Abstract
The first years in the twenty-first century have meant the inclusion of nanotechnology in most industrial sectors, from very specific sensors to construction materials. The increasing use of nanomaterials in consumer products has raised concerns about their potential risks for workers, consumers and the environment. In a comprehensive risk assessment or life cycle assessment, a life cycle schema is the starting point necessary to build up the exposure scenarios and study the processes and mechanisms driving to safety concerns. This book chapter describes the processes that usually occur at all the stages of the life cycle of the nano-enabled product, from the nanomaterial synthesis to the end-of-life of the products. Furthermore, release studies reported in literature related to these processes are briefly discussed.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
The increasing use of nanomaterials in industrial and consumer products results in a potential risk for workers, consumers and the environment.
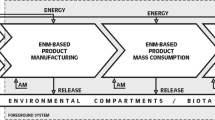
A starting point for any comprehensive risk assessment or life cycle assessment is the identification of all relevant life cycle steps, so that all scenarios with a potential risk can be evaluated. The life cycle is totally product-dependent, as each product has its own manufacturing processes, uses and waste treatment and, so, its own hotspots for nanomaterial release and associated risks. This chapter presents a brief overview of the most common processes that take place at different steps of a product life-cycle (Fig. 3.1) and highlights the potential contribution of each step to the release of nanomaterials and associated risk.
It is also important to clearly identify all the processes implied in each life cycle stage to know which mechanisms drive to release. Apart from release quantification, the form of release (isolated particles, aggregates, embedded in a matrix, surface modified by hydration or oxidation, etcetera) may be determinant for the hazard evaluation.
1.1 Production Levels of Engineered Nanomaterials
Some nanomaterials, such as carbon black and silica, have been industrially used for decades. However, during the last decade, new materials and modifications have allowed a dramatic expansion of nanotechnology. Despite the multiple materials that are being investigated at a research scale, at this moment it is estimated that nanomaterials produced at an industrial scale belong to only around 20 chemical classes [1]. At the moment, any attempt to determine nanomaterials production has to be based on estimations as they do not have to be reported. Only France and Denmark have recently regulated nanomaterials in products, so these nano-additivated products have to be registered and labelled in order to inform the consumers [2, 3].
Attempts to estimate the production levels and applications of nanomaterials have been based on information provided by industry through surveys. Sometimes the data collected relates to production capacities and sometimes to actual production amounts and the geographical area under scope also differs [4–6]. One of the most thorough recent surveys is that of Piccino et al., who send a survey to industrial representatives from companies producing or using nanomaterials to estimate the worldwide or Europe-wide production of such materials [7]. A considerable large variability among answers by different industrial representatives reflects the general uncertainties related to the actual worldwide production volumes. However, there was general agreement that silica, titanium dioxide, zinc oxide, carbon nanotubes, iron oxides, aluminium oxides, and cerium oxides are the nanomaterial types with highest production volumes. The median production quantities for each of these nanomaterials ranged between 55 and 5500 tonnes per year worldwide [7], depending on the material. These values are also consistent with estimated annual production volumes in China in 2012, which ranged from 200 to around 1300 tonnes for titanium dioxide, zinc oxide, aluminium oxide, zirconium oxide, and silver [8].
According to the French registry [9], the quantities produced and imported in 2014 are around 274,000 and 122,000 tones, respectively. Carbon black and silicon dioxide are the category of substances with largest produced or imported quantities, both above 100,000 t/year. These are followed by calcium carbonate and titanium dioxide, with volumes ranging 10,000–100,000 t/year. Other materials reported to be produced or imported in amounts above 1000 t/year are aluminium oxide, boehmite (γ-Al(OH)O), calcium 4-[(5-chloro-4-methyl-2-sulphonatophenyl)azo]-3-hydroxy-2-naphthoate, reaction mixture of cerium dioxide and zirconium dioxide, polyvinyl chloride, and magnesium silicate.
2 Engineered Nanomaterials Synthesis
The synthesis of ENMs is the step of a nano-enabled product life cycle that has received the highest attention in the literature in relation to the potential risks for human health [10–13]. By contrast, the potential release of ENMs to the environment during this step has received little attention and it is commonly assumed to be low, though this completely depends on the procedures used during the production, cleaning and maintenance [14–17].
Due to the novelty of the field and the continuous research in the development of new nanomaterials, multitude of synthetic methodologies can be found in the literature. Most of these processes are adequate for laboratory scale and even pilot scale synthesis, but completely unworkable at industrial scales. Synthetic methods are often divided in top-down and bottom-up methods [18].
-
Top-down methods. The successive cutting or slicing of bulk materials into nanomaterials play an important role in industrial synthesis of nanostructures that need specific shapes/sizes such as nanotransistors. Lithography, milling and attrition are the most common top-down processes used at the moment. The principal disadvantages of top-down approaches are the internal stress and the imperfection introduced in the surface structure due to the use of so energetic techniques. Such imperfections may have dramatic effect over surface chemistry and physical properties of such prepared nanomaterials.
-
Bottom-up methods. Atom by atom chaotic building of nanomaterials comprises most of synthetic methods as these are also the most common procedures in materials science. The main disadvantage of these methods is that usually a distribution of sizes is obtained, but compositions are more homogeneous than in top-down approaches.
Synthetic methods are usually divided in dry or wet synthesis; open or enclosed reactions; and gas-, solid- or liquid-phase reactions. Moreover, depending on the synthetic method and the material, different purification steps may be necessary and, in the case of coated particles, one or more modification steps with extra purification are needed. To facilitate comprehension, this section is organized first by nanomaterial group, and then by synthetic methods.
2.1 Carbon Based Nanomaterials Production
Sizable quantities of carbon based nanomaterials can be produced using various methods; among them, plasma based, thermal and hydrothermal syntheses processes are the most used techniques [19–22].
The two most common plasma methods in the literature are Arc Discharge and Laser Ablation. Arc Discharge Method consists in passing current between two graphite electrodes under helium, hydrogen or methane at low pressure in presence of transition metal based catalysts [23–28]. This causes vaporization of graphite that condenses over the cathode (and walls of the reactor). Carbon nanotubes (CNT) can also be produced by Laser Ablation, which is similar to Arc Discharge but the energy is provided by a laser. This laser vaporizes graphite and catalyst, so that nanocatalysts are formed and the carbon nanomaterials grow over them [29–35].
Thermal synthesis methods are also very abundant in the literature for the production of CNT. Carbon Vapour Deposition (CVD) consists in the decomposition of a carbon source (usually a hydrocarbon over a transition metal catalyst). Both type of carbon source and catalyst affect in the CNT growth. Carbon based nanomaterials can also be produced by sono- or hydrothermal methods, which consist on the heating of a hydrocarbon/water mixture under pressure in the presence of a catalyst (usually Ni) [36–38].
These methods usually produce low quantities of carbon based nanomaterials (fullerenes, SWCNT, MWCNT, etcetera) mixed with other allotropic forms of carbon. The conditions used during the synthesis favour one form over the others, but purification steps are always necessary.
Graphene, graphene oxide and derivatives are synthesized very differently [39–43]. The bottom-up approaches used for the rest of carbon based nanomaterials are modified to get 2D carbon layers over a support, which has to avoid 3D growth [44]. Graphene can be also produced by top-down approaches. The purest and most perfect graphene is produced by exfoliation of graphite [45–47], graphite oxide (followed by reduction) [48–50] and carbon nanotubes [51].
Carbon based nanomaterials surface can be modified to improve their dispersibility, their compatibility with a matrix or to functionalize them to add chemical groups that can later react or bond to any other entity, such as antibodies [52, 53], quantum dots [54] or gold nanoparticles [55]. These modifications are usually based on the following approach. In a first step, the nanomaterials are oxidized by a hydrothermal process (i.e.: sonication in presence of diluted nitric acid) that causes defects on the surface. Then, the hydroxyls and carboxylic acids formed are used for subsequent functionalization by traditional chemical reactions. Other strategies include direct arylation, carbene or nitrene addition or Friedel-Craft acylation.
Carbon nanotubes and nanofibers have centred most of the attention of hygienists and toxicologists due to their fast increase in production volume and observed dustiness [16, 56–64]. Most published studies on occupational exposure to CNT show that, as the synthesis processes take place in closed reactors, most of the exposure occurs during material recovery and during cleaning and maintenance operations [57, 65–67].
2.2 Metallic Nanomaterials Production
Metallic nanomaterials (MNM) are traditionally synthesized by the reduction of a precursor under controlled conditions and in the presence of a stabilizer [68]. The solvent, conditions and stabilizers used depend on the element of the NP and on the purpose. Wet syntheses have been traditionally considered of lower risk in terms of occupational exposure due to lower aerosol formation compared to the work with powders.
Noble and seminoble metallic nanoparticles (Ag [69], Au [70], Pt [71], Pd [72, 73], Ru [74], Rh [75], Ir [76]) are usually industrially synthesized by reduction of a precursor salt in water (such as HAuCl4 or RuCl3). Once the colloids are synthesized, they can be directly functionalized in situ or phase-transferred to an organic solvent for further surface functionalization when necessary [77]. The conditions and precursors used completely determine the results in terms of size and morphology [78]. Although less common, all these materials can be produced by other methods, such as electrochemical deposition, physical synthesis or sol-gel method.
More reactive metallic nanoparticles are synthesized by similar approaches (which can be also used with noble metals), but under more controlled conditions (air-free atmosphere, organic solvents, ionic liquids, etcetera). The most common strategy consists on direct reduction or decomposition of organometallic compounds or metallic carbonyls, such as Fe(CO)5, Co2(CO)8 or Ru(cod)(cot). Initially these syntheses used to produce polydisperse NP, but their optimization has improved the control of size and nowadays they are used for the synthesis of zerovalent nanoparticles of several metals: Fe, Co, Ru, Ir, Au, Ni or Rh, and different types of mixtures (core-shell, alloys...) [79, 80]. Surface modification of these materials is usually done in situ, and the organic modifier is used as stabilizer.
Though most metallic nanoparticles are produced by wet processes, occupational exposure cannot be neglected [81–86]. Release of metallic nanomaterials to the environment has received some attention, particularly Ag-NPs release, but most studies use assumptions to estimate the release during the MNP synthesis rather than actual measurements [87].
2.3 Oxide Based Nanomaterials Production
Several physical and chemical routes for the synthesis of nanometal oxides (NMOx) have been reported. Solution routes are the most widely used at laboratory scale, as they need more easily accessible set-ups and allow having a better control size and shape. In contrast, at the industrial scale, gas phase methods are the most commonly used as they are usually cleaner and better conversions are obtained. Moreover, they can be used for the deposition of thin films or nanostructures over a particular substrate. Both solution and gas-phase methods are widely used to produce different NMOx such as ZnO [88], TiO2 [89], FexOy [90], CeO2 [91, 92], ZrO2 [93], CuxO [94], Al2O3 [95], SiO2 [96–98], CoxOy [99], etcetera.
Solution routes are based on the decomposition of a salt or alkoxide precursor in solution, normally by means of a source of energy, to form the nanomaterial. Thereafter, this nanomaterial is separated from the solution by centrifugation, nanofiltration or other nano-appropriate techniques. Some examples of solution routes include solvo-/hydrothermal method, precipitation method, electrochemical synthesis, sonochemical method, sol-gel method and microemulsion.
In gas phase methods, metal vapour is produced by thermal, laser ablation, electron beam, ion beam, molecular beam or by vaporizing and dissociating any metal precursor. This metal vapour reacts with oxygen to produce the metal oxide that is deposited on the bottom and the internal walls of the reactor.
Different surface modification strategies exist. The most commonly reported are: (i) chemical functionalization of the surface by bonding of a silane derivate, which renders a very stable modification; (ii) addition of compounds that have affinity for NMOx surfaces, such as carboxylates or phosphates; and (iii) polymer grafting [100].
Occupational exposure to nanometal oxides in production facilities has not received much attention in the literature. In general, the reported studies show that the exposure is due to specific operations such as reactor opening, material recovery or cleaning and maintenance [86, 101–104].
2.4 Quantum Dots Production
Quantum dots (QD) can be produced by several methods, both top-down and bottom-up approaches [105–107]. The advantage of top-down processes is that very well-defined QD are produced. This is necessary, for instance, when producing nano-transistors for computing. However, these high energy methods usually result on physical and chemical damage to the particle surface. On the other hand, bottom-up approaches produce smaller and purer particles, as necessary in sensing applications.
Lithography, reactive-ion etching and wet chemical etching are the most commonly used top-down processes. The bottom-up processes are very similar to the ones explained for nanometal oxides synthesis.
The surface of most quantum dots is very easy to modify, as several functional groups have affinity for them (thiols, amines, carboxylic acids, etcetera). Thus, a multitude of papers report QD functionalization with biomolecules (DNA, RNA, proteins...) [108, 109], polymers [110] or other nanomaterials [111].
Despite of the high concern about quantum dots toxicity [106, 112–114] and their potential impact over workers and the environment, there is a lack of experimental data on the exposure to quantum dots during synthesis steps. Moreover, the only study that was found at the moment focused on a labscale synthesis [104].
2.5 Polymeric and Ceramic Nanofibres Production
Nanofibres are usually produced by the electrospinning method [115–119]. This method produces non-woven fabrics, in which the fibres are randomly oriented and connected by physical entanglements or bonds, without any knitting or stitching. In the electrospinning method, polymers are usually dissolved in a proper solvent (or molten) and the nanofiber is produced by high voltage. Recently, this technique has been extended to ceramic nanofibres synthesis. In this case, polymeric nanofibres loaded with ceramic precursors are prepared by electrospinning and, later, combusted to render the ceramic nanofiber.
Nanofibres production is usually done in closed conditions and neither occupational nor environmental exposure have been reported during this life cycle step. All the publications have focused on secondary manufacturers [120].
3 Nanocomposite Production
The incorporation of the nanomaterial in or on a matrix is a key step in nano-enabled products life cycle (except on those cases where nanomaterials are a final product by themselves, such as nanocatalysts). Nanotechnology has greatly progressed during the last two decades and, nowadays, we can find applications for almost any type of nanomaterial in any type of matrix.
A nanocomposite is a multiphase solid material that has at least one of the phases in the nanoscale. The main difference between nanocomposites and traditional composites is the high surface of contact between the phases in the first case. The addition of nanomaterials to solid matrices produces materials with enhanced, or even completely new, attributes, such as conductive polymeric matrices, electroluminescent metals, semiconductor ceramics or photo-luminescent textiles. The properties and quality of the resulting nanocomposite depend on the constituents of the composite, but also on the degree of dispersion and homogeneity of the different phases, which depend on the compatibilization between the phases and the mixing/addition methods.
In addition, nanomaterials can be also added to the surface of a material to obtain new or improved surface properties. This surface addition can be done by in situ nanocomposite formation, such as surface treatment of ceramic tiles with a solution of nanosized titania and a resin that is later dried [121]; by physical or chemical attachment of the nanomaterial to the surface, such as textiles with silver nanoparticles bonded to the fibres [122]; or by direct deposition of a thin layer over the surface, such as solar cells of nanosized TiO2 prepared by direct CVD over the cell surface [123].
Other types of nano-additivated formulations include nanomaterial dispersion in emulsions, such as paints or cosmetics, or mixtures of non-consolidated solids, such as catalytic mixtures for gas emission treatment.
The literature on this field is very broad (8,280; 88,000 and 75,000 results in Google Books, Google Scholar and Web of Science, respectively, when looking for all in title: nanocomposites).Footnote 1 Due to the scope of this book, this chapter will only provide an overview on the production processes for the main types of nanocomposites: polymeric, ceramic, metallic and textiles. If needed, the reader can expand this information in some of the existing reviews [124–126].
3.1 Polymeric-Matrix Nanocomposites
Research on polymeric nanocomposites has exponentially grown in the last decades and this has been reflected in an increase in the number of products launched to the market based on such materials, from conductive polymeric materials to artificial tissues.
Polymeric matrix nanocomposites can be synthesized by different techniques that can be divided in three major groups: solution casting, melt blending and in situ polymerization [127–130]. Solution casting consists in the dissolution of the polymer and dispersion of the nanomaterial in a solvent (usually using ultrasonication). Then, the nanocomposite is obtained by removing the solvent. In the melt blending method, the polymer and the nanomaterial are intensively mixed in an extruder or a mixer at a temperature that allows polymer mobility. In situ polymerization consists on the mixture of the nanomaterial and monomers (in solution or not) under conditions that favour the polymerization. Polymerization can be catalyzed by the nanomaterial itself (i.e. silicate layers promote intercalated monomer polymerization) or by the addition of polymerization catalysts. Moreover, the nanomaterial may be coated with vinyl moieties where polymerization can start.
The choice of the synthesis method and its conditions completely depend on the polymer and on the type of nanomaterial. A good compatibility of the polymer and the nanofiller is critical for a homogeneous physical-chemical behaviour of the composite and to reduce nanomaterial release in following life cycle phases [131]. The most common strategies used to improve such compatibility are the use of additives that act as a surfactant between the nanomaterial and the polymer [132–135], and the surface modification of the nanomaterial to make it more compatible with the polymer [135–138].
3.2 Ceramic-Matrix Nanocomposites
Nano-additivation of ceramic materials has resulted in the development of new materials with enhanced properties. The most important disadvantage of ceramic materials is their fragility, and the addition of nanomaterials is mainly used for the reinforcement of the ceramics that allows their use in new applications (i.e. armours, surgery materials or artificial bones). In addition, nanomaterials can also confer other properties that make these materials useful in fields such as optical, electronic or sensing [128, 139]. Ceramic matrices were traditionally reinforced with metallic particles [140–142], but nowadays one can find in the literature ceramics reinforced with carbon based nanomaterials [143, 144], nanometal oxides [145, 146] or quantum dots [147].
Three methodologies are basically used in the processing of ceramic matrix nanocomposites: powder process, polymer precursor process and sol-gel process [128]. Powder process consists of the mixing of the different materials that are thoroughly milled together in wet conditions; later the mixture is dried and consolidated, usually by pressure or moulding. This process is simple but results on a heterogeneous material. Polymer precursor process is similar, but the nanomaterial precursor is added to a polymer that is later pyrolized. Sol-gel process consists in the hydrolysis and condensation of molecular precursors dissolved in organic media to form a sol-gel, which is later dried and consolidated.
3.3 Metal-Matrix Nanocomposites
Particulate reinforced metal-matrix composites have been used for decades [148], but the reinforcement with nanomaterials has been developed recently and metal-matrix nanocomposites are still in their infancy [149]. The main advantages of metal matrices are their inherent thermal stability, resistance to abrasion, and thermal and electrical conductivities. But their development was strained by their cost and the difficulties of preparation [128, 149, 150]. The nano-additivation of metal matrices confers a combination of ceramic and metal properties to the material. This makes the material ideal for multiple applications, such as structural materials in the aeronautic industry or in light energy conversion.
Several methods for metal-matrix nanocomposites processing are described in the literature, including vapour phase processing, spray pyrolysis, powder metallurgy, solidification, chemical and deformation processes. The most used and cheapest method is solidification, which consists on the melting of the metal and the nano-reinforcement and rapid solidification of the melt by different processes. Liquid infiltration is similar, but in this case only the metal is melt and surrounds the nanomaterial. The homogeneity of the mixture can be improved by ultrasounds. The other methods are similar to the ones used in nanomaterial synthesis (sol-gel synthesis, CVD, spray pyrolysis, etcetera) (see Sect. 3.2).
3.4 Nano-additivated Textiles
Nanomaterials can be integrated in the textiles in different phases of their fabrication, which leads to different types of nano-additivated textiles: (i) Nanotextiles, when the nanomaterial is added once the fabric is produced, most of the products falls in this category; (ii) Nanocomposite textiles, when the material used to make the fibres is a nanocomposite; and (iii) Nanofibrous materials, when they are made from nanofibres (woven or non-woven) [151]. Nanomaterials are added to textiles to provide new or improved properties. The most common ones are antimicrobial activity and UV-filtering, but they are also used as flame retardants, water repellent, static protection, electrical conductivity, enhanced resistance or strength, photo- or electro-luminescent, self-cleaning, etcetera.
Several methods have been used for surface modification of fibre-based materials, such as textiles and membranes [152–155]. Usually they involve small modifications during the fabric processing; the nanomaterial is added as any other additive by methods such as impregnation, roll-to-roll and pad-dry-cure. The main problem of these methods is that the nanomaterials are usually not well fixed to the fabric and majorly released during the washing process [156–161]. In order to minimize the release of nanomaterials, binders and functionalized particles are used to improve particle affinity for the textiles. Ultrasounds, UV irradiation, plasma-treatment and ion-beam-assisted deposition are very effective for the surface modification of textiles, but impractical for large scale manufacturing (several preparatory steps, time-consuming and costly).
Nanocomposite fibres have emerged in the last decade as a very interesting material for nano-enabled textiles processing. In this case, nanocomposite material is produced as any other polymer-matrix nanocomposite (see Sect. 3.3.2). The main challenge is to get nano-reinforced polymers that can be processed as fibres and that, later, do not reduce the mechanical properties of the fabric.
Textiles and other non-woven products (such as filtering membranes) can be made of nanofibres. Nanofibres are produced by electrospinning (see Sect. 3.2.5) and can contain pure polymer(s) or nanocomposites. At the moment, nanofibres are not woven at industrial scale and are usually used as additive over other fibres [151]. Non-woven nanofibres are used as layer and barrier materials [115, 117, 118, 162, 163].
3.5 Occupational and Environmental Exposure During Nanocomposite Production
Most of the available studies on occupational exposure to nanomaterials focused on the nano-additivated material preparation. Nanomaterial synthesis is usually done in close reactors and workers are basically exposed during the nanomaterial recovery and during cleaning and maintenance. In contrast, weighting, pouring, and mixing of nanomaterial and bulk materials (common steps during nano-additivation of materials) are usually done in open conditions, and can involve big amounts of nanomaterials, so that the exposure during this step is potentially high [10, 12, 164–166]. This has been corroborated by exposure monitoring campaigns in workplaces as described in different reviews [167, 168] and other later studies [169–175].
On the other hand, nanomaterial release to the environment during this step is usually considered unlikely. Once the process is finished, the nanomaterials are embedded in a matrix or a mixture, so their recovery is easier and also the waste treatment [16].
4 Product Manufacturing
Product manufacturing involves a series of processes to convert the nano-additivated material into the final product. Machining is necessary to obtain final products with specified dimensions, surface finishing and tolerances. Most of the machining processes are physically aggressive and can lead to nanomaterial release. Although these processes are carried out by machines, they usually need an operator, sometimes in close and long contact to the material (i.e. sewing). Some examples of machining processes include soldering, welding, cutting, sewing, grinding, shredding, sanding, punching and drilling. Moreover, one has to consider than several of these processes may be necessary to get the final product, which may mean the product manufacturing divided in several phases that can even occur in different companies or locations.
Nanomaterial release from nanocomposites during the machining processes has received special attention in the literature in comparison to other processes during manufacturing and use stages. Indeed, almost half of the papers identified in a recent review on nanomaterial release from nanocomposites focused on machining processes [176].
Most of these studies are focused on CNT- and NMOx-based nanocomposites, with almost no attention to nanometal-based nanocomposites. Regarding the matrix, most of the studies focus on polymeric nanocomposites, probably because they are the ones with highest production volumes (Fig. 3.2) [176, 177].
Nanomaterial release from nanocomposites during the machining processes can be studied simulating real operations or using standardized protocols. Non-standard studies of cutting/sawing [120, 178, 179], grinding [120, 180], shredding [181], sanding [65, 120, 182–186], and drilling [187–190] under different conditions (wet/dry, hot/cold, etcetera) are found in the literature. Studies based on standard protocols usually focus on abrasion, using a Taber abraser [131, 185, 191–198]. Regardless on the type of simulation, most studies analyze the released material and usually conclude that part of the matrix released contains nanomaterials embedded. Only four publications report significant release of isolated nanomaterial [120, 131, 186, 192]. It is important to notice that most of these sanding/abrasion studies do not clearly distinguish between abrasions due to aging or industrial processes.
From the publications mentioned in this section, it can be concluded that the matrix play a more important role than the nano-reinforcement on the overall degradation caused by the machining processes. Moreover, good dispersion of the nanofillers in the matrix could reduce the release of isolated nanomaterials [131].
5 Use Phase
At the moment, the major usage of nanomaterials is considered to be at the industrial level. For example, they are used as catalysts, membranes, and as additives or technical components of materials in various application fields. In addition, some nano-enabled products are addressed to professionals and consumers. There is no doubt that the diversity of applications of ENMs in commercial products has grown extensively over the past decade, and continues to grow rapidly [199]. However, the actual distribution of nanomaterials over different product categories is largely unknown. According to the (US) Nanotechnology Consumer Products Inventory [200], which has been updated very recently, the number of consumer products that are claimed to contain nanomaterials has increased from 54 products in 2005 to 1628 products in 2013. Although these numbers are likely to reflect real trends, their accuracy is questionable because tracking products that contain nanomaterials is rather challenging. With a few exceptions, current labelling regulations do not require that the nanomaterial be listed specifically as an ingredient. On the other hand, some products on the market with the claim of “nano” may neither contain nanomaterials nor be produced with nanotechnology. Depending on the area of application, interest in reporting the use of nanomaterials can differ, which could result on biased estimations on the main area of application if based on reported use.
Such lack of information regarding the real use of nanomaterials in consumer products may change in the coming years. First, some regulations, such as those affecting cosmetics and food ingredients in the EU are currently already requesting producers to label nanomaterials in their products [201, 202]. And second, some countries established compulsory registries of nano-enabled products. France was the first European country to require the identification of ‘substances with nanoparticle status’ that are produced, imported, distributed, or formulated from the 1st of January 2013 (Article 185 of the French Environmental Code [3]). Since June 2014, the Danish EPA also requests the reporting into the nano-product register of mixtures and articles that are intended for sale to the general public and which contain nanomaterials. They did, however, limit the type of products that should be reported on the basis of their potential to represent a risk to the user or the environment. Therefore, reporting is only requested for products where the nanomaterial itself is released under normal or reasonably foreseeable use or where the nanomaterial itself is not released but substances in soluble form that are classified as carcinogen/mutagen/reprotoxic (CMRs) or environmentally dangerous substances are released from the nanomaterial. In addition, some type of products (mostly those covered by specific product risk assessments, such as medicines or cosmetics) are also exempt [2].
A recent report outlines the results of the two first declaration periods in France (up to 1st June 2014) [9, 203]. Table 3.1 includes the sectors of use with more than 100 declarations in 2014. The sectors with the highest number of declarations were agriculture, forestry and fishery, and formulation [mixing] of preparations and/or re-packaging (excluding alloys) with 58 and 19 of the declarations, respectively. Regarding chemical product categories, the most commonly reported are: (1) coatings and paints, thinners, paint removers, (2) cosmetics and personal care products, and (3) plant protection products, altogether accounting for almost 70 % of the chemical product categories registered (Table 3.2). Finally, among the registered articles, the most frequently reported categories were rubber articles (AC10), machinery, mechanical appliances, and electrical/electronic articles (AC2), plastic articles (AC13), vehicles (AC1), and other articles with intended release of substances (AC30).
Future updates of this registry and other registries will provide more realistic estimates of the global production of nanomaterials and their main applications.
5.1 Common Nanomaterial Applications and Potential for Release of Nanomaterials
Some applications involve the intended release of NM, either to result on an intended human exposure (e.g., application of nano-enabled sunscreen onto the skin), or to application on other surfaces (e.g., generation of a nanocoating by spraying into a glass surface). In these cases, the estimation of the direct release of NMs is rather straightforward. However, understanding which fraction of it reaches its target application point, and which is the fate of such fraction after application is still largely unknown.
In other many applications, NM are part of the product matrix and are not intended to become released during use. Nevertheless, some of the normal use processes for some products may result on such unintended release. These can be mechanical processes, such as washing, wearing, tearing, breaking, and drilling, or physical-chemical degradation processes, such as weathering and chemical abrasion. The amount of the NM released from the matrix during the use stage will depend on several factors: the amount of NM in the product, the product lifetime, the way the NM are incorporated in the material (surface applications or in matrix), the surface contact area of the product that is affected by the process inducing release, the transfer factor of the NM within the matrix, the thickness of the product, and the frequency and duration of use.
During the last years, an increasing interest has resulted on research on the release of NM during the use phase of nano-enabled products. Indeed, it is assumed that unintended emissions from diffuse sources are one of the most important sources of NM releases to the environment [14]. Nevertheless, the number of studies evaluating release of NM from solid nanocomposites is still very low (Table 3.3) [176].
In general, weathering studies with polymeric nanocomposites have shown the degradation of the polymeric matrices due to photo- and chemical degradation. As a consequence, the nanoparticles tend to accumulate in the degraded zone, at the surface of the nanocomposite. However, free released NM are barely detected and rarely freed from the matrix in which they were included, even when weathering experiments have been combined with secondary mechanical forces [204, 205].
The release of nanomaterials (embedded in organic binder, as aggregates, or as single particles) from conventional paints during run-off events has been reported [195, 206–209]. However, the amounts released greatly differ among studies.
A considerable number of studies have also focused on the release of nanomaterials (mostly silver) from textiles during washing processes [157, 160, 161, 210–212]. High releases have often been reported during the first washing event [211]. All these studies suggest that the silver particles in the textile dissolve to silver ions in the water and form secondary particles. A similar process seems to occur when textiles containing silver nanoparticles are immersed in artificial sweat [213–215].
The available research still provides a rather partial view of the potential release of nanomaterials or dissolved ions from consumer products. And further research is needed to understand and model which factors and how determine release under different processes.
6 End of Life
Products containing nanomaterials will eventually reach the end of their useful lives and, unless recycled, be discarded. In addition, waste materials containing nanomaterials are being generated during the manufacture of nanotechnology products. These waste streams generated during the life cycle of products containing nanomaterials are potential sources of nanomaterials into the environment. The handling, treatment and disposal of such wastes will determine the resulting environmental releases of nanomaterials. Therefore, the development of appropriate end-of-life management strategies for waste streams containing nanomaterials is critical.
This section provides an overview of the most common recycling and end-of-life processes for products and waste streams containing nanomaterials.
6.1 Recycling
Two categories of waste can be considered in terms of recycling processes. First, waste streams that are treated as broad waste categories, such as plastics or paper. These are typically highly heterogeneous mixtures of different products that could include multitude of different nanomaterials. And second, narrower categories, such as PET bottles, tyres, and Li-ion batteries, that are comparatively much more homogeneous. Regardless of the category, current recycling processes will handle products with and without nanomaterials in unknown proportions. Research is needed to estimate the type and quantity of nanomaterials in different material flows entering recycling systems, and on how the presence of nanomaterials alters the quality of the recycled material. Indeed some research has been published on the performance of recycled composites containing nanomaterials, and results show that the presence of nanomaterials may negatively affect the quality of the recycled composites [216, 217]. Such information could result on changes in the optimal applications for the recycled materials or on changes in the recycling processes per se. In addition, information is also needed on the potential release of nanomaterials during these processes and on technical measures that could be used for minimizing them [218]. The generation of such information is necessary to evaluate potential negative impacts on workers or the environment [219].
6.2 Incineration
Incineration is a thermal treatment, through which waste is combusted in an oxidizing ambient at temperatures in the range of 850–1200 °C [220]. There are different types of plants, which mostly differ in the off-gas treatment section. Materials (including nanomaterials) that enter an incineration plant can be totally or partially combusted or remain unaltered, depending on the local conditions in the combustion chamber, the melting point and reactivity of the materials, and additional matrix materials in which they are present. Unaltered or partly combusted materials can end up in the slag/bottom ash, retained in the particle control filters and becoming part of the fly ash, or go through such filters and be released to the environment.
Nanomaterials in the waste streams entering an incinerator may exist as free particles (i.e. a powder) or dispersed in a liquid or solid material. Based on theoretical thermodynamic considerations and on some experimental data, it is generally assumed that most nanomaterials in waste would end up in bottom ash. This would be the case for particle aggregates or particles that do not totally combust. A smaller fraction, mainly free particles and some partly combusted materials, would reach the air filtration systems, where a proportion of those would be retained [17, 220–225]. Some experimental data suggest that state-of-the-art flue gas cleaning systems (such as electrostatic precipitators and wet scrubbers) would effectively retain nanomaterials, but the efficiency of current filter techniques is still controversial [221, 222]. Further experiments are needed to fully substantiate these assumptions on the fate of nanomaterials in an incineration plant, and quantify efficiency of filter techniques for different type of nanomaterials. In addition, it remains unclear in what form the NM are present in the bottom ash. Treatment of the bottom ash depends on regional legislation, but it is usually disposed in landfills, unless originated from special waste streams that justify its further confinement. It is assumed that most of the waste streams containing nanomaterials will be considered domestic wastes, resulting in less strict regulations on the fate of resulting bottom ashes. Therefore, understanding in which form the nanomaterials are usually present in the bottom ash (i.e. whether or not enclosed in vitrified fragments) is important to understand their possible later mobility [225]. This information could be used to evaluate if current treatments are appropriate for the resulting ashes.
6.3 Landfilling
Landfill is a system of waste disposal that is based on burial of municipal solid waste (MSW) in specifically designed sites. Although landfill is one of the most exploited treatments for MSW end of life, it is not yet clear how NM behave during disposal. If NM are able to be transported through waste, then the potential for release from landfills to the surrounding environment increases. Existing studies show some degree of mobility for different NM, which depends on the NM and the composition of the leachate (organic composition, ionic strength, pH) [226, 227]. Another concern about the presence of NM in landfills is related to their capacity to influence biological activity. Very few data is available on this issue, and so far this indicates no effects on the overall biological activity [228, 229], although bacterial community structure has been shown to be sensitive to some nanomaterials [229].
6.4 Waste Water Treatment
Domestic (and some industrial) waste water containing nanomaterials will end up in sewage treatment plants and industrial waste water treatment plants.
Concerns are related to the impact of nanomaterials on the biological systems within such treatments, and on their fate. Several studies have investigated such processes (see recent review by Neale et al. [230]), but available information is still rather partial. Part of the sewage sludge, when metal concentrations are below established maximum limits, is applied on land as supplemental fertilizer of landfill cover. Current regulations establish metal content limits without consideration of particle size. Yang et al. estimated the proportion of nano-TiO2 present in a landfill and concluded that it represented around 0.1–0.2 % of the total Ti [231]. However, these values could vary regionally and with changing trends in the production of nano-TiO2. Further knowledge on the mechanisms of metal transport in soils and effects of environmental conditions and particle size are needed to evaluate the potential impact of applying sewage sludge containing nanomaterials on soils.
6.5 Current Practise and Regulations
Altogether, there is very limited information on the possible risks associated to the presence of nanomaterials in wastes. In the lack of specific evidence for concern, no specific processes are required for wastes containing nanomaterials in neither Europe nor the United States [232, 233]. In Europe, wastes are classified as hazardous or non-hazardous based on Regulation No. 1272/2008 on Classification, Labelling and Packaging of Substances and Mixtures [234]. This regulation does not include specific requirements for nanomaterials. Therefore, it is likely that nanomaterials will be classified in the same categories as their bulk form, and nano-specific hazards may be overlooked. The classification of waste as hazardous or non-hazardous is a key step as it leads to different requirements under the Waste Framework Directive. For example, mixing restrictions, labelling, and record keeping do not apply to wastes containing nanomaterials, unless they have been classified as hazardous [235].
Even when nanomaterials would be classified as hazardous, they may still be appropriate for use in some consumer products. In those cases, it is unlikely that their classification would result on specific end-of-life treatments for consumer products containing them. However, this is an issue that also applies to other type of hazardous substances.
More details on how current regulations affect wastes containing nanomaterials (and associated gaps) can be found in previous review reports [232, 233, 236].
Notes
- 1.
Search done on the 21st January 2015.
References
Luther W, Zweck A (2013) Safety aspects of engineered nanomaterials. doi: 10.4032/9789814364867
BEK (2014) BEK nr 644 af 13/06/2014. Bekendtgørelse om register over blandinger og varer, der indeholder nanomaterialer samt producenter og importørers indberetningspligt til registeret. BEK, Denmark
JORF (2010) Article 185. Prévention des risques pour la santé et l’environnement résultant de l’exposition aux substances à l’état nanoparticulaire. JORF n°0160 du 13 juillet 2010 page 12905, France
Robichaud CO, Uyar AE, Darby MR et al (2009) Estimates of upper bounds and trends in nano-TiO 2 production as a basis for exposure assessment. Environ Sci Technol 43:4227–4233. doi:10.1021/es8032549
Schmid K, Riediker M (2008) Use of nanoparticles in Swiss industry: a targeted survey. Environ Sci Technol 42:2253–2260. doi:10.1021/es071818o
Hendren CO, Mesnard X, Dröge J, Wiesner MR (2011) Estimating production data for five engineered nanomaterials as a basis for exposure assessment. Environ Sci Technol 45:2562–2569. doi:10.1021/es103300g
Piccinno F, Gottschalk F, Seeger S, Nowack B (2012) Industrial production quantities and uses of ten engineered nanomaterials in Europe and the world. J Nanopart Res 14:1109. doi:10.1007/s11051-012-1109-9
Gao Y, Luo Z, He N, Wang M (2013) Metallic nanoparticle production and consumption in China between 2000 and 2010 and associative aquatic environmental risk assessment. J Nanopart Res 15:1681. doi:10.1007/s11051-013-1681-7
ANSES (2015) R-nano.fr - Déclaration des substances à l’état nanoparticulaire
Sánchez Jiménez A, Brouwer D, van Tongeren M (2014) Workplace inhalation exposure to engineered nanomaterials. Detection, measurement, and assessment. In: Monteiro-Riviere NA, Tran CL (eds) Nanotoxicology prog. towar. nanomedicine, 2 edn. CRC Press, London, pp. 77–96
Brouwer D, Duuren-Stuurman B, Berges M et al (2009) From workplace air measurement results toward estimates of exposure? Development of a strategy to assess exposure to manufactured nano-objects. J Nanopart Res 11:1867–1881. doi:10.1007/s11051-009-9772-1
Vogel U, Savolainen K, Wu Q, et al. (2014) Handbook of nanosafety. Handb nanosafety. doi: 10.1016/B978-0-12-416604-2.00002-0
Brouwer D (2010) Exposure to manufactured nanoparticles in different workplaces. Toxicology 269:120–127. doi:10.1016/j.tox.2009.11.017
Gottschalk F, Nowack B (2011) The release of engineered nanomaterials to the environment. J Environ Monit 13:1145–1155. doi:10.1039/c0em00547a
Nowack B, Ranville JF, Diamond S et al (2012) Potential scenarios for nanomaterial release and subsequent alteration in the environment. Environ Toxicol Chem 31:50–59. doi:10.1002/etc.726
Nowack B, David RM, Fissan H et al (2013) Potential release scenarios for carbon nanotubes used in composites. Environ Int 59:1–11. doi:10.1016/j.envint.2013.04.003
Keller AA, Mcferran S, Lazareva A, Suh S (2013) Global life cycle releases of engineered nanomaterials. J Nanopart Res 15:1692–1703. doi:10.1007/s11051-013-1692-4
Biswas A, Bayer IS, Biris AS et al (2012) Advances in top-down and bottom-up surface nanofabrication: techniques, applications & future prospects. Adv Colloid Interface Sci 170:2–27. doi:10.1016/j.cis.2011.11.001
Varshney K (2014) Carbon nanotubes: a review on synthesis, properties and applications. Int J Eng Res Gen Sci 2:660–677
Prasek J, Drbohlavova J, Chomoucka J et al (2011) Methods for carbon nanotubes synthesis—review. J Mater Chem 21:15872. doi:10.1039/c1jm12254a
Purohit R, Purohit K, Rana S et al (2014) Carbon nanotubes and their growth methods. Procedia Mater Sci 6:716–728. doi:10.1016/j.mspro.2014.07.088
Rao C, Govindaraj A (2011) Nanotubes and nanowires, 2nd edn. doi:10.1039/9781847552525
Zhao X, Ohkohchi M, Wang M et al (1997) Preparation of high-grade carbon nanotubes by hydrogen arc discharge. Carbon N Y 35:775–781. doi:10.1016/S0008-6223(97)00033-X
Shimotani K, Anazawa K, Watanabe H, Shimizu M (2014) New synthesis of multi-walled carbon nanotubes using an arc discharge technique under organic molecular atmospheres. Appl Phys 73 A:451–454. doi:10.1007/s003390100821
Parkansky N, Boxman RL, Alterkop B et al (2004) Single-pulse arc production of carbon nanotubes in ambient air. J Phys D Appl Phys 37:2715–2719. doi:10.1088/0022-3727/37/19/015
Tsai YY, Su JS, Su CY, He WH (2009) Production of carbon nanotubes by single-pulse discharge in air. J Mater Process Technol 209:4413–4416. doi:10.1016/j.jmatprotec.2008.10.049
Saito Y, Nishikubo K, Kawabata K, Matsumoto T (1996) Carbon nanocapsules and single-layered nanotubes produced with platinum-group metals (Ru, Rh, Pd, Os, Ir, Pt) by arc discharge. J Appl Phys 80:3062. doi:10.1063/1.363166
Journet C, Maser WK, Bernier P et al (1997) Large-scale production of single-walled carbon nanotubes by the electric-arc technique. Nature 388:756–758. doi:10.1038/41972
Zhang Y, Gu H, Iijima S (1998) Single-wall carbon nanotubes synthesized by laser ablation in a nitrogen atmosphere. Appl Phys Lett 73:3827. doi:10.1063/1.122907
Muñoz E, Maser W, Benito A et al (2000) Gas and pressure effects on the production of single-walled carbon nanotubes by laser ablation. Carbon N Y 38:1445–1451. doi:10.1016/S0008-6223(99)00277-8
Arepalli S, Scott C (1999) Spectral measurements in production of single-wall carbon nanotubes by laser ablation. Chem Phys Lett 302:139–145. doi:10.1016/S0009-2614(99)00098-6
Kokai F, Takahashi K (2000) Laser ablation of graphite-Co/Ni and growth of single-wall carbon nanotubes in vortexes formed in an Ar atmosphere. J Phys Chem B 104:6777–6784. doi:10.1021/jp000359+
Yudasaka M, Kokai F, Takahashi K et al (1999) Formation of single-wall carbon nanotubes: comparison of CO2 laser ablation and Nd: YAG laser ablation. J Phys Chem B 103:3576–3581. doi:10.1021/jp990072g
Zhang Y, Iijima S (1999) Formation of single-wall carbon nanotubes by laser ablation of fullerenes at low temperature. Appl Phys Lett 15:3087. doi:10.1063/1.125239
Niu K, Sun J, Yang J, Du X (2012) The synthesis of carbon nanotubes by pulsed-laser ablation of a nickel/carbon composite target in ethanol or ambient air. Sci Adv Mater 4:463–466. doi:10.1166/sam.2012.1302
Hu B, Wang K, Wu L, Yu S (2010) Engineering carbon materials from the hydrothermal carbonization process of biomass. Adv Sci 22:813–828. doi:10.1002/adma.200902812
Skrabalak S (2009) Ultrasound-assisted synthesis of carbon materials. Phys Chem Chem Phys 11:4930–4942. doi:10.1039/B823408F
Manafi S, Rahimipour M, Mobasherpour I, Soltanmoradi A (2012) The synthesis of peculiar structure of springlike multiwall carbon nanofibers/nanotubes via mechanothermal method. J Nanomater 803546:8. doi:10.1155/2012/803546
Sen LH, Nainar M, Begum S (2014) Model, synthesis and applications of graphene oxide–a review. Nanomater. Energy 3:61–65. doi:10.1680/nme.13.00031
Liu W, Chai S, Mohamed A, Hashim U (2014) Synthesis and characterization of graphene and carbon nanotubes: a review on the past and recent developments. J Ind Eng Chem 20:1171–1185. doi:10.1016/j.jiec.2013.08.028
Geim AK (2009) Graphene: status and prospects. Science 324:1530–1534. doi:10.1126/science.1158877
Choi W, Lahiri I (2010) Synthesis of graphene and its applications: a review. Crit Rev Solid State Mater Sci 35:52–71. doi:10.1080/10408430903505036
Zhang Y, Petibone D, Xu Y et al (2014) Toxicity and efficacy of carbon nanotubes and graphene: the utility of carbon-based nanoparticles in nanomedicine. Drug Metab Rev. doi:10.3109/03602532.2014.883406
Muñoz R, Gómez-Aleixandre C (2013) Review of CVD synthesis of graphene. Chem Vap Depos 19:297–322. doi:10.1002/cvde.201300051
Coleman J (2012) Liquid exfoliation of defect-free graphene. Acc Chem Res 46:14–22. doi:10.1021/ar300009f
Khan U, O’Neill A, Lotya M et al (2010) High-concentration solvent exfoliation of graphene. Small 6:864–871. doi:10.1002/smll.200902066
Hernandez Y, Nicolosi V, Lotya M (2008) High-yield production of graphene by liquid-phase exfoliation of graphite. Nat Nanotechnol 3:563–568. doi:10.1038/nnano.2008.215
Kaniyoor A, Baby T, Ramaprabhu S (2010) Graphene synthesis via hydrogen induced low temperature exfoliation of graphite oxide. J Mater Chem 20:8467–8469. doi:10.1039/C0JM01876G
Zhu Y, Stoller M, Cai W et al (2010) Exfoliation of graphite oxide in propylene carbonate and thermal reduction of the resulting graphene oxide platelets. ACS Nano 4:1227–1233. doi:10.1021/nn901689k
Stankovich S, Piner R, Nguyen S, Ruoff R (2006) Synthesis and exfoliation of isocyanate-treated graphene oxide nanoplatelets. Carbon N Y 44:3342–3347. doi:10.1016/j.carbon.2006.06.004
Cano-Márquez A (2009) Ex-MWNTs: graphene sheets and ribbons produced by lithium intercalation and exfoliation of carbon nanotubes. Nano Lett 9:1527–1533. doi:10.1021/nl803585s
Erlanger B, Chen B, Zhu M, Brus L (2001) Binding of an anti-fullerene IgG monoclonal antibody to single wall carbon nanotubes. Nano Lett 1:465–467. doi:10.1021/nl015570r
McDevitt M, Chattopadhyay D, Kappel B et al (2007) Tumor targeting with antibody-functionalized, radiolabeled carbon nanotubes. J Nucl Med 48:1180–1189. doi:10.2967/jnumed.106.039131
Juárez B, Klinke C, Kornowski A, Weller H (2007) Quantum dot attachment and morphology control by carbon nanotubes. Nano Lett 7:3564–3568. doi:10.1021/nl071225b
Zanella R, Basiuk E, Santiago P et al (2005) Deposition of gold nanoparticles onto thiol-functionalized multiwalled carbon nanotubes. J Phys Chem B 109:16290–16295. doi:10.1021/jp0521454
Dahm M, Evans D (2012) Occupational exposure assessment in carbon nanotube and nanofiber primary and secondary manufacturers. Ann Occup Hyg 56:542–556. doi:10.1093/annhyg/mer110
Lee JH, Lee S-B, Bae GN et al (2010) Exposure assessment of carbon nanotube manufacturing workplaces. Inhal Toxicol 22:369–381. doi:10.3109/08958370903367359
Lam C, James J, McCluskey R et al (2006) A review of carbon nanotube toxicity and assessment of potential occupational and environmental health risks. Crit Rev Toxicol 36:189–217. doi:10.1080/10408440600570233
Liu Y, Zhao Y, Sun B, Chen C (2013) Understanding the toxicity of carbon nanotubes. Acc Chem Res 46:702–713. doi:10.1021/ar300028m
Kolosnjaj-Tabi J, Szware H, Moussa F (2012) In vivo toxicity studies of pristine carbon nanotubes: a review. Deliv Nanoparticles. doi:10.5772/34201
Aschberger K, Johnston H (2010) Review of carbon nanotubes toxicity and exposure-appraisal of human health risk assessment based on open literature. Crit Rev Toxicol 40:759–790. doi:10.3109/10408444.2010.506638
Wang J, Xu Y, Yang Z et al (2013) Toxicity of carbon nanotubes. Curr Drug Metab 14:891–899
Donaldson K, Aitken R, Tran L (2006) Carbon nanotubes: a review of their properties in relation to pulmonary toxicology and workplace safety. Toxicol Sci 92:5–22. doi:10.1093/toxsci/kfj130
Boczkowski J, Lanone S (2012) Respiratory toxicities of nanomaterials – a focus on carbon nanotubes. Adv Drug Deliv Rev 64:1694–1699. doi:10.1016/j.addr.2012.05.011
Cena LG, Peters TM (2011) Characterization and control of airborne particles emitted during production of epoxy/carbon nanotube nanocomposites. J Occup Environ Hyg 8:86–92. doi:10.1080/15459624.2011.545943
Han JH, Lee EJ, Lee JH et al (2008) Monitoring multiwalled carbon nanotube exposure in carbon nanotube research facility. Inhal Toxicol 20:741–749. doi:10.1080/08958370801942238
Fujitani Y, Kobayashi T, Arashidani K et al (2008) Measurement of the physical properties of aerosols in a fullerene factory for inhalation exposure assessment. J Occup Environ Hyg 5:380–389. doi:10.1080/15459620802050053
Masala O, Seshadri R (2004) Synthesis Routes for large volumes of nanoparticles. Annu Rev Mat Res 34:41–81. doi:10.1146/annurev.matsci.34.052803.090949
Panacek A, Kvítek L, Prucek R et al (2006) Silver colloid nanoparticles: synthesis, characterization, and their antibacterial activity. J Phys Chem B 110:16248–16253. doi:10.1021/jp063826h
Alex S, Tiwari A (2015) Functionalized gold nanoparticles: synthesis, properties and applications—a review. J Nanosci Nanotechnol 15:1869–1895. doi:10.1166/jnn.2015.9718
Chen A, Holt-Hindle P (2010) Platinum-based nanostructured materials: synthesis, properties, and applications. Chem Rev 110:3767–3804. doi:10.1021/cr9003902
Nemamcha A (2006) Synthesis of palladium nanoparticles by sonochemical reduction of palladium (II) nitrate in aqueous solution. J Phys Chem B 110:383–387. doi:10.1021/jp0535801
Xiong Y, Xia Y (2007) Shape-controlled synthesis of metal nanostructures: the case of palladium. Adv Mater 19:3385–3391. doi:10.1002/adma.200701301
Viau G, Brayner R, Poul L et al (2003) Ruthenium nanoparticles: size, shape, and self-assemblies. Chem Mater 15:486–494. doi:10.1021/cm0212109
Mévellec V, Nowicki A, Roucoux A et al (2006) A simple and reproducible method for the synthesis of silica-supported rhodium nanoparticles and their investigation in the hydrogenation of aromatic compounds. New J Chem 30:1214–1219. doi:10.1039/B605893K
Stowell C, Korgel B (2005) Iridium nanocrystal synthesis and surface coating-dependent catalytic activity. Nano Lett 5:1203–1207. doi:10.1021/nl050648f
Lista M, Liu D, Mulvaney P (2014) Phase transfer of noble metal nanoparticles to organic solvents. Langmuir 30:1932–1938. doi:10.1021/la404569h
Tao AR, Habas S, Yang P (2008) Shape control of colloidal metal nanocrystals. Small 4:310–325. doi:10.1002/smll.200701295
Philippot K, Chaudret B (2003) Organometallic approach to the synthesis and surface reactivity of noble metal nanoparticles. C R Chimie 6:1019–1034. doi:10.1016/j.crci.2003.07.010
Chaudhuri RG, Paria S (2011) Core/shell nanoparticles: classes, properties, synthesis mechanisms, characterization, and applications. Chem Rev 112:2373–2433. doi:10.1021/cr100449n
Park J, Kwak BK, Bae E et al (2009) Characterization of exposure to silver nanoparticles in a manufacturing facility. J Nanopart Res 11:1705–1712. doi:10.1007/s11051-009-9725-8
Zimmermann E, Derrough S, Locatelli D et al (2012) Results of potential exposure assessments during the maintenance and cleanout of deposition equipment. J Nanopart Res 14:1209. doi:10.1007/s11051-012-1209-6
Debia M, Beaudry C, Weichenthal S et al (2013) Report R-777. Characterization and control of occupational exposure to nanoparticles and ultrafine particles. IRSST, Montréal
Ling M-P, Lin W-C, Liu C-C et al (2012) Risk management strategy to increase the safety of workers in the nanomaterials industry. J Hazard Mater 229–230:83–93. doi:10.1016/j.jhazmat.2012.05.073
Lee JH, Ahn K, Kim SM et al (2012) Continuous 3-day exposure assessment of workplace manufacturing silver nanoparticles. J Nanopart Res 14:1134. doi:10.1007/s11051-012-1134-8
Lee J, Kwon M, Ji J, Kang C (2011) Exposure assessment of workplaces manufacturing nanosized TiO2 and silver. Inhal Toxicol 23:226–236. doi:10.3109/08958378.2011.562567
Gottschalk F, Sun T, Nowack B (2013) Environmental concentrations of engineered nanomaterials : review of modeling and analytical studies. Environ Pollut. doi:10.1016/j.envpol.2013.06.003
Fan Z, Lu J (2005) Zinc oxide nanostructures: synthesis and properties. J Nanosci Nanotechnol 5:1561–1573. doi:10.1166/jnn.2005.182
Chen X, Selloni A (2014) Introduction: titanium dioxide (TiO2) nanomaterials. Chem Rev 114:9281–9282. doi:10.1021/cr500422r
Lu A-H, Salabas EL, Schüth F (2007) Magnetic nanoparticles: synthesis, protection, functionalization, and application. Angew Chem Int Ed Engl 46:1222–1244. doi:10.1002/anie.200602866
Chen Y, Lv S, Chen C, Qiu C (2014) Controllable synthesis of ceria nanoparticles with uniform reactive {100} exposure planes. J Phys Chem C 118:4437–4443. doi:10.1021/jp410625n
Chen H, Mazzolini J, Ayers J, et al. (2013) Synthesis and characterization of nano ceria for biological applications. In: Kang SW, Park SH, Lee LP, et al (eds) Proc. SPIE 8879, Nano-bio sensing, imaging, spectrosc. Jeju, Republic of Korea p 887910. doi: 10.1117/12.2018564
Chen G, Guo C, Qiao H et al (2013) Well-dispersed sulfated zirconia nanoparticles as high-efficiency catalysts for the synthesis of bis(indolyl) methanes and biodiesel. Catal Commun 41:70–74. doi:10.1016/j.catcom.2013.07.006
Yin M, Wu C, Lou Y et al (2005) Copper oxide nanocrystals. J Am Chem Soc 127:9506–9511. doi:10.1021/ja050006u
Cai W, Yu J, Anand C et al (2011) Facile synthesis of ordered mesoporous alumina and alumina-supported metal oxides with tailored adsorption and framework properties. Chem Mater 23:1147–1157. doi:10.1021/cm102512v
Rahman I, Padavettan V (2012) Synthesis of silica nanoparticles by sol-gel: size-dependent properties, surface modification, and applications in silica-polymer nanocomposites—a review. J Nanomater 132424:15. doi:10.1155/2012/132424
Wu S, Mou C, Lin H (2013) Synthesis of mesoporous silica nanoparticles. Chem Soc Rev 42:3862–3875. doi:10.1039/C3CS35405A
Tang F, Li L, Chen D (2012) Mesoporous silica nanoparticles: synthesis, biocompatibility and drug delivery. Adv Mater 24:1504–1534. doi:10.1002/adma.201104763
Yang J, Liu H, Martens W, Frost RL (2009) Synthesis and characterization of cobalt hydroxide, cobalt oxyhydroxide, and cobalt oxide nanodiscs. J Phys Chem C 114:111–119. doi:10.1021/jp908548f
Kango S, Kalia S, Celli A et al (2013) Surface modification of inorganic nanoparticles for development of organic–inorganic nanocomposites—a review. Prog Polym Sci 38:1232–1261. doi:10.1016/j.progpolymsci.2013.02.003
Curwin B, Bertke S (2011) Exposure characterization of metal oxide nanoparticles in the workplace. J Occup Environ Hyg 8:580–587. doi:10.1080/15459624.2011.613348
Leppänen M, Lyyränen J, Järvelä M et al (2012) Exposure to CeO(2) nanoparticles during flame spray process. Nanotoxicology 6:643–651. doi:10.3109/17435390.2011.600838
Yang Y, Mao P, Wang Z, Zhang J (2012) Distribution of nanoparticle number concentrations at a nano-TiO2 plant. Aerosol Air Qual Res 12:934–940. doi:10.4209/aaqr.2012.02.0047
Methner M, Hodson L, Dames A, Geraci C (2010) Nanoparticle Emission Assessment Technique (NEAT) for the identification and measurement of potential inhalation exposure to engineered nanomaterials–Part B: Results from 12 field studies. J Occup Environ Hyg 7:163–176. doi:10.1080/15459620903508066
Bera D, Qian L, Tseng T-K, Holloway PH (2010) Quantum dots and their multimodal applications: a review. Materials (Basel) 3:2260–2345. doi:10.3390/ma3042260
Valizadeh A, Mikaeili H, Samiei M et al (2012) Quantum dots: synthesis, bioapplications, and toxicity. Nanoscale Res Lett 7:480. doi:10.1186/1556-276X-7-480
Evans C, Cass L, Knowles K (2012) Review of the synthesis and properties of colloidal quantum dots: the evolving role of coordinating surface ligands. J Coord Chem 65:2391–2414. doi:10.1080/00958972.2012.695019
Deng Z, Samanta A (2012) Robust DNA-functionalized core/shell quantum dots with fluorescent emission spanning from UV–vis to near-IR and compatible with DNA-directed self-assembly. J Am Chem Soc 134:17424–17427. doi:10.1021/ja3081023
Goswami N, Giri A, Kar S, Bootharaju M (2012) Protein-directed synthesis of NIR-emitting, tunable HgS quantum dots and their applications in metal-ion sensing. Small 8:3175–3184. doi:10.1002/smll.201200760
Wang D, Qian J, Cai F et al (2012) “Green”-synthesized near-infrared PbS quantum dots with silica–PEG dual-layer coating: ultrastable and biocompatible optical probes for in vivo animal imaging. Nanotechnology 23:245701. doi:10.1088/0957-4484/23/24/245701
Lee J, Kwon B, Park H et al (2013) Solar cells: exciton dissociation and charge-transport enhancement in organic solar cells with quantum-dot/N-doped CNT hybrid nanomaterials. Adv Mater 25:2104. doi:10.1002/adma.201370088
Hardman R (2006) A toxicologic review of quantum dots: toxicity depends on physicochemical and environmental factors. Environ Health Perspect 114:165–172. doi:10.1289/ehp.8284
Chen N, He Y, Su Y et al (2012) The cytotoxicity of cadmium-based quantum dots. Biomaterials 33:1238–1244. doi:10.1016/j.biomaterials.2011.10.070
Tsoi K, Dai Q (2012) Are quantum dots toxic? Exploring the discrepancy between cell culture and animal studies. Acc Chem Res 46:662–671. doi:10.1021/ar300040z
Huang Z-M, Zhang Y-Z, Kotaki M, Ramakrishna S (2003) A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Compos Sci Technol 63:2223–2253. doi:10.1016/S0266-3538(03)00178-7
Wu H, Pan W, Lin D, Li H (2012) Electrospinning of ceramic nanofibers: fabrication, assembly and applications. J Adv Ceram 1:2–23. doi:10.1007/s40145-012-0002-4
Panda PK, Sahoo B (2013) Synthesis and applications of electrospun nanofibers-a review. In: Navani KN, Sinha S, Govil JN (eds) Nanotechnology, vol. 1: Fundamental applications. Studiun Press LLC, Houston, TX, USA, pp 399–416
Lee YS, Im JS (2010) Preparation of functionalized nanofibers and their applications. In: Kumar A (ed) Nanofibers. InTech, Rijeka, Croatia, p 121–140
Dai Y, Liu W, Formo E (2011) Ceramic nanofibers fabricated by electrospinning and their applications in catalysis, environmental science, and energy technology. Polym Adv Technol 22:326–338. doi:10.1002/pat.1839
Methner M, Crawford C, Geraci C (2012) Evaluation of the potential airborne release of carbon nanofibers during the preparation, grinding, and cutting of epoxy-based nanocomposite material. J Occup Environ Hyg 9:308–318. doi:10.1080/15459624.2012.670790
Daoud WA (2013) Self-cleaning materials and surfaces: a nanotechnology approach. Wiley, New York
Rai M, Yadav A, Gade A (2009) Silver nanoparticles as a new generation of antimicrobials. Biotechnol Adv 27:76–83. doi:10.1016/j.biotechadv.2008.09.002
Thelakkat M, Schmitz C, Schmidt H (2002) Fully vapor-deposited thin-layer titanium dioxide solar cells. Adv Mater 14:577–581. doi:10.1002/1521-4095(20020418)14:8<577::AID-ADMA577>3.0.CO;2-S
Davim JP, Charitidis CA (2013) Nanocomposites: materials, manufacturing and engineering. De Gruyter, Berlin
Kumar CSSR (2010) Nanocomposites. John Wiley & Sons, Inc., Hoboken, NJ, USA. doi: 10.1002/9781119096122
Twardowski TE (2007) Introduction to nanocomposite materials: properties, processing, characterization, 1st edn. DEStech Publications Inc, Lancaster
Anandhan S, Bandyopadhyay S (2011) Polymer nanocomposites: from synthesis to applications. In: Cuppoletti J (ed) Nanocomposites polym. with anal. methods. InTech, Rijeka, Croatia, p 3–28
Camargo PHC, Satyanarayana KG, Wypych F (2009) Nanocomposites : synthesis, structure, properties and new application opportunities. Mater Res 12:1–39. doi:10.1590/S1516-14392009000100002
Hussain F (2006) Review article: polymer-matrix nanocomposites, processing, manufacturing, and application: an overview. J Compos Mater 40:1511–1575. doi:10.1177/0021998306067321
Mittal V (2010) Polymer nanocomposites: synthesis, microstructure, and properties. In: Mittal V (ed) Optim. polym. nanocomposite prop. Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, p 1–20. ISBN:978-3-527-32521-4
Golanski L, Guiot A, Pras M et al (2012) Release-ability of nano fillers from different nanomaterials (toward the acceptability of nanoproduct). J Nanopart Res 14:962. doi:10.1007/s11051-012-0962-x
Phua Y, Lau N, Sudesh K et al (2014) A study on the effects of organoclay content and compatibilizer addition on the properties of biodegradable poly (butylene succinate) nanocomposites under natural weathering. J Compos Mater. doi:10.1177/0021998314527328
Mistretta M, Fontana P, Ceraulo M et al (2015) Effect of compatibilization on the photooxidation behaviour of polyethylene/polyamide 6 blends and their nanocomposites. Polym Degrad Stab 112:192–197. doi:10.1016/j.polymdegradstab.2015.01.002
García-López D, Picazo O, Merino JC, Pastor JM (2003) Polypropylene–clay nanocomposites: effect of compatibilizing agents on clay dispersion. Eur Polym J 39:945–950. doi:10.1016/S0014-3057(02)00333-6
Taguet A, Cassagnau P, Lopez-Cuesta J (2014) Structuration, selective dispersion and compatibilizing effect of (nano)fillers in polymer blends. Prog Polym Sci 39:1526–1563. doi:10.1016/j.progpolymsci.2014.04.002
Sengupta R, Bhattacharya M (2011) A review on the mechanical and electrical properties of graphite and modified graphite reinforced polymer composites. Prog Polym Sci 36:638–670. doi:10.1016/j.progpolymsci.2010.11.003
Li D, You Y, Li R, Deng X (2013) Effects of nanometer-TiO2 surface modification and concentration on the mechanical performances of polypropylene/polyamide maleic anhydride-grafted. J Reinf Plast Compos 32:1807–1820. doi:10.1177/0731684413493341
Beyou E, Akbar S, Chaumont P, Cassagnau P (2013) Polymer nanocomposites containing functionalised multiwalled carbon nanoTubes: a particular attention to polyolefin based materials. Synth Appl Carbon Nanotub Their Compos. doi:10.5772/50710
Low I (2014) Advances in ceramic matrix composites. Woodhead Publishing Limited, Cambridge, UK
Chawla K (1998) Ceramic matrix composites, 2nd edn. Springer
Yeomans J (2008) Ductile particle ceramic matrix composites—Scientific curiosities or engineering materials? J Eur Ceram Soc 28:1543–1550. doi:10.1016/j.jeurceramsoc.2007.12.009
Rosso M (2006) Ceramic and metal matrix composites: Routes and properties. J Mater Process Technol 175:364–375. doi:10.1016/j.jmatprotec.2005.04.038
Samal S, Bal S (2008) Carbon nanotube reinforced ceramic matrix composites-A review. J Miner Mater Charact Eng 7:355–370
Peigney A, Laurent C, Flahaut E, Rousset A (2000) Carbon nanotubes in novel ceramic matrix nanocomposites. Ceram Int 26:677–683. doi:10.1016/S0272-8842(00)00004-3
Zhao Z, Sun R, Xin G (2013) A review: application of nanomaterials in concrete. Appl Mech Mater 405–408:2881–2884. doi:10.4028/www.scientific.net/AMM.405-408.2881
Mukhopadhyay A, Basu B (2007) Consolidation–microstructure–property relationships in bulk nanoceramics and ceramic nanocomposites: a review. Int Mater Rev 52:257–288. doi:10.1179/174328007X160281
Hedayati M, Faupel F, Elbahri M (2014) Review of plasmonic nanocomposite metamaterial absorber. Materials (Basel) 7:1221–1248. doi:10.3390/ma7021221
Ibrahim I, Mohamed F, Lavernia E (1991) Particulate reinforced metal matrix composites—a review. J Mater Sci 26:1137–1156. doi:10.1007/BF00544448
Rohatgi P, Schultz B (2007) Lightweight metal matrix nanocomposites–stretching the boundaries of metals. Mater Matters 2:16–19
He F, Han Q, Jackson M (2008) Nanoparticulate reinforced metal matrix nanocomposites–a review. Int J Nanoparticles 1:301–309. doi:10.1504/IJNP.2008.026473
Haydon B (2013) Nanomaterials and their applications in textiles, standards: domestic standardization for Canadian Manufacturers and Importers and International Standardization Developments. Waterloo, Ontario, Canada. ISBN:978-1-100-21089-6
Dastjerdi R, Montazer M (2010) A review on the application of inorganic nano-structured materials in the modification of textiles: focus on anti-microbial properties. Colloids Surf B Biointerfaces 79:5–18. doi:10.1016/j.colsurfb.2010.03.029
Zille A, Almeida L, Amorim T et al (2014) Application of nanotechnology in antimicrobial finishing of biomedical textiles. Mater Res Express 1:032003. doi:10.1088/2053-1591/1/3/032003
Coyle S, Diamond D (2010) Smart nanotextiles: materials and their application. In: Encycl. Mater. Elsevier Ltd., Atlanta, GA, USA, p 8. doi: 10.1016/B978-008043152-9.02220-X
Coyle S, Wu Y, Lau K, Rossi DD (2007) Smart nanotextiles: a review of materials and applications. MRS Bull 32:434–442. doi:10.1557/mrs2007.67
Som C, Wick P, Krug H, Nowack B (2011) Environmental and health effects of nanomaterials in nanotextiles and façade coatings. Environ Int 37:1131–1142. doi:10.1016/j.envint.2011.02.013
Geranio L, Heuberger M, Nowack B (2009) The behavior of silver nanotextiles during washing. Environ Sci Technol 43:8113–8118. doi:10.1021/es9018332
Farkas J, Peter H, Christian P et al (2011) Characterization of the effluent from a nanosilver producing washing machine. Environ Int 37:1057–1062. doi:10.1016/j.envint.2011.03.006
Windler L, Lorenz C, von Goetz N et al (2012) Release of titanium dioxide from textiles during washing. Environ Sci Technol 46:8181–8188. doi:10.1021/es301633b
Lorenz C, Windler L, von Goetz N et al (2012) Characterization of silver release from commercially available functional (nano)textiles. Chemosphere 89:817–824. doi:10.1016/j.chemosphere.2012.04.063
El-Rafie MH, Ahmed HB, Zahran MK (2014) Characterization of nanosilver coated cotton fabrics and evaluation of its antibacterial efficacy. Carbohydr Polym 107:174–181. doi:10.1016/j.carbpol.2014.02.024
Chronakis I (2005) Novel nanocomposites and nanoceramics based on polymer nanofibers using electrospinning process—a review. J Mater Process Technol 167:283–293. doi:10.1016/j.jmatprotec.2005.06.053
Leong S, Razmjou A, Wang K (2014) TiO2 based photocatalytic membranes: a review. J Membr Sci 472:167–184. doi:10.1016/j.memsci.2014.08.016
Nowack B, Brouwer C, Geertsma RE et al (2013) Analysis of the occupational , consumer and environmental exposure to engineered nanomaterials used in 10 technology sectors. Nanotoxicology 7:1152–1156. doi:10.3109/17435390.2012.711863
Wohlleben W, Kuhlbusch TAJ, Schnekenburger J, Lehr C-M (2014) Safety of nanomaterials along their lifecycle: release, exposure, and human hazards. CRC Press, London
Vance ME, Marr LC (2014) Exposure to airborne engineered nanoparticles in the indoor environment. Atmos Environ. doi:10.1016/j.atmosenv.2014.12.056
Kuhlbusch TA, Asbach C, Fissan H et al (2011) Nanoparticle exposure at nanotechnology workplaces: a review. Part Fibre Toxicol 8:22. doi:10.1186/1743-8977-8-22
Pietroiusti A, Magrini A (2014) Engineered nanoparticles at the workplace: current knowledge about workers’ risk. Occup Med (Lond) 64:319–330. doi:10.1093/occmed/kqu051
Gomez V, Irusta S, Balas F et al (2014) Unintended emission of nanoparticle aerosols during common laboratory handling operations. J Hazard Mater 279:75–84. doi:10.1016/j.jhazmat.2014.06.064
Heitbrink WA, Lo L-M, Dunn KH (2015) Exposure controls for nanomaterials at three manufacturing sites. J Occup Environ Hyg 12:16–28. doi:10.1080/15459624.2014.930559
Kim B, Lee J, Choi B, Park S (2013) Ultrafine particle characteristics in a rubber manufacturing factory. Ann Occup Hyg 57:728–739. doi:10.1093/annhyg/mes102
Kim B, Kim H, Yu IJ (2014) Assessment of nanoparticle exposure in nanosilica handling process: including characteristics of nanoparticles leaking from a vacuum cleaner. Ind Health 52:152–162. doi:10.2486/indhealth.2013-0087
Göhler D, Stintz M (2014) Granulometric characterization of airborne particulate release during spray application of nanoparticle-doped coatings. J Nanopart Res 16:2520. doi:10.1007/s11051-014-2520-1
Brouwer DH, Duuren-Stuurman B, Berges M et al (2013) Workplace air measurements and likelihood of exposure to manufactured nano-objects, agglomerates, and aggregates. J Nanopart Res 15:2090. doi:10.1007/s11051-013-2090-7
Voliotis A, Bezantakos S, Giamarelou M et al (2014) Nanoparticle emissions from traditional pottery manufacturing. Environ Sci Process Impacts 16:1489–1494. doi:10.1039/c3em00709j
Froggett SJ, Clancy SF, Boverhof DR, Canady RA (2014) A review and perspective of existing research on the release of nanomaterials from solid nanocomposites. Part Fibre Toxicol 11:17. doi:10.1186/1743-8977-11-17
Duncan TV (2015) Release of engineered nanomaterials from polymer nanocomposites: the effect of matrix degradation. ACS Appl Mater Interfaces 7:20–39. doi:10.1021/am5062757
Hsu L-Y, Chein H-M (2006) Evaluation of nanoparticle emission for TiO2 nanopowder coating materials. J Nanopart Res 9:157–163. doi:10.1007/s11051-006-9185-3
Bello D, Wardle BL, Yamamoto N et al (2008) Exposure to nanoscale particles and fibers during machining of hybrid advanced composites containing carbon nanotubes. J Nanopart Res 11:231–249. doi:10.1007/s11051-008-9499-4
Ogura I, Kotake M, Shigeta M et al (2013) Potential release of carbon nanotubes from their composites during grinding. J Phys Conf Ser 429:12049. doi:10.1088/1742-6596/429/1/012049
Raynor PC, Cebula JI, Spangenberger JS et al (2012) Assessing potential nanoparticle release during nanocomposite shredding using direct-reading instruments. J Occup Environ Hyg 9:1–13. doi:10.1080/15459624.2012.633061
Koponen IK, Jensen KA, Schneider T (2009) Sanding dust from nanoparticle-containing paints: physical characterisation. J Phys Conf Ser 151:012048. doi:10.1088/1742-6596/151/1/012048
Koponen IK, Jensen KA, Schneider T (2010) Comparison of dust released from sanding conventional and nanoparticle-doped wall and wood coatings. J Expo Sci Environ Epidemiol 21:408–418. doi:10.1038/jes.2010.32
Göhler D, Stintz M, Hillemann L, Vorbau M (2010) Characterization of nanoparticle release from surface coatings by the simulation of a sanding process. Ann Occup Hyg 54:615–624. doi:10.1093/annhyg/meq053
Wohlleben W, Brill S, Meier MW et al (2011) On the lifecycle of nanocomposites: comparing released fragments and their in-vivo hazards from three release mechanisms and four nanocomposites. Small 7:2384–2395. doi:10.1002/smll.201002054
Huang G, Park JH, Cena LG et al (2012) Evaluation of airborne particle emissions from commercial products containing carbon nanotubes. J Nanopart Res. doi:10.1007/s11051-012-1231-8
Irfan A, Sachse S, Njuguna J et al (2013) Assessment of nanoparticle release from polyamide 6- and polypropylene-silicon composites and cytotoxicity in human lung A549 cells. J Inorg Organomet Polym Mater 23:861–870. doi:10.1007/s10904-013-9856-3
Sachse S, Silva F, Zhu H et al (2012) The effect of nanoclay on dust generation during drilling of PA6 nanocomposites. J Nanomater 2012:1–8. doi:10.1155/2012/189386
Sachse S, Silva F, Irfan A et al (2012) Physical characteristics of nanoparticles emitted during drilling of silica based polyamide 6 nanocomposites. IOP Conf Ser Mater Sci Eng 40:12012. doi:10.1088/1757-899X/40/1/012012
Bello D, Wardle BL, Zhang J et al (2010) Characterization of exposures to nanoscale particles and fibers during solid core drilling of hybrid carbon nanotube advanced composites. Int J Occup Environ Health 16:434–450. doi:10.1179/107735210799159996
Wohlleben W, Meier MW, Vogel S et al (2013) Elastic CNT-polyurethane nanocomposite: synthesis, performance and assessment of fragments released during use. Nanoscale 5:369–380. doi:10.1039/c2nr32711b
Schlagenhauf L, Chu BTT, Buha J et al (2012) Release of carbon nanotubes from an epoxy-based nanocomposite during an abrasion process. Environ Sci Technol 46:7366–7372. doi:10.1021/es300320y
Guiot A, Golanski L, Tardif F (2009) Measurement of nanoparticle removal by abrasion. J Phys Conf Ser 170:12014. doi:10.1088/1742-6596/170/1/012014
Vorbau M, Hillemann L, Stintz M (2009) Method for the characterization of the abrasion induced nanoparticle release into air from surface coatings. J Aerosol Sci 40:209–217. doi:10.1016/j.jaerosci.2008.10.006
Zuin S, Gaiani M, Ferrari A, Golanski L (2013) Leaching of nanoparticles from experimental water-borne paints under laboratory test conditions. J Nanopart Res 16:2185. doi:10.1007/s11051-013-2185-1
Zhou L, Zhang Z, Xia S et al (2014) Effects of suspended titanium dioxide nanoparticles on cake layer formation in submerged membrane bioreactor. Bioresour Technol 152:101–106. doi:10.1016/j.biortech.2013.11.006
Golanski L, Guiot A, Braganza D, Tardif F (2010) New method for the characterization of abrasion-induced nanoparticle release into air from nanomaterials. In: NSTI-Nanotech 2010. Anaheim, CA, USA, p 720–723
Biswal M, Mohanty S, Nayak SK, Kumar PS (2013) Effect of functionalized nanosilica on the mechanical, dynamic-mechanical, and morphological performance of polycarbonate/nanosilica nanocomposites. Polym Eng Sci 53:1287–1296. doi:10.1002/pen.23388
Grieger KD, Laurent A, Miseljic M et al (2012) Analysis of current research addressing complementary use of life-cycle assessment and risk assessment for engineered nanomaterials: have lessons been learned from previous experience with chemicals? J Nanopart Res 14:958. doi:10.1007/s11051-012-0958-6
Project on Emerging Nanotechnologies (2014) Consumer Products Inventory
EP (2009) Regulation (EC) No 1223/2009 of the European Parliament and of the Council of 30 November 2009 on cosmetic products. Europe
EP (2011) Regulation (EU) No 1169/2011 of the European Parliament and the Council of 25 October 2011 on the provision of food information to consumers. Europe
Ministère de l’Écologie du Développement durable et de l’Énergie (2014) Éléments issus des déclarations des substances à l’état nanoparticulaire – exercice 2014. Agence nationale de sécurité sanitaire de l’alimentation, de l’environnement et du travail, Maisons-Alfort, France
Göhler D, Nogowski A, Fiala P, Stintz M (2013) Nanoparticle release from nanocomposites due to mechanical treatment at two stages of the life-cycle. J Phys Conf Ser 429:12045. doi:10.1088/1742-6596/429/1/012045
Hirth S, Cena L, Cox G et al (2013) Scenarios and methods that induce protruding or released CNTs after degradation of nanocomposite materials. J Nanopart Res 15:1504. doi:10.1007/s11051-013-1504-x
Kaegi R, Sinnet B, Zuleeg S et al (2010) Release of silver nanoparticles from outdoor facades. Environ Pollut 158:2900–2905. doi:10.1016/j.envpol.2010.06.009
Al-Kattan A, Wichser A, Vonbank R et al (2013) Release of TiO2 from paints containing pigment-TiO2 or nano-TiO2 by weathering. Environ Sci Process Impacts 15:2186–2193. doi:10.1039/c3em00331k
Kaegi R, Ulrich A, Sinnet B et al (2008) Synthetic TiO2 nanoparticle emission from exterior facades into the aquatic environment. Environ Pollut 156:233–239. doi:10.1016/j.envpol.2008.08.004
Al-Kattan A, Wichser A, Zuin S et al (2014) Behavior of TiO(2) released from Nano-TiO(2)-containing paint and comparison to pristine Nano-TiO(2). Environ Sci Technol 48:6710–6718. doi:10.1021/es5006219
Benn TM, Westerhoff P (2008) Nanoparticle silver released into water from commercially available sock fabrics. Environ Sci Technol 42:4133–4139
Benn T, Cavanagh B, Hristovski K et al (2010) The release of nanosilver from consumer products used in the home. J Environ Qual 39:1875. doi:10.2134/jeq2009.0363
Mitrano DM, Rimmele E, Wichser A et al (2014) Presence of nanoparticles in wash water from conventional silver and nano-silver textiles. ACS Nano 8:7208–7219. doi:10.1021/nn502228w
Kulthong K, Srisung S, Boonpavanitchakul K et al (2010) Determination of silver nanoparticle release from antibacterial fabrics into artificial sweat. Part Fibre Toxicol 7:8. doi:10.1186/1743-8977-7-8
von Goetz N, Lorenz C, Windler L et al (2013) Migration of Ag- and TiO2-(Nano)particles from textiles into artificial sweat under physical stress: experiments and exposure modeling. Environ Sci Technol 47:9979–9987. doi:10.1021/es304329w
Yan Y, Yang H, Li J et al (2012) Release behavior of nano-silver textiles in simulated perspiration fluids. Text Res J 82:1422–1429. doi:10.1177/0040517512439922
Sánchez C, Hortal M, Aliaga C et al (2014) Recyclability assessment of nano-reinforced plastic packaging. Waste Manag 34:2647–2655. doi:10.1016/j.wasman.2014.08.006
Touati N, Kaci M, Bruzaud S, Grohens Y (2011) The effects of reprocessing cycles on the structure and properties of isotactic polypropylene/cloisite 15 A nanocomposites. Polym Degrad Stab 96:1064–1073. doi:10.1016/j.polymdegradstab.2011.03.015
Struwe J, Schindler E, Pfirrmann O (2012) Relevance of nanomaterials in waste recycling. Hans-Böckler Foundation, Düsseldorf, Germany, AP270
Caballero-Guzman A, Sun T, Nowack B (2015) Flows of engineered nanomaterials through the recycling process in Switzerland. Waste Manag 36:33–43. doi:10.1016/j.wasman.2014.11.006
Holder AL, Vejerano EP, Zhou X, Marr LC (2013) Nanomaterial disposal by incineration. Environ Sci Process Impacts 15:1652–1664. doi:10.1039/c3em00224a
Mueller NC, Buha J, Wang J et al (2013) Modeling the flows of engineered nanomaterials during waste handling. Environ Sci Process Impacts 15:251. doi:10.1039/c2em30761h
Roes L, Patel MK, Worrell E, Ludwig C (2012) Preliminary evaluation of risks related to waste incineration of polymer nanocomposites. Sci Total Environ 417–418:76–86. doi:10.1016/j.scitotenv.2011.12.030
Walser T, Limbach LK, Brogioli R et al (2012) Persistence of engineered nanoparticles in a municipal solid-waste incineration plant. Nat Nanotechnol 7:520–524. doi:10.1038/nnano.2012.64
Vejerano EP, Leon EC, Holder AL, Marr LC (2014) Characterization of particle emissions and fate of nanomaterials during incineration. Environ Sci Nano. doi:10.1039/c3en00080j
Massari A, Beggio M, Hreglich S et al (2014) Behavior of TiO2 nanoparticles during incineration of solid paint waste: a lab-scale test. Waste Manag 34:1897–1907. doi:10.1016/j.wasman.2014.05.015
Lozano P, Berge ND (2012) Single-walled carbon nanotube behavior in representative mature leachate. Waste Manag 32:1699–1711. doi:10.1016/j.wasman.2012.03.019
Khan IA, Berge ND, Sabo-attwood T et al (2013) Single-walled carbon nanotube transport in representative municipal solid waste landfill conditions. Env Sci Technol 47:8425–8433. doi:10.1021/es401748f
Bolyard SC, Reinhart DR, Santra S, States U (2013) Behavior of engineered nanoparticles in landfill leachate. Environ Sci Technol 47:8114–8122. doi:10.1021/es305175e
Gitipour A, El Badawy A, Arambewela M et al (2013) The impact of silver nanoparticles on the composting of municipal solid waste. Environ Sci Technol 47:14385–14393. doi:10.1021/es402510a
Neale PA, Jämting ÅK, Escher BI, Herrmann J (2013) A review of the detection, fate and effects of engineered nanomaterials in wastewater treatment plants. Water Sci Technol 68:1440–1453. doi:10.2166/wst.2013.388
Yang Y, Wang Y, Westerhoff P et al (2014) Metal and nanoparticle occurrence in biosolid-amended soils. Sci Total Environ 485-486:441–449. doi:10.1016/j.scitotenv.2014.03.122
Ganzleben C, Pelsy F, Hansen SF, et al (2011) Review of environmental legislation for the regulatory control of nanomaterials. Directorate-General for Environment-European Commision, Brussels, Belgium, p. 210
Beaudrie CEH, Kandlikar M, Satterfield T (2013) From cradle-to-grave at the nanoscale: gaps in U.S. regulatory oversight along the nanomaterial life cycle. Environ Sci Technol 47:5524–5534. doi:10.1021/es303591x
EP (2008) Regulation (EC) No 1272/2008 of the European Parliament and of the Council of 16 December 2008 on classification, labelling and packaging of substances and mixtures, amending and repealing. Europe
EP (2008) Directive 2008/98/EC of the European Parliament and of the Council of 19 November 2008 on waste and repealing certain Directives. 3–30
Breggin LK, Pendergrass J (2007) Does the nano go? End-of-life regulation of nanotechnologies. Project on Emerging Nanotechnologies at the Woodrow Wilson International Center for Scholars, Washington, DC
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
González-Gálvez, D., Janer, G., Vilar, G., Vílchez, A., Vázquez-Campos, S. (2017). The Life Cycle of Engineered Nanoparticles. In: Tran, L., Bañares, M., Rallo, R. (eds) Modelling the Toxicity of Nanoparticles. Advances in Experimental Medicine and Biology, vol 947. Springer, Cham. https://doi.org/10.1007/978-3-319-47754-1_3
Download citation
DOI: https://doi.org/10.1007/978-3-319-47754-1_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-47752-7
Online ISBN: 978-3-319-47754-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)