Abstract
Objective: A drawback in the use of an external ventricular drain (EVD) originates in the fact that draining cerebrospinal fluid (CSF) (open system) and intracranial pressure (ICP) monitoring can be done at the same time but is considered to be unreliable regarding the ICP trace. Furthermore, with the more widespread use of autoregulation monitoring using blood pressure and ICP signals, the question arises of whether an ICP signal from an open EVD can be used for this purpose. Using an EVD system with an integrated parenchymal ICP probe we compared the different traces of an ICP signal and their derived parameters under opened and closed CSF drainage.
Methods: Twenty patients with either subarachnoid or intraventricular hemorrhage and indication for ventriculostomy plus ICP monitoring received an EVD in combination with an air-pouch-based ICP probe. ICP was monitored via an open ventricular catheter (ICP_evd) and ICP probe (ICP_probe) simultaneously. Neuromonitoring data (ICP, arterial blood pressure, cerebral perfusion pressure, pressure reactivity index (PRx)) were recorded by ICM+ software for the time of ICU intensive care treatment. Routinely (at least every 4 h) ICP was recorded with a closed CSF drainage system for at least 15 min. ICP, ICP amplitude, and the autoregulation parameters (PRx_probe, PRx_evd) were evaluated for every episode with closed CSF drainage and during the 3 h prior with an open drainage system.
Results: One hundred and forty-four episodes with open/closed drainage were evaluated. During open drainage, overall mean ICP_evd levels were nonsignificantly different from those of ICP_probe, with 9.8 + 3.3 versus 8.2 + 3.2 mmHg, respectively. Limits of agreement ranged between 5.2 and −8.3 mmHg. However, 51 increases of ICP >20 mmHg with a duration of 3–30 min were missed by ICP_evd, and in 101 episodes the difference between ICPs was greater than 10 mmHg. After closure of the EVD, ICP increased moderately using both methods. Mean PRx_evd was significantly higher (falsely indicating impaired autoregulation) and more subjected to fluctuations than PRx_probe.
Conclusion: The general practice of draining CSF and monitoring ICP via a (usually open) EVD plus frequently performed catheter closure for ICP reading is feasible for assessment of overall ICP trends. However, it does have clinically relevant drawbacks, namely, a significant amount of undetected increases in ICP above thresholds, and continuous assessment of cerebrovascular autoregulation is less reliable. In conclusion, all patients who need CSF drainage plus ICP monitoring due to the severity of their brain insult need either an EVD with integrated ICP probe or an EVD line plus a separate ICP probe.
Access provided by CONRICYT-eBooks. Download conference paper PDF
Similar content being viewed by others
Keywords
Introduction
When patients are admitted with intraventricular or subarachnoid hemorrhage (SAH), they frequently receive a ventriculostomy for the treatment of acute hydrocephalus by an extraventricular drainage (EVD) line. At the same time, especially if the clinical condition is poor or other reasons for continued sedation are present, continuous monitoring of intracranial pressure (ICP) and, nowadays more frequently employed, assessment of cerebrovascular autoregulation, e.g., by pressure reactivity index (PRx), is warranted [1,10,15,16,4,5,14,8,13,2,9,6,7,3].
The available ICP monitoring devices differ in the location (intraventricular, intraparenchymal, sub- and epidural) and the sensor technology for ICP detection (fluid-coupled pressure transducer, piezo-electric, stain-gauge, and fiber-optic sensors) [4,5,14,8,13,2,9,6,7]. By conventional means a continuous cerebrospinal fluid (CSF) drainage and simultaneous continuous ICP-based neuromonitoring can only be achieved by implantation of both an EVD and a separate, for example intraparenchymal ICP, probe, doubling the costs, the invasiveness, and the rate of potential complications thereafter, e.g., for infection [8].
To reduce these drawbacks, a compromise solution is often used as general practice in many neurocritical care units (NCCUs), i.e., to use the EVD for both CSF diversion and sole ICP monitoring device via connection by a fluid-filled line to a conventional pressure transducer. In this setting, there are two possible options, as outlined previously [9]:
-
1.
Monitor first: The EVD is generally kept closed and connected to the ICP transducer, allowing for continuous ICP measurement until a certain threshold is reached, then opening would enable CSF drainage for a certain time period until the ICP returns to below the threshold.
-
2.
Drainage first: The EVD is generally open, allowing for continuous CSF drainage, and closed at certain intervals to assess the “true” ICP because with an open EVD system the pressure gradient along the catheter may produce erroneous false low ICP results.
In general, both options are employed, although either the risk for underdrainage and unnecessary high ICPs or misjudgment of ICP (false low ICP) is conceivable [10,15,16,4,5,14,8,13,2,9,6,7,3,11,12].
Besides assessment and display of an accurate ICP, the calculated cerebral perfusion pressure (CPP), and its trends, modern neuromonitoring requires high-resolution recording of the ICP signal to facilitate, for example, wave form analysis, ICP amplitude analysis, and bedside monitoring of cerebrovascular autoregulation, for example by PRx, which has proven feasible via an EVD-mediated ICP signal [1, 13]. Nevertheless, it is unclear whether the aforementioned practice of alternating open/closed EVD guarantees reliable data collection. Therefore, we compared the neuromonitoring of ICP and associated parameters (ICP amplitude and PRx) by an EVD in open and closed settings with a direct intraparenchymal ICP probe measurement via a combined EVD with an air-pouch-based integrated probe.
Patients and Methods
The standard NCCU management of patients with intraventricular hemorrhage or aneurysmal SAH includes ventriculostomy in case of acute hydrocephalus and monitoring of ICP in patients who cannot be subjected to neurological assessment owing to their poor clinical condition, which mandates continuous analgosedation. Intensive care management was conducted according to our current NCCU standards. This means that mechanical ventilation is regulated to keep arterial pO2 at 110 ± 5 mmHg and arterial pCO2 between 35 and 40 mmHg, fluid balance aims at normovolemia, and catecholamines (noradrenalin) are titrated to ensure cerebral perfusion pressures of ≥70 mmHg.
Extraventricular Drainage, ICP Monitoring, and Assessment of Cerebral Autoregulation
When the decision to do a ventriculostomy and ICP monitoring was made, an extraventricular silver drain with a combined air-pouched-based ICP probe (Silverline Ventricular probe®, Spiegelberg GmbH & Co.KG, Hamburg, Germany) was implanted in the right frontal horn of the lateral ventricle via a one-lumen bolt. While the catheter tip is placed in the ventricle, the air pouch is located about 2 cm more proximal in the parenchyma of the frontal white matter. The drainage system was set at (5)–10–(15) cm above the foramen of Monro (depending on the clinically desired ICP levels that should be maintained) and permitted continuous CSF drainage (“drainage first” protocol).
ICP was continuously assessed by both the ICP probe via an air pressure–mediating line to a bedside ICP monitor (Spiegelberg GmbH & Co.KG, Hamburg, Germany) (ICP_probe) and the EVD via a fluid-coupled pressure transducer (xtrans, CODAN pvb Critical Care GmbH, Forstinning, Germany) referenced as closely as possible to the foramen of Monro (ICP_evd). Mean arterial pressure (MAP) was continuously monitored by a radial artery catheter with the transducer equally referenced to the foramen of Monro.
ICP_evd and MAP were continuously recorded on a bedside mounted device (Datalogger MPR, Raumedic AG, Helmbrechts, Germany) and transmitted to the bedside hospital monitoring system. The ICP_probe signal was not visualized on the hospital monitor but was visible only at the Spiegelberg monitor and thus indirectly accessible to the staff of the intensive care unit (ICU).
Blood pressure and ICP_evd taken from the Datalogger MPR and ICP_probe from the Spiegelberg monitor were additionally digitally sampled at a rate of 100 Hz by a notebook PC running ICM+ software (Cambridge Enterprise, Cambridge, UK). The ICM+ software was used for both online display of data and retrospective analysis of recorded neuromonitoring parameters. CPP_probe and CPP_evd were calculated as the difference between MAP and the respective ICP. ICP amplitude was calculated by the ICM+ software following Fourier transformation of the ICP signal as the ICP amplitude corresponding to the first harmonic, which is the heart rate (AMP).
The ICP/ABP-derived PRx as a parameter of cerebrovascular autoregulatory capacity was calculated as both PRx_probe and PRx_evd, as described elsewhere [14]. In short, PRx was computed as a moving Pearson correlation coefficient between averaged (60 s periods) ICP and MAP calculated over the moving window length of 5 min. PRx may vary between −1 and 1. Intact cerebral autoregulation can be assumed when index values are close to or below zero, meaning that no or a negative correlation between ICP and MAP exists.
CSF Drainage and Intermittent EVD Closure
The practice of intermittent EVD closure to assess the true ICP was investigated. For this the EVD was kept open for continuous drainage of CSF while monitoring ICP_evd, and on several occasions EVD was closed for at least 15 min. ICU nursing staff is generally required to close EVDs at least three times per 8 h shift and more frequently if that is deemed necessary. In parallel, EVD closure was marked as an event in the ICM+ record file.
Data Analysis and Statistics
The local ethics committee’s approval was obtained for computerized neuromonitoring and data collection for retrospective data analysis. The local ethics committee granted a waiver for patient consent.
For retrospective analysis ICP and MAP data were subjected to manual artifact detection and removal. Events of EVD closure were identified in the ICM+ data files, and a baseline of 3 h monitoring prior to closure and time of EVD closure were evaluated for each event. Besides the overall mean values of MAP, ICP, ICP amplitude, CPP, PRx (evd and probe) for the 3 h baseline and 15 min period of closed EVD a 1 min mean value was calculated for ICP and ICP amplitude (evd and probe) for the 3 h baseline period as well as the period of EVD closure.
For a comparison of methods for ICP monitoring and assessment of PRx, Bland-Altman plots were used [15]. Data analysis was performed using Sigmaplot 12.5 software (Systat Software GmbH, Erkrath, Germany).
Results
Patient and General ICP Monitoring Characteristics
ICP monitoring data of 20 patients with either intraventricular hemorrhage (n = 1) or SAH (n = 19) in the year 2015 was retrospectively evaluated. Mean age was 55 years, with 60% of the patients being female. Monitoring was initiated within 48 h of ICU admission, and the duration ranged from several hours to more than 23 days.
Overall, 144 episodes of open/closed EVD were recorded and evaluated.
Neuromonitoring Parameters and Cerebral Autoregulation
Open Drainage
Mean ICP_evd levels were moderately higher than ICP_probe, with 9.8 ± 3.3 and 8.2 ± 3.2 mmHg, respectively, p > 0.05. Limits of agreement according to Bland-Altman analysis ranged between 5.2 and −8.3 mmHg. ICP amplitude (AMP) did not differ significantly between the two methods, with 1.5 ± 0.6 and 1.8 ± 0.9 mmHg for ICP_evd and ICP_probe, respectively, p > 0.05.
During open EVD, ICP_evd did not detect 51 episodes of ICP_probe values above 20 mmHg. These episodes ranged between 5 and 30 min, in one case 77 min. In one case, the reason for the missed detection was EVD obstruction due to ventricular collapse (e.g., Fig. 1). For the remaining episodes ventricular drainage was considered undisturbed (e.g., Fig. 2). Furthermore, 101 episodes were identified in which the absolute difference between ICP_evd and ICP_probe was greater than 10 mmHg. In 85% of the cases, ICP_probe was higher than ICP_evd.
Assessment of pressure reactivity by PRx was feasible with both ICP_evd and ICP_probe. Cerebral autoregulation of all analyzed 3 h time segments as quantified by PRx taken from ICP_probe showed preserved vasoreactivity with mean values around zero (PRx_probe 0.01 ± 0.09). If calculated from the ICP_evd, PRx_evd was assessed with 0.12 ± 0.20, p > 0.05 vs. PRx_probe. The much greater variance for PRx_evd is also visualized by the increasing discrepancy for higher PRx values (around 0.2) in Bland-Altman analysis (Fig. 3).
Closed EVD
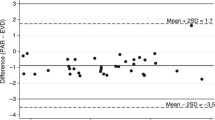
When EVD was closed, both ICP_evd and ICP_probe increased moderately, but insignificantly, to 11.3 ± 4.1 and 9.0 ± 3.1 mmHg, respectively, at 15 min after closure, compared to baseline and between each other, p > 0.05 vs. baseline and between methods (Fig. 4). Limits of agreement for 15 min mean values of ICP ranged between 4.6 and −9.0 mmHg (Fig. 5). Mean ICP amplitude did not change significantly, with 1.9 ± 0.9 and 2.0 ± 0.9 mmHg for ICP_evd and ICP_probe, respectively, p > 0.05 vs. baseline.
Autoregulation assessment was not significantly affected by EVD closure with a 15 min mean PRx_evd of 0.16 ± 0.23 and PRx_probe of 0.01 ± 0.18, p > 0.05 to baseline. Limits of agreement remain wide, with a remaining greater variance for PRx_evd.
Discussion
Our data once again demonstrate that ICP readings via a CSF pressure transducer in the setting of an opened EVD can be erroneous [10,15,16,4,5,14,8,13,2,9,6,7,3,11,12]. Even if the CSF drainage system is at a medium level, i.e., in our ICU usually 10 cm above the foramen of Monro (corresponding to 7.3 mmHg), the intracranial compliance is well preserved, and ICP is mostly below the pathological range, there are periods of temporal ICP increase, e.g., during nursing maneuvers, decreased sedation levels, or during vasogenic waves, where the induced increased CSF outflow creates a pressure gradient via the catheter, and correct ICP assessment by EVD is impossible. In 19 of our 20 patients, ICP levels were not substantially elevated during the monitoring period, which explains the high agreement of ICP assessment (between ICP_probe and ICP_evd).
Nevertheless 51 episodes were detected with temporal ICP values above 20 mmHg, in which those temporal increases in ICP were missed by the ICP_evd reading. The episodes were usually short, less than 1 h, and not life threatening, however effectively decreasing the CPP. In one of the 20 patients, however, who was suffering from progressive brain swelling, a persistent high ICP level above 30 mmHg developed. The resulting CSF outflow led to a ventricular collapse and a loss of fluid coupling within the EVD system. Therefore, ICP_evd showed constantly low values, but with a suspiciously silent and nonfluctuating ICP trace, and the high ICP values were only noticed when the EVD was closed (Fig. 1). This is a typical example of false negative ICP readings via EVD, which seems to occur especially in the most critical situation of brain edema/brain swelling.
The identification of a total of 101 episodes with an ICP difference of >10 mmHg between the open (EVD) and closed (air pouch) system underscores the dangers of false low ICU determination and, thus, false high CPP calculation using an open EVD system for ICP monitoring.
With the drainage system of the EVD closed, ICP increased only mildly within 15 min, as can be expected in patients with preserved intracranial reserve capacity at ICP values below 20 mmHg. The bias and discrepancy of the two methods (ICP_evd and ICP_probe) can be considered acceptable (Fig. 5) and correlated to other comparative studies of different ICP monitors [11], where differences of 5 mmHg are found frequently and were considered to have no significant clinical impact. Thus, with a closed drainage system, EVD-based ICP monitoring seems to be sufficient, but our study only compared 15 min intervals as opposed to 3 h intervals with an open drainage system. Since all patients had acute posthemorrhagic occlusive hydrocephalus, a monitor-first approach with closed EVD was most of the time not possible because of rapid ICP increase.
The air-pouch-based parenchymal ICP monitor provided continuous, reliable, and high-frequency assessment of ICP [16]. The failure rate for this device was determined to be zero in these 20 patients. Fluid-coupled ICP monitoring, in contrast, is susceptible to artifacts and several possible human handling errors, like undetected partial or complete catheter blockage, air bubbles in the line, or incorrect height adjustment of the transducer with false zeroing [11]. Moreover, in patients with reduced compliance, frequent EVD closure might be critical.
As other authors have pointed out, continuous bedside determination of cerebrovascular autoregulatory state has become a well-recognized tool in ICU treatment. The PRx uses the extent of correlation between slow frequency waves in the ICP and ABP signals in a closed system, i.e., the cranium, to establish a statement about the state of cerebrovascular autoregulation [3, 14]. It has been disputed whether an open EVD already abolishes the requirement for a closed system since the ICP waveform is altered in this setting. Recently, Aries et al. not only showed that autoregulation assessment by PRx is feasible in the setting of an open EVD; they also demonstrated that the ICP signal from an open EVD, otherwise corrupted for estimation of ICP, carries sufficient information, i.e., low-frequency waves, to produce a reliable PRx [1].
From the present data we can confirm that PRx can be assessed by the ICP_evd signal, and, although it is difficult to draw a definitive conclusion from 15 min of recording, EVD closure did not change the PRx_evd value significantly. Nevertheless, when comparing PRx_evd with PRx_probe, we assume that PRx_probe is the more precise variable because its variance of the 3 h mean values is much less. In parallel, PRx_probe did not differ between EVD opening and closure.
The fact that our PRx_evd variable was not as reliable and comparable to ICP_probe as in the Aries et al. study [1] might be explained by the fact that we used a longer fluid-filled line connected to the ICP pressure transducer, which is a well-known source of signal artifact. From the presented data in this specific setup, PRx_probe seems to be the preferred method for reliable continuous assessment of cerebral autoregulation.
A concern expressed previously referred to a possible dampening of the ICP signal by the air-pouch-based technique of ICP measurement, which transfers changes in ICP to an air-filled balloon and line connected to the ICP monitor. From the signal assessment of the ICP_probe wave with preserved ICP amplitude (AMP) especially compared to the signal via a closed EVD, we conclude that this presumed dampening effect can be neglected.
Conclusion
In summary, if current NCCU treatment standards require an accurate, high-resolution ICP recording, which furthermore makes continuously collected data available for online evaluation, and if the ICP signal is used for the assessment of indices for autoregulation and cerebrospinal compliance, then a combined EVD with a drainage-independent, parenchymal ICP monitoring system is most suitable.
As stated earlier, this can be achieved as well by an EVD plus a separate ICP probe. The compromise of using one EVD only requires an elaborate technique and the highest surveillance and alertness of the nursing staff to minimize false recordings and artifacts. This still has the disadvantage that a truly continuous signal recording cannot be achieved when open and closed intervals alternate.
Lastly, continuous assessment of cerebrovascular autoregulation (via PRx) by EVD gives less reliable information with a high likelihood of having false positive results, meaning that autoregulatory capacities would be considered nonfunctional at a much earlier time point.
References
Aries MJH, de Jong SF, van Dijk JMC, Regtien J, Depreitere B, Czosnyka M, Smielewski P, Elting JWJ. Observation of autoregulation indices during ventricular CSF drainage after aneurysmal subarachnoid hemorrhage: a pilot study. Neurocrit Care. 2015;23(3):347–54.
Jaeger M, Schuhmann MU, Soehle M, Nagel C, Meixensberger J. Continuous monitoring of cerebrovascular autoregulation after subarachnoid hemorrhage by brain tissue oxygen pressure reactivity and its relation to delayed cerebral infarction. Stroke. 2007;38(3):981–6.
Le Roux P, Menon DK, Citerio G, et al. Consensus summary statement of the international multidisciplinary consensus conference on multimodality monitoring in neurocritical care: a statement for healthcare professionals from the Neurocritical Care Society and the European Society of Intensive C. Neurocrit Care. 2014;21(Suppl 2):S1–26.
Citerio G, Piper I, Chambers IR, Galli D, Enblad P, Kiening K, Ragauskas A, Sahuquillo J, Gregson B, BrainIT Group. Multicenter clinical assessment of the raumedic neurovent-P intracranial pressure sensor: a report by the BrainIT group. Neurosurgery. 2008;63(6):1152–8. discussion 1158
Czosnyka M, Czosnyka Z, Pickard JD. Laboratory testing of three intracranial pressure microtransducers: technical report. Neurosurgery. 1996;38(1):219–24.
Piper I, Barnes A, Smith D, Dunn L. The Camino intracranial pressure sensor: is it optimal technology? An internal audit with a review of current intracranial pressure monitoring technologies. Neurosurgery. 2001;49(5):1158–64. discussion 1164–5
Raabe A, Stöckel R, Hohrein D, Schöche J. Reliability of intraventricular pressure measurement with fiberoptic or solid-state transducers: avoidance of a methodological error. Neurosurgery. 1998;42(1):74–9. discussion 79–80
Dimitriou J, Levivier M, Gugliotta M. Comparison of complications in patients receiving different types of intracranial pressure monitoring: a retrospective study in a single center in Switzerland. World Neurosurg. 2016;89:641–6.
Kim GS, Amato A, James ML, Britz GW, Zomorodi A, Graffagnino C, Zomorodi M, Olson DM. Continuous and intermittent CSF diversion after subarachnoid hemorrhage: a pilot study. Neurocrit Care. 2011;14(1):68–72.
Birch AA, Eynon CA, Schley D. Erroneous intracranial pressure measurements from simultaneous pressure monitoring and ventricular drainage catheters. Neurocrit Care. 2006;5(1):51–4.
Vender J, Waller J, Dhandapani K, McDonnell D. An evaluation and comparison of intraventricular, intraparenchymal, and fluid-coupled techniques for intracranial pressure monitoring in patients with severe traumatic brain injury. J Clin Monit Comput. 2011;25(4):231–6.
Wilkinson HA, Yarzebski J, Wilkinson EC, Anderson FA. Erroneous measurement of intracranial pressure caused by simultaneous ventricular drainage: a hydrodynamic model study. Neurosurgery. 1989;24(3):348–54.
Howells T, Johnson U, McKelvey T, Ronne-Engström E, Enblad P. The effects of ventricular drainage on the intracranial pressure signal and the pressure reactivity index. J Clin Monit Comput. 2016;31(2):469–78. https://doi.org/10.1007/s10877-016-9863-3.
Czosnyka M, Smielewski P, Kirkpatrick P, Laing RJ, Menon D, Pickard JD. Continuous assessment of the cerebral vasomotor reactivity in head injury. Neurosurgery. 1997;41(1):11–7. discussion 17–9
Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet (London). 1986;1(8476):307–10.
Chambers IR, Siddique MS, Banister K, Mendelow AD. Clinical comparison of the Spiegelberg parenchymal transducer and ventricular fluid pressure. J Neurol Neurosurg Psychiatry. 2001;71(3):383–5.
Conflicts of interest statement
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG
About this paper
Cite this paper
Hockel, K., Schuhmann, M.U. (2018). ICP Monitoring by Open Extraventricular Drainage: Common Practice but Not Suitable for Advanced Neuromonitoring and Prone to False Negativity. In: Heldt, T. (eds) Intracranial Pressure & Neuromonitoring XVI. Acta Neurochirurgica Supplement, vol 126. Springer, Cham. https://doi.org/10.1007/978-3-319-65798-1_55
Download citation
DOI: https://doi.org/10.1007/978-3-319-65798-1_55
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-65797-4
Online ISBN: 978-3-319-65798-1
eBook Packages: MedicineMedicine (R0)