Abstract
The bioactivity of plant natural products depends on more than just their scaffolds. The physicochemical properties of the natural products are often dramatically changed by tailoring functional groups. Addition of sugar residues (glycons) to small lipophilic molecules (aglycons) is a particularly frequent tailoring mechanism mostly conveyed by nucleoside diphosphate (NDP)-sugar dependent glycosyltransferases (GTs). Glycosylation increases solubility and stability, while also reducing the toxicity of the aglycons. Moreover, the attachment of a sugar may determine the fate of the molecule by controlling its compartmentalization. Above all, glycosylation affects bioactivity and bioavailability. Consequently, glycosides and glucose esters of bioactive natural products have become of utmost importance for the food, feed, cosmetics and pharmaceutical industry. In the past, glycosylation was achieved chemically involving flammable solvents and toxic catalysts, harsh laboratory conditions and the necessity of protecting groups, which eventually resulted in high costs. Nowadays, alternative biotechnological methods are available. Cultivation of whole-cell biocatalysts harboring GTs enables the regio- and stereo-selective production of glycosides and glucose esters at high product titers, while keeping the costs at a minimum. Due to their sedentary life style, plants have to cope with numerous stressors and, because glycosylated natural products play important roles in stress defense, are also rich sources of GTs. This chapter covers the last 10 years of plant GT research, with special emphasis upon new enzymes and glycoside/glucose ester production of plant natural products by whole-cell biocatalysts.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Glycosyltransferase
- Biotechnology
- Phenolics
- Terpenoids
- Metabolic engineering
- Whole-cell biotransformation
1 Introduction
In the biosynthesis of plant natural products, modification of their chemical structure by tailoring enzymes is pivotal. These reactions include transfer of sugar units catalyzed by carbohydrate-active enzymes (CAZymes; http://www.cazy.org/) which are proteins whose functional domains are able to form, degrade or modify glycosidic bonds [1]. Their general abundance is in accordance with their high biological significance in Nature. In fact, most of the dry weight of plant biomass on earth exists as carbohydrate polymers, which emerged from glycosyl transfer reactions [2]. Some end products of primary plant metabolism are the major base of the food and the feed industry (for example, starch in grain and tuber crops). Glycosides and glucose esters of secondary metabolites are in general less abundant, but nevertheless of equal importance. On a cellular level, glycosylation functions, among other roles, in energy metabolism, pathogen virulence, molecular defense, and storage information signaling [3]. Addition of a sugar residue also increases water solubility and stability, and thus affects the bioactivity of acceptor molecules [4]. Therefore, the interest of the pharmaceutical, cosmetics and food industry in these compounds has increased continuously. At present, glycosides are used as therapeutic drugs, functional ingredients, and dietary supplements. They can be produced either chemically, for example by the Koenigs-Knorr reaction, or biochemically by the action of glycosidases, transglycosidases, glycoside phosphorylases and glycosyltransferases [5].
Glycosyltransferases (GTs) form a vast class of CAZymes that transfer sugar moieties from activated donors (usually nucleoside diphosphate (NDP) activated monosaccharides) to specific acceptor molecules (generally alcohols and carboxylic acids) (Fig. 9.1). However, transfer to N-, S-, and C-atoms, resulting in the formation of glycosylamines, thioglycosides, and C-glycosides, respectively, have also frequently been observed [6,7,8]. Recently, family 1 GT enzymes have drawn special interest from industry, because they change the physicochemical properties and consequently the bioactivity of lipophilic small molecules, thus offering new and exciting possibilities for the biotechnological production of bioactive glycosides [4, 9]. An advantage of family 1 GTs in this context is due to their ability to catalyze glycosidic bond formation regio- and stereoselectively with high yield [10, 11]. Hence, the property of the molecule of interest can be adapted on demand. This is especially important for the application of carbohydrate-tailored drugs, where the precise identity and position of the glycosyl residue decides about the mechanism of action and the bioavailability [12, 13]. The rapidly growing number of publicly available genomes has enabled the identification of new GT sequences that encode proteins with novel activities, and the first GT enzymes have now been successfully applied in bio-catalytic processes [14]. The following chapter will cover the last 10 years of research on family 1 GT enzymes with focus on the biotechnological production of glycosides/glucose esters and special emphasis upon production in whole cell biocatalysts and applications in industry.
Reactions catalyzed by glycosyltransferase (a). Anthocyanin-storing vacuoles of Rhoeo spathacea (b). Cells have been plasmolyzed. Picture was adapted from Wikipedia (https://en.wikipedia.org/wiki/Vacuole; last accessed April, 2017). Hydrangea variety producing the anthocyanin myrtillin (delphinidin-3-O-glucoside) (c), and Delphinium variety accumulating the anthocyanin violdelphin (delphinidin 3-O-rutinoside-7-O-(6-O-(4-O-(6-O-(p-hydroxybenzoyl)-glucosyl)-oxybenzoyl)-glucoside) (d). Transport of glycosides into the vacuole (e). ABC ATP-binding cassette transporter, AVI anthocyanin vacuolar inclusions, GST glutathion S-transferase, GT glycosyltransferase, MATE multidrug and toxic extrusion protein, R alkyl or aryl
2 The Significance of Glycosylation in Plants
Glycosylation is one of the most important tailoring mechanisms of bioactive compounds in plants [15]. The addition of a glycon (sugar unit) fundamentally changes the physicochemical properties of the acceptor aglycon (non-sugar component). Through glycosylation by GTs, plants can modulate the structure and function of secondary metabolites.
2.1 Glycosylation Increases Solubility
Glycosides and glucose esters of natural products are less hydrophobic than the aglycon on its own [16]. Thus, upon glycosylation of the aglycon the water solubility increases. In particular, this is evident for hydrophobic metabolites like flavonoids that feature a complex phenolic ring structure [17]. Flavonoids are major secondary metabolites in many fruits and vegetables, where they act among others inter alia as colored pigments. Due to enhanced solubility by glycosylation, plants can accumulate glycosylated flavonoids and related anthocyanins in the lumen of the vacuole in high concentrations [18] (Fig. 9.1) (see also Chap. 3 of this book). Glycosylation of flavonoids takes place in the cytosol right after the formation of the aglycons, since some of the non-glycosylated precursor molecules are unstable under physiological conditions. Utilizing a GT enzyme from apple, the 3,5-β-D-glucoside of resveratrol was produced, thereby increasing the water-solubility of an otherwise hydrophobic compound by 1700-fold [19] (see also Chap. 3 of this book). As a currently applied dietary supplement resveratrol is administered orally to patients. However, its medical exploitation is strongly limited by the low water-solubility. Thus, the future usage of resveratrol glucosides as therapeutic agent is envisaged [20, 21]. Similarly, the biological availability of the isoflavone gentistin is limited by its insolubility in water. Consequently, water-soluble gentisin glycosides were produced whereby solubility was increased 1000–10,000-fold and antioxidant activity was maintained [22]. Likewise, quercetin glycosides were developed to improve water solubility of the flavonoid for food and other applications [23].
2.2 Glycosylation Increases Stability
Glycosides and glucose esters exhibit not only a higher hydrophilicity but also an enhanced stability. Glycosylation stabilizes and intensifies the color of plant anthocyanidins – secondary metabolites that are responsible for the red, blue, and purple pigmentation of flowers and fruits in various plant species (see also Chap. 4 of this book). This stabilization is probably further enhanced by acylation of the anthocyanin core with phenolic acids, thereby promoting intermolecular sandwich type stacking of the aromatic nuclei of the molecules [24]. However, the substitutions of the aromatic acyl groups occur at glycosyl residues. Thus, without glycosylation, a subsequent acylation with phenolic acids would be limited to the available hydroxyl groups of the aglycon. The poly-acylated anthocyanin glycoside violdelphin, one of the main color pigments of Delphinium flowers [25], is more stable and shows a stronger blue color than its less modified form myrtillin (Fig. 9.1), which is found in varieties of the genus Hydrangea [26]. Similarly, the O-glucosides of hydroxycinnamic acids are more stable than the phenolic acids, of which 20–40% degrade at room temperature during one year of storage [27]. Furthermore, the half-life of furaneol-glucoside exceeds that of the free flavor molecule by 35-fold [28].
2.3 Glycosylation Controls Sequestration/Compartmentalization
Anthocyanins are believed to be synthesized by a multi-enzyme complex located on the cytosolic side of the endoplasmic reticulum [29]. The aglycons are promptly glycosylated, and the glycosides are administered from the cytosol to the vacuole, where they accumulate to high levels [30] (see also Chap. 4 of this book). The low pH value of the vacuole suppresses oxidation reactions, and ensures anthocyanin stability. Consequently, a specific transport mechanism is required that permits the translocation of sugar-bound metabolites across the cytoplasm and through the tonoplast [31].
For transport across the cytoplasm, two main models have been proposed: the ligandin model and the vesicle-mediated transport model , both being not mutually exclusive (Fig. 9.1). The ligandin model suggests binding of cytoplasmic anthocyanins to glutathione S-transferase (GST) proteins, which function as carriers that escort/stabilize anthocyanins until they are taken up into the vacuole [32,33,34]. As an example, two GST proteins from Vitis vinifera were associated to this mode of transport as their overexpression correlated with increased anthocyanin content in grape suspension cells [33]. Furthermore, it was shown that Arabidopsis mutant seedlings deficient in expression of the GST Transparent Testa 19 (TT19) barely accumulated anthocyanins [32, 34], whereas by re-introduction of a functional TT19 the mutant phenotype could be rescued [34]. Similarly, a GST seems to be critical for anthocyanin formation in strawberry as comparative transcriptome analyses of red- and natural white-fruited strawberry genotypes uncovered GST as highly differentially expressed gene [35]. According to the vesicular transport model, anthocyanins enter the ER lumen and are subsequently transported to the vacuole in vesicles, which could be detected microscopically due to the fact that the contained anthocyanins possess autofluorescence [36]. This process leads to the vacuolar accumulation of the glycosides in anthocyanic vascular inclusions (AVIs) . AVIs have been described in more than 70 anthocyanin-producing species [31]. Since vanadate, a general inhibitor of ABC transporters, induced a dramatic increase of anthocyanin-containing sub-vacuolar structures it was suggested that cells utilize components of the protein secretory trafficking pathway for the direct transport of anthocyanins from the endoplasmic reticulum to the vacuole [37].
For transport into the vacuole two major mechanisms were suggested: On one hand, active transport was proposed that is mediated by directly energized ATP-binding cassette (ABC) transporters, such as the multidrug resistance-associated proteins (MRPs) ZmMRP3 and 4 in maize (Zea mays) [38]. Genetic loss of ZmMrp3 function in mutant plants led to a distinct pigmentation pattern, resulting from mislocalized and significantly reduced anthocyanin levels. Furthermore, yeast microsomes expressing the ZmMrp3 homologue ABCC1 from V. vinifera demonstrated the transport of anthocyanins in the presence of GSH [39]. On the other hand, a second transport involving Multidrug And Toxic Extrusion (MATE) family proteins was proposed that depends on a pre-existing, vacuolar membrane spanning H+ gradient [40,41,42]. Characterization of MATE2 from Medicago truncatula [41] and anthoMATE1 and 3 from V. vinifera [42] revealed the translocation of glycosylated pigments that have been further decorated by acylation or malonylation. However, both transport mechanisms are presumably not mutually exclusive and seem to strongly depend on the type and structure of the transported secondary metabolite and may require other, not yet known factors. As an example, abscisic acid glucosyl ester is transported by both, proton-antiport and ABC-binding cassette mechanisms [43]. Only recently, a novel way of vacuolar transport has been put forward (Fig. 9.1). The authors observed by confocal microscopy that cytoplasmic anthocyanins aggregate in AVIs in close proximity to the vacuolar surface, and are directly enclosed by the tonoplast in a microautophagy-like process [44]. The endoplasmic reticulum-to-vacuole vesicular transport of anthocyanins mediated by a trans Golgi network-independent mechanism presumably contributes to the formation of AVIs [37].
A new, putative flavonoid carrier has been found in epidermal tissues of carnation petals [45]. The amino acid sequence is similar to mammalian bilitranslocase, a plasma membrane transporter found in liver and gastric mucosa. There, the protein mediates the uptake of the pigment bilirubin, dietary anthocyanins and nicotinic acid.
2.4 Glycosylation Affects Bioactivity and -Availability
Plants can regulate the level of bioactive secondary metabolites by linking them to sugar units. The plant hormone abscisic acid (ABA), which has important functions in plant responses to abiotic stresses, seed development, and germination [46], is inactivated by esterification with glucose [47]. While “free”, unbound ABA can trigger stomatal closure and is mainly found in extra-vacuolar compartments, the ABA-glucose-ester (ABA-GE) is non-reactive and stored in the vacuole [48]. A second, oxidative mechanism of ABA inactivation has been proposed [49]. However, glucose conjugation has one decisive advantage to other modifications – it is reversible. ABA-GE is hydrolyzed by vacuolar β-glucosidases in a fast, one-step reaction to generate free cytosolic ABA [50, 51]. Therefore, ABA-GE is thought to act as an easily accessible ABA reservoir that can be tapped by plants to regulate ABA homeostasis [43]. Another example is the recent identification of xanthophyll-derived apocarotenoid glycosides (AGs) in leaves of Arabidopsis thaliana [52]. Increased carotenoid pathway flux in leaves of transgenic plants resulted in higher levels of AGs. Accordingly, the authors hypothesized that formation of AGs regulates the cellular level of carotenoids. Similarly, it has been shown that glycosylation of monoterpenes reduces the concentrations of free, aroma-active terpenols in grapes, which however, can be released again during vinification [53].
Furthermore, glycosides and glucose esters act as precursors for various biosynthetic pathways, because the glycon features several functional hydroxyl groups that enable additional metabolic reactions [25]. For example, galloylglucose ester is generally considered as precursor of ellagitannins and ellagic acid, polyphenolic antioxidants in strawberry [54], raspberry [54], grapevine [55], pedunculate oak [56], and pomegranate (Punica granatum) [57]. It is hypothesized that the galloylglucose ester functions as gallic acid donor as well as acceptor and thus, enables dimerization of the phenolic acid in the ellagitannin pathway [54].
2.5 Glycosylation Reduces Toxicity
As sedentary organisms, plants have developed strategies to mitigate the poisonous effects of toxic chemicals they are confronted with. One possible detoxification process is the conjugation of toxins to glucose molecules, making them more water soluble and enabling transport into the vacuole. Spotted knapweed (Centaurea maculosa) is able to metabolize maculosin – a host-specific toxin produced by the fungus Alternaria alternata – to the corresponding β-O-glucoside, which lacks the phytotoxicity of the aglycon [58]. Moreover, it was shown that GT enzymes are able to diminish the harmful effects of the mycotoxins zearalenone [59] and deoxynivalenol [60]. Thus, the toxins become masked but remain present in the plant tissue. Toxicological data are scarce, but several studies revealed the potential threat to consumer safety from these substances due to possible hydrolysis during mammalian digestion [61].
An interesting observation has been made by Australian wine producers. After a number of vineyards were exposed to smoke from bushfires and the berries were processed, the resulting wines exhibited a strong off-flavor [62]. Strong “smoky”, “burnt” and “ashtray” characters were reported [62, 63]. Subsequently, the presence of the β-D-glucosides of guaiacol, its conjugates, and other related phenolic smoky compounds was confirmed [64, 65]. Apparently, the plants tailor the phenols by glycosylation to cope with the toxic effect. However, during the winemaking process (e.g. fermentation) and consumption of the wine, the glycosidic bonds are hydrolyzed and the smoky compounds are released, thus causing an unpleasant taste [66, 67]. The wines are disliked due to the off-flavor, which eventually leads to a loss of income for the wine producers.
2.6 Glycosylation Affects Perception
The elucidation of the cause of the smoke-tainted wines prompted further analyses of the in-mouth hydrolysis of glycosidically bound flavor compounds [67]. It was shown that enzymes of the human saliva are able to release the volatile aglycones from their natural glycoconjugates even under low pH and elevated ethanol conditions, confirming the in-mouth breakdown of monosaccharide and disaccharide glycosides. Thus, the long, lingering aftertaste of wines, desirable or undesirable, may be due to retro-nasal perception of aromas released from glycosides, which occur naturally in grapes [67, 68].
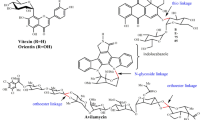
Steviol glycosides , a mixture of glycosides of the diterpene steviol, have recently been approved as sweetener in the EU [69]. Stevioside and rebaudioside A are the main diterpene glycosides present in leaf tissues, but only rebaudioside A imparts a desirable sweet taste, while stevioside produces a residual bitter aftertaste (Fig. 9.2). Glycosylation of stevioside yields rebaudioside A and can increase the ratio of rebaudioside A to stevioside in steviol glycoside products, providing a conceivable strategy to improve the organoleptic properties of steviol glycoside products. Hence, several enzymatic processes have been recently suggested to produce rebaudioside A by glucosylation of stevioside [70,71,72,73,74].
Schematic representation of the biosynthetic pathway of steviol glycoside, rebaudioside A. This biosynthetic pathway takes place in the leaf tissue of Stevia rebaudiana . The multi-step methylerythritol 4-phosphate (MEP) pathway in the chloroplast stroma converts the initial precursor pyruvate to kaurene. It is then transported to the endoplasmic reticulum (ER) where it is oxidized by kaurene oxidase (KO) and kaurenoic acid hydroxylase (KAH) to form steviol. Subsequently, steviol is glycosylated by multiple glycosyltransferases (GT) to form rebaudioside A – the sweetest and least bitter tasting glycoside – which is transported into the vacuole for storage
3 Glucoside/Glucose Ester Synthesis
Glycosidic and glucose ester bond formation is mostly achieved chemically by a series of steps involving the protection of interfering hydroxyl groups, activation of a leaving group at the anomeric carbon proceeding in an SN1 reaction mechanism, use of a heavy metal catalyst in water free medium, exclusion of light, and eventually deprotection [75]. Although decisive progress has been made in improving the methods and techniques since the classical Koenigs-Knorr reaction was published [76], chemical glycosylation has considerable drawbacks. It still suffers from low yields, high costs, usage of toxic heavy metal catalysts, and the formation of unspecific products [9, 77].
In contrast, biocatalytic reactions are a promising alternative due to the mild reaction conditions, the regio- and stereo-selectivity of the enzymatic reactions, and the ability to accept both hydrophilic and hydrophobic substrates. Furthermore, the protection of functional groups is not required. Consequently, fewer process steps are needed, which reduces production costs and ecological damage [77, 78]. Among the glycosidic bond mediating enzymes are (i) glycoside hydrolases that also efficiently catalyze the reverse hydrolytic reaction (condensation) whereby glycosides are formed, (ii) transglycosidases that are able to catalyze the transfer of glycosidic bonds within carbohydrate molecules and between glycosides, (iii) glycoside phosphorylases that use phosphate-activated glycosyl donors, and (iv) glycosyltransferases that utilize nucleotide diphosphate activated sugar donors as co-substrates to form glycosides [5].
However, successful application of these CAZymes in industrial processes is limited for various reasons, which also depend on the enzyme class [9]. Glycosidases show high promiscuity regarding their acceptor substrate specificity, yet production outcome is poor, and conversion of acceptors with multiple hydroxyl groups often results in an isomeric product mixture. Thus, expenses for the purification of the product make a considerable contribution to the overall costs. Transglycosidases and glycoside phosphorylases accept only a limited number of acceptors and exhibit poor regioselectivity, similar to glycosidases. GT enzymes on the other hand accept a wide range of hydrophobic and hydrophilic acceptors, while showing regio- and stereoselective product formation. Their disadvantage is the requirement of expensive co-substrates.
4 Family 1 Plant Glycosyltransferases
GT enzymes can be classified in families based on their reaction mechanism, sequence similarity, as well as donor, acceptor and product specificity [79,80,81,82]. Up to date, 103 GT families and approximately 316,000 annotated protein sequences are contained in the CAZY database (http://www.cazy.org, last accessed April 2017). Recently, a specific PlantCAZyme database was established, giving credit to the vast number of plant GTs [83]. Plant GT enzymes, similar to all GT proteins, catalyze the transfer of sugar units from activated nucleotide diphosphate sugar donors. Plant GTs mostly use UDP-glucose, although UDP-rhamnose, UDP-galactose, UDP-xylose, UDP-arabinose, and UDP-glucuronic acid have also been reported as donors [84,85,86,87]. Additionally, glycosides and glucose esters can be distinguished by the type of bond that is formed. Although O-glycosylation is the most common modification, N-, S-, and C-glycosides have also been described [3]. The sequence identity of plant GTs may vary, but they share distinctive characteristics, such as structural folds, stereo-chemical mechanism of glycosidic bond formation, and a conserved motif called plant secondary product glycosyltransferase (PSPG) box.
Two structural folds (Fig. 9.3) have been characterized intensively, the GT-A fold and the GT-B fold [92]. GT-A folded GTs possess a single Rossmann fold and a conserved metal-binding motif [93, 94]. In contrast, GT-B enzymes do not require metal ions and contain two Rossmann folds. These Rossmann folds are linked, facing one another, and form an active cleft between them [95, 96]. A third fold, named GT-C fold, has been proposed along with the other two folds but the distinctiveness of the GT-C fold remains controversial [97,98,99]. Recently, a new fold was reported for a bacterial GT involved in the glycosylation of serine-rich repeat streptococcal adhesins [91]. After X-ray crystallography the authors were able to identify a distinct structure, different from all known GT folds, and a new metal-binding site. Consequently, this new structure was called GT-D fold.
Representative examples of the four possible structural GT folds. GT-A: PDB 1FOA [88]; GT-B: PDB 4REL [89]; GT-C: PDB 3RCE [90], and GT-D: 4PHR [91]. The pdb-files were downloaded from RCSB Protein Data Base (http://www.rcsb.org/pdb/home/home.do; last accessed May, 2016), and visualized by the PyMOL v.1.7.4 software
GT enzymes can also be classified according to the anomeric configuration of the product. Enzymes that retain the stereo-chemistry at the anomeric center of the donor substrates are called “retaining” GTs. In contrast, enzymes that invert the stereochemistry are called “inverting” GTs [82]. Family 1 GT proteins are inverting enzymes that adopt the GT-B fold, and tailor lipophilic small-molecule acceptors [100]. Additionally, most of the family 1 GT enzymes feature the so-called PSPG box, a conserved C-terminal motif responsible for the interaction with the sugar donor [101]. An interaction between the highly conserved HCGWNS motif and UDP-glucose has been revealed [102], and the last amino acid of the PSPG box probably controls the selection of the sugar donor [103].
5 Substrates of Family 1 Plant Glycosyltransferases
Plants produce a tremendous diversity of low molecular weight metabolites, many of them being of commercial importance. The variety is mostly ensured by decoration of a limited number of common skeletons by hydroxylation, methylation, acylation and glycosylation. Glycosylation is one of the most widespread modifications.
5.1 Secondary Metabolites
Although secondary metabolites may not be essential for plant growth and development under artificial growth conditions, they are of high relevance for survival in natural environments. During evolution, when plants became sedentary, their inability to move forced them to be able to adapt to constantly changing environmental conditions. At times they are exposed to changes in temperature, UV radiation, salinity, water status, and pathogen pressure. This has led to the development of a wide variety of secondary metabolites that are constantly modified to meet the plant’s requirements. Many metabolites acting in defense responses are glycosylated for stabilization, detoxification, and sequestration.
5.1.1 Phenylpropanoids
Phenylpropanoids consist of a three carbon side chain linked to a phenyl group (Fig. 9.4). Like most phenolic secondary metabolites, their aromatic core structure is derived from phenylalanine, which in turn is derived from the shikimate pathway. Phenylalanine is converted in three reaction steps to the activated 4-coumaroyl-CoA thioester, which gives rise to simple (e.g. hydroxycinnamic acids) and more complex (e.g. flavonoids, stilbenes, lignins) phenolics; this biosynthetic pathway has been well studied and reviewed [104, 105]. The phenylpropanoid biosynthetic pathway provides metabolites acting as UV protectants (flavones) or as chemo-attractants for Rhizobia – mycorrhiza forming bacteria [106]. Furthermore, the precursors for polymeric lignin, antimicrobial phytoalexins, and pigments (anthocyanins) are derived from the general phenylpropanoid pathway [107] (see also Chap. 4 of this book). Phenylpropanoids have also drawn attention for their health-promoting properties. Anticancerogenic, antioxidative, and antimicrobial effects have been noted [108,109,110]. The already high structural diversity is further increased by the action of family 1 GTs through addition of sugar residues.
Caffeic acid (3,4-dihydroxycinnamic acid) is an example of a simple phenylpropanoid. Its esterification with glucose by GTs has been described frequently [10, 111], most recently by enzymes from the tea plant (Camellia sinensis) [112]. Strawberry, raspberry and grape GTs that produce phenylpropanoyl glucose esters show substrate promiscuity as they form glucose esters of a variety of (hydroxyl)cinnamic acids and (hydroxyl)benzoic acids, including gallic acid, and might also to be involved in the biosynthesis of ellagitannins [54].
Monolignols are the precursors of lignins, which are integrated into the cell walls of higher plants to enhance shoot stabilization. They are converted to glycosides by GTs, as has been shown for UGT72B1 from Arabidopsis , which catalyzes the glucosylation of coniferyl alcohol and coniferyl aldehyde [113]. Over-expression of PtGT1 from poplar (Populus tomentosa) in tobacco plants led to increased lignin content and an early flowering phenotype [114], indicating that monolignol glucosides might play a role in the formation of lignin in plant cell walls (although their specific functions remain elusive) [115].
Coumarins are yet another group of phenylpropanoids relevant for industry. Coumarin (1-benzopyran-2-one), the eponym of this class of metabolites, is commonly used in perfumes and fragrances because of its vanilla-like odor. In planta, coumarins are hydroxylated, which enables glycosylation of these functional groups, and these metabolites rarely occur in aglycon form. Two GTs from tobacco were found to glucosylate scopoletin (6-methoxy-7-hydroxycoumarin), which is considered a phytoalexin because it possesses antimicrobial properties and is accumulated by plant tissues upon pathogen infection [116]. Glycosylation of umbelliferone (7-hydroxycoumarin), another hydroxycoumarin with phytoalexin activity, was recently reported to be mediated by PNgt1 and 2, two GTs from Ipomoea morning glory (Pharbitis nil) [117].
5.1.2 Flavonoids, Anthocyanins
Flavonoids , which arise from phenylpropanoids by condensation with three molecules of malonyl CoA, are characterized by a 15-carbon flavan skeleton (Fig. 9.4) (see also Chap. 4 of this book). Among them are anthocyanins – glycosylated plant pigments responsible for fruit color [118]. The first plant GT gene was identified in a study on the genetic instability of transponsons in maize . Bronze 1, the gene responsible for the dark pigmentation of maize grains later turned out to encode an anthocyanidin GT [101]. In most cases, UDP-D-glucose serves as the preferred sugar donor for plant GTs, which is the reason why glucosides are so abundant in the plant kingdom. However, in red-fleshed kiwifruit (Actinidia chinensis) cyanidin 3′-O-xylo-3-O-galactoside is the main anthocyanin. It is formed by two sequential glycosylation steps. The first sugar (galactose) is transferred by F3GT1, whereas xylose is transferred by F3GGT1 [87]. Similarly, malvidin-3,5-O-bis-glucoside is synthesized by two sequential glycosylation reactions, but the monosaccharides are transferred to different positions of the anthocyanidin skeleton. The second glycosylation step is catalyzed by 5-O-glucosyltransferase Va5GT from grape (Vitis amurensis), which converts malvidin-3-O-glucoside to the corresponding 3,5-O-bis-glucoside [119, 120]. RhGT1, a dual function anthocyanidin 3,5-O-glucosyltransferase from Rosa hybrida, produces the 3,5-O-bis-glucoside directly from anthocyanidins and is able to glycosylate a wider spectrum of flavonoid metabolites including apigenin (flavone) and galangin (flavonol) [121]. The diversity of plant natural products originates from such combinatorial modifications.
5.1.3 Dihydrochalcones , Acylphloroglucinol , Stilbenes , Curcumin
Phloretin derivatives are classified as dihydrochalcones and belong to the group of acylphloroglucinols (Fig. 9.4). They are the main phenolic metabolites in apple (Malus × domestica) and pear (Pyrus communis) leaves, where they are thought to act in pathogen resistance [122]. They mainly occur in glycosylated form, with phloridzin (phloretin 2′-O-glucoside) being the most abundant product [123]. Phloridzin is produced by a GT in apple and pear that is specific for the acceptor substrate phloretin but shows relaxed activity for the donor substrate. MdPGT1 can glycosylate phloretin in the presence of three sugar donors: UDP-glucose, UDP-xylose and UDP-galactose [124,125,126]. Trilobatin (phloretin-4′-O-glucoside) is formed by MdPh-4′-OGT in apple [127].
Only recently, acylphloroglucinols have also been discovered in strawberry fruit [128]. They are synthesized by a dual functional chalcone/valerophenone synthase, which readily catalyzes the condensation of two intermediates in the branched-chain amino acid metabolism, isovaleryl-Coenzyme A (CoA) and isobutyryl-CoA, with three molecules of malonyl-CoA to form phlorisovalerophenone and phlorisobutyrophenone, respectively. Glucosylation is finally catalyzed by the promiscuous UGT71K3 enzyme [129], which also participates in the glucosylation of the key strawberry flavor compound 4-hydroxy-2,5-dimethyl-3(2H)-furanone [130].
Similar to coumarins, stilbenes are considered as phytoalexins but are also thought to act in defense response signaling and protection against UV-radiation [131]. Trans-resveratrol (trans-3,5,4′-trihydroxystilbene) has received attention lately, as it lowered blood pressure and insulin resistance when administered to rodents [132]. Glucosylation of resveratrol significantly increases its solubility and is catalyzed by PaGT3 (in Phytolacca americana) [133] or a bi-functional resveratrol/hydroxycinnamic acid glucosyltransferase (in Vitis labrusca) [134]. Four different glucosides of resveratrol, namely resveratrol 3-O-β-D-glucoside, resveratrol 4′-O-β-D-glucoside, resveratrol 3,5-O-β-D-diglucoside, and resveratrol 3,5,4′-O-β-D-triglucoside are produced by an UDP glucosyltransferase from Bacillus licheniformis [21] (see also Chap. 3 of this book).
Curcumin is the yellow pigment of turmeric, the dried rhizome of Curcuma longa. It is used primarily as food colorant, but also supposedly possesses pharmacological activity. However, its low water solubility limits further pharmacological exploration and practical application. Thus, glucosylation of curcumin was analyzed in cultured Catharanthus roseus cells and two GTs were characterized. CaUGT2 catalyzed the formation of curcumin monoglucoside from curcumin and also the conversion of curcumin monoglucoside to curcumin diglucoside [135], whereas UCGGT catalyzed the 1,6-glucosylation of curcumin 4′-O-glucoside to yield curcumin 4′-O-gentiobioside [136].
5.1.4 Terpenoids
Terpenoids are synthesized in-vivo via two biosynthetic pathways, either by the mevalonate or the 2-C-methyl-D-erythritol 4-phosphate/1-deoxy-D-xylulose 5-phosphate (MEP/DOXP) pathway [137]. In plants, terpenoids are involved in defense and stress response, and plant insect interaction [138]. Many terpenoids are fragrant volatile metabolites used in many different industrial applications, primarily in the fragrance and food sectors. The monoterpene alcohol geraniol is a commercially highly relevant terpenoid, as it possesses an odor commonly associated to rose flowers [139]. In addition, it is also a crucial flavor contributor in various grapevine varieties, and several monoterpenol GTs mediating gluco-conjugation of geraniol among other terpenoid alcohols have been reported recently [53, 140]. Similarly, AdGT4 from the kiwi plant (Actinidia deliciosa) glycosylates a range of terpenes and primary alcohols which are found as glycosides in ripe kiwifruit. Two of the enzyme’s preferred primary alcohol aglycones, hexanol and (Z)-hex-3-enol, contribute strongly to the ‘grassy-green’ aroma notes of ripe kiwifruit [141]. In tea plants (C. sinensis) terpenoid alcohols are stored as β-primeverosides, diglycosides formed from glucose and xylose, by the sequential action of CsGT1 and CsGT2 [142].
5.2 Plant Hormones
Phytohormones are bioactive compounds of plant origin that function as messenger molecules and play essential roles in germination, growth, development, and defense reactions. They are thought to be glycosylated by GTs to sustain cellular homeostasis, in other words to maintain an equilibrium of active and inactive (non-glycosylated and glycosylated) products [143]. Brassinosteroids, a family of steroid hormones, regulate cell division and elongation. In A. thaliana it was shown that homologous UGT73C5 and UGT73C6 catalyze the glucosylation of the brassinosteroid castasterone, thereby causing its inactivation [144, 145]. Likewise, cytokinins are involved in plant growth but also environmental responses, and GT activity on cytokinins such as cis-zeatin has been verified [146]. Similarly, GT mediated glucose esterification of auxins [147] and abscisic acid [148] has been shown, and a promiscuous GT enzyme from the immature seeds of morning glory (Ipomoea nil) glucosylates 2-trans-abscisic acid, indole-3-acetic acid, salicylic acid (SA) and (+/−)-jasmonic acid [149].
5.3 Miscellaneous Substrates (Alkaloids, Benzoxazinoids, Furanones, and Xenobiotics)
Alkaloids are bioactive nitrogen-containing plant metabolites derived from amino acids (see also Chap. 5 of this book). Capsaicinoids are branched or straight-chain alkylvanillylamides produced by species of the genus Capsicum, which are important sources of foods, spices, and medicines. Capsaicin (8-methyl-N-vanillyl-6-nonenamide) is the most poignant compound among naturally-occurring capsaicinoids and shows interesting bioactivity. However, capsaicinoids exhibit low water-solubility and are consequently only poorly absorbed after oral administration [150]. The search for capsaicinoid GTs yielded PaGT3, which was isolated from pokeweed (P. americana) and converted capsaicin and 8-nordihydrocapsaicin to their corresponding glucosides [150].
Benzoxazinoids (Bx) are defensive metabolites in various species of the Poaceae and are derived from tryptophan. DIBOA (2,4-dihydroxy-2H-1,4-benzoxazin-3(4H)-one) and its C-7-methoxy derivative DIMBOA are the predominant benzoxazinoids in maize, and were reported to be glucosylated by the GT Co-BX8 from larkspur (Consolida orientalis) [151]. The Bx-glucosides possess reduced toxicity compared to the aglycons and are stored in the vacuole. When the cells are disrupted upon wounding and/or infection, the toxic aglycons are released by a pre-existing β-glucosidase (Glu) that accumulates in plastids, defining them as phytoanticipins [152].
Furanones such as the key strawberry flavor compound 4-hydroxy-2,5-dimethyl-3(2H)-furanone (HDMF, furaneol®) are downstream metabolites of fructose-1,6-diphosphate [153]. HDMF is synthesized during strawberry development but becomes glucosylated by UGT71K3a and UGT71K3b, rendering the volatile odorless [130]. UGT71K3 isozymes are promiscuous and also accept acylphloroglucinols and vanillin as acceptor molecules, probably due to structural similarities with HDMF [129].
Xenobiotics are man-made chemical substances, such as pesticides and industrial chemicals that form residues in plants. Some of these have cytotoxic effects, but plants can prevent these by conjugating xenobiotic breakdown products to sugar residues. The explosive 2,4,6-trinitrotoluene (TNT) is one such chemical contaminating soil and groundwater today. Six GTs from A. thaliana were found to glucosylate hydroxylaminodinitrotoluenes and aminodinitrotoluenes, degradation products of the TNT-metabolism [154], while 44 GTs catalyzed the O-glucosylation of chlorinated phenols, but only one, UGT72B1, showed appreciable N-glucosylating activity toward chloroanilines [155]. Similarly, FaGT2, a multifunctional GT enzyme from strawberry fruits, is involved in the metabolism of natural and xenobiotic compounds, such as the herbicide 2,4,5-trichlorophenol and the herbicide analogue 3,5-dichloro-4-hydroxybenzoic acid [156].
6 Glucoside Production by Whole Cell Biocatalysts
Natural product glycosides/carbohydrate esters are new and promising bioactive substances with prospective in functional foods, drug development, cosmetics, and many other applications [157, 158]. The bio-catalytic synthesis of glucosides using GT enzymes has many advantages over classic chemical approaches [159]. The production can be carried out either in-vitro, employing purified, heterologously produced enzyme [160,161,162], or in-vivo, utilizing a whole-cell biotransformation system [74, 133, 163, 164]. Production of glycosides using living cells provides major advantages (Fig. 9.5) [77, 165, 166], as the cells can be grown to high density and cultivation is cost-efficient compared to chemical approaches or in-vitro systems. Furthermore, if supplied with appropriate nutrients, the cells will be able to take up the substrates by diffusion or active transport. Consequently, endogenous or exogenous GTs glycosylate the substrates and the products are excreted into the medium, where they can be easily collected as the biocatalyst can readily be removed. Moreover, addition of co-factors is not required, since the cells provide their own recycling machinery [162]. Since many plant genomes and consequently GT sequences are publicly available, the next step in the development of whole-cell biocatalysts, currently applied in newest research, is the creation of transgenic cells overexpressing the genes of interest (Table 9.1). However, the whole-cell approach has also some limitations [189]. Often the availability of the substrate is restricted by its solubility, and too high concentrations can be toxic for the cells. Furthermore, some substrates are not able to enter the cells [159].
Possible starting points for optimization of the whole cell biocatalyst (host cell). Utilization of improved plasmids to regulate heterologous GT expression (a) (ori: origin of replication, ABR: antibiotic resistance). Amino acid sequence mutation for enhanced protein expression, increased enzyme stability, modified substrate specificity and enzymatic activity (b). Host improvement through deletions and insertions/additions to the host genome, including metabolic engineering to improve the metabolic flux towards glycoside formation (c). R alkyl or aryl
6.1 Production System
Glycoside production using whole-cell biocatalysts can be performed in two technical production systems; shaking flasks and stirred-tank reactors.
Production in partitioned shaking flasks, as described for the production of β-glucosides employing plant GTs, consists of three sequential steps [162, 166]. (I) Biomass production : Recombinant cells are grown to a high cell density using a complex medium. (II) GT production : The culture medium is changed to a defined minimal medium. This is done to reduce unwanted byproducts and to simplify the downstream applications. Subsequently, the expression of the recombinant GT is actively induced. (III) Glycosylation : The aglycon is added to the medium and the conversion takes place. Although the use of shaking flasks is simple, it offers very little process control and is not suitable for up-scaling.
The stirred-tank reactor on the other hand offers superior aeration and can be scaled up [166]. Furthermore, it provides online data like, among others, pH value, precise temperature, and dissolved O2 concentration. This enables a more precise control of the process by, for example, using intelligent software and protocols [190]. The production of 3-O-xylosyl quercetin using an engineered E. coli strain was improved through up-scaling . By changing the cultivation vessel from 5-mL culture tubes to a 3-L bioreactor, the production of quercetin 3-O-xylose was boosted from 55–98% [170].
6.2 Types of Whole-Cell Biocatalysts
Presently, E. coli is the biocatalyst of choice in most natural product glycol-diversification approaches, as it is a well-studied bacterium with many genetic tools already available [191] (Table 9.1). A broad range of natural plant products has already been successfully glycosylated in transgenic E. coli cells expressing GTs [192]. The flavonol quercetin was transformed to the 3-O-rhamoside and 3-O-N-acetylglucosamine by E. coli cells expressing GTs from A. thaliana [172, 174], and anthocyanidins [167], kaempferol [171] and 2(4-hydroxyphenyl)ethanol [182] were glycosylated employing various GT enzymes in transgenic E. coli cells (see also Chap. 4 of this book). C-glucosides of flavonoids and related compounds (2-hydroxyflavanone, dihydrochalcone, and trihydroxyacetophenone) were produced by E. coli expressing a buckwheat C-glucosyltransferase [178].
However, depending on the substrate compatibility or the final application, a different host organism might be of advantage. For example, Saccharomyces cerevisiae cells expressing GTs from Dianthus caryophyllus (carnation) or Scutellaria baicalensis have been successfully applied as whole-cell biocatalyst for the production of naringenin glucosides [177] and scutellarein 7-O-glucoside [164], respectively. Furthermore, yeast expressing an Arabidopsis GT was used for the production of zearalenone 4-O-glucoside [60], and Schizosaccharomyces pompe cells expressing human GTs were utilized for the generation of drug metabolites [180]. Additionally, the GT-mediated production of rebaudioside A from stevioside was catalyzed by an engineered S. cerevisiae strain [74].
Plant cells have also been used as biocatalysts in glycosylation processes. Glucose esters of cinnamic acid, 4-coumaric acid, caffeic acid and ferulic acid were produced by suspension-cultured cells of Eucalyptus perriniana [193], and biotransformation of raspberry ketone and zingerone to the correspondent glucosides was achieved by cultured suspension cells of P. americana [194]. β-Thujaplicin (hinokitiol), a tropolone derivative present in the heartwood of cupressaceous plants and used as medicine, food additive, and preservative, is transformed by cultured plant cells of Nicotiana tabacum to two glucosides and two gentiobiosides [195].
6.3 Process Optimization
Although living cells as biocatalysts provide advantages over in vitro cell-free systems, there are also limitations. The harvested product suspension may contain cell debris, secretion products of the host metabolism, and other by-products, which result in more intensive downstream purification. Some organisms also express their own glycosyltransferases potentially interfering by drawing co-factors and UDP-sugars, and produce unwanted side-products. Depending on the choice of host organism, only specific metabolic pathways employing specific carbon sources are available for glucoside production. However, decisive progress has been made to overcome these hindrances. Nowadays, many properties can be added to or removed from the employed host or enzyme using strategies such as those shown in Fig. 9.5.
6.3.1 Vector Conveyed Process Optimization
Many studies engaged in optimizing the product formation use vector construct transformation strategies (Fig. 9.5a). Glycosides produced by E. coli strains are mostly conjugates of glucose or galactose. However, more and more UDP-sugar modifying enzymes are being identified and investigated. Microorganisms containing plasmids that harbor one or more of these enzymes can produce a broader range of different activated sugars and corresponding glycosides. Bioactive flavonol 3-O-rhamnosides have been successfully synthesized via co-transformation of two vectors in E. coli containing a flavonol rhamnosyltransferase and a rhamnose synthase both from A. thaliana [172]. The heterologously expressed rhamnosyltransferase produced rhamnosides and the rhamnose synthase ensured the conversion of UDP-glucose into UDP-rhamnose, thereby increasing the product formation rate. The NDP-sugar biosynthesis circuit of E. coli was also shifted towards the production of flavonoid glucosides and rhamnosides by employing a multi-monocistronic synthetic vector containing multiple genes [183]. Another study employed GTs and a sucrose synthase in enzymatic cascade reactions to enable utilization of sucrose as a carbon source, and to facilitate in-situ recycling of the NTP-sugar donor from the NDP leaving group [162]. Correspondingly, shifting of the nucleotide sugar pathways of E. coli towards the production of UDP-xylose and -arabinose for GT-conveyed synthesis of flavonoid O-pentosides was demonstrated [86]. To produce flavonoids attached to sugars such as glucuronic acid and galactoside, E. coli was genetically modified to express GTs specific for UDP-glucuronic acid (AmUGT10 from Antirrhinum majus and VvUGT from V. vinifera) and UDP-galactose (PhUGT from Petunia hybrida), along with the appropriate nucleotide biosynthetic genes to enable simultaneous production of their substrates, UDP-glucuronic acid and UDP-galactose [169]. Using these strategies, luteolin-7-O-glucuronide, quercetin-3-O-glucuronide, and quercetin 3-O-galactoside were successfully synthesized.
Overall, the success of metabolic engineering depends on a balanced expression of the biosynthesis enzymes. Factors that influence protein levels include gene copy number, promotor strength, ribosomal binding site (RBS), inducer concentration and codon usage [191, 196]. Besides, coupling to a fusion partner like GST (glutathione S-transferase), NusA (N-utilization substance protein A), MBP (maltose-binding protein), Trx (thioredoxin) or SUMO (small ubiquitin-related modifier) can increase enzyme solubility and prevent accumulation of inactive protein aggregates, thus providing enhanced biocatalytic activity [191].
6.3.2 UGT Optimization
Another strategy to improve the product yield and diversity is to optimize enzyme function (Fig. 9.5b). Usually, the GT is chosen for a specific activity or for its promiscuity towards a spectrum of aglycon substrates. However, if an enzyme with the desired substrate specificity is not available, known GTs can be modified. For example, the protein sequence of the GT OleD from Streptomyces antibioticus was mutated to broaden the accepted substrate spectrum. A more than 180-fold higher activity towards the therapeutically important acceptor 7-hydroxycoumarin-4-acetic acid was achieved [197]. The most common approach to achieve improved or altered substrate specificity, usually involves mutating amino acids positioned in the active cleft of the enzyme. For example, the activity of GTs from Panax ginseng towards ginsenosides, bioactive natural glycosides from the ginseng plant, was altered by this approach [184]. However, other researchers demonstrated that also amino acid substitutions outside of the active cleft, but in its vicinity, can procure changed product conversion rates [53, 54], and alteration of sugar donor specificities of plant GTs [103]. The catalytic mechanism and basis for O- and N-glucosylation specificity was also probed by mutagenesis and domain shuffling [198]. Mutation of an O-specific GT at just two positions installed high levels of N-GT activity, whereas molecular modeling revealed the connectivity of these residues to the catalytically active histidine-19 on UGT72B1, with its mutagenesis exclusively defining N-GT activity in UGT72B1.
Furthermore, it is imperative to secure a balanced protein expression, as overexpressed enzymes are a metabolic burden for the cell. This can lead to protein aggregation or incorrect folding, in addition to subsequent formation of enzymatically inactive inclusion bodies. Accumulation of inactive protein aggregates can be prevented by co-expression of proteins that assist in folding. It has been demonstrated that co-expression of recombinant GT enzymes with chaperones resulted in an increased enzymatic activity and a lower aggregation rate [199].
6.3.3 Host Genome Optimization – Metabolic Engineering
Along with process optimization through extrachromosomal gene expression, modification of the host metabolism can also be achieved by direct insertion/deletion of sequences into/from the genome (Fig. 9.5c). Thereby, futile metabolic cycles can be shut down, and product utilization by the host and nonessential metabolic effluxes can be avoided. Although plasmids are a quick and easy way to test out new enzyme additions without having to design a whole new expression cassette, permanent changes by gene deletion/insertion secure a stable metabolism within the cell population. However, in most cases, a combined approach of extra- and intra-chromosomal modifications achieves the best results. Figure 9.6 illustrates how the metabolic carbon flow of E. coli Waksman was modified to exclusively use sucrose and fructose as cheap carbon sources for the production of phenolic glucose esters [186]. The vector conveyed introduction of the sucrose phosphorylase from Bifidobacterium adolescentis, and the permanent deletion of the endogenous phosphoglucomutase gene created a split in the usage of sucrose. In this manner, half of the carbon was utilized for the production of phenolic glucose esters, while the second half was spared for other cell metabolism. Another strategy to increase GT product formation is the deletion of UDP-glucose consuming pathways, e.g. the UDP-glucose hydrolase (ushA) in E. coli, to prevent metabolization of the co-substrate UDP-glucose and to promote glycosylation reactions [163, 175] (Fig. 9.7a). The production of 3-O-xylosyl quercetin was improved in mutant strains deficient in phosphoglucoisomerase (pgi), D-glucose-6-phosphate dehydrogenase (zwf) and ushA genes, paired with over-expressed UDP-xylose biosynthetic genes phosphoglucomutase (pgm), glucose 1-phosphate uridylyltransferase (galU), UDP-glucose dehydrogenase (calS8) and UDP-glucuronic acid decarboxylase (calS9) [170]. Thereby, the level of glucose-1-phosphate, UDP-glucose and consequently of UDP-xylose was increased (Fig. 9.7). A similar engineered E. coli mutant , lacking the over-expressed UDP-xylose biosynthetic genes, was further developed for the efficient whole-cell biocatalysis of flavone 7-O-β-D-glucopyranosides [168], phloretin glucosides [187] and isoflavonoid glucosides [188].
Optimization strategy in E. coli Waksman for improved production of phenolic glucose esters. Introduction of sucrose phosphorylase facilitates direct formation of glucose-1-phosphate, and deletion of phosphoglucomutase prevents its degradation [186]. Blue: Endogenous E. coli Waksman pathway. Orange: Change procured by gene deletion. Green: Extrachromosomal gene additions
UDP-glucose consuming enzymes of competing metabolic pathways (a). Schematic diagram of the UDP-glucose biosynthesis pathway showing the mutations of the engineered E. coli strain used for glycoside production (b) [170]. Up-regulated and deleted enzymes are shown in red and blue, respectively
The deletion of galU can also be used to shift the metabolic pathway of E. coli towards the formation of alternative sugar donors, such as UDP-N-acetyl-D-glucosamine [174] and dTDP-6-deoxytalose [173], thereby increasing the production of novel quercetin glycosides employing extrachromosomal GTs.
Combinatorial intra- and extrachromosomal optimization approaches have recently been introduced as a successful strategy to increase product yields [167]. The authors made use of previously published advances, such as the optimization of the medium pH and the UDP-glucose supply, as well as introducing gene expression cassettes [176, 179, 200]. They combined these conditions with (i) optimized culture conditions and induction parameters, (ii) a bicistronic expression cassette for balanced co-expression of a GT and a precursor forming enzyme, and (iii) overexpression of transporter proteins for improved transportation of substrate and product across the host cell membrane. In doing so, a final titer of 350 mg/L of cyanidin 3-O-glucoside was achieved [167].
Aside from E. coli, other biocatalysts have been successfully engineered as well. For example, the metabolism of yeast strains was optimized by integration of multiple genes including GTs into the genome for de novo biosynthesis of ginsenosides and vanillin glucosides [14, 181, 184, 185] (see also Chap. 1 of this book).
7 Recent Applications of Glycosyltransferases for Production of Small Molecule Glycosides
Numerous remarkable characteristics arise from the glycosylation of natural products providing auspicious applications in new drug development, making it a hotspot in natural product biosynthesis and modification. Natural and biological approaches for glycosylation of aglycons to form glycosides have attracted substantial attention and interest, as it involves the formation of novel compounds under mild conditions. In comparison to ‘traditional’ chemical synthesis methods, biotransformation implements an environmentally friendly option for the synthesis of fine chemicals. GT enzymes that partake in the biosynthesis of natural products have demonstrated to be advantageous for the chemo-enzymatic synthesis or biosynthesis of functional compounds with new bioactivities. This enzymatic process influences their biological and chemical properties by the attachment or alteration of sugar moieties in natural or synthetic compounds. The common strategy utilizes the promiscuity feature of natural product GTs by transferring various sugar moieties to different aglycons in-vivo or in-vitro. Precise regio/stereo-selectivity and moderate reaction conditions lay the groundwork to manipulate and apply the enzymatic glycosylation of natural product GTs. In the recent years, the composed research and known features aid in the comprehensive understanding and has shed light on many GT sequences, which have been correlated with novel glycosylation reactions and perspective applications of GTs.
7.1 Glycosyltransferases and Glycosylation in Product for Consumer Consumption
In the 1960s, the discovery and extraction of glycol-conjugated forms of monoterpene alcohols has unraveled a new expanse of nonvolatile aroma precursors where researchers can indulge in the study of scents and flavor [4]. In general, nonvolatile, glycosylated aroma precursors produce an aroma when the glycosidic bond is removed and the molecule becomes volatile, and is then able to interact with the olfactory receptors [4, 67, 201,202,203]. The applications of fragrance and flavor chemical compounds have been vastly applied in food, medicine, tobacco, textiles, leather, papermaking, cosmetics, and further products for consumer consumption [4]. Among the many aromatic compounds, vanillin, phenylethanol, benzyl acetate, linalool, menthol, and geraniol are economically important aroma chemicals, which exceed annual consumption rates of 5000 tonnes [4, 137] (see also Chap. 1 of this book).
Terpenes are volatile, unsaturated hydrocarbons that serve as essential oil constituents of many plants and are often used as natural flavor additives for food, fragrances in perfumery, as well as medicine, and aromatherapy [4, 204]. A number of volatile hemiterpenoids (C5), monoterpenoids (C10), and sesquiterpenoids (C15) greatly contribute to the odor of a product. At the same time, higher terpenoids can contribute to the perception of taste. As an example, steviosides are sweet tasting glycosides of the diterpenoid steviol extracted from Stevia rebaudiana, a herb from the Asteraceae family [205]. These glycosylated secondary metabolites are bioactive constituents of the commonly used sweetener, Stevia. Aside from other metabolites found and glycosylated in the fruit tissue, the steviol glycosides are biosynthesized in the leaf tissue [206]. Steviol glycoside synthesis commences with steviol, which is a product from the MEP pathway (Fig. 9.2). The subsequent four steps include the addition of sugar molecules to the carbon backbone catalyzed by GTs. The final product of this multi-step process is the glycosylated rebaudioside A, which is transported to the vacuole for storage [207]. Previous studies of the various enzymes involved in steviol glucosylation led to the isolation of two protein fractions from S. rebaudiana leaves revealing significant GT activity [207, 208]. Each enzyme possessed a unique activity; one that exhibited high specificity and ability to catalyze the transfer of UDP-glucose to steviol and subsequent glycosides, and a second that exhibited low specificity and activity. Interestingly, both enzymes were found to also accept flavonol substrates, such as kaempferol, quercetin, and hydroquinone [206]. The different properties of the individual glycosides can vary significantly, and their characteristics are determined by the type of sugar and pattern of glycosylation. Among various tested steviolglycosides, rebaudioside A was ascertained as the sweetest tasting and least bitter glycoside [73]. Thus, several biotechnological processes involving GTs have already been suggested to manipulate the glycosylation status of steviosides [70,71,72,73,74].
Similarly, a plant GT partially purified from pomelo fruits has been immobilized and used in bioprocessing for de-bittering citrus juice by converting the astringent triterpene limonoid into glycosides [209].
Monoterpenes (linalool, menthol, and geraniol) are volatile and predominantly poorly water-soluble flavor and fragrance compounds, thus limiting their utility for industrial applications. The glycosylation of these monoterpenes enhances their water-solubility, thus rendering them odorless. Therefore, they are of interest for the flavor and fragrance industry due to the possibility of a controlled release of the bound aroma compound upon cleavage of the glycosidic bond. Moreover, monoterpene glycosides have been proposed to inhibit unpleasant odor for various products, enhance the quality of cigarette smoke, augment the aroma of freshly cut flowers, and improve longer-lasting deodorants. Besides, they can be utilized in personal hygiene products for continuous release of scents and as air fresheners [4]. Utilizing odorless volatiles bound via a glycosidic bond as fragrance materials was exemplified by incubating skin microflora with numerous glycosides. The majority of the glycosidically bound volatiles, in particular β-D-glucosides of monoterpenes, are hydrolyzed by glucosidases excreted by the skin microbiome, thereby releasing the aglycones as fragrance ingredients [210, 211].
GTs are mainly involved in natural product glycosylations via O-glycosidic bonds. In contrast, glycosylation at the carbon atom (C-glycosylations) is quite rare, and only a few C-GT enzymes are therefore available [155]. Aryl glycosides are derived from aglycons resembling flavonoids, which denote either an O- or C-glycosidic bond [212]. These glycosides have been proposed to show significant biological activities, including antioxidant properties, antiviral, and cytotoxic impacts [213, 214]. Commonly, direct isolation from plants is impractical and chemical methodologies involve a series of steps resulting in poor yield and toxic byproducts. Therefore, a single-step reaction catalyzed by GTs in-vitro represents a powerful tool for the synthesis of aryl C-glycosides [215]. Nothofagin (3-C-glucoside of phloretin) is a natural secondary metabolite found in redbush herbal tea with applications in the food industry [216]. For a proficient synthesis of nothofagin, a C-GT reaction was coupled to an enzymatic supply of glucosyl donor substrate in-situ. This step-by-step reaction results in efficient and high-yielding bio-catalytic production of nothofagin [215].
7.2 Glycorandomization
GT enzymes with broad substrate specificity have been utilized in the development of powerful tools for glycorandomization [217]. This has led to the development of an E. coli platform for the combinational biosynthesis of antibiotics where numerous new glycoderivates were generated, some of which may become valuable drug candidates [218]. Another ‘in-vitro glycorandomization’ experiment is based on the flexibility of glycosyltransferases fD and fE (GtfD, GtfE). These two GTs were used on NDP-sugar libraries to generate glycorandomized natural products and then the applied method of chemo-selective ligation produced monoglycosylated vancomycins. The obtained products’ bioactivity varied significantly and at the same time possessed notably improved antibacterial properties [219].
7.3 Glycosyltransferases and Their Role in Cancer Therapies
In the 1970s, mitoxantrole (MXT) , a synthetic anthracenedione, was discovered and developed for the treatment of various human cancers [220, 221]. Additionally, MXT has been proposed to exhibit in-vivo activity against rheumatoid arthritis in several animal models [222]. Moreover, MXT supposedly acts as anti-tuberculosis agent, functioning through the inhibition of a specific mycobacterial kinase (PknB) controlling pathogen growth and evolvement [223]. However, MXT treatments are associated with various serious side effects such as irreversible cardiomyopathy. Therefore, stimulating research to generate analogues with diverse therapeutic effects, such as increased potency and reduced cardiotoxicity, has been conducted [224,225,226]. The recently identified GT OleD produced by S. antibioticus catalyzes the transfer of a glucose moiety from UDP-Glucose to various macrolide antibiotics [227]. Protein engineering of OleD and subsequent screening for variants capable of executing extended glycosylation reactions has led to the discovery of a promiscuous triple mutant, OleD-ASP [228]. Initial liquid chromatography mass-spectrometry (LC-MS) evaluation of the OleD-ASP acceptor flexibility revealed its ability to glycosylate a range of pharmaceuticals including anthraquinones, indolocarbozoles, polyenes, cardenolides, steroids, macrolides, beta-lactams, and enediyenes [217]. As engineered GT with unique promiscuity, OleD-ASP, was shown to regio- and stereo-selectively modify and glycosylate also MXT, thus providing an asymmetric MXT 4′-β-D-glucoside [228]. Interestingly, OleD-ASP is the first engineered GT that is able to asymmetrically glycosylate an anticancer drug whilst retaining its activity. At the same time, this is a single-step reaction requiring no protecting groups or sugar activation guidance. The single glucoside of MXT may potentially offer a beneficial toxicity profile leading to a reduction of side effects with the potential to be further optimized [228].
8 Conclusions and Future Prospects
Synthetic biology has been driven by the development of new powerful tools for DNA synthesis, sequencing and genome editing, which enable microbial engineering for the production of pharmaceuticals and other high-value chemicals. There is great diversity in the type and number of sugar units that could be added to naturally occurring aglycones, and the biological relevance remains elusive [229]. It is anticipated, that the attachment of sugars to natural products, or altering an existing sugar moiety, can improve pharmacological properties and specificity at multiple levels.
Glycotechnologies have been used for the generation of novel glycosylated compounds either in in-vitro or in-vivo experiments. Several GTs are suitable for altering glycosylation patterns, but strict substrate specificity remains a limiting factor in natural product diversification. Engineering GTs is the most promising way to discover and develop new GTs with clearly defined specificities. Suitable high-throughput screening systems will support glycodiversification technologies. Further structure elucidation of GTs will help to understand the mode of action of these enzymes [229]. Modifying the specificity of current natural product GTs and enhancing the biosynthetic technologies in the discovery of new GTs to improve the yields of applicable natural products, is increasingly becoming of utmost importance for researchers.
Abbreviations
- ABA:
-
Abscisic acid
- ABA-GE:
-
ABA-glucose ester
- ABC:
-
ATP-binding cassette
- AG:
-
Apocarotenoid glycoside
- AVI:
-
Anthocyanin vacuolar inclusion
- CAZymes:
-
Carbohydrate-Active enZymes
- CRC:
-
Colorectal cancer
- E. coli :
-
Escherichia coli
- ER:
-
Endoplasmatic reticulum
- GALNT12:
-
Polypeptide N-acetylgalactosaminyltransferase 12
- galU:
-
Glucose-1-phosphate uridylyltransferase
- GST:
-
Glutathione S-transferase
- GT:
-
Glycosyltransferase
- KAH:
-
Kaurenoic acid 13-hydroxylase
- KO:
-
Kaurine oxidase
- LC-MS:
-
Liquid chromatography mass-spectrometry
- MATE:
-
Multidrug and toxic extrusion
- MEP/DOXP:
-
2-C-methyl-D-erythritol 4-phosphate/1-deoxy-D-xylulose 5-phosphate
- MXT:
-
Mitoxantrole
- pgi:
-
Phosphoglucose isomerase
- PSPG box:
-
Plant secondary product glycosyltransferase box
- SG:
-
Steviol glycoside
- ushA:
-
UDP-glucose hydrolase
- zwf:
-
D-glucose-6-phosphate dehydrogenase
References
André I, Potocki-Véronèse G, Barbe S, Moulis C, Remaud-Siméon M. CAZyme discovery and design for sweet dreams. Curr Opin Chem Biol. 2014;19:17–24. https://doi.org/10.1016/j.cbpa.2013.11.014.
Huber GW, Chheda JN, Barrett CJ, Dumesic JA. Production of liquid alkanes by aqueous-phase processing of biomass-derived carbohydrates. Science. 2005;308:1446–50. https://doi.org/10.1126/science.1111166.
Singh S, Phillips GN Jr, Thorson JS. The structural biology of enzymes involved in natural product glycosylation. Nat Prod Rep. 2012;29:1201–37. https://doi.org/10.1039/c2np20039b.
Schwab W, Fischer TC, Giri A, Wüst M. Potential applications of glucosyltransferases in terpene glucoside production: impacts on the use of aroma and fragrance. Appl Microbiol Biotechnol. 2014;99:165–74. https://doi.org/10.1007/s00253-014-6229-y.
Desmet T, Soetaert W, Bojarová P, Křen V, Dijkhuizen L, Eastwick-Field V, et al. Enzymatic glycosylation of small molecules: challenging substrates require tailored catalysts. Chem Eur J. 2012;18:10786–801. https://doi.org/10.1002/chem.201103069.
Wang J, Ma X, Kojima M, Sakakibara H, Hou B. N-Glucosyltransferase UGT76C2 is involved in cytokinin homeostasis and cytokinin response in Arabidopsis thaliana. Plant Cell Physiol. 2011;52:2200–13. https://doi.org/10.1093/pcp/pcr152.
Kopycki J, Wieduwild E, Kohlschmidt J, Brandt W, Stepanova AN, Alonso JM, et al. Kinetic analysis of Arabidopsis glucosyltransferase UGT74B1 illustrates a general mechanism by which enzymes can escape product inhibition. Biochem J. 2013;450:37. https://doi.org/10.1042/BJ20121403.
Hirade Y, Kotoku N, Terasaka K, Saijo-Hamano Y, Fukumoto A, Mizukami H. Identification and functional analysis of 2-hydroxyflavanone C-glucosyltransferase in soybean (Glycine max). FEBS Lett. 2015;589:1778–86. https://doi.org/10.1016/j.febslet.2015.05.010.
Schwab W, Fischer T, Wüst M. Terpene glucoside production: improved biocatalytic processes using glycosyltransferases. Eng Life Sci. 2015;15:376–86. https://doi.org/10.1002/elsc.201400156.
Lim E, Higgins GS, Li Y, Bowles DJ. Regioselectivity of glucosylation of caffeic acid by a UDP-glucose:glucosyltransferase is maintained in planta. Biochem J. 2003;373:987–92. https://doi.org/10.1042/BJ20021453.
Jackson R, Knisley D, McIntosh C, Pfeiffer P. Predicting flavonoid UGT regioselectivity. Adv Bioinforma. 2011:506583. https://doi.org/10.1155/2011/506583.
Graefe EU, Wittig J, Mueller S, Riethling A, Uehleke B, Drewelow B, et al. Pharmacokinetics and bioavailability of quercetin glycosides in humans. J Clin Pharmacol. 2001;41:492–9. https://doi.org/10.1177/00912700122010366.
Makino T, Shimizu R, Kanemaru M, Suzuki Y, Moriwaki M, Mizukami H. Enzymatically modified isoquercitrin, α-oligoglucosyl quercetin 3-O-glucoside, is absorbed more easily than other quercetin glycosides or aglycone after oral administration in rats. Biol Pharm Bull. 2009;32:2034–40. https://doi.org/10.1248/bpb.32.2034.
Brochado AR, Matos C, Møller BL, Hansen J, Mortensen UH, Patil KR. Improved vanillin production in baker’s yeast through in silico design. Microb Cell Factories. 2010;9:84. https://doi.org/10.1186/1475-2859-9-84.
Yang C, Tanaka O. Advances in plant glycosides, chemistry and biology: proceedings of the international symposium on plant glycosides, Aug 12–15, 1997 Kunming, China. 1st edn. Amsterdam/New York: Elsevier; 1999.
Woo H, Kang H, Nguyen TTH, Kim G, Kim Y, Park J, et al. Synthesis and characterization of ampelopsin glucosides using dextransucrase from Leuconostoc mesenteroides B-1299CB4: glucosylation enhancing physicochemical properties. Enzym Microb Technol. 2012;51:311–8. https://doi.org/10.1016/j.enzmictec.2012.07.014.
Hofer B. Recent developments in the enzymatic O-glycosylation of flavonoids. Appl Microbiol Biotechnol. 2016;100:4269–81. https://doi.org/10.1007/s00253-016-7465-0.
Grotewold E. The science of flavonoids. New York: Springer; 2006.
Lepak A, Gutmann A, Kulmer ST, Nidetzky B. Creating a water-soluble resveratrol-based antioxidant by site-selective enzymatic glucosylation. Chembiochem. 2015;16:1870–4. https://doi.org/10.1002/cbic.201500284.
Fuggetta M, Mattivi F. The immunomodulating activities of resveratrol glucosides in humans. Recent Pat Food Nutr Agric. 2011;3:81–90.
Pandey RP, Parajuli P, Shin JY, Lee J, Lee S, Hong Y, et al. Enzymatic biosynthesis of novel resveratrol glucoside and glycoside derivatives. Appl Environ Microbiol. 2014;80:7235–43. https://doi.org/10.1128/AEM.02076-14.
Chung MJ, Kang A, Lee KM, Oh E, Jun H, Kim S, et al. Water-soluble genistin glycoside isoflavones up-regulate antioxidant metallothionein expression and scavenge free radicals. J Agric Food Chem. 2006;54:3819–26. https://doi.org/10.1021/jf060510y.
Haskins AH, Su C, Engen A, Salinas VA, Maeda J, Uesaka M, et al. Data for induction of cytotoxic response by natural and novel quercetin glycosides. Data Brief. 2016;6:262–6. https://doi.org/10.1016/j.dib.2015.11.066.
Yonekura-Sakakibara K, Nakayama T, Yamazaki M, Saito K. Modification and stabilization of anthocyanins. In: Winefield C, Davies K, Gould K, editors. Anthocyanins: biosynthesis, functions, and applications. New York: Springer; 2009. p. 169–90. https://doi.org/10.1007/978-0-387-77335-3_6.
Sasaki N, Nishizaki Y, Ozeki Y, Miyahara T. The role of acyl-glucose in anthocyanin modifications. Molecules. 2014;19:18747–66. https://doi.org/10.3390/molecules191118747.
Yoshida K, Mori M, Kondo T. Blue flower color development by anthocyanins: from chemical structure to cell physiology. Nat Prod Rep. 2009;26:884–915. https://doi.org/10.1039/b800165k.
Mäkilä L, Laaksonen O, Alanne A, Kortesniemi M, Kallio H, Yang B. Stability of hydroxycinnamic acid derivatives, flavonol glycosides, and anthocyanins in black currant juice. J Agric Food Chem. 2016;64:4584–98. https://doi.org/10.1021/acs.jafc.6b01005.
Roscher R, Schwab W, Schreier P. Stability of naturally occurring 2,5-dimethyl-4-hydroxy-3 [2H]-furanone derivatives. Z Lebensm Unters F A. 1997;204:438–41. https://doi.org/10.1007/s002170050109.
Saslowsky D, Winkel-Shirley B. Localization of flavonoid enzymes in Arabidopsis roots. Plant J. 2001;27:37–48. https://doi.org/10.1046/j.1365-313x.2001.01073.x.
Grotewold E, Davies K. Trafficking and sequestration of anthocyanins. Nat Prod Commun. 2008;3:1251–8.
Petrussa E, Braidot E, Zancani M, Peresson C, Bertolini A, Patui S, et al. Plant flavonoids – biosynthesis, transport and involvement in stress responses. Int J Mol Sci. 2013;14:14950–73. https://doi.org/10.3390/ijms140714950.
Kitamura S, Shikazono N, Tanaka A. TRANSPARENT TESTA 19 is involved in the accumulation of both anthocyanins and proanthocyanidins in Arabidopsis. Plant J. 2004;37:104–14. https://doi.org/10.1046/j.1365-313X.2003.01943.x.
Conn S, Curtin C, Bézier A, Franco C, Zhang W. Purification, molecular cloning, and characterization of glutathione S-transferases (GSTs) from pigmented Vitis vinifera L. cell suspension cultures as putative anthocyanin transport proteins. J Exp Bot. 2008;59:3621–34. https://doi.org/10.1093/jxb/ern217.
Sun Y, Li H, Huang J. Arabidopsis TT19 functions as a carrier to transport anthocyanin from the cytosol to tonoplasts. Mol Plant. 2012;5:387–400. https://doi.org/10.1093/mp/ssr110.
Härtl K, Denton A, Franz-Oberdorf K, Hoffmann T, Spornraft M, Usadel B, et al. Early metabolic and transcriptional variations in fruit of natural white-fruited Fragaria vesca genotypes. Sci Rep. 2017;7:45113. https://doi.org/10.1038/srep45113.
Gomez C, Conejero G, Torregrosa L, Cheynier V, Terrier N, Ageorges A. In vivo grapevine anthocyanin transport involves vesicle-mediated trafficking and the contribution of anthoMATE transporters and GST. Plant J. 2011;67:960–70. https://doi.org/10.1111/j.1365-313X.2011.04648.x.
Poustka F, Irani NG, Feller A, Lu Y, Pourcel L, Frame K, et al. A trafficking pathway for anthocyanins overlaps with the endoplasmic reticulum-to-vacuole protein-sorting route in Arabidopsis and contributes to the formation of vacuolar inclusions. Plant Physiol. 2007;145:1323–35. https://doi.org/10.1104/pp.107.105064.
Goodman CD, Casati P, Walbot V. A multidrug resistance–associated protein involved in anthocyanin transport in Zea mays. Plant Cell. 2004;16:1812–26. https://doi.org/10.1105/tpc.022574.
Francisco RM, Regalado A, Ageorges A, Burla BJ, Bassin B, Eisenach C, et al. ABCC1, an ATP binding cassette protein from grape berry, transports anthocyanidin 3-O-glucosides. Plant Cell. 2013;25:1840–54. https://doi.org/10.1105/tpc.112.102152.
Marinova K, Pourcel L, Weder B, Schwarz M, Barron D, Routaboul J, et al. The Arabidopsis MATE transporter TT12 acts as a vacuolar flavonoid/H+-antiporter active in proanthocyanidin-accumulating cells of the seed coat. Plant Cell. 2007;19:2023–38. https://doi.org/10.1105/tpc.106.046029.
Gomez C, Terrier N, Torregrosa L, Vialet S, Fournier-Level A, Verriès C, et al. Grapevine MATE-type proteins act as vacuolar H+-dependent acylated anthocyanin transporters. Plant Physiol. 2009;150:402–15. https://doi.org/10.1104/pp.109.135624.
Zhao J, Huhman D, Shadle G, He X, Sumner LW, Tang Y, et al. MATE2 mediates vacuolar sequestration of flavonoid glycosides and glycoside malonates in Medicago truncatula. Plant Cell. 2011;23:1536–55. https://doi.org/10.1105/tpc.110.080804.
Burla B, Pfrunder S, Nagy R, Francisco RM, Lee Y, Martinoia E. Vacuolar transport of abscisic acid glucosyl ester is mediated by ATP-binding cassette and proton-antiport mechanisms in Arabidopsis. Plant Physiol. 2013;163:1446–58. https://doi.org/10.1104/pp.113.222547.
Chanoca A, Kovinich N, Burkel B, Stecha S, Bohorquez-Restrepo A, Ueda T, et al. Anthocyanin vacuolar inclusions form by a microautophagy mechanism. Plant Cell. 2015;27:2545–59. https://doi.org/10.1105/tpc.15.00589.
Passamonti S, Cocolo A, Braidot E, Petrussa E, Peresson C, Medic N, et al. Characterization of electrogenic bromosulfophthalein transport in carnation petal microsomes and its inhibition by antibodies against bilitranslocase. FEBS J. 2005;272:3282–96. https://doi.org/10.1111/j.1742-4658.2005.04751.x.
Cutler SR, Rodriguez PL, Finkelstein RR, Abrams SR. Abscisic acid: emergence of a core signaling network. Annu Rev Plant Biol. 2010;61:651–79.
Nambara E, Marion-Poll A. Abscisic acid biosynthesis and catabolism. Annu Rev Plant Biol. 2005;56:165–85. https://doi.org/10.1146/annurev.arplant.56.032604.144046.
Xu Z, Kim DH, Hwang I. ABA homeostasis and signaling involving multiple subcellular compartments and multiple receptors. Plant Cell Rep. 2013;32:807–13. https://doi.org/10.1007/s00299-013-1396-3.
Kushiro T, Okamoto M, Nakabayashi K, Yamagishi K, Kitamura S, Asami T, et al. The Arabidopsis cytochrome P450 CYP707A encodes ABA 8′-hydroxylases: key enzymes in ABA catabolism. EMBO J. 2004;23:1647–56. https://doi.org/10.1038/sj.emboj.7600121.
Wang P, Liu H, Hua H, Wang L, Song C. A vacuole localized β-glucosidase contributes to drought tolerance in Arabidopsis. Chin Sci Bull. 2011;56:3538–46. https://doi.org/10.1007/s11434-011-4802-7.
Xu ZY, Lee KH, Dong T, Jeong JC, Jin JB, Kanno Y, et al. A vacuolar beta-glucosidase homolog that possesses glucose-conjugated abscisic acid hydrolyzing activity plays an important role in osmotic stress responses in Arabidopsis. Plant Cell. 2012;24:2184–99. https://doi.org/10.1105/tpc.112.095935.
Lätari K, Wust F, Hubner M, Schaub P, Beisel KG, Matsubara S, et al. Tissue-specific apocarotenoid glycosylation contributes to carotenoid homeostasis in Arabidopsis leaves. Plant Physiol. 2015;168:1550–62. https://doi.org/10.1104/pp.15.00243.
Bönisch F, Frotscher J, Stanitzek S, Ruhl E, Wust M, Bitz O, et al. A UDP-glucose:monoterpenol glucosyltransferase adds to the chemical diversity of the grapevine metabolome. Plant Physiol. 2014;165:561–81. https://doi.org/10.1104/pp.113.232470.
Schulenburg K, Feller A, Hoffmann T, Schecker JH, Martens S, Schwab W. Formation of β-glucogallin, the precursor of ellagic acid in strawberry and raspberry. J Exp Bot. 2016;67:2299–308. https://doi.org/10.1093/jxb/erw036.
Khater F, Fournand D, Vialet S, Meudec E, Cheynier V, Terrier N. Identification and functional characterization of cDNAs coding for hydroxybenzoate/hydroxycinnamate glucosyltransferases co-expressed with genes related to proanthocyanidin biosynthesis. J Exp Bot. 2012;63:1201–14. https://doi.org/10.1093/jxb/err340.
Mittasch J, Böttcher C, Frolova N, Bonn M, Milkowski C. Identification of UGT84A13 as a candidate enzyme for the first committed step of gallotannin biosynthesis in pedunculate oak (Quercus robur). Phytochemistry. 2014;99:44–51. https://doi.org/10.1016/j.phytochem.2013.11.023.
Ono NN, Qin X, Wilson AE, Li G, Tian L. Two UGT84 family glycosyltransferases catalyze a critical reaction of hydrolyzable tannin biosynthesis in pomegranate (Punica granatum). PLoS One. 2016;11:e0156319. https://doi.org/10.1371/journal.pone.0156319.
Park HS, Stierle A, Strobel GA. Metabolism of maculosin, a host-specific phytotoxin produced by Alternaria alternata on spotted knapweed (Centaurea maculosa). Phytochemistry. 1993;35:101–6. https://doi.org/10.1016/S0031-9422(00)90516-8.
Poppenberger B, Berthiller F, Lucyshyn D, Sieberer T, Schuhmacher R, Krska R, et al. Detoxification of the Fusarium mycotoxin deoxynivalenol by a UDP-glucosyltransferase from Arabidopsis thaliana. J Biol Chem. 2003;278:47905–14. https://doi.org/10.1074/jbc.M307552200.
Poppenberger B, Berthiller F, Bachmann H, Lucyshyn D, Peterbauer C, Mitterbauer R, et al. Heterologous expression of Arabidopsis UDP-glucosyltransferases in Saccharomyces cerevisiae for production of zearalenone-4-O-glucoside. Appl Environ Microbiol. 2006;72:4404–10. https://doi.org/10.1128/AEM.02544-05.
Berthiller F, Crews C, Dall’Asta C, Saeger SD, Haesaert G, Karlovsky P, et al. Masked mycotoxins: a review. Mol Nutr Food Res. 2013;57:165–86. https://doi.org/10.1002/mnfr.201100764.
Høj P, Pretorius I, Blair R. The Australian wine research institute, Annual Report. Eds; The Australian wine research institute: Adelaide, Australia 2003:7–38.
Kennison KR, Wilkinson KL, Williams HG, Smith JH, Gibberd MR. Smoke-derived taint in wine: effect of postharvest smoke exposure of grapes on the chemical composition and sensory characteristics of wine. J Agric Food Chem. 2007;55:10897–901. https://doi.org/10.1021/jf072509k.
Hayasaka Y, Baldock GA, Pardon KH, Jeffery DW, Herderich MJ. Investigation into the formation of guaiacol conjugates in berries and leaves of grapevine Vitis vinifera L. Cv. cabernet sauvignon using stable isotope tracers combined with HPLC-MS and MS/MS analysis. J Agric Food Chem. 2010;58:2076–81. https://doi.org/10.1021/jf903732p.
Hayasaka Y, Dungey KA, Baldock GA, Kennison KR, Wilkinson KL. Identification of a β-D-glucopyranoside precursor to guaiacol in grape juice following grapevine exposure to smoke. Anal Chim Acta. 2010;660:143–8. https://doi.org/10.1016/j.aca.2009.10.039.
Kelly D, Zerihun A, Hayasaka Y, Gibberd M. Winemaking practice affects the extraction of smoke-borne phenols from grapes into wines. Aust J Grape Wine Res. 2014;20:386–93.
Mayr CM, Parker M, Baldock GA, Black CA, Pardon KH, Williamson PO, et al. Determination of the importance of in-mouth release of volatile phenol glycoconjugates to the flavor of smoke-tainted wines. J Agric Food Chem. 2014;62:2327–36. https://doi.org/10.1021/jf405327s.
Parker M, Osidacz P, Baldock GA, Hayasaka Y, Black CA, Pardon KH, et al. Contribution of several volatile phenols and their glycoconjugates to smoke-related sensory properties of red wine. J Agric Food Chem. 2012;60:2629–37. https://doi.org/10.1021/jf2040548.
Hellfritsch C, Brockhoff A, Stähler F, Meyerhof W, Hofmann T. Human psychometric and taste receptor responses to steviol glycosides. J Agric Food Chem. 2012;60:6782–93. https://doi.org/10.1021/jf301297n.
Wang Y, Chen L, Li Y, Li Y, Yan M, Chen K, et al. Efficient enzymatic production of rebaudioside A from stevioside. Biosci Biotechnol Biochem. 2016;80:67–73. https://doi.org/10.1080/09168451.2015.1072457.
Olsson K, Carlsen S, Semmler A, Simón E, Mikkelsen MD, Møller BL. Microbial production of next-generation stevia sweeteners. Microb Cell Factories. 2016;15:207. https://doi.org/10.1186/s12934-016-0609-1.
Yang Y, Huang S, Han Y, Yuan H, Gu C, Zhao Y. Base substitution mutations in uridinediphosphate-dependent glycosyltransferase 76G1 gene of Stevia rebaudiana causes the low levels of rebaudioside A: mutations in UGT76G1, a key gene of steviol glycosides synthesis. Plant Physiol Biochem. 2014;80:220–5. https://doi.org/10.1016/j.plaphy.2014.04.005.
Madhav H, Bhasker S, Chinnamma M. Functional and structural variation of uridine diphosphate glycosyltransferase (UGT) gene of Stevia rebaudiana–UGTSr involved in the synthesis of rebaudioside A. Plant Physiol Biochem. 2013;63:245–53. https://doi.org/10.1016/j.plaphy.2012.11.029.
Li Y, Li Y, Wang Y, Chen L, Yan M, Chen K, et al. Production of rebaudioside A from stevioside catalyzed by the engineered Saccharomyces cerevisiae. Appl Biochem Biotechnol. 2016;178:1586–98. https://doi.org/10.1007/s12010-015-1969-4.
Demchenko AV. General aspects of the glycosidic bond formation. In: Demchenko AV, editor. Handbook of chemical glycosylation: advances in stereoselectivity and therapeutic relevance. Weinheim: Wiley-VCH Verlag GmbH & Co. KGaA; 2008. p. 1–27.
Koenigs W, Knorr E. Ueber einige Derivate des Traubenzuckers und der Galactose. Ber Dtsch Chem Ges. 1901;34:957–81. https://doi.org/10.1002/cber.190103401162.
Křen V, Thiem J. Glycosylation employing bio-systems: from enzymes to whole cells. Chem Soc Rev. 1997;26:463–73. https://doi.org/10.1039/CS9972600463.
Lim E. Plant glycosyltransferases: their potential as novel biocatalysts. Chem Eur J. 2005;11:5486–94. https://doi.org/10.1002/chem.200500115.
Sinnott ML. Catalytic mechanism of enzymic glycosyl transfer. Chem Rev. 1990;90:1171–202. https://doi.org/10.1021/cr00105a006.
Campbell JA, Davies GJ, Bulone V, Henrissat B. A classification of nucleotide-diphospho-sugar glycosyltransferases based on amino acid sequence similarities. Biochem J. 1997;326:929–39.
Coutinho PM, Deleury E, Davies GJ, Henrissat B. An evolving hierarchical family classification for glycosyltransferases. J Mol Biol. 2003;328:307–17. https://doi.org/10.1016/S0022-2836(03)00307-3.
Lairson LL, Henrissat B, Davies GJ, Withers SG. Glycosyltransferases: structures, functions, and mechanisms. Annu Rev Biochem. 2008;77:521–55. https://doi.org/10.1146/annurev.biochem.76.061005.092322.
Ekstrom A, Taujale R, McGinn N, Yin Y. PlantCAZyme: a database for plant carbohydrate-active enzymes. Database (Oxford). 2014. https://doi.org/10.1093/database/bau079.
Mackenzie PI, Rogers A, Treloar J, Jorgensen BR, Miners JO, Meech R. Identification of UDP glycosyltransferase 3A1 as a UDP N-acetylglucosaminyltransferase. J Biol Chem. 2008;283:36205–10. https://doi.org/10.1074/jbc.M807961200.
Shibuya M, Nishimura K, Yasuyama N, Ebizuka Y. Identification and characterization of glycosyltransferases involved in the biosynthesis of soyasaponin I in Glycine max. FEBS Lett. 2010;584:2258–64. https://doi.org/10.1016/j.febslet.2010.03.037.
Han SH, Kim BG, Yoon JA, Chong Y, Ahn J. Synthesis of flavonoid O-pentosides by Escherichia coli through engineering of nucleotide sugar pathways and glycosyltransferase. Appl Environ Microbiol. 2014;80:2754–62. https://doi.org/10.1128/AEM.03797-13.
Montefiori M, Espley RV, Stevenson D, Cooney J, Datson PM, Saiz A, et al. Identification and characterisation of F3GT1 and F3GGT1, two glycosyltransferases responsible for anthocyanin biosynthesis in red-fleshed kiwifruit (Actinidia chinensis). Plant J. 2011;65:106–18. https://doi.org/10.1111/j.1365-313X.2010.04409.x.
Unligil UM, Zhou S, Yuwaraj S, Sarkar M, Schachter H, Rini JM. X-ray crystal structure of rabbit N-acetylglucosaminyltransferase I: catalytic mechanism and a new protein superfamily. EMBO J. 2000;19:5269–80. https://doi.org/10.1093/emboj/19.20.5269.
Hiromoto T, Honjo E, Tamada T, Noda N, Kazuma K, Suzuki M, et al. Crystal structure of UDP-glucose:anthocyanidin 3-O-glucosyltransferase from Clitoria ternatea. J Synchrotron Radiat. 2013;20:894–8. https://doi.org/10.1107/S0909049513020712.
Lizak C, Gerber S, Numao S, Aebi M, Locher KP. X-ray structure of a bacterial oligosaccharyltransferase. Nature. 2011;474:350–5. https://doi.org/10.1038/nature10151.
Zhang H, Zhu F, Yang T, Ding L, Zhou M, Li J, et al. The highly conserved domain of unknown function 1792 has a distinct glycosyltransferase fold. Nat Commun. 2014;5:4339. https://doi.org/10.1038/ncomms5339.
Liang D, Liu J, Wu H, Wang B, Zhu H, Qiao J. Glycosyltransferases: mechanisms and applications in natural product development. Chem Soc Rev. 2015;44:8350–74. https://doi.org/10.1039/c5cs00600g.
Busch C, Hofmann F, Selzer J, Munro S, Jeckel D, Aktories K. A common motif of eukaryotic glycosyltransferases is essential for the enzyme activity of large clostridial cytotoxins. J Biol Chem. 1998;273:19566–72. https://doi.org/10.1074/jbc.273.31.19566.
Charnock SJ, Davies GJ. Structure of the nucleotide-diphospho-sugar transferase, SpsA from Bacillus subtilis, in native and nucleotide-complexed forms. Biochemistry. 1999;38:6380–5. https://doi.org/10.1021/bi990270y.
Vrielink A, Rüger W, Driessen HP, Freemont PS. Crystal structure of the DNA modifying enzyme beta-glucosyltransferase in the presence and absence of the substrate uridine diphosphoglucose. EMBO J. 1994;13:3413–22.
Morera S, Lariviere L, Kurzeck J, Aschke-Sonnenborn U, Freemont PS, Janin J, et al. High resolution crystal structures of T4 phage β-glucosyltransferase: induced fit and effect of substrate and metal binding. J Mol Biol. 2001;311:569–77. https://doi.org/10.1006/jmbi.2001.4905.
Lovering AL, de Castro LH, Liza H, Lim D, Strynadka NCJ. Structural insight into the transglycosylation step of bacterial cell-wall biosynthesis. Science. 2007;315:1402–5. https://doi.org/10.1126/science.1136611.
Yuan Y, Barrett D, Zhang Y, Kahne D, Sliz P, Walker S. Crystal structure of a peptidoglycan glycosyltransferase suggests a model for processive glycan chain synthesis. Proc Natl Acad Sci U S A. 2007;104:5348–53. https://doi.org/10.1073/pnas.0701160104.
Igura M, Maita N, Kamishikiryo J, Yamada M, Obita T, Maenaka K, et al. Structure-guided identification of a new catalytic motif of oligosaccharyltransferase. EMBO J. 2008;27:234–43. https://doi.org/10.1038/sj.emboj.7601940.
Bowles D, Lim E, Poppenberger B, Vaistij FE. Glycosyltransferases of lipophilic small molecules. Annu Rev Plant Biol. 2006;57:567–97. https://doi.org/10.1146/annurev.arplant.57.032905.105429.
Gachon CMM, Langlois-Meurinne M, Saindrenan P. Plant secondary metabolism glycosyltransferases: the emerging functional analysis. Trends Plant Sci. 2005;10:542–9. https://doi.org/10.1016/j.tplants.2005.09.007.
Hans J, Brandt W, Vogt T. Site-directed mutagenesis and protein 3D-homology modelling suggest a catalytic mechanism for UDP-glucose-dependent betanidin 5-O-glucosyltransferase from Dorotheanthus bellidiformis. Plant J. 2004;39:319–33. https://doi.org/10.1111/j.1365-313X.2004.02133.x.
Kubo A, Arai Y, Nagashima S, Yoshikawa T. Alteration of sugar donor specificities of plant glycosyltransferases by a single point mutation. Arch Biochem Biophys. 2004;429:198–203. https://doi.org/10.1016/j.abb.2004.06.021.
Dixon RA, Achnine L, Kota P, Liu C, Reddy MSS, Wang L. The phenylpropanoid pathway and plant defence-a genomics perspective. Mol Plant Pathol. 2002;3:371–90. https://doi.org/10.1046/j.1364-3703.2002.00131.x.
Umezawa T. The cinnamate/monolignol pathway. Phytochem Rev. 2010;9:1–17. https://doi.org/10.1007/s11101-009-9155-3.
Mandal SM, Chakraborty D, Dey S. Phenolic acids act as signaling molecules in plant-microbe symbioses. Plant Signal Behav. 2010;5:359–68. https://doi.org/10.4161/psb.5.4.10871.
Ferrer J, Austin MB, Stewart C Jr, Noel JP. Structure and function of enzymes involved in the biosynthesis of phenylpropanoids. Plant Physiol Biochem. 2008;46:356–70. https://doi.org/10.1016/j.plaphy.2007.12.009.
Castelluccio C, Paganga G, Melikian N, Paul Bolwell G, Pridham J, Sampson J, et al. Antioxidant potential of intermediates in phenylpropanoid metabolism in higher plants. FEBS Lett. 1995;368:188–92. https://doi.org/10.1016/0014-5793(95)00639-Q.
Barber MS, McConnell VS, DeCaux BS. Antimicrobial intermediates of the general phenylpropanoid and lignin specific pathways. Phytochemistry. 2000;54:53–6. https://doi.org/10.1016/S0031-9422(00)00038-8.
Bruyere C, Genovese S, Lallemand B, Ionescu-Motatu A, Curini M, Kiss R, et al. Growth inhibitory activities of oxyprenylated and non-prenylated naturally occurring phenylpropanoids in cancer cell lines. Bioorg Med Chem Lett. 2011;21:4174–9. https://doi.org/10.1016/j.bmcl.2011.05.089.
Lunkenbein S, Bellido M, Aharoni A, Salentijn EMJ, Kaldenhoff R, Coiner HA, et al. Cinnamate metabolism in ripening fruit. Characterization of a UDP-glucose:cinnamate glucosyltransferase from strawberry. Plant Physiol. 2006;140:1047–58. https://doi.org/10.1104/pp.105.074955.
Cui L, Yao S, Dai X, Yin Q, Liu Y, Jiang X, et al. Identification of UDP-glycosyltransferases involved in the biosynthesis of astringent taste compounds in tea (Camellia sinensis). J Exp Bot. 2016;67:2285–97. https://doi.org/10.1093/jxb/erw053.
Lin J, Huang X, Li Q, Cao Y, Bao Y, Meng X, et al. UDP-glycosyltransferase 72B1 catalyzes the glucose conjugation of monolignols and is essential for the normal cell wall lignification in Arabidopsis thaliana. Plant J. 2016;n/a. https://doi.org/10.1111/tpj.13229.
Wang Y, Wang W, Jin S, Wang J, Wang B, Hou B. Over-expression of a putative poplar glycosyltransferase gene, PtGT1, in tobacco increases lignin content and causes early flowering. J Exp Bot. 2012;63:2799–808. https://doi.org/10.1093/jxb/ers001.
Le Roy J, Huss B, Creach A, Hawkins S, Neutelings G. Glycosylation is a major regulator of phenylpropanoid availability and biological activity in plants. Front Plant Sci. 2016;7:132. https://doi.org/10.3389/fpls.2016.00735.
Chong J, Baltz R, Schmitt C, Beffa R, Fritig B, Saindrenan P. Downregulation of a pathogen-responsive tobacco UDP-glc:phenylpropanoid glucosyltransferase reduces scopoletin glucoside accumulation, enhances oxidative stress, and weakens virus resistance. Plant Cell. 2002;14:1093–107. https://doi.org/10.1105/tpc.010436.
Kanoh H, Kawauchi M, Kuroyanagi M, Arima T. Molecular cloning and characterization of coumarin glucosyltransferase in hairy roots of Pharbitis nil (Ipomoea nil). Plant Biotechnol. 2014;31:21–8. https://doi.org/10.5511/plantbiotechnology.13.1203a.
Griesser M, Hoffmann T, Bellido ML, Rosati C, Fink B, Kurtzer R, et al. Redirection of flavonoid biosynthesis through the down-regulation of an anthocyanidin glucosyltransferase in ripening strawberry fruit. Plant Physiol. 2008;146:1528–39. https://doi.org/10.1104/pp.107.114280.
He F, Chen W, Yu K, Ji X, Duan C, Reeves MJ, et al. Molecular and biochemical characterization of the UDP-glucose: anthocyanin 5-O-glucosyltransferase from Vitis amurensis. Phytochemistry. 2015;117:363–72. https://doi.org/10.1016/j.phytochem.2015.06.023.
Jánváry L, Hoffmann T, Pfeiffer J, Hausmann L, Töpfer R, Fischer TC, et al. A double mutation in the anthocyanin 5-O-glucosyltransferase gene disrupts enzymatic activity in Vitis vinifera L. J Agric Food Chem. 2009;57:3512–8. https://doi.org/10.1021/jf900146a.
Wang L, Han W, Xie C, Hou J, Fang Q, Gu J, et al. Comparing the acceptor promiscuity of a Rosa hybrida glucosyltransferase RhGT1 and an engineered microbial glucosyltransferase OleDPSA toward a small flavonoid library. Carbohydr Res. 2013;368:73–7. https://doi.org/10.1016/j.carres.2012.12.012.
Gosch C, Halbwirth H, Stich K. Phloridzin: biosynthesis, distribution and physiological relevance in plants. Phytochemistry. 2010;71:838–43. https://doi.org/10.1016/j.phytochem.2010.03.003.
Gosch C, Halbwirth H, Schneider B, Hölscher D, Stich K. Cloning and heterologous expression of glycosyltransferases from Malus x domestica and Pyrus communis, which convert phloretin to phloretin 2′-O-glucoside (phloridzin). Plant Sci. 2010;178:299–306. https://doi.org/10.1016/j.plantsci.2009.12.009.
Zhang T, Liang J, Wang P, Xu Y, Wang Y, Wei X, et al. Purification and characterization of a novel phloretin-2′-O-glycosyltransferase favoring phloridzin biosynthesis. Sci Rep. 2016;6:35274. https://doi.org/10.1038/srep35274.
Jugdé H, Nguy D, Moller I, Cooney JM, Atkinson RG. Isolation and characterization of a novel glycosyltransferase that converts phloretin to phlorizin, a potent antioxidant in apple. FEBS J. 2008;275:3804–14. https://doi.org/10.1111/j.1742-4658.2008.06526.x.
Gutmann A, Bungaruang L, Weber H, Leypold M, Breinbauer R, Nidetzky B. Towards the synthesis of glycosylated dihydrochalcone natural products using glycosyltransferase-catalysed cascade reactions. Green Chem. 2014;16:4417–25. https://doi.org/10.1039/C4GC00960F.
Yahyaa M, Davidovich-Rikanati R, Eyal Y, Sheachter A, Marzouk S, Lewinsohn E, et al. Identification and characterization of UDP-glucose: phloretin 4′-O-glycosyltransferase from Malus x domestica Borkh. Phytochemistry. 2016;130:47–55. https://doi.org/10.1016/j.phytochem.2016.06.004.
Song C, Ring L, Hoffmann T, Huang F, Slovin JP, Schwab W. Acylphloroglucinol biosynthesis in strawberry fruit. Plant Physiol. 2015. https://doi.org/10.1104/pp.15.00794.
Song C, Zhao S, Hong X, Liu J, Schulenburg K, Schwab W. A UDP-glucosyltransferase functions in both acylphloroglucinol glucoside and anthocyanin biosynthesis in strawberry (Fragaria × ananassa). Plant J. 2016;85:730–42. https://doi.org/10.1111/tpj.13140.
Song C, Hong X, Zhao S, Liu J, Schulenburg K, Huang F, et al. Glucosylation of 4-hydroxy-2,5-dimethyl-3(2H)-furanone, the key strawberry flavor compound in strawberry fruit. Plant Physiol. 2016;171:139–51. https://doi.org/10.1104/pp.16.00226.
Kiselev KV, Aleynova OA, Grigorchuk VP, Dubrovina AS. Stilbene accumulation and expression of stilbene biosynthesis pathway genes in wild grapevine Vitis amurensis Rupr. Planta. 2016:1–9. https://doi.org/10.1007/s00425-016-2598-z.
Mozafari M, Nekooeian AA, Mashghoolozekr E, Panjeshahin MR. The cardioprotective effects of resveratrol in rats with simultaneous type 2 diabetes and renal hypertension. Nat Prod Commun. 2015;10:335–8.
Ozaki S, Imai H, Iwakiri T, Sato T, Shimoda K, Nakayama T, et al. Regioselective glucosidation of trans-resveratrol in Escherichia coli expressing glucosyltransferase from Phytolacca americana. Biotechnol Lett. 2012;34:475–81. https://doi.org/10.1007/s10529-011-0784-4.
Hall D, de Luca V. Mesocarp localization of a bi-functional resveratrol/hydroxycinnamic acid glucosyltransferase of concord grape (Vitis labrusca). Plant J. 2007;49:579–91. https://doi.org/10.1111/j.1365-313X.2006.02987.x.
Kaminaga Y, Sahin FP, Mizukami H. Molecular cloning and characterization of a glucosyltransferase catalyzing glucosylation of curcumin in cultured Catharanthus roseus cells. FEBS Lett. 2004;567:197–202. https://doi.org/10.1016/j.febslet.2004.04.056.
Oguchi Y, Masada S, Kondo T, Terasaka K, Mizukami H. Purification and characterization of UDP-glucose: curcumin glucoside 1,6-glucosyltransferase from Catharanthus roseus cell suspension cultures. Plant Cell Physiol. 2007;48:1635–43. https://doi.org/10.1093/pcp/pcm138.
Schwab W, Davidovich-Rikanati R, Lewinsohn E. Biosynthesis of plant-derived flavor compounds. Plant J. 2008;54:712–32. https://doi.org/10.1111/j.1365-313X.2008.03446.x.
Gershenzon J, Dudareva N. The function of terpene natural products in the natural world. Nat Chem Biol. 2007;3:408–14. https://doi.org/10.1038/nchembio.2007.5.
Chen W, Viljoen AM. Geraniol — a review of a commercially important fragrance material. S Afr J Bot. 2010;76:643–51. https://doi.org/10.1016/j.sajb.2010.05.008.
Bönisch F, Frotscher J, Stanitzek S, Ruhl E, Wust M, Bitz O, et al. Activity-based profiling of a physiologic aglycone library reveals sugar acceptor promiscuity of family 1 UDP-glucosyltransferases from grape. Plant Physiol. 2014;166:23–39. https://doi.org/10.1104/pp.114.242578.
Yauk Y, Ged C, Wang MY, Matich AJ, Tessarotto L, Cooney JM, et al. Manipulation of flavour and aroma compound sequestration and release using a glycosyltransferase with specificity for terpene alcohols. Plant J. 2014;80:317–30. https://doi.org/10.1111/tpj.12634.
Ohgami S, Ono E, Horikawa M, Murata J, Totsuka K, Toyonaga H, et al. Volatile glycosylation in tea plants: sequential glycosylations for the biosynthesis of aroma β-primeverosides are catalyzed by two Camellia sinensis glycosyltransferases. Plant Physiol. 2015;168:464–77. https://doi.org/10.1104/pp.15.00403.
Dong T, Hwang I. Contribution of ABA UDP-glucosyltransferases in coordination of ABA biosynthesis and catabolism for ABA homeostasis. Plant Signal Behav. 2014;9:e28888. https://doi.org/10.4161/psb.28888.
Poppenberger B, Fujioka S, Soeno K, George GL, Vaistij FE, Hiranuma S, et al. The UGT73C5 of Arabidopsis thaliana glucosylates brassinosteroids. Proc Natl Acad Sci U S A. 2005;102:15253–8. https://doi.org/10.1073/pnas.0504279102.
Husar S, Berthiller F, Fujioka S, Rozhon W, Khan M, Kalaivanan F, et al. Overexpression of the UGT73C6 alters brassinosteroid glucoside formation in Arabidopsis thaliana. BMC Plant Biol. 2011;11:51. https://doi.org/10.1186/1471-2229-11-51.
Kudo T, Makita N, Kojima M, Tokunaga H, Sakakibara H. Cytokinin activity of cis-zeatin and phenotypic alterations induced by overexpression of putative cis-zeatin-O-glucosyltransferase in rice. Plant Physiol. 2012;160:319–31. https://doi.org/10.1104/pp.112.196733.
Tognetti VB, van Aken O, Morreel K, Vandenbroucke K, van de Cotte B, de Clercq I, et al. Perturbation of indole-3-butyric acid homeostasis by the UDP-glucosyltransferase UGT74E2 modulates Arabidopsis architecture and water stress tolerance. Plant Cell. 2010;22:2660–79. https://doi.org/10.1105/tpc.109.071316.
Liu Z, Yan J, Li D, Luo Q, Yan Q, Liu Z, et al. UDP-glucosyltransferase71C5, a major glucosyltransferase, mediates abscisic acid homeostasis in Arabidopsis. Plant Physiol. 2015;167:1659–70. https://doi.org/10.1104/pp.15.00053.
Suzuki H, Hayase H, Nakayama A, Yamaguchi I, Asami T, Nakajima M. Identification and characterization of an Ipomoea nil glucosyltransferase which metabolizes some phytohormones. Biochem Biophys Res Commun. 2007;361:980–6. https://doi.org/10.1016/j.bbrc.2007.07.147.
Noguchi A, Kunikane S, Homma H, Liu W, Sekiya T, Hosoya M, et al. Identification of an inducible glucosyltransferase from Phytolacca americana L. cells that are capable of glucosylating capsaicin. Plant Biotechnol. 2009;26:285–92. https://doi.org/10.5511/plantbiotechnology.26.285.
Dick R, Rattei T, Haslbeck M, Schwab W, Gierl A, Frey M. Comparative analysis of benzoxazinoid biosynthesis in monocots and dicots: independent recruitment of stabilization and activation functions. Plant Cell. 2012;24:915–28. https://doi.org/10.1105/tpc.112.096461.
Sue M, Nakamura C, Nomura T. Dispersed benzoxazinone gene cluster: molecular characterization and chromosomal localization of glucosyltransferase and glucosidase genes in wheat and rye. Plant Physiol. 2011;157:985–97. https://doi.org/10.1104/pp.111.182378.
Schwab W. Natural 4-hydroxy-2,5-dimethyl-3(2H)-furanone (Furaneol(R)). Molecules. 2013;18:6936–51. https://doi.org/10.3390/molecules18066936.
Gandia-Herrero F, Lorenz A, Larson T, Graham IA, Bowles DJ, Rylott EL, et al. Detoxification of the explosive 2,4,6-trinitrotoluene in Arabidopsis: discovery of bifunctional O- and C-glucosyltransferases. Plant J. 2008;56:963–74. https://doi.org/10.1111/j.1365-313X.2008.03653.x.
Brazier-Hicks M, Evans KM, Gershater MC, Puschmann H, Steel PG, Edwards R. The C-glycosylation of flavonoids in cereals. J Biol Chem. 2009;284:17926–34. https://doi.org/10.1074/jbc.M109.009258.
Landmann C, Fink B, Schwab W. FaGT2: a multifunctional enzyme from strawberry (Fragaria × ananassa) fruits involved in the metabolism of natural and xenobiotic compounds. Planta. 2007;226:417–28. https://doi.org/10.1007/s00425-007-0492-4.
Yu B, Sun J, Yang X. Assembly of naturally occurring glycosides, evolved tactics, and glycosylation methods. Acc Chem Res. 2012;45:1227–36. https://doi.org/10.1021/ar200296m.
Grynkiewicz G, Szeja W. Synthetic glycosides and glycoconjugates of low molecular weight natural products. Curr Pharm Des. 2016;22:1592–627.
Thibodeaux CJ, Melançon CE, Liu H. Natural-product sugar biosynthesis and enzymatic glycodiversification. Angew Chem Int Ed. 2008;47:9814–59. https://doi.org/10.1002/anie.200801204.
Masada S, Kawase Y, Nagatoshi M, Oguchi Y, Terasaka K, Mizukami H. An efficient chemoenzymatic production of small molecule glucosides with in situ UDP-glucose recycling. FEBS Lett. 2007;581:2562–6. https://doi.org/10.1016/j.febslet.2007.04.074.
Terasaka K, Mizutani Y, Nagatsu A, Mizukami H. In situ UDP-glucose regeneration unravels diverse functions of plant secondary product glycosyltransferases. FEBS Lett. 2012;586:4344–50. https://doi.org/10.1016/j.febslet.2012.10.045.
Huang FC, Hinkelmann J, Hermenau A, Schwab W. Enhanced production of β-glucosides by in-situ UDP-glucose regeneration. J Biotechnol. 2016;224:35–44. https://doi.org/10.1016/j.jbiotec.2016.02.022.
de Bruyn F, van Brempt M, Maertens J, van Bellegem W, Duchi D, de Mey M. Metabolic engineering of Escherichia coli into a versatile glycosylation platform: production of bio-active quercetin glycosides. Microb Cell Factories. 2015;14:138. https://doi.org/10.1186/s12934-015-0326-1.
Wang H, Yang Y, Lin L, Zhou W, Liu M, Cheng K, et al. Engineering Saccharomyces cerevisiae with the deletion of endogenous glucosidases for the production of flavonoid glucosides. Microb Cell Factories. 2016;15:134. https://doi.org/10.1186/s12934-016-0535-2.
Rivas F, Parra A, Martinez A, Garcia-Granados A. Enzymatic glycosylation of terpenoids. Phytochem Rev. 2013;12:327–39. https://doi.org/10.1007/s11101-013-9301-9.
Caputi L, Lim E, Bowles DJ. Discovery of new biocatalysts for the glycosylation of terpenoid scaffolds. Chem Eur J. 2008;14:6656–62. https://doi.org/10.1002/chem.200800548.
Lim CG, Wong L, Bhan N, Dvora H, Xu P, Venkiteswaran S, et al. Development of a recombinant Escherichia coli strain for overproduction of the plant pigment anthocyanin. Appl Environ Microbiol. 2015;81:6276–84. https://doi.org/10.1128/AEM.01448-15.
Thuan NH, Park JW, Sohng JK. Toward the production of flavone-7-O-β-D-glucopyranosides using Arabidopsis glycosyltransferase in Escherichia coli. Process Biochem. 2013;48:1744–8. https://doi.org/10.1016/j.procbio.2013.07.005.
Kim SY, Lee HR, Park K, Kim B, Ahn J. Metabolic engineering of Escherichia coli for the biosynthesis of flavonoid-O-glucuronides and flavonoid-O-galactoside. Appl Microbiol Biotechnol. 2015;99:2233–42. https://doi.org/10.1007/s00253-014-6282-6.
Pandey RP, Malla S, Simkhada D, Kim B, Sohng JK. Production of 3-O-xylosyl quercetin in Escherichia coli. Appl Microbiol Biotechnol. 2013;97:1889–901. https://doi.org/10.1007/s00253-012-4438-9.
Pei J, Dong P, Wu T, Zhao L, Fang X, Cao F, et al. Metabolic engineering of Escherichia coli for astragalin biosynthesis. J Agric Food Chem. 2016;64:7966–72. https://doi.org/10.1021/acs.jafc.6b03447.
Kim B, Kim HJ, Ahn J. Production of bioactive flavonol rhamnosides by expression of plant genes in Escherichia coli. J Agric Food Chem. 2012;60:11143–8. https://doi.org/10.1021/jf302123c.
Yoon J, Kim B, Lee WJ, Lim Y, Chong Y, Ahn J. Production of a novel quercetin glycoside through metabolic engineering of Escherichia coli. Appl Environ Microbiol. 2012;78:4256–62. https://doi.org/10.1128/AEM.00275-12.
Kim B, Sung SH, Ahn J. Biological synthesis of quercetin 3-O-N-acetylglucosamine conjugate using engineered Escherichia coli expressing UGT78D2. Appl Microbiol Biotechnol. 2012;93:2447–53. https://doi.org/10.1007/s00253-011-3747-8.
Malla S, Pandey RP, Kim B, Sohng JK. Regiospecific modifications of naringenin for astragalin production in Escherichia coli. Biotechnol Bioeng. 2013;110:2525–35. https://doi.org/10.1002/bit.24919.
Yan Y, Li Z, Koffas MAG. High-yield anthocyanin biosynthesis in engineered Escherichia coli. Biotechnol Bioeng. 2008;100:126–40. https://doi.org/10.1002/bit.21721.
Werner SR, Morgan JA. Expression of a Dianthus flavonoid glucosyltransferase in Saccharomyces cerevisiae for whole-cell biocatalysis. J Biotechnol. 2009;142:233–41. https://doi.org/10.1016/j.jbiotec.2009.05.008.
Ito T, Fujimoto S, Shimosaka M, Taguchi G. Production of C-glucosides of flavonoids and related compounds by Escherichia coli expressing buckwheat C-glucosyltransferase. Plant Biotechnol. 2014;advpub. doi:https://doi.org/10.5511/plantbiotechnology.14.1016a.
Yan Y, Chemler J, Huang L, Martens S, Koffas MAG. Metabolic engineering of anthocyanin biosynthesis in Escherichia coli. Appl Environ Microbiol. 2005;71:3617–23. https://doi.org/10.1128/AEM.71.7.3617-3623.2005.
Drăgan C, Buchheit D, Bischoff D, Ebner T, Bureik M. Glucuronide production by whole-cell biotransformation using genetically engineered fission yeast Schizosaccharomyces pombe. Drug Metab Dispos. 2010;38:509. https://doi.org/10.1124/dmd.109.030965.
Hansen EH, Møller BL, Kock GR, Bünner CM, Kristensen C, Jensen OR, et al. De novo biosynthesis of vanillin in fission yeast (Schizosaccharomyces pombe) and baker’s yeast (Saccharomyces cerevisiae). Appl Environ Microbiol. 2009;75:2765–74. https://doi.org/10.1128/AEM.02681-08.
Xue F, Guo H, Hu Y, Liu R, Huang L, Lv H, et al. Expression of codon-optimized plant glycosyltransferase UGT72B14 in Escherichia coli enhances salidroside production. Biomed Res Int. 2016:9845927. https://doi.org/10.1155/2016/9845927.
Parajuli P, Pandey RP, Trang NTH, Chaudhary AK, Sohng JK. Synthetic sugar cassettes for the efficient production of flavonol glycosides in Escherichia coli. Microb Cell Factories. 2015;14:76. https://doi.org/10.1186/s12934-015-0261-1.
Wei W, Wang P, Wei Y, Liu Q, Yang C, Zhao G, et al. Characterization of Panax ginseng UDP-glycosyltransferases catalyzing protopanaxatriol and biosyntheses of bioactive ginsenosides F1 and Rh1 in metabolically engineered yeasts. Mol Plant. 2015;8:1412–24. https://doi.org/10.1016/j.molp.2015.05.010.
Wang P, Wei Y, Fan Y, Liu Q, Wei W, Yang C, et al. Production of bioactive ginsenosides Rh2 and Rg3 by metabolically engineered yeasts. Metab Eng. 2015;29:97–105. https://doi.org/10.1016/j.ymben.2015.03.003.
de Bruyn F, de Paepe B, Maertens J, Beauprez J, de Cocker P, Mincke S, et al. Development of an in vivo glucosylation platform by coupling production to growth: production of phenolic glucosides by a glycosyltransferase of Vitis vinifera. Biotechnol Bioeng. 2015;112:1594–603. https://doi.org/10.1002/bit.25570.
Pandey RP, Li TF, Kim E, Yamaguchi T, Park YI, Kim JS, et al. Enzymatic synthesis of novel phloretin glucosides. Appl Environ Microbiol. 2013;79:3516–21. https://doi.org/10.1128/AEM.00409-13.
Pandey RP, Parajuli P, Koirala N, Lee JH, Park YI, Sohng JK. Glucosylation of isoflavonoids in engineered Escherichia coli. Mol Cells. 2014;37:172–7. 10.14348/molcells.2014.2348.
Straathof AJJ, Panke S, Schmid A. The production of fine chemicals by biotransformations. Curr Opin Biotechnol. 2002;13:548–56. https://doi.org/10.1016/S0958-1669(02)00360-9.
Takors R. Scale-up of microbial processes: impacts, tools and open questions. J Biotechnol. 2012;160:3–9. https://doi.org/10.1016/j.jbiotec.2011.12.010.
Rosano GL, Ceccarelli EA. Recombinant protein expression in Escherichia coli: advances and challenges. Front Microbiol. 2014;5:172. https://doi.org/10.3389/fmicb.2014.00172.
Kim BG, Yang SM, Kim SY, Cha MN, Ahn J. Biosynthesis and production of glycosylated flavonoids in Escherichia coli: current state and perspectives. Appl Microbiol Biotechnol. 2015;99:2979–88. https://doi.org/10.1007/s00253-015-6504-6.
Katsuragi H, Shimoda K, Kubota N, Nakajima N, Hamada H, Hamada H. Biotransformation of cinnamic acid, p-coumaric acid, caffeic acid, and ferulic acid by plant cell cultures of Eucalyptus perriniana. Biosci Biotechnol Biochem. 2010;74:1920–4. https://doi.org/10.1271/bbb.100335.
Shimoda K, Harada T, Hamada H, Nakajima N, Hamada H. Biotransformation of raspberry ketone and zingerone by cultured cells of Phytolacca americana. Phytochemistry. 2007;68:487–92. https://doi.org/10.1016/j.phytochem.2006.11.030.
Kwon S, Shimoda K, Hamada H, Ishihara K, Masuoka N. High production of β-thujaplicin glycosides by immobilized plant cells of Nicotiana tabacum. Acta Biol Hung. 2008;59:347–55. https://doi.org/10.1556/ABiol.59.2008.3.8.
Jones JA, Koffas, M. A. G. Optimizing metabolic pathways for the improved production of natural products, chap. 8. In: Sarah E. O’Connor, editor. Methods in enzymology: synthetic biology and metabolic engineering in plants and microbes part A: metabolism in microbes. Academic; 2016. p. 179–193. doi:https://doi.org/10.1016/bs.mie.2016.02.010.
Williams GJ, Zhang C, Thorson JS. Expanding the promiscuity of a natural-product glycosyltransferase by directed evolution. Nat Chem Biol. 2007;3:657–62. https://doi.org/10.1038/nchembio.2007.28.
Brazier-Hicks M, Offen WA, Gershater MC, Revett TJ, Lim E, Bowles DJ, et al. Characterization and engineering of the bifunctional N- and O-glucosyltransferase involved in xenobiotic metabolism in plants. Proc Natl Acad Sci U S A. 2007;104:20238–43. https://doi.org/10.1073/pnas.0706421104.
Choi SH, Ryu M, Yoon YJ, Kim D, Lee EY. Glycosylation of various flavonoids by recombinant oleandomycin glycosyltransferase from Streptomyces antibioticus in batch and repeated batch modes. Biotechnol Lett. 2012;34:499–505. https://doi.org/10.1007/s10529-011-0789-z.
Leonard E, Yan Y, Fowler ZL, Li Z, Lim C, Lim K, et al. Strain improvement of recombinant Escherichia coli for efficient production of plant flavonoids. Mol Pharm. 2008;5:257–65. https://doi.org/10.1021/mp7001472.
Rowan DD. Volatile metabolites. Meta. 2011;1:41–63.
Marks LE, Veldhuizen MG, Shepard TG, Shavit AY. Detecting gustatory–olfactory flavor mixtures: models of probability summation. Chem Senses. 2012;37:263–77. https://doi.org/10.1093/chemse/bjr103.
Dunkel A, Steinhaus M, Kotthoff M, Nowak B, Krautwurst D, Schieberle P, et al. Nature’s chemical signatures in human olfaction: a foodborne perspective for future biotechnology. Angew Chem Int Ed. 2014;53:7124–43. https://doi.org/10.1002/anie.201309508.
Caputi L, Aprea E. Use of terpenoids as natural flavouring compounds in food industry. Recent Pat Food Nutr Agric. 2011;3:9–16. https://doi.org/10.1016/j.tibtech.2005.02.003.
Geuns JMC. Stevioside. Phytochemistry. 2003;64:913–21. https://doi.org/10.1016/S0031-9422(03)00426-6.
Richman A, Swanson A, Humphrey T, Chapman R, McGarvey B, Pocs R, et al. Functional genomics uncovers three glucosyltransferases involved in the synthesis of the major sweet glucosides of Stevia rebaudiana. Plant J. 2005;41:56–67. https://doi.org/10.1111/j.1365-313X.2004.02275.x.
Shibata H, Sonoke S, Ochiai H, Nishihashi H, Yamada M. Glucosylation of steviol and steviol-glucosides in extracts from Stevia rebaudiana Bertoni. Plant Physiol. 1991;95:152–6. https://doi.org/10.1104/pp.95.1.152.
Shibata H, Sawa Y, Oka T, Sonoke S, Kim KK, Yoshioka M. Steviol and steviol-glycoside: glucosyltransferase activities in Stevia rebaudiana Bertoni – purification and partial characterization. Arch Biochem Biophys. 1995;321:390–6. https://doi.org/10.1006/abbi.1995.1409.
Karim MR, Hashinaga F. Preparation and properties of immobilized pummelo limonoid glucosyltransferase. Process Biochem. 2002;38:809–14. https://doi.org/10.1016/S0032-9592(02)00233-9.
Ikemoto T, Okabe B, Mimura K, Kitahara T. Formation of fragrance materials from odourless glycosidically-bound volatiles by skin microflora (part 1). Flavour Fragr J. 2002;17:452–5. https://doi.org/10.1002/ffj.1127.
Ikemoto T, Mimura K, Kitahara T. Formation of fragrant materials from odourless glycosidically-bound volatiles on skin microflora (part 2). Flavour Fragr J. 2003;18:45–7. https://doi.org/10.1002/ffj.1150.
Veitch NC, Grayer RJ. Flavonoids and their glycosides, including anthocyanins. Nat Prod Rep. 2011;28:1626–95.
Harborne JB, Williams CA. Advances in flavonoid research since 1992. Phytochemistry. 2000;55:481–504. https://doi.org/10.1016/S0031-9422(00)00235-1.
Pietta P. Flavonoids as antioxidants. J Nat Prod. 2000;63:1035–42. https://doi.org/10.1021/np9904509.
Bungaruang L, Gutmann A, Nidetzky B. Leloir glycosyltransferases and natural product glycosylation: biocatalytic synthesis of the C-glucoside nothofagin, a major antioxidant of redbush herbal tea. Adv Synth Catal. 2013;355:2757–63. https://doi.org/10.1002/adsc.201300251.
Krafczyk N, Glomb MA. Characterization of phenolic compounds in rooibos tea. J Agric Food Chem. 2008;56:3368–76. https://doi.org/10.1021/jf703701n.
Gantt RW, Goff RD, Williams GJ, Thorson JS. Probing the aglycon promiscuity of an engineered glycosyltransferase. Angew Chem Int Ed. 2008;47:8889–92. https://doi.org/10.1002/anie.200803508.
Jiang M, Zhang H, Park S, Li Y, Pfeifer BA. Deoxysugar pathway interchange for erythromycin analogues heterologously produced through Escherichia coli. Metab Eng. 2013;20:92–100. https://doi.org/10.1016/j.ymben.2013.09.005.
Fu X, Albermann C, Jiang J, Liao J, Zhang C, Thorson JS. Antibiotic optimization via in vitro glycorandomization. Nat Biotech. 2003;21:1467–9. https://doi.org/10.1038/nbt909.
Weiss RB. Mitoxantrone: its development and role in clinical practice. Oncology (Williston Park). 1989;3:135–41. discussion 141–3, 147–8
van der Graaf WTA, de Vries EG. Mitoxantrone: bluebeard for malignancies. Anti-Cancer Drugs. 1990;1(2):109–25.
Verdrengh M, Isaksson O, Tarkowski A. Topoisomerase II inhibitors, irrespective of their chemical composition, ameliorate experimental arthritis. Rheumatology. 2005;44:183–6. https://doi.org/10.1093/rheumatology/keh444.
Wehenkel A, Fernandez P, Bellinzoni M, Catherinot V, Barilone N, Labesse G, et al. The structure of PknB in complex with mitoxantrone, an ATP-competitive inhibitor, suggests a mode of protein kinase regulation in mycobacteria. FEBS Lett. 2006;580:3018–22. https://doi.org/10.1016/j.febslet.2006.04.046.
Cavalletti E, Crippa L, Mainardi P, Oggioni N, Cavagnoli R, Bellini O, et al. Pixantrone (BBR 2778) has reduced cardiotoxic potential in mice pretreated with doxorubicin: comparative studies against doxorubicin and mitoxantrone. Investig New Drugs. 2007;25:187–95. https://doi.org/10.1007/s10637-007-9037-8.
Evison BJ, Mansour OC, Menta E, Phillips DR, Cutts SM. Pixantrone can be activated by formaldehyde to generate a potent DNA adduct forming agent. Nucleic Acids Res. 2007;35:3581–9. https://doi.org/10.1093/nar/gkm285.
Fulbright JM, Huh W, Anderson P, Chandra J. Can anthracycline therapy for pediatric malignancies be less cardiotoxic? Curr Oncol Rep. 2010;12:411–9. https://doi.org/10.1007/s11912-010-0129-9.
Quirós LM, Carbajo RJ, Salas JA. Inversion of the anomeric configuration of the transferred sugar during inactivation of the macrolide antibiotic oleandomycin catalyzed by a macrolide glycosyltransferase. FEBS Lett. 2000;476:186–9. https://doi.org/10.1016/S0014-5793(00)01721-X.
Zhou M, Thorson JS. Asymmetric enzymatic glycosylation of mitoxantrone. Org Lett. 2011;13:2786–8. https://doi.org/10.1021/ol200977u.
Härle J, Bechthold A. The power of glycosyltransferases to generate bioactive natural compounds, chap. 12. In: Hopwood DA, editor. Methods in enzymology : complex enzymes in microbial natural product biosynthesis, part A: overview articles and peptides, vol. 458. Amsterdam: Academic; 2009. p. 309–33. https://doi.org/10.1016/S0076-6879(09)04812-5.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG
About this chapter
Cite this chapter
Härtl, K., McGraphery, K., Rüdiger, J., Schwab, W. (2018). Tailoring Natural Products with Glycosyltransferases. In: Schwab, W., Lange, B., Wüst, M. (eds) Biotechnology of Natural Products. Springer, Cham. https://doi.org/10.1007/978-3-319-67903-7_9
Download citation
DOI: https://doi.org/10.1007/978-3-319-67903-7_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-67902-0
Online ISBN: 978-3-319-67903-7
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)