Abstract
Sorghum bicolor, one of the world’s five most important crops, originated in Africa. While this has long been clear, accumulating data from both archaeobotany and genetics , provides the basis for a new overview on the domestication process, racial evolution, and geographical dispersal of sorghum . Archaeobotanical finds from 113 sites in Africa and Eurasia are reviewed and mapped. Of these only 16 provide identifications of probable morphological races . Domestication is evidently taking place more than 3000 years BC in the eastern Sudan near the Atbara and Gash rivers. Early domesticated race bicolor then spread to South Asia around 2000 BC and to the Niger Basin in West Africa after 1000 BC. The framework of five cultivated races remains useful, with the original domesticated race bicolor being characterized by tight-fitting hulls requiring dehusking and the other races representing subsequent parallel evolution for free-threshing and larger-grained cultivars. This took place at least three times, including race ‘caudatum’ focused initially on the Sahelian region race ‘durra’ that evolved probably in India , and race ‘guinea’ that evolved in forested West Africa. Early race guinea in turn produced an even more forest adapted ‘mageritiferum’ type that appears to be ancestral to southern African guinea and ‘kafir’ sorghums, implying a dispersal across the central African rainforests. In contrast other eastern African caudatums and ‘bicolor’ types presumably followed a savannah dispersal. In addition to the early dispersal of race bicolor from Africa to India , which was ancestral to East Asian sorghums, a later dispersal of guinea types is inferred to have taken place from southeastern Africa across the Indian Ocean.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Alongside wheat, barley, maize and rice, sorghum forms one of the five dominant staple cereal crops, and with pearl millet is one of the two main cereal crops to emerge from Africa. For much of Africa, the history of sorghum is the history of early farming. Yet despite its significance it is the only one of the five major cereals whose domestication is still relatively little understood in terms of the timing, location(s) of domestication , ecological environment, and the nature and length of this process.
Some 40 years ago Harlan and Stemler (1976) wrote a seminal paper “The races of Sorghum in Africa”, and together with “Variability in Sorghum bicolor” (de Wet et al. 1976), formed an important step in our understanding of sorghum domestication . Here, we revisit these papers presenting an update of the current state of knowledge, utilizing a database of archaeobotanical finds across Africa and Eurasia alongside recent insights from molecular genetics . After summarizing the variability of sorghum and genetic evidence for the relationships between different morphological and geographical populations we summarize the archaeobotanical evidence. We summarize new evidence for early cultivation and the domestication process in eastern Sudan , followed by a review of the chronology of the spread of sorghum across Africa and Asia, and the evidence for the evolution and dispersal of advanced free-threshing races of sorghum .
One of the challenges to documenting the cultural and evolutionary history of sorghum is a paucity of hard evidence in terms of archaeobotanical remains. Undoubtedly the biggest contributor to this problem has been the limited application of large scale and systematic archaeobotanical sampling programs in Africa, although these are now becoming increasingly common, with important results (e.g. Bigga and Kahlheber 2011; Giblin and Fuller 2011; Logan 2012; Crowther et al. 2016). Nevertheless, preservation on African sites is often poor, which is likely acerbated by both patterns of occupational mobility associated with the importance of pastoralism as well as high bioturbation in tropical soils. One important line of evidence has proved to be the impressions in ceramics from the use from plant harvesting or processing waste as temper (e.g. Stemler 1990; Manning and Fuller 2014; McClatchie and Fuller 2014; Beldados et al. 2018; Winchell et al. 2017). Such studies of ceramic plant fossils offers a method by which to gain some plant evidence from even the most deflated pastoral sites and can be studied from existing archives of ceramics of excavations or surveys in regions that may be less accessible to modern re-sampling.
The State of Knowledge 40 Years Ago
Research into the domestication of sorghum prior to the late 1960’s had undoubtedly been hampered by complications within the taxonomy of the species. The first significant attempt to decipher the cultivated sorghums was made by Dr Otto Stapf (1917), an Austrian born botanist and pioneer of archaeobotany (Stapf 1886), during his time at the Royal Botanical Gardens in Kew. However, it was soon clear on the completion of this work that further inquiry was needed and to this effect Hugh Charles Sampson, Kew’s first economic botanist, wrote to various Directors of Agriculture across the former British Empire requesting flowering and fruiting sorghum specimens that were sent to Kew (see Hill, in Snowden 1936, p. iii). The task to classify this material was handed to Joseph Davenport Snowden a recently retired economic botanist within the Department of Agriculture in Uganda. The culmination of this work was the “Cultivated Races of Sorghum ” (Snowden 1936) a meticulously detailed work that divided cultivated sorghums into 6 sub-series, comprising 28 cultivated species, and 156 varieties.
This work was later revised and simplified by Harlan and de Wet (1972) into five basic cultivated races and 10 intermediate races , based upon their seeds , glumes and inflorescence shape (see Table 1). The taxonomy outlined by Harlan and colleagues (Harlan and de Wet 1972; de Wet et al. 1976; Harlan and Stemler 1976) also proposed that wild, weedy and cultivated sorghums were treated as a single species, Sorghum bicolor, with five races of the domesticated subspecies bicolor, and four African wild races in the subspecies arundinaceum (now subsp. verticilliflorum). A wild sorghum , currently Sorghum propinquum (Kunth) Hitchc. was also classified as a separate race, but was noted to be the only race found outside Africa (de Wet et al. 1976). In Table 2 we provide a simplified comparison of a binomial taxonomy, in which wild forms and domesticated forms are kept as separate species (a simpler system to follow archaeobotanically), and the single species taxonomy de Wet (1977), updated by Wiersema and Dahlberg (2007). In this paper we will follow the binomial system.Footnote 1
The domestication of sorghum was proposed by Harlan and Stemler (1976) to have occurred in the savannah between western Ethiopia and eastern Chad, e.g. in the Republic of the Sudan from subsp. arundinaceum (correctly subsp. verticilliflorum (Steud.) de Wet ex Wiersema and Dahlberg 2007), presumably from the wild race aethiopicum, which is adapted to drier semi-desert/Sahel conditions. The oldest race they identified as bicolor on the basis of it being the least morphologically specialized, having the widest geographical distribution, its similarities to arundinaceum and that back-crossing the other races with wild sorghums resulted in hybrids displaying many of the bicolor characteristics (see de Wet et al. 1976).
The other four races are characterized by being free-threshing, in contrast to the tightly hulled grains of S. arundinaceum or race bicolor. Thus sorghum evolution parallels wheat and barley in the post-domestication selection for free-threshing forms (see Zohary et al. 2012; Fuller and Lucas 2014). Race guinea they saw as emerging in West Africa from primitive bicolors selected for tolerance of high rainfall, after which guinea spread back eastwards. Snowden (1955) speculated that wild race arundinaceum ( Sorghum arundinaceum (Desv.) Stapf.), found in the coastal regions of Guinea and Congo, hybridized with race bicolor to give rise to the local West African Sorghums (now classed as guinea). The origin of caudatum they saw as overlapping in range with that of bicolor, broadly in the Sudan region. They associated the dispersal of caudatum with “Chari-Nile ” languages (an internal grouping of Nilo-Saharan languages including East Sudanic, Central Sudanic), found today in South Sudan , parts of Ethiopia , Eritrea, Darfur and Nubia . The distribution of durra, being historically the dominant sorghum within both India and the Near East, as well as the north-eastern coastal regions of Africa and southern Sahara, suggested to Harlan and Stemler that the durra race originated outside Africa, within India or the Near East, from primitive bicolors that had been transported east in prehistory, adapting to drier regions, dispersing later back to eastern Africa. The final race kafir is largely grown by Bantu speaking peoples south of the equator. As such the race was identified as having arisen within southern Africa, potentially through hybridization with local, geographically separate wild race verticilliflorum sorghums (Shechter and de Wet 1975).
Chronologically, while bicolor was identified as the earliest race, appearing before 1000 BC they then hypothesised that guinea was most likely the second race to emerge due to its wide distribution. The third, durra, was seen as having emerged in India by the first millennium BC, and then spreading back to Africa. Guinea they speculated was around 2000 years ago taken directly from Ethiopia to north-west India , utilizing well established existing trade networks. That kafir was absent from India led them to postulate that this race emerged in southern Africa less than 2000 years ago, after guinea had spread from Africa to India . The most recent of the races , was suggested to be caudatum, due to its very limited distribution. At some time, probably by the early first millennium AD, they proposed that the Indian derived durra race came back west into northeast Africa via Egypt and the Sinai, a movement that led the writers of ancient Greece and Rome to speculate that sorghum came from India . The spread of race bicolor from India into China was seen to be via Burma (modern Myanmar) spreading into Indonesia and northwards into China by at least the mid first millennium AD (Hagerty 1941; Harlan and Stemler 1976). This last saw the emergence of the Chinese kaoliang, a variety of race bicolor (Snowden’s Sorghum nervosum Bess ex. Schult.). However, there was little archaeological evidence in the 1970s with which to corroborate this framework.
The Races of Sorghum as seen from Genetics
While Snowden (1936) originally envisaged multiple domestications, Harlan and colleagues proposed a single origin for bicolor with later separation of guinea, durra, kafir and caudatum, each involving some degree of introgressions with local wild populations. A schema for understanding sorghum evolution in these terms is illustrated in Fig. 1. In recent years a large number of genetic studies have examined the division of the five main races . In the majority of studies some clear differentiation has been seen between the later races ; guinea, durra, kafir and caudatum, with race bicolor showing much less clear differentiation (Deu et al. 1994, 2006; Casa et al. 2008; Mace et al. 2008; Brown et al. 2011). An earlier study demonstrated that cultivars were most closely aligned with wild populations of sorghum within central-northeast Africa (Aldrich and Doebley 1992). This study and others also note two to three strongly differentiated haplotype groups highlighting the problems of differentiating between potentially separate domestication events and later migration events with introgression from local wild populations (de Alencar Figueiredo et al. 2008; cf. Lin et al. 2012).
The sorghum evolutionary framework relating wild, domesticated and the major domesticated races . Horizontal axis show seed dispersal from wild shattering to free‐ threshing; vertical axis the development of panicle form/density. Solid arrows indicate the main direction of evolution and improvement. Dashed lines indicate hypothetical introgression from wild populations.
Genetic studies, while recognizing the separation of the races , also note divisions within them. Guinea showed separation into four clusters; two closely related in West Africa, and two related clusters in Southern Africa and Asia (Deu et al. 1994, 2006). Further the populations of sub-race margaritiferum of West Africa, with small spherical grains, clearly separated from other guineas (Deu et al. 1994, 2006; Casa et al. 2008; de Alencar Figueiredo et al. 2008; Brown et al. 2011; Ramu et al. 2013; Luo et al. 2016), showing close affinity to local wild and weedy sorghums (Billot et al. 2013; Morris et al. 2013) of wild race arundinaceum. Other studies also demonstrate a genetic affinity between guinea populations of southern and Eastern Africa with those of South Asia (Folkertsma et al. 2005), supporting a hypothesis suggesting the migration of this crop during Indian Ocean maritime trade networks from the 8th century AD onwards (see Boivin et al. 2014). Other studies show Indian guineas clustering more closely with durras (Morris et al. 2013), which might be accounted for by ongoing introgression across Indian crop populations, or both having introgression from wild Sorghum propinquum.
These studies demonstrate the need to reclassify some of the sub-races . One clear example is the West African kaura group (Snowden’s S. caudatum var. kerstingianum) which clusters with guineas, not with durra-caudatums into which they are traditionally ascribed (Brown et al. 2011). Genetically kaura sorghum is then derived from race guinea, but later evolved tighter, compacted panicles and shorter glumes, characteristic of the durra and caudatum races respectively, perhaps in response to the drier regions of northeast Nigeria , where they are most often found.
While the general implication is that genetic studies broadly support the morphological separation of the races , one of the largest and most recent studies by Billot et al. (2013) highlights the importance of geographic distribution with respect to genetic differentiation. For example, eastern Asian caudatums, durras and bicolors appear more closely related to each other than to these same races in Africa (Billot et al. 2013). What remains unclear is whether this implies that free-threshing forms evolved many times in parallel or whether the movement of free-threshing races was followed by extensive introgression with locally adapted landraces of bicolor. The latter is perhaps more parsimonious, requiring fewer selection events for free-threshing morphotypes. What is needed is an understanding of the particular alleles that create the various free-threshing morphotypes.
Durra showed strong separation in most studies (Deu et al. 2006; Perumal et al. 2007; Casa et al. 2008; Mace et al. 2008; Brown et al. 2011). Within the study conducted by Brown et al. (2011) Asian durra sorghums clustered together, but were most closely related to durras from Somalia, and Ethiopia . Other studies demonstrate similarities between northern Chinese Kaoliangs (traditionally classified as bicolor due to their persistent husks; Snowden’s S. nervosum) with durra types, especially from Yemen (Morris et al. 2013). This might suggest that durra evolved from the same South Asian populations that dispersed to China, a process still poorly documented archaeologically, but probably dated to the early to mid-first millennium AD from northeast India via Yunnan (Hagerty 1941; Bonjean 2010).
Those assigned to the unfortunately named kafir (Snowden’s S. cafrorum) exhibited strong separation in all studies (Deu et al. 1994, 2006; Casa et al. 2008; Mace et al. 2008; Brown et al. 2011). The low diversity seen within kafir, along with West African guineas, suggests bottlenecks relating to geographical isolation (Casa et al. 2008; see Deu et al. 2006). A study on photoperiod indicated that guinea types brought from West Africa into southern Africa, potentially gave rise to kafirs, adapted to longer-day ecosystems. Perhaps associated with Bantu-speaking groups that crossed the central African rainforest and migrated slowly southwards (cf. Klein et al. 2015). This would represent the west and southwest Bantu stream via the Sangha River Interval (sensu Grollemund et al. 2015), implying an arrival in southern Africa later than the initial sorghum associated with early Iron Age Eastern Bantu migrants.
Caudatums split into two clusters, the main one geographically centred on the Great Lakes Region of East Africa e.g. Tanzania, Malawi, Zambia, Kenya, Uganda (Deu et al. 1994, 2006), but stretching into Ethiopia and Sudan (Brown et al. 2011). However, many studies demonstrated sorghum lines assigned to caudatum outside this geographical area falling into separate groups (see Deu et al. 1994, 2006; Mace et al. 2008; Billot et al. 2013). Studies also separated out zerazera-caudatums (Snowden assigned as a form of S. caudatum var. durum), found mainly in the border region between Sudan and South Sudan but stretching southeast, from West African and Central Sudanese guinea-caudatums (probably genetically guineas) and other caudatums centred mainly on South Sudan , Uganda and northeast Congo, with some further groups in South Africa (Sukumaran et al. 2012; Casa et al. 2008).
Many studies imply at least two separate introductions of sorghum into Asia (Deu et al. 2006). The first was an early bicolor that evolved into durra and Chinese kaoliang (S. nervosum). The second presumably was an east African guinea variety traded from the Swahili world. The origins of these bicolors in northern Sudan would explain the genetic affinities to some central African caudatums (cf. Billot et al. 2013), which also evolved in northern Sudan . Guinea sorghums often have a hard, corneous endosperm, making the grains suited to whole-grain boiling, and these forms are popular in the tribal zones of eastern and central India (Appa Rao et al. 1996). Whether or not caudatum sorghums in India represent a third ancient introduction, a recent introduction, or rather are just morphologically similar adaptions from durra and guinea/kafir types remains to be determined. Notably, one caudatum type from India examined by Brown et al. (2011) was genetically closer to kafir, implying that the transition between guinea/kafir and caudatum morphology (i.e. a c. 45° twist in the grain primordium) represents a simple genetic shift that occurred locally in Indian guinea sorghum .
While most genetic research is congruent with the conventional view of a single major domestication episode of sorghum , followed by differentiation into races during and after geographical dispersal, a high profile study of non-shattering genetics argued for three independent domestications, two in northern Africa and one in the southeast (Olsen 2012). This is based on the identification of three alternative dominant mutations to the sorghum gene Sh1 on chromosome 1 that turn off the abscission zone at the base of the spikelet, and have differing geographical distributions. One of these mutations (Tx623) is found in kafir, bicolor and guinea sorghum races from southern Africa, another (Tx430) is largely restricted to caudatums throughout Africa, and a third (SC265) is found across all races except kafir, in both Asia and Africa (Lin et al. 2012: Table S1). Before considering the implications of this study, some caution is warranted, as early claims to have identified the key mutation for non-shattering in rice domestication (e.g. Li et al. 2006, 2007) have been overturned with evidence that the interaction of several loci is involved and that these evolved sequentially, some probably only in limited post-domestication lineages (e.g. Oryza qsh1) (Ishii et al. 2013; Htun et al. 2014). If multiple initial domestication episodes of race bicolor had involved other loci that also caused reduced shattering, then a higher degree of non-shattering might be expected to be seen as advanced free-threshing races evolved, much as the free-threshing Q gene was added to the non-shattering Br1 mutations that was involved in hulled wheat domestication (Li and Gill 2006; Fuller and Allaby 2009: 251). Thus we predict that sorghum Sh1 was involved in post-domestication improvements to non-shattering rather than to the initial evolution of non-shattering during race bicolor domestication . This may be further supported by the many race bicolor accessions reported as having only “moderate” non-shattering phenotypes in the USDA database (NPGS 2016), whereas only strongly shattering domesticated and non-shattering wild accessions were included in the study of Lin et al. (2012). Looking more closely at these data (Lin et al. 2012: Table S1), they can be reduced to just two pathways as the kafir type (Tx623) variant could be derived from further mutations (mainly a large deletion and 2 SNPs) to the caudatum type (Tx430) haplotype. By contrast the third variant (SC265) appears to be caused by a small number of different SNPs, shared across a range of races and regions, including widespread race bicolor accessions. This could be congruent with evolution of Sh1 twice, once within the western, the other towards the eastern parts of the zone of initial north Sudanese sorghum domestication (see archaeobotanical discussion, below). These mutations were plausibly additive to other alleles, not yet characterized, that are associated with earlier moderate non-shattering phenotypes (of which there are many varieties among Sudanese race bicolor, but none of them were included in the genetic study of Lin et al. 2012).
An Updated Archaeobotanical Framework of Sorghum Domestication
Domestication represents a number of adaptations on the part of a plant to being cultivated and harvested by people (see Harlan et al. 1973; Fuller et al. 2010, 2014). Key changes include loss of natural seed dispersal (visible archaeologically in spikelet bases), changes in germination traits (not easily visible archaeologically), and increase in grain size, most notably grain width (measurable archaeologically). To date, however, few archaeobotanical assemblages have allowed these domestication traits to be documented for sorghum .
To begin wild sorghum was likely gathered with baskets as stands of wild-type shattering plants matured. Such spikelets would still have had tight fitting husks and hence required dehusking, then winnowing, to obtain clean grain. With the evolution of non-shattering (domesticated) type plants, threshing and a further winnowing stage would be required, in order to break the rachises and secondary branches and hence separate the spikelets from the harvested panicles. Possibly winnowing/sieving practices already existed following the basket gathering of wild spikelets, in order to remove lighter plant parts and seeds from other wild species collected at the same time; the spatial patterning in wild sorghum versus other grasses in Nabta Playa contexts could be indicative of such practices (cf. Wasylokowa and Dahlberg 1999, 2001).
The development of non-shattering (domesticated) plants would have necessitated more labour input for processing, while new techniques and technologies of harvesting, such as sickles, would have been promoted. Following the trends for other cereals, cultivation would likely have selected for larger grains, leading to increased yields (Fuller et al. 2010). Subsequent evolution of the free-threshing sorghum races would have reduced and shifted the labour investment in processing. Grains would no longer have needed to be routinely dusked and winnowed, but the removal of chaff during threshing would potentially have prolonged winnowing operations following the harvest. As such practices were likely conducted seasonally and in the field, free-threshing sorghum may be less prone to preservation by charring than race bicolor. The dense panicles in some advanced races also facilitated harvesting, as well as increasing yields. Thus when considered in terms of harvesting, processing and related aspects of morphology, we can outline the morphological variation of sorghum , represented by its domestication and racial evolution (Fig. 1).
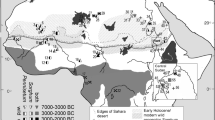
The first step in studying domestication is reconstructing the distribution of wild progenitor populations for the period in the past when cultivation is likely to have begun (Fig. 2). However, the distribution of these populations today is quite different from what they would have been at the start of the Holocene . Under the wetter conditions of the early to middle Holocene , the era of a Green Sahara (Kröpelin et al. 2008; Manning and Timpson 2014), the savannahs with sorghum were located much further north, approximately midway across the modern Sahara. This is supported by archaeobotanical finds of morphologically wild sorghum in the Western Desert of Egypt and southwest Libya (Fig. 2), dating to the early to middle Holocene , the earliest at Nabta Playa E-75-6, c. 8000 BC (Wasylikowa and Dahlberg 1999), with later finds from Farafra, c. 5800 BC, and Abu Ballas, c. 3750 BC (Barakat and Fahmy 1999). These sites provide no evidence for cultivation but it is likely that wild grains were collected (see Wasylikowa and Dahlberg 2001). Continued wild sorghum use in the 5th and 4th millennium BC, is indicated by impressions in pottery from central Sudan (Magid 1989, 2003; Stemler 1990) in the Khartoum region. These comprise Neolithic societies with evidence for domesticated sheep, goat and cattle (Gautier and van Neer 2006; Chaix and Honegger 2015). This shift in the evidence is congruous with a southerly retreat of wild sorghum stands as the Sahara dried and expanded. Possibly this involved southward migrations, and would fit with Nilo-Saharan proto-language reconstructions (Ehret 2014) and evidence for the southward spread of pastoralism. The evidence presumably relates to exploitation of S. verticilliflorum race aethiopicum, while other wild sorghums so far lack evidence for exploitation. Wild Sorghum sp. identified in first millennium BC Yemen (de Moulins et al. 2003) seems most likely race verticilliflorum on geographical grounds.
Distribution of wild modern and archaeological sorghum . Hatched area represents approximate/potential modern distributions of wild sorghum races (S. verticilliflorum) after Harlan and Stemler (1976). Triangles indicate archaeological sites with finds of morphologically wild sorghum : 1. Takarkori (7000–6200 BC), 2. Adrar Bous (3500–1500 BC), 3. Kursakata (1000–200 BC), 4. Farafra Oasis (6060–5560 BC), 5. Amarna (1365–1345 BC), 6. Dalkhleh Oasis sites (8300–7000 BC), 7. Abu Ballas (4500–3000 BC), 8. Tutankhamun ’s tomb (1328 BC), 9. Nabta Playa E‐75‐6 (8100–7900 BC), 10. Qasr Ibrim (1000–300 BC), 11. Rabak (4500–4300 BC), 12. Sabir (1000–800 BC), 13. El Kadada (4000–3000 BC), 14. Shaheinab (4100–3300 BC), 15. Shaqadud (3500–2200 BC), 16. El Zakiab (4300–4000 BC), 17. Kadero (3800–3600 BC), 18. Um Direiwa (5000–3800 BC), 19. El Mahalab (6650–5800 BC), 20. Sheikh el‐Amin (7000–6450 BC), 21. Sheikh Mustafa (4400–3300 BC), 22. Mahal Teglinos K1 (2000–1700 BC), 23. Khashm el Girba KG23 (3500–3000 BC).
While Stemler (1990) identified a single impression of a possible “domesticated-type” spikelet with a torn rachilla from Um Direiwa (> 3800 BC) this might still relate to the harvesting of green or near-ripe wild sorghum . However, a larger dataset of sorghum pottery impressions has recently come to light dating to the mid to late fourth millennium BC at Khashm el Girba KG23 near Kassala (Winchell et al. 2017). These new data consist of 265 plant impressions of sorghum grains and spikelets preserved in sherds of Khordhag plain pottery of the Butana group period from site KG23 (see Winchell 2013 for archaeological details). Among these are 16 impressions from 9 sherds, that clearly preserve evidence for non-shattering sorghum spikelet bases with torn rachilla (Fig. 3a, b), while 18 other impressions spread across 13 sherds preserve wild type smooth spikelet bases (Fig. 3c, d). A further 17 impressions were identified as recognizably immature. Taken together these data suggest the harvesting of cultivated populations undergoing domestication .
Examples of archaeological sorghum relating to early domestication and racial differentiation: A. modern wild shattering spikelet of S. verticilliflorum; B. wild‐type sorghum spikelet impression in ceramics, Khashm el Girba KG23 (studied by the authors; Winchell et al. 2017); C. domesticated, sorghum spikelet base impression with rachilla, Khashm el Girba KG23 (studied by the authors; Winchell et al. 2017); D. modern domesticated spikelet base, race bicolor, after grain removal; E. race bicolor type grain, Early Historic Paithan, Maharashtra, India (Fuller in press); F. race caudatum type grain, SEM/sketch of dorsal and lateral, Tinda B, Libya (after Pelling 2005); G. caudatum(?) type grain, Kawa, Nubia , dorsal and lateral view (after Fuller 2004); H. caudatum type grain, poorly preserved surface in dorsal and lateral views, Early Iron Age Kabusanze, Rwanda (after Giblin and Fuller 2011); I. caudatum type grain, Middle Iron Age Panga Ya Saidi, Kenya (after Shipton et al. 2013); J. durra type grain, Early Historic Paithan, Maharashtra, India (Fuller in press); K. durra type grain, Meroitic Hamadab, Sudan (authors, unpublished); L. bicolor type grain, Meroitic Hamadab, Sudan .
These sorghum spikelets, comprising mature non-shattering types, mature shattering wild types, and immature “green” types, are consistent with the harvesting of populations still displaying asynchronous ripening. Hence, during these early stages of pre-domestication cultivation, harvesting was conducted before all plants had matured to avoid grain loss caused by the still high presence of wild-type shattering plants. This same practice has been observed during the earlier stages of pre-domestication cultivation for rice in the Lower Yangtze, where the “green” harvesting of rice resulted in charred archaeobotanical assemblages that included immature spikelet bases, wild type spikelet bases and non-shattering domesticated types (Fuller et al. 2010). These finds, together with those from the early second millennium BC at Kassala K1 (Beldados and Costantini 2011; Beldados et al. 2018) suggest a protracted period of pre-domestication sorghum cultivation from before 3500 BC to perhaps c. 1700 BC. These data are consistent with the protracted domestication episodes of 2000–4000 years documented for other cereals, including wheats, barley and rice (Fuller et al. 2014).
These new finds indicate that morphologically domesticated sorghum was present in eastern Sudanese populations, even if these types were not fully dominant before 3000 BC. They fit with the region of domestication hypothesized by Clark (1984), lying within the confluence of the Blue and White Nile , and the Atbara River, but are earlier than he anticipated. Of particular importance the Kassala evidence pre-dates finds in India , where domesticated sorghums are well established as being present by at least the early second millennium BC (Fuller 2003a; Fuller and Boivin 2009). Although grains from the Harappan site of Kunal are considered earlier (c. 2400 BC) (Saraswat and Pokharia 2003), these are not-directly dated and full details of their context are not available, so caution is warranted in accepting them as mid third millennium BC. It should be noted also that older reports of sorghum from the Arabian Peninsula are problematic. Impressions from the site of al-Raqlah in Yemen, 3000–2500 BC (Costantini 1990) are poorly illustrated and hard to accept as legitimate (Rowley-Conwy et al. 1997; Fuller 2002, 281–2; de Moulins et al. 2003; Fuller and Boivin 2009): these are too round for an early bicolor form that would be expected at such an age. Clearly mis-identified are also the often cited impressions of sorghum from third millennium BC Hili in Oman (Cleuziou and Costantini (1980), Fig. 2; first questioned by Willcox (1992)), which show fruits attached on the jointed ends of flared pedicels, that are consistent with Asphodelus sp. (Liliaceae), a common weed of Arabian wheat and barley cultivation in macroremains assemblages of the period (Tengberg 2003). Taken together these data suggest that cultivated, at least partially domesticated, if not near fully domesticated sorghum was rapidly transferred from eastern Sudan /Eritrea to western India (Gujarat) by the start of the second millennium BC. It is not clear whether there was a geographically intermediate stage of sorghum cultivation in Yemen or southern Oman (Dhofar) (for further discussions of the mechanisms and cultural context, see Boivin and Fuller 2009). Once separated from wild gene flow and adopted into the established wheat, barley or millet cultivation regimes it is probable that the non-shattering traits became fixed in populations relatively quickly, thereby reaching full domestication more or less upon its arrival in Western India . Therefore contrary to the Haaland (1995) hypothesis there is no reason to postulate the transfer of a morphologically wild sorghum to India and a return voyage of domesticated race bicolor. Nevertheless, further systematic study of early Indian sorghum is needed to trace domestication traits and racial evolution within South Asia, although most early finds are plausibly race bicolor (e.g. Figure 3e).
Indian historical linguistics are unable to shed further light on this issue. Indic terms for sorghum (e.g. Prakrit gajja, Sanskrit yavanala) are likely derived from older roots for barley. Those in Dravidian languages possibly represent a semantic shift from an older millet term, e.g. for Brachiaria ramosa (Southworth 2006), as sorghum appears to reconstruct to an earlier stage of the Dravidian languages than either pearl millet or wheat (Fuller 2003b).
Several hypotheses derived from historical linguistic inferences suggest the migration of sorghum -growing farmers into various parts of Africa, although discrepancies with available archaeobotanical data remain, especially in terms of the inferred date of these dispersals. For example, while early Nilo-Saharan speakers focused on pastoralism in the eastern Sahara and Sahel, probably alongside wild sorghum harvesting, new terms for cultivation appear later in the Proto-Saharo-Sahelian stage (Ehret 2014), potentially attributed to the later middle Holocene , e.g. c. 4000 BC. As reviewed above archaeobotancial finds from the central and eastern Sudan suggest cultivation was established by 3500 BC with morphological domesticated types already present. As such sorghum cultivators a millennium or more earlier might be sought further north. However, it is hard to determine whether sorghum cultivation in this region, which generally shows local continuity through much of the earlier to middle Holocene (Winchell 2013), was brought by immigrants arriving from the north, although cattle, sheep and goat must have spread this way. Ehret (2011) also inferred that Afroasiatic speakers, from the Cushitic lineage probably had sorghum cultivation when they moved south from the Southern Atbai region into the Ethiopian highlands (by c. 3500 BC). However, it is hard at present to reconcile this with the, albeit limited, archaeobotanical data that initially show just wheat and barley in the 1st millennium BC, and only later finds of sorghum and the indigenous Ethiopian tef (D’Andrea and Wadge 2011). Nevertheless the new evidence for sorghum cultivation in the 4th millennium BC, and presumably through the early second millennium BC, in eastern Sudan might support this. Further, the arrival of domesticated sorghum in India by c. 1700 BC (Fuller 2003a; Fuller and Boivin 2009), makes it highly plausible that during the third millennium BC sorghum -growing agropastoralists were expanding through the Eritrean region and hence potentially further south into parts of Ethiopia .
Dispersal and Diversification of Sorghum: The Archaeobotany of the Races
After its establishment as a crop in parts of Sudan , e.g. Bayuda to Southern Atbai belt, morphologically domesticated sorghum dispersed and eventually diversified. Archaeobotanical finds, although still few and far between in Africa allow us to sketch the arrival time of sorghum into various regions and thereby infer how quickly it spread (Fig. 4). In some cases sorghum is likely to have diffused as part of a package of crops and animals with migrating farmers, although sorghum was also transferred across language families.
Map of the distribution of archaeological domesticated sorghum in broad time slices, 1. Alibori SIII, 2. Mege, 3. Kawa, 4. Um Dereiwa, 5. Khashm el Girba, 6. Kasala K1, 7. Rojdi, 8. Pirak, 9. Ojiyana, 10. Banawali & Kunal, 11. Rohira, 12. Sanghol, 13. Hulas, 14. Imlidh-Khurd, 15. Malhar and Raja-Nala-Ka-Tila, 16. Senuwar, 17. Kaothe (intrusive?), 18. Tuljapur Garhi, 19. Daimabad, 20. Inamgaon, 21. Piklihal, 22. Miranduolo, 23. Ferrara, 24. Ingiliz, 25. Jarma & Tinda, 26. Kom el-Nana, 27. Karga Oasis, 28. Mon Claudianus, 29. Quesir al-Qadim, 30. Berenike & Shenshef, 31. Wadi Qitna, 32. Qasr Ibrim, 33. Faras East, 34. Umm Muri, 35. Essouk, 36. Dangeil, 37. Hamdab & Meroe, 38. Soda, 39. Jebel Qeili, 40. Jebel Tomat, 41. Abu Geili, 42. Axum, 43. Tuwatey Zonga Cave, 44. Sanghol, 45. Sanchankot/Ramkot, 46. Wari-Bateshwar, 47. Nevasa, 48. Paithan, 49. Bhokardan, 50. Bhon & Paturda, 51. Bhatkuli, 52. Paunar, 53. Mantai, 54. Kabusanze, 55. Kabuye (possible pollen ), 56. Engaruka, 57. Mgombani, 58. Panga Ya Saidi, 59. Tumbe, 60. Fukuchani, 61. Unguka Ukuu, 62. Juani Primary School & Kilwa & Songo Mnara, 63. Mikindani sites, 64. Old Sima, 65. M’teteshi & Mondake, 66. Xakota, 67. Nqoma, 68. Leopard’s Kopje, 69. Kgaswe, 70. Matlhapaneng, 71. Mapela, 72. Schroda, 73. Magogo, 74. Dia-Mara & Sorotomo, 75. Dia-Shoma, 76. Jenne-jeno, 77. Oursi hu-beero & Kolel Nord, 78. Banda 13 & 27 & 41 & Kuulo Kataa & Ngre Kataa, 79. Yohongu, 80. Pekinga, 81. Tintin, 82. Birnin Lafiya & Kantoro, 83. Madekali, 84. Elkido North & Dorota, 85. Mege & Kursakata, 86. Daima, 87. Kayam & Jidderi Saoudjo & Balda Tagamre & Tchere & Goray & Salak & Mowo & Louggero, 88. Douloumi & Be. Key sources in addition to those cited in the text, include: (Fuller 2014; Mitchell 2002; Logan 2012; Nixon et al. 2011; Ruas et al. 2011; Delneuf 1995).
The westward dispersal of sorghum has not been tied to linguistically inferred ethnic migrations, but evidence for sorghum in northern Benin prior to the local Iron Age (c. 700 BC, see Champion and Fuller, 2018), and probable evidence for sorghum at Mege, near Lake Chad, at 800–400 BC (Bigga and Kahlheber 2011), indicate a westward diffusion in the first millennium BC. Evidence for pearl millet as early as sorghum in India , i.e. 1900–1700 BC, and possible cowpea (Fuller 2003a; Fuller and Boivin 2009) indicate that established cultural networks allowed pearl millet to diffuse eastward from West Africa probably shortly after the mid third millennium BC. Pearl millet is a crop highly suited to mobile agro-pastoralists with a short growing season and low water requirements, which may have facilitated its rapid spread eastwards over less than a millennium along the southern margins of the Sahara (Manning and Fuller 2014). These same social networks are likely to have been responsible for the less rapid spread of caudatum westwards, after free-threshing caudatum evolved in the central Sudan .
Two distinct westward trajectories can be advocated. Race bicolor likely dispersed westwards across the southern savannah, towards Nigeria , Cameroon and Benin, early in the first millennium BC, and some grains from Birnin Lafiya in Benin suggest local evolution of guinea types by the early centuries AD (Table 3), likely involving gene flow from wild race arundinceum sorghums. By contrast more northerly and Sahelian dispersal for caudatum can be postulated, presumably from origins in Sudan . This is indicated by finds at Jarma in the Fezzan in Libya by 100 BC (Fig. 3e; Pelling 2005, 2007), Jenne-Jeno, Mali by the 6th/7th century AD, and Daima near Lake Chad in the 9th or 10th century (Connah 1967, see Table 3). The still un-replicated evidence from Kawa in Nubia , 800–400 BC, might yet hint at earlier origins for race caudatum (Fig. 3f). Meanwhile the caudatum like grains from Kaothe in India probably should be regarded as intrusive, as suggested by Kajale (1990).
Moving to the south of the Ethiopian highlands evidence for sorghum is much later. Securely identified and dated finds come only from the early centuries AD in East Africa, including third or fourth century AD finds from Kabusanze (Fig. 3g) (Giblin and Fuller 2011), and from Zimbabwe, Leopard’s Kopje, in the 10th or 11th Century AD (Huffman 1974; Mitchell 2002, 274). De Wet (1977) suggested the latter, based on geography rather than morphology, could represent an early southern African guinea. Along the East African coast and near shore islands, sorghum is present in Middle Iron Age levels from the second half of the first millennium AD, e.g. at Panga Ya Saidi in southeast Kenya, Pemba, Zanzibar and Mafia Islands (Walshaw 2010; Boivin et al. 2013; Crowther et al. 2014, 2016). Many of these finds include grains resembling caudatum (Fig. 3H; Table 3), fitting with the hypotheses that caudatum, evolved in the Sudan (Harlan and Stemler 1976; Brown et al. 2011), and was transferred from Nilo-Saharan speaking farmers to the early dispersing Bantu near the southern Sudanese region as Bantu farmers moved around the rainforest (Ehret 1973; Schoenbrun 1993; Boivin et al. 2013).
The general pattern in all of these areas of East Africa is that the earliest farming is associated with an eastern Bantu crop package, including pearl millet , cowpea, baobab, and sorghum , with finger millet appearing later (Crowther et al. 2016). Small quantities of crops derived from Asia across the Indian Ocean arrived on African islands from the 8th century AD, including rice (Oryza sativa), mungbean (Vigna radiata), cotton (Gossypium sp.), and coconut (Cocos nucifera). Therefore, we lack any firm evidence for contact between tropical Asia and eastern Africa south of Ethiopia earlier than c. AD 700, despite the distribution of taro, Asian yam, and bananas in continental Africa that have been suggested to relate to much earlier trans-Indian Ocean transfers (e.g. Blench 2009; see also Fuller and Boivin 2009; Boivin et al. 2014). The presence of apparent race guinea in parts of India , has however suggested Indian Ocean transfers out of southeast Africa, later than the posited Bronze Age circum-Arabian transfer of race bicolor (see Fuller 2003a). Genetic data indicate southeast African guineas derive from margaritiferum types in the West African rainforests, which imply a dispersal from western to southern African and then across the Indian Ocean.
Archaeobotanical finds referred to as durra are restricted to regions where durra is prominent today, including peninsular India and Nubia (Sudan ). Durra types are present at the early historic city of Paithan, Maharashtra (Fig. 3i) from nearly 2000 years ago, while finds from Meroe and nearby Hamdab (Fig. 3j, k) suggest a broadly similar age in Nubia (Table 3). The northerly dispersal of durra down the Nile to Lower Nubia is seen from probable intermediate durra-bicolor morphotypes at late post-Meroitic Qasr Ibrim (c. AD 500), eventually dominating in the Christian and Medieval periods (Rowley-Conwy et al. 1999; Clapham and Rowley-Conwy 2007). Nevertheless, race bicolor remained important in this region, as indicated by 12th century AD finds at Nauri near the Third cataract (Fuller and Edwards 2001), and Quesir on the Red Sea Coast (van der Veen 2011); the illustrated sorghum from Byzantine Kom el-Nana is also race bicolor (see Smith 2003). The hulled grains of race bicolor are likely more resilient under various storage conditions, and less prone to bird predation, hence still have advantages over the free-threshing races . The transfer of sorghum into Mediterranean agriculture , in central and northern Italy (e.g. Bosi et al. 2009; Buonincontri et al. 2014), notably also involved race bicolor having similar cultivation, storage and processing requirements to Setaria italica and Panicum miliaceum that were already well-established.
Sorghum Diversification and Dispersal: A Revised Framework
We are now in an improved position to outline the history of sorghum diversification and dispersal (Fig. 5) as a revision to the schema of Harlan and Stemler (1976). While genetics has revealed a more complex series of interregional relationships than was originally envisaged in the five race classification, archaeobotanical specimens that can be referred to various advanced free-threshing races , or as plausibly free-threshing, provide a framework of dates for when races differentiated and arrived in different regions (Table 3). We assume that bicolor spread along all of these paths, either accompanying or preceding the advanced free-threshing races (Fig. 5). The overall pattern suggests sorghum domestication and subsequent evolution of caudatum in the central/eastern Sudan region with dispersals east, west and southward. These dispersals likely led to introgression with local wild races , providing the genetic materials for various local ecological adaptations, including some of the variants in photoperiodicity genes that helped to alter and broaden the seasonality of sorghum . This overall pattern illustrates the importance of introgressive capture (sensu Larson and Fuller 2014) in structuring the genetic geography of domesticated sorghum to resemble pre-existing geographical patterning in wild diversity. It is this introgression that likely lies behind erroneous claims for multiple domestications (e.g. Shechter and de Wet 1975; Olsen 2012), as well the clustering of South and East Asian sorghum germplasm, regardless of race (Billot et al. 2013), probably through introgression of domesticated sorghum with wild Sorghum propinquum. The extent to which S. propinquum provided key adaptive attributes to cultivars requires genetic research, but it can be hypothesized to have contributed to race durra or to kaoliang sorghums (Snowden’s S. nervosum) that extend north to c. 40º latitude in China, Korea and Japan.
A revised framework of the dispersal and diversification history of domesticated sorghum , based on the archaeobotanical and genetic evidence reviewed in this paper. Solid oval indicates zone of domestication of early race bicolor and subsequent evolution of race caudatum; dashed ovals indicate hypothetical zones for the evolution of other races or varieties. Solid arrows indicate trajectories inferred from archaeobotanical evidence, dashed arrows indicate hypothetical dispersal inferred from genetics without archaeobotanical support. Numbers indicate approximate age in years before AD 2000 for arrival time or evolutionary event. Letters on arrows indicate races or varietal groups inferred for each dispersal trajectory: B = bicolor race, BK = Chinese Kaoliang sorghum group within race bicolor, C = caudatum race, D = durra race, GW = race guinea, west African group, GM = race guinea, margaritiferum varieties (= S. margaritiferum Stapf.), GSE = race guinea, southeast African group, GI = race guinea, Indian group, K = race kafir (= S. caffrorum Snowden). Note that race bicolor types should be regarded as co‐dispersing along most, if not all trajectories, but these are only indicated where no advanced races are involved.
The schema of sorghum dispersal and differentiation takes into account our current understanding of sorghum genetics , as well as available archaeobotanical finds, but also fits with several linguistic inferences. The later adoption of sorghum into established pearl millet farming systems across west Africa in the past 2000–2500 years is evident in linguistic evidence for a widespread cognate term for sorghum , “kVn-”, which seems to have spread as a loan across several distinct language families and subfamilies, including West/Central Afro-Asiatic, Songhay and Saharan and several subgroups of Niger-Congo (Mande, Atlantic, Adamawa, and some West Benue-Congo languages) (Blench 2006: 215), but apparently after Bantoid languages had already diverged and moved southward with a focus on rainforest subsistence (cf. Bostoen 2014). These more woodland-focused Bantu groups (Grollemund et al. 2015), picked up pearl millet by 2500 years ago, in keeping with traditions of food preparation that included a stiff porridge of a starchy staple and production of flour, and related cooking terms (Ricquier and Bosteon 2011). The lines of margaritiferum guinea sorghums that gave rise to kafirs might also have moved via the same routes as pearl millet through the Congo, i.e. the Sangha River Interval, or, given the lack of archaeobotaical evidence, have diffused secondarily through these same societies after farming was already established, much as Asian cultivars probably did. Once established in southeast Africa, this lineage was carried via Indian Ocean trade to India to become Indian guineas. That these are popular in forested hill tracts through central and eastern Indian probably reflects the higher rainfall adaptations that ultimately derived from wild race arundinaceum, as well as their suitability to whole grain cooking as opposed to flour cuisine (Appa Rao et al. 1996).
As farmers of the eastern Bantu stream spread to the eastern/northeastern African rainforest margins, new cereals were incorporated into their subsistence, evident in loan words into the Eastern Bantu proto-language, or its early decedents, including a widespread pearl millet term (*-bèdé), most likely from Eastern Sudanic languages around the margins of what is today South Sudan or the Central Africa Republic (Ricquier and Bosteon 2011). Similarly, terms for sorghum (*–saka; *-pemba; *-pila) were adopted into Eastern Bantu or some of its subgroups in the great lakes region of eastern Africa from Central Sudanic languages (Ehret 1973; cf. Philippson and Bahuchet 1994). This is congruent with sorghum , including both races bicolor and caudatum being adopted into eastern African agriculture in the early first millennium AD.
Thus the history of early agriculture in Africa is not merely a narrative of migrating farmers but also borrowing across ethnolinguistic groups and local adaptations in part arising from capturing genetic diversity from local wild sources. Some crop varieties moved long-distances, sometimes with highly mobile economies, like those posited for the Neolithic Sahel, but also sometimes via long-distance trade , around the Bronze Age Arabian sea and later Indian Ocean.
For most of Africa sorghum is central to agriculture , overlapping with and extending beyond the range of other African cereals. It has forms nearly as tolerant of low rainfall as pearl millet and others that, like finger millet, range into higher rainfall and mountainous zones. Compared to both it is relatively more productive. In traditional East African agriculture , for example, sorghum yields from 550–1700 kg/ha, compared to 450–900 kg/ha for finger millet, and on average c. 450 kg/ha for pearl millet (Acland 1971), a range of 270–900 kg/ha is reported for West African pearl millet (Irvine 1969). Under rainfed cultivation African rice ( Oryza glaberrima ) yields 450–900 kg/ha, although 1000–3000 kg is reported for wet fields (Borlaug et al. 1996). In general then, sorghum is the most productive rainfed cereal in most African environments, at least prior to the introduction of American maize. Thus it is not surprising that sorghum cultivation is recurrently associated with sedentary farming and urbanisation in many different cultural traditions of pre-historic Africa, from the Inland Niger Delta (McIntosh 1995) to Meroitic Nubia (Fuller 2014), from Swahili islands (Boivin et al. 2013) to Engaruka (Sassoon 1967) and Great Zimbabwe (Chirikure et al. 2014); and it is unique amongst the African cereals in not just flourishing in similar environments in India but making inroads into northern temperate farming systems, in the Mediterranean or northeastern Asia.
Notes
- 1.
It is worth noting that there remain some nomenclature differences between published taxonomy and that listed as accepted on www.theplantlist.org (accessed 28 Feb. 2016).
References
Acland JD (1971) East African crops. Longman, London
Aldrich PR, Doebley J (1992) Restriction fragment variation in the nuclear and chloroplast genomes of cultivated and wild Sorghum bicolor. Theor Appl Genet 85:293–302
Appa Rao S, Prasada Rao KE, Mengesha MH et al (1996) Morphological diversity in sorghum germplasm from India. Genet Resour Crop Evol 43(6):559–567
Barakat H, Fahmy el-Din AG (1999) Wild grasses as ‘Neolithic’ food resources in the eastern Sahara: a review of the evidence from Egypt. In: van der Veen M (ed) The exploitation of plant resources in ancient Africa. Plenum Press, New York, pp 33–46
Beldados A, Costantini L (2011) Sorghum exploitation at Kassala and its environs, Northeastern Sudan in the second and first millennium BC. Nyame Akuma 75:33–39
Beldados A, Manzo A, Murphy C et al. (2018) Evidence of sorghum cultivation and introduced West Africa crops in the second millennium BCE at Kassala, Eastern Sudan
Bigga G, Kahlheber S (2011) From gathering to agricultural intensification: archaeobotanical remains from Mege, Chad Basin, NE Nigeria. In: Fahmy AG, Kahlheber S, D’Andrea AC (eds) Windows on the African Past: current approaches to African archaeobotany. Africa Magna Verlag, Frankfurt, pp 19–45
Billot C, Ramu P, Bouchet S et al (2013) Massive Sorghum collection genotyped with SSR Markers to enhance use of global genetic resources. PLoS ONE 8(4):e59714. https://doi.org/10.1371/journal.pone.0059714
Blench RM (2006) Archaeology language and the African past. Altamira Press, Lanham
Blench RM (2009) Bananas and plantains in Africa: re-interpreting the linguistic evidence. Ethnobotany Res Appl 7:363–380
Boivin N, Crowther A, Helm R et al (2013) East Africa and Madagascar in the Indian Ocean world. J World Prehist 26(3):213–281
Boivin N, Crowther A, Prendergast M et al (2014) Indian Ocean food globalisation and Africa. Afr Archaeol Rev 31(4):547–581
Bonjean APA (2010) Origins and historical diffusion in China of major native and alien cereals. In: He Z, Bonjean APA (eds) Cereals in China. Mexico, CIMMYT, pp 1–14
Borlaug NE, Axtell J, Burton GW et al (1996) Lost crops of Africa Grains, vol I. National Academy Press, Washington DC
Bosi G, Mercuri AM, Guarnieri C et al (2009) Luxury food and ornamental plants at the 15th century AD Renaissance court of the Este family (Ferrara, northern Italy). Veg Hist Archaeobot 18(5):389–402
Bostoen K (2014) Wild trees in the subsistence economy of early Bantu speech communities: a historical-linguistic approach. In: Stevens CJ, Nixon S, Murray MA, Fuller DQ (eds) Archaeology of African Plant Use. Left Coast Press, Walnut Creek, pp 129–140
Brown PJ, Myles S, Kresovich S (2011) Genetic support for phenotype-based racial classification in Sorghum. Crop Sci 51:224–230
Buonincontri M, Moser D, Allevato E et al (2014) Farming in a rural settlement in central Italy: cultural and environmental implications of crop production through the transition from Lombard to Frankish influence (8th–11th centuries ad). Veg Hist Archaeobot 23(6):775–788
Casa AM, Pressoir G, Brown PJ et al (2008) Community resources and strategies for association mapping in sorghum. Crop Sci 48(1):30–40
Chaix L, Honegger M (2015) New data on animal exploitation from the Mesolithic to the Neolithic periods in Northern Sudan. In: Kerner S, Dann RJ, Bangsgaard P (eds) Climate and ancient societies. Museum of Tusculanum Press, Copenhagen, pp 197–209
Champion L, Fuller DQ (2018) New evidence on the development of millet and rice economies in the Niger river basin: archaeobotanical results from Benin. Plants and People in the African Past 529–547
Chirikure S, Manyanga M, Pollard AM et al (2014) Zimbabwe culture before Mapungubwe: new evidence from Mapela Hill South-Western Zimbabwe. PLoS ONE 9(10):e111224. https://doi.org/10.1371/journal.pone.0111224
Clapham AJ, Rowley-Conwy PA (2007) New discoveries at Qasr Ibrim, Lower Nubia. In: Cappers RTJ (ed) Fields of change: progress in African archaeobotany. The Netherlands: Barkhuis & Groningen University Library, Groningen, pp 157–164
Clark JD (1984) Prehistoric cultural continuity and the economic change in the Central Sudan in the early Holocene. In: Clark JD, Brandt SA (eds) From hunters to farmers, the causes and consequences of food production in Africa. University of California Press, Berkley, pp 113–126
Cleuziou S, Costantini L (1980) Premiers éléments sur l’agriculture protohistorique de l’Arabie Orientale. Paléorient 6:245–251
Connah G (1967) Progress report on archaeological work in Bornu 1964–1966 with particular reference to the excavations at Daima mound. In: Northern History Research Scheme (ed), Second Interim Report. Ahmadu Bello University, Zaria, pp 20–31
Costantini L (1990) Harappan agriculture in Pakistan: the evidence of Naursharo. In: Taddei M (ed) South Asian archaeology 1987. Istituto Italiano per il Medio ed Estremo Oriente, Rome, pp 321–332
Crowther A, Horton M, Kotarba-Morley A et al (2014) Iron age agriculture, fishing and trade in the Mafia Archipelago, Tanzania: new evidence from Ukunju Cave. Azania 49(1):21–44
Crowther A, Lucas L, Helm R et al (2016) Ancient crops provide first archaeological signature of the westward Austronesian expansion. Proc Nat Acad Sci 113 (24):6635–6640. https://doi.org/10.1073/pnas.1522714113
D’Andrea AC, Wadge P (2011) T’ef (Eragrostis tef): A Legacy of Pastoralism? In: Fahmy AG, Kahlheber S, D’Andrea AC (eds) Windows on the African past: current approaches to African archaeobotany. Africa Magna Verlag, Frankfurt, pp 225–241
de Alencar Figueiredo LF, Calatayud C, Dupuits C et al (2008) Phylogeographic evidence of crop neodiversity in sorghum. Genetics 179(2):997–1008
de Moulins D, Phillips C, Durrani N (2003) The archaeological records of Yemen and the question of Afro-Asian contact. In: Neumann K, Buttler A, Kahlheber S (eds) Food, fuels and fields: progress in African archaeobotany. Heinrich Barth-Institut, Cologne, pp 281–299
de Wet JML (1977) Domestication of African cereals. Afr Econ Hist 3:15–32
de Wet JML, Harlan JR, Price EG (1976) Variability in Sorghum bicolor. In: Harlan JR, de Wet JMJ, Stemler ABL (eds) Origins of African plant domestication. Mouton Press, The Hague, pp 453–463
Delneuf MOT (1995) L’environment et les usages alimentaires en vigneur a l’époque protohistorique dans l’extreme-nord du Cameroun. In: Marliac A (ed) Milieux, Societés et Archéologues. Office de la Recherche scientifique et technique de outre-mer, Paris, pp 213–226
Deu, MD. Gonzalez-de-Leon J-C, Glaszmann I et al (1994) RFLP diversity in cultivated sorghum in relation to racial differentiation. Theor Appl Genet 88:838–844
Deu M, Rattunde H, Chantereau J (2006) A global view of genetic diversity in cultivated sorghums using a core collection. Genome 49:168–180
Ehret C (1973) Patterns of Bantu and central Sudanic settlement in central and southern Africa (1000 BC–500AD) Transafrican J Hist 3:1–71
Ehret C (2011) History and the testimony of language. University of California, Berkley
Ehret C (2014) Linguistic evidence and the origins of food production in Africa. Where are we now? In: Stevens CJ, Nixon S, Murray MA, Fuller DQ (eds) Archaeology of African plant use. Left Coast Press, Walnut Creek, pp 233–242
Folkertsma R, Rattunde HFW, Chandra S et al (2005) The pattern of genetic diversity of guinea race Sorghum bicolor (L.) Moench landraces as revealed with SSR markers. Theor Appl Genet 111:399–409
Fuller DQ (2002) Fifty Years of Archaeobotanical Studies in India: Laying a Solid Foundation. In: Settar S, Korisettar R (eds) Indian archaeology in retrospect, vol III. Archaeology and interactive disciplines. Publications of the Indian Council for Historical Research Manohar, New Dehli, pp 247–364
Fuller DQ (2003a) African crops in prehistoric South Asia: a critical review. In: Neumann K, Butler A, Kahlheber S (eds) Food, Fuel and Fields. Progress in Africa Archaeobotany, Africa Praehistorica 15. Heinrich-Barth-Institut, Cologne, pp 239–271
Fuller DQ (2003b) An agricultural perspective on Dravidian historical linguistics: archaeological crop packages, livestock and Dravidian crop vocabulary. In: Bellwood P, Renfrew C (eds) Assessing the languaging/farming dispersal hypothesis. McDonald Institute, Cambridge, pp 191–213
Fuller DQ (2004) Early Kushite agriculture: archaeobotanical evidence from Kawa. Sudan Nubia 8:70–74
Fuller DQ (2014) Agricultural innovation and state collapse in Meroitic Nubia: the impact of the savannah package. In: Stevens CJ, Nixon S, Murray MA, Fuller DQ (eds) Archaeology of African plant use. Left Coast Press, Walnut Creek, pp 165–178
Fuller DQ (in press) Archaeobotany. In: Kennet D, Rao JVP (eds.) Paithan Excavations. Memoirs of the Archaeological Survey of India, Delhi, pp 289–327
Fuller DQ, Allaby R (2009) Seed dispersal and crop domestications: shattering, germination and seasonality in evolution under cultivation. Ann Plant Rev 38:238–295
Fuller DQ, Boivin N (2009) Crops, cattle and commensals across the Indian Ocean: current and potential archaeobiological evidence. In: Lefevre G (ed) Plantes et Societes, Etudes Ocean Indien 42-43. Institut National des Langues et Civilisations Orientales, Paris, pp 13–46
Fuller DQ, Edwards DN (2001) Medieval plant economy in Middle Nubia: preliminary archaeological evidence from Nauri. Sudan Nubia 5:97–103
Fuller DQ, Lucas L (2014) Wheats: origins and development. In: Smith C (ed) Encyclopedia of global archaeology. Springer, New York, pp 7812–7817
Fuller DQ, Allaby RG, Stevens CJ (2010) Domestication as innovation: the entanglement of techniques, technology and chance in the domestication of cereal crops. World Archaeol 42(1):13–28
Fuller DQ, Denham T, Arroyo-Kalin M et al (2014) Convergent evolution and parallelism in plant domestication revealed by an expanding archaeological record. Proc Nat Acad Sci 111(17):6147–6152
Gautier A, Van Neer W (2006) Animal remains from Mahal Teglinos (Kassala, Sudan) and the arrival of pastoralism in the Southern Atbai. J Afr Archaeol 4(2):223–233
Giblin JD, Fuller DQ (2011) First and second millennium AD agriculture in Rwanda: archaeobotanical finds and radiocarbon dates from seven sites. Veg Hist Archaeobot 20(4):253–265
Grollemund R, Branford S, Bostoen K et al (2015) Bantu expansion shows that habitat alters the route and pace of human dispersals. Proc Nat Acad Sci 112(43):13296–13301
Haaland R (1995) Sedentism, cultivation and plant domestication in the Holocene middle Nile region. J Field Archaeol 22(2):157–173
Hagerty MH (1941) Comments on writings concerning Chinese sorghums. Harvard J Asia Stud 5(3–4):234–260
Harlan JR, De Wet JML (1972) A simplified classification of cultivated sorghum. Crop Sci 12:172–176
Harlan JR, Stemler ABL (1976) The races of sorghum in Africa. In: Harlan JR, de Wet J, Stemler A (eds) Origins of African plant domestication. Mouton Press, The Hague, pp 465–478
Harlan JR, De Wet JML, Price EG (1973) Comparative evolution of cereals. Evolution 277:311–325
Helm R, Crowther A, Shipton C et al (2012) Exploring agriculture, interaction and trade on the eastern African littoral: preliminary results from Kenya. Azania 47(1):39–63
Htun TM, Inoue C, Chhourn O et al (2014) Effect of quantitative trait loci for seed shattering on abscission layer formation in Asian wild rice Oryza rufipogon. Breed Sci 64(3):199–205
Huffman TN (1974) The Leopard’s Kopje Tradition. Salisbury: National Museums and Monuments (Memoir 6)
Irvine FR (1969) West African Agriculture, 3 edn, vol 2. West African Crops. Oxford University Press, Oxford
Ishii T, Numaguchi K, Miura K et al (2013) OsLG1 regulates a closed panicle trait in domesticated rice. Nat Genet 45:462–465
Kajale MD (1990) Observations on the plant remains from excavation at Chalcolithic Kaothe, District Dhule, Maharashtra with cautionary remarks on their interpretations. In: Dhavalikar MK, Shinde VS, Atre SM (eds) Excavations at Kaothe. Decan College Postgraduate Research Institute, Pune, pp 265–280
Klein RR, Miller FR, Dugas DV et al (2015) Allelic variants in the PRR37 gene and the human-mediated dispersal and diversification of sorghum. Theor Appl Genet 128(9):1669–1683
Kröpelin S, Verschuren D, Lézine A-M et al (2008) Climate-driven ecosystem succession in the Sahara: the past 6000 years. Science 320(5877):765–768
Larson G, Fuller DQ (2014) The evolution of animal domestication. Ann Rev Ecology Evolution Systematics 45:115–136
Li WL, Gill BS (2006) Multiple pathways for seed shattering in the grasses. Funct Integr Genomics 6:300–309
Li C, Zhou A, Sang T (2006) Rice domestication by reduced shattering. Science 311:1936–1939
Lin Z, Griffith ME, Li X et al (2007) Origin of seed shattering in rice (Oryza sativa L.). Planta 226:11–20
Lin Z, Li X, Shannon LM et al (2012) Parallel domestication of the Shattering1 genes in cereals. Nat Genet 44(6):720–724
Logan AL (2012) A history of food without history: food, trade, and environment in west-central Ghana in the second millennium AD. Unpublished PhD dissertation. University of Michigan, Ann Arbor
Luo H, Zhao W, Wang Y et al (2016) SorGSD: a sorghum genome SNP database. Biotechnol Biofuels 9:6. https://doi.org/10.1186/s13068-015-0415-8
Mace ES, Xia L, Jordan DR et al (2008) DArT markers: diversity analyses and mapping in Sorghum bicolor. BMC Genom 9:26
Magid AA (1989) Plant Domestication in the Middle Nile Basin: an Archaeobotanical Case Study. Cambridge Monographs in African Archaeology 35. BAR S523. British Archaeological Reports, Oxford
Magid AA (2003) Exploitation of food-plants in the early and middle Holocene Blue Nile area, Sudan and neighbouring areas, Complutum 14. Universidad Complutense de Madrid Servicio de Publicaciones, Madrid
Manning K, Fuller DQ (2014) Early millet farmers in the lower Tilemsi Valley, Northeastern Mali. In: Stevens CJ, Nixon S, Murray MA, Fuller DQ (eds) Archaeology of African plant use. Left Coast Press, Walnut Creek, CA, pp 73–81
Manning K, Timpson A (2014) The demographic response to Holocene climate change in the Sahara. Quat Sci Rev 101:28–35
McClatchie M, Fuller DQ (2014) Leaving a lasting impression: arable economies and cereal impressions in Africa and Europe. In: Stevens CJ, Nixon S, Murray MA, Fuller DQ (eds) Archaeology of African plant use. Left Coast Press, Walnut Creek, pp 259–266
McIntosh SK (1995) Paleobotanical and human osteological remains. In: McIntosh SK (ed) Excavations at Jenné-Jeno, Hambarketolo, and Kaniana (Inland Niger Delta, Mali): the 1981 season. University of California Press, Berkley, pp 348–359
Mitchell PJ (2002) The archaeology of Southern Africa. Cambridge University Press, Cambridge
Morris G, Ramu P, Deshpande SP (2013) Population genomic and genome-wide association studies of agroclimatic traits in Sorghum. Proc Nat Acad Sci 110(2):453–458
Nixon S, Murray MA, Fuller DQ (2011) Plant use at an early Islamic merchant town in the West African Sahel: the archaeobotany of Essouk-Tadmakka (Mali). Ved Hist Archaeobot 20(3):223–239
NPGS (2016) National plant germplasm system. Germplasm Resources Information Network (GRIN), Washington, USDA. http://www.ars-grin.gov/npgs/index.html. Accessed 29 Mar 2015
Olsen KM (2012) One gene’s shattering effects. Nat Genet 44:616–617
Pelling R (2005) Garamantian agriculture and its significance in a wider North African context: the evidence of the plant remains from the Fazzan project. J N Afr Stud 10(3/4):397–412
Pelling R (2007) Agriculture and trade amongst the Garamantes and the Fezzanese: 3000 years of archaeobotanical data from the Sahara and its margins. PhD Thesis. UCL, London
Perumal R, Krishnaramanujam R, Menz MA et al (2007) Genetic diversity among sorghum races and working groups based on AFLPs and SSRs. Crop Sci 47:1375–1383
Philippson G, Bahuchet S (1994) Cultivated crops and Bantu migrations in central and eastern Africa: a linguistic approach. Azania 29–30(1):103–120
Ramu P, Billot C, Rami J et al (2013) Assessment of genetic diversity in the sorghum reference set using EST-SSR markers. Theor Appl Genet 126(8):2051–2064
Ricquier B, Bosteon K (2011) Stirring up the porridge: how early Bantu speakers prepared their cereals. In: Fahmy AG, Kahlheber S, D’Andrea AC (eds) Windows on the African past: current approaches to African archaeobotany. Africa Magna Verlag, Frankfurt, pp 209–224
Rowley-Conwy P (1991) Sorghum from Qasr Ibrim, Egyptian Nubia, c. 800 BC AD 1811: a preliminary study. In: Renfrew J (ed) New light on early farming. Edinburgh University Press, Edinburgh, pp 191–212
Rowley-Conwy P, Deakin W, Shaw CH (1997) Ancient DNA from archaeological sorghum (Sorghum bicolor) from Qasr Ibrim, Nubia: implications for domestication and evolution and a review of the archaeological evidence. Sahara 9:23–34
Rowley-Conwy P, Deakin W, Shaw CH (1999) Ancient DNA from Sorghum. In: van der Veen M (ed) The exploitation of plant resources in ancient Africa. Plenum, New York, pp 55–61
Ruas MP, Tengberg M, Ettahiri AS et al (2011) Archaeobotanical research at the medieval fortified site of Îgîlîz (anti-atlas, morocco) with particular reference to the exploitation of the argan tree. Vegetation History Archaeobotany 20:419–433
Saraswat KS, Pokharia AK (2003) Palaeoethnobotanical investigations at early Harappan Kunal. Pragdhara 13:105–140
Sassoon H (1967) New views on Engaruka, northern Tanzania. J African History 8(02):201–217
Schoenbrun DL (1993) We are what we eat: ancient agriculture between the Great lakes. J Afr Hist 34:1–31
Shechter Y, De Wet JMJ (1975) Comparative electrophoresis and isozyme analysis of seed proteins from cultivated races of Sorghum. Am J Bot 62:254–261
Shinnie PL, Anderson J (2004) The capital of kush 2: meroe excavations, 1973–1984, Meroitica 20. Harrassowitz, Wiesbaden
Shipton C, Helm R, Boivin N et al (2013) Intersections, networks and the genesis of social complexity on the Nyali coast of east Africa. Afr Archaeol Rev 30:427–453
Smith W (2003) Archaeobotanical investigations of agriculture at late antique Kom el-Nana (Tell el-Amarna). Egypt Exploration Soc, London
Snowden JD (1936) The cultivated races of Sorghum. Allard and Son, London
Snowden JD (1955) The wild fodder Sorghums of the secion Eu-Sorghum. J Linn Soc Bot (London) 55:191–260
Southworth FC (2006) Proto-Dravidian agriculture. In: Osada T (ed), Proceedings of the Pre-symposium of RIHN and 7th ESCA Harvard- Kyoto Roundtable. Research Institute for Humanity and Nature, Kyoto, pp 121–150
Stapf O (1886) Die Pflanzenreste des Hallstätter Heidengebirges. Zool -Bot Ges, Österreich
Stapf O (1917) Sorghum. In: Prain D (ed) Flora of Tropical Africa, vol 9. Gramineae (Maydeae-Paniceae). L. Reeve, Asford, pp 104–154
Stemler AB (1990) A scanning electron microscopic analysis of plant impressions in pottery from sites of Kadero, El Zakiab, Um Direiwa and El Kadada. Archaeologie du Nil Moyen 4:87–106
Sukumaran S, Xiang W, Bean S et al (2012) Associated mapping for grain quality in a diverse sorghum collection. Plant Genome 5:126–135
Tengberg M (2003) Archaeobotany in the Oman Peninsula and the role of eastern Arabia in the spread of African crops. In: Neumann K, Butler A, Kahlheber S (eds) Food, fuel and fields progress in African archaeobotany. Africa Praehistorica. Monographien zur Archäologie und Umwelt Afrikas. Heinrich-Barth-Institut, Köln, pp 229–237
van der Veen M (2011) Consumption, trade and innovation: exploring the botanical remains from the Roman and Islamic ports at Quseir al-Qadim. Egypt, Africa Magna Verglag, Frankfurt
van der Veen M, Lawrence T (1991) The plant remains. In: Welsby DA, Daniels CM (eds) Soba: archaeological research at a medieval capital on the Blue Nile British institute in Eastern Africa memoirs, London, pp 264–274
Walshaw SC (2010) Converting to rice: urbanization, Islamization and crops on Pemba Island, Tanzania, AD 700–1500. World Archaeology 42:137–154
Wasylikowa K, Dahlberg JA (1999) Sorghum in the economy of the early Neolithic nomadic tribes at Nabta Playa, southern Egypt. In: van der Veen M (ed) The exploitation of plant resources in ancient Africa. Plenum Press, New York, pp 11–32
Wasylokowa K, Dahlberg JA (2001) Sorghum remains from site E-75-6. In: Wendorf F, Schild R (eds) Holocene settlement of the Egyptian Sahara: Volume 1: the archaeology of Nabta Playa Springer/Plenum, New York, pp 578–587
Wiersema JH, Dahlberg J (2007) The nomenclature of Sorghum bicolor (L.) Moench (Gramineae). Taxon 56(3):941–946
Willcox G (1992) Some differences between crops of near Eastern origin and those from the tropics. In: Jarrige C (ed) South Asian archaeology 1989. Monographs in World Archaeology, vol 14, Wisconsin, pp 291–300
Winchell F (2013) The Butana group ceramics and their place in the Neolithic and post-neolithic of northeast Africa. Cambridge monographs in African archaeology 83, British archaeological report series 2459. Archaeopress, Oxford
Winchell F, Stevens CJ, Murphy C et al (2017) Evidence for sorghum domestication in fourth millennium BC eastern Sudan: spikelet morphology from ceramic impressions of the Butana Group. Curr Anthropol 58(7):673–683
Zohary D, Hopf M, Weiss E (2012) Domestication of plants in the Old World. Oxford University Press, Oxford
Acknowledgements
This research has been carried out as part of the “Comparative Pathways to Agriculture” project, funded by a European Research Council advanced grant (No. 323842). We are grateful to our colleagues who have contributed to our Old World Crops Archaeobotanical Databse (OWCAD), including Leilani Lucas, Charlene Murphy, Louise Champion and Fabio Silva. We also thank Alem Beldados, Andrea Manzo, and Frank Winchell for brining to our attention new evidence from Sudan, and Alison Crowther and Nicole Boivin for bringing to our attention new samples from eastern Africa. Lastly, we thank peer-reviewers and editors for their helpful suggestions.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Fuller, D.Q., Stevens, C.J. (2018). Sorghum Domestication and Diversification: A Current Archaeobotanical Perspective. In: Mercuri, A., D'Andrea, A., Fornaciari, R., Höhn, A. (eds) Plants and People in the African Past. Springer, Cham. https://doi.org/10.1007/978-3-319-89839-1_19
Download citation
DOI: https://doi.org/10.1007/978-3-319-89839-1_19
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-89838-4
Online ISBN: 978-3-319-89839-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)