Abstract
In this study, we analyzed the effect of fluoride ions on photocatalytic activity of titanium dioxide . Synthesized and calcined F-doped materials exhibit photocatalytic activity in the both UV and visible light range. The photocatalytic activity induced by UV exposure is higher than that of commercial material Degussa P25, which is pure titanium dioxide . Also the research on influence of fluoride ions on anatase -to-rutile phase transition was carried out by X-ray powder diffraction. The introduction of fluoride ions was found to increase the thermal stability of anatase modification of titanium dioxide .
Access provided by CONRICYT-eBooks. Download conference paper PDF
Similar content being viewed by others
1 Introduction
Recently, the issue concerning the purification of wastewater from organic pollutants have attracted much attention. The method based on photocatalytic degradation of organic substances is the most viable approach, since the reaction results in mineralization of organics to CO2, H2O and inorganic acids [1, 2]. Although various semiconductors can be used as photocatalysts [3], the most promising material is titanium dioxide (TiO2) due to its high photocatalytic activity, photochemical and thermal stability, low cost, nontoxicity, and biocompatibility [4,5,6]. To increase the efficiency of heterogeneous photocatalytic process, nanosized titanium dioxide with a large total surface area is applied. TiO2 has three polymorphs: rutile, anatase and brookite [7]. Rutile is the most stable form of TiO2, anatase and brookite convert to rutile upon heating above 600 °C. With a particle size of less than 14 nm, anatase is known to be more thermodynamically stable than rutile [8]. Anatase is considered the most photocatalytic active modification.
The application of TiO2 as a photocatalyst is limited by its large band gap of 3.2 eV, due to which the activation of titanium dioxide requires UV radiation, thereby hindering the use of visible light [9]. To achieve the photocatalytic response of TiO2 in the visible light range, the metal and nonmetal dopants are successfully employed [2, 9,10,11].
Nonmetals are used more often because it is believed that although metals decrease the band gap they are not always successfully introduced into the structure of titanium dioxide and the remaining metals on the surface of TiO2 shelter photoreaction sites [12].
As for nonmetal dopants, the various ways to introduce nitrogen and subsequent characterization of resulting compounds are under intensive consideration [9, 13, 14]; the doping with sulfur, boron and halogens is also described in the literature [2, 9,10,11].
The introduction of fluorine is of interest, since the fluoride dopants increase the surface acidity and cause the formation of reduced ions Ti3+ due to the charge compensation between Ti4+ and F−. Thus, the charge separation and enhancement of photoinduced processes can be achieved, whilst avoiding enlargement of band gap. The introduction of fluoride ions also enhances adsorptivity of molecules and increases the temperature of anatase -to-rutile phase transition [9]. The synthesized F-doped TiO2 flower-like nanomaterials possess good crystallinity and exhibit high photoelectrochemical activity for water-splitting and photodegradation of organic pollutants compared with P-25, which is currently considered to be one of the best commercial TiO2 photocatalysts [15]. The doping with fluoride ions increases thermostability, and the morphological features of F-doped titanium dioxide aggregates offer high photocatalytic activity of powders in a wide range of processing temperatures including 900–1000 °C. Additionally, the modified powders remain highly photocatalytically active being involved in cyclic photocatalytic process, while the activity of unmodified material drops from cycle to cycle [16].
In this paper, we report on soft chemistry synthesis of nanocrystalline powder based on titanium dioxide doped with fluoride ions and discuss its properties, including photocatalytic activity in the visible light range.
2 Experimental Methods
2.1 Materials
All reagents used were commercially obtained and used without any further purification. All solutions were prepared using deionized water.
2.2 Photocatalyst Preparation
TiO2 nanocrystallites were synthesized from aqueous solutions of titanium chloride (pure TiCl4 liquid was carefully diluted with ice water to a transparent colorless aqueous solution) with concentration [Ti4+] = 0.1 M. Ammonia solution (10%) was added to these solutions drop wise until white precipitates (gels) were obtained and the pH = 7. The precipitates were washed with deionized water until chlorine ions were not detected. After the gel was filtered, hydrofluoric acid was introduced with the following molar ratios: 1 mol% (material 1), 5 mol% (material 2), or sodium fluoride (1, 3, 5 mol% for materials 3, 4, 5 respectively). Then the gels were dried and calcined for 120 min at different temperatures selected in accordance with DTA/TG-analysis and results of previous studies [17].
The materials 1, 2, 3, 4, 5 were calcined for 2 h at 500 °C (resulting materials 1.1, 2.1, 3.1, 4.1, 5.1), 600 °C (materials 1.2, 2.2, 3.2, 4.2, 5.2), 800 °C (materials 1.3, 2.3, 3.3, 4.3, 5.3), 900 °C (materials 1.4, 2.4, 3.4, 4.4, 5.4), and 1000 °C (materials 1.5, 2.5, 3.5, 4.5, 5.5). Degussa P25 (P25) was used as a reference for comparison.
2.3 Materials Characterization
X-ray powder diffraction (XRD) analysis was carried out using an ARL X’TRA diffractometer equipped with a high-intensity Kα1 irradiation (λ = 1.540562 Å) operated at 40 kV and 30 mA. Typical scans were performed in a wide range of Bragg angle (20° ≤ 2θ ≤ 60°). The XRD patterns were analyzed using the standard JCPDS files. Qualitative analysis of phase composition was performed using PDF-2 database and PCPDFWIN software.
Coherent scattering region was calculated from the X-ray line broadening, according to the Debye-Scherrer equation.
Thermogravimetry (TGA) and Differential Thermal Analysis (DTA) were carried out using a thermal analyzer (STA 449-S/4G Jupiter Jupted) at a heating rate of 10 °C/min.
The morphological characteristics were analyzed with transmission electron microscopy utilizing a TEM Tecnai G2 Spirit Bio TWIN operating at 120 kV.
2.4 Photocatalytic Activity Measurements
The photocatalytic activity of the prepared samples in the aqueous media was evaluated through monitoring of the discoloration of organic azo dye methylene blue (MB, C16H18N3SCl). In a typical measurement TiO2 power (1 g/l) was suspended in MB aqueous solution (20 mg/l) by stirring. Experiments were carried out at room temperature in quartz glass beakers. 125 W medium pressure mercury lamp was used as the UV light source. For modeling the photodegradation in the visible light range, a fluorescent light lamp was used (6400 K, 40 W). Residual concentration of the MB solutions was analyzed by mass spectrometry (Spectrophotometer UNICO 1201). Recycling test was subsequently performed three times.
3 Results and Discussion
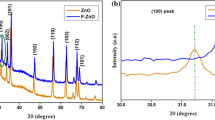
As evidenced by XRD analysis, the titanium dioxide gel obtained from the diluted solutions of inorganic precursors at low temperatures is X-ray amorphous. After thermal exposure, the XRD patterns showed the peaks of anatase and/or rutile phase (Figs. 2.1 and 2.2). However, the anatase modification remain unchanged in all F-doped materials processed for 2 h at temperatures up to 800 °C (Fig. 2.2). When the materials were processed at 900 °C for 2 h, we observed a mixture of anatase and rutile phases, and at 1000 °C the anatase modification had fully converted to the rutile phase. It is worth noting that, for pure titanium dioxide , the anatase-to-rutile phase transition starts at temperatures of above 700 °C under the same synthesis conditions. Consequently, the introduction of fluoride ions into TiO2-based materials offers the stabilization of anatase modification of titanium dioxide . This is consistent with the results reported in the literature [16].
Transmission electron microscopy revealed that all materials synthesized through this method contain nanoparticles . The characteristic particle shape is close to spherical, an average particle size is of about 10–30 nm, depending on the synthesis conditions (Fig. 2.3). The TEM data on average crystallite size coincide with the average size of corresponding coherent scattering areas calculated by the Debye-Scherrer equation. Among the factors influencing the crystallite size are the synthesis conditions for titanium hydroxide precursor phase, temperature and processing time for titanium dioxide . Thus, the crystallite size grows with increasing processing temperature and time.
Photocatalytic activity was studied both in the UV and visible light range. The photodegradation of standard methylene blue dye under UV radiation was carried out first. Among the materials containing 5 mol% fluoride ions, the material 2.2 calcined at 600 °C exhibits the best photocatalytic activity (Fig. 2.4). The same pattern was observed both for materials of other series containing a smaller amount of fluoride ions and pure TiO2 obtained through this method. Therefore, this temperature was optimal for fabrication of F-doped TiO2 photocatalytic materials. There is a general trend towards an increase in particle size and decrease in total surface area with increasing temperature. At lower processing temperatures, the particles of the material are poorly crystallized and do not show photocatalytic activity, even though the particle size is small and the total surface area is large.
All the synthesized materials that were calcined at 600 °C exhibit better photocatalytic activity than commercial Degussa P25 (Fig. 2.5). It is worth noting that amount of fluoride ions in a range of 1–5 mol% practically has no effect on the photocatalytic properties of obtained materials in the UV range.
The F-doped materials show high photocatalytic response not only under UV exposure but also in the visible light range (Fig. 2.6). The coverage of the visible light range offers significant increase in capacity of TiO2-based materials as photocatalysts . The reference sample, pure titanium dioxide synthesized through the same method under the same conditions, shows no photocatalytic activity in the visible light range.
4 Conclusions
The synthesized F-doped materials calcined at 600 °C exhibit photocatalytic activity in the both UV and visible light range. The photocatalytic activity induced by UV exposure is higher than that of commercial material Degussa P25, which is pure titanium dioxide . The introduction of fluoride ions was found to increase the thermal stability of anatase modification of titanium dioxide .
References
V. Etacheri, C. Di Valentin, J. Schneider, D. Bahnemann, S.C. Pillai, J. Photochem. Photobiol. C: Photochem. Rev. 25, 1 (2015)
M. Pelaez, N.T. Nolan, S.C. Pillai et al., Appl. Catal. B 125, 331 (2012)
M.R. Hoffmann, S.T. Martin, W. Choi et al., Chem. Rev. 95(1), 69 (1995)
C. Wang, H. Liu, Y. Qu, J. Nanomaterials 1, 2013 (2013)
A. Buthiyappan, A. Aziz, A. Raman, W. Daud, W.M. Ashri, Rev. Chem. Eng. 32(1), 1 (2016)
Q. Guo, C. Zhou, Z. Ma, Z. Ren, H. Fan, X. Yang, Chem. Soc. Rev. 45(13), 3701 (2016)
U. Diebold, Surf. Sci. Rep. 48(5), 53 (2003)
J. Banfield, J. Mater. Chem. 8(9), 2073 (1998)
L.G. Devi, R. Kavitha, Appl. Catal. B 140, 559 (2013)
M. Hamadanian, S. Karimzadeh, V. Jabbari, D. Villagrán, Mater. Sci. Semicond. Process. 41, 168 (2016)
N. Karanasios, J. Georgieva, E. Valova, S. Armyanov, G. Litsardakis, S. Sotiropoulos, Curr. Org. Chem. 19(6), 512 (2015)
R. Fagan, D.E. MacCormack, D.D. Dionysiou, S.C. Pillai, Mater. Sci. Semicond. Process. 42, 2 (2016)
S.A. Bakar, C. Ribeiro, J. Photochem. Photobiol., C 27, 1 (2016)
S.A. Ansari, M.M. Khan, M.O. Ansari, M.H. Cho, New J. Chem. 40(4), 3000 (2016)
G. Wu, J. Wang, D.F. Thomas, A. Chen, Langmuir 24(7), 3503 (2008)
T.A. Sednieva, E.P. Lokshin, A.T. Belyaev, V.T. Kalinnikov, Adv. Mater. 6, 49 (2007)
E.M. Bayan, T.G. Lupeiko, L.E. Pustovaya, A.G. Fedorenko, in, Advanced Materials Manufacturing, Physics, Mechanics and Applications. Springer Proceedings in Physics, vol. 175, eds. by I.A. Parinov, S.-H. Chang, V.Y. Topolov (Springer, Heidelberg, 2016), p. 51
Acknowledgements
This research was supported by the Project Part of the State Assignment for Research (Ref. No.: 4.2592.2014/K).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this paper
Cite this paper
Bayan, E.M., Lupeiko, T.G., Kolupaeva, E.V., Pustovaya, L.E., Fedorenko, A.G. (2017). Fluorine-Doped Titanium Dioxide: Synthesis, Structure, Morphology, Size and Photocatalytic Activity. In: Parinov, I., Chang, SH., Jani, M. (eds) Advanced Materials. Springer Proceedings in Physics, vol 193. Springer, Cham. https://doi.org/10.1007/978-3-319-56062-5_2
Download citation
DOI: https://doi.org/10.1007/978-3-319-56062-5_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-56061-8
Online ISBN: 978-3-319-56062-5
eBook Packages: Physics and AstronomyPhysics and Astronomy (R0)