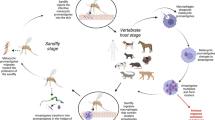
Abstract
Purpose of review
This paper wishes to provide updated information on different entities of leishmaniasis endemic in the Mediterranean region, concerning parasite taxonomy, disease burden and treatment options, and the status of vectors and reservoirs. Challenges associated with monitoring and surveillance of the leishmaniases are also highlighted.
Recent findings
The burden of the main human clinical forms, visceral (VL) and cutaneous leishmaniasis (CL), was recently estimated in each Mediterranean country. Significant VL emergence was reported in restricted areas or retrospectively recognized at country level. High CL incidences (240,000–390,000 cases/year) were estimated in North Africa and Middle East. Phenological aspects associated with potential leishmaniasis exposure were determined for six phlebotomine vectors of Leishmania infantum through a 3-year survey involving eight countries. The extent of canine leishmaniasis spread and prevalence was analyzed in ∼500,000 dogs from southwest Europe.
Summary
Mediterranean leishmaniases are widespread neglected diseases which suffer from poor awareness and reporting, difficult case management, and lack of effective control measures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Leishmaniasis is a complex of vector-borne protozoan diseases caused by members of the genus Leishmania, parasites infecting numerous mammal species including humans, and transmitted by the bite of phlebotomine sand flies (Phlebotomus spp and Lutzomyia spp in the Old and New Worlds, respectively). Clinical manifestations of human leishmaniasis, caused by some 20 Leishmania species, are largely diverse and can be grouped into two main clinical forms: visceral leishmaniasis (VL), a severe condition resulting from the dissemination of Leishmania in the phagocytes of the reticuloendothelial system and that is fatal in almost all cases if left untreated; and cutaneous leishmaniasis (CL), a benign but often disfiguring condition caused by the multiplication of Leishmania in the phagocytes of the skin with a tendency towards spontaneous resolution. The leishmaniases are endemic in some 100 countries with more than 350 million people at risk [1]. It is now estimated that 0.2–0.4 million of VL cases and 0.7–1.2 million of CL cases occur every year [2]. India, Bangladesh, Sudan, South Sudan, and Ethiopia, which are countries endemic for anthroponotic VL caused by L. donovani, account for more than 90% of global VL burden. Several entities of CL are widely distributed in countries of Latin America, Mediterranean Basin, Middle East, and Central Asia.
Nosogeographical Entities of Mediterranean Leishmaniasis
According to ancient medical literature, it can be argued that leishmaniases have been endemic in the Mediterranean region for centuries. Four indigenous Leishmania species causing VL, CL, or both are currently found in countries of the region.
Zoonotic VL is the most widespread entity, being endemic in all the countries of southern Europe, North Africa, and Middle East. The agent is L. infantum, which also causes sporadic CL in the same territories. It is now well established that Mediterranean L. infantum was imported to Central and South America about 500 years ago, where the parasite adapted to local Lutzomyia sand fly species and settled to cause an endemic cycle of zoonotic VL whose agent was named L. chagasi in 1937 [3]. Some thousands of human VL cases, mostly in Brazil, are currently recorded [2].
Zoonotic CL caused by L. major is typically found in arid areas of North Africa and Middle East, often causing epidemics in rural territories. This parasite has been recently detected at the gates of southern Europe having been identified for the first time as an agent of CL in several territories of Turkey [4].
Anthroponotic CL due to L. tropica is endemic in scattered urban foci of North Africa and Middle East including Southeast Turkey. Greece is the only European country endemic for this parasite, detected in CL cases from both mainland and insular territories, for example, in Crete [5]. A zoonotic cycle of L. tropica has recently been reported from a focus of human CL in north Israel [6].
Finally, the occurrence of a few VL and CL cases due to L. donovani was recently recorded from Cyprus and parts of Turkey [7].
Disease Burden
The leishmaniases are dynamic diseases because they rapidly reflect changes in transmission conditions, which are determined by environmental, demographic, human behavior, and co-morbidity parameters. For example, the incidence of zoonotic VL in humans is usually low and tends to be stable in most of the endemic territories; on average, 1200 to 2000 clinical cases are reported to occur each year from all the countries bordering the Mediterranean Basin [2]. However, significant case clusters, fluctuations, or even genuine outbreaks have been reported during the years 2000s from some endemic territories. In the Madrid region, Spain, the incidence rate of L. infantum leishmaniases (VL and CL) rose from 2.4/100,000 inhabitants in 2009 to 54.2/100,000 inhabitants in 2013 in one municipality [8•]. In Italy, a multi-annual VL epidemic peaking in 2000–2004 with more than 200 cases/year was detected retrospectively [9]. In Tbilisi, Georgia, an emerging urban VL focus was reported in 2011 [10]. On the other hand, the incidence of HIV-Leishmania co-infections, which had been very common in southwest Europe (Portugal, Spain, France, and Italy) during the 1990s, has declined considerably in recent years [11].
Age-related clinical VL varies between countries. Where general standards of living have improved considerably in the past decades (e.g., in southern Europe), young children and adults—the latter patients often presenting conditions of immunosuppression—are similarly affected [9]. In North Africa and Middle East or in the poorest territories of Balkans, young children represent the vast majority of clinical VL cases [12].
CL incidences are largely diverse depending on environmental and social differences which characterize the countries of the Mediterranean area. They are low or very low in southern Europe (L. infantum and/or L. tropica CL), whereas they are elevated or very high in countries of North Africa and Middle East including Turkey (L. major and/or L. tropica CL). Although current estimates do not distinguish between different etiological entities of CL, the annual range estimated for the whole Mediterranean region accounts for 240,000 to 394,000 cases, the highest number of cases being recorded in the Maghreb area (Algeria, Morocco, Tunisia, and Libya) and in Syria [2]. With the start of the Syrian war, the frequency and magnitude of CL outbreaks increased alarmingly in neighboring countries such as Lebanon and Jordan [13]. One out of three adult refugees from Syria has been diagnosed as having CL at GeoSentinel clinics worldwide in 2013–2015 [14•].
The reported rates of asymptomatic L. infantum carriers are elevated and represent an indicator of the spread of infections in the Mediterranean. Prevalence rates in a range as high as 10–47% were recorded in healthy population from VL/CL endemic areas of Croatia, France, Greece, Italy, and Spain using serological and/or molecular methods [15, 16].
Phlebotomine Sand Fly Vectors
The vectorial status of phlebotomine sand fly vectors of Leishmania in countries of the Mediterranean area was recently reviewed [17]. Twelve indigenous Phlebotomus species have been implicated in the transmission of Mediterranean VL/CL caused by L. infantum, of which eight have been fully incriminated as competent vectors according to standard criteria recommended by the World Health Organization [1]: Phlebotomus ariasi, P. balcanicus, P. kandelakii, P. langeroni, P. neglectus, P. perfiliewi, P. perniciosus, and P. tobbi. These species are members of the Larroussius subgenus except P. balcanicus which is classified in the Adlerius subgenus. On the contrary, P. papatasi is the sole vector of L. major throughout the Mediterranean region, whereas P. sergenti is the main vector of L. tropica in classical urban foci of anthroponotic CL. Other proven or suspected vectors of L. tropica in limited areas are P. arabicus (Israel) and P. similis (Greece), respectively.
The recorded northward spread of Mediterranean L. infantum vectors is probably due to the current climate changes [18, 19]. It can also be argued that generalized warming of the region could affect seasonality of sand flies. To address this issue, entomologists from eight Mediterranean countries have performed periodical vector collections from 2011 to 2013, following a common standard protocol [20••]. The temporal density trends of 56,000 specimens belonging to 6 vector species and collected from 37 sites of active L. infantum transmission were recorded and analyzed, resulting in a seasonal dynamics description throughout a vast area from Portugal to Georgia. Significant positive correlation was found between mean annual temperature and timing of sand fly appearance (ranging from April to June) or the number of seasonal density peaks (from one to three, over the May-September period). The duration of the potential exposure to L. infantum in the Mediterranean region was associated to a density trend encompassing the April-November period and peaking from July through September. Basically, only 4 months (from December to March) were shown to be without risk of leishmaniasis transmission during the investigated years.
Domestic and Wild Reservoirs
Dogs, which may suffer from a severe disease, are the primary domestic reservoir hosts of zoonotic VL. Canine infections are widespread in the Mediterranean region, representing both a public health threat and a veterinary problem. In 2011, Leishmania seroprevalence data from a total of 504,369 dogs examined in 947 surveys carried out from 1971 to 2006 were collated and analyzed in 4 endemic countries of western Europe (France, Italy, Portugal, and Spain). The overall median seroprevalence was 10%, with a range of 5.9 to 17.7% among the countries. Such elevated cumulative rate was found almost unchanged during the last 26 years of the study: 10.2% in 1981–1990; 11.7% in 1991–2000; and 11.1% in 2001–2006 [21]. Interest by the vet community in the canine leishmaniasis management (e.g., diagnosis, infection/disease staging, treatment, and prevention) is shown by the increasing number of guidelines produced by expert consortia [22,23,24].
With the geographical spreading of competent vectors and the increasing travel of infected pets, available measures aimed at avoiding the establishment of endemic cycles in L. infantum-free countries (especially in the fringe areas between endemic and non-endemic countries) have been reviewed and some of them recommended to the European authorities [25•]. Vaccination with a canine leishmaniasis vaccine available in Europe, which reduces disease progression [26] in combination with topical treatment using synthetic pyrethroids with proven efficacy against sand fly bites [27], showed the best predicted performance for prevention.
A number of other indigenous mammal species have been found infected with L. infantum, although their infectiousness potential was not demonstrated, and include Mus spretus (Algerian mouse), Apodemus sylvaticus (European wood mouse), Rattus rattus (black rat), Rattus norvegicus (brown rat), Meles meles (European badger), Martes martes (European pine marten), Mustela nivalis (weasel), and Geneta geneta (common genet) [5]. In addition to domestic dogs, the ability to transmit infection to vectors has been confirmed through xenodiagnosis (i.e., the deliberate exposure of the host to bites from laboratory-reared female sand flies) for Vulpes vulpes (red fox), Lepus granatensis (Iberian hare), black rats, and domestic cats, suggesting that they may represent a secondary reservoir host for L. infantum [28].
The fat sand rat Psammomys obesus is the natural host of L. major in most of the CL foci of North Africa and Middle East. This gerbil feeds exclusively on Chenopodiaceae plants which grow in salty lands throughout North Africa up to the Jordan Valley. In Syria, P. obesus breeds in burrows made in non-saline soil where a different chenopod grows. The natural rodent reservoir/sand fly vector system is very efficient for transmission (humans being only accidental hosts): during summer, the infected proportion of P. obesus increases when also densities of the vector P. papatasi, which breeds and rests into the rodent burrows, are higher [29]. In some areas of L. major distribution where P. obesus is absent, the main reservoir hosts are gerbils of the genus Meriones (for example, M. shawi in southern Morocco and M. tristami in northern Israel) or voles of the species Microtus guentheri (northern Israel) [30].
CL caused by L. tropica is universally believed to be anthroponotic because it is prevalent in urban settings where the vector P. sergenti breeds successfully. However, some proven or putative zoonotic foci have been described, such in north Israel where rock hyraxes (Procavia capensis) have been incriminated as L. tropica reservoir hosts [6], or in the Maghreb area where Ctenodactylus gundi rodents have been found infected with members of the L. tropica complex [31].
Treatment
Current approaches for Mediterranean VL treatment have not changed much over the past decade. Recently-introduced antileishmanial drugs or drug formulations (e.g., miltefosine or injectable paromomycin) developed for L. donovani VL treatment in India and East Africa, have not been validated for the therapy of VL caused by L. infantum [32].
Pentavalent antimony has been the only drug employed for decades in treatment of zoonotic VL throughout the Mediterranean region, with excellent cure rates in hospitalized patients. From the mid 1990s, the emergence of antimony resistance in the Indian subcontinent and the availability of alternative drugs have induced a slow but steady change in the therapeutic approaches for the disease. Information collected from 11 endemic countries of southern Europe, North Africa, and the Middle East in 2008 (Cyprus, France, Greece, Israel, Italy, Morocco, Palestinian National Authority, Portugal, Spain, Tunisia, and Turkey) showed that current therapeutic approaches for VL differ in the region [33]: in North Africa and parts of Middle East, pentavalent antimonials are still the first-line drug. In a few European countries and in Israel, both antimonials and liposomal amphotericin B (L-AmB) are used, but in different patient populations. Finally, in most countries of Europe, only L-AmB is employed. Drug cost (very high for L-AmB) and hospital treatment duration (quite long for pentavalent antimonials, thus impacting on costs in high-standard health facilities) are the main factors affecting the national drug policy, despite differences in drug toxicity.
A standard single treatment regimen for Mediterranean CL is not available. The therapeutic decision is based on the patient’s benefit/risk evaluation and it takes into account of disease severity, drug toxicity, and, where possible, the identification of causative Leishmania species, which are the main intrinsic determinant for rapid spontaneous resolution versus chronic evolution and dissemination of lesions. In general, systemic treatment is less commonly required in Mediterranean entities of CL as compared with New World diseases. An attempt to standardize CL treatment in different etiological/clinical situations was recently made by the Eastern Mediterranean Office of World Health Organization [34]. Localized CL of minor severity (i.e., infection with L. major; lesions are small in number and size, potentially non-disfiguring, and they are not periorificial or close to small joints) can be treated with only wound dressing care. Localized CL of mild severity (i.e., any Mediterranean Leishmania agent at any stage; lesions are small in number and size which might be disfiguring—e.g., on face—and are not periorificial or close to small joints) should be treated with intralesional pentavalent antimony alone or in combination with cryotherapy, or with thermotherapy alone. Severe CL cases (high number and/or large-sized lesions or lesions complicated by lymphatic dissemination) should be treated with systemic treatment using pentavalent antimony, oral miltefosine, or liposomal AmB (however, there is a low grade of evidence for the last two drugs). Oral fluconazole or itraconazole can also be used for severe L. major and L. tropica CL lesions, respectively [34].
Challenges in Epidemiological Surveillance of Mediterranean Leishmaniases
Delay in diagnosis or misdiagnosis of leishmaniases are frequent in all Mediterranean countries, especially in areas where cases occur rarely. In low endemic settings, rise of medical awareness is needed to avoid aggravation of VL or disfiguring CL in patients seeking for care. In the fringe areas between endemic and non-endemic territories, travelers may even get infected in places not recorded as endemic by the local health authorities [35]. In such cases, the apparent lack of risk factors may further delay diagnosis, which would suggest the implementation of systems for transnational information when unexpected cases of leishmaniasis have been identified in a territory.
Deaths due to VL are not uncommon. Fatal cases are adults with severe underlying conditions [11] or young children who are malnourished and in whom VL diagnosis was delayed [12]. Deaths due to the improper use of systemic drugs (employed for VL but also for severe complicated CL) may also occur [1]. In many Mediterranean countries, antimonial drugs are largely employed, for they are much cheaper than the safe L-AmB. Overdosage of pentavalent antimony, especially in adults, may cause severe acute pancreatitis and heart failures [1].
Challenges concerning epidemiological surveillance of Mediterranean leishmaniases have been recently highlighted [36], the main ones being: (i) A well-structured system for mandatory reporting of leishmaniasis is not available all over the Mediterranean region; (ii) The existing surveillance systems are not sufficiently harmonized as regards case definition, detection strategies, clinical management, and indicators of diagnostic and treatment performance. The last indicator is particularly ignored, so that emerging drug-resistance issues cannot be quickly monitored and contrasted; (iii) Even in countries with efficient notification systems, mild under-reporting of VL is the rule—estimated to be 1.2–4-folds (2). Furthermore, not always CL has a separate notification route (i.e., VL and CL may be notified together as “leishmaniasis”). Even when notified separately, CL under-reporting remains dramatically severe—estimated to be 2.8–4.6-folds (2). This occurs because CL cases are often diagnosed in private dermatology clinics and not in public hospitals; (iv) Travelers are usually not informed about leishmaniasis risk by consultant physicians.
Conclusions
There is large amount of evidence that Leishmania infections are widespread in the Mediterranean, both in humans and in animals. Ecological and clinical features of different leishmaniasis entities vary according to geographic and social characteristics of the endemic areas. A low incidence of clinical VL is observed throughout the Mediterranean; however, the prevalence of asymptomatic human infections and that of canine infection/disease has been shown exceptionally high, thus representing a potential threat for endemic populations. Entomological work has disclosed a long period of potential exposure to the agent of zoonotic VL by the bite of several vector species, which explains the wide diffusion of this parasite. Southernmost and easternmost territories of the Mediterranean region are affected by CL agents which are difficult to prevent and control and continue to cause thousands of clinical cases among urban (anthroponotic CL) and rural (zoonotic CL) populations. It can be concluded that Mediterranean leishmaniases are still neglected diseases which suffer from poor awareness and reporting, difficult case management, and lack of effective control measures.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
World Health Organization. Control of the leishmaniases. WHO Tech Rep Ser 949. Geneva: World Health Organization; 2010.
Alvar J, Vélez ID, Bern C, Herrero M, Desjeux P, Cano J, et al. Leishmaniasis worldwide and global estimates of its incidence. PLoS ONE. 2012;7:e35671.
Leblois R, Kuhls K, François O, Schönian G, Wirth T. Guns, germs and dogs: on the origin of Leishmania chagasi. Infect Genet Evol. 2011;11:1091–5.
Özbilgin A, Çulha G, Uzun S, Harman M, Topal SG, Okudan F, et al. Leishmaniasis in Turkey: first clinical isolation of Leishmania major from 18 autochthonous cases of cutaneous leishmaniasis in four geographical regions. Trop Med Int Health. 2016;21:783–91.
Antoniou M, Gramiccia M, Molina R, Dvorak V, Volf P. The role of indigenous phlebotomine sandflies and mammals in the spreading of leishmaniasis agents in the Mediterranean region. Euro Surveill 2013;18:pii = 20540.
Talmi-Frank D, Jaffe CL, Nasereddin A, Warburg A, King R, Svobodova M, et al. Leishmania tropica in rock hyraxes (Procavia capensis) in a focus of human cutaneous leishmaniasis. Am J Trop Med Hyg. 2010;82:814–8.
Gouzelou E, Haralambous C, Amro A, Mentis A, Pratlong F, Dedet JP, et al. Multilocus microsatellite typing (MLMT) of strains from Turkey and Cyprus reveals a novel monophyletic L. donovani sensu lato group. PLoS Negl Trop Dis. 2012;6:e1507.
• Arce A, Estirado A, Ordobas M, Sevilla S, García N, Moratilla L, et al. Reemergence of leishmaniasis in Spain: community outbreak in Madrid, Spain, 2009 to 2012. Euro Surveill 2013;18:pii = 20546. This paper reports on a recent epidemic of leishmaniasis in a southern European country.
Gramiccia M, Scalone A, Di Muccio T, Orsini S, Fiorentino E, Gradoni L. The burden of visceral leishmaniasis in Italy from 1982 to 2012: a retrospective analysis of the multi-annual epidemic that occurred from 1989 to 2009. Euro Surveill 2013;18:pii = 20535.
Giorgobiani E, Chitadze N, Chanturya G, Grdzelidze M, Jochim RC, Machablishvili A, et al. Epidemiologic aspects of an emerging focus of visceral leishmaniasis in Tbilisi. Georgia PLoS Negl Trop Dis. 2011;5:e1415.
Monge-Maillo B, Norman FF, Cruz I, Alvar J, López-Vélez R. Visceral leishmaniasis and HIV coinfection in the Mediterranean region. PLoS Negl Trop Dis. 2014;8:e3021.
Petrela R, Kuneshka L, Foto E, Zavalani F, Gradoni L. Pediatric visceral leishmaniasis in Albania: a retrospective analysis of 1,210 consecutive hospitalized patients (1995–2009). PLoS Negl Trop Dis. 2010;4:e814.
Alawieh A, Musharrafieh U, Jaber A, Berry A, Ghosn N, Bizri AR. Revisiting leishmaniasis in the time of war: the Syrian conflict and the Lebanese outbreak. Int J Infect Dis. 2014;29:115–9.
• Mockenhaupt FP, Barbre KA, Jensenius M, Larsen CS, Barnett ED, Stauffer W, et al. Profile of illness in Syrian refugees: a GeoSentinel analysis, 2013 to 2015. Euro Surveill 2016;21:pii = 30160. This brief note reports on the alarming cutaneous leishmaniasis incidence in Syrian refugees.
Michel G, Pomares C, Ferrua B, Marty P. Importance of worldwide asymptomatic carriers of Leishmania infantum (L. chagasi) in human. Acta Trop. 2011;119:69–75.
Šiško-Kraljević K, Jerončić A, Mohar B, Punda-Polić V. Asymptomatic Leishmania infantum infections in humans living in endemic and non-endemic areas of Croatia, 2007 to 2009. Euro Surveill 2013;18:pii = 20533.
Maroli M, Feliciangeli MD, Bichaud L, Charrel RN, Gradoni L. Phlebotomine sandflies and the spreading of leishmaniases and other diseases of public health concern. Med Vet Entomol. 2013;27:123–47.
Morosetti G, Bongiorno G, Beran B, Scalone A, Moser J, Gramiccia M, et al. Risk assessment for canine leishmaniasis spreading in the north of Italy. Geospat Health. 2009;4:115–27.
Fischer D, Moeller P, Thomas SM, Naucke TJ, Beierkuhnlein C. Combining climatic projections and dispersal ability: a method for estimating the responses of sandfly vector species to climate change. PLoS Negl Trop Dis. 2011;5:e1407.
•• Alten B, Maia C, Afonso MO, Campino L, Jiménez M, González E, et al. Seasonal dynamics of Phlebotomine sand fly species proven vectors of Mediterranean leishmaniasis caused by Leishmania infantum. PLoS Negl Trop Dis. 2016;10:e0004458. This is the most comprehensive study on leishmaniasis vectors in southern Europe as a whole.
Franco AO, Davies CR, Mylne A, Dedet JP, Gállego M, Ballart C, et al. Predicting the distribution of canine leishmaniasis in western Europe based on environmental variables. Parasitology. 2011;138:1878–91.
Solano-Gallego L, Miró G, Koutinas A, Cardoso L, Pennisi MG, Ferrer L, et al. LeishVet guidelines for the practical management of canine leishmaniosis. Parasit Vectors. 2011;4:86.
Roura X, Fondati A, Lubas G, Gradoni L, Maroli M, Oliva G, et al. Prognosis and monitoring of leishmaniasis in dogs: a working group report. Vet J. 2013;198:43–7.
Paltrinieri S, Gradoni L, Roura X, Zatelli A, Zini E. Laboratory tests for diagnosing and monitoring canine leishmaniasis. Vet Clin Pathol 2016.
• EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare). Scientific opinion on canine leishmaniosis. EFSA J. 2015;13:4075. It represents the first official European scientific document on the burden and control of canine leishmaniasis.
Oliva G, Nieto J, Foglia Manzillo V, Cappiello S, Fiorentino E, Di Muccio T, et al. A randomised, double-blind, controlled efficacy trial of the LiESP/QA-21 vaccine in naïve dogs exposed to two Leishmania infantum transmission seasons. PLoS Negl Trop Dis. 2014;8:e3213.
Maroli M, Gradoni L, Oliva G, Castagnaro M, Crotti A, Lubas G, et al. Guidelines for prevention of leishmaniasis in dogs. J Am Vet Med Assoc. 2010;236:1200–6.
Millán J, Ferroglio E, Solano-Gallego L. Role of wildlife in the epidemiology of Leishmania infantum infection in Europe. Parasitol Res. 2014;113:2005–14.
Postigo JA. Leishmaniasis in the World Health Organization Eastern Mediterranean Region. Int J Antimicrob Agents. 2010;36(S1):S62–5.
Faiman R, Abbasi I, Jaffe C, Motro Y, Nasereddin A, Schnur LF, et al. A newly emerged cutaneous leishmaniasis focus in northern Israel and two new reservoir hosts of Leishmania major. PLoS Negl Trop Dis. 2013;7:e2058.
Jaouadi K, Haouas N, Chaara D, Gorcii M, Chargui N, Augot D, et al. First detection of Leishmania killicki (Kinetoplastida, Trypanosomatidae) in Ctenodactylus gundi (Rodentia, Ctenodactylidae), a possible reservoir of human cutaneous leishmaniasis in Tunisia. Parasit Vectors. 2011;4:159.
Monge-Maillo B, López-Vélez R. Therapeutic options for visceral leishmaniasis. Drugs. 2013;73:1863–88.
Gradoni L, Soteriadou K, Louzir H, Dakkak A, Toz SO, Jaffe C, et al. Drug regimens for visceral leishmaniasis in Mediterranean countries. Trop Med Int Health. 2008;13:1272–6.
World Health Organization, Regional Office for the Eastern Mediterranean. Manual for case management of cutaneous leishmaniasis in the WHO Eastern Mediterranean Region. WHO Regional Publications, Eastern Mediterranean Ser 2014;35.
Faber WR, Hoekzema R, Bart A, Zeegelaar JE, de Vries HJ. Cutaneous leishmaniasis acquired in Jura, France. Emerg Infect Dis. 2012;18:183–4.
Gradoni L. Epidemiological surveillance of leishmaniasis in the European Union: operational and research challenges. Euro Surveill 2013;18:pii = 20539.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Luigi Gradoni declares that he has no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by the author.
Additional information
This article is part of the Topical Collection on Tropical Medicine in the Mediterranean Region
Rights and permissions
About this article
Cite this article
Gradoni, L. The Leishmaniases of the Mediterranean Region. Curr Trop Med Rep 4, 21–26 (2017). https://doi.org/10.1007/s40475-017-0099-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40475-017-0099-1