Abstract
Salalah plain aquifer, located in the southeast of Sultanate Oman, has been extensively used for agricultural, industrial and municipal purposes since the early 1970s. Over abstraction to satisfy the growing need of freshwater demand has contributed to the groundwater salinisation by seawater intrusion. The aim of this study is to evaluate the impact of this phenomenon on the groundwater quality in the Salalah plain. The occurrence of seawater intrusion in this plain was analysed using a hydrodynamic and hydrochemical approaches. The first approach was based on the analysis of groundwater table depth and salinity data available during the period from 1984 to 2009. The groundwater piezometric heads recorded in the upstream and intermediate regions of the plain showed seasonal fluctuations due to the monsoon. In the coastal region, the piezometric heads showed small fluctuations, which are indicative of a quasi-static regime. The groundwater salinity showed large variations and generally increased in the flow direction from north to south. Near the shoreline, the groundwater salinity has increased by 8420 µS/cm during the period from 1984 to 1994. In the study area, 11 groundwater samples were collected during October 2015 and analysed for EC, pH and major ions. Results indicate that groundwater chemistry was classified as Na–Cl type in one well, Ca–Mg–Cl type in three wells and Ca–Cl type in seven wells. The first two water types indicate the effect of seawater intrusion and comparatively the Ca–Cl water type highlights the effect of ion exchange reactions on the mineralisation of groundwater. The high concentrations of nitrate recorded especially in the Hafah agricultural area confirm the impact of fertilizers application on groundwater quality.
Access provided by CONRICYT-eBooks. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Saltwater intrusion is a global environmental issue that degrades groundwater quality by increasing salinisation in many coastal aquifers (Park et al. 2005; Bahar and Reza 2010; Askri 2015). This process occurs due to groundwater overexploitation to meet the growing requirement of freshwater for agricultural, industrial and drinking purposes (Singh 2014; Yüce 2005). In coastal regions, the excessive groundwater abstraction may cause a decline of piezometric heads and induce a modification of natural flow system (Kouzana et al. 2009). The effect of seawater intrusion on groundwater salinisation has been studied extensively during the last two decades using different approaches. Sherif et al. (2011) showed that the recently seawater intrusion has become the principal factor of water salinity in the aquifer of wadi Ham, UAE. Arslan (2013) classified the groundwater in Bafra coastal plain, Turkey, into three groups affected by saltwater intrusion of different levels. Kuzana et al. (2009) indicated that saltwater intrusion from the Mediterranean Sea is the main cause of high groundwater salinity observed in the Korba aquifer, northern Tunisia. In this coastal aquifer, Zghibi et al. (2013) highlighted the occurrence of direct cation exchange associated with saltwater intrusion and dissolution processes linked to cations exchange. Agoubi et al. (2013) studied the causes of groundwater salinisation in the marine Jeffara aquifer, southern Tunisia. They showed that the saltwater mixing with groundwater is the consequence of seawater intrusion.
Coastal groundwater aquifers in Sultanate of Oman are supplying about 99% of freshwater demand (Rajmohan et al. 2009). The rapid growth of population in Salalah city along with economic growth enhanced the groundwater salinisation through over abstraction of groundwater for drinking, municipal, agricultural and industrial purposes. Clarck et al. (1987) indicated significant drops of piezometric heads in the Salalah plain aquifer since the early 1980s. As consequence, evidence of seawater intrusion due to the excessive groundwater abstraction became apparent mainly in the coastal sector (D&MI 1992). This study concerns the impact of seawater intrusion on the groundwater quality in the Salalah plain. The objectives are (i) to analyse the dynamics of seawater intrusion in the shallow groundwater aquifer, (ii) to explore the hydrochemical processes regulating the groundwater mineralization and (iii) to evaluate the degree of suitability of groundwater for irrigation purpose.
2 Materials and Methods
2.1 Study Area
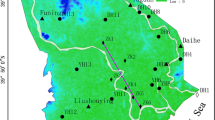
Salalah is the capital of Dhofar Governorate in southeastern Sultanate of Oman. It is the second largest city in the country with a population of 134,000 inhabitants and a yearly population growth rate of 2.3% (Oman Census 2003). The study area is a sector of the Salalah coastal plain (Fig. 1). Jabal Al-Qara (mountain) and Arabian Sea represent, respectively, the northern and southern boundaries of the area. Wadis exist in the plain and flow to the sea. Two recharge and flood-protecting dams have been built in 1993 and 2007 on Sahalnawt and Zarsis wadis, respectively. Since 2003, treated wastewater was injected into the groundwater to create a hydraulic water barrier to reduce the amount of saltwater that can penetrate into the freshwater (Shammas 2007).
Location map of the study area filled circle represents sampling wells in October 2015, filled triangle represents monitoring wells from May 1982 to February 2007. Line A–B is a cross section clarified in Fig. 2
Most of precipitation in Salalah region results from the monsoon occurring between late June and early September. It is a combination of rain, fog, drizzle and mist. The annual rainfall amounts vary from 45 and 154 mm in the plain and from 230 to 450 mm in Jabal AL-Qara (Shammas 2007). Cyclonic storms occur once every 3–7 years, affect the plain and cause loss of life and extensive damage of infrastructures. Daily temperature ranges from 27 °C in January to 32 °C in June. Humidity varies from 40% in February to 97% in July. The evaporation is very high and ca reach 1700 mm/year.
The Salalah plain is an intensively irrigated area. Fruits such as carica papaya, bananas and coconut palms, grass and vegetables are cultivated mainly in the Hafa agricultural area. Recently, large-scale grass cultivation has been implemented in Sahalnawt and Jarziz farms.
The study area is a part of the down-faulting of the Salalah plain of Oman. This plain resulted from the major tectonic activity, which was occurring in Dhofar region during the refting between the Arabian and African plates (Roger and Platel 1987). It has two distinct formations: the Fars Group of the Tertiary age (middle to early Miocene age) and the Mushsayl Formation of the Tertiary age (Early Miocene to Oligocene) (Fig. 2). The Fars group has a maximum saturated thickness of 120 m, while the permeable and saline Mughsayl Formation has a thickness of about 800 m (Shammas and Jacks 2007). The Fars group comprises Nar and Adawnib Formations. The main aquifer in the plain contains carbonate and conglomeratic marine deposits of Adawnib Formation. The aquifer thickness is varying from 60 to 70 m. Wadi alluvium including gravel, gravely clays and calcarenite deposits are overlying the aquifer.
Hydrogeological cross-section of Salalah plain and adjacent Jabal Al-Qara (after Geo Resources Consultancy 2004)
The groundwater is considered confined aquifer in Jabal Al-Qara and unconfined aquifer in the Salalah plain. It is characterised by a storage coefficient of 3%, hydraulic conductivity ranging from of 10 to 1000 m/day and transmissivity varying from 1000 to 200,000 m2/day (MAF-ICBA 2012). In general, the groundwater is flowing from north to south with piezometric heads at about 10 m above mean sea level near the piedmont of Jabal Al-Qara, declining to about sea level near the coast (Fig. 3). The low hydraulic gradients of this aquifer (about 1 m/km) can be explained by its hydraulic connection to the sea and its high transmissivity. In this case, any depletion caused by the groundwater pumping can be compensated by the inflows from the sea.
Contour of groundwater piezometric heads in the study area (Askri et al. 2016)
The domestic, municipal, agricultural and industrial water needs in the Salalah plain are provided by the groundwater. The potential water resources of this aquifer are about 874 Mm3 of which 42% is considered as fresh groundwater and the remaining 58% as brackish groundwater (MAF-ICBA 2012). The main sources of recharge are the underflow from Jabal Al-Qara to the plain, waters mobilised in Sahalnawt and Zarsis dams, infiltrations of precipitation falling on the plain, infiltrations of floodwater flowing through the wadis and injection of treated wastewater. The groundwater aquifer is exploited by about 1500 wells and 17 boreholes. The total groundwater abstraction is estimated at 69 Mm3, out of which 46% is used for irrigation (Askri et al. 2016).
2.2 Sampling and Data Collections
Historical data of groundwater table depth and electrical conductivity (EC) recorded in the Salalah plain were collected from the Ministry of Regional Municipalities and Water Resources, Sultanate of Oman. The hydraulic heads in the groundwater aquifer were measured as the height of the groundwater table above mean sea level. They were calculated by subtracting the groundwater table depth from the land surface altitudes. These data were used to investigate the dynamic of seawater intrusion in the plain aquifer by means of spatial and temporal variations of the hydraulic heads and salinity of the groundwater aquifer.
In October 2015, eleven groundwater samples were collected in the study area for major anion and cation analyses. The concentrations of major ions were measured using titration method at the Laboratory of Water in Salalah city, Directorate General of Water, Sultanate of Oman. Assuming chloride to be a conservative ion, the fraction of seawater in the mixed water was estimated as follow (Appelo and Postma 2005):
where CCl(sample), CCl(freshwater) and CCl(seawater) are the chloride concentrations of groundwater sample, freshwater and seawater, respectively.
PHREEQC software was used as a specification program to calculate the saturation indices (SI) (Parkhurst and Appelo 1999). This parameter is showing if water will trend to precipitate (SI ˃ 0) or dissolve (SI ˂ 0) a given mineral (Hamzaoui-Azaza et al. 2011). Specific calculations were run for calcite, aragonite, dolomite and halite minerals In order to understand the effect of saline sources on groundwater quality. The processes inducing groundwater salinisation were identified using the following molar ratios: Na/Cl, Ca/Mg and (Ca + Mg)/HCO3.
The electrical conductivity (EC) and the sodium adsorption ratio (SAR) were used to assess the degree of groundwater suitability for irrigation use. Indeed, the water availability to the plants decreases with the increase of these two parameters values. The sodium hazard is expressed by the SAR, which is calculated using the following equation:
where units are expressed in milli-equivalent per liter.
The assessment of irrigation waters was made using the diagram of US Salinity Laboratory Staff (US Salinity Lab Staff 1954), where SAR is plotted against EC.
3 Results and Discussion
3.1 Temporal Variations of Piezometric Heads and Salinity of Groundwater
Data of monthly groundwater table depth in three irrigation wells located in upstream (F-63), intermediate (F-68) and downstream (F-56) regions of the study area were available from May 1984 to February 2007. The piezometric heads of the groundwater show seasonal and long-term fluctuations (Fig. 4). The high piezometric heads observed during monsoon were usually followed by decreasing trend during post monsoon. The small declines are explained by the high transmissivity of the karstic aquifer and its hydraulic connection to the sea. The long-term fluctuations of the groundwater piezometric heads show different patterns with respect to well location. The piezometric heads recorded in the wells F-63 and F-68 declined until June 1998, after which they increased until February 2007. The decreasing trend observed during the first period may be explained by the increasing number of wells implemented in the intermediate and upstream regions of the study area. The piezometric head recorded in the well F-56 was approximately constant until June 1995. Then, it decreased continuously until April 2002. In May 2002, this piezometric head increased by 40 cm after which it was maintained approximately constant until August 2006. The groundwater regime in this well seems to be in equilibrium, which means that the water abstraction by pumping was compensated by recharge through the rainwater infiltration and lateral flow from the sea.
Data of monthly groundwater salinity in the wells F56, F-63 and F-68 were available from 1984 to 1994. During this period, the EC increased by 8420, 152 and 83 µS/cm for the wells F-56, F-68 and F-63, respectively (Fig. 5). These results show that the groundwater salinity has increased in the study area at different rates. This rate was very high in the coastal area probably due to a strong seawater intrusion, which has been favored by overpumping. The groundwater salinity increased slowly in the upstream and intermediate of the study area. The location of these regions far from the sea and from the agricultural areas suggest the mineral dissolution as the main source of the groundwater salinisation.
3.2 Spatial Variability of Groundwater Salinity
Figure 6 shows the spatial distributions of the groundwater salinity in the study area in 1998, 2004, 2006 and 2009. The EC of the groundwater showed large variations and generally increased in the flow direction from north to south. Comparing the maps for the years 1998 and 2004, it is evident that the area of fresh waters has increased and the area of saline waters has decreased mainly in eastern corner of the study area. This amelioration of the groundwater quality can be explained by the positive effect of the groundwater injection by the treated wastewater. The comparison between the salinity maps recorded during the years 2004 and 2009 shows an opposite trend as the area of fresh waters has decreased, while the area of saline waters has increased mainly in eastern corner of the study area. The deterioration of the groundwater quality during this period was observed despite the increase in the artificial recharge from the treated water from 2.0 to about 7.0 mm3 per year during the period from 2003 to 2009 (Shammas 2007). During the last year, only 53 mm of rainfall was recorded in the Salalah plain. Thus, the groundwater salinisation can be explained by the evapo-concentration of some salts, which is the consequence of high rate of evaporation and low quantity of rainfall.
3.3 Major Ion Trends
In October 2015, the pH values in the study area were in the range of 7.1–7.8, showing that the samples are neutral to slightly alkaline (Table 1). In this case, the dissolution of carbonate minerals may be the origin of the groundwater alkalinity. The EC of the waters varied from 1360 to 9310 µS/cm with an average of 3124 µS/cm. Only one sample collected in the center of the Hafah agricultural area (SAL-9) was classified into fresh water category with EC of ˂1000 µS/cm (Mondal et al. 2010). Four water samples (SAL-3, SAL-4, SAL-5 and SAL-10) were classified into saline category with EC ˃ 3000 µS/cm. The first three wells are located in the Awqad region in southwestern the study area and the fourth well is located in the Dahariz region, southeastern the study area. These two regions are located near the shoreline and the high EC maybe explained by the high rate of seawater intrusion. TDS contents varied from 884 to 6051 mg/l with an average of 2030 mg/l. About 91% of the water samples had TDS value higher than the recommended limit for drinking water as per WHO (2004) standard, i.e., 1000 mg/l.
The order of relative abundance of major cations was (molar basis): Na+ > Ca2+ > Mg2+ > K+ in four wells (SAL-4, SAL-5, SAL-10 and SAL-11), Ca2+ > Na2+ > Mg2+ > K+ in four wells (SAL-1, SAL-3, SAL-6 and SAL-8), Ca2+ > Mg2+ > Na+ > K+ in two wells (SAL-2 and SAL-7) and Mg2+ > Ca2+ > Na+ > K+ in the well SAL-9. The Na level constituted on average about 39.0% of the total cations concentration and varied from 3.92 to 36.98 meq/l. About 55% of total samples fall above the suitable limit of Na (8.7 meq/l) for drinking water (WHO 2004). The concentrations of Ca and Mg ranged from 5.69 to 14.02 meq/l and from 4.94 to 12.35 meq/l, respectively. Their concentrations represented on average 33 and 27% of all the cations, respectively. Higher concentrations of Na, Ca and Mg were found in the well SAL-10. The K concentrations represented on average only 0.5% of all the cations and ranged from 0.05 to 0.44 meq/l. The low concentration of this cation would be explained by its tendency to be fixed by clay minerals.
The order of anion abundance are Cl− > HCO3 − > SO4 2− > NO3 − in two wells (SAL-11 and SAL-5), Cl− > NO3 − > HCO3 − > SO4 2− in eight wells (SAL-1, SAL-2, SAL-3, SAL-4, SAL-6, SAL-8, SAL-9 and SAL-10), and Cl− > SO4 2− > HCO3 − > NO3 − in one well (SAL-7). The Cl level represented about 58% of all the anions and ranged from 9.53 to 25.66 meq/l. Higher Cl concentration in Dahariz region (SAL-10) suggests seawater intrusion. However, the relatively low concentrations of this anion in the upstream region (SAL-1 and SAL-11) indicate the lateral recharge from Jabal Al-Qara. The suitable limit of chloride (250 mg/l) for drinking water (WHO 2004) has been exceeded in total water samples. The SO4 and HCO3 concentrations ranged from 0.81 to 4.31 meq/l and 3.33 to 6.63 meq/l, respectively. Their average concentrations represent 10 and 14% of all the anions, respectively. Total water samples had SO4 concentration below WHO standard, i.e., 250 mg/l. The increase of SO4 concentration from the inland to the shoreline can be explained by a possible seawater intrusion, which has a high SO4 concentration. This interpretation is also justified by the absence of geological formations containing SO4 in the study area. The HCO3 can come from the following three sources: dissolution of carbonate minerals, soil CO2 or bacterial degradation of the organic contamination (Bahar and Reza 2010). The NO3 concentrations varied from 2.71 to 8.57 meq/l. Its average concentration represent about 17% of all the anions. About 64% of total waters exceeded the drinking water standard for nitrate (50 mg/l). The highest NO3 concentrations recorded in Hafah agricultural area shows an anthropogenic pollution, which may be introduced by agricultural activities (crops residue, use of nitrogen fertilizers and animal manure).
The seawater fraction in the groundwater varied from 1.3 to 4.3% in the wells SAL-11 and SAL-10, respectively (Fig. 7). The first well is located 9.3 km far from the coastline, while the second one is located 1.2 km far from the coastline.
3.4 Water Types
The hydrochemical processes operating in the groundwater aquifer were analysed using the Piper diagram (Fig. 8). Trilinear diagram analysis indicates that all brackish waters are Ca, Mg, Na, Cl or Ca, Na, Mg, Cl which means high groundwater episodes are washing salinised groundwater from Na–Cl to Ca and Mg that coming from carbonate aquifer in the study area.
Three water types were found in the study area. They are in the order of Ca–Cl ˃ mixed Ca–Mg–Cl ˃ Na–Cl. The low mineralised Ca–Cl type freshwater and brackish water was identified in seven wells (SAL-1, SAL-2, SAL-3, SAL-6, SAL-7, SAL-8 and SAL-9) located mainly in northwestern the study area. Cl and Ca were the dominant anion and cation, respectively. This water type was influenced by the reverse ion exchange process. Water types (mixed Ca–Mg–Cl and Na–Cl) are present in four wells (SAL-4, SAL-5, SAL-10 and SAL-11) and indicate the mixing of high salinity water caused from seawater intrusion with existing water. These wells seem to be influenced by a strong seawater intrusion. These results indicate an active saltwater intrusion followed by reverse ion exchange reactions in the study area.
3.5 Effects of Mineral Dissolution
Approximately the total water samples were under-saturated with respect to halite and supersaturated with respect to araganite, calcite and dolomite minerals (Table 2). These results show that these mineral phases are contributing to the groundwater mineralisation in the study area. Thus, the precipitation of calcite, araganite and dolomite minerals, and dissolution of halite are probable. Precipitation of the first three minerals may occur during the cation exchange process (Mtoni et al. 2012).
3.6 Sources of Groundwater Salinisation
Some molar ratios can be used to determine the sources of groundwater salinisation in coastal regions (Leboeuf et al. 2003; Yüce 2005; Mondal et al. 2010; Arslan 2013). In this context, the Ca/Mg and Na/Cl molar ratios reflect the degree of seawater intrusion. This process is indicated by a high Ca/Mg (>1) molar ratio and relatively low Na/Cl molar ratio (<0.86) (Vengosh et al. 1997). Table 2 shows the selected molar ratios of the groundwater in the study area. The Na/Cl ratio was less than 0.86 in 82% of total water samples, while the Ca/Mg ratio was higher than 1 in 91% of the total water samples. These results show that the process of seawater intrusion has a predominant effect on groundwater quality in the study area.
The processes of mineral dissolution-precipitation in the study area were alos analysed using some molar ratios. For example, a Na/Cl molar ratio close to 1 shows that halite dissolution is responsible for sodium, whereas the Na/Cl molar ratio greater than 1 typically shows that the reactions of silicate weathering are the main origin of sodium (Meybeck 1987). The (Ca + Mg)/HCO3 molar ratio can determine the sources of Ca and Mg in groundwater. These two cations originate from the dissolution of carbonate minerals if this ratio is equal to 0.5 (Sami 1992). The release of sodium from silicate weathering reactions may be a source of sodium in two wells situated in the intermediate region of the Salalah plain. Na/Cl molar ratio was less than 1 in 82% of total samples, indicating that the reverse ion exchange process is the main cause of the significant reduction of Na concentration. Potassium or sodium in seawater replaces magnesium or calcium adsorbed onto the surface of clays during the occurrence of the seawater intrusion. This phenomenon caused a relative depletion of sodium in groundwater. The (Ca + Mg)/HCO3 molar ratio was higher than 3.04 in total water samples showing that an excess of alkalinity in the groundwater was balanced by the sodium and potassium. The excess of calcium and magnesium over bicarbonates indicates extra sources of these cations.
3.7 Suitability of Groundwater for Irrigation Use
The SAR values varied in the study area from 1.6 to 10.2 with a mean of 3.6. Three water samples fall into the category C3-S1 (high salinity with low sodium) and one water sample falls into the category C4-S4 (very high salinity with very high sodium). The other water samples fall into the categories C4-S3, C4-S2, S4-S1 and C3-S2 (Fig. 9).
Generally, the water samples show high to very high salinity hazard with medium to low alkali hazard. The most of these samples fall into the category high salinity with low to medium sodium. Thus, the most of the water samples may not be suitable for irrigation under ordinary conditions. They can be used occasionally if soils are permeable, drainage is adequate, irrigation water is applied in excess to provide leaching, and salt tolerant crops are selected.
4 Conclusions
Available data of groundwater table depth and salinity in the Salalah plain aquifer were analysed to understand the dynamics of seawater intrusion in this plain. The piezometric heads recorded in the upstream and intermediate regions had a decreasing trend during the period from 1984 to 1998, which may be explained by the increasing number of wells implemented in these regions. The groundwater regime in the coastal area was in equilibrium in terms of recharge-discharge. However, the rate of seawater intrusion was very high.
In October 2015, the groundwater chemistry in the Salalah plain was strongly influenced by seawater intrusion, reverse ion exchange process and anthropogenic pollutants. Three water types were identified in the study area: Ca–Cl type in seven wells, mixed Ca–Mg–Cl type in three wells and Na–Cl type in one well. The first seven wells were under the influence of the reverse ion exchange, while the last four wells were affected by a strong seawater intrusion. The occurrence of this phenomenon was also demonstrated by Na/Cl and Ca/Mg molar ratios. The high nitrate concentrations recorded in the Hafah agricultural area highlights the impact of agricultural activities on the groundwater quality. The use of groundwater in Salalah plain for irrigation purpose require certain conditions related to the soil texture, implementation of a drainage system and selection of salt tolerant crops.
References
Agoubi B, Kharroubi A, Abichou T, Abida H (2013) Hydrochemical and geoelectrical investigation of Marine Jeffara Aquifer, southeastern Tunisia. Appl Water Sci 3:415–429
Appelo CAJ, Postma D (2005) Geochemistry, groundwater and pollution, seconded. Balkema, Rotterdam
Arslan H (2013) Application of multivariate statistical techniques in the assessment of groundwater quality in seawater intrusion area in Bafra Plain, Turkey. Environ Monit Assess 185:2439–2452
Askri B (2015) Hydrochemical processes regulating groundwater quality in the coastal plain of Al Musanaah, Sultanate of Oman. J Afr Earth Sci 106:87–98
Askri B, Abdelkader TA, Razan AS, Rachida B, Khater KF (2016) Isotopic and geochemical identifications of groundwater salinisation processes in Salalah coastal plain, Sultanate of Oman. Chem Erde 76:243–255
Bahar MM, Reza MS (2010) Hydrochemical characteristics and quality assessment of shallow groundwater in a coastal area of Southwest Bangladesh. Environ Earth Sci 61:1065–1073
Clark ID, Fritz P, Quinn OP, Rippon PW, Nash H, Al-Said SB (1987) Modern and fossil groundwater in an arid environment: a look at the hydrogeology of southern Oman. In: Proceedings of use of stable isotopes in water resources development, IAEA-SM-299, Vienna, Austria, pp 167–187
D&MI (1992) Water and wastewater master plan for Salalah: Office of the Minister of State and Governor of Dhofar; Salalah, Sultanate of Oman
Geo Resources Consultancy (2004) Detailed water resources management and planning study for the Salalah region (Part A, B & C), final report prepared for MRMEWR, Sultanate of Oman
Hamzaoui-Azaza F, Ketata M, Bouhlila R, Gueddari M, Riberio L (2011) Hydrogeochemical characteristics and assessment of drinking water quality in Zeuss-Koutine aquifer, southeastern Tunisia. Environ Monit Assess 174:283–298
Kouzana L, Mammou AA, Felfoul M (2009) Seawater intrusion and associated processes: case of the Korba aquifer (Cap-Bon, Tunisia). C R Geosci 341:21–35
Leboeuf PP, Bosh AP, Calvaches ML, Vallejos A, Andreu JM (2003) Strontium, SO4/Cl amd Mg/Ca ratios as tracers for the evolution of seawater in coastal aquifers: the example of Castell de Ferro Aquifer (SE Spain). Academie des Sci Geosci 335(14):1039–1048
MAF-ICBA (2012) Oman salinity strategy, Ministry of Agriculture and Fisheries. Sultanate of Oman. International Center for Biosaline Agriculture, Dubai, UAE
Meybeck M (1987) Global chemical weathering of surficial rocks estimated from river dissolved loads. Am J Sci 287:401–428
Mondal NC, Singh VP, Singh VS, Saxena VK (2010) Determining the interaction between groundwater and saline water through groundwater major ions chemistry. J Hydrol 388:100–111
Mtoni Y, Mjemah IC, Bakundukize C, Camp MV, Martens K, Walraevens K (2012) Saltwater intrusion and nitrate pollution in the coastal aquifer of Dar es Salaam, Tanzania. Environ Earth Sci. doi:10.1007/s12665-012-2197-7
Oman Census (2003) Ministry of National Economic, Sultanate of Oman
Park SC, Yun ST, Chae GT, Yoo IS, Shin KS, Heo CH, Lee SK (2005) Regional hydrochemical study on salinization of coastal aquifers, western coastal area of South Korea. J Hydrol 313:182–194
Parkhurst DL, Appelo CAJ (1999) User’s guide to PHREEQC (version 2)—a computer program for speciation, batch-reaction, one-dimensional transport, and inverse geochemical calculations: U.S. geological survey water-resources investigations report 99-4259, 312 pp
Rajmohan N, Al-Futaisi A, Al Touqi S (2009) Geochemical process regulating groundwater quality in a coastal region with complex contamination sources: Barka, Sultanate of Oman. Environ Earth Sci 59:385–398
Roger J, Platel JP (1987) Geological mapping and mineral exploration programme in Southern Dhofar. Final Report. Bureau de Recherches Geologiques et minieres, 87 OMN 091. Ministry of petroleum and minerals, directorate general of minerals
Sami K (1992) Recharge mechanisms and geochemical processes in a semi-arid sedimentary basin, Eastern cape, South Africa. J Hydrol 139:27–48
Shammas MI (2007) Sustainable management of the Salalah coastal aquifer in Oman using an integrated approach. Ph.D. thesis, Department of Land and Water Resources Engineering, Royal Institute of Technology (KTH), Sweden
Shammas MI, Jacks G (2007) Seawater intrusion in the Salalah plain aquifer, Oman. Environ Geol 53:575–587
Sherif M, Mohamed M, Kacimov A, Shetty A (2011) Assessment of groundwater quality in the northeastern coastal area of UAE as precursor for desalination. Desalination 273:436–446
Singh A (2014) Optimization modelling for seawater intrusion management. J Hydrol 508:43–52
USSL (1954) Diagnosis and improvement of saline and alkali soils, handbook, vol 60. USDA, Washington, 147 pp
Vengosh A, Gill J, Reyes A, Thoresberg K (1997) A multi-isotope investigation of the origin of groundwater salinity in Salinas valley, California. American Geophysical Union, San Francisco, California
World Health Organization (2004) Guidelines for drinking water quality, vol. 1 recommendations (3rd). WHO, Geneva
Yüce G (2005) Determination of the recharge area and salinization degree of karst springs in the Lamas Basin (Turkey). Isot Environ Health Stud 41(4):391–404
Zghibi A, Tarhouni L, Zouhri L (2013) Assessment of seawater intrusion and nitrate contamination on the groundwater quality in the Korba coastal plain of Cap-Bon (North-east of Tunisia). J Afr Earth Sci 87:1–12
Acknowledgements
The authors are thankful to the Department of Monitoring of Water Resources, Ministry of Regional Municipalities and Water Resources, Sultanate of Oman for providing necessary data. The authors are also thankful to the anonymous reviewers for their helpful comments.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this paper
Cite this paper
Askri, B., Ali Al-Shanfari, R. (2017). Assessment of Hydro-chemical Processes Inducing the Groundwater Salinisation in Coastal Regions: Case Study of the Salalah Plain, Sultanate of Oman. In: Abdalla, O., Kacimov, A., Chen, M., Al-Maktoumi, A., Al-Hosni, T., Clark, I. (eds) Water Resources in Arid Areas: The Way Forward. Springer Water. Springer, Cham. https://doi.org/10.1007/978-3-319-51856-5_20
Download citation
DOI: https://doi.org/10.1007/978-3-319-51856-5_20
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-51855-8
Online ISBN: 978-3-319-51856-5
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)