Abstract
Regulation of chromatin structures is important for the control of DNA processes such as gene expression, and misregulation of chromatin is implicated in diverse diseases. Covalent post-translational modifications of histones are a prominent way to regulate chromatin structure and different chromatin regions bear their specific signature of histone modifications. The composition of centromeric chromatin is significantly different from other chromatin structures and mainly defined by the presence of the histone H3-variant CENP-A. Here we summarize the composition of centromeric chromatin and what we know about its differential regulation by post-translational modifications.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
1 Chromatin
In the nuclei of all eukaryotic cells, genomic DNA is highly folded, constrained, and compacted by both histone and non-histone proteins in a dynamic polymer called chromatin. Chromatin is organized in the dynamic structuring of nucleosomes, which represent the basic repeating unit of the chromatin fiber. Each nucleosome is formed by 146–147 bp of chromosomal DNA tightly wrapped around an octamer of proteins comprising two subunits each of the canonical histones H3, H4, H2A, and H2B, or variants of these histones (Davey et al. 2002; Luger et al. 1997).
Histones are small basic proteins consisting of a globular domain, called the histone fold domain (HFD), and a more flexible and charged NH2-terminus (histone tail). These flexible N-terminal tails of the four core histones undergo a range of post-translational modifications (PTMs), including acetylation, methylation, phosphorylation, ubiquitination, sumoylation, ribosylation, and many others (Hatakeyama et al. 2016). These covalent modifications reveal a “histone code” that is involved in generating epigenetic information, the code that is ‘above’ (greek ‘epi’) the genetic code by influencing the state of chromatin structure and function (Jenuwein and Allis 2001; Kouzarides 2007). The two most distinct chromatin states are euchromatin, which is mainly found on chromosome arms and comprises most of the protein-coding genes. In contrast, heterochromatin is more compact, highly condensed, and most prominently found at telomeres and in vicinity to centromeres (Grewal and Elgin 2007).
The transcriptional consequences of histone modifications are revealed either as a result of the direct biophysical consequences of the modification, or through the catalytic activities of proteins and complexes that recognize and bind these specifically modified histones. For instance, acetylation, which reduces the net positive charge on the nucleosome, results in decreased stability of the histone associations with the negatively charged DNA, facilitating access of chromatin associating factors and promoting, most prominently, transcription. Therefore, by balancing levels and activities of histone acetyltransferases (HATs) and deacetylases (HDACs), which, respectively, add and remove acetyl groups from histones (and other proteins), transcription can be activated or repressed (Bannister and Kouzarides 2011). In contrast, the effects of histone methylation appear to be transmitted more indirectly, as the addition of a methyl group does not modify histone chain charge. Methylated histones rather seem to serve as a recognition platform for protein complexes that bind to chromatin and remodel its compaction, localization, and transcriptional activity (Cohen et al. 2011).
Apart from covalent modifications occurring at histone tails, the DNA sequence can also be chemically modified. A detailed description of DNA modifications is beyond the scope of this chapter, but it is important to mention that there is significant crosstalk between histone PTMs and DNA modifications, and that these two gene expression regulatory phenomena can be dependent on each other. For instance, DNA methylation has been proposed to serve as a template for establishing particular PTMs on newly synthesized histones after DNA replication (Cedar and Bergman 2009). DNA methylation is the classic example of a heritable epigenetic mark, specifically at CpG islands, which are maintained in a semi-conservative manner by the activity of the DNA methyltransferase 1 (Dnmt1) (Jones and Liang 2009). DNA methylation is found symmetrically on parental strands, each daughter strand therefore contains one methylated strand acting as a template for faithfully maintaining the methylation pattern at the newly synthesized DNA strand.
2 Machineries of Histone Post-translational Modifications (PTMs)
The addition and removal of histone PTMs are considered key regulatory processes of chromatin function. Enzymes which catalyze the addition of PTMs to histones are often referred to as “writers.” The added modifications are then recognized by so-called “readers” (proteins which are sensitive to the PTMs presented by the histones), and, ultimately, some of them by “erasers” (enzymes which catalyze the removal of these marks) (Falkenberg and Johnstone 2014). The actions of these proteins are crucial for the dynamic regulation of histone modification levels on the chromatin fiber, since most PTMs have a writer/eraser pair with opposing effects. For example, histone acetylation is regulated by the opposing actions of HATs and HDACs, and phosphorylation is regulated by the activity of kinases and phosphatases. The fine regulation of these enzyme activities determine whether histone PTMs contribute to short-term chromatin regulation (immediate functions) or long-term chromatin changes (heritable function). In cellular signaling events, rapid responses to environmental stimuli necessitate a high turnover rate of histone PTMs, whereas defining and maintaining chromatin structures, such as constitutive heterochromatin, throughout the cell cycle or from one cell generation to the next, requires constant PTMs with little dynamics (Turner 2007).
3 PTMs in Epigenetic Inheritance
Epigenetics is generally defined as heritable changes in gene activity and expression that occur without altering the underlying DNA sequence (Bird 2007; Goldberg et al. 2007). Epigenetics is therefore often considered a link between genotype and phenotype. Besides DNA methylation (which is not found in all species), histone PTMs are a major regulatory entity for epigenetic chromatin regulation and function, since they determine heritable differences in chromatin states. Important players at all stages of histone and chromatin regulation (including PTM modulation) are histone chaperones. They associate with newly synthesized histones, help transport them into the nucleus, and associate them to DNA (Avvakumov et al. 2011).
Epigenetic inheritance and the transmission of information beyond the DNA sequence during cell division is crucial for maintaining differential gene expression patterns during development and differentiation, and also for the development of many diseases (Heard and Martienssen 2014). The known association of histone chaperones and chromatin-modifying enzymes to the replication fork highlights that, at least for some of these marks, their inheritance is of crucial importance for cell viability and function.
An epigenetic role for histones was first proposed in 1980 (Stein 1980), but it was almost 20 years later, in 1997, when the first mechanistic understanding of how histone PTMs mediate the inheritance of silenced chromatin was reported, using budding yeast as a model (Sherman and Pillus 1997). More and more examples of histone-mediated inheritance were discovered in higher eukaryotes, including humans, resulting in more effort to unravel the epigenetic roles of PTMs. Today, the information on different epigenetic roles of PTMs is overwhelming and summarized elsewhere (Allis and Jenuwein 2016).
4 Maintenance of PTMs Throughout Cell Cycle
Chromatin in proliferating cells is highly dynamic. During cell cycle, there are two major events involving a global chromatin reconstruction. First, during S-phase, newly synthesized histones must be correctly incorporated with nascent DNA, and histone PTMs must be reestablished (Annunziato 2005). Second, major chromatin remodeling and distributional changes of chromatin-associated factors occur during mitosis; most prominent is the condensation of chromosomes and the phosphorylation of histone H3S10 (Hsu et al. 2000).
In the case of histone modifications, canonical histones are displaced and replaced during S-phase and DNA replication. During DNA replication, chromatin is disassembled prior to the replication fork and reassembled on the two daughter strands. How PTMs of recycled and newly incorporated histones are faithfully passed from one generation to the next is not yet fully understood. Alabert et al. have recently examined the maintenance of histone PTMs after DNA replication in HeLa cells and propose two modes for the transmission of histone modifications at the replication fork: (i) most of the PTMs on parental old histones are retained and newly synthesized histones collect PTMs to become identical within the same cell cycle; and (ii) gradual modification of both new and parental histones. In the case of the H3K9me3 and H3K27me3 marks on new H3, more than one generation is required to complete the trimethylation. As these marks are therefore diluted in half after replication, a potential loss of cellular memory could result (Apostolou and Hochedlinger 2013). However, this is counterbalanced by the continual modification of both new and old histones.
The results of Alabert et al. suggest that histone PTMs are transmitted at the replication fork and would be in contrast to the observation of complete deletion of PTMs in experiments using Drosophila embryos, where the TrxG and PcG histone-modifying enzyme complexes remain associated during replication and reestablish the histone modifications after S-phase (Petruk et al. 2012).
5 Centromeric Chromatin: CENP-A, the Epigenetic Marker for Centromere Identity
The proper segregation of genetic information during cell division is crucial to maintain genomic integrity. Errors in segregation can lead to abnormal chromosome number—known as aneuploidy—which is linked to human disease (Kops et al. 2005). Accurate segregation during mitosis is mediated by the centromere, a chromosomal chromatin region of each sister chromatid that serves as a chromatin foundation for kinetochore formation and as a chromosome attachment to the mitotic microtubule spindle (Przewloka and Glover 2009).
Despite differences in complexity, one common centromere feature is conserved among species: the replacement of the canonical histone H3 with the histone H3 variant CENP-A in a subset of centromeric nucleosomes (Allshire and Karpen 2008). The underlying DNA sequence is fast evolving and not conserved between species (Murphy et al. 2005). With the exception of some yeast species such as Saccharomyzes cerevisiae and Kluyveromyces lactis, the centromeric DNA sequence alone seems insufficient to confer centromeric identity, and it is, therefore, widely accepted that centromeres are regulated epigenetically (Karpen and Allshire 1997).
CENP-A is structurally similar to the canonical histone H3. The C-terminus contains a globular HFD that shares 62% sequence homology with the HFD of canonical H3 (Sullivan et al. 1994). The HFD of CENP-A, like all histone proteins, consists of three α-helices linked by two loops (Arents et al. 1991). In addition to mediating the interaction with histone H4, CENP-A’s HFD contains the critical structural features that are needed to deposit CENP-A to centromeres, i.e., loop1 (L1) and α-helix 2, which build up the CENP-A targeting domain (CATD), a region that is necessary and sufficient to promote centromeric targeting (Black et al. 2004). In contrast to the HFD, the N-terminal tail of CENP-A is very diverse and varies in length between different species as discussed later (Smith 2002). X-ray crystallography has revealed that CENP-A and canonical nucleosomes are structurally very similar, and both types of nucleosomes wrap their DNA in a left-handed manner (Tachiwana et al. 2011). The precise composition of centromeric nucleosomes has been a subject of controversy over the past years, however, most evidence points to an octamer as the predominant centromeric structure (Dunleavy et al. 2013). Differences in the octameric structure of CENP-A-containing nucleosomes may be mediated by the binding of CENP-C, which can reshape these nucleosomes into a more rigid structure (Falk et al. 2015).
6 PTMs of Centromeric Chromatin
Despite the essential role of CENP-A for most centromeres, the chromatin environment created by the presence of specific PTMs on all histone species at centromeres is just as important. In most species, centromeres are organized with a central region that is defined by the presence of CENP-A-containing nucleosomes, surrounded on both sides by flanking heterochromatin (pericentric chromatin) (Blower and Karpen 2001; Blower et al. 2002; Partridge et al. 2000). The PTMs on histones present at human centromeres indicate that centromeric chromatin is neither heterochromatic nor euchromatic. This unique mixture of repressive and permissive histone marks has been termed “centrochromatin” (Sullivan and Karpen 2004).
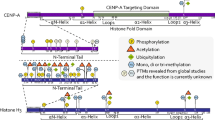
The PTMs of centromeric chromatin can be sorted in four different categories depending on their localization: PTMs (i) of the pericentromeric region, (ii) of nucleosomes adjacent to CENP-A containing nucleosomes, (iii) of CENP-A containing nucleosomes, and (iv) of CENP-A itself (Fig. 1).
6.1 Modifications of the Pericentromeric Region
As mentioned before, chromatin is generally divided into distinct types: transcriptionally ‘open’ euchromatin and tightly packaged, ‘closed’ heterochromatin. It is now clear that this “open” or “closed” classification is an oversimplification, and more precise subdivisions of chromatin structures have been proposed based on specific PTMs and the protein associated with these modifications (Filion et al. 2010). Early studies have shown that centromeres are surrounded by heterochromatin (Lima de Faria 1949), and following studies have found that these pericentromeric regions are indeed containing hypermethylated H3K9 that is dependent on the Suv39h histone methyltransferases as a typical repressive mark of heterochromatin (Peters et al. 2003; Rice et al. 2003). Similar to centromeres, the surrounding pericentromeric heterochromatin is characterized by hypoacetylated canonical histones. However, in contrast to centromeres, pericentric chromatin is characterized by dimethylation (flies and fission yeast) or trimethylation (in mammalian cells) of H3K9 (Noma et al. 2001). Another repressive mark present at the pericentromeric region is H3K27me3, which may serve slightly different or additional functions for instance a compensatory mechanism for the loss of K9 methylation, causing redistribution of this mark to the region, preserving in this manner the repressive state (Lam et al. 2006). Trimethylation of H4K20 is another marker of constitutive heterochromatin that is present in DNA repetitive regions, focally enriched at pericentric heterochromatin. All these repressive marks index pericentric heterochromatin in a sequential model. After H3K9 and H3K27 methylation occurs, the heterochromatin protein 1 (HP1) binds, further recruiting Suv4-20h enzymes, which trimethylate H4K20, reinforcing the heterochromatic state of pericentromeric chromatin (Schotta et al. 2004). HP1 dissociation from heterochromatin is regulated by the Aurora B phosphorylation of H3S10 in M-phase (Fischle et al. 2005). The levels of HP1 dissociation, however, seem to vary in different model systems. In Drosophila cultured cells, for instance, HP1 is still detectable on metaphase chromosomes (Rosic et al. 2014).
The presence of heterochromatin in pericentromeric regions is also required to ensure recruitment of cohesin protein complex, which holds sister chromatids together until anaphase onset (Sakuno et al. 2009; Yamagishi et al. 2008). Moreover, heterochromatin of pericentromeric regions is restricted to a particular portion as shown in Schizosaccharomyces pombe, where centromeres are surrounded by chromatin barriers containing tRNA genes (Scott et al. 2006). It is also important to note that there is a correlation between heterochromatin and neocentromere establishment at least in some species: neocentromere formation is reduced in mutants of the S. pombe H3K9 methyltransferase Clr4, suggesting that heterochromatin positively influences neocentromere formation (Ishii et al. 2008). Consistently, stable hotspots of overexpressed CENP-A in Drosophila cells are preferentially established at euchromatin/heterochromatin boundaries (Olszak et al. 2011).
While repressive marks on the canonical histones H3 or H4 play a role in establishing centromeric chromatin, histone variants (apart from CENP-A) can also do so but are less well understood. For instance, the H3 variant H3.3 replaces the canonical H3.1 in nucleosomes of pericentromeres, telomeres, and regions of active transcription in a replication-independent manner (Chow et al. 2005; Wirbelauer et al. 2005). During mitosis, H3.3S31 is phosphorylated by CHK1 at pericentromeric regions (Hake et al. 2005). H3.3S31ph then spreads along the chromosome arm in lagging or misaligned chromosomes, causing p53-dependent cell cycle arrest. Mitotic H3.3S31ph has been unraveled recently, as a sensor to promote nuclear p53 accumulation in aneuploidy daughter cells, thereby preventing and suppressing aneuploidy (Hinchcliffe et al. 2016).
6.2 Modifications of H3 Nucleosomes Adjacent to CENP-A Containing Nucleosomes
Examination of extended chromatin fibers has revealed that CENP-A nucleosomes occupy discrete domains that are interspersed with chromatin containing canonical histone H3 (Blower et al. 2002; Sullivan and Karpen 2004; Zinkowski et al. 1991). In contrast to the repressive marks at pericentromeres, canonical histone H3 within centromeric chromatin contains some marks that are usually specific for open chromatin, e.g., K36me2 (Bergmann et al. 2011). At the same time, other typical euchromatic modifications, such as acetylation of H3 and H4, are missing, but so are typically silent chromatin marks, such as H3K9me3 (Sullivan and Karpen 2004). The inhibition of HDAC activity by trichostatin A (TSA) leads to hyperacetylated centromeres and chromosome segregation defects in S. pombe, and prolonged mitotic arrest in HeLa cells (Shin et al. 2003), suggesting that histone marks are crucial for cell cycle progression and accurate segregation.
Using human artificial chromosomes (HACs), Bergmann et al. found H3K36me as a new centrochromatin modification. This modification is normally associated with transcription elongation, supporting observations that centromeres are transcriptionally active (Bergmann et al. 2011). This study also found that H3K4me2 plays a role in CENP-A maintenance. H3K4me2 depletion at the alphoidtetO centromere of the HAC by tethering the lysine-specific demethylase 1 (LSD1) causes a reduction of CENP-A incorporation as a result of the loss of the CENP-A chaperone HJURP at centromeres, suggesting that this modification is involved in the recruitment of HJURP to centromeres.
A more recent study shows that CENP-A-proximal nucleosomes containing canonical histones are not uniformly modified, but bear complex combinations of PTMs. They confirm the presence of H3K4me2 and H3K36me2/3, and show that these modifications exist in combination with methylation (and some low levels of acetylation) on different lysines of the same histone, predominantly H3K9 (mono-, di-, and trimethylations), and H3K27 (mono, di, and trimethylation) (Bailey et al. 2015). Regulating the balance between H3K9ac, which promotes CENP-A assembly, and H3K9me3, which inhibits it, may be crucial not only for kinetochore assembly but also for genome stability (Ohzeki et al. 2012). In conclusion, the distinct combination of histone modifications associated with centrochromatin distinguishes it from bulk chromatin, thereby creating a chromatin environment crucial for facilitating the centromere function and its propagation.
In addition to establishing a unique chromatin environment, some marks established only during specific processes such as mitosis are also important for centromere function. For instance, the mitotic kinase haspin is responsible for H3T3 phosphorylation and this mark is specifically enriched at H3 nucleosomes of the centromeric core of mitotic chromosomes (Kelly et al. 2010; Wang et al. 2010; Yamagishi et al. 2010) and has been proposed to guarantee proper chromosome congression to the metaphase plate for faithful segregation of sister chromatids during anaphase (Dai and Higgins 2005).
6.3 Modifications of CENP-A Containing Nucleosomes
X-ray crystallography studies showed that human CENP-A and H4 interact to form a heterotetramer (Sekulic et al. 2010; Tachiwana et al. 2011). H4 associated with pre-nucleosomal CENP-A is acetylated in a manner that is essentially identical to H4 in complex with pre-nucleosomal H3. The three predominant acetylation sites of H4 are the α-N-terminus, which is modified constitutively during translation (Hole et al. 2011), and lysines K5 and K12, which are acetylated by histone acetyltransferase B (HAT B) (Chang et al. 1997). Acetylation of H4 at K5 and K12 is found within the pre-nucleosomal CENP-A-H4-HJURP complex and requires RbAp46/48 for its subsequent successful localization of CENP-A to centromeres (Shang et al. 2016).
In contrast to chromatin-associated centromeric H4, pre-nucleosomal CENP-A associated histone H4 lacks K20me (Bailey et al. 2015). H4K20me1 has been reported to be enriched at centromeres and essential for correct kinetochore assembly (Hori et al. 2014). Bailey et al. also found that the most abundant form of centromeric H4 in cycling cells bore H4K20me2. However, as they discussed, H4K20me2 is a common modification within general chromatin and the ubiquitous nature of H4K20me2 makes it unlikely to play a unique role in centromere identity.
In S. cerevisiae hypoacetylation of H4K16 at centromeres has been reported to be important for kinetochore function, since its deregulation leads to failures in chromosome segregation (Choy et al. 2011).
6.4 Modifications of CENP-A Itself
The CENP-A N-terminal tail is enriched in arginines and lacks most of the well-characterized lysines of histone H3 that are targets for modification. A divergence is not only found between CENP-A and other canonical histones or other variants, but also between CENP-A orthologs from different species. The N-terminal tails of CENP-A orthologs vary significantly in length (for instance, 20 aa in S. pombe versus 200 aa in Caenorhabditis elegans) (Smith 2002). Like other histones, CENP-A is also subjected to post-translational modifications. Depending on the modification, the effect will influence CENP-A stability, structure, or positioning. What is special to CENP-A modifications is that many of these PTMs affect the recruitment of kinetochore components. However, for most of the so far discovered PTMs of CENP-A we know very little about their catalysis, dynamics, and function.
Phosphorylation. The CENP-A N-terminus is phosphorylated on S16 and S18 already in prenucleosomal CENP-A, and these marks are important for reliable chromosome portioning during division (Bailey et al. 2013). The phosphorylation state of Drosophila CENP-A varies with its subnuclear localizations since mass spectrometry analysis detected cytoplasmic CENP-A peptides in unmodified, mono-, and dephosphorylated form (most prominently at S20ph and S75ph), while nucleoplasmic CENP-A peptides were only detected as unmodified and monophosphorylated (S77ph) peptide (Boltengagen et al. 2015).
Apart from regulating the passage of the newly synthesized protein through different pre-assembly complexes, phosphorylation of CENP-A can impair its deposition at centromeres. CDK1 phosphorylates CENP-A at S68, which interferes with CENP-A binding to its loading factor HJURP and, therefore, with its deposition to centromeric chromatin prior to mitotic exit (Yu et al. 2015; Zhao et al. 2016). At the time of CENP-A loading onto centromeric chromatin this phosphorylation is removed by the phosphatase PP1α. However, in long-term cell survival assays, S68 phosphorylation seems dispensable for CENP-A function and cellular survival, challenging the finding that S68 phosphorylation is necessary for CENP-A recognition by HJURP and therefore faithful loading (Fachinetti et al. 2017).
CENP-A is also phosphorylated by Aurora A and B at S7 and this modification is required for mitotic progression and proper kinetochore function. CENP-A-S7ph is initially established by Aurora A in prophase and this is required for Aurora B restriction to the inner centromere, the maintenance of CENP-A phosphorylation at S7 by Aurora B from late prophase to metaphase, and for recruiting the inner kinetochore protein CENP-C (Goutte-Gattat et al. 2013; Kunitoku et al. 2003). The maize ortholog of CENP-A is phosphorylated at S50 during chromosome segregation, in a temporal pattern very similar to the S7ph in human CENP-A (Zhang et al. 2005).
Methylation. CENP-A N-terminus not only bears phosphorylation sites, but is also α-trimethylated on Gly1 by the N-terminal RCC1 methyltransferase NRMT, though it is unclear how NRMT activity is regulated (Bailey et al. 2013). The α-N-methylation has previously been reported to facilitate the chromatin localization of the regulator of chromatin condensation 1 (RCC1), a key player in nucleocytoplasmic transport, mitosis, and nuclear envelope assembly (Chen et al. 2007). The addition of three methyl groups on CENP-A implies the introduction of a conformational change that mediates DNA interactions. The conservation of the CENP-A N-terminal motifs among different species and the fact that most of CENP-A nucleosomes carry G1me3 points to the importance of this modification.
In S. cerevisiae a single nucleosome defines the centromeric region and therefore constitutes a so-called point centromere (Morey et al. 2004). In the essential N-terminal domain of Cse4 (CENP-A ortholog), R37 is methylated and this modification is proposed to positively regulate the recruitment of the complete kinetochore complex and consequently control proper chromosome segregation (Samel et al. 2012).
Acetylation. Apart from the phosphorylations already discussed that affect CENP-A localization within the cell, CENP-A-K105ac has been described in Drosophila cytosolic prenucleosomal CENP-A but not in nuclear extracts (Boltengagen et al. 2015). Therefore, this prenucleosomal modification might be important for its association with chaperones and/or for its import into the nucleus, as has been shown for H4 acetylation at K5 and K12 (Lassallette et al. 2011). Human CENP-A has also been reported to be acetylated at K124 in G1/S-phase-derived cells, a residue located within the HFD closer to the C terminus. Adding an acetyl group to K124 neutralizes the positively charged lysine surface, supposedly loosening the DNA-histone interface and increasing the accessibility of the CENP-A nucleosomal interior to non-histone proteins or to chromatin remodelers (Bui et al. 2012). It was proposed that this CENP-A K124ac functions in “priming” or “blocking” CENP-A K124 for ubiquitylation until the M-phase.
Ubiquitylation. At the same residue as the previously discussed acetylation, CENP-A can be ubiquitylated (K124ub) by the CUL4A-RBX1-COPS8 complex in vivo and in vitro. Acetylation of CENP-A serves as a signal for its deposition at centromeres. The ubiquitylation at this residue occurs in the M and G1 phases and is required for efficient interaction with HJURP to properly localize CENP-A at centromeres and is, therefore, essential for CENP-A loading onto chromatin (Niikura et al. 2015). This study has recently been contradicted by Fachinetti et al. who found no evidence for CENP-A-K124ub to be important for loading or maintenance of CENP-A (Fachinetti et al. 2017). CENP-A mono-ubiquitylation seems epigenetically inherited through dimerization between cell divisions and this inheritance is important for the control of CENP-A deposition and maintenance at centromeres (Niikura et al. 2016). Similar to the human K124ub, mono-ubiquitylation of Drosophila CENP-A by the E3 ligase CUL3/RDX has been reported (Bade et al. 2014). Mono-ubiquitylation stabilizes CENP-A that is bound to its loading factor CAL1. The CAL1 interaction to the ubiquitin machinery mediates the mono-ubiquitylation of CENP-A and, therefore, its accurate loading, securing that chromosomes segregate correctly.
Mono-ubiquitylation of CENP-A seems to be important for its stability, but ubiquitin is normally used to poly-ubiquitylate proteins, thereby marking them for degradation by the proteasome. In the case of CENP-A, proteolysis of residual, spare, or overproduced CENP-A helps to prevent its spreading into euchromatin in several organisms and restrict loading to centromeric chromatin only (Hewawasam et al. 2010; Moreno-Moreno et al. 2006, 2011; Ranjitkar et al. 2010).
Taken together, post-translational modifications present on centromeric chromatin at different levels impact the prenucleosomal assembly, nuclear import, and the pre-loading states of CENP-A, as well as the formation of centromeric chromatin and kinetochore formation, allowing centromeres to mediate faithful mitosis and meiosis (Fig. 2).
PTMs of centromeric chromatin. Summary of the PTMs present at histones composing the core centromere and the pericentromeric region, and at CENP-A itself. Mitosis-specific PTMs are labeled with asterisk and PTMs specific of certain species are also marked (Dmel Drosophila melanogaster, Sc Saccharomyzes cerevisiae, Sp Schizosaccharomyzes pombe, Zm Zea mays)
7 Phosphorylation of Non-histone Proteins, Regulatory Effect of Kinetochore Activity
As already mentioned, the interaction between the centromere-attached kinetochore and the microtubules ensures the precise segregation of chromosomes in mitosis as well as in meiosis. For accurate regulation, kinetochore components are also post-translationally modified in large numbers. The most commonly known PTM of kinetochore proteins is phosphorylation. Two prominent kinase families at the kinetochore are Polo and Aurora that phosphorylate many proteins at centromeric regions during the cell cycle. Nevertheless, other kinases such as mps1, haspin, Cdk1, or bub1 are key regulators of mitotic progression that function at or near centromeric chromatin and are essential for correct cell cycle progression in mitosis (Bayliss et al. 2012).
The Polo-like kinases (Plk) compose a family of structurally related Ser/Thr kinases that are highly conserved from yeast to humans. They have multiple cell cycle functions, e.g., coordinating the entry into M-phase by the activation and control of cyclin-dependent kinase 1 (CDK1) (Archambault and Glover 2009). Strikingly, Polo kinases localize to the centromeric and kinetochore regions and have been shown to be required for initial CENP-A deposition in human cells. Plk1, which is the most extensively studied among the four mammalian Plks, is required downstream of CENP-C localization, and its substrate is M18BP1, a subunit of the kinetochore protein family Mis18 complex. The phosphorylation of M18BP1 by Plk1 promotes the centromeric localization of Mis18 complex, thereby licensing centromeres for CENP-A deposition (McKinley and Cheeseman 2016). Plk1 localization has been suggested to be dependent on phosphorylation of inner centromere protein (INCENP) by CDK1. However, it is difficult to rule out whether these modifications directly mediate Plk1 localization to the kinetochore, or whether it is a secondary effect of other aspects in kinetochore assembly. Furthermore, Plk1 seems to act as a sensor of tension at the kinetochore. Plk1 phosphorylates BubR1 at S676 and thereby stabilizes kinetochore–microtubule interactions during mammalian mitosis (Elowe et al. 2007).
A second important family of conserved kinases involved in chromosome segregation is the family of Aurora protein kinases. This Ser/Thr-direct kinase family encompasses Aurora A, B, and C. Aurora B has been implicated in many cell cycle processes, including chromosome condensation, segregation, sister chromatid cohesion, and cytokinesis (Carmena et al. 2009). Aurora B is part of the chromosomal passenger complex (CPC), that it is also composed of INCENP, Borealin, and Survivin. Localization of the CPC is dynamic during mitosis and is an indication of the multiple roles of the CPC. Aurora B, along with CDK1, contributes to sister chromatid’s resolution by phosphorylating the cohesion-stabilizing protein Soronin (Nishiyama et al. 2013). CPC also regulates kinetochore to microtubule attachments and activation of the mitotic checkpoint until chromosomes become bi-oriented (Muñoz-Barrera and Monje-Casas 2014). Whether and how these phosphorylated proteins interact with PTMs of centromeric histones or of other enzymes that act on histones is likely to be an area of intense research in the future.
Remarkably, DNA processes can also regulate kinase function. For instance, transcription at the centromere plays an important role in kinetochore assembly, since noncoding RNAs are required for regulating the activation and localization of Aurora B (Blower 2016; Ferri et al. 2009; Jambhekar et al. 2014).
8 Conclusion and Perspectives
In summary, a vast array of PTMs regulate centromeric chromatin and centromere function. Like the rest of the genome, modifications of centromeric chromatin exist in a unique pattern that specifies centromere identity. While previous research on PTMs relied on site-specific antibodies, these methods are replete with technical obstacles. As a result of the progress in protein mass spectrometry, many new aspects of these modifications have been unraveled. Single-cell epigenomic methods are also very rapidly developing, which have the potential to polish our understanding of histone modifications in a more detailed manner. Single-cell DamID could also support genome wide analysis of histone modifications by using Dam fusion with specific histone readers or modifiers.
Improving and refining our knowledge about histone modifications that occur in particular cells at defined moments will improve our understanding of how epigenetic processes crosstalk with one another, and their role in stemness, development, and disease.
Abbreviations
- aa:
-
Amino acid
- C. elegans :
-
Caenorhabditis elegans
- CAL1:
-
Chromosome alignment defect 1
- CID:
-
Centromere identifier (Drosophila)
- Dam:
-
Deoxyadenosine methylase
- D. melanogaster :
-
Drosophila melanogaster
- Dnmt:
-
DNA methyltransferase
- HACs:
-
Human artificial chromosomes
- HAT B:
-
Histone acetyltransferase B
- HDACs:
-
Histone Deacetylases
- HFD:
-
Histone fold domain
- HJURP:
-
Holliday junction-recognition protein
- H3:
-
Histone 3
- LSD1:
-
Lysine-specific demethylase 1
- PTM:
-
Post-translational modifications
- PcG:
-
Polycomb group
- Plk:
-
Polo-like kinase
- RCC1:
-
Regulator of chromatin condensation 1
- Rdx:
-
Roadkill
- S. cerevisiae :
-
Saccharomyces cerevisiae
- S. pombe :
-
Schizosaccharomyces pombe
- TrxG:
-
Trithorax group
- Z. may :
-
Zea mays
References
Allis CD, Jenuwein T (2016) The molecular hallmarks of epigenetic control. Nat Rev Genet 17:487–500
Allshire RC, Karpen GH (2008) Epigenetic regulation of centromeric chromatin: old dogs, new tricks? Nature reviews. Genetics 9:923–937
Annunziato AT (2005) Split decision: what happens to nucleosomes during DNA replication? J Biol Chem 280:12065–12068
Apostolou E, Hochedlinger K (2013) Chromatin dynamics during cellular reprogramming. Nature 502:462–471
Archambault V, Glover DM (2009) Polo-like kinases: conservation and divergence in their functions and regulation. Nat Rev Mol Cell Biol 10:265–275
Arents G, Burlingame RW, Wang BC, Love WE, Moudrianakis EN (1991) The nucleosomal core histone octamer at 3.1 A resolution: a tripartite protein assembly and a left-handed superhelix. Proc Natl Acad Sci USA 88:10148–10152
Avvakumov N, Nourani A, Cote J (2011) Histone chaperones: modulators of chromatin marks. Mol Cell 41:502–514
Bade D, Pauleau AL, Wendler A, Erhardt S (2014) The E3 ligase CUL3/RDX controls centromere maintenance by ubiquitylating and stabilizing CENP-A in a CAL1-dependent manner. Dev Cell 28:508–519
Bailey AO, Panchenko T, Sathyan KM, Petkowski JJ, Pai P-J, Bai DL, Russell DH, Macara IG, Shabanowitz J, Hunt DF et al (2013) Posttranslational modification of CENP-A influences the conformation of centromeric chromatin. PNAS 110:11827–11832
Bailey AO, Panchenko T, Shabanowitz J, Lehman SM, Bai DL, Hunt DF, Black BE, Foltz DR (2015) Identification of the posttranslational modifications present in centromeric chromatin. Mol Cell Proteomics 15:918–931
Bannister AJ, Kouzarides T (2011) Regulation of chromatin by histone modifications. Cell Res 21:381–395
Bayliss R, Fry A, Haq T, Yeoh S (2012) On the molecular mechanisms of mitotic kinase activation. Open Biol 2:120–136
Bergmann JH, guez MGOMRI, Martins NMC, Kimura H, Kelly DA, Masumoto H, Larionov V, Jansen LET, Earnshaw WC (2011) Epigenetic engineering shows H3K4me2 is required for HJURP targeting and CENP-A assembly on a synthetic human kinetochore. EMBO J 30:328–340
Bird A (2007) Perceptions of epigenetics. Nature 447:396–398
Black BE, Foltz DR, Chakravarthy S, Luger K, Woods VL, Cleveland DW (2004) Structural determinants for generating centromeric chromatin. Nature 430:578–582
Blower MD (2016) Centromeric transcription regulates Aurora-B localization and activation. Cell Rep 15(8):1624–1633
Blower MD, Karpen GH (2001) The role of Drosophila CID in kinetochore formation, cell-cycle progression and heterochromatin interactions. Nat Cell Biol 3:730–739
Blower MD, Sullivan BA, Karpen GH (2002) Conserved organization of centromeric chromatin in flies and humans. Dev Cell 2:319–330
Boltengagen M, Huang A, Boltengagen A, Trixl L, Lindner H, Kremser L, Offterdinger M, Lusser A (2015) A novel role for the histone acetyltransferase Hat1 in the CENP-A/CID assembly pathway in Drosophila melanogaster. Nucl Acids Res 1–15
Bui M, Dimitriadis EK, Hoischen C, An E, Quénet D, Giebe S, Nita-Lazar A, Diekmann S, Dalal Y (2012) Cell-cycle-dependent structural transitions in the human CENP-A nucleosome in vivo. Cell 150:317–326
Carmena M, Ruchaud S, Earnshaw WC (2009) Making the Auroras glow: regulation of Aurora A and B kinase function by interacting proteins. Curr Opin Cell Biol 21:796–805
Cedar H, Bergman Y (2009) Linking DNA methylation and histone modification: patterns and paradigms. Nat Rev Genet 10:295–304
Chang L, Loranger SS, Mizzen C, Ernst SG, Allis CD, Annunziato AT (1997) Histones in transit: cytosolic histone complexes and diacetylation of H4 during nucleosome assembly in human cells. Biochemistry 36:469–480
Chen T, Muratore TL, Schaner-Tooley CE, Shabanowitz J, Hunt DF, Macara IG (2007) N-terminal alpha-methylation of RCC1 is necessary for stable chromatin association and normal mitosis. Nat Cell Biol 9:596–603
Chow CM, Georgiou A, Szutorisz H, Maia e Silva A, Pombo A, Barahona I, Dargelos E, Canzonetta C, Dillon N (2005) Variant histone H3.3 marks promoters of transcriptionally active genes during mammalian cell division. EMBO Rep 6:354–360
Choy JS, Acuna R, Au WC, Basrai MA (2011) A role for histone H4K16 hypoacetylation in Saccharomyces cerevisiae kinetochore function. Genetics 189:11–21
Cohen I, Poreba E, Kamieniarz K, Schneider R (2011) Histone modifiers in cancer: friends or foes? Genes Cancer 2:631–647
Dai J, Higgins JM (2005) Haspin: a mitotic histone kinase required for metaphase chromosome alignment. Cell Cycle 4:665–668
Davey CA, Sargent DF, Luger K, Maeder AW, Richmond TJ (2002) Solvent mediated interactions in the structure of the nucleosome core particle at 1.9 a resolution. J Mol Biol 319:1097–1113
Dunleavy EM, Zhang W, Karpen GH (2013) Solo or doppio: how many CENP-As make a centromeric nucleosome? Nat Struct Mol Biol 20:648–650
Elowe S, Hummer S, Uldschmid A, Li X, Nigg EA (2007) Tension-sensitive Plk1 phosphorylation on BubR1 regulates the stability of kinetochore microtubule interactions. Genes Dev 21:2205–2219
Fachinetti D, Logsdon GA, Abdullah A, Selzer EB, Cleveland DW, Black BE (2017) CENP-A modifications on Ser68 and Lys124 are dispensable for establishment, maintenance, and long-term function of human centromeres. Dev Cell 40:104–113
Falk SJ, Guo LY, Sekulic N, Smoak EM, Mani T, Logsdon GA, Gupta K, Jansen LET, Van Duyne GD, Vinogradov SA et al (2015) CENP-C reshapes and stabilizes CENP-A nucleosomes at the centromere. Science 348:699–703
Falkenberg KJ, Johnstone RW (2014) Histone deacetylases and their inhibitors in cancer, neurological diseases and immune disorders. Nat Rev Drug Discovery 13:673–691
Ferri F, Bouzinba-Segard H, Velasco G, Hube F, Francastel C (2009) Non-coding murine centromeric transcripts associate with and potentiate Aurora B kinase. Nucleic Acids Res 37:5071–5080
Filion GJ, Bemmel JGV, Braunschweig U, Talhout W, Kind J, Ward LD, Castro JD, Kerkhoven RM, Bussemaker HJ, Steensel BV et al (2010) Systematic protein location mapping reveals five principal chromatin types in Drosophila cells. Cell 143:212–224
Fischle W, Tseng BS, Dormann HL, Ueberheide BM, Garcia BA, Shabanowitz J, Hunt DF, Funabiki H, Allis CD (2005) Regulation of HP1-chromatin binding by histone H3 methylation and phosphorylation. Nature 438:1116–1122
Goldberg AD, Allis CD, Bernstein E (2007) Epigenetics: a landscape takes shape. Cell 128:635–638
Goutte-Gattat D, Shuaib M, Ouararhni K, Gautier T, Skoufias DA, Hamiche A, Dimitrov S (2013) Phosphorylation of the CENP-A amino-terminus in mitotic centromeric chromatin is required for kinetochore function. Proc Natl Acad Sci USA 110:8579–8584
Grewal SIS, Elgin SCR (2007) Transcription and RNA interference in the formation of heterochromatin. Nature 447:399–406
Hake SB, Garcia Ba, Kauer M, Baker SP, Shabanowitz J, Hunt DF, Allis CD (2005) Serine 31 phosphorylation of histone variant H3.3 is specific to regions bordering centromeres in metaphase chromosomes. Proc Natl Acad Sci USA 102:6344–6349
Hatakeyama A, Hartmann B, Travers A, Nogues C, Buckle M (2016) High-resolution biophysical analysis of the dynamics of nucleosome formation. Sci Rep 6:1–14
Heard E, Martienssen RA (2014) Transgenerational epigenetic inheritance: myths and mechanisms. Cell 157:95–109
Hewawasam G, Shivaraju M, Mattingly M, Venkatesh S, Martin-Brown S, Florens L, Workman JL, Gerton JL (2010) Psh1 is an E3 ubiquitin ligase that targets the centromeric histone variant Cse4. Mol Cell 40:444–454
Hinchcliffe EH, Day CA, Karanjeet KB, Fadness S, Langfald A, Vaughan KT, Dong Z (2016) Chromosome missegregation during anaphase triggers p 53 cell cycle arrest through histone H3.3 Ser31 phosphorylation. Nat Cell Biol 18:668–675
Hole K, van Damme P, Dalva M, Aksnes H, Glomnes N, Varhaug JE, Lillehaug JR, Gevaert K, Arnesen T (2011) The human N-Alpha-acetyltransferase 40 (hNaa40p/hNatD) is conserved from yeast and N-terminally acetylates histones H2A and H4. PLoS One 6:1–11
Hori T, Shang WH, Toyoda A, Misu S, Monma N, Ikeo K, Molina O, Vargiu G, Fujiyama A, Kimura H et al (2014) Histone H4 Lys 20 monomethylation of the CENP-A nucleosome is essential for kinetochore assembly. Dev Cell 29:740–749
Hsu J-Y, Sun Z-W, Li X, Reuben M, Tatchell K, Bishop DK, Grushcow JM, Brame CJ, Caldwell JA, Hunt DF et al (2000) Mitotic phosphorylation of histone H3 is governed by Ipl1/Aurora kinase and Glc7/PP1 phosphatase in budding yeast and nematodes. Cell 102:279–291
Ishii K, Ogiyama Y, Chikashige Y, Soejima S, Masuda F, Kakuma T, Hiraoka Y, Takahashi K (2008) Heterochromatin integrity affects chromosome reorganization after centromere dysfunction. Science 321:1088–1091
Jambhekar A, Emerman AB, Schweidenback CT, Blower MD (2014) RNA stimulates Aurora B kinase activity during mitosis. PLoS One 9:e100748
Jenuwein T, Allis CD (2001) Translating the histone code. Science 293:1074–1081
Jones PA, Liang G (2009) Rethinking how DNA methylation patterns are maintained. Nat Rev Genet 10:805–811
Karpen GH, Allshire RC (1997) The case for epigenetic effects on centromere identity and function. Trends Genet: TIG 13:489–496
Kelly AE, Ghenoiu C, Xue JZ, Zierhut C, Kimura H, Funabiki H (2010) Survivin reads phosphorylated histone H3 threonine 3 to activate the mitotic kinase Aurora B. Science 330:235–239
Kops GJPL, Weaver BAA, Cleveland DW (2005) On the road to cancer: aneuploidy and the mitotic checkpoint. Nat Rev Cancer 5:773–785
Kouzarides T (2007) Chromatin modifications and their function. Cell 128:693–705
Kunitoku N, Sasayama T, Marumoto T, Zhang D, Honda S, Kobayashi O, Hatakeyama K, Ushio Y, Saya H, Hirota T (2003) CENP-A phosphorylation by Aurora-A in prophase is required for enrichment of Aurora-B at inner centromeres and for kinetochore function. Dev Cell 5:853–864
Lam AL, Boivin CD, Bonney CF, Rudd MK, Sullivan BA (2006) Human centromeric chromatin is a dynamic chromosomal domain that can spread over noncentromeric DNA. Proc Natl Acad Sci USA 103:4186–4191
Lassallette AE, Mocquard E, Arnaud M-C, Thiriet C, Matera AG (2011) H4 replication-dependent diacetylation and Hat1 promote S-phase chromatin assembly in vivo. Mol Biol Cell 2011(22):245–255
Lima de Faria A (1949) Genetics, origin and evolution of kinetochores. Hereditas 35:422–444
Luger K, Mader AW, Richmond RK, Sargent DF, Richmond TJ (1997) Crystal structure of the nucleosome core particle at 2.8 A resolution. Nature 389:251–260
McKinley KL, Cheeseman IM (2016) The molecular basis for centromere identity and function. Nat Rev Mol Cell Biol 17:16–29
Moreno-Moreno O, Medina-Gir S, Torras-Llort M, Azorn NF (2011) The F box protein partner of paired regulates stability of drosophila centromeric histone H3, CenH3 CID. Curr Biol 21:1488–1493
Moreno-Moreno O, Torras-Llort MN, Azor NF (2006) Proteolysis restricts localization of CID, the centromere-specific histone H3 variant of Drosophila, to centromeres. Nucl Acids Res 34:6247–6255
Morey L, Barnes K, Chen Y, Fitzgerald-Hayes M, Baker RE (2004) The histone fold domain of Cse4 is sufficient for CEN targeting and propagation of active centromeres in budding yeast. Eukaryot Cell 3:1533–1543
Muñoz-Barrera M, Monje-Casas F (2014) Increased Aurora B activity causes continuous disruption of kinetochore-microtubule attachments and spindle instability. Proc Natl Acad Sci USA 111:E3996–E4005
Murphy WJ, Larkin DM, Everts-van der Wind A, Bourque G, Tesler G, Auvil L, Beever JE, Chowdhary BP, Galibert F, Gatzke L et al (2005) Dynamics of mammalian chromosome evolution inferred from multispecies comparative maps. Science 309:613–617
Niikura Y, Kitagawa R, Kitagawa K (2016) CENP-A ubiquitylation is inherited through dimerization between cell divisions. Cell Rep 15:61–76
Niikura Y, Kitagawa R, Ogi H, Abdulle R, Pagala V, Kitagawa K (2015) CENP-A K124 ubiquitylation is required for CENP-A deposition at the centromere. Dev Cell 32(5):589–603
Nishiyama T, Sykora MM, Mechtler K, Peters J-M (2013) Aurora B and Cdk1 mediate WapI activation and release of acetylated cohesin from chromosomes by phosphorylating Sororin. Proc Natl Acad Sci 110:13404–13409
Noma KI, Allis CD, Grewal SIS (2001) Transitions in distinct histone H3 methylation patterns at the heterochromatin domain boundaries. Science 293:1150–1155
Ohzeki J-I, Bergmann JH, Kouprina N, Noskov VN, Nakano M, Kimura H, Earnshaw WC, Larionov V, Masumoto H (2012) Breaking the HAC barrier: histone H3K9 acetyl/methyl balance regulates CENP-A assembly. EMBO J 31:2391–2402
Olszak AM, van Essen D, Pereira AJ, Diehl S, Manke T, Maiato H, Saccani S, Heun P (2011) Heterochromatin boundaries are hotspots for de novo kinetochore formation. Nat Cell Biol 13:799–808
Partridge JF, Borgstrøm B, Allshire RC (2000) Distinct protein interaction domains and protein spreading in a complex centromere. Genes Dev 14:783–791
Peters AHFM, Kubicek S, Mechtler K, O’Sullivan RJ, Derijck AAHA, Perez-Burgos L, Kohlmaier A, Opravil S, Tachibana M, Shinkai Y et al (2003) Partitioning and plasticity of repressive histone methylation states in mammalian chromatin. Mol Cell 12:1577–1589
Petruk S, Sedkov Y, Johnston DM, Hodgson JW, Black KL, Kovermann SK, Beck S, Canaani E, Brock HW, Mazo A (2012) TrxG and PcG proteins but not methylated histones remain associated with DNA through replication. Cell 150:922–933
Przewloka MR, Glover DM (2009) The kinetochore and the centromere: a working long distance relationship. Annu Rev Genet 43:439–465
Ranjitkar P, Press MO, Yi X, Baker R, MacCoss MJ, Biggins S (2010) An E3 ubiquitin ligase prevents ectopic localization of the centromeric histone H3 variant via the centromere targeting domain. Mol Cell 40:455–464
Rice JC, Briggs SD, Ueberheide B, Barber CM, Shabanowitz J, Hunt DF, Shinkai Y, Allis CD (2003) Histone methyltransferases direct different degrees of methylation to define distinct chromatin domains. Mol Cell 12:1591–1598
Rosic S, Kohler F, Erhardt S (2014) Repetitive centromeric satellite RNA is essential for kinetochore formation and cell division. J Cell Biol 207:335–349
Sakuno T, Tada K, Watanabe Y (2009) Kinetochore geometry defined by cohesion within the centromere. Nature 458:852–858
Samel A, Cuomo A, Bonaldi T, Ehrenhofer-Murray AE (2012) Methylation of CenH3 arginine 37 regulates kinetochore integrity and chromosome segregation. Proc Natl Acad Sci 109:9029–9034
Schotta G, Lachner M, Sarma K, Ebert A, Sengupta R, Reuter G, Reinberg D, Jenuwein T (2004) A silencing pathway to induce H3-K9 and H4-K29 trimethylation at constitutive heterochromatin. Genes Dev 18:1251–1262
Scott KC, Merrett SL, Willard HF (2006) A heterochromatin barrier partitions the fission yeast centromere into discrete chromatin domains. Curr Biol 16:119–129
Sekulic N, Bassett EA, Rogers DJ, Black BE (2010) The structure of (CENP-A-H4)(2) reveals physical features that mark centromeres. Nature 467:347–351
Shang WH, Hori T, Westhorpe FG, Godek KM, Toyoda A, Misu S, Monma N, Ikeo K, Carroll CW, Takami Y et al (2016) Acetylation of histone H4 lysine 5 and 12 is required for CENP-A deposition into centromeres. Nat Commun 7:13465
Sherman JM, Pillus L (1997) An uncertain silence. Trends Genet 13:308–313
Shin H-J, Baek K-H, Jeon A-H, Kim S-J, Jang K-L, Sung Y-C, Kim C-M, Lee C-W (2003) Inhibition of histone deacetylase activity increases chromosomal instability by the aberrant regulation of mitotic checkpoint activation. Oncogene 22:3853–3858
Smith MM (2002) Centromeres and variant histones: what, where, when and why? Curr Opin Cell Biol 14:279–285
Stein WD (1980) The epigenetic address: a model for embryological development. J Theor Biol 82:663–677
Sullivan BA, Karpen GH (2004) Centromeric chromatin exhibits a histone modification pattern that is distinct from both euchromatin and heterochromatin. Nat Struct Mol Biol 11:1076–1083
Sullivan KF, Hechenberger M, Masri K (1994) Human CENP-A contains a histone H3 related histone fold domain that is required for targeting to the centromere. J Cell Biol 127:581–592
Tachiwana H, Kagawa W, Shiga T, Osakabe A, Miya Y, Saito K, Hayashi-Takanaka Y, Oda T, Sato M, Park S-Y et al (2011) Crystal structure of the human centromeric nucleosome containing CENP-A. Nature 476:232–235
Turner BM (2007) Defining an epigenetic code. Nat Cell Biol 9:2–6
Wang F, Dai J, Daum JR, Niedzialkowska E, Banerjee B, Stukenberg PT, Gorbsky GJ, Higgins JM (2010) Histone H3 Thr-3 phosphorylation by haspin positions Aurora B at centromeres in mitosis. Science 330:231–235
Wirbelauer C, Bell O, Schubeler D (2005) Variant histone H3.3 is deposited at sites of nucleosomal displacement throughout transcribed genes while active histone modifications show a promoter-proximal bias. Genes Dev 19:1761–1766
Yamagishi Y, Honda T, Tanno Y, Watanabe Y (2010) Two histone marks establish the inner centromere and chromosome bi-orientation. Science 330:239–243
Yamagishi Y, Sakuno T, Shimura M, Watanabe Y (2008) Heterochromatin links to centromeric protection by recruiting shugoshin. Nature 455:251–255
Yu Z, Zhou X, Wang W, Deng W, Fang J, Hu H, Wang Z, Li S, Cui L, Shen J et al (2015) Dynamic phosphorylation of CENP-A at Ser68 orchestrates its cell-cycle-dependent deposition at centromeres. Dev Cell 32:68–81
Zhang X, Li X, Marshall JB, Zhong CX, Dawe RK (2005) Phosphoserines on maize centromeric histone H3 and histone H3 demarcate the centromere and pericentromere during chromosome segregation. Plant Cell 17:572–583
Zhao H, Bui M, Dalal Y, Papoian GA (2016) Promiscuous histone mis-assembly is actively prevented by chaperones. J Am Chem Soc 138:13207
Zinkowski RP, Meyne J, Brinkley BR (1991) The centromere-kinetochore complex: a repeat subunit model. J Cell Biol 113:1091–1110
Acknowledgements
We apologize to those authors whose work we did not cite because of space limitations. We thank Anne-Laure Pauleau, Engin Demirdizen, and Aubry K. Miller for comments on the manuscript and the entire Erhardt lab for fruitful discussions. Work in the Erhardt laboratory is funded by the Deutsche Forschungsgemeinschaft (EXC81, SFB1036, and ER576/2-2) and the European Research Council (ERC-CoG-682496). AGA is a member of the Hartmut Hoffmann-Berling International Graduate School of Molecular and Cellular Biology.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Glossary
- Histone code
-
It describes the hypothesis that the genetic information encoded in the DNA with a four-letter code is controlled by diverse post-translational modifications of histones which act in combination to provide binding sites for specific regulatory proteins depending on the combinatorial use of histone modifications.
- PTM
-
Post-translational modifications are covalent modifications of proteins catalyzed by enzymes, which occur after proteins translation is completed.
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
García del Arco, A., Erhardt, S. (2017). Post-translational Modifications of Centromeric Chromatin. In: Black, B. (eds) Centromeres and Kinetochores. Progress in Molecular and Subcellular Biology, vol 56. Springer, Cham. https://doi.org/10.1007/978-3-319-58592-5_9
Download citation
DOI: https://doi.org/10.1007/978-3-319-58592-5_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-58591-8
Online ISBN: 978-3-319-58592-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)