Abstract
The kinetochore is the multi-protein complex that drives chromosome segregation in eukaryotes. It assembles onto centromeric DNA and mediates attachment to spindle microtubules. Kinetochore research over the last several decades has been focused on a few animal and fungal model organisms, which revealed a detailed understanding of the composition and organization of their kinetochores. Yet, these traditional model organisms represent only a small fraction of all eukaryotes. To gain insights into the actual degree of kinetochore diversity, it is critical to extend these studies to nontraditional model organisms from evolutionarily distant lineages. In this chapter, we review the current knowledge of kinetochores across diverse eukaryotes with an emphasis on variations that arose in nontraditional model organisms. In addition, we also review the literature on species, in which the subcellular localization of kinetochores has changed from the nucleoplasm to the nuclear membrane. Finally, we speculate on the organization of the chromosome segregation machinery in an early eukaryotic ancestor to gain insights into fundamental principles of the chromosome segregation machinery, which are common to all eukaryotes.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Chromosome Segregation Machinery
- Non-traditional Model Organisms
- Last Eukaryotic Common Ancestor (LECA)
- Extranuclear Spindle
- Ndc80 Complex
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Mitosis is the process that partitions newly replicated chromosomes from the mother cell into the two emerging daughter cells (McIntosh 2016). Fundamental to this process is the kinetochore, a macromolecular protein complex that assembles onto specialized chromosomal regions called centromeres to mediate the attachment of sister chromatids to spindle microtubules (Cheeseman and Desai 2008; Santaguida and Musacchio 2009). Kinetochores also promote the recruitment of cohesin complexes around centromeres to hold duplicated sister chromatids together until anaphase (Nasmyth and Haering 2009). At their DNA-binding interface, kinetochores need to ensure stable attachment to tolerate the pulling forces exerted by kinetochore microtubules (Allshire and Karpen 2008; Fukagawa and Earnshaw 2014; Westhorpe and Straight 2015; McKinley and Cheeseman 2016). In contrast to this more static attachment, the binding to spindle microtubules must be dynamically regulated (Foley and Kapoor 2013; Cheerambathur and Desai 2014; London and Biggins 2014; Etemad and Kops 2016). Faithful chromosome segregation requires that sister kinetochores form bioriented attachments to spindle microtubules emanating from opposite poles (Nicklas 1997). Biorientation is necessary for the accurate distribution of sister chromatids into daughter cells during anaphase.
Research on kinetochores has mainly been performed on a few model organisms, such as fungi, worms, flies, and vertebrates. Their studies have been instrumental in informing us about the basic composition and organization of kinetochores among these species. However, these “traditional” model organisms only represent a small fraction of the entire eukaryotic biodiversity. In fact, both animals and fungi are members of the Opisthokonta, that is only one out of six major supergroups of eukaryotes (Fig. 1) (Walker et al. 2011; Adl et al. 2012). While extensive analyses have not been performed on kinetochores in non-opisthokonts, glances into kinetochores from additional species scattered across the eukaryotic phylogenetic tree have revealed extraordinary levels of variations in kinetochore composition and subcellular location. This stands in sharp contrast to many other cell cycle machines that are highly conserved among diverse eukaryotes (e.g., Cyclin/CDK, cohesin, condensin, the anaphase promoting complex, and proteasomes). In this chapter, we will first discuss the extent of similarity and variation in kinetochore composition among animals and fungi. We will then review kinetochores in select organisms from different supergroups, as well as unique kinetochores that evolved in kinetoplastids. Following up on that, we will highlight membrane-bound kinetochores found in some unicellular organisms. Finally, we will speculate on the organization of the chromosome segregation machinery in early eukaryotes.
2 The Kinetochore Complex in Animals and Fungi
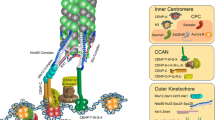
Genetic and biochemical analyses in fungi and vertebrates have led to the identification of more than 80 proteins that are part of the kinetochore (Biggins 2013; Cheeseman 2014). The structural core of the kinetochore consists of an inner and an outer complex. The inner kinetochore complex binds centromeric chromatin. It serves as a platform for the recruitment of the outer kinetochore complex that binds spindle microtubules during mitosis and meiosis. Both complexes are characterized by a network of several protein complexes that work in concert to regulate the proper attachment of kinetochore microtubules to centromeric DNA.
2.1 Similarities and Variations of the Inner Kinetochore in Animals and Fungi
In vertebrates and fungi, the inner kinetochore consists of ~16 members that are commonly referred to as the Constitutive Centromere Associated Network or CCAN (Cheeseman and Desai 2008; Westermann and Schleiffer 2013; Westhorpe and Straight 2013; Fukagawa and Earnshaw 2014) (Table 1). It is generally agreed that the recruitment of all CCAN members in these species depends on a specialized centromeric histone H3 variant, CENP-A (also known as CenH3—see the Note on nomenclature at the end of this chapter) (Black and Cleveland 2011; Müller and Almouzni 2014; Earnshaw 2015) and its direct DNA-binding partner CENP-C (Carroll et al. 2010; Basilico et al. 2014). In addition to CENP-A and CENP-C, other CCAN components also make DNA contacts, including the histone-fold proteins CENP-T and CENP-W as well as CENP-UAme1 and CENP-QOkp1 in budding yeast (Hori et al. 2008; Hornung et al. 2014).
Given their central role in kinetochore function, it is surprising that several inner kinetochore components undergo rapid evolution at the amino acid level, which complicates homology-based predictions even in well-sequenced species (Henikoff et al. 2001; Talbert et al. 2009; Malik and Henikoff 2009). While sequence similarity of several CCAN components between vertebrates and budding yeast was revealed early on (Meraldi et al. 2006), the identification of phylogenetic relationship for other CCAN components often required advanced bioinformatics tools due to limited sequence similarities (Schleiffer et al. 2012; Westermann and Schleiffer 2013). For example, the budding yeast CENP-TCnn1 was only identified using a combination of proteomic approaches and remote homology predictions (Schleiffer et al. 2012). Thus, experimental approaches as well as advanced bioinformatics are required to obtain a comprehensive picture of kinetochores.
While most CCAN components are conserved between vertebrates and fungi, CCAN proteins appear to be absent in Caenorhabditis elegans and Drosophila melanogaster except for CENP-C (Table 1). While it is formally possible that these species have highly divergent CCAN members, the wealth of extensive genetic screens for chromosome segregation defects and biochemical purifications of kinetochore components makes this unlikely (Cheeseman et al. 2004; Goshima et al. 2007; Przewloka et al. 2007, 2011). Therefore, it appears that nematodes and Diptera have “simpler” inner kinetochore complexes that just consist of CENP-C, which connects the CENP-A-containing chromatin to outer kinetochore proteins. The reason for this potential reduction in kinetochore complexity remains unknown. In contrast to D. melanogaster, homologous CCAN members have been identified in other insects (see below), showing that the near-complete loss of CCAN is not common to all insects and instead occurred in a dipteran ancestor around 250 Mya (Hedges et al. 2006).
While CENP-A was thought to be essential for kinetochore assembly in all animals and fungi, recent studies showed that a number of insects have recurrently lost CENP-A (Drinnenberg et al. 2014). Intriguingly, all CENP-A-deficient insects analyzed are derived from independent transitions from monocentric chromosomes (where microtubules attach to a single chromosomal region) to holocentric chromosomes (where microtubules attach along the entire length of the chromosome) (Melters et al. 2012; Drinnenberg et al. 2014). This strong correlation between the change in centromeric architecture and the loss of CENP-A supports a causal relationship between the two events in that the transition to holocentromeres facilitated the loss of CENP-A or vice versa. While CENP-A and its binding partner CENP-C are lost, several of the CCAN components continue to be present even in CENP-A-deficient insects (e.g., the silkworm Bombyx mori (Table 1)). These findings suggest that the assembly of the inner kinetochore has been altered in CENP-A-deficient insects, allowing CENP-A-independent kinetochore formation. Whether or not new kinetochore components have evolved to compensate for the loss of CENP-A is an open question. It is important to note that other holocentric organisms including nematodes (e.g., C. elegans) have retained CENP-AHCP-3. Thus, despite the usage of the generic term “holocentromere”, the basic architecture and regulation of holocentromeres is likely to be diverse among different species.
2.2 The Composition of the Outer Kinetochore Is Highly Conserved in Animals and Fungi
The outer kinetochore complex is recruited to centromeres upon the onset of mitosis to connect to spindle microtubules. This interaction is accomplished by the ~10-subunit KMN network that consists of the Knl1, Mis12, and Ndc80 complexes (Cheeseman et al. 2006; Petrovic et al. 2014). In contrast to the inner kinetochore, the composition of the outer kinetochore is widely conserved across animals and fungi (Meraldi et al. 2006; Tromer et al. 2015). Even CENP-A-deficient insects encode the same repertoire of outer kinetochore components, implying similar means of attaching to microtubules while utilizing alternate inner kinetochore assembly pathways (Drinnenberg et al. 2016). A notable exception to the otherwise conserved composition of the KMN network is found in Diptera. D. melanogaster has lost Dsn1, a subunit of the Mis12 complex (Przewloka and Glover 2009). In addition, the Nnf1 subunit of the Mis12 complex underwent a duplication event giving rise to two paralogs, Nnf1a and Nnf1b, that are part of two distinct Mis12 complexes with similar biochemical behaviors (Przewloka et al. 2007; Schittenhelm et al. 2007; Liu et al. 2016; Richter et al. 2016; Blattner et al. 2016). The loss of Dsn1 could have been compensated by the C-terminal part of the Drosophila Knl1 homolog (Przewloka et al. 2009). Indeed, the overall organization of this complex appears to resemble the human and yeast counterparts (Hornung et al. 2011; Przewloka et al. 2011; Screpanti et al. 2011). Whether these changes have any functional consequences on the Drosophila KMN complex is currently unclear.
3 Glimpses into Kinetochore Compositions in Diverse Eukaryotes
While research on kinetochores in fungi and animals has revealed a paradigm for the basic organization of kinetochores, it remains unclear whether other eukaryotes have similar kinetochores. Comparative studies in additional eukaryotic lineages are a key to revealing the degree of conservation and divergence of kinetochores among eukaryotes. Although bioinformatic analyses have identified some homologous kinetochore proteins in diverse eukaryotes (Table 1), very few studies have characterized the function of individual kinetochore proteins. Furthermore, extensive proteomic screens have not been carried out in most organisms, leaving open the possibility of lineage-specific evolution of additional kinetochore proteins. Below we summarize the current knowledge of kinetochores in select eukaryotes from different supergroups to highlight their peculiarities.
3.1 Supergroup Amoebozoa
The only kinetochore protein that has been characterized in the supergroup Amoebozoa is the centromere-specific histone H3 variant in Dictyostelium discoideum (Dubin et al. 2010). In contrast to nearly all other characterized CENP-A proteins that have at least one extra amino acid in the loop 1 region within the histone fold compared to histone H3 (Malik and Henikoff 2003), D. discoideum CENP-ACenH3 does not have a longer loop 1. While alterations or shortening of residues in loop 1 in other species can impair centromere targeting (Vermaak et al. 2002), cytological studies of D. discoideum CENP-ACenH3 revealed incorporation into centromeric DNA (Dubin et al. 2010). Therefore, the insertion of extra amino acids in loop 1 is not an obligatory feature of CENP-A.
3.2 Supergroup Archaeplastida
Several kinetochore proteins have been characterized in land plants (e.g., Arabidopsis, maize, and barley) (Dawe et al. 1999; ten Hoopen et al. 2000; Sato et al. 2005). For example, homologous kinetochore proteins (such as CENP-C and Mis12) identified by bioinformatics searches were analyzed by means of cytological and mutational studies, confirming their importance for chromosome segregation in mitosis and meiosis. Although most eukaryotes have a single CENP-A protein, multiple CENP-ACENH3 variants are found in Arabidopsis halleri, A. lyrata (Kawabe et al. 2006), Brassica sp. (Wang et al. 2011), Mimulus monkeyflowers (Finseth et al. 2015), barley (Ishii et al. 2015), and Fabeae sp. (Neumann et al. 2012; Neumann et al. 2015). While it is currently unclear whether the individual CENP-ACENH3 variants are functionally distinct, it has been hypothesized that CENP-ACENH3 duplications occurred to counteract the evolutionary force from centromere drive (Finseth et al. 2015) (centromere drive is discussed in the chapter “Cell Biology of Cheating—Transmission of Centromeres and Other Selfish Elements Through Asymmetric Meiosis” by Chmátal et al.).
Compared to land plants, much less is known about kinetochores in other Archaeplastida species. Cyanidioschyzon merolae is a thermoacidiphilic red alga that is thought to be one of the most primitive photosynthetic eukaryotes. Its simple cellular architecture and reduced genome make it an attractive organism for cell biological study (Matsuzaki et al. 2004). Among several homologous kinetochore proteins identified (Table 1), only CENP-ACENH3 has been experimentally characterized to date (Maruyama et al. 2007; Kanesaki et al. 2015). Given its hot and acidic living habitats, it will be interesting to test for potential adaptations of kinetochore components that evolved to cope with such extreme environments.
3.3 Supergroup SAR
The supergroup SAR (Stramenopiles, Alveolates, and Rhizaria: also referred to as Harosa) includes diatoms, ciliates, apicomplexans, and dinoflagellates (discussed later). Ciliates have a somatic macronucleus with highly amplified genes for RNA synthesis as well as several germline micronuclei for genome maintenance. The number of chromosomes in the somatic macronucleus can be as high as 16,000 in some species (Swart et al. 2013). While the germline micronucleus has CENP-ACNA1 and segregates its chromosomes accurately, the somatic macronucleus does not have CENP-ACNA1 and segregates its chromosomes randomly (Cervantes et al. 2006; Cui and Gorovsky 2006).
Apicomplexans include a number of important human pathogens, including Plasmodium and Toxoplasma (Francia and Striepen 2014). Several kinetochore proteins have been identified and functionally characterized in Plasmodium falciparum and Toxoplasma gondii including CENP-ACENH3, CENP-C, and members of the Ndc80 complex (Brooks et al. 2011; Verma and Surolia 2013; Farrell and Gubbels 2014). While the domain architecture appears to be conserved, the T. gondii Nuf2 homolog contains a conserved amino acid motif that appears specific to apicomplexan (Farrell and Gubbels 2014). The functional relevance of this motif, however, remains unclear.
3.4 Supergroup CCTH
Very little is known about kinetochores in the supergroup CCTH (Cryptophytes, Centrohelids, Telonemids, and Haptophytes: also called Hacrobia). Cryptophyte algae are thought to have evolved by engulfing a red alga that contained a primary plastid (Tanifuji and Archibald 2014). In the cryptomonad Guillardia theta, the secondary plastid has retained the red algal-derived relict nucleus (called nucleomorph) (Curtis et al. 2012). How the nucleomorph genome is maintained during cell division remains unknown. While the nucleomorph genome encodes for a putative CENP-A homolog (Douglas et al. 2001) (Table 1), this protein lacks the hallmark of an extended loop 1 region. It will therefore be necessary to experimentally confirm whether it indeed functions as the centromeric histone variant for the segregation of the nucleomorph genome.
3.5 Supergroup Excavata
Excavata is a group of predominantly flagellated species (Walker et al. 2011; Adl et al. 2012). It is divided into Metamonads and Discoba. A number of human parasites belong to this supergroup, such as Giardia, Trichomonas vaginalis, Naegleria fowleri, and Trypanosoma brucei.
Giardia intestinalis (Metamonads) has two histone H3-like molecules that have a longer loop 1. Cytological studies have revealed that only one H3 variant incorporates into centromeres, while the other variant localizes to pericentric heterochromatin (Dawson et al. 2007), underlining the need for experimental approaches to corroborate the identity of the centromeric histone H3 variant. As in Dictyostelium (see above), another metamonad Trichomonas vaginalis has a CENP-ACenH3 protein that does not have a longer loop 1, but its localization pattern is suggestive of centromeric incorporation (Zubácová et al. 2012).
Discoba (also called JEH for Jakobids, Euglenozoa, Heterolobosea) includes Naegleria, Euglena, and kinetoplastids. Although canonical kinetochore proteins have been identified in Naegleria gruberi and Euglena gracilis (Table 1), none has been identified in the genome of kinetoplastids.
4 Unconventional Kinetoplastid Kinetochores
Identification of at least a fraction of canonical kinetochore proteins (especially CENP-A and the Ndc80 complex) in diverse eukaryotes led to a notion that all eukaryotes may build the structural core of the kinetochore using a conserved set of kinetochore proteins (Meraldi et al. 2006). However, none of the canonical kinetochore proteins were identified in the genome of kinetoplastids (Lowell and Cross 2004; Berriman et al. 2005), a group of unicellular eukaryotes defined by the presence of kinetoplast (a large structure in the mitochondrion that contains mitochondrial DNA) (Vickerman 1962). They belong to the supergroup Excavata, Discoba group, Euglenozoa. Euglenozoa is a diverse group of flagellates that include euglenids, diplonemids, symbiontids, and kinetoplastids (Walker et al. 2011; Cavalier-Smith 2016).
To uncover the repertoire of kinetoplastid kinetochores, recent studies utilized proteomic and functional approaches and identified 20 kinetochore proteins in Trypanosoma brucei, named KKT1–20 (Akiyoshi and Gull 2014; Nerusheva and Akiyoshi 2016). The majority of these proteins are conserved among kinetoplastids, including the free-living Bodo saltans. However, obvious orthologs of KKT proteins were not found even in euglenids, which instead have canonical kinetochore proteins (Akiyoshi 2016). The unique KKT-based kinetochores are therefore not conserved across Euglenozoa but are apparently restricted to kinetoplastids. It remains unclear why kinetoplastids possess a unique set of kinetochore proteins (discussed below).
4.1 Domain Architectures of Kinetoplastid Kinetochore Proteins
Sequence analyses of kinetoplastid kinetochore proteins have revealed the following conserved domains: a BRCT (BRCA1 C terminus) domain in KKT4, FHA (Forkhead-associated) domain in KKT13, WD40-like domain in KKT15, divergent polo boxes (DPB) in KKT2, KKT3 and KKT20, unique protein kinase domain in KKT2 and KKT3, and CLK (cdc2-like kinase) kinase domain in KKT10 and KKT19. While orthologs of any of the KKT proteins have not been identified in non-kinetoplastid species, the domain architecture and sequence similarity of KKT2, KKT3, and KKT20 suggest that these proteins may share common ancestry with a Polo-like kinase (PLK) (Nerusheva and Akiyoshi 2016). Consistent with this possibility, although the kinase domain of KKT2/3 is apparently unique (Parsons et al. 2005), the next closest kinase domain is that of PLK (Akiyoshi 2016). Furthermore, putative DNA-binding motifs are present in KKT2 and KKT3, suggesting that these proteins likely bind DNA and play a critical role in establishing unique kinetochores in kinetoplastids. Although PLK localizes at the kinetochore in some species, it is not considered to be a structural kinetochore protein in any eukaryote. Substrates of these KKT kinases have yet to be identified.
BRCT, FHA, or CLK-like kinase domains are not present in canonical kinetochore proteins. Domains found in canonical kinetochore proteins such as CH (calponin homology) and RWD (RING finger, WD repeat, DEAD-like helicases) domains have not been identified in KKT proteins. Although KKT proteins do not have similarity to canonical kinetochore proteins at the primary sequence level, high-resolution structural data are necessary to reveal if there is any similarity at the tertiary level.
4.2 Common Features
Although components of the core kinetoplastid kinetochore appear to be distinct from canonical kinetochore proteins present in other eukaryotes, various regulatory proteins that are known to be important for chromosome segregation are conserved, including Aurora B, Cyclin/CDK, cohesin, condensin, separase, and the anaphase promoting complex (Berriman et al. 2005; Akiyoshi and Gull 2013). Aurora B apparently localizes at the kinetochore during prometaphase and metaphase in Trypanosoma brucei (Li et al. 2008), suggesting that its kinetochore regulatory function may be conserved. It is known that the kinase–phosphatase balance is important for regulating kinetochore functions in other eukaryotes. For example, the KNL1 outer kinetochore protein recruits the PP1 phosphatase (Liu et al. 2010; Rosenberg et al. 2011; Meadows et al. 2011; Espeut et al. 2012). Interestingly, a conserved PP1-binding motif is present in KKT7, suggesting that PP1 may regulate kinetochore functions in kinetoplastids. It is therefore possible that kinetoplastid kinetochores, while being structurally distinct, may still utilize a conserved mechanism for the regulation of kinetochore functions.
4.3 Implications from Kinetoplastid Kinetochores
The discovery of KKT-based kinetochores in kinetoplastids challenged a widely held assumption that the core of the kinetochore would be composed of proteins conserved throughout eukaryotes (e.g., CENP-A and Ndc80). A corollary is that eukaryotic chromosome segregation can be achieved using proteins distinct from CENP-A or Ndc80. Understanding how KKT proteins carry out the conserved kinetochore functions will likely provide important insights into fundamental principles of the kinetochore. It also raises a possibility that there might be as yet different types of kinetochores to be discovered in eukaryotes.
5 Membrane-Embedded Kinetochores
In addition to compositional variations, the subcellular location of kinetochores has also been altered in some lineages. In all eukaryotes, chromosomes are enclosed inside the nuclear envelope during most of the cell cycle. This keeps chromosome-based activities physically separated from the cytoplasm where protein synthesis and metabolic processes take place (Martin and Koonin 2006; Koumandou et al. 2013). This separation necessitates proper nuclear remodeling to be coordinated with the chromosome segregation apparatus. There are mainly three types of mitoses depending on the extent of nuclear envelope breakdown: open, semi-open, and closed (Sazer et al. 2014; Makarova and Oliferenko 2016). In open mitosis, the nuclear envelope breaks down completely during mitosis, facilitating access for cytoplasmic spindle microtubules to chromosomes. Semi-open mitosis involves a partial breakdown of the nuclear envelope, allowing transport of material while keeping chromosomes inside the nucleus. In this case, the spindle assembles either inside or outside of the nucleus. In the latter case, spindle microtubules appear to fenestrate through the nuclear envelope and capture chromosomes that are located inside the nucleus. Finally, in closed mitosis, the nuclear envelope does not break down. To enable capturing of sister chromatids, most eukaryotes with closed mitosis assemble an intranuclear spindle. Some eukaryotes, however, assemble an extranuclear spindle where spindle microtubules are located outside of the nucleus. This type of mitosis, though not very common, is found in some Alveolata (dinoflagellates and Perkinsozoa) and Parabasalids (Trichomonads and Hypermastigia), suggesting that it arose independently. To enable attachments between spindle microtubules and kinetochores, these organisms embed their kinetochores in the nuclear envelope. Below we will summarize the current literature on these organisms as well as their sister species and then discuss potential adaptations and implications from such kinetochores.
5.1 Dinoflagellates
Dinoflagellates are a highly diverse group of flagellates, including photosynthetic free-living and parasitic species (Taylor et al. 2007). They belong to the supergroup SAR, Alveolata group, and their sister groups include Perkinsozoa and Apicomplexa (Saldarriaga et al. 2004) (Fig. 2). Dinoflagellates are characterized by large genome sizes in the range of 1,500 Mbp to 185,000 Mbp (Wisecaver and Hackett 2011). Despite having all core histone genes, histones are not involved in packaging the majority of nuclear DNA (Hackett et al. 2005; Marinov and Lynch 2015). In addition, other basic nuclear proteins including Dinoflagellates/Viral NucleoProteins (DVNPs) and HU-like proteins might substitute major histone functions in some of these organisms (Sala-Rovira et al. 1991; Chan and Wong 2007; Gornik et al. 2012; Talbert and Henikoff 2012; Bachvaroff et al. 2014). Their chromosomes are permanently condensed, showing a characteristic liquid crystalline state even in interphase. Interestingly, some, but not all, dinoflagellates have kinetochores embedded in the nuclear envelope with an extranuclear spindle (Leadbeater and Dodge 1967; Kubai and Ris 1969; Spector and Triemer 1981).
Membrane-bound kinetochores have independently evolved at least twice. The diagram shows the evolutionary transition to membrane-bound kinetochores in Perkinsozoa and Parabasalids indicated by the blue star and thick branches. Two black stars indicate the reversion to non-membrane-bound kinetochores
Dinoflagellates are divided into core dinoflagellates, Syndiniales, and early diverging Oxyrrhinales (Fig. 2) (Wisecaver and Hackett 2011). Electron microscopy revealed that kinetochores are embedded in the nuclear envelope in core dinoflagellates [e.g., Amphidinium (Oakley and Dodge 1974) and Crypthecodinium cohnii (Bhaud et al. 2000) (Fig. 3)] as well as in Syndiniales (e.g., Syndinium sp. (Ris and Kubai 1974)). Due to their large genome sizes, genome sequence data are limited in dinoflagellates. In fact, the only dinoflagellate genome sequence available to date is for Symbiodinium minutum (Shoguchi et al. 2013), which revealed putative CENP-A and outer kinetochore components (Table 1) as well as a spindle assembly checkpoint protein (Mad3/BubR1: symbB.v1.2.026514.t1). These findings suggest that this organism still utilizes canonical kinetochore components and the spindle checkpoint. Indeed, a microtubule inhibitor nocodazole delayed mitotic exit in Crypthecodinium cohnii, showing that the spindle checkpoint is functional in core dinoflagellates (Yeung et al. 2000).
Membrane-embedded kinetochores in dinoflagellates. Top Electron microscopy micrograph of mitotic Crypthecodinium cohnii cells. Note that the kinetochore-like structure embedded in the nuclear membrane makes contact with extranuclear spindle microtubules (arrows). Bars 0.8 µm (left), 0.3 µm (right). Reproduced from Bhaud et al. (2000) with permission from the Company of Biologists Limited. Bottom Simplified schematic of images on top
In contrast, a member of the early diverging Oxyrrhinales, Oxyrrhis marina, has an intranuclear spindle, and its chromosomes are not attached to the nuclear envelope (Triemer 1982; Gao and Li 1986; Kato et al. 2000). These studies show that the extranuclear spindle is not a ubiquitous feature of dinoflagellates.
5.2 Perkinsozoa
Perkinsozoa is one of the closest relatives of dinoflagellates (Fig. 2). Like core dinoflagellates and Syndiniales, Perkinsozoa undergoes a closed mitosis with an extranuclear spindle, suggesting that its kinetochores are embedded in the nuclear envelope (e.g., Perkinsus marinus (Perkins 1996) and Cryptophagus (Brugerolle 2002)). Unlike dinoflagellates, however, Perkinsozoa has a smaller genome size that is packaged into nucleosomes (58 Mbp in Perkinsus marinus (Gornik et al. 2012)), and its chromosomes are not permanently condensed. Taken together, the observations in Perkinsozoa suggest that extranuclear spindles and membrane-embedded kinetochores are not necessarily the consequence of an expanded genome or the diminution of packaging histones.
In contrast to Perkinsozoa, its sister group Apicomplexa (e.g., Plasmodium and Toxoplasma gondii) undergoes a closed mitosis with an intranuclear spindle as is the case for many other species in the SAR supergroup (Francia and Striepen 2014). These observations suggest that the nuclear envelop-embedded kinetochores and the extranuclear spindle appeared at or before the emergence of Perkinsozoa (Cavalier-Smith and Chao 2004) (Fig. 2). Therefore, the intranuclear spindle in Oxyrrhis is most likely a derived feature, i.e., back to a more canonical state. The driving forces underlying the switch to the extranuclear spindle or back, however, remain unclear.
5.3 Parabasalids
Membrane-bound kinetochores have also evolved in Parabasalids that belong to the supergroup Excavata, Metamonads group. They are characterized by a unique parabasal apparatus (Honigberg 1963). Electron microscopy studies showed that Trichomonads (Tritrichomonas foetus and Trichomonas vaginalis) and Hypermastigia (Trichonympha agilis) undergo a closed mitosis with an extranuclear spindle, and have kinetochores embedded in the nuclear envelope (Kubai 1973; Ribeiro et al. 2002) (Fig. 4). As in dinoflagellates and Perkinsozoa, canonical kinetochore proteins are found in the genome of Trichomonas vaginalis (Carlton et al. 2007; Zubácová et al. 2012) (Table 1). Because other members of metamonads such as Giardia have intranuclear spindles (Sagolla et al. 2006), membrane-embedded kinetochores and extranuclear spindles in Parabasalids appear to be a derived feature that independently evolved in this lineage.
Bipolar organization of an extranuclear mitotic spindle in Parabasalids. Left Electron microscopy image of Tritrichomonas foetus. Note that some extranuclear spindle microtubules terminate outside the nuclear membrane. Bars 560 and 320 nm (inset). Reproduced from Ribeiro et al. (2002) with permission from John Wiley and Sons. Right Simplified schematic of the images on left
5.4 Implications from Membrane-Bound Kinetochores
The findings of nuclear envelope-embedded kinetochores raise several questions.
It is likely that the change in the location required adaptations of the kinetochore due to the change in biophysical environment. What modifications are necessary to allow kinetochores to be embedded in the nuclear envelope and what are possible consequences? Although the exact position of kinetochores/centromeres within the lipid bilayer of nuclear membranes remains unclear, electron microscopy data indicate that microtubules likely interact with kinetochores in the cytoplasm rather than in the nuclear envelope. This implies that the microtubule-binding domain of the Ndc80 complex is located outside of the nuclear envelope. Other kinetochore proteins that bridge between the Ndc80 complex and CENP-A-containing centromeric chromatin within the nucleus must therefore be embedded within the nuclear envelope. Transmembrane domains have so far not been identified in any of Symbiodinium minutum and Trichomonas vaginalis kinetochore proteins. It is possible that their kinetochores insert into the lipid bilayer by interacting with other nuclear envelope-embedded components such as the nuclear pore complex as previously suggested (Kubai 1973; Ris and Kubai 1974; Cachon and Cachon 1977; Drechsler and McAinsh 2012).
Another question is how membrane-embedded kinetochores form biorientation and regulate cell cycle progression. Can kinetochores move freely in the nuclear envelope or do they require new membrane synthesis? And once biorientation is achieved, how do nuclear and cytoplasmic environments communicate to promote the transition to anaphase, activating the anaphase promoting complex to disrupt cohesion (in the nucleus) and Cyclin B (in the nucleus or cytoplasm), while coordinating the elongation of spindle microtubules (in the cytoplasm)? Finally, what was the evolutionary driving force that underlies the assembly of kinetochores within the nuclear envelope? To address these unknowns, new tools and model systems need to be developed. Importantly, genetic manipulations have already been established in some dinoflagellates (Te and Lohuis 1998; Radakovits et al. 2010) and Trichomonas vaginalis (Delgadillo et al. 1997). Studies on these membrane-embedded kinetochores will likely shed new light onto the diverse mechanism of kinetochore assembly and chromosome segregation in eukaryotes.
6 Speculation of Kinetochores in Early Eukaryotes
Chromosome segregation in the last eukaryotic common ancestor (LECA) was likely driven by tubulin-based polymers because microtubules are a universal feature of the chromosome segregation machinery in all known eukaryotes (McIntosh et al. 2010; Yutin and Koonin 2012; Findeisen et al. 2014). In addition, the LECA likely used condensins to compact chromosomes and cohesins to connect duplicated sister chromatids until anaphase (Nasmyth and Haering 2009; Hirano 2016). Furthermore, the presumed presence of cyclin-dependent kinases and the anaphase promoting complex suggests that chromosome segregation was probably already regulated in the cell cycle dependent manner (Nasmyth 1995; Cavalier-Smith 2010a; Garg and Martin 2016).
In contrast to these components, no obvious ortholog for any of the kinetochore proteins has been identified in prokaryotes, including Lokiarchaeota that is considered to be the closest sister group to eukaryotes (Spang et al. 2015). Therefore, it is unclear whether the LECA utilized canonical kinetochore components, such as CENP-A and Ndc80 that are found in nearly all extant species. It is formally possible that the LECA utilized a KKT-based complex that has later been replaced by canonical kinetochore components in most eukaryotic lineages. This model is consistent with the controversial hypothesis that kinetoplastids might represent the earliest branching eukaryotes (Cavalier-Smith 2010b; Cavalier-Smith 2013; Akiyoshi and Gull 2014). Alternatively, the KKT-based kinetochore may be a derived feature that replaced early eukaryotic kinetochores at some point during the kinetoplastid evolution. A third possibility is that either canonical kinetochores or the KKT-based kinetochores had not yet evolved in the LECA. In this case, chromosome segregation in early eukaryotes might have been similar to the plasmid-partitioning systems found in Bacteria (Gerdes et al. 2010; Reyes-Lamothe et al. 2012) and Archaea (Barillà 2016) where specific DNA elements are recognized by DNA-binding proteins that connect to filament-forming proteins to drive segregation. In such a system, chromosome movement and DNA attachment in the LECA could have been mediated by kinesin or dynein motor proteins. In fact, motor proteins that transport cargo along microtubules and chromokinesins that are capable of connecting chromosomes to microtubules were likely already present in the LECA (Wickstead and Gull 2007; Wickstead et al. 2010).
Kinetochores in all extant eukaryotes are highly complex and consist of many components. Gene duplication likely played an important role in increasing the structural complexity in both canonical and kinetoplastid kinetochores, as evident by the presence of multiple kinetochore proteins that apparently share common ancestry (Schmitzberger and Harrison 2012; Nerusheva and Akiyoshi 2016; Dimitrova et al. 2016; Petrovic et al. 2016). To ensure proper assembly and biorientation of kinetochores, the invention of the Aurora kinase could have been a key evolutionary step that likely had occurred before the emergence of the LECA (Lampson and Cheeseman 2011; Carmena et al. 2012; Hochegger et al. 2013). Error correction by Aurora and direct stabilization of kinetochore-microtubule attachment by tension likely increased the fidelity of chromosome segregation (Akiyoshi et al. 2010; Miller et al. 2016).
7 Conclusions
Most cell biological research over the last several decades has focused on a limited number of model organisms that were selected largely based on historical, not necessarily biological, reasons. Although these studies revealed insights into basic principles of kinetochore organization, a number of differences have been noted even among traditional animal and fungal model organisms. In addition, the unconventional kinetochore in kinetoplastids, the absence of CENP-A in holocentric insects, and nuclear envelope-embedded kinetochores in some eukaryotic lineages all suggest that kinetochores are more plastic than previously thought. The advance of sequencing and genome editing techniques combined with experimental approaches should enable researchers to characterize kinetochores in nontraditional model organisms in a relatively short space of time (Warren 2015; Kobayashi et al. 2015; Gladfelter 2015; Goldstein and King 2016). Insights into kinetochores from diverse species outside of our current catalog of model organisms have a potential to reveal fundamental design and working principles of the eukaryotic segregation machines.
Note to Nomenclature
In different organisms, the centromeric histone H3 variant is referred to with different names (Earnshaw et al. 2013; Talbert and Henikoff 2013). To be consistent with other chapters, we generally refer to the centromeric histone as CENP-A across species. To account for the differences in nomenclature in specific organisms, we donate the superscript of the original name wherever appropriate (for example, CENP-AHCP-3 for C. elegans).
References
Adl SM, Simpson AGB, Lane CE et al (2012) The revised classification of eukaryotes. J Eukaryot Microbiol 59:429–514. doi:10.1111/j.1550-7408.2012.00644.x
Akiyoshi B (2016) The unconventional kinetoplastid kinetochore: from discovery toward functional understanding. Biochem Soc Trans 44:1201–1217. doi:10.1042/BST20160112
Akiyoshi B, Gull K (2013) Evolutionary cell biology of chromosome segregation: insights from trypanosomes. Open Biol 3:130023. doi:10.1098/rsob.130023
Akiyoshi B, Gull K (2014) Discovery of unconventional kinetochores in kinetoplastids. Cell 156:1247–1258. doi:10.1016/j.cell.2014.01.049
Akiyoshi B, Sarangapani KK, Powers AF et al (2010) Tension directly stabilizes reconstituted kinetochore-microtubule attachments. Nature 468:576–579. doi:10.1038/nature09594
Allshire RC, Karpen GH (2008) Epigenetic regulation of centromeric chromatin: old dogs, new tricks? Nat Rev Genet 9:923–937. doi:10.1038/nrg2466
Bachvaroff TR, Gornik SG, Concepcion GT et al (2014) Dinoflagellate phylogeny revisited: using ribosomal proteins to resolve deep branching dinoflagellate clades. Mol Phylogenet Evol 70:314–322. doi:10.1016/j.ympev.2013.10.007
Barillà D (2016) Driving apart and segregating genomes in archaea. Trends Microbiol. doi:10.1016/j.tim.2016.07.001
Basilico F, Maffini S, Weir JR et al (2014) The pseudo GTPase CENP-M drives human kinetochore assembly. Elife 3:e02978
Berriman M, Ghedin E, Hertz-Fowler C et al (2005) The genome of the African trypanosome Trypanosoma brucei. Science 309:416–422. doi:10.1126/science.1112642
Bhaud Y, Guillebault D, Lennon J et al (2000) Morphology and behaviour of dinoflagellate chromosomes during the cell cycle and mitosis. J Cell Sci 113(Pt 7):1231–1239
Biggins S (2013) The composition, functions, and regulation of the budding yeast kinetochore. Genetics 194:817–846. doi:10.1534/genetics.112.145276
Black BE, Cleveland DW (2011) Epigenetic centromere propagation and the nature of CENP-A nucleosomes. Cell 144:471–479. doi:10.1016/j.cell.2011.02.002
Blattner AC, Aguilar-Rodríguez J, Kränzlin M et al (2016) Drosophila Nnf1 paralogs are partially redundant for somatic and germ line kinetochore function. Chromosoma. doi:10.1007/s00412-016-0579-4
Brooks CF, Francia ME, Gissot M et al (2011) Toxoplasma gondii sequesters centromeres to a specific nuclear region throughout the cell cycle. Proc Natl Acad Sci U S A 108:3767–3772. doi:10.1073/pnas.1006741108
Brugerolle G (2002) Cryptophagus subtilis: a new parasite of cryptophytes affiliated with the Perkinsozoa lineage. Eur J Protistol 37:379–390. doi:10.1078/0932-4739-00837
Cachon J, Cachon M (1977) Observations on the mitosis and on the chromosome evolution during the lifecycle of Oodinium, a parasitic dinoflagellate. Chromosoma 60:237–251
Carlton JM, Hirt RP, Silva JC et al (2007) Draft genome sequence of the sexually transmitted pathogen Trichomonas vaginalis. Science 315:207–212. doi:10.1126/science.1132894
Carmena M, Wheelock M, Funabiki H, Earnshaw WC (2012) The chromosomal passenger complex (CPC): from easy rider to the godfather of mitosis. Nat Rev Mol Cell Biol 13:789–803. doi:10.1038/nrm3474
Carroll CW, Milks KJ, Straight AF (2010) Dual recognition of CENP-A nucleosomes is required for centromere assembly. J Cell Biol 189:1143–1155. doi:10.1083/jcb.201001013
Cavalier-Smith T (2010a) Origin of the cell nucleus, mitosis and sex: roles of intracellular coevolution. Biol Direct 5:7. doi:10.1186/1745-6150-5-7
Cavalier-Smith T (2010b) Kingdoms Protozoa and Chromista and the eozoan root of the eukaryotic tree. Biol Lett 6:342–345. doi:10.1098/rsbl.2009.0948
Cavalier-Smith T (2013) Early evolution of eukaryote feeding modes, cell structural diversity, and classification of the protozoan phyla Loukozoa, Sulcozoa, and Choanozoa. Eur J Protistol 49:115–178. doi:10.1016/j.ejop.2012.06.001
Cavalier-Smith T (2016) Higher classification and phylogeny of Euglenozoa. Eur J Protistol 56:250–276. doi:10.1016/j.ejop.2016.09.003
Cavalier-Smith T, Chao EE (2004) Protalveolate phylogeny and systematics and the origins of Sporozoa and dinoflagellates (phylum Myzozoa nom. nov.). EUR J PROTISTOL 40:185–212. doi:10.1016/j.ejop.2004.01.002
Cervantes MD, Xi X, Vermaak D et al (2006) The CNA1 histone of the ciliate Tetrahymena thermophila is essential for chromosome segregation in the germline micronucleus. Mol Biol Cell 17:485–497. doi:10.1091/mbc.E05-07-0698
Chan Y-H, Wong JTY (2007) Concentration-dependent organization of DNA by the dinoflagellate histone-like protein HCc3. Nucleic Acids Res 35:2573–2583. doi:10.1093/nar/gkm165
Cheerambathur DK, Desai A (2014) Linked in: formation and regulation of microtubule attachments during chromosome segregation. Curr Opin Cell Biol 26:113–122. doi:10.1016/j.ceb.2013.12.005
Cheeseman IM (2014) The kinetochore. Cold Spring Harb Perspect Biol 6:a015826. doi:10.1101/cshperspect.a015826
Cheeseman IM, Desai A (2008) Molecular architecture of the kinetochore-microtubule interface. Nat Rev Mol Cell Biol 9:33–46. doi:10.1038/nrm2310
Cheeseman IM, Niessen S, Anderson S et al (2004) A conserved protein network controls assembly of the outer kinetochore and its ability to sustain tension. Genes Dev 18:2255–2268. doi:10.1101/gad.1234104
Cheeseman IM, Chappie JS, Wilson-Kubalek EM, Desai A (2006) The conserved KMN network constitutes the core microtubule-binding site of the kinetochore. Cell 127:983–997. doi:10.1016/j.cell.2006.09.039
Cui B, Gorovsky MA (2006) Centromeric histone H3 is essential for vegetative cell division and for DNA elimination during conjugation in Tetrahymena thermophila. Mol Cell Biol 26:4499–4510. doi:10.1128/MCB.00079-06
Curtis BA, Tanifuji G, Burki F et al (2012) Algal genomes reveal evolutionary mosaicism and the fate of nucleomorphs. Nature 492:59–65. doi:10.1038/nature11681
Dawe RK, Reed LM, Yu HG et al (1999) A maize homolog of mammalian CENPC is a constitutive component of the inner kinetochore. Plant Cell 11:1227–1238
Dawson SC, Sagolla MS, Cande WZ (2007) The cenH3 histone variant defines centromeres in Giardia intestinalis. Chromosoma 116:175–184. doi:10.1007/s00412-006-0091-3
Delgadillo MG, Liston DR, Niazi K, Johnson PJ (1997) Transient and selectable transformation of the parasitic protist Trichomonas vaginalis. Proc Natl Acad Sci U S A 94:4716–4720
Dimitrova YN, Jenni S, Valverde R et al (2016) Structure of the MIND complex defines a regulatory focus for yeast kinetochore assembly. Cell 167(1014–1027):e12. doi:10.1016/j.cell.2016.10.011
Douglas S, Zauner S, Fraunholz M et al (2001) The highly reduced genome of an enslaved algal nucleus. Nature 410:1091–1096. doi:10.1038/35074092
Drechsler H, McAinsh AD (2012) Exotic mitotic mechanisms. Open Biol 2:120140. doi:10.1098/rsob.120140
Drinnenberg IA, deYoung D, Henikoff S, Malik HS (2014) Recurrent loss of CenH3 is associated with independent transitions to holocentricity in insects. Elife 3:e03676. doi:10.7554/eLife.03676
Drinnenberg IA, Henikoff S, Malik HS (2016) Evolutionary turnover of kinetochore proteins: a Ship of Theseus? Trends Cell Biol 26:498–510. doi:10.1016/j.tcb.2016.01.005
Dubin M, Fuchs J, Gräf R et al (2010) Dynamics of a novel centromeric histone variant CenH3 reveals the evolutionary ancestral timing of centromere biogenesis. Nucleic Acids Res 38:7526–7537. doi:10.1093/nar/gkq664
Earnshaw WC (2015) Discovering centromere proteins: from cold white hands to the A, B, C of CENPs. Nat Rev Mol Cell Biol 16:443–449. doi:10.1038/nrm4001
Earnshaw WC, Allshire RC, Black BE et al (2013) Esperanto for histones: CENP-A, not CenH3, is the centromeric histone H3 variant. Chromosome Res 21:101–106. doi:10.1007/s10577-013-9347-y
Espeut J, Cheerambathur DK, Krenning L et al (2012) Microtubule binding by KNL-1 contributes to spindle checkpoint silencing at the kinetochore. J Cell Biol 196:469–482. doi:10.1083/jcb.201111107
Etemad B, Kops GJPL (2016) Attachment issues: kinetochore transformations and spindle checkpoint silencing. Curr Opin Cell Biol 39:101–108. doi:10.1016/j.ceb.2016.02.016
Farrell M, Gubbels M-J (2014) The Toxoplasma gondii kinetochore is required for centrosome association with the centrocone (spindle pole). Cell Microbiol 16:78–94. doi:10.1111/cmi.12185
Findeisen P, Mühlhausen S, Dempewolf S et al (2014) Six subgroups and extensive recent duplications characterize the evolution of the eukaryotic tubulin protein family. Genome Biol Evol 6:2274–2288. doi:10.1093/gbe/evu187
Finseth FR, Dong Y, Saunders A, Fishman L (2015) Duplication and adaptive evolution of a key centromeric protein in Mimulus, a genus with female meiotic drive. Mol Biol Evol 32:2694–2706. doi:10.1093/molbev/msv145
Foley EA, Kapoor TM (2013) Microtubule attachment and spindle assembly checkpoint signalling at the kinetochore. Nat Rev Mol Cell Biol 14:25–37. doi:10.1038/nrm3494
Francia ME, Striepen B (2014) Cell division in apicomplexan parasites. Nat Rev Micro 12:125–136. doi:10.1038/nrmicro3184
Fukagawa T, Earnshaw WC (2014) The centromere: chromatin foundation for the kinetochore machinery. Dev Cell 30:496–508. doi:10.1016/j.devcel.2014.08.016
Gao XP, Li JY (1986) Nuclear division in the marine dinoflagellate Oxyrrhis marina. J Cell Sci 85:161–175
Garg SG, Martin WF (2016) Mitochondria, the cell cycle, and the origin of sex via a syncytial eukaryote common ancestor. Genome Biol Evol 8:1950–1970. doi:10.1093/gbe/evw136
Gerdes K, Howard M, Szardenings F (2010) Pushing and pulling in prokaryotic DNA segregation. Cell 141:927–942. doi:10.1016/j.cell.2010.05.033
Gladfelter AS (2015) How nontraditional model systems can save us. Mol Biol Cell 26:3687–3689. doi:10.1091/mbc.E15-06-0429
Goldstein B, King N (2016) The future of cell biology: emerging model organisms. Trends Cell Biol 26:818–824. doi:10.1016/j.tcb.2016.08.005
Gornik SG, Ford KL, Mulhern TD et al (2012) Loss of nucleosomal DNA condensation coincides with appearance of a novel nuclear protein in dinoflagellates. Curr Biol 22:2303–2312. doi:10.1016/j.cub.2012.10.036
Goshima G, Wollman R, Goodwin SS et al (2007) Genes required for mitotic spindle assembly in Drosophila S2 cells. Science 316:417–421. doi:10.1126/science.1141314
Hackett JD, Scheetz TE, Yoon HS et al (2005) Insights into a dinoflagellate genome through expressed sequence tag analysis. BMC Genom 6:80. doi:10.1186/1471-2164-6-80
Hedges SB, Dudley J, Kumar S (2006) TimeTree: a public knowledge-base of divergence times among organisms. Bioinformatics 22:2971–2972. doi:10.1093/bioinformatics/btl505
Henikoff S, Ahmad K, Malik HS (2001) The centromere paradox: stable inheritance with rapidly evolving DNA. Science 293:1098–1102. doi:10.1126/science.1062939
Hirano T (2016) Condensin-based chromosome organization from bacteria to vertebrates. Cell 164:847–857. doi:10.1016/j.cell.2016.01.033
Hochegger H, Hégarat N, Pereira-Leal JB (2013) Aurora at the pole and equator: overlapping functions of Aurora kinases in the mitotic spindle. Open Biol 3:120185. doi:10.1098/rsob.120185
Honigberg BM (1963) Evolutionary and systematic relationships in the flagellate order Trichomonadida Kirby. J Protozool 10:20–63
Hori T, Amano M, Suzuki A et al (2008) CCAN makes multiple contacts with centromeric DNA to provide distinct pathways to the outer kinetochore. Cell 135:1039–1052. doi:10.1016/j.cell.2008.10.019
Hornung P, Maier M, Alushin GM et al (2011) Molecular architecture and connectivity of the budding yeast Mtw1 kinetochore complex. J Mol Biol 405:548–559. doi:10.1016/j.jmb.2010.11.012
Hornung P, Troc P, Malvezzi F et al (2014) A cooperative mechanism drives budding yeast kinetochore assembly downstream of CENP-A. J Cell Biol 206:509–524. doi:10.1083/jcb.201403081
Ishii T, Karimi-Ashtiyani R, Banaei-Moghaddam AM et al (2015) The differential loading of two barley CENH3 variants into distinct centromeric substructures is cell type- and development-specific. Chromosome Res 23:277–284. doi:10.1007/s10577-015-9466-8
Kanesaki Y, Imamura S, Matsuzaki M, Tanaka K (2015) Identification of centromere regions in chromosomes of a unicellular red alga, Cyanidioschyzon merolae. FEBS Lett 589:1219–1224. doi:10.1016/j.febslet.2015.04.009
Kato KH, Moriyama A, Itoh TJ et al (2000) Dynamic changes in microtubule organization during division of the primitive dinoflagellate Oxyrrhis marina. Biol Cell 92:583–594. doi:10.1016/S0248-4900(00)01106-0
Kawabe A, Nasuda S, Charlesworth D (2006) Duplication of centromeric histone H3 (HTR12) gene in Arabidopsis halleri and A. lyrata, plant species with multiple centromeric satellite sequences. Genetics 174:2021–2032. doi:10.1534/genetics.106.063628
Kobayashi N, Suzuki Y, Schoenfeld LW et al (2015) Discovery of an unconventional centromere in budding yeast redefines evolution of point centromeres. Curr Biol 25:2026–2033. doi:10.1016/j.cub.2015.06.023
Koumandou VL, Wickstead B, Ginger ML et al (2013) Molecular paleontology and complexity in the last eukaryotic common ancestor. Crit Rev Biochem Mol Biol 48:373–396. doi:10.3109/10409238.2013.821444
Kubai DF (1973) Unorthodox mitosis in Trichonympha agilis: kinetochore differentiation and chromosome movement. J Cell Sci 13:511–552
Kubai DF, Ris H (1969) Division in the dinoflagellate Gyrodinium Cohnii (schiller). J Cell Biol 40:508–528. doi:10.1083/jcb.40.2.508
Lampson MA, Cheeseman IM (2011) Sensing centromere tension: Aurora B and the regulation of kinetochore function. Trends Cell Biol 21:133–140. doi:10.1016/j.tcb.2010.10.007
Leadbeater B, Dodge JD (1967) An electron microscope study of nuclear and cell division in a dinoflagellate. Arch Mikrobiol 57:239–254
Li Z, Umeyama T, Wang CC (2008) The chromosomal passenger complex and a mitotic kinesin interact with the Tousled-like kinase in trypanosomes to regulate mitosis and cytokinesis. PLoS ONE 3:e3814. doi:10.1371/journal.pone.0003814
Liu D, Vleugel M, Backer CB et al (2010) Regulated targeting of protein phosphatase 1 to the outer kinetochore by KNL1 opposes Aurora B kinase. J Cell Biol 188:809–820. doi:10.1083/jcb.201001006
Liu Y, Petrovic A, Rombaut P et al (2016) Insights from the reconstitution of the divergent outer kinetochore of Drosophila melanogaster. Open Biol 6:150236. doi:10.1098/rsob.150236
London N, Biggins S (2014) Signalling dynamics in the spindle checkpoint response. Nat Rev Mol Cell Biol 15:736–747. doi:10.1038/nrm3888
Lowell JE, Cross GAM (2004) A variant histone H3 is enriched at telomeres in Trypanosoma brucei. J Cell Sci 117:5937–5947. doi:10.1242/jcs.01515
Makarova M, Oliferenko S (2016) Mixing and matching nuclear envelope remodeling and spindle assembly strategies in the evolution of mitosis. Curr Opin Cell Biol 41:43–50. doi:10.1016/j.ceb.2016.03.016
Malik HS, Henikoff S (2003) Phylogenomics of the nucleosome. Nat Struct Biol 10:882–891. doi:10.1038/nsb996
Malik HS, Henikoff S (2009) Major evolutionary transitions in centromere complexity. Cell 138:1067–1082. doi:10.1016/j.cell.2009.08.036
Marinov GK, Lynch M (2015) Diversity and divergence of dinoflagellate histone proteins. G3 (Bethesda) 6:397–422. doi:10.1534/g3.115.023275
Martin W, Koonin EV (2006) Introns and the origin of nucleus-cytosol compartmentalization. Nature 440:41–45. doi:10.1038/nature04531
Maruyama S, Kuroiwa H, Miyagishima S et al (2007) Centromere dynamics in the primitive red alga Cyanidioschyzon merolae. Plant J 49:1122–1129. doi:10.1111/j.1365-313X.2006.03024.x
Matsuzaki M, Misumi O, Shin-I T et al (2004) Genome sequence of the ultrasmall unicellular red alga Cyanidioschyzon merolae 10D. Nature 428:653–657. doi:10.1038/nature02398
McIntosh JR (2016) Mitosis. Cold Spring Harb Perspect Biol. doi:10.1101/cshperspect.a023218
McIntosh JR, Volkov V, Ataullakhanov FI, Grishchuk EL (2010) Tubulin depolymerization may be an ancient biological motor. J Cell Sci 123:3425–3434. doi:10.1242/jcs.067611
McKinley KL, Cheeseman IM (2016) The molecular basis for centromere identity and function. Nat Rev Mol Cell Biol 17:16–29. doi:10.1038/nrm.2015.5
Meadows JC, Shepperd LA, Vanoosthuyse V et al (2011) Spindle checkpoint silencing requires association of PP1 to both Spc7 and kinesin-8 motors. Dev Cell 20:739–750. doi:10.1016/j.devcel.2011.05.008
Melters DP, Paliulis LV, Korf IF, Chan SWL (2012) Holocentric chromosomes: convergent evolution, meiotic adaptations, and genomic analysis. Chromosome Res 20:579–593. doi:10.1007/s10577-012-9292-1
Meraldi P, McAinsh AD, Rheinbay E, Sorger PK (2006) Phylogenetic and structural analysis of centromeric DNA and kinetochore proteins. Genome Biol 7:R23. doi:10.1186/gb-2006-7-3-r23
Miller MP, Asbury CL, Biggins S (2016) A TOG protein confers tension sensitivity to kinetochore-microtubule attachments. Cell 165:1–12. doi:10.1016/j.cell.2016.04.030
Müller S, Almouzni G (2014) A network of players in H3 histone variant deposition and maintenance at centromeres. Biochim Biophys Acta 1839:241–250. doi:10.1016/j.bbagrm.2013.11.008
Nasmyth K (1995) Evolution of the cell cycle. Philos Trans R Soc Lond B Biol Sci 349:271–281. doi:10.1098/rstb.1995.0113
Nasmyth K, Haering CH (2009) Cohesin: its roles and mechanisms. Annu Rev Genet 43:525–558. doi:10.1146/annurev-genet-102108-134233
Nerusheva OO, Akiyoshi B (2016) Divergent polo box domains underpin the unique kinetoplastid kinetochore. Open Biol 6:150206. doi:10.1098/rsob.150206
Neumann P, Navrátilová A, Schroeder-Reiter E et al (2012) Stretching the rules: monocentric chromosomes with multiple centromere domains. PLoS Genet 8:e1002777. doi:10.1371/journal.pgen.1002777
Neumann P, Pavlíková Z, Koblížková A et al (2015) Centromeres off the hook: massive changes in centromere size and structure following duplication of cenh3 gene in Fabeae species. Mol Biol Evol 32:1862–1879. doi:10.1093/molbev/msv070
Nicklas RB (1997) How cells get the right chromosomes. Science 275:632–637
Oakley BR, Dodge JD (1974) Kinetochores associated with the nuclear envelope in the mitosis of a dinoflagellate. J Cell Biol 63:322–325
Parsons M, Worthey EA, Ward PN, Mottram JC (2005) Comparative analysis of the kinomes of three pathogenic trypanosomatids: Leishmania major, Trypanosoma brucei and Trypanosoma cruzi. BMC Genom 6:127. doi:10.1186/1471-2164-6-127
Perkins FO (1996) The structure of Perkinsus marinus (Mackin, Owen and Collier, 1950) Levine 1978 with comments on taxonomy and phylogeny of Perkinsus spp. J Shellfish Res 6:65–87
Petrovic A, Mosalaganti S, Keller J et al (2014) Modular assembly of RWD domains on the Mis12 complex underlies outer kinetochore organization. Mol Cell 53:591–605. doi:10.1016/j.molcel.2014.01.019
Petrovic A, Keller J, Liu Y et al (2016) Structure of the MIS12 complex and molecular basis of its interaction with CENP-C at human kinetochores. Cell 167(1028–1040):e15. doi:10.1016/j.cell.2016.10.005
Przewloka MR, Glover DM (2009) The kinetochore and the centromere: a working long distance relationship. Annu Rev Genet 43:439–465. doi:10.1146/annurev-genet-102108-134310
Przewloka MR, Zhang W, Costa P et al (2007) Molecular analysis of core kinetochore composition and assembly in Drosophila melanogaster. PLoS ONE 2:e478. doi:10.1371/journal.pone.0000478
Przewloka MR, Venkei Z, Glover DM (2009) Searching for Drosophila Dsn1 kinetochore protein. Cell Cycle 8:1292–1293. doi:10.4161/cc.8.8.8159
Przewloka MR, Venkei Z, Bolanos-Garcia VM et al (2011) CENP-C is a structural platform for kinetochore assembly. Curr Biol 21:399–405. doi:10.1016/j.cub.2011.02.005
Radakovits R, Jinkerson RE, Darzins A, Posewitz MC (2010) Genetic engineering of algae for enhanced biofuel production. Eukaryot Cell 9:486–501. doi:10.1128/EC.00364-09
Reyes-Lamothe R, Nicolas E, Sherratt DJ (2012) Chromosome replication and segregation in bacteria. Annu Rev Genet. doi:10.1146/annurev-genet-110711-155421
Ribeiro KC, Pereira-Neves A, Benchimol M (2002) The mitotic spindle and associated membranes in the closed mitosis of trichomonads. Biol Cell 94:157–172
Richter MM, Poznanski J, Zdziarska A et al (2016) Network of protein interactions within the Drosophila inner kinetochore. Open Biol 6:150238. doi:10.1098/rsob.150238
Ris H, Kubai DF (1974) An unusual mitotic mechanism in the parasitic protozoan Syndinium sp. J Cell Biol 60:702–720
Rosenberg JS, Cross FR, Funabiki H (2011) KNL1/Spc105 recruits PP1 to silence the spindle assembly checkpoint. Curr Biol 21:942–947. doi:10.1016/j.cub.2011.04.011
Sagolla MS, Dawson SC, Mancuso JJ, Cande WZ (2006) Three-dimensional analysis of mitosis and cytokinesis in the binucleate parasite Giardia intestinalis. J Cell Sci 119:4889–4900. doi:10.1242/jcs.03276
Sala-Rovira M, Geraud ML, Caput D et al (1991) Molecular cloning and immunolocalization of two variants of the major basic nuclear protein (HCc) from the histone-less eukaryote Crypthecodinium cohnii (Pyrrhophyta). Chromosoma 100:510–518
Saldarriaga JF, “Max” Taylor FJR, Cavalier-Smith T et al (2004) Molecular data and the evolutionary history of dinoflagellates. EUR J PROTISTOL 40:85–111. doi:10.1016/j.ejop.2003.11.003
Santaguida S, Musacchio A (2009) The life and miracles of kinetochores. EMBO J 28:2511–2531. doi:10.1038/emboj.2009.173
Sato H, Shibata F, Murata M (2005) Characterization of a Mis12 homologue in Arabidopsis thaliana. Chromosome Res 13:827–834. doi:10.1007/s10577-005-1016-3
Sazer S, Lynch M, Needleman D (2014) Deciphering the evolutionary history of open and closed mitosis. Curr Biol 24:R1099–R1103. doi:10.1016/j.cub.2014.10.011
Schittenhelm RB, Heeger S, Althoff F et al (2007) Spatial organization of a ubiquitous eukaryotic kinetochore protein network in Drosophila chromosomes. Chromosoma 116:385–402. doi:10.1007/s00412-007-0103-y
Schleiffer A, Maier M, Litos G et al (2012) CENP-T proteins are conserved centromere receptors of the Ndc80 complex. Nat Cell Biol 14:604–613. doi:10.1038/ncb2493
Schmitzberger F, Harrison SC (2012) RWD domain: a recurring module in kinetochore architecture shown by a Ctf19–Mcm21 complex structure. EMBO Rep 13:216–222. doi:10.1038/embor.2012.1
Screpanti E, De Antoni A, Alushin GM et al (2011) Direct binding of Cenp-C to the Mis12 complex joins the inner and outer kinetochore. Curr Biol 21:391–398. doi:10.1016/j.cub.2010.12.039
Shoguchi E, Shinzato C, Kawashima T et al (2013) Draft assembly of the Symbiodinium minutum nuclear genome reveals dinoflagellate gene structure. Curr Biol 23:1399–1408. doi:10.1016/j.cub.2013.05.062
Spang A, Saw JH, Jørgensen SL et al (2015) Complex archaea that bridge the gap between prokaryotes and eukaryotes. Nature 521:173–179. doi:10.1038/nature14447
Spector DL, Triemer RE (1981) Chromosome structure and mitosis in the dinoflagellates: an ultrastructural approach to an evolutionary problem. BioSystems 14:289–298
Swart EC, Bracht JR, Magrini V et al (2013) The Oxytricha trifallax macronuclear genome: a complex eukaryotic genome with 16,000 tiny chromosomes. PLoS Biol 11:e1001473. doi:10.1371/journal.pbio.1001473
Talbert PB, Henikoff S (2012) Chromatin: packaging without nucleosomes. Curr Biol 22:R1040–R1043. doi:10.1016/j.cub.2012.10.052
Talbert PB, Henikoff S (2013) Phylogeny as the basis for naming histones. Trends Genet 29:499–500. doi:10.1016/j.tig.2013.06.009
Talbert PB, Bayes JJ, Henikoff S (2009) Evolution of centromeres and kinetochores: a two-part fugue. In: De Wulf P, Earnshaw WC (eds) The Kinetochore. Springer, New York, pp 1–37
Tanifuji G, Archibald JM (2014) Nucleomorph comparative genomics. In: Löffelhardt W (ed) Endosymbiosis. Springer, Vienna, pp 197–213
Taylor FJR, Hoppenrath M, Saldarriaga JF (2007) Dinoflagellate diversity and distribution. Biodivers Conserv 17:407–418. doi:10.1007/s10531-007-9258-3
Te MR, Lohuis Miller DJ (1998) Genetic transformation of dinoflagellates (Amphidinium and Symbiodinium): expression of GUS in microalgae using heterologous promoter constructs. Plant J 13:427–435. doi:10.1046/j.1365-313X.1998.00040.x
ten Hoopen R, Manteuffel R, Dolezel J et al (2000) Evolutionary conservation of kinetochore protein sequences in plants. Chromosoma 109:482–489
Triemer RE (1982) A unique mitotic variation in the marine dinoflagellate Oxyrrhis marina (pyrrophyta)1. J Phycol 18:399–411. doi:10.1111/j.1529-8817.1982.tb03202.x
Tromer E, Snel B, Kops GJPL (2015) Widespread recurrent patterns of rapid repeat evolution in the kinetochore scaffold KNL1. Genome Biol Evol 7:2383–2393. doi:10.1093/gbe/evv140
Verma G, Surolia N (2013) Plasmodium falciparum CENH3 is able to functionally complement Cse4p and its, C-terminus is essential for centromere function. Mol Biochem Parasitol 192:21–29. doi:10.1016/j.molbiopara.2013.11.002
Vermaak D, Hayden HS, Henikoff S (2002) Centromere targeting element within the histone fold domain of Cid. Mol Cell Biol 22:7553–7561
Vickerman K (1962) The mechanism of cyclical development in trypanosomes of the Trypanosoma brucei sub-group: an hypothesis based on ultrastructural observations. Trans R Soc Trop Med Hyg 56:487–495
Walker G, Dorrell RG, Schlacht A, Dacks JB (2011) Eukaryotic systematics: a user’s guide for cell biologists and parasitologists. Parasitology 138:1638–1663. doi:10.1017/S0031182010001708
Wang G, He Q, Liu F et al (2011) Characterization of CENH3 proteins and centromere-associated DNA sequences in diploid and allotetraploid Brassica species. Chromosoma 120:353–365. doi:10.1007/s00412-011-0315-z
Warren G (2015) In praise of other model organisms. J Cell Biol 208:387–389. doi:10.1083/jcb.201412145
Westermann S, Schleiffer A (2013) Family matters: structural and functional conservation of centromere-associated proteins from yeast to humans. Trends Cell Biol 23:260–269. doi:10.1016/j.tcb.2013.01.010
Westhorpe FG, Straight AF (2013) Functions of the centromere and kinetochore in chromosome segregation. Curr Opin Cell Biol 25:334–340. doi:10.1016/j.ceb.2013.02.001
Westhorpe FG, Straight AF (2015) The centromere: epigenetic control of chromosome segregation during mitosis. Cold Spring Harb Perspect Biol 7:a015818. doi:10.1101/cshperspect.a015818
Wickstead B, Gull K (2007) Dyneins across eukaryotes: a comparative genomic analysis. Traffic 8:1708–1721. doi:10.1111/j.1600-0854.2007.00646.x
Wickstead B, Gull K, Richards TA (2010) Patterns of kinesin evolution reveal a complex ancestral eukaryote with a multifunctional cytoskeleton. BMC Evol Biol 10:110. doi:10.1186/1471-2148-10-110
Wisecaver JH, Hackett JD (2011) Dinoflagellate genome evolution. Annu Rev Microbiol 65:369–387. doi:10.1146/annurev-micro-090110-102841
Yeung PK, New DC, Leveson A et al (2000) The spindle checkpoint in the dinoflagellate Crypthecodinium cohnii. Exp Cell Res 254:120–129. doi:10.1006/excr.1999.4749
Yutin N, Koonin EV (2012) Archaeal origin of tubulin. Biol Direct 7:10. doi:10.1186/1745-6150-7-10
Zubácová Z, Hostomská J, Tachezy J (2012) Histone H3 variants in Trichomonas vaginalis. Eukaryot Cell 11:654–661. doi:10.1128/EC.00006-12
Acknowledgements
We thank Paul Talbert, Kim Nasmyth, Geert Kops, and members of the Akiyoshi and Drinnenberg groups for comments on the manuscript. I.A.D. was supported by funds from the CNRS (Atip Avenir) and Institut Curie. B.A. was supported by a Sir Henry Dale Fellowship jointly supported by the Wellcome Trust and the Royal Society (grant number 098403/Z/12/Z), as well as a Wellcome-Beit Prize Fellowship (grant number 098403/Z/12/A).
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Drinnenberg, I.A., Akiyoshi, B. (2017). Evolutionary Lessons from Species with Unique Kinetochores. In: Black, B. (eds) Centromeres and Kinetochores. Progress in Molecular and Subcellular Biology, vol 56. Springer, Cham. https://doi.org/10.1007/978-3-319-58592-5_5
Download citation
DOI: https://doi.org/10.1007/978-3-319-58592-5_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-58591-8
Online ISBN: 978-3-319-58592-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)