Abstract
A multimodal pattern of hazard of relapse among early stage breast cancer patients has been identified in multiple databases from US, Europe and Asia. What began as a simple investigation of these anomalous data has taken the authors on a path through a diverse variety of clinical topics involving a number of medical specialties and now proposing a clinical trial in Nigeria. We have been studying these data to determine if this can lead to new ideas on how to prevent relapse in breast cancer. Using computer simulation and access to a very high quality database from Milan for patients treated with mastectomy, we proposed that relapses within 3 years of surgery are stimulated somehow by the surgical procedure. Most relapses in breast cancer are in this early category. Retrospective data from a Brussels anesthesiology group suggests a plausible mechanism. Use of ketorolac, a common NSAID analgesic used in surgery was associated with far superior disease-free survival in the first 5 years after surgery. The expected prominent early relapse events in months 9–18 are reduced fivefold. Transient systemic inflammation accompanying surgery (identified by IL-6 in serum) could facilitate angiogenesis of dormant micrometastases, proliferation of dormant single cells, and seeding of circulating cancer stem cells (perhaps in part released from bone marrow) resulting in early relapse and could have been effectively blocked by the perioperative anti-inflammatory agent. If this observation holds up to further scrutiny, it could mean that the simple use of this safe, inexpensive and effective anti-inflammatory agent at surgery might eliminate early relapses. We suggest this would be most effective for triple negative breast cancer and be especially valuable in low and middle income countries. Similar multimodal patterns have been identified in other cancers suggesting a general effect.
Addressing the perioperative window in breast cancer; a 2000 year old unsolved problem in oncology.
Authors’ note: Some figures and text are taken from our 2013 review [1] with kind permission from Bentham Science Publishers.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Breast cancer
- Surgery
- Inflammation
- Bimodal relapse
- Early relapse
- Dormancy
- Ketorolac
- Triple negative breast cancer
- High quality data
- Computer simulation
- Mammography
- Racial disparity
- Adjuvant chemotherapy
- Nigeria
2.1 Introduction
The earliest known recorded description of breast cancer is from Imhotep in the Egyptian pyramid age of 3000–2500 BC. He wrote “If thou examinest a man having tumors on his breast, (and) thou findest that swelling have spread over his breast” and you place your hand upon his breast tumors and you find “like touching a ball of wrappings, or they may be compared to the unripe hemat fruit, which is hard and cool to the touch” they have no granulations; contain no fluid; give rise to no liquid discharge, “thou shouldst say concerning him: One having tumors. An ailment against which I will fight.” Imhotep describes an early differential diagnosis that rules out abscesses or inflammatory diseases. He further writes concerning treatment “There is no treatment.” His comment regarding treatment remained accurate for many centuries. The earliest reports of successful treatment we could find are from Celsus (30 BC–38 AD) and Galen (131–203 AD) [2, 3].
Just as Imhotep described, the initial obvious presentation of breast cancer is usually a breast tumor. In modern times at least in developed countries, breast tumors are removed surgically. Surgeons are very good at removing the breast tumor and leaving no trace of cancer cells behind—at least in the breast. However that is not the end of the problem. The most serious sequential event is that it can return afterwards elsewhere in the body. This could happen any time in the next 15–20 years, yet with different time dependent probabilities. In breast cancer the common pathway from the original tumor to mortality from the disease is after metastatic relapse. Advances have been made but there is much room for improvement since 230,815 new cases of invasive breast cancer were diagnosed and 40,860 females died from breast cancer in the US in 2013 (latest data available from US Center for Disease Control) [4]. If a way could be found to prevent relapse from early stage cancer where the tumor is confined to the primary breast and resectable to late stage cancer where the tumor progresses to much less treatable sites such as liver, lung, bone, and brain, this would dramatically reduce the breast cancer problem. We are very impressed with recent exciting developments to treat metastatic cancer. However a far better solution in our opinion would be to prevent relapses rather than treat after they occur. We aim high in this project and intend to try to solve the problem upstream. Specifically, in this main chapter we will describe our studies that address the relapse problem that has been known for a very, very long time and we will propose a significant partial solution [1].
We have already mentioned in the Introduction and Baum chapter [5] how our investigation into this project began. In this main chapter, we will describe the research over the past 24 years that led us to narrowly studying what may be a simple, inexpensive and non-toxic method to reduce relapse and mortality by 25–50% in developed countries and perhaps more in developing countries. This therapy can be conducted anywhere in the world. In particular it can be done in low and middle income countries where they have 70% of the world’s cancer burden and 5% of the resources [6].
We will make the case that this should be tested where the incidence of triple negative breast cancer (TNBC) is very high. That is where the benefit will appear best and where it is most needed.
2.2 Milan Data Showing Early Bimodal Relapse Pattern That Started This Investigation
Milan data are particularly well known for quality. Persons in Italy typically reside in the same towns and cities for generations so few are lost to follow-up and patients are known to be compliant with physician guidelines. The specific data from Milan that triggered this project were originally presented by Romano Demicheli. These data will be shown and discussed in detail but just as an overview, as shown in Table 2.1, there were 1173 patients treated well before the routine use of adjuvant chemotherapy and with long follow-up. There was a sharp peak in relapses at 18 months post surgery, a minimum at about 50 months and then a broad shallow peak with maximum at approximately 60 months and extending to 10 and more years. Once we knew what to look for, patterns very similar to this have now been identified in over 21 independent databases from Europe, US and Asia. There are more but we stopped counting at 21 [9].
Figure 2.1 shows Demicheli’s Milan data for postmenopausal patients. The sharp peak is seen at 18 months, the minimum at approximately 50 months and broad shallow peak at about 60 months. That small bump at about 100 months is at present poorly understood due to the small number of events at that follow-up time.
Hazard of relapse for postmenopausal patients treated at Istituto Nazionale Tumori in Milan, Italy. Hazard is the number of events that occur in a time interval divided by the number of patients who enter that time as event free. Patients were treated by mastectomy well before the routine use of adjuvant therapy. The time interval in all hazard figures used here is 3 months. Average and standard deviations are indicated as diamonds and bars. The curve was obtained by a kernel-like smoothing procedure
Figure 2.2 shows equivalent data for the premenopausal patients. It can be seen that the 18 month peak now seems to have split into two peaks at 10 months and 30 months. There again is the minimum at 50 months and the shallow broad peak at 60 or so months with a long tail extending to 10 years or more. It needs to be noted that the temporal resolution of these hazard data is 3 months while the more familiar disease-free survival presentation form is typically much longer. It should be emphasized that observing subtle structures of the recurrence dynamics needs large databases and accurate reporting of recurrence times.
Hazard of relapse for premenopausal patients treated at Istituto Nazionale Tumori in Milan, Italy. (Same as Fig. 2.1 except these are premenopausal patients)
Figure 2.3 shows these same Milan data but in the disease-free survival form. These data were previously published by Bonadonna et al. [10]. Bonadonna who unfortunately died in late 2015 was the clinician and the first person to use multiple drugs in adjuvant therapy to prevent relapse in breast cancer. Valagussa was the database manager since the study began. Retsky has had much good and bad experience with breast cancer databases [11]. He trust these Milan data and Pinuccia Valagussa is a major reason for that. As can be seen in this figure there is a rapid fall in disease-free survival in the first few years and then it seems to slow at 4 years before it abruptly begins to fall again extending to 10 and more years. That short plateau is not an artifact and corresponds to the minimum seen in Figs. 2.1 and 2.2.
Milan data for patients treated with mastectomy are presented in the more conventional format as disease free survival. The percentage disease free starts at 100% and rapidly drops until approximately 4 years where there seems to be a short plateau. Relapses start to happen again at about 5 years and slowly continue thereafter tapering off gradually at about 15 years. The plateau at 4 years corresponds to the end of the early relapses seen in Figs. 2.1 and 2.2. Bonadonna, Valagussa et al. NEJM 1995
Figure 2.4 is taken from Fisher et al. [12]. These data are also from the period before the routine use of adjuvant chemotherapy and thus consist of data from patients treated with mastectomy. Retsky was attending an American Society for Clinical Oncology (ASCO) conference after a period when he had been intensely studying the Milan data. Someone flashed this figure as part of a talk. Retsky could immediately identify the same bimodal relapse pattern. Let us explain. These Fisher et al. data are disease free survival grouped by nodes positive with zero nodes on the top and 12 and more nodes on the bottom. For the zero node population, surgery alone cures 80% of patients. Of the 20% who relapse, it can be seen that 10% are in the first 3 years and after a period with few if any relapses, the other 10% appear in the period after 50 or 60 months. For the 12 plus nodes population, it can be seen that essentially all patients eventually relapse. Of these, approximately 90% relapse in the first 3 years then there are few and afterwards the remainder relapse after 60 months. The timing of the relapses is equal from the zero node population to the 12 plus node population. It seems clear to us that similar early and late events are happening in the two groups but the early events are far more frequent for the 12 plus node group.
Something apparently happens to about 10% of the patients with zero nodes positive that results in relapse 1–3 years after surgery. For the patients with 12+ nodes, it seems that 90% of the patients undergo something identical. That apparently is the basic difference between the two populations of patients with widely different number of positive lymph nodes and prognosis. The magnitude of the early relapses is apparent in this figure. It seems that the label of poor prognosis is a result of the high number of early relapses and has far less to do with late relapses. This figure was published by Fisher et al. over 30 years ago but that it showed a bimodal relapse pattern was only known after we reported it as so a few years ago.
2.3 Multipeak Relapse Data Suggest Continuous Growth Is Not a Correct Description of Spontaneous Cancers
These multipeak relapse data are not consistent with the continuous Gompertzian or exponential growth model that has guided breast cancer therapy and early detection for many years [13,14,15,16,17]. The continuous growth model failed to explain growth dynamics of local recurrences after mastectomy suggesting the occurrence of tumor dormancy at clinical level [18]. These various peaks must indicate some clinically important and relevant biological activity but at the time we were unaware of what that was. Demicheli and Retsky began to collaborate.
Retsky visited the Milan National Cancer Institute in 1994. First, Pinuccia Valagussa put a computer disk containing the Milan data in his hand. Then Retsky sat in Demicheli’s office who then described a simple model of tumor growth. Demicheli has both M.D. and Ph.D. degrees. In addition to knowledge of medical oncology, he did much research and published a number of papers on animal model growth. Demicheli then discussed what he considered to be a basic description of metastatic tumor growth. This will seem obvious now but 23 years ago that was definitely not the case. Demicheli said a cancer metastasis starts off as a single cell with malignant potential. It may remain in a non-dividing state for a variable period of time before it begins to divide. After this happens, it can grow to a size limited by lack of blood supply that corresponds to approximately one or a few mm in size. It can remain at this size for another variable period of time until angiogenesis occurs and then it can grow to a detectable size and is diagnosed as a metastatic lesion with lethal potential.
Retsky’s task was to take Valagussa’s data and Demicheli’s model and try and understand what happened to cause the multimodal relapse patterns for pre- and postmenopausal patients treated with surgery only (Fig. 2.5).
Retsky obtained a small grant in 1995 to develop an expert system for clinical breast cancer based on these Milan data. Given his unusual background, Retsky was sufficiently experienced with using computer simulation and stochastic methods to attempt such a project. At the time Retsky was at Harvard Medical School and on Judah Folkman’s staff—another important advantage. Retsky arranged for Demicheli to visit the Folkman lab and speak with him and his staff during 1995. Although preliminary at that time, lab findings and clinical data showed a general agreement.
Retsky was more than willing to use Demicheli’s model in the computer simulation but arrived at that viewpoint from a totally different perspective. Retsky did not have Demicheli’s clinical viewpoint and experimental experience with animal models but the model was consistent with his prior research into the old literature.
2.4 Library Research at University of Texas–San Antonio
In 1989 while Retsky was Professor of Biology at University of Colorado–Colorado Springs he was invited to be Visiting Professor alternate weeks for 6 months in the Department of Oncology at University of Texas–San Antonio. This was in the department of William McGuire, M.D. who played a lead role in introducing estrogen and progesterone receptor values as prognostic markers in breast cancer [19]. They had a database of thousands of patients with receptor values and clinical outcome. McGuire had one of his staff two levels down extract a few columns from their data and provide it electronically to Retsky for his project. Retsky’s task was to integrate these receptor values into a computer simulation he had previously developed and modify it to provide a relevant prognostic report for individual patients. This project did not work well at all. However as was discovered months later, the columns given to Retsky were mislabeled causing what appeared to be significant discrepancies between simulation results and actual clinical outcome. (It is interesting to compare this flawed data transfer process with Valagussa personally placing a computer disk with her Milan data in Retsky’s hand.)
With the planned project results so discouraging, Retsky used his time in San Antonio to pursue other scientific interests. He then spent much time in the well equipped University of Texas Briscoe Medical Library reading papers from prior decades when tumors were not always immediately resected from patients and experimental tumors in animals were allowed to grow larger than in current times when animal rights are more respected. This was also at a time before libraries were digitized. Retsky could have ten volumes open on the table and look from one paper to another. He ended up writing a paper describing old published data on tumor growth in humans and animals [20]. Retsky was particularly interested in the mathematical description of tumor growth. Specifically did tumors grow continually according to the exponential or damped exponential Gompertzian equation as conventionally thought or did tumors grow with occasional periods of temporary dormancy as suggested in Retsky’s first paper in cancer research in 1984? [21]. There are published clinical data reporting dormancy for periods of a year and more in primary breast, primary colon and metastatic pulmonary cancers. This Medical Hypotheses 1990 paper with much relevant information is available free open access.
2.5 Ingleby and Moore Data
One particular paper from 1956 [22] had what appeared to be very distinctive data showing dormancy followed by growth for an untreated primary breast cancer. A 78 year old patient had recently spent their family life savings on what proved to be futile treatment for her husband (carcinoma of the larynx) and stubbornly refused to have her recently discovered breast tumor resected. She did however allow occasional X-rays. Data reported by Ingleby and Moore based on X-ray shadows showed virtually no growth for 11 months (tumor grew from 7 to 8 cm3 with volume doubling time of 20 years) followed by ordinary growth for another 11 months (tumor grew from 8 to 38 cm3 which is a relatively normal 180 day volume doubling time). (1 cm3 is approximately 109 cells.) There were skin changes during that period during which there was infinitesimal change in tumor dimensions. After this growth, the tumor was resected. Subsequent pathology report showed unremarkable adenocarcinoma. This was the only report Retsky could find in the literature showing more than two size measurements of an undisturbed primary breast tumor over a period of more than 1 year (there were 4 measurements taken over 22 months). It could not have grown with 20 year doubling time to the size when first measured. That would have been impossible since the tumor would have had to start growing before the patient was born. It must have grown faster previously and then reached a temporary dormant state for at least 11 months and then growth resumed.
These data were the subject of a polite but intense published argument with Larry Norton of Memorial Sloan Kettering Cancer Center who was the chief proponent of the Gompertzian equation as the general description of breast cancer growth [23, 24]. At least from our point of view, the Gompertzian growth model is clearly inconsistent with Ingleby and Moore data (Fig. 2.6).
Ingleby and Moore [22] tumor size data for an untreated primary breast tumor. Data were originally presented as area but converted to volume for this figure. Growth curve of untreated primary breast cancer
By 1994 Retsky and Demicheli were kindred spirits when it came to possible dormancy in cancer growth—particularly for breast cancer.
The computer simulation went well but the results were unexpected. To summarize the results Fig. 2.7 is shown superimposed upon the Milan data for premenopausal women. The early dominant peak consists of two previously unreported mastectomy-induced relapse modes. Avascular micrometastases that were previously dormant are induced into angiogenesis and produce detectable size lesions at about 10 months post surgery. Also single dormant cells are induced into division and then stochastically undergo angiogenesis to produce relapses at about 30 months post surgery. Together these two relapse modes comprise about 50–80% of all relapses in breast cancer increasing with poor prognosis based on tumor size and the number of nodes positive. The mastectomy-induced angiogenesis mode is most prominent for young patients (premenopausal) with node positive status. The specific ratios are 5:1 node positive to node negative and 2:1 pre- to postmenopausal. Approximately 20% of premenopausal node positive patients experience surgery-induced angiogenesis. We speculated that the late peak with shallow maximum at about 60 months might represent what may be considered the natural history of metastatic growth with no stimulation of growth from surgery. The top of the late shallow peak might indicate the point at which the benefit of surgery first appears. Without surgery, the relapses would continue rising instead of starting to decline at about 60 months. That implies the unstimulated metastatic pipeline is 5 years in duration but much shorter in the presence of intervention. These results were not well received. Nobody was pleased to see these findings.
A 2 day meeting was held in fall 1996 at the University of Colorado - Colorado Springs (UCCS) to discuss the counterintuitive results of this project. Attending were Robert Wardwell, Dr. Jack Speer, Douglas Swartzendruber, Paul Bame, Pinuccia Valagussa, Michael Retsky, and Romano Demicheli. At the time, Retsky was Professor of Biology and Swartzendruber was Dean of Letters, Arts and Sciences at UCCS. After the meeting Wardwell and Retsky took Valagussa and Demicheli on a driving tour to Taos, New Mexico where we spent some time exploring the Southwest US and American Indian culture. As one cultural highlight, Wardwell arranged a lunch meeting with a US citizen of Italian descent. This person was put through law school by the local Indians and lived among them for years. He was trained specifically to represent the Indian community in building and operating casinos on their land. Many questions were asked about the Indian culture. It was very educational.
During this trip Retsky became very impressed with Valagussa as having a perfect personality for a database manager. Valagussa has been a principle speaker at ASCO with several thousand oncologists in the audience so she is very knowledgeable about the subject. She would be the first person to know about a problem in a randomized controlled clinical trial. A classic example of such a problem would be more persons with positive nodes in the intervention arm than in the control arm of a mammography trial. Retsky had seen such a situation before and had met the (mild mannered) database manager. The problem did not get discussed until years after the trial was over when it was too late to do anything except argue about whether the trial was flawed against mammography [25, 26]. Valagussa would not be intimidated and be on the phone screaming to some physician in a distant city to fix their problem.
Retsky et al. [27] paper was published reporting the computer simulation results (with a cautionary label of “Hypothesis” inserted by the editor) and a parallel Demicheli et al. [28] paper was published describing the biological implication and relevance to breast cancer.
In November 1994 Retsky was diagnosed with Stage IIIc colon cancer. Overlapping this scientific investigation Retsky was diagnosed with stage IIIc colon cancer, the result of a routine colonoscopy. However based on his prior research in cancer growth, instead of the usual maximum tolerated adjuvant chemotherapy protocol, he used a previously untested low dose virtually non-toxic, long term (2.5 years) adjuvant chemotherapy that is now called metronomic chemotherapy. The therapy was designed by William Hrushesky, M.D. who is now one of our research colleagues. The reasoning behind this and other details have been described a number of times [29,30,31] including in a biography of Judah Folkman by Robert Cooke in 2001 [32] and an online report on ProPublica by Jake Bernstein in 2014 [33]. A more detailed discussion here would be a distraction from the main theme of the chapter. However it should be noted that the continuous growth Gompertzian kinetics that underlies the scientific basis of maximum tolerated chemotherapy can trace its roots to a mathematically flawed study from 1965 that includes data from only 18 rodents and one rabbit.
Ironically Retsky was under this treatment while conducting the computer simulation. Of note, there was no cognitive dysfunction or “chemo-brain” as it is commonly called by patients.
We have seen similar bimodal relapse patterns in other cancer sites. These include pancreatic, melanoma, non-small-cell lung cancer, prostate, bladder, esophageal, head and neck, osteosarcoma, and renal. This list includes combinations of large databases and case reports. A recent paper by Hamard et al. [34] addressed the possibility that glioblastoma might be another cancer site in which relapse is stimulated by surgery.
Demicheli was working with a group from Duke University and had access to their lung cancer database. He found a multipeak relapse pattern in non-small-cell lung cancer [35]. The timing was different from breast and there was a male to female difference but otherwise it looked very similar to breast. A paper by Maniwa et al. in 1998 shows what appears to be a clear clinical example of surgery induced angiogenesis in lung cancer [36]. Another clinical example of surgery induced angiogenesis can be seen in osteosarcoma (Fig. 2.4 in Retsky et al. Medical Hypotheses 1990) [20]. Data are from Smithers [37].
2.6 It Seems We Have Rediscovered a Problem That Was Known to Surgeons 2000 Years Ago
Remarkably physicians 2000 years ago were able to remove breast cancers without benefit of anesthesia and antibiotics and patients survived the treatment and some did not die from cancer. Celsus (30 BC–38 AD) wrote about cacoethes, carcinoma without ulcerations and then fungating ulcer as a staging sequence. He then wrote that only the cacoethes could be removed; all others are irritated by every method. The more violent the operation, the more angry they grow. Today we often hear about cancer as “angry”. Galen (131–203 AD) wrote they have often cured the tumor growth disease when tumors were small but no one has successively treated it when tumors were large [2]. This information seems to have been overlooked or ignored since the time of Celsus and Galen. We are apparently late-comers to a 2000 year old unsolved problem in oncology.
2.7 Clinical Correlations to Suggested Surgery Induced Metastatic Activity
The surgery induced effects we describe are so large that they must be apparent in clinical cancer. Thus, looking for evidence of these effects we began to examine clinical breast cancer observations to see if and where they may appear. There are more [9, 11] but four such situations are presented below.
-
1.
Adjuvant chemotherapy
Adjuvant chemotherapy works best by far for premenopausal node positive patients. Indeed the curative benefit of adjuvant chemotherapy is approximately 12% for premenopausal node-positive patients and in the 2–6% range for all other categories [38]. This is consistent with our metastasis model. Surgery induces single cell activity that would produce a certain level of chemosensitivity for all patients. However, for premenopausal node positive patients, surgery induced angiogenesis produces very intense micrometastatic cancer growth just after surgery which corresponds to when the clinicians empirically found adjuvant chemotherapy is most effective. We published this hypothesis in 2004 [39]. Note the authors include Judah Folkman with 30 years experience in angiogenesis research and Gianni Bonadonna with 30 years experience in adjuvant chemotherapy research. Figure 2.8 shows Milan data for patients treated with the Bonadonna classic cyclophosphamide methotrexate 5-fluorouracil (CMF) chemotherapy compared to mastectomy only patients. It can be seen that the effect of adjuvant chemotherapy is mostly to reduce early relapses with far less effect on late relapses. That is consistent with our theory.
-
2.
Mammography paradox for women age 40–49
Very disturbing when initially reported, mammography works better for women age 50–59 than it does for women age 40–49. In the first few years of trials to measure the benefit of early detection, more women age 40–49 died of breast cancer in the intervention arm than in the control arm. This was apparent in large trials conducted in US, UK and Sweden. We calculated that because of surgery induced angiogenesis 1/10,000 apparently healthy screened women age 40–49 in the screened population would relapse and die of metastatic breast cancer 3 years after the start of trials of early detection. (Relapse occurs approximately 1 year after detection and surgery and results in mortality 2 years later.) That is just what was found. Thus based on our analysis and Milan data, surgery induced angiogenesis for premenopausal node positive women could quantitatively explain the mammography paradox for young women. Data are shown in our prior publications [40].
Our studies suggest that early detection sometimes results in earlier recruitment of an unfavorable event. This is particularly the case for premenopausal women and even more so for those that have triple negative breast cancer (TNBC) and those that have early onset. We published papers from 2001 to 2013 on this subject. A paper in 2005 got some publicity in Wall Street Journal [41] and as a result we received some very helpful feedback that ultimately led to an explanation for the racial disparity in outcome. Isaac Gukas (who unfortunately died of cancer in 2013) was a surgeon in UK but was originally from Nigeria and practiced oncology there for over 10 years. Gukas reported in a letter to the editor that cancer of the breast commonly occurred in early age 40s in Nigeria and patients relapsed very soon after surgery. Thus Nigerian women would usually avoid surgery and see a traditional herbal therapist since they thought surgery “provoked” the cancer. Gukas coauthored several important papers with us over the next 6 years. His name as one of the authors of our papers was a major asset when we later presented our science in Nigeria.
-
3.
Racial disparity in outcome
This information from Gukas led us to propose an explanation for the racial disparity in outcome in the US. In the US there is a 1.5–2.2-fold excess mortality of Americans of African descent (AA) compared to Americans of European descent (EA). It turns out that breast cancer in AA appears at average age 46 while breast cancer in EA appears at average age 57. It is also apparent that there is an inversion in racial disparity outcome at age 57. That is, for AA of age less than 57 there is a racial disadvantage while for age greater than 57 there is a racial advantage according to a report by Jatoi et al. [42]. Thus it is hard to explain the racial disparity by considering reduced access to health care. We published a number of papers on this subject [43,44,45]. Demicheli et al. Cancer 2007 is perhaps the most pertinent [43].
Since the average age of diagnosis of breast cancer in Nigeria is 42.7 years, this could also explain the very poor outcome in sub-Saharan Africa. It is shown that early relapses are highly associated with TNBC and especially with early onset breast cancer. These two factors that are very common in Nigeria could account for the very poor outcome in that country.
-
4.
“Aggressiveness” in young women
It is a commonly noted trait that breast cancer in young women is “aggressive”. From our perspective this is a result of surgery induced angiogenesis and relapses at approximately 10 months post surgery. While this would appear to the cancer clinician as “aggressive”, from our perspective it is clockwork relapse for premenopausal women at 10 months. Since this occurs in 20% of premenopausal node positive women, breast cancer would appear as “aggressive” for young women.
Summarizing results to this point, it is clear to us that something happens at or about the time of primary tumor surgical removal to induce two previously unreported modes of early relapse in mastectomy treated breast cancer. Surgery induced angiogenesis, most prominent for premenopausal node positive patients, occurs at approximately 10 months post surgery and surgery induced single cell division from dormancy is apparent at 30 months post surgery. These relapse modes comprise the majority of relapses and are most common for poor prognosis patients.
This is most likely a general effect and not just limited to breast cancer. It is more apparent for patients with markers for relapse such as tumor size and number of positive lymph nodes. Prior to 2010 we had a hypothesis to explain this but, compared to what was to follow, it was far less interesting [46].
2.8 Surprising Development from a Belgian Anesthesiology Group
A paper that led to an explanation of these data was published in 2010 by a Belgian anesthesiologist group. Forget et al. [47] retrospectively considered 327 consecutive breast cancer patients treated by mastectomy and conventional adjuvant therapy. The same surgeon conducted the mastectomies. This was done at a teaching hospital so they use a variety of analgesics to train the residents. The paper reported outcome for these patients grouped by what drug was given as anesthetic. One drug resulted in far fewer early relapses. That drug ketorolac was the only NSAID used. Forget et al. data are shown in Fig. 2.9 below.
Data from Forget et al. show disease free survival for patients given perioperative NSAID ketorolac vs. any other analgesic. Forget P, Vandenhende J, Berliere M, Machiels JP, Nussbaum B, Legrand C, De Kock M; Do intraoperative analgesics influence breast cancer recurrence after mastectomy? A retrospective analysis. Anesth Analg. 2010 Jun 1;110(6):1630–5
Demicheli and Retsky visited the hospital in Brussels. Presentations were made by both groups and their data were then updated by resident Sarah Amar and analyzed by Demicheli (Fig. 2.10). (Unfortunately we do not have access to these data to provide more current updates.)
What could explain the Forget et al. data (Table 2.2)?
2.9 Our Attention Is Drawn to Inflammation
We were aware that some intervention that started before surgery would be necessary in order to prevent surgery-induced tumor activity but we were unaware of what could explain the data of Forget et al. A paper published along with the Forget et al. study by Gottschalk et al. [49] presented a long list of possible effects of surgery and the use of anesthesia that might impact tumor growth. These include immunosuppression, transfusion, pain, stress, hypothermia, inflammation and a few others. More recently Horowitz et al. [50] also discuss the perioperative influence on cancer growth. These are all possible however our interest was directed to inflammation as we knew there was a long historical and extensive literature correlating and connecting inflammation and cancer growth.
An earlier event also triggered our interest in inflammation. In 2005 a paper was submitted by El Saghir et al. [51] to Bio-Med-Central Cancer and Retsky was asked to review it. The paper was a case report of a 54 year old smoker from Lebanon who was diagnosed with non-resectable non-small-cell lung cancer. This patient was treated with radiation and while his prognosis was extremely poor, he was at least for a while able to function relatively normally. While driving his car, he bumped his head on the sun visor and within 30 days a 7 cm tumor grew at that spot. The paper was submitted and it was suggested that this might be a result of surgery-induced angiogenesis as we had reported in our papers. Having a quantitative understanding of surgery-induced growth, we were able to consider the numbers from the case report and quickly ruled out any possibility that surgery induced angiogenesis or anything that we have described as surgery induced tumor growth could explain this case. But then Retsky was at a loss and was wandering around the Folkman lab asking if anyone had any ideas on how to explain the case report. Taturo Udagawa suggested he look at inflammation and recommended a paper by Mina Bissell’s group [52]. That paper described an avian model of virus-induced cancer. The curious thing was that tumor grew at any point where the bird was wounded and that this could be fully correlated with an anti-inflammatory intervention. If inflammation was prevented, tumor would not develop at the point of wounding and if inflammation was not so prevented, tumor would grow anywhere the bird was wounded. That could explain the El Saghir et al. paper. Retsky discussed this with Demicheli and then wrote in a published comment to the case report paper: “The unusual isolated and exaggerated situation allowed El Saghir et al. to observe what may be a new and possibly important hematologic metastatic pathway: inflammation as a facilitating precursor to tumor [53]. Metastasis is a very inefficient process. (Tens of thousands of viable) cancer cells might be found in a patient’s blood but only a few metastases occur. The Martins-Green et al. discussion of an inflammation sequence would certainly increase metastatic efficiency since it bypasses extravasation through an intact vessel wall and it provides growth factors in the microenvironment. In the context of Paget’s 1889 “seed and soil” metaphor [54], we have a situation here where many viable seeds and good fertilizer is applied in a large vacant field. The possible connection between inflammation and tumor growth might also help explain why anti-inflammatory drugs such as aspirin, prednisone and Celebrex are often used in cancer prevention and therapy.”
Inflammation is a host response to insult of tissue. Damage to tissue either by pathogen or trauma from physical action triggers a cascade of complex events [55,56,57]. Inflammatory cells and associated complexes collaborate to reconstitute the extracellular matrix, clear debris, attack pathogens, and participate in the transfer and proliferation of healthy cells to the area insulted. This may be considered as a particular aspect of host homeostasis processes and the stability of tissues of the body. This is also considered the hard wired natural reaction to trauma and the resulting host repair process. Many factors including the host condition determine the extent, timing and magnitude of this process. Mast cells in particular can intensify the inflammatory response by releasing histamine which can increase capillary diameter thus increasing its permeability.
Balkwill et al. write that if genetic damage is the “match that lights the fire” of cancer, then inflammation is the “fuel that feeds the flames” and that the survival and proliferation of already initiated cancer cells is affected by inflammation [58]. Inflammation is known to be a significant component of the tumor microenvironment.
Inflammatory oncotaxis, a term used to describe tumor growth at a site of inflammation, has long been seen in persons with known or occult cancer and who have local trauma [59]. As an example, in 1914 when it was more common for persons to walk around with known cancer, Jones and Rous stated: “The localization of secondary tumors at points of injury has been so often remarked upon that it is unnecessary to cite specific instances. The cause for the phenomenon is unknown.” [60].
Systemic inflammation can occur after surgery in which a primary tumor was resected for breast cancer and colon cancer. Regarding colon cancer, Pascual et al. [61] measured proinflammatory cytokine interleukin-6 (IL-6) in serum prior to open or laparoscopic colectomy to establish the baseline levels. To determine the temporal trends, the cytokine was measured again at 4, 12, 24 and 48 h and at 4 days after surgery. It appears that IL-6 level in serum was elevated in open surgery by a factor of 2 compared to laparoscopic surgery and would gradually return to baseline in a week or so. For breast cancer, there are data from Chow et al. [62] and from Perez-Rivas et al. [63] providing an association between primary tumor removal and inflammation that was transient for several days.
In their investigation of the effect of clarithromycin on transient inflammation post mastectomy, Chow et al. used three markers of inflammation that are found useful in predicting outcome in renal disease. These markers are IL-6, tumor necrosis factor-alpha (TNF-alpha) and C-reactive protein (CRP). With 54 patients, they measured inflammation in peripheral blood daily, starting the day prior to surgery and each day for 3 days after surgery. For both control and clarithromycin treated patients Chow et al. found no significant change in TNF-alpha but 60% increase in CRP and 50% increase in IL-6 for several days after surgery. Extrapolating their data it would seem that inflammation was elevated for approximately 1 week. In addition, platelets decreased by about 10% with the same temporal trends as CRP and IL-6.
In their study, Perez-Rivas et al. [63] compared serum markers before and after surgery for early stage breast cancer patients (56 with invasive disease and 7 with ductal carcinoma in situ) and healthy women (16 with benign fibroadenoma). Samples were collected 8 h before mastectomy or lumpectomy and 24 h after surgery. For the general population, they reported that concentration increased for Thrombospondin-2 (THSB2), Colony Stimulating Factor (CSF1), IL-6, IL-7, IL-16, Vascular Endothelial Growth Factor B (VEGF-B), Human Epidermal Growth Factor Receptor 2 (HER2), and Fas Ligand (FasL). These include markers of inflammation and angiogenesis promoters. For invasive breast cancer they report IL-16 and VEGF-A show high velocity after surgery and it is suggested that IL-16 is a factor in dormancy escape.
2.10 Possible Mechanisms for a Post-surgical Systemic Inflammatory Reaction to Drive Tumor Growth
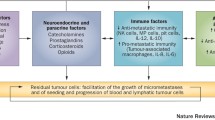
In this following section we paraphrase and quote liberally from our 2013 review on possible mechanisms. A possible new mechanism involves mobile and attracted neutrophils and is described in the accompanying chapter by Bonnelykke-Behrndtz ML et al. [64]. This is possibly also related to discussion in the Forget and DeKock chapter [65] on the importance of neutrophil to lymphocyte ratio (NLR) and a report from Egawa et al. [66] on enhanced capillary leakage from vesicle in presence of inflammation. Such leakage increased to the extent that particles 2000 kDa could passage instead of only 70 kDa in absence of inflammation. Attention is made to the Schmidt et al. chapter and the Forget and DeKock chapter. At the time of writing this chapter we have not seen all submitted invited chapters so we will refrain from additional comments here (Fig. 2.11).
Early relapses are assumed to be related, at least in part, to the inflammatory process due to primary tumor surgical removal, directly or indirectly eliciting peritumoral endothelial cell and single cell proliferation. A few possible mechanisms are explained. Angiogenic factors, like VEGF and bFGF, are directly released by degranulated platelets or even produced via IL-6. Bone marrow derived CXCR-4 positive cells, acting both on tumor foci and on the inflammatory process, are mobilized by SDF-1 directly released or even produced via COX-2. Perioperative ketorolac would restrict both endocrine and cellular pathways, thus impairing the metastatic process. CTC refers to circulating tumor cells.
The suggestion that inflammation can spur angiogenesis and tumor growth is supported by a number of reports [58, 67,68,69,70]. Inflammation could activate growth of dormant single cells or avascular micrometastases thus occasioning early recurrences. A reasonable hypothesis advocates that decrease of inflammatory response to the surgical maneuver may hamper the angiogenesis switch.
There are a few mechanisms that we can propose. At steady state conditions in adult mammals, the majority of endothelial cells are in a quiescent state and this is thought to play a role in the homeostasis of organs and the dormancy of tumors [71]. Inflammation may cause an upregulation possibly including the release of factors that stimulate endothelial cells to proliferate. Secretion of specific cytokines from endothelial cells could then support the regeneration of normal cells and malignant stem cells.
It appears that tumor stem cells like normal stem cells are able to change state from proliferation or quiescence with control of a supporting “niche”, i.e., a specialized microenvironment including both cells and extra-cellular matrix (ECM). A metastatic “vascular niche”, involving endothelial cells, where angiogenesis dependent dormancy could result from interaction between tumor cells and endothelial cells has been suggested, perhaps involving Notch signaling [72]. If so, it is reasonable to assume that the endothelial cells, under an angiogenic spike by the surgical approach to primary tumor, may contribute to dormancy interruption [73]. Thus, reducing inflammation could impair dormant foci awakening.
Additionally, an inflammatory stimulus could modify circulating tumor cell phenotype. When circulating tumor cell expression profile related to activation and ability to adhere, which is a prerequisite for metastasis formation, was analysed, changes in the expression of nanog, a sign of stem cell properties enabling the cells to self renew and grow, was observed. Moreover, EpCAM, HER2, and the adhesion molecule vimentin are risk factors of proliferation possibly influenced by inflammation [74, 75].
Mobilization of bone marrow derived cells that respond to chemo-attractant signals from various organs, undergo a homing process and release several chemokines was induced by tissue lesions [76]. This phenomenon is particularly marked during neovascularization of wounded tissues and is thought to result from direct or paracrine activity inducing capillary formation. Cell trafficking is a common basis of the above-mentioned processes [76]. Indeed, mobilization of normal stem cells from their natural niche and homing in a given tissue is regulated by definite signals. For example, hematopoietic stem cells express the chemokine receptor CXCR4 and selectively respond to SDF-1α. The SDF-1/CXCR4 axis is a main regulator of the normal cell trafficking underlying tissue homeostasis. It is also involved in tumor cell trafficking. CXCR4 overexpression has been detected in more than 20 human tumor types, including ovarian, prostate, esophageal, melanoma, neuroblastoma, and renal cell carcinoma [77]. Therefore, one could reasonably hypothesize that NSAIDs may interfere with SDF1 levels via the pathway COX-2 → PGE → SDF-1, thus impairing processes underlying metastasis development.
Even if an NSAID class effect is plausible, a specific effect of ketorolac remains possible. As already stated, whereas all NSAIDs act against the growth of tumors, they are probably not equivalent for this antitumoral effect [47]. Alternative targets, such as the tumor-associated NADH oxydase (tNOX), are possibly involved in this anticancer effect. The existence of tNOX explains the fact that some cancer cell lines lacking COX-2 respond to certain NSAIDs but not to others, suggestive of additional COX-2 independent antitumor activities [78].
Another possible explanation for the lack of surgery-induced angiogenesis when ketorolac is used involves inflammation induced platelet degranulation and that platelets are known to sequester angiogenesis regulating proteins including VEGF [79]. This is especially interesting in view of Chow et al. findings that platelets decrease by about 10% in the few days post-surgery. There is also a report that NSAIDs are antiangiogenic and another report that transcript of stem cell marker CD133 that is correlated with poor prognosis in a number of solid tumors was lower in patients treated preoperatively with NSAIDs [80, 81].
It is well established that many cancer patients have circulating tumor cells [82,83,84] and there are cells released as a result of surgery [85]. Camara et al. data show a surge in circulating epithelial cells after primary breast cancer surgery, but intriguingly, that surge occurs 3–7 days after surgery. Such a delayed increase in what may be circulating tumor cells after breast cancer surgery was also reported by Daskalakis et al. [86]. This phenomenon recalls the surge of CD34+ progenitor cells 3–5 days after tissue damage (e.g., myocardial infarction) [87]. Also it has been recently reported in an animal model, where mice with subcutaneous implantation of Lewis lung carcinoma were subjected to an operative injury, that surgery induced the release of cytokines/chemokines and mobilized bone marrow–derived cells (BMDC) [88]. These mobilized cells were then recruited into tumor tissue with concomitant enhancement of angiogenesis, thereby accelerating tumor growth. Furthermore, blocking recruitment of bone marrow stem cells by disrupting SDF/CXCR signals completely negated the accelerated tumor growth. Many questions arise. Are these surged cells reported by Camara [85] shed or spilled into circulation during surgery and if so why are they delayed by a few days? Or perhaps are these cells released from the bone marrow as part of the programmed wound healing process? Is this a connection to Dvorak’s comment that cancer is “wound healing gone awry” [89]? What exactly controls this effect? Can a perioperative NSAID stop this process?
It further suggests that tumors may share physiological mechanisms with normal tissues and, moreover, that inhibiting the inflammatory process might reduce late metastases as well as can be seen in a recent report of daily use of aspirin [90]. Interestingly, the benefit of daily aspirin does not appear until after 2 years of use. This would be consistent with the possibility that late relapses are the result of a variety of inflammation driven events that induce single cell growth and that result in relapses approximately 30 months hence. If late relapses were the result of some type of induced angiogenesis, we would expect to see a benefit of daily aspirin at 10 or so months after starting.
Blood flow in capillaries is only 0.05 cm/s [91] which would make leaky capillary venules a relatively efficient way for circulating tumor cells to get trapped or enter tissue. It may be that what we previously called dormant single cells induced into metastatic growth were at least in some cases residing not at the site of eventual relapse. Rather, circulating tumor cells or BMDC released into circulation by a host response to surgery in an inflammatory environment extravasate, resulting months later in a metastatic tumor. It is thereby logical to expect that an effective perisurgical anti-inflammatory strategy may affect surgery-induced and possibly angiogenesis-mediated cancer spread.
The metastatic process is highly inefficient. A clonal malignant cell injected into the circulation has approximately 0.0001 probability to result in a growing metastatic site [92]. Inflammation bypasses the need for extravasation through an intact vessel wall and also provides growth factors to the microenvironment. We estimate using our computer simulation that the metastatic seeding process is amplified 100-fold during the few days after primary surgery.
This inflammation driven capillary leakage of CTCs theory has much appeal and seems to address all information presented above but there are other data that cannot be explained. In a 1984 paper Tarin et al. discussed 30 patients with peritoneovenous shunting for the alleviation of abdominal pain and distension in malignant ascites due to inoperable cancer [93]. This procedure returns the fluid to the circulation via a one way valved anastomosis between the peritoneum and the jugular vein. Surprisingly, although the patients treated with this technique receive direct infusions of malignant tumor cells into the blood, this study of 29 patients, 15 of whom came to autopsy, shows that they did not all develop metastases, some being completely free of such lesions despite long survival. Even when metastases do form, they are small and clinically asymptomatic, and the technique is therefore not hazardous. In some patients, inert tumor cells identifiable by natural markers were recognized in the tissues, but no growing metastases were observed. In others, the distribution of secondary deposits was unexpected in that metastases did not form in the organ containing the first capillary bed encountered, although hematogenous metastases had formed in other organs. Despite the fact that various factors such as (a) the small numbers of patients treated with the technique; (b) the sensitive nature of studies on terminally ill patients; and (c) the absence of consistency in the sample population with regard to factors such as length of survival and site of neoplasm, combine to reduce the number of suitable cases for study, the approach has unrivaled power and interest for those seeking to understand mechanisms underlying tumor metastasis in humans.
It is not known if the shunt insertion procedure produces systemic inflammation since the process is done under local anesthesia and there is no mention of inflammatory markers in serum such as IL-6. Nonetheless it appears that the Tarin et al. report does not support the conjecture that systemic inflammation in presence of CTCs leads to distant relapses within 3 years.
Are the missing early relapses in Forget et al. data never to happen or are they merely postponed to become late relapses? Whatever their source and shedding timing, cancer cells in circulation may have half-life of a few hours. Cancer cells are approximately 15 μm in diameter and capillaries are approximately 7 μm in diameter. A series of papers by Weiss et al. [94, 95] several decades ago discussed that the deformation to pass through the capillaries kills 80% or so of cancer cells each passage. The circulation period is several minutes so it is easy to understand why cancer cells have short half-life in the body. Unless injected into more hospitable surroundings such as tissue, these cells will likely harmlessly die off. These data and our analysis suggest that at least for some patients the early relapses apparently avoided in the Forget et al. data do not show up later.
Lastly, the reduced recurrence risk for patients receiving perioperative NSAID may be attributed, at least in part, to the reduced usage of opioids for pain management with ketorolac [96, 97]. It cannot be excluded that all the above mentioned mechanisms could act together impacting relapses within the subsequent few years.
2.11 TNBC and Early Relapses: Possibly an Ideal Group for Testing Perioperative Ketorolac
We now turn our attention to methods of testing this new hypothesis. Animal studies would be very important; however in view of the analyses and data presented we think this should be tested prospectively in a clinical trial. The next question that arises is what patient group would be a good candidate for a trial. Most breast cancer clinical trials, at least in the US, focus on distinct patient subgroups based on recurrence risk levels. The triple negative subgroup attracted our attention for several reasons [98]. Lacking markers for HER2, Estrogen or Progesterone receptors that strongly suggest that there is benefit of targeted therapy, triple negative breast cancer is looked upon by clinicians as a “bad tumor” with high recurrence rate in spite of adjuvant chemotherapy. That pessimistic viewpoint seems justified since TNBC has 12% incidence but accounts for approximately 20% of mortality in breast cancer.
We had access to a triple negative breast cancer data base from Milan that we analyzed with our hazard methods. The relapse hazard (Fig. 2.12 below) looks remarkably similar to the no-ketorolac group in the Forget et al. study shown in Fig. 2.9. Triple negative breast cancer therefore appears to be the ideal study group with which to test benefit of perioperative ketorolac in a clinical trial.
The incidence of TNBC is 12% in US population (as mentioned), 25% among African Americans, and 25–35% among patients from India and Korea [99,100,101]. In sub-Saharan Africa the TNBC incidence is apparently about 70%. (There may be other as yet unexamined groups also with high incidence of TNBC.) Locations with relatively high incidence of TNBC would be ideal places to conduct a clinical trial in order to make it easier to show an improvement in early relapse.
As noted by Wallace et al., the racial disparity in breast cancer outcome is due primarily to deaths within the first few years after diagnosis providing an additional motivation to test at the earliest opportunity what we report here [102]. That would be consistent with the information just noted.
2.12 Current Activities
2.12.1 Focus on India and Nigeria
After speaking at an important conference on Challenging Dogma in Cancer in Mumbai, India at the Tata Memorial Centre in March 2016, Retsky was invited to visit a hospital in Surat, India. He gave the Tata presentation to the surgical staff and suggested a clinical trial is needed. The chief of surgery agreed and expressed intent to submit a proposal to their Institutional Review Board. They suggested including neighboring hospitals in such a trial. Ketorolac is already used in breast cancer for pain relief after mastectomy at the Surat Hospital so this would not be a major change for them. Health care is provided very inexpensively for all citizens by the government in India. The chief of surgery is now retired so a trial may not occur in that hospital but based on that discussion, it would seem reasonably feasible to conduct a trial of perioperative NSAID in India.
Retsky visited Abuja, Nigeria in September 2015 and May 2016. That is another good opportunity for a clinical trial of perioperative ketorolac. We knew that TNBC is very common in sub-Saharan Africa and particularly Nigeria. Considering our contacts in Nigeria, we were very interested in proposing a clinical trial there. However first we needed to recommend some means of recruiting patients with early stage breast cancer since we knew that most patients there present with locally advanced or late stage disease. What can we suggest as a method to increase women with breast lumps to seek medical care before it is too late for our therapy to make a difference? Before Retsky visited Nigeria in May 2016 to give an invited talk, our group had a very interesting discussion that lasted for about a week on email. A face to face meeting was not possible since we are scattered in four different countries. Retsky started the meeting with an email message to all. He said that Michael Baum is probably the world’s authority on advantages and disadvantages of mammography. Should we recommend mammography to detect early stage breast cancer in Nigeria? They have essentially no infrastructure to conduct early detection. It is a blank slate. We knew from our collaboration with the late Isaac Gukas that women with a breast lump are reluctant to see a surgeon since it is common folklore that the cancer spreads after surgery or words to that effect. The word Isaac used was that the surgery “provoked” the cancer. Despite the known problem of over-diagnosis with use of mammography in US and Europe, Retsky was proposing to use mammography to detect early stage cancer in Nigeria. Baum argued instead that a country like Nigeria could not afford to train a new generation of radiologists and to purchase and maintain expensive mammography equipment. Perhaps there are other options. One of us found Corbex et al. and Devi et al. papers [103, 104]) and we immediately recognized that their description of what was done in Malaysia could be an excellent solution for Nigeria. The following abstract was written after our meeting. This was presented in Abuja, Nigeria in February 2016 and describes our current suggestion for Nigeria:
Access to low cost treatment and team work among healthcare workers is key to the effective diagnosis and management of Cancers in Developing Countries. [105].
Michael Retsky, Ph.D.
Staff-Harvard TH Chan School of Public Health and Honorary Faculty—University College London.
Breast cancer is a major health concern in many countries in the world. My research is based in US and UK and while we share a serious breast cancer problem with Nigeria, there are differences that may help us propose methods to improve outcome in general. The US has a multi-racial population and we know, while the incidence is lower, there is a racial disparity in outcome for African Americans (AA) compared to European Americans (EA) of 1.5–2.2-fold. However this racial disparity inverts as age of diagnosis increases. That is, AA present at average age 46 while EA present at average age 57. However AA who happen to present at or above age 57 have superior outcome compared to EA. Thus there must be a biological explanation to racial disparity likely related to age at onset rather than reduced access to quality medical care as one might suspect. It is easier to solve a biological problem than a socioeconomic problem. What is biologically different about breast cancer in AA compared to EA? The main differences are that breast cancer in AA presents early, is typically triple negative (TNBC) and metastatic relapses occur within a few years of surgery. There are no known biological receptors for TNBC that would indicate the effectiveness of any known targeted therapy. The only therapy that is used in treating TNBC is conventional chemotherapy. These drugs have been available for decades, are toxic and are only slightly effective.
As would be expected, breast cancer in Nigeria is very similar to AA, diagnosed at average age 43 years, is very commonly TNBC, and relapses occur shortly after tumor removal.
My colleagues and I have proposed that the surgery to remove a primary breast cancer causes a systemic inflammatory response in the host that initiates early metastatic activity. We think this is most common in young women and could be prevented if there was a method to prevent inflammation from surgery. Based on a retrospective study by my Belgian colleague, ketorolac, a very low cost non-toxic common non-steroidal anti-inflammatory drug (NSAID) that is sometimes used just before surgery to prevent pain seems to prevent early relapses. Nigeria would be an ideal place to confirm this result. If it works as we suspect, it could be used anywhere. A protocol for a clinical trial in Nigeria was written by Demicheli [106].
We think this perioperative NSAID therapy needs to be used in patients before the cancer is locally advanced. However, more often than not, breast cancer patients in Nigeria first see a physician only after the cancer has already become locally advanced (inoperable). This same late presentation problem has been dramatically reduced in Malaysia by reducing the time between first abnormal breast appearance and seeing a physician [103, 104]. This clinical down-staging program covering a population of 1.1 million women managed to reduce late presentation from 60 to 35% in less than 5 years. The program consisted of training by a teaching team (3 medical staff and 6 nurses) and about 400 first line health staff throughout the state and cost less than US $34,000. To conduct a clinical trial of perioperative NSAID in Nigeria as we propose, something similar needs to be conducted here.
See Osaro Nelson’s chapter on breast cancer in Nigeria [107] and we coauthored a book on treatment of breast cancer in Nigeria [108]. As mentioned, a protocol was published for treating patients with perioperative NSAID ketorolac in Nigeria. There are two key features that should be noted. First the anti-cancer action of perioperative ketorolac has been separated from the analgesic process. That is, ketorolac is used to try preventing relapses, with other standard measures to prevent pain from the primary surgery. The second is that ketorolac is scheduled to be used perioperatively as expected but additionally continued every 8 h for the 3 days post surgery to expand its anti-inflammatory action within its appropriate use. Despite the many concerns fully documented by the Erhabor et al. chapter, we find Nigeria to be an excellent place to conduct a clinical trial of perioperative ketorolac.
2.13 Analysis of Data from Norway on Relapse Hazard After Delayed Reconstruction
Demicheli and coauthors analyzed data on 312 patients who had reconstruction after mastectomy but the reconstruction was delayed (median time to reconstruction 33 months after original mastectomy). Thus they could look at relapse hazard counting time either from the day of mastectomy or from the day of reconstruction. Both ways of setting the clock to zero produced the same two waves of relapses. In each case, the first wave was at 18 months and the second wave was at 50–60 months. The magnitude of the 18 month peak was related to the extent of surgery. For simple implant the peak was not large but for the more extensive Tram-Flap procedure, the magnitude was large. The conclusion was that a surgical maneuver seems to be the initiating step in the metastatic activity from dormancy. It seems that this eliminates the possibility of cancer cells physically released during the incision as a cause of early relapse—at least in this situation (acknowledgement to Robert Weinberg for this observation). The notion that gives a minor role, if any, to CTCs released by primary tumor is in keeping with the above reported findings on pre-surgical CTC detection and on peritoneovenous shunting for malignant ascites [109].
2.14 Concerns About Bleeding Complications
One of the issues related to the perioperative use of ketorolac has been concern about bleeding complications. There are mixed reports of excessive bleeding with use of perioperative ketorolac [110]. In our opinion, these concerns, while real, pale in significance compared to the concerns about surgery induced inflammation that can lead to over half of all relapses in breast cancer.
2.15 Summary
Careful analysis of breast cancer recurrences suggests a paradigm where early recurrences, i.e. the majority of adverse events resulting in poor prognosis, are induced by angiogenic switching of avascular micrometastases and single cell activation. Both events are triggered by primary tumor surgical removal.
Results reported by Forget et al. analysis of retrospective data, suggesting perioperative NSAID ketorolac significantly reduces early relapses, may be deciphered in the light of this model. Indeed, post-surgical transient systemic inflammation might be the precipitating factor and common denominator for early relapses. In particular, inflammation would be important for angiogenesis induction of avascular distant micrometastases.
A few points need further investigations. First, the Forget et al. findings need to be confirmed in randomized clinical trials. Such investigations are imperative not only from the scientific point of view but more so for their possible clinical consequences, resulting from the fact that breast cancer mortality could be reduced by 25 to over 50% at low cost and toxicity. A subset of patients for a randomized clinical trial should be characterized by unfavorable prognostic factors resulting in early recurrences covering the first 2–3 years. We suggest that the best breast cancer population for such a trial may be triple negative breast cancer and particularly when onset is early as exists in sub-Saharan Africa.
In spite of the fact that breast cancer is known as a disease that runs its course in a decade or more, most of the relevant events resulting in recurrences apparently occur shortly after primary surgery. Investigations focused on events occurring during the first few days and weeks following primary tumor removal are strongly warranted.
There have been very exciting recent developments in methods to prolong life with metastatic cancer. That is welcome news indeed. We are proposing upstream improvements in cancer care that together with the downstream improvements should result in major reductions in the worldwide cancer problem. The new path outlined here could be a revolutionary break (“Something for nothing” rarely if ever happened in cancer therapy) from the past and should also be explored in other neoplasias. High priority should be given to test this hypothesis as it is implementable regardless of state of socio-economic development because of its low cost.
2.16 Conclusions
To be clear, we are discussing an unsolved 2000 year old problem in oncology to which we are late-comers (Table 2.3).
References
Retsky M, Demicheli R, Hrushesky WJ, Forget P, De Kock M, Gukas I, Rogers RA, Baum M, Sukhatme V, Vaidya JS (2013) Reduction of breast cancer relapses with perioperative non-steroidal anti-inflammatory drugs: new findings and a review. Curr Med Chem 20(33):4163–4176. Review. PubMed PMID: 23992307; PubMed Central PMCID: PMC3831877. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3831877/
Ariel I (1994) A historical review of breast cancer treatment. In: Johnson H, Wise L (eds) Breast cancer: controversies in management. Futura, Armonk
University of Chicago Oriental Institute. https://oi.uchicago.edu/research/publications/oip/edwin-smith-surgical-papyrus-volume-1-hieroglyphic-transliteration
US Center for Disease Control. https://www.cdc.gov/cancer/breast/statistics
Retsky M, Demicheli R (2017) Introduction. Springer, New York, p xvii
Sikora K (1999) Developing a global strategy for cancer. Eur J Cancer 35:1870–1877
Demicheli R, Abbattista A, Miceli R, Valagussa P, Bonadonna G (1996) Time distribution of the recurrence risk for breast cancer patients undergoing mastectomy: further support about the concept of tumor dormancy. Breast Cancer Res Treat 41(2):177–185. PubMed PMID: 8944336
Baum M (1996) Does surgery disseminate or accelerate cancer? Lancet 347(8996):260. PubMed PMID: 8551898
Retsky MW, Demicheli R, Hrushesky WJ, Baum M, Gukas ID (2008) Dormancy and surgery-driven escape from dormancy help explain some clinical features of breast cancer. APMIS 116(7–8):730–741. doi:10.1111/j.1600-0463.2008.00990.x. Review. PubMed PMID: 18834415
Bonadonna G, Valagussa P, Moliterni A, Zambetti M, Brambilla C (1995) Adjuvant cyclophosphamide, methotrexate, and fluorouracil in node-positive breast cancer: the results of 20 years of follow-up. N Engl J Med 332(14):901–906. PubMed PMID: 7877646
Retsky M, Demicheli R, Hrushesky W, Baum M, Gukas I (2010) Surgery triggers outgrowth of latent distant disease in breast cancer: an inconvenient truth? Cancers (Basel) 2(2):305–337. doi:10.3390/cancers2020305. PubMed PMID: 24281072; PubMed Central PMCID: PMC3835080
Fisher ER, Sass R, Fisher B (1984) Pathologic findings from the National Surgical Adjuvant Project for breast cancers (protocol no. 4). X. Discriminants for tenth year treatment failure. Cancer 53:712–723
Laird AK (1965) Dynamics of tumour growth: comparison of growth rates and extrapolation of growth curve to one cell. Br J Cancer 19:278–291
Laird AK (1969) Dynamics of growth in tumors and in normal organisms. Natl Cancer Inst Monogr 30:15–28. PubMed PMID: 5351826
Pittello RF, Schabel FM, Skipper HE (1970) The “sensitivity” of resting and dividing cells. Cancer Chemother Rep Part 1 54(3):137–142
Norton L (1988) A Gompertzian model of human breast cancer growth. Cancer Res 48(24 Pt 1):7067–7071. PubMed PMID: 3191483
Norton L. Cancer log-kill revisited. Am Soc Clin Oncol Educ Book 2014:3–7. doi:10.14694/EdBook_AM.2014.34.3. Review. PubMed PMID: 24857052
Demicheli R et al (1994) Local recurrences following mastectomy: support for the concept of tumor dormancy. J Natl Cancer Inst 86:45–48
Sunderland MC, McGuire WL (1990) Prognostic indicators in invasive breast cancer. Surg Clin North Am 70(5):989–1004. Review. PubMed PMID: 2218829
Retsky MW, Swartzendruber DE, Wardwell RH, Bame PD (1990) Is Gompertzian or exponential kinetics a valid description of individual human cancer growth? Med Hypotheses 33(2):95–106. PubMed PMID: 2259298. https://www.researchgate.net/publication/20897370_Is_Gompertzian_kinetics_a_valid_description_of_individual_tumor_growth
Speer J, Petrosky V, Retsky M, Wardwell R (1984) A stochastic numerical model of breast cancer growth that simulates clinical data. Cancer Res 44:4124–4130
Ingleby H, Moore L (1956) Periodic roentgenographic studies of a growing human mammary cancer. Cancer 9(4):749–752. PubMed PMID: 13356257
Norton L, Simon R (1986) The Norton-Simon hypothesis revisited. Cancer Treat Rep 70:163–169
Retsky M, Swartzendruber D, Wardwell R, Bame P (1989) Petrosky V Correspondence re: Larry Norton, a Gompertzian model of human breast cancer growth. Cancer Res 49:6443–6444. Cancer Res 1988 48:7067–7071
Fletcher SW (1997) Whither scientific deliberation in health policy recommendations? Alice in the wonderland of breast-cancer screening. N Engl J Med 336(16):1180–1183. PubMed PMID: 9099666
Retsky M, Demicheli R, Hrushesky W (2001) Breast cancer screening for women aged 40-49 years: screening may not be the benign process usually thought. J Natl Cancer Inst 93(20):1572. PubMed PMID: 11604483
Retsky MW, Demicheli R, Swartzendruber DE, Bame PD, Wardwell RH, Bonadonna G, Speer JF, Valagussa P (1997) Computer simulation of a breast cancer metastasis model. Breast Cancer Res Treat 45(2):193–202. PubMed PMID: 9342444
Demicheli R, Retsky MW, Swartzendruber DE, Bonadonna G (1997) Proposal for a new model of breast cancer metastatic development. Ann Oncol 8(11):1075–1080. Review. PubMed PMID: 9426326
Retsky M (2011) Metronomic chemotherapy was originally designed and first used in 1994 for early stage cancer—why is it taking so long to proceed? J Bioequiv Availab 3: 00i–0iv doi:10.4172/jbb.100000e6. https://dash.harvard.edu/bitstream/handle/1/5111470/Retsky_MetronomicChemotherapy.pdf?sequence=1
Retsky M (2013) How long should adjuvant chemotherapy be given in early stage colon cancer? J Clin Exp Pathol 3(1):136. doi:10.4172/2161- 0681.1000136. https://dash.harvard.edu/bitstream/handle/1/12559516/Retsky_HowLong.pdf?sequence=1
Retsky MW et al (2014) Ecancermedicalscience 8:ed38
Cooke R (2001) Dr. Folkman’s war—angiogenesis and the struggle to defeat cancer. Random House, New York, pp 346–348
Bernstein J. MIA in the war on cancer: where are the low-cost treatments? http://www.propublica.org/article/where-are-the-low-cost-cancer-treatments
Hamard L, Ratel D, Selek L, Berger F, van der Sanden B, Wion D (2016) The brain tissue response to surgical injury and its possible contribution to glioma recurrence. J Neurooncol 128(1):1–8. doi:10.1007/s11060-016-2096-y. Epub 2016 Mar 9. Review. PubMed PMID: 26961772.
Demicheli R, Fornili M, Ambrogi F, Higgins K, Boyd JA, Biganzoli E, Kelsey CR (2012) Recurrence dynamics for non-small cell lung cancer: effect of surgery on the development of metastases. J Thorac Oncol 7(4):723–730
Maniwa Y, Okada M, Ishii N, Kiyooka K (1998) Vascular endothelial growth factor increased by pulmonary surgery accelerates the growth of micrometastases in metastatic lung cancer. Chest 114(6):1668–1675. PubMed PMID: 9872204
Smithers DW (1968) Clinical assessment of growth-rate in human tumors. Clin Radiol 19:113
Bergh J, Jönsson PE, Glimelius B, Nygren P, SBU-Group (2001) Swedish council of technology assessment in health care a systematic overview of chemotherapy effects in breast cancer. Acta Oncol 40:253–281
Retsky M, Bonadonna G, Demicheli R, Folkman J, Hrushesky W, Valagussa P (2004) Hypothesis: induced angiogenesis after surgery in premenopausal node-positive breast cancer patients is a major underlying reason why adjuvant chemotherapy works particularly well for those patients. Breast Cancer Res 6(4):R372-4. PubMed PMID: 15217504; PubMed Central PMCID: PMC468653
Retsky M, Demicheli R, Hrushesky W (2001) Premenopausal status accelerates relapse in node positive breast cancer: hypothesis links angiogenesis, screening controversy. Breast Cancer Res Treat 65:217–224
Amy Dockser Marcus, The Wall Street Journal, September 13, 2005; Page D1 probing surgery’s link to cancer recurrence. Some researchers say removing a tumor can trigger a process that leads to new growth
Jatoi I, Anderson WF, Rao SR, Devesa SS (2005) Breast cancer trends among black and white women in the United States. J Clin Oncol 23:7836–7841
Demicheli R, Retsky MW, Hrushesky WJ, Baum M, Gukas ID, Jatoi I (2007) Racial disparities in breast cancer outcome: insights into host-tumor interactions. Cancer 110:1880–1888
Retsky M, Demicheli R, Gukas I, Hrushesky W (2007) Enhanced surgery-induced angiogenesis among premenopausal women may partially explain the breast cancer excess mortality of blacks compared to whites. Int J Surg 5:300–304
Gukas I, Jatoi I, Demicheli R, Retsky M, Hrushesky W, Baum M (2009) Complex interplay between race and breast cancer: should this affect breast cancer diagnostic and therapeutic strategies? Curr Med Literature 21:1–8
Retsky MW, Hrushesky WJ, Gukas ID (2009) Hypothesis: primary antiangiogenic method proposed to treat early stage breast cancer. BMC Cancer 9:7. PubMed PMID: 19133151; PMCID: PMC2633344
Forget P, Vandenhende J, Berliere M, Machiels JP, Nussbaum B, Legrand C, De Kock M (2010) Do intraoperative analgesics influence breast cancer recurrence after mastectomy? A retrospective analysis. Anesth Analg 110(6):1630–1635. http://www.ncbi.nlm.nih.gov/pubmed/20435950
Karhade M, Hall C, Mishra P, Anderson A, Kuerer H, Bedrosian I, Krishnamurthy S, Lucci A (2014) Circulating tumor cells in non-metastatic triple-negative breast cancer. Breast Cancer Res Treat 147(2):325–333. doi:10.1007/s10549-014-3103-7. PubMed PMID: 25164970; PubMed Central PMCID:PMC4149877
Gottschalk A, Sharma S, Ford J, Durieux ME, Tiouririne M (2010) Review article: the role of the perioperative period in recurrence after cancer surgery. Anesth Analg 110(6):1636–1643
Horowitz M, Neemna E, Ben Eliyahu S (2015) Nat Rev Clin Oncol 12:213–226; published online 20 January 2015. doi:10.1038/nrclinonc.2014.224
El Saghir NS, Elhajj II, Geara FB, Hourani MH (2005) Trauma associated growth of suspected dormant micrometastasis. BMC Cancer 5:94
Martins-Green M, Boudreau N, Bissell MJ (1994) Inflammation is responsible for the development of wound-induced tumors in chickens infected with Rous sarcoma virus. Cancer Res 54(16):4334–4341. [PubMed]
Retsky M (2005) Comment—what would explain the sudden growth of lung cancer after minor trauma reported by El Saghir et al? BMC Cancer 5:94. https://bmccancer.biomedcentral.com/articles/10.1186/1471-2407-5-94/comments
Paget S (1889) The distribution of secondary growth in cancer of the breast. Lancet 1:571–573
Martin P, Parkhurst SM (2004) Parallels between tissue repair and embryo morphogenesis. Development 131:3021–3034
Werner S, Grose R (2003) Regulation of wound healing by growth factors and cytokines. Physiol Rev 83:835–870
Badiavas EV, Abedi M, Butmarc J, Falanga V, Quesenberry P (2003) Participation of bone marrow derived cells in cutaneous wound healing. J Cell Physiol 196:245–250
Balkwill F, Mantovani A (2001) Inflammation and cancer: back to Virchow? Lancet 357(9255):539–545. [PubMed]
Walter ND, Rice PL, Redente EF, Kauvar EF, Lemond L, Aly T, Wanebo K, Chan ED (2011) Wound healing after trauma may predispose to lung cancer metastasis: review of potential mechanisms. Am J Respir Cell Mol Biol 44(5):591–596. [PubMed]
Jones FS, Rous P (1914) On the cause of the localization of secondary tumors at points of injury. J Exp Med 20(4):404–412. [PMC free article] [PubMed]
Pascual M, Alonso S, Parés D, Courtier R, Gil MJ, Grande L, Pera M (2011) Randomized clinical trial comparing inflammatory and angiogenic response after open versus laparoscopic curative resection for colonic cancer. Br J Surg 98(1):50–59. [PubMed]
Chow LW, Yuen KY, Woo PC, Wei WI (2000) Clarithromycin attenuates mastectomy-induced acute inflammatory response. Clin Diagn Lab Immunol 7(6):925–931. [PMC free article] [PubMed]
Perez-Rivas LG, Jerez JM, Fernandez-De Sousa CE, de Luque V, Quero C, Pajares B, Franco L, Sanchez-Muñoz A, Ribelles N, Alba E (2012) Serum protein levels following surgery in breast cancer patients: a protein microarray approach. Int J Oncol 41(6):2200–2206. [PubMed]
Bonnelykke-Behrndtz ML et al (2017) The impact of wound inflammation on cancer progression; studies in fish and patients. Springer, New York
Forget P, DeKock M (2017) Long term consequences of acute inflammation in cancer surgery: new findings and perspectives. Springer, New York
Egawa G, Nakamizo S, Natsuaki Y, Doi H, Miyachi Y, Kabashima K (2013) Intravital analysis of vascular permeability in mice using two-photon microscopy. Sci Rep 3:1932. doi:10.1038/srep01932. PubMed PMID: 23732999; PubMed Central PMCID: PMC3671357
Keibel A, Singh V, Sharma MC (2009) Inflammation, microenvironment, and the immune system in cancer progression. Curr Pharm Des 15(17):1949–1955. [PubMed]
McAllister SS, Weinberg RA (2014) The tumour-induced systemic environment as a critical regulator of cancer progression and metastasis. Nat Cell Biol 16(8):717–727. doi:10.1038/ncb3015. Review. PubMed PMID: 25082194
Allavena P, Mantovani A (2012) Immunology in the clinic review series; focus on cancer: tumour-associated macrophages: undisputed stars of the inflammatory tumour microenvironment. Clin Exp Immunol 167(2):195–205. [PMC free article] [PubMed]
Mantovani A, Marchesi F, Porta C, Sica A, Allavena P (2007) Inflammation and cancer: breast cancer as a prototype. Breast 16(Suppl 2):S27–S33. [PubMed]
Carmeliet P, Jain RK (2000) Angiogenesis in cancer and other tissues. Nature 407:249–257. [PubMed]
Favaro E, Amadori A, Indraccolo S (2008) Cellular interaction in the vascular niche: implications in the regulation of tumour dormancy. APMIS 116:648–659. [PubMed]
Butler JM, Kobayashi H, Rafii S (2010) Instructive role of the vascular niche in promoting tumour growth and tissue repair by angiocrine factors. Nat Rev Cancer 10(2):138–146. [PMC free article] [PubMed]
Bao B, Ahmad A, Kong D, Ali S, Azmi AS, Li Y, Banerjee S, Padhye S, Sarkar FH (2012) Hypoxia induced aggressiveness of prostate cancer cells is linked with deregulated expression of VEGF, IL-6 and miRNAs that are attenuated by CDF. PLoS One 7(8):e43726. [PMC free article] [PubMed]
Tveito S, Andersen K, Kåresen R, Fodstad Ø (2011) Analysis of EpCAM positive cells isolated from sentinel lymph nodes of breast cancer patients identifies subpopulations of cells with distinct transcription profiles. Breast Cancer Res. 13(4):R75. [PMC free article] [PubMed]
Kavanagh DP, Kalia N (2011) Hematopoietic stem cell homing to injured tissues. Stem Cell Rev 7(3):672–682. [PubMed]
Domanska UM, Kruizinga RC, Nagengast WB, Timmer-Bosscha H, Huls G, de Vries EGE, Walenkamp AME (2013) A review on CXCR4/CXCL12 axis in oncology: no place to hide. Eur J Cancer 49(1):219–230. http://dx.doi.org/10.1016/j.ejca.2012.05.005. [PubMed]
Morré DJ, Morre DM (2006) tNOX, an alternative target to COX-2 to explain the anticancer activities of non-steroidal anti-inflammatory drugs (NSAIDS). Mol Cell Biochem 283(1–2):159–167. [PubMed]
Klement GL, Yip TT, Cassiola F, Kikuchi L, Cervi D, Podust V, Italiano JE, Wheatley E, Abou-Slaybi A, Bender E, Almog N, Kieran MW, Folkman J (2009) Platelets actively sequester angiogenesis regulators. Blood 113(12):2835–2842. [PMC free article] [PubMed]
Pakneshan P, Birsner AE, Adini I, Becker CM, D’Amato RJ (2008) Differential suppression of vascular permeability and corneal angiogenesis by nonsteroidal anti-inflammatory drugs. Invest Ophthalmol Vis Sci 49(9):3909–3913. [PubMed]
Lönnroth C, Andersson M, Nordgren S, Lundholm K (2012) Downregulation of Prominin 1/CD133 expression in colorectal cancer by NSAIDs following short-term preoperative treatment. Int J Oncol 41(1):15–23. [PubMed]
Krebs MG, Hou JM, Ward TH, Blackhall FH, Dive C (2010) Circulating tumour cells: their utility in cancer management and predicting outcomes. Ther Adv Med Oncol 2(6):351–365. [PMC free article] [PubMed]
Zhe X, Cher ML, Bonfil RD (2011) Circulating tumor cells: finding the needle in the haystack. Am J Can Res 1(6):740–751. [PMC free article] [PubMed]
Meng S, Tripathy D, Frenkel EP, Shete S, Naftalis EZ, Huth JF, Beitsch PD, Leitch M, Hoover S, Euhus D, Haley B, Morrison L, Fleming TP, Herlyn D, Terstappen LW, Fehm T, Tucker TF, Lane N, Wang J, Uhr JW (2004) Circulating tumor cells in patients with breast cancer dormancy. Clin Cancer Res 10(24):8152–8162. [PubMed]
Camara O, Kavallaris A, Nöschel H, Rengsberger M, Jörke C, Pachmann K (2006) Seeding of epithelial cells into circulation during surgery for breast cancer: the fate of malignant and benign mobilized cells. World J Surg Oncol 4:67. [PMC free article] [PubMed]
Daskalakis M, Mavroudis D, Sanidas E, Apostolaki S, Askoxylakis I, de Bree E, Georgoulias V, Melissas J (2011) Assessment of the effect of surgery on the kinetics of circulating tumour cells in patients with operable breast cancer based on cytokeratin-19 mRNA detection. Eur J Surg Oncol 37(5):404–410. [PubMed]
GrØgaard HK, Solheim S, Landsverk KS, Seljeflot I, Hoffmann P, Arnesen H, Ilebekk A (2011) Circulating CD34+ progenitor cells and growth factors in patients treated with PCI for acute myocardial infarction or stable angina pectoris. Scand J Clin Lab Invest 71:322–329. [PubMed]
Takemoto Y, Li TS, Kubo M, Ohshima M, Ueda K, Harada E, Enoki T, Okamoto M, Mizukami Y, Murata T, Hamano K (2011) Operative injury accelerates tumor growth by inducing mobilization and recruitment of bone marrow-derived stem cells. Surgery 149(6):792–800. [PubMed]
Dvorak HF (1986) Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med 315(26):1650–1659. [PubMed]
Rothwell PM, Wilson M, Price J, Belch JF, Meade TW, Mehta Z (2012) Effect of daily aspirin on risk of cancer metastasis: a study of incident cancers during randomised controlled trials. Lancet 379(9826):1591–1601. [PubMed]
Stucker M, Baier V, Reuther T, Hoffmann K, Kellam K, Altmeyer P (1996) Capillary blood velocity in human skin capillaries located perpendicularly to the skin surface: measured by a new laser Doppler anemometer. Microvasc Res 52:188–192. [PubMed]
Mehlen P, Puisieux A (2006) Metastasis: a question of life or death. Nat Rev Cancer 6(6):449–458. Review. PubMed PMID: 16723991. [PubMed]
Tarin D, Price JE, Kettlewell MG, Souter RG, Vass AC, Crossley B (1984) Mechanisms of human tumor metastasis studied in patients with peritoneovenous shunts. Cancer Res 44(8):3584–3592
Barbera-Guillem E, Weiss L (1993) Cancer-cell traffic in the liver. III lethal deformation of B16 melanoma cells in liver sinusoids. Int J Cancer 54(5):880–884. PubMed PMID: 8325713
Barbera-Guillem E, Smith I, Weiss L (1993) Cancer-cell traffic in the liver. II. Arrest, transit and death of B16F10 and M5076 cells in the sinusoids. Int J Cancer 53(2):298–301. PubMed PMID: 8425768
Burke JP, Pestotnik SL, Classen DC, Lloyd JF (1996) Evaluation of the financial impact of ketorolac tromethamine therapy in hospitalized patients. Clin Ther 18(1):197–211. [PubMed]
Gach K, Wyrębska A, Fichna J, Janecka A (2011) The role of morphine in regulation of cancer cell growth. Naunyn Schmiedebergs Arch Pharmacol 384(3):221–230. [PMC free article] [PubMed]
Elias, A,D (2010) Triple-negative breast cancer: a short review. Am J Clin Oncol 33(6):637–645
Manjunath S, Prabhu JS, Kaluve R, Correa M, Sridhar TS (2011) Estrogen receptor negative breast cancer in India: do we really have higher burden of this subtype? Indian J Surg Oncol 2(2):122–125
Keam B, Im SA, Kim HJ, Oh DY, Kim JH, Lee SH, Chie EK, Han W, Kim DW, Moon WK, Kim TY, Park IA, Noh DY, Heo DS, Ha SW, Bang YJ (2007) Prognostic impact of clinic pathologic parameters in stage II/III breast cancer treated with neoadjuvant docetaxel and doxorubicin chemotherapy: paradoxical features of the triple negative breast cancer. BMC Cancer 7:203
Patil VW, Singhai R, Patil V, Gurav PD (2011) Triple-negative (ER, PgR, HER-2/neu) breast cancer in Indian women. Breast Cancer 3:9–19
Wallace TA, Martin DN, Ambs S (2011) Interactions among genes, tumor biology and the environment in cancer health disparities: examining the evidence on a national and global scale. Carcinogenesis 32(8):1107–1121
Corbex M, Burton R, Sancho-Garnier H (2012) Breast cancer early detection methods for low and middle income countries, a review of the evidence. Breast 21(4):428–434. doi:10.1016/j.breast.2012.01.002. Epub 2012 Jan 30. Review. PubMed PMID: 22289154
Devi BC, Tang TS, Corbex M (2007) Reducing by half the percentage of late-stage presentation for breast and cervix cancer over 4 years: a pilot study of clinical downstaging in Sarawak, Malaysia. Ann Oncol 18(7):1172–1176. Epub 2007 Apr 13
Retsky M (2016) Abuja, Nigeria
Demicheli R, Osaro E, Retsky M, Patrice F, Vaidya JS, Bello SO (2016) Protocol for a randomised, multicentre, double blinded phase iii study of perioperative ketorolac in women of African descent with operable breast cancer. J Intern Med 2(1):017. http://internalmedicine.jacobspublishers.com/images/Internal_Med/J_J_Intern_Medicine_2_1_017.pdf
Osaro E (2017) Randomized clinical trials on breast cancer in Nigeria and other developing countries—challenges and constraints. Springer, New York
Challenges associated with the diagnosis and management of breast cancer in Nigeria—the need for affordable and accessible care, Erhabor O, Abdulrahaman Y, Retsky M, Forget P, Vaidya JS, Bello O, Adias TC, Dagana A, Egenti BN, Mainasara AS, Sahabi SM, Rilwanu TI, Ahmed Y, Hassan M, Ifedayo A, Okara GC, Lori J, Ibiang L. AuthorHouse™ UK 1663 Liberty Drive Bloomington, IN 47403 USA. www.authorhouse.co.uk
Dillekås H, Demicheli R, Ardoino I, Jensen SA, Biganzoli E, Straume O (2016) The recurrence pattern following delayed breast reconstruction after mastectomy for breast cancer suggests a systemic effect of surgery on occult dormant micrometastases. Breast Cancer Res Treat 158:169–178
Gobble RM, Hoang HL, Kachniarz B, Orgill DP. Ketorolac does not increase perioperative bleeding: a meta-analysis of randomized controlled trials. Plast Reconstr Surg. 2014 Mar;133(3):741-55. doi: 10.1097/01.prs.0000438459.60474.b5. PubMed PMID: 24572864 http://www.druglib.com/abstract/go/gobble-rm1_plast-reconstr-surg_20140000.html
Acknowledgements
Many people other than the authors have participated in this project. Contributions were made by Robert Wardwell, Jack Speer, Douglas Swartzendruber, Victor Petrosky, Paul Bame, Vikas Sukhatme, Patrice Forget, Rick Rogers, Katherina Pachmann, Marc DeKock, Gianni Bonadonna, Judah Folkman, Pinuccia Valagussa, Isaac Gukas, Jayant Vaidya, and Michael Baum. The authors wish to acknowledge the seminal work of friends and mentors Judah Folkman and Bernard Fisher. Much of this work was supported by a grant from The Komen Foundation 2010-2014. An SBIR grant from NIH in 1995 was used to develop the computer simulation reported in 1997. A number of figures and several sections of text were taken from Retsky et al. CMC 2013 [1] with permission of Bentham Science Publishers.
Conflicts of interest Michael Retsky is on the board of directors of the Colon Cancer Alliance. No other COIs to report.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Retsky, M.W., Demicheli, R. (2017). Perioperative Inflammation as Triggering Origin of Metastasis Development. In: Retsky, M., Demicheli, R. (eds) Perioperative Inflammation as Triggering Origin of Metastasis Development. Springer, Cham. https://doi.org/10.1007/978-3-319-57943-6_2
Download citation
DOI: https://doi.org/10.1007/978-3-319-57943-6_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-57942-9
Online ISBN: 978-3-319-57943-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)