Abstract
The role of mitochondria in diabetic complications has been viewed as a source of excess superoxide production leading to cell dysfunction. However, with the lack of benefit of non-specific anti-oxidant approaches this view needs to be re-evaluated. With recent studies using real-time imaging of superoxide, metabolomics, flux studies, transcriptomics and proteomics a new appreciation for the role of mitochondria in the evolution of diabetic kidney disease has emerged. Ongoing studies to further unravel the time course and mechanisms that reduce mitochondrial function will be relevant to novel therapies that could have a major impact on diabetic kidney disease and other diabetic complications.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
The pathobiology of diabetic kidney disease has primarily focused on a variety of metabolic pathways linked to glucose metabolism and inflammatory mediators [1]. However, many of the pathways implicated including the poly-ol pathway, hexosamine formation, glycosylation, protein kinase C have not translated to interventions showing clinical utility [1–3]. Beginning with the work from Brownlee et al., the concept that mitochondrial superoxide production played an upstream role in regulating a variety of pathways has held sway [4, 5]. Unfortunately, clinical approaches to reduce superoxide production have not had promising results [6, 7]. However, recent work has not found evidence of an increase in mitochondrial superoxide production in the diabetic kidney [8, 9]. In contrast, an opposing theory stating that reduced mitochondrial function (or dysfunction) is a major upstream consequence of hyperglycemia and diabetes has been gaining traction [10]. In support of this newer view, an approach to augment mitochondrial function may provide novel therapeutics to delay and arrest diabetic complications.
Mitochondrial Function in the Kidney
Apart from the heart, the kidney is considered to contain the greatest density of mitochondria per mass of tissue [11]. An explanation for this observation may be that apart from the heart, the kidney is constantly required to be functioning to maintain adequate homeostasis of water, electrolytes, minerals and many other organic and inorganic ions within the organism. Therefore, a loss of mitochondrial function or content could be the basis or consequence of reduced organ function. The role of mitochondria in organ dysfunction may be best understood in the setting of ischemia-reperfusion syndrome in the heart and kidney. The normal heart has to continuously perform a coordinated function linking electrical activity to diastolic and systolic activity of the right and left ventricles. The energy demands amount to about 30 kg of ATP production on a daily basis and much of the ATP generation is considered to be derived from ox-phos activity. However, it is exceedingly difficult to quantitate the amount and function of mitochondria in an organ. One approach is to quantify the supramolecular complexes or respirasomes [12]. Recently, respirasomes were found to be reduced in heart failure [12] setting up a paradigm that reduced mitochondrial energy output could contribute to the progressive failure of heart function in CHF [13].
Similarly, the kidney tubular cells are considered to require ATP for the enormous requirements of the proximal tubular cell to retain water, sodium and electrolytes. The TAL cell also requires a large amount of ATP to pump sodium, potassium and chloride into the interstitium and generate the counter-current gradient. In models of ischemia-reperfusion injury and septic kidney disease there appears to be a marked depletion of mitochondrial function [11, 14], which may underlie the basis of reduced renal function despite normal appearing glomeruli and restoration of renal blood flow. Whether mitochondrial dysfunction plays a role in human diabetic kidney disease was not comprehensively evaluated until quite recently.
Metabolomic Studies Implicating Mitochondrial Dysfunction in Diabetes
Recently, several groups have applied both urine and serum metabolomics to characterize biochemical disturbances in diabetic kidney disease. Blood studies revealed a role for pseudouridine as a biomarker for diabetic kidney disease [15, 16], however the role of pseudouridine remains unclear. Other metabolites that have been identified include indoxylsulfate [17] which may be a marker of renal dysfunction and has been recognized as a uremic metabolite and partly controlled by gut bacteria [16]. Kynurenine has also been found to be elevated in patients and may predispose to kidney disease [18]. Additionally, plasma sphingomyelin has been found to be elevated in patients with diabetes and may pre-date kidney disease [19].
Urine metabolomics studies have been plagued by lack of quality control in collecting timed samples of urine [3]. A well conducted study using both GC-MS and LC-MS based analysis of urine [20] identified that acyl-carnitines, acyl-glycines and tryptophan metabolites discriminated patients with DKD. In our study of subjects with diabetes and evidence of kidney disease, characterized by reduced eGFR (<75 ml/min/1.73 m2) using 24 h urine collections, there was a consistent reduction in a panel of 13 metabolites [21]. These 13 metabolites were found to be reduced in subjects from many parts of the USA and Europe with diabetes and CKD. Using a network analysis approach it was found that the 13 metabolites were all linked in a large network and that 12/13 metabolites were controlled by enzymes localized to mitochondria. As there was a significant reduction of the metabolites it was concluded that overall mitochondrial function was reduced in patients with diabetic kidney disease. Support from this conclusion came from studies of kidney biopsies which indicated a reduction in mitochondrial protein (complex IV) and reduction in PGC1α the master regulator of biogenesis.
A reduction in mitochondria in patients with diabetic kidney disease was also demonstrated by a reduction of urine exosomal mtDNA [21] and a reduction in blood levels of mtDNA [22]. These studies demonstrate from a variety of approaches that diabetic kidney disease is marked by reduced mitochondrial content and function. Of major interest is that non-diabetic kidney disease is also marked by reduction of circulating mtDNA [23] and reduced PGC1α in kidney tissues [24].
Mitochondrial Content and Function in Experimental DKD
Mitochondrial function has been considered to be at the heart of understanding diabetic complications as glucose oxidation fundamentally involves the mitochondrial TCA cycle and oxidative phosphorylation. However, the response of mitochondria to excess glucose in many cells and organs still remain unclear. Using a targeted metabolomics approach, Mootha’s group studied the metabolic response to glucose [25]. In healthy volunteers the ingestion of an oral glucose challenge resulted in a remarkable increase in glycolytic intermediates and an increase in bile acids in the blood. Surprisingly, there was a marked reduction in many amino acids, including the branched chain amino acids, leucine, isoleucine and valine. There was also a marked reduction in the ketone body, β-OH butyrate. The overall response was considered a switch from catabolism to anabolism. Once mitochondrial function analysis was developed initial studies focused on the skeletal muscle response in patients with diabetes. As skeletal muscle is considered to contain the greatest content of mitochondria, it is logical to assess the skeletal muscle mitochondrial response in the diabetic milieu. A series of studies found that mitochondrial content and function were reduced in the skeletal muscle [26, 27]. However, therapeutic approaches to improve mitochondrial function in patients with diabetes have not been pursued until very recently.
The kidney is considered to have the greatest amount of mitochondria per tissue mass, and is only second to the heart [28]. With hyperglycemia from type 1 diabetes, the kidney response was found to reduce the content of mitochondria per mass of tissue [8]. Electron chain complex activity was measured with classical methods and found to be generally reduced across all of the complexes [8]. There may be a reduction of pyruvate entry into the mitochondria as the pyruvate dehydrogenase complex was phosphorylated [8]. Despite the reduction in mitochondrial electron chain complex activity and PDH, several studies have found an increase in TCA metabolites in the urine of mouse models of type 1 diabetes. Recent studies have also addressed mitochondrial function and content with type 1 diabetic animal models. With STZ-induced diabetes in the rat, mitochondrial fragmentation was found at 4 weeks of diabetes in association with reduced ATP content and these disturbances preceded albuminuria and elevation in urinary KIM1 levels [9]. Reduced mitochondrial electron chain complex III activity was found in the diabetic rat kidney by a separate group [29]. Mitochondrial DNA content was found to be increased with diabetes in this model. Mitochondrial DNA was found to be elevated in mesangial cells exposed to high glucose in the first 4 days before reduction was noted with longer durations [22].
Several groups have also found marked differences in mitochondria with type 2 diabetes. In the db/db mouse model, there is a fragmentation and reduced function of mitochondria in the podocytes [30]. There was an increase in mitochondrial fission which was mediated via Rho-associated coiled coil-containing protein kinase 1 (ROCK1) activation. The role of dynamin related protein (Drp1) was recently implicated as mice with podocyte specific inducible reduction of Drp1 had improved mitochondrial structure and function [31]. Rap1b, a small GTPase, was also found to improve mitochondrial function in renal tubular cells treated with high glucose and Rap1b was recently found to be reduced in tubular cells in biopsy tissue from patients with diabetic nephropathy [32]. Using electron microscopy, there was evidence of reduction of elongated mitochondria in tubular cells of diabetic nephropathy specimens [32].
In patients with diabetic kidney disease our group initially found a reduction in mitochondrial DNA content in urine exosomes [21]. As urine exosomes are largely derived from podocytes and tubular epithelial cells, the reduction in urine exosomal mtDNA was considered to reflect a reduction in renal epithelial mitochondrial content. Support for this hypothesis was found with reduction of mitochondrial complex protein levels in kidney biopsies from patients with diabetic nephropathy. The reduction of mitochondria is likely due in large part due to reduction of PGC1α, the master regulator of mitochondrial biogenesis.
Role of PGC1α in CKD and DKD
The dependence of mitochondrial function for recovery of kidney function may be exemplified by the role of PGC1α. As the master regulator of mitochondrial biogenesis, PGC1α has been recognized to be critically important in a variety of tissues including muscle tissue [33]. With diabetes, it was recognized that PGC1α was reduced in the skeletal muscle of patients with type 2 diabetes. However, the role of PGC1α in the muscle remains unclear as overexpression of PGC1α leads to insulin resistance (see Chap. 25). A recent study found that upregulation of PGC1α was associated with stimulation of a specific metabolite, 3-hydroxyisobutyrate (3-HIB), a catabolic intermediate of valine [34]. 3-HIB mediated enhanced trans-endothelial fatty acid transport and may play a role in the link with branched chain amino acids and development of insulin resistance and type 2 diabetes [34]. Interestingly, one of the 13 metabolites that were reduced in the urine of patients with DKD is 3-HIB and we were the first to recognize that PGC1α was reduced in kidney disease [21].
The role of PGC1α was found to play a major role initially in acute kidney injury. In studies by Parikh and colleagues, tubular reduction of PGC1α made mice susceptible to ischemic and sepsis related injury [35] whereas over expression of PGC1α in tubular cells conferred protection [14]. The benefit of upregulating PGC1α in ischemic renal injury may partly be due to regulation of NAD. In a model of chronic kidney disease and renal fibrosis with folic acid-induced nephropathy, tubular upregulation of PGC1α was found to be remarkably protective [24].
In animal models of diabetic kidney disease, PGC1α has been found to be reduced and with stimulation of AMPK, PGC1α protein levels were restored along with markers of mitochondrial content and electron transport chain activity [8]. Several groups have now demonstrated a similar relationship in diabetic kidney disease [36, 37]. The regulation of PGC1α and mitochondrial biogenesis in DKD appears to be primarily be regulated by AMPK.
AMPK, Mitochondrial Function and ROS in DKD
PGC1α can be affected by numerous signaling pathways as well as potentially by epigenetic regulation of the PGC1α promoter. In the context of diabetes, a major upstream pathway appears to be AMPK, the master energy sensor. 5′-adenosine monophosphate (AMP)-activated protein kinase (AMPK) is an evolutionarily conserved serine/threonine kinase that is a key player in maintaining cellular energy homeostasis. AMPK is highly regulated by the AMP/ATP ratio and is activated under conditions of low ATP levels. In this context activation of AMPK serves to improve energy efficiency by reducing protein synthesis and enhancing glucose entry into cells and stimulation of PGC1α and mitochondrial biogenesis. AMPK is also now recognized to have a variety of functional roles and is also regulated by a variety of kinases such as liver kinase B1 (LKB1), calcium−/calmodulin-dependent kinase kinase 2 (CAMKK2), TGF-β activated kinase 1 (TAK1) as well as a variety of phosphatases, such as protein phosphatase 2A (PP2A), protein phosphatase 2C (PP2C) and Mg2+/Mn2+ dependent protein phosphatase 1E (PPM1E) [38]. Several groups have identified that AMPK is reduced in models of type 1 and type 2 diabetic kidney disease as well as in the diet-induced obesity model of kidney disease [8, 39–41].
The regulation of AMPK in diabetic kidney disease remains unclear. Studies by Kasinath’s group identified that LKB1 activity was reduced by high glucose due to acetylation of LKB1 and contributed to reduced AMPK activity [42]. Similar results were found by Susztak’s group as deletion of LKB1 in tubular cells led to AMPK reduction and apoptosis whereas stimulation of AMPK was able to rescue the phenotype [43]. Recently, our group evaluated the regulation of ATP, ADP and AMP in the diabetic kidney using mass spec imaging [44]. As ATP is easily metabolized in harvested tissue and AMP is of very low levels, accurate measurements are difficult to perform. With the advent of mass spec imaging on frozen tissues, the aspect of tissue processing is limited and the likelihood that ATP levels are similar to the in vivo condition is higher. Using MALDI-TOF approach with targeted mass spec imaging, we found that glomerular levels of ATP/AMP or ATP/ADP were elevated in diabetic kidneys. Thus, it is likely that at the glomerular level, the reduction of AMPK is at least in part due to an elevation in the ATP/AMP ratio.
Stimulation of AMPK in diabetic kidney disease was found to have a remarkable effect to reduce albuminuria, mesangial matrix expansion and the stimulation of matrix molecules and TGF-β. AMPK activation also reduced a key producer of reactive oxygen species in the kidney, NADPH oxidase. The benefit of AMPK activation with AICAR was found to be dependent on AMPK, as mice deficient in AMPKα2 had increased albuminuria but no beneficial effect with AICAR. AMPK activation also markedly reduced mTOR activation in the diabetic kidney and restored PGC1α levels in the diabetic kidney independent of lowering blood glucose or changes in body weights in type 1 models of diabetic kidney disease.
A key insight was the effect of AMPK activation on markers of mitochondrial biogenesis and electron chain activity. The diabetic kidney was found to have a reduction of mitochondrial content and reduced activity of the electron chain complexes. This was completely restored with AMPK activation, likely via stimulation of PGC1α. Therefore, this study provided compelling evidence that stimulation of mitochondrial biogenesis and mitochondrial electron transport chain activity led to beneficial outcomes in diabetic kidney disease. This study also provided new insight into the role of mitochondrial ROS in the context of diabetic kidney disease.
It has been widely believed that overproduction of superoxide from mitochondria played a key role in causing diabetic complications and diabetic kidney disease. However, this theory had not been supported by direct in vivo measurement of mitochondrial superoxide. We developed a DHE-based in vivo assessment of superoxide in mice and found that the normal kidney had a high level of superoxide production [8]. Surprisingly, with type 1 diabetes there was a marked reduction of renal superoxide production in association with reduced mitochondrial electron transport chain activity. Therefore, we concluded that the reduction of mitochondrial electron transport chain activity was reflected by reduced production of superoxide. As the mitochondrial contribution of superoxide in the baseline state is widely believed to be 80% of basal superoxide production, our DHE-based studies support the idea that mitochondrial superoxide is reduced in the diabetic kidney. Additional studies using electron paramagnetic resonance with kidney extracts also found a reduced level of superoxide from the diabetic kidney. Finally, isolated mitochondria from the diabetic kidney also exhibited reduced levels of ROS production.
To further evaluate the role of mitochondrial superoxide, studies were also performed in the SOD2+/− mouse. The reduced levels of mitochondrial SOD were associated with a high level of DHE-based superoxide production in the kidney. However, despite this high level of superoxide the SOD2+/− mouse has no evidence of kidney disease [8]. Even with diabetes, this mouse does not develop any worsening of diabetic kidney disease. These results indicate that mitochondrial superoxide are neither necessary nor sufficient for development of diabetic kidney disease. The major detrimental source of ROS in the diabetic kidney is likely to be non-mitochondrial and possibly arising from NADPH oxidase. Indeed, stimulation of mitochondrial electron transport chain activity is associated with an enhancement of superoxide production and amelioration of renal inflammation and fibrosis. This concept is captured in the theory of mitochondrial hormesis in the context of diabetic complications. At present, several pharmaceutical companies are pursuing strategies to improve mitochondrial function for many chronic conditions, including diabetic nephropathy.
New Concepts Linking Reduced Mitochondrial Function to Diabetic Kidney Disease
A major impediment to advancing novel treatments for diabetic kidney disease is the lack of insightful biomarkers. Recently an inhibitor of Nox4 was found to benefit diabetic kidney disease by several groups, however the downstream target of Nox4 activity has not been identified. Using urine metabolomics, we found that inhibition of Nox4 was found to dose-dependently reduce levels of urine fumarate in type 1 diabetic micei [45]. Tissue levels of fumarate were also increased in the diabetic kidney and reduced with the Nox4 inhibitor.
Fumarate is a key metabolite within the TCA cycle and arises from conversion of succinate to fumarate by succinate dehydrogenase (SDH). Fumarate itself is converted to malate by fumarate hydratase. As fumarate was increased in the diabetic tissue, we hypothesized that FH activity would be reduced in the diabetic kidney. Indeed there was a marked reduction of FH activity and FH protein in the diabetic kidney. FH appears to be a key target of Nox4, as overexpression of Nox4 led to marked reduction of FH levels and Nox4 inhibition restored FH levels. Importantly, FH was also found to be reduced in the human diabetic kidney.
Thus, there is now supportive data that a key mitochondrial enzyme, FH, is affected in diabetic kidney disease and likely contributes to an upregulation of tissue and urine fumarate levels. A recent study using patients with diabetes found that urine levels of fumarate were increased in patients with diabetes who had progressive renal disease. Further studies are ongoing with larger prospective cohorts to determine the role of urine fumarate as a biomarker of progressive diabetic kidney disease.
Fumarate itself has been identified as a “fibrogenic metabolite”. Addition of fumarate to renal cells leads to stimulation of TGF-β and gene expression of extracellular matrix molecules. Fumarate is also a potent stimulator of HIF1α accumulation by inhibiting prolyl-hydroxylase activity. The combination of increased HIF1α and TGF-β may work in concert to suppress mitochondrial ox-phos activity, suppress mitochondrial biogenesis and promote matrix accumulation and fibrosis.
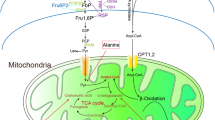
Another major insight has been the recognition that reduced fatty acid oxidation and impaired cholesterol export could contribute to progressive kidney disease, including diabetic kidney disease. With reduced mitochondrial biogenesis, there could be reduced fatty acid oxidation leading to accumulation of fatty acids. In addition, reduced mitochondrial biogenesis may contribute to reduced cholesterol export pathways and lipotoxicity. Stimulation of fatty acid oxidation, possibly via PPARα stimulation is an attractive target for future therapeutics.
Concluding Remarks
In the past 5 years there has been a remarkable resurgence of interest in the role of mitochondrial function in diabetic kidney disease. In contrast to the prevailing theory, accumulating evidence suggests that mitochondrial dysfunction and suppression are consequence of diabetes and not causative for disease progression. By restoring mitochondrial biogenesis, reducing mitochondrial fragmentation and improving mitochondrial activity there is marked improvement in disease parameters. Future studies to specifically target pathways of mitochondrial biogenesis via ROCK1 inhibitors, AMPK activation and PGC1α stimulation would provide novel approaches. Newer targets such as fumarate hydratase activators and pathways to enhance fatty acid oxidation may also provide potent therapeutic approaches. With the parallel improvements in metabolomics, a coordinated companion diagnostic approach would be of added value to provide informative biomarkers to assure that disease pathways are being engaged by the therapeutic and efficacy will hopefully be predicted.
References
Filla LA, Edwards JL. Metabolomics in diabetic complications. Mol BioSyst. 2016;12:1090–105.
Hallan S, Sharma K. The role of mitochondria in diabetic kidney disease. Curr Diabetes Rep. 2016;16:61.
Darshi M, Van Espen B, Sharma K. Metabolomics in diabetic kidney disease: unraveling the biochemistry of a silent killer. Am J Nephrol. 2016;44:92–103.
Nishikawa T, Edelstein D, Du XL, Yamagishi S, Matsumura T, Kaneda Y, et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000;404:787–90.
Nishikawa T, Brownlee M, Araki E. Mitochondrial reactive oxygen species in the pathogenesis of early diabetic nephropathy. J Diabetes Investig. 2015;6:137–9.
de Zeeuw D, Akizawa T, Audhya P, Bakris GL, Chin M, Christ-Schmidt H, et al. Bardoxolone methyl in type 2 diabetes and stage 4 chronic kidney disease. N Engl J Med. 2013;369:2492.
Alkhalaf A, Kleefstra N, Groenier KH, Bilo HJ, Gans RO, Heeringa P, et al. Effect of benfotiamine on advanced glycation end products and markers of endothelial dysfunction and inflammation in diabetic nephropathy. PLoS One. 2012;7:e40427.
Dugan LL, You YH, Ali SS, Diamond-Stanic M, Miyamoto S, DeCleves AE, et al. AMPK dysregulation promotes diabetes-related reduction of superoxide and mitochondrial function. J Clin Invest. 2013;123:4888–99.
Coughlan MT, Nguyen TV, Penfold SA, Higgins GC, Thallas-Bonke V, Tan SM, et al. Mapping time-course mitochondrial adaptations in the kidney in experimental diabetes. Clin Sci (Lond). 2016;130:711–20.
Sharma K. Mitochondrial hormesis and diabetic complications. Diabetes. 2015;64:663–72.
Parikh SM, Yang Y, He L, Tang C, Zhan M, Dong Z. Mitochondrial function and disturbances in the septic kidney. Semin Nephrol. 2015;35:108–19.
Rosca MG, Vazquez EJ, Kerner J, Parland W, Chandler MP, Stanley W, et al. Cardiac mitochondria in heart failure: decrease in respirasomes and oxidative phosphorylation. Cardiovasc Res. 2008;80:30–9.
Santulli G, Xie W, Reiken SR, Marks AR. Mitochondrial calcium overload is a key determinant in heart failure. Proc Natl Acad Sci USA. 2015;112:11389–94.
Tran MT, Zsengeller ZK, Berg AH, Khankin EV, Bhasin MK, Kim W, et al. PGC1alpha drives NAD biosynthesis linking oxidative metabolism to renal protection. Nature. 2016;531:528–32.
Solini A, Manca ML, Penno G, Pugliese G, Cobb JE, Ferrannini E. Prediction of declining renal function and albuminuria in patients with type 2 diabetes by metabolomics. J Clin Endocrinol Metab. 2016;101:696–704.
Niewczas MA, Sirich TL, Mathew AV, Skupien J, Mohney RP, Warram JH, et al. Uremic solutes and risk of end-stage renal disease in type 2 diabetes: metabolomic study. Kidney Int. 2014;85:1214–24.
Atoh K, Itoh H, Haneda M. Serum indoxyl sulfate levels in patients with diabetic nephropathy: relation to renal function. Diabetes Res Clin Pract. 2009;83:220–6.
Rhee EP, Thadhani R. New insights into uremia-induced alterations in metabolic pathways. Curr Opin Nephrol Hypertens. 2011;20:593–8.
Makinen VP, Tynkkynen T, Soininen P, Forsblom C, Peltola T, Kangas AJ, et al. Sphingomyelin is associated with kidney disease in type 1 diabetes (The FinnDiane Study). Metabolomics. 2012;8:369–75.
van der Kloet FM, Tempels FW, Ismail N, van der Heijden R, Kasper PT, Rojas-Cherto M, et al. Discovery of early-stage biomarkers for diabetic kidney disease using ms-based metabolomics (FinnDiane study). Metabolomics. 2012;8:109–19.
Sharma K, Karl B, Mathew AV, Gangoiti JA, Wassel CL, Saito R, et al. Metabolomics reveals signature of mitochondrial dysfunction in diabetic kidney disease. J Am Soc Nephrol. 2013;24:1901–12.
Czajka A, Ajaz S, Gnudi L, Parsade CK, Jones P, Reid F, et al. Altered mitochondrial function, mitochondrial DNA and reduced metabolic flexibility in patients with diabetic nephropathy. EBioMedicine. 2015;2:499–512.
Tin A, Grams ME, Ashar FN, Lane JA, Rosenberg AZ, Grove ML, et al. Association between mitochondrial DNA copy number in peripheral blood and incident CKD in the atherosclerosis risk in communities study. J Am Soc Nephrol. 2016;27:2467–73.
Kang HM, Ahn SH, Choi P, Ko YA, Han SH, Chinga F, et al. Defective fatty acid oxidation in renal tubular epithelial cells has a key role in kidney fibrosis development. Nat Med. 2015;21:37–46.
Shaham O, Wei R, Wang TJ, Ricciardi C, Lewis GD, Vasan RS, et al. Metabolic profiling of the human response to a glucose challenge reveals distinct axes of insulin sensitivity. Mol Syst Biol. 2008;4:214.
Kelley DE, He J, Menshikova EV, Ritov VB. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes. 2002;51:2944–50.
Dobson AW, Kelley MR, Wilson GL, LeDoux SP. Targeting DNA repair proteins to mitochondria. Methods Mol Biol. 2002;197:351–62.
Pagliarini DJ, Calvo SE, Chang B, Sheth SA, Vafai SB, Ong SE, et al. A mitochondrial protein compendium elucidates complex I disease biology. Cell. 2008;134:112–23.
Mustata GT, Rosca M, Biemel KM, Reihl O, Smith MA, Viswanathan A, et al. Paradoxical effects of green tea (Camellia sinensis) and antioxidant vitamins in diabetic rats: improved retinopathy and renal mitochondrial defects but deterioration of collagen matrix glycoxidation and cross-linking. Diabetes. 2005;54:517–26.
Wang W, Wang Y, Long J, Wang J, Haudek SB, Overbeek P, et al. Mitochondrial fission triggered by hyperglycemia is mediated by ROCK1 activation in podocytes and endothelial cells. Cell Metab. 2012;15:186–200.
Ayanga BA, Badal SS, Wang Y, Galvan DL, Chang BH, Schumacker PT, et al. Dynamin-related protein 1 deficiency improves mitochondrial fitness and protects against progression of diabetic nephropathy. J Am Soc Nephrol. 2016;27:2733–47.
Xiao L, Zhu X, Yang S, Liu F, Zhou Z, Zhan M, et al. Rap1 ameliorates renal tubular injury in diabetic nephropathy. Diabetes. 2014;63:1366–80.
Leone TC, Lehman JJ, Finck BN, Schaeffer PJ, Wende AR, Boudina S, et al. PGC-1alpha deficiency causes multi-system energy metabolic derangements: muscle dysfunction, abnormal weight control and hepatic steatosis. PLoS Biol. 2005;3:e101.
Jang C, Oh SF, Wada S, Rowe GC, Liu L, Chan MC, et al. A branched-chain amino acid metabolite drives vascular fatty acid transport and causes insulin resistance. Nat Med. 2016;22:421–6.
Tran M, Tam D, Bardia A, Bhasin M, Rowe GC, Kher A, et al. PGC-1alpha promotes recovery after acute kidney injury during systemic inflammation in mice. J Clin Invest. 2011;121:4003–14.
Hong YA, Lim JH, Kim MY, Kim TW, Kim Y, Yang KS, et al. Fenofibrate improves renal lipotoxicity through activation of AMPK-PGC-1alpha in db/db mice. PLoS One. 2014;9:e96147.
Wu L, Wang Q, Guo F, Zhou Y, Ji H, Liu F, et al. Activation of FoxO1/PGC-1alpha prevents mitochondrial dysfunction and ameliorates mesangial cell injury in diabetic rats. Mol Cell Endocrinol. 2015;413:1–12.
Jeon SM. Regulation and function of AMPK in physiology and diseases. Exp Mol Med. 2016;48:e245.
Eid AA, Ford BM, Block K, Kasinath BS, Gorin Y, Ghosh-Choudhury G, et al. AMP-activated protein kinase (AMPK) negatively regulates Nox4-dependent activation of p53 and epithelial cell apoptosis in diabetes. J Biol Chem. 2010;285:37503–12.
Lee MJ, Feliers D, Mariappan MM, Sataranatarajan K, Mahimainathan L, Musi N, et al. A role for AMP-activated protein kinase in diabetes-induced renal hypertrophy. Am J Physiol Renal Physiol. 2007;292:F617–27.
Decleves AE, Mathew AV, Cunard R, Sharma K. AMPK mediates the initiation of kidney disease induced by a high-fat diet. J Am Soc Nephrol. 2011;22:1846–55.
Lee MJ, Feliers D, Sataranatarajan K, Mariappan MM, Li M, Barnes JL, et al. Resveratrol ameliorates high glucose-induced protein synthesis in glomerular epithelial cells. Cell Signal. 2010;22:65–70.
Han SH, Malaga-Dieguez L, Chinga F, Kang HM, Tao J, Reidy K, et al. Deletion of Lkb1 in renal tubular epithelial cells leads to CKD by altering metabolism. J Am Soc Nephrol. 2016;27:439–53.
Miyamoto S, Hsu CC, Hamm G, Darshi M, Diamond-Stanic M, Decleves AE, et al. Mass spectrometry imaging reveals elevated glomerular ATP/AMP in diabetes/obesity and identifies sphingomyelin as a possible mediator. EBioMedicine. 2016;7:121–34.
You YH, Quach T, Saito R, Pham J, Sharma K. Metabolomics reveals a key role for fumarate in mediating the effects of NADPH oxidase 4 in diabetic kidney disease. J Am Soc Nephrol. 2016;27(2):466–81.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Sharma, K. (2017). Mitochondrial Dysfunction in the Diabetic Kidney. In: Santulli, G. (eds) Mitochondrial Dynamics in Cardiovascular Medicine. Advances in Experimental Medicine and Biology, vol 982. Springer, Cham. https://doi.org/10.1007/978-3-319-55330-6_28
Download citation
DOI: https://doi.org/10.1007/978-3-319-55330-6_28
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-55329-0
Online ISBN: 978-3-319-55330-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)