Abstract
India has a large network of textile industries of varying capacity and textile effluent is one of the main contributors of water pollution and it adversely affects fauna and flora. Phytoremediation technology can be effective approach for remediating contaminated sites of such textile dyeing effluents. The objective of this research is to study the efficiency of aquatic macrophytes plant contributed for remediation of textile waste water. In this study, lab scale wetland was constructed and tested with Alternanthera sessilis, sand and gravel as filter. Results indicated that, textile effluent degradation and significant reductions in TS (78%), TDS (83%), TSS (38%), COD (65%), BOD5 (66%), chloride (44%), hardness (76%) and TDS (59%) were observed by the combined use of plants within 24 hrs. The removal efficiency of unplanted were TS (50%), TDS (50%), TSS (29%), COD (27%), Hardness (57%), Chloride (26%), BOD5 (27%) respectively. When compared the removal efficiency of planted with unplanted, planted shows a maximum removal the results showed that the removal efficiencies of the organics TDS, COD and BOD5 were improved significantly with the extension of HRT. This objective of study and efficiency of plants and proposed mechanism of aquatic macrophytes plant contributed for remediation of textile waste water.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Industrial revolution, the beginning has accelerated toxic metal in polluted water is a major problem and is leading cause of diseases/death worldwide (Sayyed and Sayadi 2011). Textile industrial production uses different chemicals and dyes according to textile wastewater practices, their dilution after production and wastewater management. Worldwide, textile industries commercially produce around 10,000 different dyes with an annual production more than 7 × 105 metric tons. The textile processing produce wastewater that in different stages of dyeing, fixing, washing and other processing, which contain suspended solids, high amount of dissolved solids, unreacted dyestuffs and other chemicals (Mohan et al. 2005). Textile effluents are dark color, high turbidity of the receiving water body due to usage of dyes and chemical. Improper disposal of textile wastewater causes direct contamination of ground water and surface water (Thilakar et al. 2012).
Treatment process include physical, chemical and biological methods for achieving color removal and reduction of TDS, BOD5, COD, which have merits and economical limitations (Prajapati et al. 2012). The physical, chemical dye removal methods are expensive and have limited versatility (Zee and Villaverde 2005). Recycling the wastewater from the industries involve various methods, including aerobic and anaerobic microbial degradation, coagulation (Guendy 2010), absorption (Sivakumar 2014; Sivakumar et al. 2014), activated carbon (Syafalni et al. 2012), electrochemical process (Dogan and Haluk 2012), reverse osmosis (Ramesh Kumar et al. 2013), ozonation (Guendy 2007), adsorption (Shankar and Sreenivasa 2012; Sivakumar and Shankar 2012), catalytic oxidation (Hussein and Abass 2010) and membrane processes (Abdulraheem and Abiodun 2012) etc. for color removal from wastewater, these processes have high operational costs and limited applicability.
Phytoremediation is one of the waste water treatment methods by using plant based systems for removing the contaminants from various natural sources. To clean up the contaminated water, selection of an appropriate and efficient plant system is highly essential. Those plant systems should have high uptake of both organic and inorganic pollutants, grow well in polluted water and be easily controlled in quantitatively propagated dispersion (Roongtanakiat et al. 2007). Phytoremediation is easier to manage because it is an autotrophic system of large biomass that requires little nutrient input. Moreover, plants offer protection against water and wind erosion, and prevents contaminants from spreading (Cluis 2004). Phytoremediation treatment is used for treating many classes of contaminants like petroleum hydrocarbons, chlorinated solvents, pesticides, explosives heavy metals and radionuclides and landfill leachates. These technologies involve moderately relatively high capital expenditure and manpower as well as long-term operating costs (Shenbagavalli 2007; Prithabai 2008). In recent years, considerable attention has been focused on absorption process using aquatic plants because of its advantage over conventional treatment methods include low cost, high efficiency, minimization of chemical and biological sludge (Roy et al. 2010). In India, terrestrial plants like Helianthus annuus, Phragmites karka, Datura innoxia, Brassica juncea, Alternanthera sessilis and Zea mays have been used to treat different metals contaminating effluent soil and sludge from various types of industries (Meagher 2000; Kramer and Chardonnens 2001; Peuke and Rennenberg 2005; Prithabai 2008). The objective of study is to examine the proposed mechanism of aquatic macrophytes plant contribution for remediation of textile waste water.
2 Materials and Methods
2.1 Experimental Setup
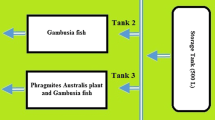
The lab-scale vertical-flow container beds with 44, 34 and 14.2 cm (length, width and height) dimension were constructed 2 in number. The beds were white plastic tray and planted with Alternanthera sessilis (Fig. 1). Each bed was filled with the media consisting 5 cm of gravel layer and sand on the top as the supporting layer. Approximately 10 L of raw effluent from factories was brought to the laboratory in plastic containers and the experiments were setup in plastic containers. The experiment plants were initially subject to stabilization in tanks containing tap water for one month for acclimatization.
Ten liters of the particularly diluted effluents were prepared and then transferred to plastic tank. For each experimental set, one control was maintained in stock tanks and were collected, cleaned, introduced in the experimental tanks. Approximately 250 g each experimental plant used for the study, each occupying half of tanks. Triplicate of each experimental setup was maintained and 500 ml from each effluent were examined. The wastewater was treated at different hydraulic retention time for 24 h. The textile effluent was brought to the laboratory, diluted with tap water and placed in the storage tank with control flow to feed 0.3 L wastewater to the bed in a one minute interval on a daily basis.
2.2 Analytical Methods
The effluent samples were collected in a sterile 500 ml plastic bottles, stored at 4 °C and physico-chemical parameters like temperature, pH, electrical conductivity (EC), total solids (TS), total dissolved solids, (TDS), total suspended solids, chemical oxygen demand (COD) and biological oxygen demand (BOD5) were analyzed as described by APHA (1998). The percentage concentration decrease efficiency was evaluated according to Eq. (1) proposed by the International Water Association.
where, E = percentage concentration decrease efficiency (%), Ci = inlet concentration (mg/l) and Co = outlet concentration (mg/l).
3 Result and Discussions
3.1 Physico-Chemical Characterization of Textile Effluents
The characteristics of textile effluent collected from Tirupur common effluent treatment plant was brought to laboratory and was diluted with tape water. The physico-chemical parameters are showed in Table 1.
3.2 Effect on Temperature
The temperatures determine the solubility of dissolved oxygen in water, its concentration and its availability to aquatic organisms (Anon 1996). The effect of the textile effluent, on the phytoremediation was studied at temperature values ranging from 24.5 to 25.5 °C (Fig. 2). The effluent from the bed planted with A. sessilis was effectively removed (14%) at temperature of 24.5 °C during hydraulic retention time (24 h) when compared to control.
3.3 Effect on PH
The pH of textile effluent was 8.25, the alkaline nature might be due to dyes and other chemicals. The effluent from the planted A. sessilis had a mean pH of 7.76 and unplanted bed had a mean pH of 7.8 during hydraulic retention time of 24 h (Fig. 3). This result is consistent with the behaviour of pH in other treatment wetlands (Belmont et al. 2004). The pH reduction in effluents is due to nitrification that produces the hydrogen ion in constructed wetlands (Lin et al. 2005).
3.4 Effect on Electrical Conductivity
The EC of textile effluent was observed (Fig. 4), the A. sessilis planted bed had mean value 0.89 μS/cm, whereas the unplanted bed had a mean of 1.32 μS/cm during retention time 24 h. EC was reduced due to evapotranspiration and/or movement of substrate by plant roots (Hench et al. 2003) and earlier reports for pilot-container experiments in papyrus-based treatments showed higher reductions (Kyambadde et al. 2004).
3.5 Effect on Total Solids
Total solids of textile effluent 6814 mg/l, when treated with A. sessilis, had a mean of 1500 mg/l whereas unplanted bed had mean of 3500 mg/l during HRT of 24 h. In the present study, plants removed TS in 78% textile effluent whereas in the unplanted it is 49% (Fig. 5).
3.6 Effect on Total Dissolved Solids
The TDS removal by A. sessilis was good in almost all concentrations (Fig. 6) while the textile effluent was observed with 577.48 mg/l TDS. The effluent from the planted had a mean TDS of 73.26 mg/l and unplanted bed had a mean 115.14 mg/l during the HRT of 24 h. There occurred an 83% increase in planted while lowest occured at 50% in unplanted.
3.7 Effect on Total Suspended Solids
The TSS of textile effluent was 814 mg/l, after treated with A. sessilis the mean was 300 mg/l, whereas unplanted bed had a mean TSS 714 mg/l during the HRT of 24 h (Fig. 7). TSS removal efficiencies were reported in similar studies (Neralla et al. 2000; Steer et al. 2002). In the present study the performance efficiency was 38% which is higher than the control (12%).
3.8 Effect on Total Alkalinity
The average TA concentration of textile effluent was 250 mg/l, after treatment with A. sessilis it reduced to 90 mg/l, during the HRT of 24 h (Fig. 8) which is 64% effective whereas in the unplanted bed TA reduced at 18% rate in 24 h.
3.9 Effect on Total Hardness
The hardness of textile effluent was 272 mg/l and reduced to 90 mg/l by phytoremediation during the HRT of 24 h and 160 mg/l was found in control (Fig. 9).
3.10 Effect on Chloride
The chloride content in textile effluent was 648 mg/l which are alarmingly high values. The effluent from the treatment had a mean chloride of 425 mg/l and unplanted bed had a mean chloride of 551 mg/l during the HRT of 24 h (Fig. 10). In the present study it has been found that A. sessilis removed 44% of chloride whereas in unplanted it reduced to 26%.
3.11 Effect on Dissolved Oxygen
The dissolved oxygen was increased (5.85 mg/l) with in 24 h by A. sessilis treatment than the 2.5 mg/l of textile effluent (Fig. 11). According to Reddy (1981), it might be the reason that the presence of plants in wastewater depletes dissolved CO2 during the period of photosynthetic activity and an increase in DO of water, thus creating aerobic conditions in wastewater.
3.12 Effect on Biological Oxygen Demand
The BOD5 of textile effluent was 162 mg/l and increased by A. sessilis treatment to 110 mg/l which is efficient than unplanted bed which had a mean BOD5 of 130 mg/l during the HRT of 24 h (Fig. 12). BOD5 removal between planted and unplanted wetlands may be due to microbial degradation of organics coupled with root zone oxygen input (Klomjek and Nitisoravut 2005). The BOD reduction study by Steer et al. (2002) reported that the sedimentation, adsorption and microbial metabolism are considered to be the primary mechanisms for BOD removal, it is likely that the plant roots and falling residues provide a more effective settling medium than gravel alone, while at the same time increasing surface area for attachment and food sources for the microbial populations. In the present study the plant removed BOD5 at maximum of 66% and minimum of 27% in textile effluent.
3.13 Effect on Chemical Oxygen Demand
The COD was reduced by A. sessilis to 180 mg/l (65%) from the observed 460 (20%) mg/l in the textile effluent (Fig. 13). In control the COD was reduced to 280 mg/l during the HRT 24 h, this data agrees with the finding of Mashauri et al. (2000) who found that longer retention time would reduce COD. The removal of COD is attributed to microbial degradation of substrate by the plants roots (Greenway and Woolley 1999; Vymazal 2002; Steer et al. 2002). This is in agreement with the observations made by Lim et al. (2001) but also in contrast to the results reported by Tanner et al. (2005). However, daytime photosynthes is done by plants could actually generate oxygen to balance the oxygen used for organic mineralization and nitrification (Lin et al. 2005).
4 Conclusion
There are various methods employed for effluent treatment in textile industry. But, all the methods are not significantly used in textile industry because of various difficulties such as time, cost, raw material unavailability, etc. In this study, the investigation was carried out in the textile dye effluent by natural adsorbents namely phytoremdiation. The results indicated that the removal of organic pollutant was enhanced by the presence of plants. It is possible that the presence of plants roots with rhizobacteria had an advantageous effect on improving the organic pollutant degradation. It is observed that these adsorbents are active in removal of dye and harmful pathogenic bacteria. From this study, the promising attributes of Alternanthera sessilis includes its tolerance to dye and dye absorption along with good root development, low maintenance and ready availability in contaminated regions. These characteristics helps to prove that the suitability of the plant in dyeing industry effluent treatment ponds. Hence, it is concluded that A. sessilis has the potential in removal of dye and harmful pathogenic bacteria and more useful for waste water treatment applications.
References
Abdulraheem G, Abiodun O (2012) The applications of membrane operations in the textile industry: a review. Br J Appl Sci Technol 2(3):296–310
American Public Health Association (APHA) (1998) Greenburg AE, Conners JJ, and Jenkins D (eds) Standard methods for the examination of water and wastewater, 20th edn. American water works association, Water pollution control federation, Washington, DC, p 1325
Anon (1996) South African water quality guidelines, 2nd Edn. Domestic Use 1, Department of Water Affairs and Forestry, South African
Belmont MA, Cantellano E, Thompson S, Williamson M, Sanchez A, Metcalfe CD (2004) Treatment of domestic wastewater in a pilot-scale natural treatment system in central Mexico. Eco Eng 23:299–311
Cluis C (2004) Junk-greedy greens: phytoremediation as a new option for soil decontamination. Biotechnol J 2:61–67
Dogan D, Haluk T (2012) Electrochemical treatment of actual textile indigo dye effluent. Pol J Environ Stud 21(5):1185–1190
Greenway M, Wolley A (1999) Constructed wetlands in Queensland: performance efficiency and nutrient bioaccumulation. Eco Eng 12(1–2):39–55
Guendy HR (2010) Treatment and reuse of wastewater in the textile industry by means of coagulation and adsorption techniques. J Appl Sci Res 6(8):964–972
Guendy HR (2007) Ozone treatment of textile wastewater relevant to toxic effect elimination in marine environment. Egypt J Aqua Res 33(1):98–115
Hench KR, Bissonnette GK, Sexstone AJ, Coleman JG, Garbutt K, Skousen JG (2003) Fate of physical, chemical, and microbial contaminants in domestic wastewater following treatment by small constructed wetlands. Water Res 37:921–927
Hussein FH, Abass TA (2010) Photocatalytic treatment of textile industrial wastewater. Int J Chem Sci 8(3):1353–1364
Klomjek P, Nitisoravut S (2005) Constructed treatment wetland: a study of eight plant species under saline conditions. Chemosphere 58(5):585–593
Kramer U, Chardonnens AN (2001) The use of transgenic plants in the bioremediation of soils contaminated with trace elements. Appl Microbio Biotech 55:661–672
Kyambadde J, Kansiime F, Gumaelius L, Dalhammar G (2004) A comparative study of Cyperus papyrus and Miscanthidium violaceum-based constructed wetlands for wastewater treatment in a tropical climate. Water Res 38(2):475–485
Lim PE, Wong TF, Lim DV (2001) Oxygen demand, nitrogen and copper removal by free–water-surface and subsurface-flow constructed wetlands under tropical conditions. Environ Int 26(5–6):425
Lin YF, Jing SR, Lee DY, Chang YF, Chen YM, Shih KC (2005) Performance of a constructed wetland treating intensive shrimp aquaculture wastewater under high hydraulic loading rate. Environ Pollut 134:411–421
Mashauri DA, Mulungu DMM, Abdulhussein BS (2000) Constructed wetland at the University of Dar Es Salaam. Water Res 34:1135–1144
Meagher RB (2000) Bioremediation of toxic elemental and organic pollutants. Curr Opin Plant Biol 3:153–162
Mohan S, Prasad K, Rao NC, Sarma PN (2005) Acid azo dye degradation by free and immobilized horseradish peroxidase (HRP) catalyzed process. Chemosphere 58:1097–1105
Neralla S, Weaver EW, Lesikar BJ, Persyn AP (2000) Improvement of domestic wastewater quality by subsurface flow constructed wetlands. Bioresour Technol 75:19–25
Peuke AD, Rennenberg H (2005) Phytoremediation. EMBO Rep 6:497–501
Prajapati SK, Meravi N, Singh S (2012) Phytoremediation of chromium and cobalt using Pistia stratiotes: a sustainable approach. Proc Int Acad Ecol Environ Sci 2(2):136–138
Prithabai C(2008). Phytoremediation: a cost-effective plant based technology for the removal of metals from the environment. Dissertation, Annamalai University, Tamil Nadu
Ramesh Kumar M, Koushik CV, Saravanan K (2013) Textile wastewater treatment using reverse osmosis and SDI. Elixir Chem Eng 54A:12713–12717
Reddy KR (1981) Diel variations of certain physico-chemical parameters of water in selected aquatic systems. Hydrobiologia 85:201
Roongtanakiat N, Tangruangkiat S, Meesat R (2007) Utilization of vetiver grass (vetiveria zizanioides) for removal of heavy metals from industrial wastewaters. Sci Asia 33:397–403
Roy R, Fakhruddin ANM, Khatun R, Islam MS (2010) Reduction of COD and pH of textile industrial wastewaters by aquatic macrophytes and algae. J Bangladesh Acad Sci 34(1):9–14
Sayyed MRG, Sayadi MH (2011) Variations in the heavy metal accumulations within the surface soils from the Chitgar industrial area of Tehran. Proc Int Acad Ecol Environ Sci 1(1):36–46
Shankar DS, Sreenivasa RB (2012) Kinetic models in adsorption—a review Asian. J Res Chem 5(1):8–13
Shenbagavalli T (2007) Studies on the influence of Eichhonia Crassipes on the removal of metallic pollutants by phytoremediation in the river Uppanar near Kudikadu, Cuddalore, District, Tamil Nadu, M. Phil., Thesis, Annamalai University, Tamil Nadu
Sivakumar D, Shankar D (2012) Colour removal from textile industry wastewater using low cost adsorbents. Int J Chem Environ Pharma Res 3(1):52–57
Sivakumar D (2014) Role of Lemna minor lin. in treating the textile industry wastewater. Int J of Environ Earth Sci Eng 8(3):55–59
Sivakumar D, Shankar D, Dhivya P, Balasubramanian K (2014) Bioaccumulation study by Lemna Gibba lin. Pollut Res 33(3):531–536
Steer D, Fraser L, Boddy J, Seibert B (2002) Efficiency of small constructed wetlands for subsurface treatment of single-family domestic effluent. Ecol Eng 18:429–440
Syafani S, Abustan I, Dahlan I, Chan K, Umar G (2012) Treatment of dye wastewater using granular activated carbon and zeolite filter. Mod Appl Sci 6(2):37–51
Tanner CC, Clayton JS, Upsdel MP (1995) Effect of loading rate and planting on treatment of dairy farm wastewaters in constructed wetlands: removal of oxygen demand, suspended solids and faecal coliforms. Water Res 29:17–26
Tanner CC, Nguyen ML, Sukias JPS (2005) Nutrient removal by a constructed wetland treating subsurface drainage from grazed dairy pasture. Agr Ecosystems Envirn 105:145–162
Thilakar RJ, Rathi J, Pillai PM (2012) Phytoaccumulation of chromium and copper by Pistia stratiotes L. and Salvinia natans (L.). J Nat Prod Plant Resour 2(6):725–730
Vymazal J (2002) The use of sub-surface constructed wetlands for wastewater treatment in the Czech Republic: 10 years experience. Ecol Eng 18:633–646
Zee VFP, Villaverde S (2005) Combined anaerobic-aerobic treatment of azo dyes—a short review of bioreactor studies. Water Res 39:1425
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Arivoli, A., Sathiamoorthi, T., Satheeshkumar, M. (2017). Treatment of Textile Effluent by Phytoremediation with the Aquatic Plants: Alternanthera sessilis . In: Prashanthi, M., Sundaram, R., Jeyaseelan, A., Kaliannan, T. (eds) Bioremediation and Sustainable Technologies for Cleaner Environment. Environmental Science and Engineering(). Springer, Cham. https://doi.org/10.1007/978-3-319-48439-6_15
Download citation
DOI: https://doi.org/10.1007/978-3-319-48439-6_15
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-48438-9
Online ISBN: 978-3-319-48439-6
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)