturbances were all short in duration and had minimal influence on quality of life or activities of daily living.
-
Nausea occurred in approximately 50 % of patients, diarrhea and vomiting occurred too. Again, these disturbances were mild and of short duration.
-
Further frequent side effects were peripheral edema, constipation, fatigue, decreased appetite, and dizziness (Xalkori 2012).
-
In 1.3 % of cases, administration of crizotinib can result in prolongation of the QT interval in the electrocardiogram (ECG) (Xalkori 2012). Patients already showing a prolonged QT interval or taking drugs known to prolong it should be monitored periodically with an ECG (Curran 2012).
5 Drug Interactions
Crizotinib is primarily metabolized in the liver by CYP3A which can result in drug interactions with CYP3A inducers like rifampicin, resulting in decreased plasma levels of crizotinib. CYP3A inhibitors like ketoconazole can lead to increased plasma levels of crizotinib. Finally, crizotinib itself may act as a CYP3A inhibitor, raising plasma levels of other substrates like midazolam (Curran 2012).
6 Biomarkers
The efficacy of crizotinib was tested in small subsets of patients with NSCLC, who are clearly defined on a molecular basis.
By now, three targets for crizotinib are identified: rearrangements in ALK and ROS as well as c-Met mutations. The gold standard for detection of ALK or ROS rearrangements is fluorescence in situ hybridization (FISH). For ALK, 15 % of cells must show this rearrangement for being classified as “ALK positive.” This number is not chosen arbitrary but represents the double standard deviation from the number of ALK rearrangements found in normal tissue. FISH is a time-consuming procedure and can only be done by specially trained personnel. A valid screening test is thus needed. Overexpression of ALK measured by immunohistochemistry (IHC) is a promising candidate tool as shown by Park and colleagues: In a series of 262 patients representing an enriched cohort (no EGFR mutation) of patients, IHC and ALK FISH were performed on formalin-fixed, paraffin-embedded tissue. ALK protein was expressed in 28 (10.7 %) tumors in 262 patients. ALK FISH was positive in 25 (9.5 %) cases. All patients with IHC score of 3 (n = 9) were FISH positive, and all patients with score of 0 (n = 234) were FISH negative. Among patients with IHC scores of 1 and 2, five (83.3 %, 5/6) and eleven (84.6 %, 11/13) were FISH positive, respectively. The sensitivity and specificity of ALK IHC with intensity score of 1 or more were 100 and 98.7 %, respectively. So maybe in the future, people with an IHC score of 3 do not need a FISH for confirmation (and can be classified as “ALK positive”) as do patients with a score of 0 (“ALK negative”), preserving the method for patients with an indeterminate IHC score of 1 or 2 (Park et al. 2012).
7 Summary and Perspectives
Crizotinib revealed to be a potent drug for a very small subset of patients with NSCLC, i.e., those with an ALK or ROS rearrangement. Its role in patients with a c-MET amplification remains to be clarified. The major challenge is the management of crizotinib resistance which will inevitably occur in almost every patient. Interestingly, many patients developing clinical progression under treatment with crizotinib do not show a resistance mutation on rebiopsy and are successfully treated with another ALK inhibitor with an even higher affinity to its target, LDK-378 (Shaw et al. 2013b).
Hopefully, in the future, we will be able to treat the majority of patients suffering from NSCLC on a molecular targeted base with similar success as those with ALK and ROS rearrangements (Table 2).
References
Camidge DR, Bang YJ, Kwak EL, Iafrate AJ, Varella-Garcia M, Fox SB et al (2012) Activity and safety of crizotinib in patients with ALK-positive non-small-cell lung cancer: updated results from a phase 1 study. Lancet Oncol 13(10):1011–1019
Choi YL, Soda M, Yamashita Y, Ueno T, Takashima J, Nakajima T et al (2010) EML4-ALK mutations in lung cancer that confer resistance to ALK inhibitors. N Engl J Med 363(18):1734–1739
Christensen JG, Zou HY, Arango ME, Li Q, Lee JH, McDonnell SR et al (2007) Cytoreductive antitumor activity of PF-2341066, a novel inhibitor of anaplastic lymphoma kinase and c-Met, in experimental models of anaplastic large-cell lymphoma. Mol Cancer Ther 6(12):3314–3322
Cilloni D, Carturan S, Bracco E, Campia V, Rosso V, Torti D et al (2013) Aberrant activation of ROS1 represents a new molecular defect in chronic myelomonocytic leukemia. Leuk Res 37(5):520–530
Costa DB, Kobayashi S, Pandya SS, Yeo WL, Shen Z, Tan W et al (2011) CSF concentration of the anaplastic lymphoma kinase inhibitor crizotinib. J Clin Oncol 29(15):e443–e445
Cui JJ, Tran-Dube M, Shen H, Nambu M, Kung PP, Pairish M et al (2011) Structure based drug design of crizotinib (PF-02341066), a potent and selective dual inhibitor of mesenchymal-epithelial transition factor (c-MET) kinase and anaplastic lymphoma kinase (ALK). J Med Chem 54(18):6342–6363
Curran MP (2012) Crizotinib: in locally advanced or metastatic non-small cell lung cancer. Drugs 72(1):99–107
Fleuren ED, Roeffen MH, Leenders WP, Flucke UE, Vlenterie M, Schreuder HW et al (2013) Expression and clinical relevance of MET and ALK in Ewing sarcomas. Int J Cancer 133(2):427–436
Hirsch FR, Janne PA, Eberhardt WE, Cappuzzo F, Thatcher N, Pirker R et al (2013) Epidermal growth factor receptor inhibition in lung cancer: status 2012. J Thorac Oncol 8(3):373–384
Kwak EL, Bang YJ, Camidge DR, Shaw AT, Solomon B, Maki RG et al (2010) Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med 363(18):1693–1703
Lee J, Lee SE, Kang SY, Do IG, Lee S, Ha SY et al (2013) Identification of ROS1 rearrangement in gastric adenocarcinoma. Cancer 119(9):1627–1635
Matthay KK, George RE, Yu AL (2012) Promising therapeutic targets in neuroblastoma. Clin Cancer Res 18(10):2740–2753
Okamoto W, Okamoto I, Arao T, Kuwata K, Hatashita E, Yamaguchi H et al (2012) Antitumor action of the MET tyrosine kinase inhibitor crizotinib (PF-02341066) in gastric cancer positive for MET amplification. Mol Cancer Ther 11(7):1557–1564
Ordemann R, Stohlmacher J, Beuthien-Baumann B, Platzek I, van den Hoff J, Kroschinsky F et al (2013) Use of targeted therapy for refractory ALK-positive anaplastic large cell lymphoma as a bridging strategy prior to allogeneic transplantation. Ann Hematol 92(1):125–127
Ou SH, Kwak EL, Siwak-Tapp C, Dy J, Bergethon K, Clark JW et al (2011) Activity of crizotinib (PF02341066), a dual mesenchymal-epithelial transition (MET) and anaplastic lymphoma kinase (ALK) inhibitor, in a non-small cell lung cancer patient with de novo MET amplification. J Thorac Oncol 6(5):942–946
Ou SH, Bang YJ, Camidge DR, Riely G (2013) Efficacy and safety of crizotinib in patients with advanced ROS1-rearranged non-small cll lung cancer (NSCLC). J Clin Oncol 31:8032
Park HS, Lee JK, Kim DW, Kulig K, Kim TM, Lee SH et al (2012) Immunohistochemical screening for anaplastic lymphoma kinase (ALK) rearrangement in advanced non-small cell lung cancer patients. Lung Cancer 77(2):288–292
Rodig SJ, Shapiro GI (2010) Crizotinib, a small-molecule dual inhibitor of the c-Met and ALK receptor tyrosine kinases. Curr Opin Invest Drugs 11(12):1477–1490
Salgia R, Solomon B, Shaw AT, Camidge DR (2011) Visual effects in anaplastic lymphoma kinase (ALK)-positive advanced non-small cell lung cancer (NSCLC) treated with crizotinib. J Clin Oncol 30:7596
Sasaki T, Okuda K, Zheng W, Butrynski J, Capelletti M, Wang L et al (2010) The neuroblastoma-associated F1174L ALK mutation causes resistance to an ALK kinase inhibitor in ALK-translocated cancers. Cancer Res 70(24):10038–10043
Shaw AT, Yeap BY, Solomon BJ, Riely GJ, Gainor J, Engelman JA et al (2011) Effect of crizotinib on overall survival in patients with advanced non-small-cell lung cancer harbouring ALK gene rearrangement: a retrospective analysis. Lancet Oncol 12(11):1004–1012
Shaw AT, Kim DW, Nakagawa K, Seto T, Crino L, Ahn MJ et al (2013a) Crizotinib versus Chemotherapy in advanced ALK-positive lung cancer. N Engl J Med 368:2385–2394
Shaw AT, Mehra R, Kim DW, Felip E (2013b) Clinical activity of the ALK inhibitor LDK378 in advanced, ALK-positive NSCLC. J Clin Oncol 31(suppl), abstr 8010. 2013
Takeda M, Okamoto I, Nakagawa K (2013) Clinical impact of continued Crizotinib administration after isolated central nervous system progression in patients with lung cancer positive for ALK rearrangement. J Thorac Oncol 8(5):654–657
Tanizaki J, Okamoto I, Okamoto K, Takezawa K, Kuwata K, Yamaguchi H et al (2011) MET tyrosine kinase inhibitor crizotinib (PF-02341066) shows differential antitumor effects in non-small cell lung cancer according to MET alterations. J Thorac Oncol 6(10):1624–1631
Tothova Z, Wagner AJ (2012) Anaplastic lymphoma kinase-directed therapy in inflammatory myofibroblastic tumors. Curr Opin Oncol 24(4):409–413
Xalkori (crizotinib) prescribing information. Pfizer Inc. 2012
Yasuda H, de Figueiredo-Pontes LL, Kobayashi S, Costa DB (2012) Preclinical rationale for use of the clinically available multitargeted tyrosine kinase inhibitor crizotinib in ROS1-translocated lung cancer. J Thorac Oncol 7(7):1086–1090
Zou HY, Li Q, Lee JH, Arango ME, McDonnell SR, Yamazaki S et al (2007) An orally available small-molecule inhibitor of c-Met, PF-2341066, exhibits cytoreductive antitumor efficacy through antiproliferative and antiangiogenic mechanisms. Cancer Res 67(9):4408–4417
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Heigener, D.F., Reck, M. (2014). Crizotinib. In: Martens, U. (eds) Small Molecules in Oncology. Recent Results in Cancer Research, vol 201. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-54490-3_11
Download citation
DOI: https://doi.org/10.1007/978-3-642-54490-3_11
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-54489-7
Online ISBN: 978-3-642-54490-3
eBook Packages: MedicineMedicine (R0)