Abstract
Leishmaniases are worldwide vector-borne diseases with diverse clinical manifestations caused by protozoa belonging to genus Leishmania. About 20 named Leishmania species are pathogenic for humans and are annually responsible for 0.7–1.2 million cases of cutaneous and 0.2–0.4 million cases of visceral forms of the disease. According to the transmission cycle involving animals or humans, leishmaniasis can be categorized in two main epidemiological groups: zoonotic (representing the large majority of such entities) and anthroponotic. Leishmaniases have re-emerged in recent years showing a wider geographic distribution and increased global prevalence. Environmental, demographic and human behavioural factors contribute to the changing epidemiology of the disease and to its recent spread throughout the world. Control strategies against anthroponotic leishmaniases should combine case management and vector control in order to reduce or eliminate parasite transmission. For zoonotic leishmaniases control of reservoir hosts has also been recommended, but few advances have been made, namely, in the case of cutaneous forms in both the Old and New Worlds due to the sylvatic nature of the reservoir hosts. On the other hand, strategies to control L. infantum in the domestic canine reservoir host have been developed, namely, topical insecticides against parasite infection and vaccines against disease evolution. Nevertheless and since Leishmania parasites have been found in a variety of wild and domestic animals around the world, it will be important to determine their role in the local epidemiology of leishmaniasis.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
4.1 Introduction
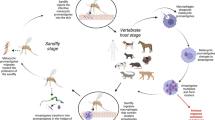
Leishmaniases are parasitic diseases caused by protozoa belonging to the genus Leishmania (order Kinetoplastida, family Trypanosomatidae), which infects several mammal species, including humans. These parasites are primarily transmitted by the bite of an insect vector, the phlebotomine sand fly (order Diptera, family Psychodidae; subfamily Phlebotominae) of the genera Phlebotomus (Old World) and Lutzomyia (New World) (Killick-Kendrick 1999; WHO 2010; Maroli et al. 2013). Human leishmaniases have diverse clinical manifestations. Visceral leishmaniasis (VL) caused by parasites of the Leishmania donovani complex (L. donovani in the Old World and L. infantum in both the Old and New Worlds) is a severe disease of humans and other mammals, which leads to death if left untreated. A number of different Leishmania spp. cause localized cutaneous (LCL) or diffuse cutaneous (DCL) or mucocutaneous (MCL) leishmaniasis, which are responsible for considerable morbidity of a vast number of people in endemic foci. Leishmaniases are endemic in 98 countries on 4 continents, with more than 350 million people at risk. Published figures indicate an estimated incidence of 0.2–0.4 million VL cases and 0.7–1.3 million cutaneous leishmaniasis (CL) cases (WHO 2010; Alvar et al. 2012). These figures are, most probably, underestimated as official data and are often obtained through passive case detection, and the extent of underreporting in most leishmaniasis endemic countries (even in those where the disease is of compulsory notification) is substantial (Desjeux 2004; Dujardin et al. 2008; WHO 2010; Alvar et al. 2012).
Leishmaniases are dynamic diseases, and the circumstances of transmission are continually changing in relation to environmental, demographic and human behavioural factors. In most endemic regions, leishmaniases are characterized by a patchy distribution with discrete transmission foci due to microecological conditions that affect the vector, the parasite and the reservoir host. Changes in the habitat of the natural host and vector, immunosuppressive conditions (e.g. HIV infection or organ transplantation-associated therapies) and the consequences of military conflicts, all contribute to the changing leishmaniasis landscape, which can result in either an increase or a decrease in the incidence of the disease (Gramiccia and Gradoni 2005; WHO 2010; Alvar et al. 2012; Antoniou et al. 2013).
Leishmaniases can be grouped into two broad epidemiological categories according to the source of human infection: zoonotic leishmaniases, in which the reservoir hosts are wild or domestic animals and humans play a role of an accidental host, and anthroponotic leishmaniases, in which man is the sole reservoir host and source of vector’s infection (Desjeux 2004; Gramiccia and Gradoni 2007; WHO 2010).
A reservoir host of leishmaniases is an animal in which an infectious agent survives persistently in a way that the animal may serve as a source of parasites to the vectors. The mere presence of the infection in a particular mammal species, even in large numbers, does not necessarily indicate that this mammal is a reservoir host. In order to incriminate a reservoir host formally, it is necessary to demonstrate that the parasite population depends on that particular mammal for its long-term maintenance (Ashford 1996; WHO 2010). A “good” reservoir should be susceptible to the parasites, live in close contact with man, and it should be a good source of parasites to the vectors. The proportion of individuals that become infected during their lifetime should be considerable, although the incidence can vary greatly with season. A good reservoir should provide a significant food source for the phlebotomine sand fly, and both should rest and breed in the same habitat. Infection should present a chronic evolution allowing the animal to survive at least until the next transmission season (Bray 1982; Ashford 1996; WHO 2010). Leishmania parasites identified in reservoir hosts must be biochemically and genetically the same as those in humans.
When more than one host species can be infected, they are often divided on epidemiological grounds into primary and secondary (or minor) reservoir hosts and accidental (or incidental) hosts: primary reservoir host is a host that is responsible for maintaining the parasite indefinitely in nature. In these hosts, the infection is normally without clinical signs; secondary reservoir host is a host that can transmit infection but cannot maintain parasite transmission in the absence of the primary host(s), while accidental host is a host that although infected plays no role in the maintenance of the transmission cycle (Silva et al. 2005; WHO 2010; Quinnell and Courtenay 2009).
4.2 Anthroponotic Leishmaniases
Human beings are directly involved as a principal reservoir host in two forms of the disease: VL caused by L. donovani in Indian subcontinent (Bangladesh, India and Nepal) and East Africa (Djibouti, Ethiopia, Eritrea, Kenya, Somalia, South Sudan, Sudan and Uganda) and CL caused by L. tropica in semiarid subtropical regions from south-east Turkey to north-west of India. Small foci have also been described in Arabia, Ethiopia, Greece, Namibia, North Africa (Algeria, Egypt, Morocco and Tunisia) and in Central Asia (Gramiccia and Gradoni 2007; WHO 2010).
L. donovani-infected animals have been increasingly reported in several foci, despite the predominant anthroponotic transmission pattern, where post-kala-azar dermal leishmaniasis patients might constitute the main interepidemic reservoir host (WHO 2010). In certain districts of Sudan, rodents from the Arvicanthis genus (Hoogstraal and Heyneman 1969) and the Egyptian mongoose (Herpestes ichneumon) were suspected to be reservoir hosts of the parasite (Elnaiem et al. 2001). Anti-Leishmania antibodies have also been detected in donkeys, cows, goats and sheep in a kala-azar endemic region in Sudan, suggesting exposure of these animals to L. donovani infection (Mukhtar et al. 2000). In addition, canine leishmaniasis (CanL) seroprevalence between 42.9 and 74.3% and the same zymodemes were found in both humans and dogs in an endemic VL focus in Eastern Sudan (Dereure et al. 2000, 2003). However, in a more recent study performed in the same geographic region, a low number of dogs were found to have specific antibodies against Leishmania or to harbour parasites (Hassan et al. 2009).
In north-western Ethiopia, antibodies to and/or DNA of L. donovani complex have been detected in the blood of several domestic animals such as goats, sheep, cows, dogs and donkeys (Kalayou et al. 2011; Kenubih et al. 2015; Rohousova et al. 2015). L. donovani has also been molecularly amplified from the bone marrow of dogs (Bashaye et al. 2009) and from the spleen, bone marrow or liver of wild Ethiopian rodents (Arvicanthis, Gerbilliscus and Mastomys genera) (Kassahun et al. 2015a; Lemma et al. 2017). Finally, in the Indian subcontinent, L. donovani DNA has been detected in the blood of goats, cows and buffaloes in Nepal (Bhattarai et al. 2010), in goats (Singh et al. 2013) and domestic dogs (Jambulingam et al. 2017) in India and in stray dogs from Bangladesh (Akter et al. 2016).
Similarly, and despite L. tropica is considered to depend on humans for its survival, at least in long-established endemic foci in urban settings (WHO 2010; Antoniou et al. 2013), in foci with few or sporadic cases, the disease is known or suspected to be zoonotic (Ashford 2000; WHO 2010). CanL due to L. tropica have been reported in Morocco (Dereure et al. 1991; Guessous-Idrissi et al. 1997), in Iran (Hajjaran et al. 2013; Bamorovat et al. 2015), in Israel (Baneth et al. 2014) and in Crete (Ntais et al. 2014). In addition, L. tropica promastigotes have recently been isolated from the blood of a young stray dog from Israel admitted to a veterinarian hospital with a complaint of lethargy (Baneth et al. 2017). The isolation of the same zymodemes from dogs as those found in man in the same focus raised a potential role of dogs as reservoir hosts of this dermotropic Leishmania species. Nevertheless, the small number of canine cases and the short duration of the lesions in dogs make it difficult to define the precise role of this mammal in the epidemiological cycle (Dereure et al. 1991). In a broader geographical context of the Mediterranean region, several zoonotic foci have been described, with rock hyraxes (Procavia capensis) as reservoir hosts in Israel (Svobodova et al. 2006; Talmi-Frank et al. 2010) and the North African gundi (Ctenodactylus gundi) as probably serving as reservoir host of Leishmania killicki (synonym of L. tropica, Pratlong et al. 2009) in the area of Maghreb (Jaouadi et al. 2011; Bousslimi et al. 2012). In addition, L. tropica DNA has recently been detected in the spleen of wild rodents (Acomys, Arvicanthis, Gerbillus genera) (Kassahun et al. 2015a) and of one heart-nosed bat (Cardioderma cor) in Ethiopia (Kassahun et al. 2015b) as well as in the blood of stray cats from Izmir, Turkey (Can et al. 2016).
Despite these recent findings, more extensive studies to clarify the role of domestic animals in maintenance and transmission of L. donovani and L. tropica focusing on isolation and typing of the parasite and xenodiagnosis should be advocated.
4.3 Zoonotic Visceral Leishmaniasis
Leishmania infantum (synonymous of L. chagasi) is the etiological agent for zoonotic VL in several countries of Central and South America, the Mediterranean Basin, Middle East and Asia. The main vector in the New World is Lutzomyia longipalpis, while in the Old World, several species belonging to the subgenus Phlebotomus (Larrossius) (e.g. Phlebotomus ariasi, Phlebotomus perniciosus, Phlebotomus tobbi) are involved in L. infantum transmission (Maroli et al. 2013). Domestic dogs are the main domestic reservoir hosts for human infection.
In the Old and New Worlds, several indigenous wild mammal species have been found infected by or exposed to L. infantum (Table 4.1).
The role of foxes (Vulpes spp. and Cerdocyon thous), jackals (Canis aureus), wolves (Canis lupus) and raccoon dogs (Nyctereutes procyonoides) as sylvatic reservoir hosts has been suggested (Abranches 1989; WHO 2010). The existence of an autonomous or semi-autonomous sylvatic cycle in the Mediterranean Basin maintained by red foxes (Vulpes vulpes) has been proposed (Abranches et al. 1984), but the dependence level and the direction of parasite transmission (i.e. if foxes are inoculated with L. infantum through the bite of competent vectors that become infected after feeding on dogs harbouring parasites or vice versa) between these animal species were not evaluated. In fact, there is no strong evidence that wild carnivores are an important source of infection stressing the need of further quantitative studies to confirm their infectiousness to the vectors (Quinnell and Courtenay 2009). On the other hand, the ability to transmit infection has been confirmed by xenodiagnosis in black rats (Rattus rattus), hares and wild rabbits suggesting that they may represent a secondary reservoir host for L. infantum (Gradoni et al. 1983; Molina et al. 2012; Jiménez et al. 2014). The evidence that hares and, to a lesser extent, rabbits can play a role as reservoir hosts of L. infantum in a new focus in Fuenlabrada, Spain, linked to the urbanization of a sylvatic transmission cycle due to the creation of an urban periphery where both lagomorphs and phlebotomine sand fly vectors have the optimal conditions to increase in numbers, is an example that leishmaniasis can emerge due to environmental changes induced by man (Molina et al. 2012; Jiménez et al. 2014).
Among reports on domestic animals recurrently found infected with L. infantum, those regarding cats deserve attention for the potential implications to public health. L. infantum infection has been reported in domestic cats from several endemic countries in Europe, the Middle East and Brazil (Ozon et al. 1998; Martín-Sánchez et al. 2007; Nasereddin et al. 2008; Hatam et al. 2010; Vides et al. 2011; Pennisi et al. 2012; Chatzis et al. 2014; Maia et al. 2014; Can et al. 2016; Attipa et al. 2017). Thus, an increasing trend to regard cats as a potential domestic reservoir host of L. infantum exists as they seem to be:
-
1.
Naturally susceptible to infection by this species, normally without development of clinical signs (these, when present are usually cutaneous but systemic involvement has also been recorded)
-
2.
A blood source for some Leishmania vectors
-
3.
Present parasites in an available way to infect the vector
-
4.
Among the most popular pet animals around the world, often present in areas where the peridomestic and domestic transmission cycles of the parasite occur (Colmenares et al. 1995; Maroli et al. 2007; Martín-Sánchez et al. 2007; da Silva et al. 2010; Maia et al. 2010; Vides et al. 2011; Pennisi et al. 2012; Chatzis et al. 2014)
In addition, parasites isolated from infected cats seem to be biochemically and genetically identical to the ones obtained from humans and dogs with leishmaniases (Maroli et al. 2007; Pennisi et al. 2012; Maia et al. 2015). Despite this evidence, the epidemiological importance of cats in leishmaniasis is still poorly understood (Gramiccia and Gradoni 2007; Gramiccia 2011; Maia and Campino 2011; Pennisi et al. 2015). Therefore, from an epidemiological and control perspective it would be very important to evaluate the proportion of transmission in endemic areas attributable to cats in order to clarify if these animals are reservoir hosts sustaining and spreading Leishmania infection (Maia and Campino 2011). The dependence level and the direction of parasite transmission (i.e. if cats are inoculated with L. infantum through the bite of competent vectors that become infected after feeding on dogs harbouring parasites or vice versa) between these animal species are also important issues (Maia and Campino 2011).
Antibodies to L. infantum or its DNA have also been detected in horses in endemic areas from the Old and New Worlds (Solano-Gallego et al. 2003; Rolão et al. 2005; Fernández-Bellon et al. 2006; Lopes et al. 2013; Soares et al. 2013; Gama et al. 2014; Aharonson-Raz et al. 2015) and in nonendemic areas (i.e. Switzerland and Germany) close to the border of the limit of leishmaniasis distribution in Southern Europe (Koehler et al. 2002). Clinical cases of equine leishmaniasis have been described as self-limiting nodular or ulcerated skin lesions, isolated or disseminated (Koehler et al. 2002; Portús et al. 2002; Rolão et al. 2005; Gama et al. 2014; Baneth et al. 2015). Previous experimental data did not identified Equus asinus as a L. infantum reservoir host since the lesions of the experimentally infected donkeys spontaneously disappeared and xenodiagnosis performed using the vector L. longipalpis was negative (Cerqueira et al. 2003). Nevertheless, the dogma that domestic equines seem to display clinical and immunological responses of the resistant type (Fernández-Bellon et al. 2006) has recently been challenged as the concomitant cutaneous and visceral L. infantum infection was described in three horses from Belo Horizonte, Brazil (Soares et al. 2013). In addition, in northern Israel, facial lesions due to L. infantum in two horses progressively proliferated and needed to be treated with intralesional injections of meglumine antimoniate (Baneth et al. 2015), which together with the presence of L. infantum DNA in P. perniciosus sand flies that fed on two parasitaemic subclinically infected horses from the same stable allowed the authors to suggest that horses may serve as secondary reservoir hosts for this Leishmania species. Nevertheless, more research is required to elucidate the role, if any, of horses in L. infantum epidemiology, namely, the isolation for a more refined genetic, biological and biochemical characterization of the parasites infecting horses and the infectiousness of horses to vectors from nature and in horse populations.
Epidemiological studies conducted worldwide in endemic areas of VL caused by L. infantum strongly suggest that asymptomatic human infections are common (Costa et al. 2002; Michel et al. 2011). Risk factors for progression to disease include age, malnutrition, HIV coinfection and other immunosuppressive conditions (Gramiccia and Gradoni 2007; Boelaert and Sundar 2014). Parasite transmission by blood transfusion has also been reported (Michel et al. 2011). Therefore and despite the very low parasitaemia level, at least in immunocompetent asymptomatic carriers, their potential role as reservoir hosts should be addressed (Michel et al. 2011). It would also be important to screen patients from endemic areas for Leishmania infection before starting an immunosuppressive treatment (Basset et al. 2005).
Leishmania siamensis (nomen nudum) is referred in literature as the causative agent of several recent human cases of VL and CL with and without other co-immunosuppressive states in Thailand (Sukmee et al. 2008; Suankratay et al. 2010; Bualert et al. 2012; Chusri et al. 2012) and Myanmar (Noppakun et al. 2014). A putative vector, Sergentomyia gemmea, has recently been proposed (Kanjanopas et al. 2013). This so-called species, which belongs to the Leishmania enrietti complex, has not been formally named and described and therefore is not taxonomically valid (Pothirat et al. 2014; Akhoundi et al. 2016). In fact, it was recently showed that the majority of the ITS-1 and RNAPolII sequences that have been previously identified as L. siamensis in Thailand may actually be Leishmania martiniquensis (Pothirat et al. 2014). The geographical distribution of these novel Leishmania strains seems to be wide, as sporadic autochthonous equine and bovine CL have been reported in Germany, in Switzerland and in the USA (Müller et al. 2009; Lobsiger et al. 2010; Reuss et al. 2012). The zoonotic potential of L. siamensis has been suggested, as its DNA was amplified from liver and spleen samples of two black rats collected from the affected geographical area where VL in Thai patients have been reported (Chusri et al. 2014).
4.4 Zoonotic Cutaneous Leishmaniases
4.4.1 Old World
Leishmania aethiopica shows a geographical distribution limited to the highlands of East Africa (Ethiopia, Kenya and Uganda), and stable foci of low endemicity are maintained by hyraxes (Procavia capensis and Heterohyrax brucei) (Ashford et al. 1973; Saliba and Oumeish 1999; Tonui 2006; WHO 2010; Alvar et al. 2012). Phlebotomus longipes, P. pedifer and P. sergenti are the proven vectors (Killick-Kendrick 1999; Maroli et al. 2013; Akhoundi et al. 2016). Rock hyrax is also suspected of being the reservoir host of L. aethiopica in Saudi Arabia (Morsy et al. 1997; WHO 2010). Human LCL cases, and less frequently DCL or MCL, occur mostly in rural villages built on rock hills or river banks, associated with proximity to hyrax colonies. However, human cases have also been reported in and near Ethiopian urban centres, including Addis Ababa suggesting that this parasite is probably not so uncommon at lower altitudes (Negera et al. 2008; Lemma et al. 2009). L. aethiopica has also been isolated from a goat in Kenya (Williams et al. 1991) and from a ground squirrel (Xerus rutilus) in Ethiopia (Abebe et al. 1990).
Sporadic cases of LCL due to L. infantum are seen throughout the Mediterranean Basin (WHO 2010). This parasite is the most frequent cause of CL in Southern Europe (Gramiccia and Gradoni 2007). As mentioned before, several phlebotomine sand flies of Larrossius subgenus are the proven vectors of this parasite, and dogs are the main reservoir hosts for human infection. Nevertheless, in the recent focus of VL and CL in Fuenlabrada, Spain, the role of lagomorphs as potential sylvatic reservoir hosts has been raised up (Molina et al. 2012; Jiménez et al. 2014).
Leishmania major is the main cause of zoonotic CL in an area that stretches from India through Central Asia, the Middle East, to North and West Africa (WHO 2010). CL due to this Leishmania species, which is transmitted by several Phlebotomus species of Paraphlebotomus and Phlebotomus subgenera (Killick-Kendrick 1999; Maroli et al. 2013; Akhoundi et al. 2016), is widely distributed in rural arid areas with proneness to epidemic pattern with seasonal occurrence of cases (WHO 2010; Aoun and Bouratbine 2014). Several rodent species have been identified as reservoir hosts: the great gerbil (Rhombomys opimus) in Central Asia, Northern Afghanistan and Iran, the Indian desert jird (Meriones hurrianae) in India, the fat sand rat (Psammomys obesus) and Sundevall’s jird (Meriones crassus) in Northern Africa and Middle East, Libyan jird (Meriones libycus) in the Arabian Peninsula and Central Asia, the short-tailed bandicoot rat (Nesokia indica) in Iran and several rodent species (Arvicanthis, Tatera, Mastomys or Xerus spp.) in sub-Saharan Africa (Ashford 2000; Gramiccia and Gradoni 2005; Pourmohammadi et al. 2008; WHO 2010; Aoun and Bouratbine 2014; Chaara et al. 2014). The Shaw’s jird (Meriones shawi) also seems to play an important role in the transmission of L. major in Morocco (Rioux et al. 1982) and Tunisia (Ghawar et al. 2011a). The voles of the species Microtus tristrami and Microtus guentheri have recently been implicated as L. major reservoir hosts in a CL focus in northern Israel (Faiman et al. 2013). The sympatric occurrence of both vector (Phlebotomus papatasi) and M. guentheri in Turkey, Central Asia and Southern Europe suggests a threat for the spread of L. major into these regions (Antoniou et al. 2013; Faiman et al. 2013).
Leishmania major DNA has also been detected in internal organs of North African hedgehogs (Atelerix algirus) collected in Algeria (Tomás-Pérez et al. 2014) and North-Western Tunisia (Chemkhi et al. 2015), in the liver and spleen of Baluchistan gerbils (Gerbillus nanus) and brown rats (Rattus norvegicus) (Motazedian et al. 2010) and in the ears of long-eared hedgehogs (Hemiechinus auritus) in Iran (Azizi et al. 2011; Rouhani et al. 2014). The parasite was also detected by molecular techniques in the spleen of a hairy slit-faced bat (Nycteris hispida) in Ethiopia (Kassahun et al. 2015b), in the blood of domestic cats in the Ege Region of Turkey (Paşa et al. 2015) and in two dogs with dermal lesions from Israel (Baneth et al. 2016, 2017). L. major was isolated from an ear ulcer of a dog in Saudi Arabia (Elbihari et al. 1987) and from the spleen of an emaciated dog and from the blood of a dog with mild generalized alopecia, both from Egypt (Morsy et al. 1987). Isolation of parasites has also been made from cutaneous lesions in a vervet monkey (Chlorocebus aethiops) in Kenya (Binhazim et al. 1987) and in a least weasel (Mustela nivalis) in Tunisia (Ghawar et al. 2011b). In Kenya, specific antibodies to L. major have been reported in feral nonhuman primates: vervet monkeys, olive baboons (Papio cynocephalus anubis) and Sykes’ Monkeys (Cercopithecus albogularis) (Gicheru et al. 2009). Nevertheless and despite the detection of this Leishmania species in a variety of mammals, most of them are probably accidental hosts as they are rarely infected.
4.4.2 New World
Most of Leishmania species responsible for CL in the Americas are native to tropical rainforests, where a variety of wild animal species and phlebotomine sand flies maintain the enzootic cycle (Table 4.2).
Leishmania amazonensis (syn. Leishmania garnhami) is endemic in Argentina, Bolivia, Brazil, Colombia, Costa Rica, Ecuador, French Guyana, Peru, Suriname and Venezuela (Lainson 2010; WHO 2010; Alvar et al. 2012). The main clinical human forms are localized or DCL, although this last form of the disease, an anergic variant of LCL, as well as a visceralization of infection in immunocompetent people have also been documented (WHO 2010; Boelaert and Sundar 2014). Lutzomyia flaviscutellata, the major vector of this dermotropic Leishmania species, feeds predominantly on ground-dwelling rodents, the primary reservoir hosts of L. amazonensis. Several other wild mammals (Table 4.2) are suspected of being secondary reservoir hosts (Ashford 2000; Gramiccia and Gradoni 2005; Lainson 2010; WHO 2010). This parasite has also been documented in dogs and cats (de Souza et al. 2005; Tolezano et al. 2007; reviewed by Dantas-Torres 2009 and Pennisi et al. 2015; Ferreira et al. 2015; Ramirez et al. 2016; Sanches et al. 2017). In addition, L. amazonensis DNA has recently been detected in the skin and/or spleen of different species of insectivores, frugivorous or haematophagous bats captured in non-urban and urban areas of São Paulo state, Brazil (Savani et al. 2010; de Oliveira et al. 2015).
Leishmania braziliensis is reported in almost all countries of Central and South America (WHO 2010; Alvar et al. 2012). The usual clinical form caused by the parasite is a localized CL, although diffused CL has also been reported. In addition, about 5% of the patients evolve towards a severe mucocutaneous disease (WHO 2010; Alvar et al. 2012). Visceralizing disease has also been reported for L. braziliensis in HIV coinfected patients (Boelaert and Sundar 2014) and in dogs coinfected with Hepatozoon canis (Morgado et al. 2016). Several Lutzomyia species of the Lutzomyia, Nyssomyia, Psathyromyia, Psychodopygus and Verrucarum subgenera are implicated in its transmission (Killick-Kendrick 1999; Maroli et al. 2013; Akhoundi et al. 2016). Albeit L. braziliensis is primarily associated to tropical forests and several bats, edentates, marsupials, opossums and wild rodents have been found infected (Table 4.2), this parasite has adapted to human-modified environments, being frequently found in the peridomestic environment of rural houses. In these settings, domestic animals (i.e. horses, donkeys, mules, dogs and cats) may act not only as blood sources to phlebotomine sand flies but might also participate in the transmission cycle (Bonfante-Garrido et al. 1981, 1992; Aguilar et al. 1984; Passos et al. 1996; Schubach et al. 2004; Madeira et al. 2006; Vedovello et al. 2008; Rougeron et al. 2011; Santaella et al. 2011; Truppel et al. 2014). Nevertheless, their role as reservoir hosts is still considered circumstancial (Reithinger and Davies 1999; Dantas-Torres 2007; Truppel et al. 2014; Pennisi et al. 2015). In order to prove that these animals can act as domestic reservoirs in the peridomestic environment, it will be necessary to conduct infectivity tests on phlebotomine sand flies and perform the isolation and characterization of the parasites from samples accessible to the vectors. In addition, insights derived from recent research suggest that humans might be important domestic reservoir hosts of L. braziliensis, at least during outbreaks (Dantas-Torres 2007; WHO 2010).
Leishmania colombiensis, which is responsible for single or multiple cutaneous lesions, is endemic in Colombia, Panama and Venezuela (WHO 2010; Alvar et al. 2012). Lutzomyia gomezi, Lutzomyia hartmanni and Lutzomyia panamensis are the proven or suspected vectors, and the Hoffmann’s two-toed sloth (Choloepus hoffmanni) is the reservoir host in Panama (Killick-Kendrick 1999; Lainson 2010; WHO 2010; Maroli et al. 2013; Akhoundi et al. 2016). This parasite has also been isolated from the bone marrow of a dog in Venezuela (Delgado et al. 1993).
Leishmania guyanensis is responsible for LCL, and less frequently by DCL, being endemic in Argentina, Bolivia, Brazil (Acre, Amapá, Amazonas, Pará and Roraima states), Colombia, Ecuador, French Guiana, Guyana, Peru, Suriname and Venezuela (Lainson 2010; WHO 2010; Alvar et al. 2012). Transmission is associated with activities in forests (WHO 2010). The parasite can cause mucocutaneous lesions in a small proportion of cases (WHO 2010). The main vector is Lutzomyia umbratilis (Lainson 2010). Linnaeus’s two-toed sloth (Choloepus didactylus) is a major reservoir host of L. guyanensis in Brazil and in French Guiana maintaining the zoonosis in the forest canopy (Table 4.2). The southern tamandua (Tamandua tetradactyla) has been suggested as responsible for dispersal of the parasite due to its nomadic behaviour (WHO 2010). Occasional infections in rodents and opossums have been documented (Ashford 2000; Lainson 2010; WHO 2010). The DNA of the parasite has also been detected in one dog from Colombia (Santaella et al. 2011), but the contribution of domestic dogs in the life cycle of L. guyanensis seems limited.
Leishmania lainsoni causes CL, usually presenting as a single ulcer. The disease is found in Bolivia (subtropical areas), Brazil (Acre, Amapa, Pará and Rondônia states), Ecuador, French Guiana, Peru (tropical areas) and Suriname (Silveira et al. 1987; WHO 2010; Alvar et al. 2012; Kato et al. 2016). The vectors are Lutzomyia ubiquitalis in Brazil and Peru and Lutzomyia nuneztovari anglesi in Bolivia (Silveira et al. 1991a; Killick-Kendrick 1999; WHO 2010; Maroli et al. 2013; Akhoundi et al. 2016). The lowland paca (Cuniculus paca) is said to be the reservoir host (Silveira et al. 1991b; WHO 2010).
Leishmania lindenbergi causes CL in Brazil (Pará state) (Silveira et al. 2002; Lainson 2010; WHO 2010; Alvar et al. 2012). The suspected vector is Lutzomyia antunesi, and the reservoir host remains unknown (Silveira et al. 2002; WHO 2010; Maroli et al. 2013; Akhoundi et al. 2016).
Leishmania mexicana (syn. Leishmania pifanoi) is endemic in Belize, Colombia, Costa Rica, Ecuador, Guatemala, Mexico, Southern USA and Venezuela (WHO 2010; Alvar et al. 2012). Localized CL is the most common clinical form in humans, although diffuse CL has also been reported (WHO 2010). Many species of sylvatic ground-dwelling rodents (Heteromys, Neotoma, Nyctomys, Ototylomys and Sigmodon spp.) and marsupials have been implicated in the transmission cycle of L. mexicana (Table 4.2). In Texas, USA, cases of feline CL due to parasites belonging to the L. mexicana complex have been reported in the same areas where human cases occurred (Craig et al. 1986; Barnes et al. 1993; Trainor et al. 2010). L. mexicana DNA has also been detected in skin biopsies taken from a stray dog in Texas (Kipp et al. 2016) and in different tissues (i.e. heart, liver, skin and spleen) of several species of bats collected in six states of Mexico (Berzunza-Cruz et al. 2015). This dermotropic species has also been isolated from the liver aspirate of a dog from Ecuador (Hashiguchi et al. 1991). Lutzomyia olmeca olmeca is the main vector, and various other species are suspected to be involved in the life cycle of the parasite (Killick-Kendrick 1999; Lainson 2010; WHO 2010; Maroli et al. 2013; Akhoundi et al. 2016).
Leishmania naiffi causes a single, small, self-limiting lesion. It is found in Brazil (Rondônia state), Ecuador, Suriname and French Guiana (WHO 2010; van Thiel et al. 2010; Alvar et al. 2012; Kato et al. 2013). The proven vector is Lutzomyia ayrozai, while several other species are suspected to be involved in the transmission (Killick-Kendrick 1999; Maroli et al. 2013; Akhoundi et al. 2016); the reservoir host is the nine-banded armadillo (Dasypus novemcinctus) (Lainson and Shaw 1989; Naiff et al. 1991; WHO 2010).
Leishmania panamensis is responsible for LCL with some patients developing diffuse or mucocutaneous disease. This species is endemic in Colombia, Costa Rica, Ecuador (Pacific littoral), Guatemala, Honduras, Nicaragua and Panama (WHO 2010; Alvar et al. 2012). The major vector is considered to be Lutzomyia trapidoi, but several other species (e.g. Lutzomyia gomezi and Lutzomyia panamensis) have also been found to be naturally infected (Killick-Kendrick 1999; Maroli et al. 2013; Akhoundi et al. 2016). Lutzomyia trapidoi prefers to feed in the canopy, on arboreal mammals, such as sloths, which are the primary hosts of L. panamensis. Various wild mammalian species, including monkeys and several rodent species (Table 4.2), have been found to be infected, but their role as possible reservoir hosts is poorly known. According to WHO (2010), humans seem to play a reservoir role in some outbreaks caused by this Leishmania species. Dogs have also found infected with L. panamensis, but there is no evidence that they can play a role as reservoir hosts (Dereure et al. 1994; Vélez et al. 2012; Ramírez et al. 2016).
Leishmania peruviana distribution is limited to the Peruvian Andes, confined to areas with scant vegetation of the Western slopes between 800 and 3000 m altitude (Lainson 2010; WHO 2010). The clinical form is a localized ulcerative CL, and Lutzomyia ayacuchensis, Lutzomyia peruensis and Lutzomyia verrucarum are the proven vectors (Killick-Kendrick 1999; Maroli et al. 2013; Akhoundi et al. 2016). The natural reservoir hosts are probably wild marsupials and rodents (Table 4.2). Dogs are reputed to be the principal peridomestic reservoir hosts (Llanos-Cuentas et al. 1999). This assumption is based on a positive correlation observed between the risk of human CL and CanL prevalence in Huanuco, Peru. However, the scarcity of parasites in cutaneous lesions together with the high serorecovery rates suggest that dogs are able to control infection and thus may not be the main reservoir host of the parasite (Reithinger et al. 2003). Therefore, the role of dogs as reservoir hosts of L. peruviana should be confirmed by experimental transmission studies (Dantas-Torres 2007).
Leishmania shawi found in Brazil (Atlantic Forest of Pará state) causes localized CL (WHO 2010). In primary forest, the vector is Lutzomyia whitmani (Lainson et al. 1989; Killick-Kendrick 1999; Maroli et al. 2013; Akhoundi et al. 2016). The sylvatic reservoir hosts are monkeys, coatis and sloths (Lainson et al. 1989) (Table 4.2).
Leishmania venezuelensis is responsible for localized and DCL in Venezuela (Bonfante-Garrido et al. 1996; WHO 2010). Lutzomyia olmeca bicolor is suspected of being the vector (Killick-Kendrick 1999; Lainson 2010; Maroli et al. 2013; Akhoundi et al. 2016), while domestic cats are suspected to be the reservoir hosts (Bonfante-Garrido et al. 1991).
Leishmania waltoni is a recently described species associated with cases of DCL in humans in Dominican Republic (Shaw et al. 2015). This species belongs to the L. mexicana complex, and its reservoir hosts and vectors are still unknown.
4.5 Control of Reservoir Hosts
In 2010, a WHO Expert Committee defined that control strategies of leishmaniases should combine case management, integrated vector control and, in the case of zoonotic transmission, animal reservoir host control (WHO 2010).
For the control of anthroponotic leishmaniasis, an effective strategy for active case detection, surveillance and effective treatment of patients with clinical forms of leishmaniasis, accompanied by measures for preventing reinfection, should reduce or eliminate the parasite load and reduce transmission (WHO 2010). In fact, better tools have been made available to developing countries, such as: improvement of VL diagnosis (e.g. recombinant antigen (K39)-dipstick tests for in-field diagnosis), (ii) affordable VL treatment (e.g. the first oral antileishmanial drug, miltefosine; short course of therapy) and (iii) a more efficient phlebotomine sand fly control for both anthroponotic VL and CL (e.g. long-lasting insecticide-treated bed nets) (Desjeux 2004; Gramiccia and Gradoni 2005).
Control of reservoir hosts has been recommended for zoonotic VL and CL. Due to the exophilic habit of the phlebotomine vectors and the sylvatic nature of the reservoir hosts, the control of the zoonotic CL forms in both the Old and New Worlds is not easy and may even not be feasible, as it would require expensive environmental management difficult to implement and sustain (Gramiccia and Gradoni 2005; WHO 2010; Boelaert and Sundar 2014). As there is currently no vaccine for human use, the ways to protect individuals from contracting the infection include avoiding intrusion in natural zoonotic foci as well as the adoption of personal protective measures against phlebotomine sand fly bites with repellents and other devices (WHO 2010; Boelaert and Sundar 2014).
In the case of zoonotic CL caused by L. major, where the reservoir hosts are peridomestic rodent species, their elimination could be achieved by the destruction of the burrow systems by deep ploughing followed by planting. Another approach is by poisoning the colonies of rodents with wheat grains mixed with zinc phosphide along with the prior treatment of burrows with the anticoagulant dicoumarol (Saliba and Oumeish 1999; Ashford 2000; WHO 2010; Boelaert and Sundar 2014). This method of control may be effective against Rhombomys and Meriones rodents that feed on grains but not against Psammomys obesus. Because zinc phosphide is very toxic to man and other animals, care should be taken during its application (Saliba and Oumeish 1999; WHO 2010). The removal of chenopod plants, the only ones that P. obesus feed on, from areas close to inhabitants would also lead to the reduction of their numbers (Desjeux 1996; Saliba and Oumeish 1999; WHO 2010). Transmission of zoonotic CL due to L. aethiopica could also be reduced by controlling hyraxes around villages. Elimination of hyraxes within 1 km of settlements is thought to be effective in reducing transmission. As reinvasion is likely, control must be continuous. In some countries, hyraxes are protected animals, and their control is illegal and prohibited (Saliba and Oumeish 1999; Ashford 2000; WHO 2010).
In the New World, where most of Leishmania cycles are maintained by edentates, procyonids, arboreal or ground sylvatic rodents, an integrated environmental management approach, combining clearance of primary forest around villages and spraying of the cleared areas with insecticides to remove both the reservoir hosts and the vector, thus creating a “vector- and reservoir-free” zone around villages, might be effective for the control of zoonotic CL (WHO 2010). However, even clearing forest around villages may not reach the objective, as various Leishmania species (e.g. L. braziliensis) have proved to be remarkably adaptable to environmental degradation leading to peridomestic transmission rather to the elimination of the infections (Brandão-Filho et al. 1999; Ashford 2000; Boelaert and Sundar 2014).
Regarding zoonotic VL, infection in the canine domestic reservoir host should be monitored, and the management of infected dogs should be treatment or elimination (WHO 2010). Albeit test-and-treat strategies are performed in several Mediterranean countries, treating infected dogs alone may not be an effective control measure as relapses are frequent, and because despite clinical cure, dogs can recover infectivity weeks after treatment (Gradoni et al. 1987; Alvar et al. 1994; Miró et al. 2011); therefore, the use of repellents on dogs during and after treatment is imperative. In addition, the widespread use of the available anti-Leishmania drugs for both canine and human treatment might contribute to the generation and spread of drug-resistant parasites (Campino and Maia 2012). On the other hand, and despite culling dogs infected with L. infantum has been recommended by WHO, the implementation of this measure in countries where dogs are considered part of the family is impracticable. In Brazil, seropositive dogs are eliminated as part of a control programme, although its effectiveness in the control of infection is not clear-cut and it has not been tested in trials measuring clinical disease (González et al. 2015). Failure may occur due to several reasons (e.g. poor sensitivity of diagnostic methods, delay between diagnosis and culling and rapid replacement of culled dogs by new susceptible animals).
Leishmania life cycle can be interrupted through the use of impregnated dog collars and topical application of insecticide with repellent effect against phlebotomine sand flies (Killick-Kendrick et al. 1997; Mencke et al. 2003; Liénard et al. 2013; Dumont et al. 2015; Franc et al. 2015). In fact, a significant decrease in the incidence of zoonotic VL in children (Gavgani et al. 2002) and dogs has been observed in areas where most dogs used deltamethrin collars or have been treated with permethrin-based spot-on formulations (Maroli et al. 2001; Manzillo et al. 2006; Courtenay et al. 2009; Otranto et al. 2010). The impact of this type of control measure is dependent on the correct application and frequency of reapplication of the topical insecticides and in the loss rate of collars. In addition, the application of insecticides/repellents would have less impact on disease transmission if not integrated with stray dog control (Gramiccia and Gradoni 2005). Additional measures to control phlebotomine sand flies include reducing microhabitats favourable to them in the vicinity of the house and in other locations where dogs spend time, housing pets at dusk and indoor insecticide spraying of homes and animal shelters (Alexander and Maroli 2003; Maroli et al. 2010; Solano-Gallego et al. 2011).
Vaccination could be another strategy to reduce both CanL and the incidence in humans (Alvar et al. 2004). An effective vaccine would control both infection progression and the parasite transmissibility via the vector (Gradoni 2015). In Brazil, two canine vaccines (Leishmune® and Leishtec®) have been commercialized. Leishmune® was shown to induce a significant, long-lasting and strong protective effect against CanL in phase III of clinical trials (Silva et al. 2000; Borja-Cabrera et al. 2002). Although this vaccine was also proposed to be used as immunotherapeutic in infected dogs and as a transmission-blocking vaccine (Borja-Cabrera et al. 2004; Saraiva et al. 2006), in 2014 the Brazilian Ministry of Agriculture, Livestock and Food Supply suspended its commercialization due to non-compliance with all the requirements for phase III studies (http://www.agricultura.gov.br/assuntos/politica-agricola/arquivos/nota-tecnica-dfip-38-14-leishmune.pdf/view). Leish-Tec® conferred a significant reduction in the number of cases of CanL with an efficacy of 71.4% estimated according to parasitological results (i.e. imprinting, culture, or histopathology of dog tissues) (Regina-Silva et al. 2016). The infectiousness to reared L. longipalpis of vaccinated dogs presenting antibodies against the A2 antigen was 46.6% lower in comparison with non-vaccinated animals (Regina-Silva et al. 2016). In Europe, a vaccine consisting of purified excreted-secreted proteins of L. infantum and with QA-21 saponin as adjuvant (CaniLeish®) has provided a significant reduction in the risk of progressing to active infection or overt disease, with a clinical efficacy of 68% (Oliva et al. 2014). In vaccinated dogs that developed disease and that were exposed to the bites of reared P. perniciosus, the reduction in parasite transmission was found significant when compared to matched controls (Bongiorno et al. 2013). More recently (in 2017), a second vaccine (Letifend®) consisting of a recombinant Protein Q from L. infantum MON-1 has been commercialized in Europe. According to the product information available at the European Medicines Agency, a vaccinated dog has five times less risk to develop clinical disease than a non-vaccinated dog (https://ec.europa.eu/health/documents/communityregister/2016/20160420134483/anx_134483_en.pdf).
In last years, CanL expanded northwards in Europe, mainly due to movement of infected dogs from endemic to previously nonendemic areas (Maia and Cardoso 2015). Therefore, control of CanL should also include the compulsory certification by veterinarians of the non-infective state of animals moving from one place to another to avoid the introduction of infected dogs in areas previously nonendemic, especially in those having competent vectors which might result in the persistence of L. infantum (WHO 2010; Maia and Cardoso 2015).
4.6 Final Remarks
The development of efficient tools for reservoir host control depends on proper understanding of the local epidemiology of leishmaniasis (including whether transmission is anthroponotic or zoonotic). Apart from the proven reservoir hosts, Leishmania parasites have been found in a variety of wild and domestic animals around the world, but their role in sustaining the life cycle of the parasite is unknown. In some instances, the parasites have been isolated and formally characterized, but in many cases, the infection status and parasite species have been inferred based on the detection of DNA fragments of the parasite through PCR-based tools. Therefore, it would be crucial to isolate and formally identify Leishmania parasites infecting any suspected reservoir host. As in many cases, information about food sources, breeding season, movement and migration activities and longevity of the potential reservoir host(s) is lacking; further work along these lines should also be performed.
References
Abebe A, Evans DA, Gemetchu T. The isolation of Leishmania aethiopica from the ground squirrel Xerus rutilus. Trans R Soc Trop Med Hyg. 1990;84(5):691.
Abranches P. Reservoirs of visceral leishmaniasis. In: Hart DT, editor. Leishmaniasis: the current status and new strategies for control. New York: Plenum Press; 1989. p. 61–9.
Abranches P, et al. Kala-azar in Portugal. V. The sylvatic cycle in the enzootic endemic focus of Arrabida. J Trop Med Hyg. 1984;87(5):197–200.
Aguilar C, et al. Study of an outbreak of cutaneous leishmaniasis in Venezuela. The role of domestic animals. Mem Inst Oswaldo Cruz. 1984;79:181–95.
Aharonson-Raz K, et al. Low seroprevalence of Leishmania infantum and Toxoplasma gondii in the horse population in Israel. Vector Borne Zoonotic Dis. 2015;15(12):726–31.
Akhoundi M, et al. A historical overview of the classification, evolution, and dispersion of Leishmania parasites and sandflies. PLoS Negl Trop Dis. 2016;10(3):e0004349.
Akter S, et al. Molecular and serological evidence of Leishmania infection in stray dogs from visceral leishmaniasis-endemic areas of Bangladesh. Am J Trop Med Hyg. 2016;95(4):795–9.
Alexander B, Maroli M. Control of phlebotomine sandflies. Med Vet Entomol. 2003;17(1):1–18.
Alvar J, et al. Canine leishmaniasis: clinical, parasitological and entomological follow-up after chemotherapy. Ann Trop Med Parasitol. 1994;88(4):371–8.
Alvar J, et al. Canine leishmaniasis. Adv Parasitol. 2004;57:1–88.
Alvar J, et al. Leishmaniasis worldwide and global estimates of its incidence. PLoS One. 2012;7(5):e35671.
Antoniou M, et al. The role of indigenous phlebotomine sandflies and mammals in the spreading of leishmaniasis agents in the Mediterranean region. Euro Surveill. 2013;18(30):20540.
Aoun K, Bouratbine A. Cutaneous leishmaniasis in North Africa: a review. Parasite. 2014;21:14.
Ashford RW. Leishmaniasis reservoirs and their significance in control. Clin Dermatol. 1996;14(5):523–32.
Ashford RW. The leishmaniases as emerging and reemerging zoonoses. Int J Parasitol. 2000;30(12–13):1269–81.
Ashford RW, et al. The epidemiology of cutaneous leishmaniasis in Ethiopia. Trans R Soc Trop Med Hyg. 1973;67(4):568–601.
Attipa C, et al. Prevalence study and risk factor analysis of selected bacterial, protozoal and viral, including vector-borne, pathogens in cats from Cyprus. Parasit Vectors. 2017;10(1):130.
Azizi K, et al. Gerbillus nanus (Rodentia: Muridae): a new reservoir host of Leishmania major. Ann Trop Med Parasitol. 2011;105(6):431–7.
Bamorovat M, et al. Leishmania tropica in stray dogs in southeast Iran. Iran J Public Health. 2015;44(10):1359–66.
Baneth G, et al. Mucocutaneous Leishmania tropica infection in a dog from a human cutaneous leishmaniasis focus. Parasit Vectors. 2014;7:118.
Baneth G et al. (2015) A cluster of naturally occurring equine leishmaniosis with persistent progressive clinical manifestations, sub-clinical infection and evaluation of the horse as a reservoir host. In: 25th international conference of the world association for the advancement of veterinary parasitology. p. 100.
Baneth G, et al. Leishmania major infection in a dog with cutaneous manifestations. Parasit Vectors. 2016;9(1):246.
Baneth G, et al. Canine leishmaniosis caused by Leishmania major and Leishmania tropica: comparative findings and serology. Parasit Vectors. 2017;10(1):113.
Barnes JC, Stanley O, Craig TM. Diffuse cutaneous leishmaniasis in a cat. J Am Vet Med Assoc. 1993;202(3):416–8.
Bashaye S, et al. Risk factors for visceral leishmaniasis in a new epidemic site in Amhara Region, Ethiopia. Am J Trop Med Hyg. 2009;81(1):34–9.
Basset D, et al. Visceral leishmaniasis in organ transplant recipients: 11 new cases and a review of the literature. Microbes Infect. 2005;7(13):1370–5.
Berzunza-Cruz M, et al. Leishmania (L.) mexicana infected bats in Mexico: novel potential reservoirs. PLoS Negl Trop Dis. 2015;9(1):e0003438.
Bhattarai N, et al. Domestic animals and epidemiology of visceral leishmaniasis, Nepal. Emerg Infect Dis. 2010;16(2):231–7.
Binhazim AA, et al. Isolation of Leishmania major from a naturally infected vervet monkey (Cercopithecus aethiops) caught in Kiambu District, Kenya. J Parasitol. 1987;73(6):1278–9.
Boelaert M, Sundar S. Leishmaniasis. In: Farrar J, Hotez P, Junghanss T, Kang G, Lalloo D, White N, editors. Manson’s tropical diseases. 23rd ed. Beijing: Elsevier Saunders; 2014. p. 631–51.
Bonfante-Garrido R, et al. Enzootic equine cutaneous leishmaniasis in Venezuela. Trans R Soc Trop Med Hyg. 1981;75(3):471.
Bonfante-Garrido R, et al. Natural infection of cats with Leishmania in Barquisimeto, Venezuela. Trans R Soc Trop Med Hyg. 1991;85(1):53.
Bonfante-Garrido R, et al. Cutaneous leishmaniasis in western Venezuela caused by infection with Leishmania venezuelensis and L. braziliensis variants. Trans R Soc Trop Med Hyg. 1992;86(2):141–8.
Bonfante-Garrido R, et al. Disseminated American cutaneous leishmaniasis. Int J Dermatol. 1996;35(8):561–5.
Bongiorno G, et al. Vaccination with LiESP/QA-21 (CaniLeish®) reduces the intensity of infection in Phlebotomus perniciosus fed on Leishmania infantum infected dogs—a preliminary xenodiagnosis study. Vet Parasitol. 2013;197(3–4):691–5.
Borja-Cabrera GP, et al. Long lasting protection against canine kala-azar using the FML-QuilA saponin vaccine in an endemic area of Brazil (São Gonçalo do Amarante, RN). Vaccine. 2002;20(27–28):3277–84.
Borja-Cabrera GP, et al. Effective immunotherapy against canine visceral leishmaniasis with the FML-vaccine. Vaccine. 2004;22(17–18):2234–43.
Bousslimi N, et al. Natural infection of North African gundi (Ctenodactylus gundi) by Leishmania tropica in the focus of cutaneous leishmaniasis, Southeast Tunisia. Am J Trop Med Hyg. 2012;86(6):962–5.
Brandão-Filho SP, et al. Epidemiological surveys confirm an increasing burden of cutaneous leishmaniasis in north-east Brazil. Trans R Soc Trop Med Hyg. 1999;93(5):488–94.
Brandão-Filho SP, et al. Hospedeiros reservatórios de Leishmania spp. associados à leishmaniose tegumentar americana com especial ênfase no Brasil. In: Barral A, Costa J, editors. Leishmanias e a leishmaniose tegumentar nas Américas. Salvador: Cyted, CNPq Fiocruz-BA; 2011. p. 65–74.
Bray R. The zoonotic potential of reservoirs of leishmaniasis in the old world. Ecol Dis. 1982;1(4):257–67.
Bualert L, et al. Autochthonous disseminated dermal and visceral leishmaniasis in an AIDS patient, southern Thailand, caused by Leishmania siamensis. Am J Trop Med Hyg. 2012;86(5):821–4.
Caldart ET, et al. Leishmania in synanthropic rodents (Rattus rattus): new evidence for the urbanization of Leishmania (Leishmania) amazonensis. Rev Bras Parasitol Vet. 2017;26(1):17–27.
Campino L, Maia C. The role of reservoirs: canine leishmaniasis. In: Ponte-Sucre A, Padron-Nieves M, Diaz E, editors. Drug resistance in Leishmania parasites—consequences, molecular mechanism and possible treatments. Wien: Springer; 2012. p. 45–64.
Can H, et al. Seroprevalence of Leishmania infection and molecular detection of Leishmania tropica and Leishmania infantum in stray cats of İzmir, Turkey. Exp Parasitol. 2016;167:109–14.
Carreira J, et al. Leishmania in marsupials—an overview of infection records in the Americas and Australia. Open J Anim Sci. 2017;7(3):315–43.
Cerqueira EJ, et al. Experimental infection of Equus asinus with Leishmania chagasi Cunha & Chagas, 1937. Rev Soc Bras Med Trop. 2003;36(6):695–701.
Chaara D, et al. Leishmaniases in Maghreb: an endemic neglected disease. Acta Trop. 2014;132:80–93.
Chatzis MK, et al. Cytological and molecular detection of Leishmania infantum in different tissues of clinically normal and sick cats. Vet Parasitol. 2014;202(3–4):217–25.
Chemkhi J, et al. Natural infection of Algerian hedgehog, Atelerix algirus (Lereboullet 1842) with Leishmania parasites in Tunisia. Acta Trop. 2015;150:42–51.
Chusri S, et al. Consecutive cutaneous and visceral leishmaniasis manifestations involving a novel Leishmania species in two HIV patients in Thailand. Am J Trop Med Hyg. 2012;87(1):76–80.
Chusri S, et al. Animal reservoirs and potential vectors of Leishmania siamensis in southern Thailand. Southeast Asian J Trop Med Public Health. 2014;45(1):13–9.
Colmenares M, et al. Identification of blood meals of Phlebotomus perniciosus (Diptera: Psychodidae) in Spain by a competitive enzyme-linked immunosorbent assay biotin/avidin method. J Med Entomol. 1995;32(3):229–33.
Costa CH, et al. Asymptomatic human carriers of Leishmania chagasi. Am J Trop Med Hyg. 2002;66(4):334–7.
Courtenay O, et al. A long-lasting topical deltamethrin treatment to protect dogs against visceral leishmaniasis. Med Vet Entomol. 2009;23(3):245–56.
Craig TM, et al. Dermal leishmaniasis in a Texas cat. Am J Trop Med Hyg. 1986;35(6):1100–2.
da Silva SM, et al. First report of infection of Lutzomyia longipalpis by Leishmania (Leishmania) infantum from a naturally infected cat of Brazil. Vet Parasitol. 2010;174(1–2):150–4.
Dantas-Torres F. The role of dogs as reservoirs of Leishmania parasites, with emphasis on Leishmania (Leishmania) infantum and Leishmania (Viannia) braziliensis. Vet Parasitol. 2007;149(3–4):139–46.
Dantas-Torres F. Canine leishmaniosis in South America. Parasit Vectors. 2009;2(Suppl 1):S1.
de Castro Ferreira E, et al. Leishmania (V.) braziliensis infecting bats from Pantanal wetland, Brazil: first records for Platyrrhinus lineatus and Artibeus planirostris. Acta Trop. 2017;172:217–22.
de Oliveira FM, et al. First detection of Leishmania spp. DNA in Brazilian bats captured strictly in urban areas. Acta Trop. 2015;150:176–81.
de Rezende MB, et al. Detection of Leishmania spp. in bats from an area of Brazil endemic for visceral leishmaniasis. Transbound Emerg Dis. 2017;64(6):e36–42. https://doi.org/10.1111/tbed.12597.
de Souza AI, et al. Feline leishmaniasis due to Leishmania (Leishmania) amazonensis in Mato Grosso do Sul state, Brazil. Vet Parasitol. 2005;128(1–2):41–5.
Delgado O, et al. Leishmania colombiensis in Venezuela. Am J Trop Med Hyg. 1993;48(1):145–7.
Dereure J, et al. Leishmania tropica in Morocco: infection in dogs. Trans R Soc Trop Med Hyg. 1991;85(5):595.
Dereure J, et al. Leishmaniose en Equateur. 4. Infestation naturelle du chien par Leishmania panamensis. Ann Soc Belg Med Trop. 1994;74(1):29–33.
Dereure J, et al. Visceral leishmaniasis in Sudan: first identifications of Leishmania from dogs. Trans R Soc Trop Med Hyg. 2000;94(2):154–5.
Dereure J, et al. Visceral leishmaniasis in eastern Sudan: parasite identification in humans and dogs; host-parasite relationships. Microbes Infect. 2003;5(12):1103–8.
Desjeux P. Leishmaniasis. Public health aspects and control. Clin Dermatol. 1996;14(5):417–23.
Desjeux P. Leishmaniasis: current situation and new perspectives. Comp Immunol Microbiol Infect Dis. 2004;27(5):305–18.
Dujardin JC, et al. Spread of vector-borne diseases and neglect of leishmaniasis, Europe. Emerg Infect Dis. 2008;14(7):1013–8.
Dumont P, et al. Repellent and insecticidal efficacy of a new combination of fipronil and permethrin against the main vector of canine leishmaniosis in Europe (Phlebotomus perniciosus). Parasit Vectors. 2015;8:49.
Ebani VV, et al. Serological survey on some pathogens in wild brown hares (Lepus europaeus) in Central Italy. Asian Pac J Trop Med. 2016;9(5):465–9.
Elbihari S, Cheema AH, el-Hassan AM. Leishmania infecting man and wild animals in Saudi Arabia. 4. Canine cutaneous leishmaniasis in the Eastern Province. Trans R Soc Trop Med Hyg. 1987;81(6):925–7.
Elnaiem DA, et al. The Egyptian mongoose, Herpestes ichneumon, is a possible reservoir host of visceral leishmaniasis in eastern Sudan. Parasitology. 2001;122(5):531–6.
Faiman R, et al. A newly emerged cutaneous leishmaniasis focus in northern Israel and two new reservoir hosts of Leishmania major. PLoS Negl Trop Dis. 2013;7(2):e2058.
Ferreira Ede C, et al. Mixed infection of Leishmania infantum and Leishmania braziliensis in rodents from endemic urban area of the New World. BMC Vet Res. 2015;11:71.
Fernández-Bellon H, et al. Immune response to Leishmania infantum in healthy horses in Spain. Vet Parasitol. 2006;135(2):181–5.
Franc M, et al. Efficacy of a new combination of fipronil and permethrin (Effitix®) against Phlebotomus perniciosus in dogs. Vet Parasitol. 2015;212(3–4):156–60.
Gama A, et al. Cutaneous leishmaniosis in a horse from northern Portugal. Vet Parasitol. 2014;200(1–2):189–92.
Gavgani AS, et al. Effect of insecticide-impregnated dog collars on incidence of zoonotic visceral leishmaniasis in Iranian children: a matched-cluster randomised trial. Lancet. 2002;360(9330):374–9.
Ghawar W, et al. Leishmania major infection among Psammomys obesus and Meriones shawi: reservoirs of zoonotic cutaneous leishmaniasis in Sidi Bouzid (central Tunisia). Vector Borne Zoonotic Dis. 2011a;11(12):1561–8.
Ghawar W, et al. First report of natural infection of least weasel (Mustela nivalis Linnaeus, 1776) with Leishmania major in Tunisia. Vector Borne Zoonotic Dis. 2011b;11(11):1507–9.
Gicheru MM, et al. Prevalence of antibodies and cell mediated immune response against Leishmania major in feral nonhuman primates from Kenya. Acta Trop. 2009;109(2):136–40.
González U, et al. Vector and reservoir control for preventing leishmaniasis. Cochrane Database Syst Rev. 2015;8:CD008736.
Gradoni L, et al. Leishmaniasis in Tuscany (Italy): VII. Studies on the role of the black rat, Rattus rattus, in the epidemiology of visceral leishmaniasis. Trans R Soc Trop Med Hyg. 1983;77(4):427–31.
Gradoni L, et al. Leishmania infantum infection rates in Phlebotomus perniciosus fed on naturally infected dogs under antimonial treatment. Med Vet Entomol. 1987;1(4):339–42.
Gradoni L. Canine Leishmania vaccines: still a long way to go. Vet Parasitol. 2015;208(1–2):94–100.
Gramiccia M. Recent advances in leishmaniosis in pet animals: epidemiology, diagnostics and anti-vectorial prophylaxis. Vet Parasitol. 2011;181(1):23–30.
Gramiccia M, Gradoni L. The current status of zoonotic leishmaniases and approaches to disease control. Int J Parasitol. 2005;35(11–12):1169–80.
Gramiccia M, Gradoni L. The leishmaniases in Southern Europe. In: Takken W, Knols BGJ, editors. Emerging pests and vector-borne diseases, ecology and control of vectorborne diseases, vol. 1. Wageningen: Wageningen Academic Publishers; 2007. p. 75–95.
Grimaldi G Jr, Tesh RB. Leishmaniases of the new world: current concepts and implications for future research. Clin Microbiol Rev. 1993;6(3):230–50.
Guessous-Idrissi N, et al. Short report: Leishmania tropica: etiologic agent of a case of canine visceral leishmaniasis in northern Morocco. Am J Trop Med Hyg. 1997;57(2):172–3.
Hajjaran H, et al. Molecular identification and polymorphism determination of cutaneous and visceral leishmaniasis agents isolated from human and animal hosts in Iran. Biomed Res Int. 2013;2013:789326.
Hashiguchi Y, et al. Andean leishmaniasis in Ecuador caused by infection with Leishmania mexicana and L. major-like parasites. Am J Trop Med Hyg. 1991;44(2):205–17.
Hassan M, et al. Role of the domestic dog as a reservoir host of Leishmania donovani in eastern Sudan. Parasit Vectors. 2009;2(1):26.
Hatam GR, et al. First report of natural infection in cats with Leishmania infantum in Iran. Vector Borne Zoonotic Dis. 2010;10(3):313–6.
Hoogstraal H, Heyneman D. Leishmaniasis in the Sudan Republic. 30. Final epidemiological report. Am J Trop Med Hyg. 1969;18(6):1087–210.
Jambulingam P, et al. Domestic dogs as reservoir hosts for Leishmania donovani in the southernmost western Ghats in India. Acta Trop. 2017;171:64–7.
Jaouadi K, et al. First detection of Leishmania killicki (Kinetoplastida, Trypanosomatidae) in Ctenodactylus gundi (Rodentia, Ctenodactylidae), a possible reservoir of human cutaneous leishmaniasis in Tunisia. Parasit Vectors. 2011;4:159.
Jiménez M, et al. Could wild rabbits (Oryctolagus cuniculus) be reservoirs for Leishmania infantum in the focus of Madrid, Spain? Vet Parasitol. 2014;202(3–4):296–300.
Kalayou S, et al. Serological evidence of Leishmania donovani infection in apparently healthy dogs using direct agglutination test (DAT) and rk39 dipstick tests in Kafta Humera, north-west Ethiopia. Transbound Emerg Dis. 2011;58(3):255–62.
Kanjanopas K, et al. Sergentomyia (Neophlebotomus) gemmea, a potential vector of Leishmania siamensis in southern Thailand. BMC Infect Dis. 2013;13:333.
Kassahun A, et al. Detection of Leishmania donovani and L. tropica in Ethiopian wild rodents. Acta Trop. 2015a;145:39–44.
Kassahun A, et al. Natural infection of bats with Leishmania in Ethiopia. Acta Trop. 2015b;150:166–70.
Kato H, et al. First human cases of Leishmania (Viannia) naiffi infection in Ecuador and identification of its suspected vector species. Acta Trop. 2013;128(3):710–3.
Kato H, et al. First human cases of Leishmania (Viannia) lainsoni infection and a search for the vector sand flies in Ecuador. PLoS Negl Trop Dis. 2016;10(5):e0004728.
Kenubih A, et al. Preliminary survey of domestic animal visceral leishmaniasis and risk factors in north-west Ethiopia. Trop Med Int Health. 2015;20(2):205–10.
Killick-Kendrick R. The biology and control of phlebotomine sand flies. Clin Dermatol. 1999;17(3):279–89.
Killick-Kendrick R, et al. Protection of dogs from bites of phlebotomine sandflies by deltamethrin collars for control of canine leishmaniasis. Med Vet Entomol. 1997;11(2):105–11.
Kipp EJ, et al. Genetic evidence of enzootic leishmaniasis in a stray canine and Texas mouse from sites in west and central Texas. Mem Inst Oswaldo Cruz. 2016;111(10):652–4.
Koehler K, et al. Cutaneous leishmaniosis in a horse in southern Germany caused by Leishmania infantum, Vet Parasitol. 2002;109(1–2):9–17.
Lainson R. The neotropical Leishmania species: a brief historical review of the discovery, ecology and taxonomy. Rev Pan-Amaz Saude. 2010;1:13–32.
Lainson R, Shaw JJ. Leishmania (Viannia) naiffi sp. n., a parasite of the armadillo, Dasypus novemcinctus (L.) in Amazonian Brazil. Ann Parasitol Hum Comp. 1989;64(1):3–9.
Lainson R, et al. Leishmania (Viannia) shawi sp. n., a parasite of monkeys, sloths and procyonids in Amazonian Brazil. Ann Parasitol Hum Comp. 1989;64(3):200–7.
Lainson R, Shaw JJ. New world leishmaniasis. In: Cox FEG, Kreier JP, Wakelin D, editors. Topley & Wilson’s microbiology and microbial infections. London: Arnold; 2005. p. 313–49.
Lemma W, et al. A zoonotic focus of cutaneous leishmaniasis in Addis Ababa, Ethiopia. Parasit Vectors. 2009;2(1):60.
Lemma W, et al. Preliminary study on investigation of zoonotic visceral leishmaniasis in endemic foci of Ethiopia by detecting Leishmania infections in rodents. Asian Pac J Trop Med. 2017;10(4):418–22.
Liénard E, et al. Efficacy of dinotefuran, permethrin and pyriproxyfen combination spot-on on dogs against Phlebotomus perniciosus and Ctenocephalides canis. Parasitol Res. 2013;112(11):3799–805.
Llanos-Cuentas EA, et al. Natural infections of Leishmania peruviana in animals in the Peruvian Andes. Trans R Soc Trop Med Hyg. 1999;93(1):15–20.
Lobsiger L, et al. An autochthonous case of cutaneous bovine leishmaniasis in Switzerland. Vet Parasitol. 2010;169(3–4):408–14.
Lopes AP, et al. Prevalence of antibodies to Leishmania infantum and Toxoplasma gondii in horses from the north of Portugal. Parasit Vectors. 2013;6:178.
Madeira MF, et al. Mixed infection with Leishmania (Viannia) braziliensis and Leishmania (Leishmania) chagasi in a naturally infected dog from Rio de Janeiro, Brazil. Trans R Soc Trop Med Hyg. 2006;100(5):442–5.
Maia C, Campino L. Can domestic cats be considered reservoir hosts of zoonotic leishmaniasis? Trends Parasitol. 2011;27(8):341–4.
Maia C, Cardoso L. Spread of Leishmania infantum in Europe with dog travelling. Vet Parasitol. 2015;213(1–2):2–11.
Maia C, et al. Feline Leishmania infection in a canine leishmaniasis endemic region, Portugal. Vet Parasitol. 2010;174(3–4):336–40.
Maia C, et al. Bacterial and protozoal agents of feline vector-borne diseases in domestic and stray cats from southern Portugal. Parasit Vectors. 2014;7:115.
Maia C, et al. First case of feline leishmaniosis caused by Leishmania infantum genotype E in a cat with concurrent nasal squamous cell carcinoma. J Feline Med Surg Open Rep. 2015;1(2):2055116915593969.
Manzillo V, et al. Deltamethrin-impregnated collars for the control of canine leishmaniasis: evaluation of the protective effect and influence on the clinical outcome of Leishmania infection in kennelled stray dogs. Vet Parasitol. 2006;142(1–2):142–5.
Marcelino AP, et al. Molecular detection of Leishmania braziliensis in Rattus norvegicus in an area endemic for cutaneous leishmaniasis in Brazil. Vet Parasitol. 2011;183:54–8.
Maroli M, et al. Evidence for an impact on the incidence of canine leishmaniasis by the mass use of deltamethrin-impregnated dog collars in southern Italy. Med Vet Entomol. 2001;15(4):358–63.
Maroli M, et al. Infection of sandflies by a cat naturally infected with Leishmania infantum. Vet Parasitol. 2007;145(3–4):357–60.
Maroli M, et al. Guidelines for prevention of leishmaniasis in dogs. J Am Vet Med Assoc. 2010;236(11):1200–6.
Maroli M, et al. Phlebotomine sandflies and the spreading of leishmaniases and other diseases of public health concern. Med Vet Entomol. 2013;27(2):123–47.
Martín-Sánchez J, et al. Infection by Leishmania infantum in cats: epidemiological study in Spain. Vet Parasitol. 2007;145(3–4):267–73.
Mencke N, et al. Repellent efficacy of a combination containing imidacloprid and permethrin against sand flies (Phlebotomus papatasi) in dogs. Parasitol Res. 2003;90(Suppl 3):S108–11.
Michel G, et al. Importance of worldwide asymptomatic carriers of Leishmania infantum (L. chagasi) in human. Acta Trop. 2011;119(2–3):69–75.
Millán J, Ferroglio E, Solano-Gallego L. Role of wildlife in the epidemiology of Leishmania infantum infection in Europe. Parasitol Res. 2014;113(6.):2005–2014.
Miró G, et al. Infectivity to Phlebotomus perniciosus of dogs naturally parasitized with Leishmania infantum after different treatments. Parasit Vectors. 2011;4:52.
Molina R, et al. The hare (Lepus granatensis) as potential sylvatic reservoir of Leishmania infantum in Spain. Vet Parasitol. 2012;190(1–2):268–71.
Montoya A, et al. Leishmania infantum infection in Bennett’s wallabies (Macropus rufogriseus rufogriseus) in a Spanish wildlife park. J Zoo Wildl Med. 2016;47(2):586–393.
Morgado FN, et al. Hepatozoon canis and Leishmania spp. coinfection in dogs diagnosed with visceral leishmaniasis. Rev Bras Parasitol Vet. 2016;25(4):450–8.
Morsy T, et al. Natural infections of Leishmania major in domestic dogs from Alexandria, Egypt. Am J Trop Med Hyg. 1987;37(1):49–52.
Morsy TA, al Dakhil MA, el Bahrawy AF. Characterization of Leishmania aethiopica from rock hyrax, Procavia capensis trapped in Najran, Saudi Arabia. J Egypt Soc Parasitol. 1997;27(2):349–53.
Motazedian MH, et al. First detection of Leishmania major in Rattus norvegicus from Fars Province, southern Iran. Vector Borne Zoonotic Dis. 2010;10(10):969–75.
Mukhtar MM, et al. Detection of antibodies to Leishmania donovani in animals in a kala-azar endemic region in eastern Sudan: a preliminary report. Trans R Soc Trop Med Hyg. 2000;94(1):33–6.
Müller N, et al. Occurrence of Leishmania sp. in cutaneous lesions of horses in Central Europe. Vet Parasitol. 2009;166(3–4):346–51.
Naiff RD, et al. Epidemiological and nosological aspects of Leishmania naiffi Lainson Shaw, 1989. Mem Inst Oswaldo Cruz. 1991;86(3):317–21.
Nasereddin A, Salant H, Abdeen Z. Feline leishmaniasis in Jerusalem: serological investigation. Vet Parasitol. 2008;158(4):364–9.
Negera E, et al. Outbreak of cutaneous leishmaniasis in Silti District, Ethiopia: risk factor assessment and causative agent identification. Trans R Soc Trop Med Hyg. 2008;102(9):883–90.
Noppakun N, Kraivichian K, Siriyasatien P. Disseminated dermal leishmaniasis caused by Leishmania siamensis in a systemic steroid therapy patient. Am J Trop Med Hyg. 2014;91(5):869–70.
Ntais P, et al. Will the introduction of Leishmania tropica MON-58, in the island of Crete, lead to the settlement and spread of this rare zymodeme? Acta Trop. 2014;132:125–30.
Oliva G, et al. A randomised, double-blind, controlled efficacy trial of the LiESP/QA-21 vaccine in naïve dogs exposed to two Leishmania infantum transmission seasons. PLoS Negl Trop Dis. 2014;8(10):e3213.
Otranto D, et al. Prevention of endemic canine vector-borne diseases using imidacloprid 10% and permethrin 50% in young dogs: a longitudinal field study. Vet Parasitol. 2010;172(3–4):323–32.
Ozon C, et al. Disseminated feline leishmaniosis due to Leishmania infantum in southern France. Vet Parasitol. 1998;75(2–3):273–7.
Paşa S, et al. Detection of Leishmania major and Leishmania tropica in domestic cats in the Ege region of Turkey. Vet Parasitol. 2015;212(3–4):389–92.
Passos VM, et al. Natural infection of a domestic cat (Felis domesticus) with Leishmania (Viannia) in the metropolitan region of Belo Horizonte, state of Minas Gerais, Brazil. Mem Inst Oswaldo Cruz. 1996;91(1):19–20.
Pennisi MG, et al. Serological and molecular prevalence of Leishmania infantum infection in cats from southern Italy. J Feline Med Surg. 2012;14:656–7.
Pennisi MG, et al. LeishVet update and recommendations on feline leishmaniosis. Parasit Vectors. 2015;8:302.
Pourmohammadi B, et al. Rodent infection with Leishmania in a new focus of human cutaneous leishmaniasis, in northern Iran. Ann Trop Med Parasitol. 2008;102:127–33.
Pourmohammadi B, et al. Natural infection of Nesokia indica with Leishmania major and Leishmania infantum parasites in Damghan city, northern Iran. Acta Trop. 2017;170:134–9.
Portús M, et al. Wild and domestic mammals in the life cycle of Leishmania infantum in Southwest Europe. A literature review and studies performed in Catalonia (Spain). Rev Iber Parasitol. 2002;62:72–6.
Pothirat T, et al. First isolation of Leishmania from northern Thailand: case report, identification as Leishmania martiniquensis and phylogenetic position within the Leishmania enriettii complex. PLoS Negl Trop Dis. 2014;8(12):e3339.
Pratlong F, et al. Geographical distribution and epidemiological features of old world cutaneous leishmaniasis foci, based on the isoenzyme analysis of 1048 strains. Trop Med Int Health. 2009;14(9):1071–85.
Quinnell R, Courtenay O. Transmission, reservoir hosts and control of zoonotic visceral leishmaniasis. Parasitology. 2009;136(14):1915–34.
Ramírez J, et al. Taxonomy, diversity, temporal and geographical distribution of cutaneous leishmaniasis in Colombia: a retrospective study. Sci Rep. 2016;6:28266.
Regina-Silva S, et al. Field randomized trial to evaluate the efficacy of the Leish-Tec® vaccine against canine visceral leishmaniasis in an endemic area of Brazil. Vaccine. 2016;34(19):2233–9.
Reithinger R, Davies CR. Is the domestic dog (Canis familiaris) a reservoir host of American cutaneous leishmaniasis? A critical review of the current evidence. Am J Trop Med Hyg. 1999;61(4):530–41.
Reithinger R, Espinoza J, Davies C. The transmission dynamics of canine American cutaneous leishmaniasis in Huánuco, Peru. Am J Trop Med Hyg. 2003;69(5):473–80.
Reuss SM, et al. Autochthonous Leishmania siamensis in horse, Florida, USA. Emerg Infect Dis. 2012;18(9):1545–7.
Rioux JA, et al. Meriones shawi (Duvernoy, 1842) [Rodentia, Gerbillidae] a reservoir of Leishmania major, Yakimoff and Schokhor, 1914 [Kinetoplastida, Trypanosomatidae] in South Morocco. C R Seances Acad Sci III. 1982;294(11):515–7.
Rohousova I, et al. Exposure to Leishmania spp. and sand flies in domestic animals in northwestern Ethiopia. Parasit Vectors. 2015;8:360.
Rolão N, et al. Equine infection with Leishmania in Portugal. Parasite. 2005;12(2):183–6.
Roque AL, Jansen AM. Wild and synanthropic reservoirs of Leishmania species in the Americas. Int J Parasitol Parasites Wildl. 2014;3(3):251–62.
Rougeron V, et al. First clinical case of cutaneous leishmaniasis due to Leishmania (Viannia) braziliensis in a domestic cat from French Guiana. Vet Parasitol. 2011;181(2–4):325–8.
Rouhani S, et al. Novel identification of Leishmania major in Hemiechinus auritus and molecular detection of this parasite in Meriones libycus from an important foci of zoonotic cutaneous leishmaniasis in Iran. J Infect Public Health. 2014;7(3):210–7.
Saliba EK, Oumeish OY. Reservoir hosts of cutaneous leishmaniasis. Clin Dermatol. 1999;17(3):275–7.
Sanches LD, et al. Natural canine infection by Leishmania infantum and Leishmania amazonensis and their implications for disease control. Rev Bras Parasitol Vet. 2017;25(4):465–9.
Santaella J, et al. Leishmania (Viannia) infection in the domestic dog in chaparral, Colombia. Am J Trop Med Hyg. 2011;84(5):674–80.
Saraiva EM, et al. The FML-vaccine (Leishmune) against canine visceral leishmaniasis: a transmission blocking vaccine. Vaccine. 2006;24(13):2423–31.
Savani ES, et al. Detection of Leishmania (Leishmania) amazonensis and Leishmania (Leishmania) infantum chagasi in Brazilian bats. Vet Parasitol. 2010;168(1–2):5–10.
Schubach TM, et al. American cutaneous leishmaniasis in two cats from Rio de Janeiro, Brazil: first report of natural infection with Leishmania (Viannia) braziliensis. Trans R Soc Trop Med Hyg. 2004;98:165–7.
Shapiro JT, et al. First record of Leishmania braziliensis presence detected in bats, Mato Grosso do Sul, southwest Brazil. Acta Trop. 2013;128:171–4.
Shaw J, et al. Characterization of Leishmania (Leishmania) waltoni n.sp. (Kinetoplastida: Trypanosomatidae), the parasite responsible for diffuse cutaneous leishmaniasis in the Dominican Republic. Am J Trop Med Hyg. 2015;93(3):552–8.
Silva ES, Gontijo CM, Melo MN. Contribution of molecular techniques to the epidemiology of neotropical Leishmania species. Trends Parasitol. 2005;21(12):550–2.
Silva VO, et al. A phase III trial of efficacy of the FML-vaccine against canine kala-azar in an endemic area of Brazil (São Gonçalo do Amaranto, RN). Vaccine. 2000;19(9–10):1082–92.
Silveira FT, et al. Dermal leishmaniasis in the Amazon region of Brazil: Leishmania (Viannaia) lainsoni sp.n., a new parasite from the state of Pará. Mem Inst Oswaldo Cruz. 1987;82(2):289–91.
Silveira FT, et al. Cutaneous leishmaniasis in the Amazon region: natural infection of the sandfly Lutzomyia ubiquitalis (Psychodidae: Phlebotominae) by Leishmania (Viannia) lainsoni in Pará state, Brazil. Mem Inst Oswaldo Cruz. 1991a;86(1):127–30.
Silveira FT, et al. Leishmaniose cutânea na Amazônia: isolamento de Leishmania (Viannia) lainsoni do roedor Agouti paca (Rodentia: Dasyproctidae), no Estado do Pará, Brasil. Rev Inst Med Trop Sao Paulo. 1991b;33(1):18–22.
Silveira FT, et al. An outbreak of cutaneous leishmaniasis among soldiers in Belém, Pará state, Brazil, caused by Leishmania (Viannia) lindenbergi n. sp. A new leishmanial parasite of man in the Amazon region. Parasite. 2002;9(1):43–50.
Singh N, et al. Animal reservoirs of visceral leishmaniasis in India. J Parasitol. 2013;99(1):64–7.
Soares IR, et al. First evidence of autochthonous cases of Leishmania (Leishmania) infantum in horse (Equus caballus) in the Americas and mixed infection of Leishmania infantum and Leishmania (Viannia) braziliensis. Vet Parasitol. 2013;197(3–4):665–9.
Solano-Gallego L, et al. Cutaneous leishmaniosis in three horses in Spain. Equine Vet J. 2003;35(3):320–3.
Solano-Gallego L, et al. LeishVet guidelines for the practical management of canine leishmaniosis. Parasit Vectors. 2011;4:86.
Suankratay C, et al. Autochthonous visceral leishmaniasis in a human immunodeficiency virus (HIV)-infected patient: the first in thailand and review of the literature. Am J Trop Med Hyg. 2010;82(1):4–8.
Sukmee T, et al. A suspected new species of Leishmania, the causative agent of visceral leishmaniasis in a Thai patient. Int J Parasitol. 2008;38(6):617–22.
Svobodova M, et al. Distinct transmission cycles of Leishmania tropica in 2 adjacent foci, northern Israel. Emerg Infect Dis. 2006;12(12):1860–8.
Talmi-Frank D, et al. Leishmania tropica in rock hyraxes (Procavia capensis) in a focus of human cutaneous leishmaniasis. Am J Trop Med Hyg. 2010;82(5):814–8.
Tolezano JE, et al. The first records of Leishmania (Leishmania) amazonensis in dogs (Canis familiaris) diagnosed clinically as having canine visceral leishmaniasis from Araçatuba County, São Paulo state, Brazil. Vet Parasitol. 2007;149(3–4):280–4.
Tomás-Pérez M, et al. First report of natural infection in hedgehogs with Leishmania major, a possible reservoir of zoonotic cutaneous leishmaniasis in Algeria. Acta Trop. 2014;135:44–9.
Tonui WK. Situational analysis of leishmaniases research in Kenya. Afr J Health Sci. 2006;13(1–2):7–21.
Trainor KE, et al. Eight cases of feline cutaneous leishmaniasis in Texas. Vet Pathol. 2010;47(6):1076–81.
Truppel JH, et al. Can equids be a reservoir of Leishmania braziliensis in endemic areas? PLoS One. 2014;9(4):e93731.
van Thiel PP, et al. First cases of cutaneous leishmaniasis caused by Leishmania (Viannia) naiffi infection in Surinam. Am J Trop Med Hyg. 2010;82(4):588–90.
Vedovello FD, et al. American cutaneous leishmaniasis in horses from endemic areas in the northcentral mesoregion of Paraná state, Brazil. Zoonoses Public Health. 2008;55(3):149–55.
Vélez ID, et al. An epidemic outbreak of canine cutaneous leishmaniasis in Colombia caused by Leishmania braziliensis and Leishmania panamensis. Am J Trop Med Hyg. 2012;86(5):807–11.
Vides J, et al. Leishmania chagasi infection in cats with dermatologic lesions from an endemic area of visceral leishmaniosis in Brazil. Vet Parasitol. 2011;178(1–2):22–8.
WHO. Control of the Leishmaniasis: Report of the WHO Expert Committee on the Control of Leishmaniases. World Health Organization; 2010. http://www.who.int/neglected_diseases/resources/who_trs_949/en. Accessed Sept 2016.
Williams AO, Mutinga J, Rodgers M. Leishmaniasis in a domestic goat in Kenya. Mol Cell Probes. 1991;5(5):319–25.
Acknowledgements
The authors wish to thank A. Pereira for his work on the references. C. Maia holds a FCT Investigator Starting Grant (IF/01302/2015) from Fundação para a Ciência e a Tecnologia, Ministério da Ciência, Tecnologia e Ensino Superior, Portugal.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG
About this chapter
Cite this chapter
Maia, C., Dantas-Torres, F., Campino, L. (2018). Parasite Biology: The Reservoir Hosts. In: Bruschi, F., Gradoni, L. (eds) The Leishmaniases: Old Neglected Tropical Diseases. Springer, Cham. https://doi.org/10.1007/978-3-319-72386-0_4
Download citation
DOI: https://doi.org/10.1007/978-3-319-72386-0_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-72385-3
Online ISBN: 978-3-319-72386-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)