Abstract
The diagnosis and treatment of colorectal cancer (CRC) have improved greatly over recent years; however, CRC is still one of the most common cancers and a major cause of cancer death worldwide. Several recently developed drugs and treatment strategies are currently in clinical trials; however, there is still a compelling need for novel, highly efficacious therapies. MicroRNAs (miRNAs) are short non-coding RNAs consisting of 20–25 nucleotides that regulate post-transcriptional gene expression by binding to the 3′-untranslated region of mRNAs. miRNAs are known to regulate cancer pathways and to be expressed aberrantly in cancer. Since their initial discovery, a large number of miRNAs have been identified as oncogenes, whereas others function as tumor suppressors. Furthermore, signaling pathways that are important in CRC (e.g. the WNT, MAPK, TGF-β, TP53 and PI3K pathways) are regulated by miRNAs. A single miRNA can simultaneously regulate several target genes and pathways, indicating the therapeutic potential of miRNAs in CRC. However, significant obstacles remain to be overcome, such as an efficient miRNA delivery system, and the assessment of safety and side effects. Thus, miRNA therapy is still developing and possesses great potential for the treatment of CRC. In this chapter, we focus on miRNAs related to CRC and summarize previous studies that emphasize the therapeutic aspects of miRNAs in CRC.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
The alterations of miRNA expressions can influence global gene expression networks, leading to drastic changes of cell fates including cancer initiation and progression. The aberrant miRNA expressions are observed in a wide variety of human malignancies, indicating a potential use of miRNAs as diagnostic markers and therapeutic targets. The natural endogenous expression and its remarkable stability make miRNAs a safe and efficient treatment option in cancer treatment. Now the global pharmaceutical market of miRNA-related therapy is huge and rapidly growing. It is predicted to reach 6 hundred million US dollars in 2014 and 10 hundred million in 2019. In this decade miRNA-targeting drugs have been developed all over the world, and some of them are already under investigation in preclinical randomized controlled trials. For examples, MRX34 , a double-stranded RNA mimic of miR-34a encapsulated in a liposomal nanoparticle formulation, has already been in clinical trials in patients with primary liver cancer or other selected solid tumors or hematologic malignancies [1]. Moreover, miravirsen and RG-101, effective inhibitors of liver specific miR-122 that the hepatitis C virus requires for replication, have also been in clinical trials. Miravirsen is a Locked Nucleic Acid (LNA)-modified oligonucleotide complementary to miR-122, and RG-101 is Regulus’ wholly-owned GalNAc-conjugated anti-miR-122 for the treatment of HCV.
However, systemic delivery technology of miRNAs as therapeutic targets/therapeutics for solid tumors has been obstructed by many limitations [2], including drug delivery systems , low specificity, adverse effects and miRNA instability.
This chapter focused on the molecular background and its clinical application of candidate miRNAs in colorectal cancer (CRC) (Table 13.1).
2 MicroRNAs Studied as Therapeutic Targets in Colorectal Cancer
2.1 miR-34a
Mutation of tumor suppressor p53 is observed in 50–75 % of CRCs [3]. Some miRNAs are known to be transcriptionally activated by p53 and exert its tumor suppressive effect through regulating a various kinds of targets [4]. miR-34a is one of the representative downstream molecules of p53. Target genes of miR-34a are associated with almost all kinds of biological processes including cell-cycle progression, apoptosis, DNA repair and angiogenesis . Upon DNA damage p53 directly activates miR-34a, and subsequent inhibition of miR-34a targets leads to a global cell protective response including cell cycle arrest and induction of apoptosis [5]. These anti-proliferative effect are disadvantage for cancers, therefore the pathway should be inactivated in tumors. Indeed, downregulation of miR-34a is a common feature of human malignancies including CRC.
Recent evidence suggests that p53-dependent expression of miR-34a blocks IL-6R/STAT3 /miR-34 feedback loop and consequently inhibit tumor progression in CRC [6]. As STAT3 and IL-6R play a central role in cancer proliferation , the restoration of miR-34a could be a useful treatment strategy for CRC. Nugent et al. have shown that the expression levels of miR-34a significantly decreased in CRC patients compared with healthy individuals, suggesting that miR-34a could be a useful biomarker as well as a therapeutic target in CRC [7, 8].
Notch signaling pathway is a critical regulator of asymmetric cell division, in which stem cells simultaneously generate both a daughter stem cell for self-renewal and a differentiated daughter cell to create cellular diversity [9–11]. Interestingly, recent study demonstrated that expression levels of miR-34a might define a cell division as symmetric or asymmetric [12]. High expression levels of miR-34a inhibit Notch signaling pathway and promote daughter cells to create non-CCSCs, whereas its low expression levels facilitate Notch signaling and promote daughter cells to remain CCSC. Because non-CCSCs are likely to susceptible to chemotherapy and irradiation, induction of miR-34a could be a useful therapeutic strategy through promoting asymmetric division rather than maintaining CSCs .
2.2 miR-135b
MiR-135b plays an important role as a key downstream effector of oncogenic pathways and could be a crucial therapeutic target in CRC [13]. Furthermore, anti-miR-135b therapy shows a promise because miR-135b expression in normal colorectal tissue and other organs is very low, in contrast to other miRNAs (e.g., miR-21). Another research showed that miR-135a/b target the 3′ untranslated region of APC , suppress its expression, and induce downstream Wnt pathway activity. This study showed a considerable up-regulation of miR-135a/b expressions in colorectal adenomas and carcinomas, which correlated with low APC mRNA levels [14]. Moreover, a recent study showed that miR-135b overexpression was triggered in mice and humans by APC loss, PTEN /PI3K pathway deregulation, and SRC overexpression and promoted tumor transformation and progression [13]. This study also demonstrated that miR-135b up-regulation was common in sporadic and inflammatory bowel disease-associated human CRCs and correlates with tumor stage and poor clinical outcome. Inhibition of miR-135b in CRC mouse models reduced tumor growth by controlling genes involved in proliferation , invasion , and apoptosis. These observations suggest that miR-135b is a key downstream effector of oncogenic pathways and a potential target for CRC treatment.
2.3 miR-143 , 145
Michael et al. first studied microRNAs changed in the adenomatous and cancer stages of colorectal neoplasia and identified that miR-143 and miR-145 act as potential tumor suppressors [15]. Consistent with this notions, the upregulation of the tumor suppressor miR-143 and miR-145 in post-therapeutic tumor tissue stand in line with the antitumor properties of the chemotherapy. This suggests that the expression levels of these miRs may be associated with prognosis or therapeutic outcome in CRC [16].
Both miR-143 and -145 have been shown to inhibit cell proliferation in vitro [17]. Moreover, it was reported that miR-143 directly binds to and suppresses KRAS , DNMT3A , and ERK5 and that miR-145 targets IRS-1, c-Myc, YES1, STAT1 and FLI1 [18]. In particular, administration of miR-143 potently inhibits colorectal tumor growth in xenograft mice models. miR-143 may be a promising option as potential miRNA therapeutics for colorectal tumors [17].
2.4 miR-101
The Wnt/β-catenin pathway is known to play a central role in an early colorectal carcinogenesis, where inactivation of the adenomatous polyposis coli (APC) gene is one of the major tumor initiating events. More than 60 % of colorectal adenomas and carcinomas, carries inactivating mutation in APC gene, which results in a stimulation of the Wnt/β-catenin pathway [3]. Recent evidence suggests that miRNAs represent a novel mechanism for WNT regulation in CRC. For example, miR-93 suppresses colorectal cancer development via downregulating Wnt/β-catenin pathway by partially targeting Smad7. It has been reported that activation of the Wnt/β-catenin pathway significantly induced miR-101 repression, which was reverted by blocking β-catenin activity [19]. Interestingly, miR-101 overexpression in CRC cells impaired β-catenin nuclear localization and inhibited the expression of stem/EMT-related genes, while miR-101 silencing exerted opposite effects in normal colon epithelial cells. These findings suggest that pharmacological restoration of miR-101 may inhibit the aggressive behavior of CRC.
2.5 miR-21
miR-21 is overexpressed in a wide variety of cancers including CRC [20, 21]. Recent meta-analysis revealed that circulating miR-21 is a useful diagnostic marker for CRC with adequate sensitivity and specificity [22]. Importantly, the expression levels of miR-21 in serum is elevated even in early diseases, indicating the possible use of miR-21 in early diagnosis [23, 24]. Mechanistically, miR-21 negatively regulates PDCD4 , which inhibits transformation and invasion in cancer. Asangani et al. identified a specific binding site for miR-21 in the PDCD4 3′-UTR at nucleotide position 228–249. Indeed, antisense oligonucleotides against miR-21 (Anti-miR-21) restored the expression levels of PDCD4 protein, leading to a remarkable inhibition of cancer migration, whereas overexpression of miR-21 promotes the invasive behavior of CRC cell lines [25]. A recent study also demonstrated that miR-21 is associated with invasive capacity of colorectal cancer cells through promoting nuclear translocation of β-catenin . Interestingly, this was only observed in adenomatous polyposis coli (APC) -mutated cells but not in APC-wild-type cells. CRC patients with high expression levels of serum miR-21 exhibit poorer prognosis in APC mutated cases, while this correlation was not observed in APC-wild type CRC patients [26]. Furthermore, Valeri et al. revealed that miR-21 confers resistance to 5-fluorouracil (5-FU) through downregulation of human MutS homolog 2 (MSH2). They also performed cell-cycle analysis and showed that G2/M arrest and apoptosis induced by 5-FU was decreased by overexpression of miR-21 [27]. miR-21 inhibitor (2′-F and 2′-MOE bicyclic sugar-modified antisense inhibitor) against hepatocellular carcinoma is currently being developed by Regulus Therapeutics [28]. Although the possible adverse effects of systemic induction of antisense oligonucleotides need to be overcome [29], anti-miR-21 therapy could be a promising therapeutic option in many types of cancers including CRC.
2.6 miRNAs Related to EGFR Signaling Pathway (KRAS and PI3K Pathways)
The epidermal growth factor receptor (EGFR) pathways including KRAS and PI3K contribute to promotion and progression of broad spectrum of solid tumors and it is a promising target for anticancer therapy [30]. The emerging role of EGFR signaling in cancers has led the development of anti-EGFR agents, including tyrosine kinase inhibitors (TKIs) and monoclonal antibodies against EGFR. Previously, it was considered that only patients with KRAS mutations in codons 12 and 13 of exon 2 did not have a response to anti-EGFR therapy . However, recent clinical studies revealed that other mutations in genes of the RAS family (KRAS exon 3 and 4 and NRAS exon 2, 3 and 4) are also associated with reduced response to anti-EGFR agents [31, 32]. In addition, it is estimated that 19.9 % of KRAS exon 2 wild-type tumors harbor at least one of these new RAS mutations [33]. Therefore, novel therapeutic strategies are urgently needed to treat CRC patients with RAS mutation. In this context, increasing numbers of evidence indicates that miRNAs are correlated with the drug resistance to anti-EGFR agents and regulate the EGFR signaling. For example, let-7 miRNA family has been reported to directly target KRAS oncogene [34]. Let-7 miRNA post-transcriptionally downregulates KRAS, and let-7 administration reduced tumor formation in animal cancer models expressing activating KRAS mutations . Higher let-7a expression was significantly associated with better survival outcomes in patients with mutant KRAS CRC who received salvage cetuximab (an anti-EGFR monoclonal antibody) plus irinotecan. These findings suggest that high let-7a microRNA levels in KRAS-mutated CRCs may rescue anti-EGFR therapy effects in patients with chemotherapy-refractory metastatic CRC [35].
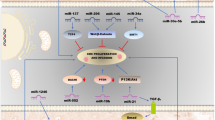
Another central signaling pathway downstream from EGFR and important in CRC development is the phosphatidylinositol-3-kinase (PI3K)-AKT pathway. Recent study revealed that KRAS, PIK3CD and BCL2 were identified as direct and functional targets of miR-30b. Moreover, miR-30b promoted G1 arrest and induced apoptosis, suppressing CRC cell proliferation in vitro and tumor growth in vivo. Expression analyses using CRC clinical samples showed that a low expression level of miR-30b was closely related to poor differentiation , advanced TNM stage and poor prognosis of CRC [36]. According to other recent studies, the p85β regulatory subunit involved in stabilizing and propagating the PI3K signal was demonstrated to be a direct target of miR-126 [37]. Furthermore, this p85β reduction mediated by miR-126 was accompanied by a substantial reduction in phosphorylated AKT levels in the cancer cells, suggesting a suppression of PI3K signaling. MiR-612 was also identified to directly target AKT2, which in turn inhibited the downstream epithelial-mesenchymal transition-related signaling pathway [38]. Comprehensive microarray profiled analysis identified miR-4689 as one of the significantly down-regulated miRNAs in mutated KRAS (G12V)- overexpressing cells [39]. MiR-4689 was found to exhibit potent growth-inhibitory and pro-apoptotic effects both in vitro and in vivo. Further analysis revealed that miR-4689 expression was significantly down-regulated in cancer tissues compared to normal mucosa, and it was particularly decreased in mutant KRAS CRC tissues. MiR-4689 directly targets both KRAS and AKT1, suggesting KRAS overdrives this signaling pathway through inhibition of miR-4689. These observations suggested that miR-4689 might be a promising therapeutic agent in mutant KRAS CRC (Fig. 13.1). Another important regulatory component of PI3K signaling pathway is a tumor suppressor gene PTEN (phosphatase and tensin homologue). Recent study revealed that PTEN was a direct target of miR-17-5p in CRC cells [40]. Overexpression of miR-17-5p promoted chemo-resistance and tumor metastasis of CRC by repressing PTEN expression. Gain and loss -of-function studies revealed that miR-32 directly target PTEN, suggesting that miR-32 was crucially involved in tumorigenesis of CRC at least in part by suppressing PTEN [41].
2.7 MiRNAs in TGF-β /Smad Signaling Pathway
The epithelial to mesenchymal transition (EMT) is a critical process in tumor invasion , metastasis , and tumorigenesis. Various signaling pathways can induce EMT and include key molecules such as transforming growth factor beta (TGF-\( \beta \)), platelet-derived growth factor (PDGF) , and the proteins nuclear factor kappa-light-chain-enhancer of activated B cells (NF-\( k \)B), Wnt, Notch and hedgehog proteins [42]. Among them, TGF-\( \beta \) is one of the major inducers of EMT. TGF-\( \beta \) binds to its receptors (TGF-\( \beta \)R), leading to the activation through phosphorylation of Smad. The complex is translocated into the nucleus where it regulates the expression of DNA binding factors, such as Snail , ZEB, and Twist . miRNAs are important regulators in controlling the TGF-\( \beta \)/Smad signaling pathway. Recently, miRNAs have been suggested to be involved in the acquisition of stem-cell-like properties for cancer cells by regulating EMT signaling. It is reported that TGF-\( \beta \)2 is a predominant target of the miR-200 family . Further study has demonstrated that miR-200c aberrantly expressed in metastatic colon tumor tissues and colon cancer cells [43]. This upregulated miR-200c was correlated with a reduction of the expression of its target genes: zinc finger E-box binding homeobox 1 (ZEB1) , which resulted in increased E-cadherin and reduced vimentin expression, sequentially led to an inhibition of EMT signaling pathway. In CRC cell lines, transfection of miR-200c precursors resulted in increased cell proliferation but reduced invasion and migration. Therefore, TGF-\( \beta \)/ZEB/miR-200 signaling regulatory network controls the plasticity between the epithelial and mesenchymal states of the CRC cells [42, 43]. Recent clinical cohort study revealed that miR-1269a expression was up-regulated in late-stage CRC and was associated with relapse and metastasis of disease-free 100 stage II CRC patients [44]. In vivo and in vitro experiments, SW480 cells treated with miR-1269a promoted CRC cells to undergo EMT and to metastasize. Furthermore, miR-1269a directly targeted Smad7 and HOXD10 to enhance TGF-β signaling, which in turn caused TGF-β mediated up-regulation of miR-1269a via Sox4. These indicate that TGF-β and miR-1269a constitute a positive feedback loop. Taken together, miR-1269a could be a potential marker for CRC patients as well as a potential therapeutic target to suppress metastasis.
Other kinds of upregulated miRNAs in CRCs, miR-130a/301a/454 family is also shown to regulate TGF-β signaling pathway through inhibiting SMAD4. Overexpression of these miRNAs enhanced cell proliferation and migration in HCT116 and SW480 colon cancer cells, while an inhibition decreased cell survival [45].
Another study demonstrated that miR-21 is involved in the maintenance of cancer stem cells by modulating transforming growth factor beta receptor 2 (TGFβR2) expression in colorectal cancer cells. Cell lines with increased fraction of cancer stem cells exhibit a relatively high expression of miR-21 [46].
3 Future Perspectives
Since the first study of miRNAs, a huge number of miRNAs have been studied as biomarkers and prognostic factors. However, only a small number of miRNAs are available as therapeutic tools. Against this background, a clinical trial of miR-34 mimics (MRX34 ) against hepatocellular carcinoma and metastatic liver cancer is now in phase I (ClinicalTrials.gov identifier: NCT01829971). The limited number of miRNAs available as therapeutic tools might be due to several factors. First, since miRNAs are short noncoding RNAs of 20–25 nucleotides, one miRNA could regulate several target genes transcriptionally, indicating the difficulty of targeting specific genes. At the same time, this nonspecificity leads to the possibility that one miRNA could regulate several targets and pathways simultaneously. To overcome this issue, further studies are necessary to elucidate the real therapeutic target miRNAs, which might avoid side effects of this therapy. Second, the optimal system for delivering miRNAs has not been established yet. In some in vivo studies, nanomolecules were used and their efficacy was reported (e.g. polymer nanoparticles, lipid nanoparticles , and liposomes ). Recently, a new anti-miR delivery system was reported, which showed that anti-miRNAs with a low-pH-induced transmembrane structure (pHLIP) were efficiently delivered to the tumor in lymphoma cases [47]. This method could transport anti-miRNAs through the plasma membrane under acidic conditions and then deliver miRNAs specifically to tumors. Additionally, two clinical trials using Dicer substrate short-interfering RNA (DsiRNATM) are ongoing (ClinicalTrials.gov identifiers: NCT02110563 and NCT02314052). DsiRNAs are synthesized 27mer RNA duplexes that are processed by Dicer into 21mer siRNAs . This new treatment related to microRNA biogenesis is also thought to improve the delivery of miRNAs to specific targets. Thus, the systems for delivering miRNAs are continuing to advance, but further investigations are necessary for their actual use in clinical practice.
On the other hand, as mentioned previously, several target miRNAs for the therapy of CRC were elucidated and directly used for anti-miRNA therapy in vivo. Furthermore, some miRNAs (e.g. miR-17-5p, miR-140, and miR-192) have also been reported to be associated with chemotherapy resistance, which indicates the possibility of combination therapy with miRNAs and anticancer drugs. Thus, miRNA therapy has great potential to expand the therapeutic options for CRC. Although several obstacles to this still remain, miRNA therapy should lead to novel discoveries relevant to the diagnosis and treatment of CRC.
References
Misso G, Di Martino MT, De Rosa G, Farooqi AA, Lombardi A, Campani V, et al. Mir-34: a new weapon against cancer? Mol Ther Nucleic Acids. 2014;3:e194.
Decuzzi P, Causa F, Ferrari M, Netti PA. The effective dispersion of nanovectors within the tumor microvasculature. Ann Biomed Eng. 2006;34:633–41.
Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759–67.
Hermeking H. MicroRNAs in the p53 network: micromanagement of tumour suppression. Nat Rev Cancer. 2012;12:613–26.
Chang TC, Wentzel EA, Kent OA, Ramachandran K, Mullendore M, Lee KH, et al. Transactivation of miR-34a by p53 broadly influences gene expression and promotes apoptosis. Mol Cell. 2007;26:745–52.
Rokavec M, Oner MG, Li H, Jackstadt R, Jiang L, Lodygin D, et al. IL-6R/STAT3/miR-34a feedback loop promotes EMT-mediated colorectal cancer invasion and metastasis. J Clin Invest. 2014;124:1853–67.
Nugent M, Miller N, Kerin MJ. Circulating miR-34a levels are reduced in colorectal cancer. J Surg Oncol. 2012;106:947–52.
Hahn S, Jackstadt R, Siemens H, Hunten S, Hermeking H. SNAIL and miR-34a feed-forward regulation of ZNF281/ZBP99 promotes epithelial-mesenchymal transition. EMBO J. 2013;32:3079–95.
Neumuller RA, Knoblich JA. Dividing cellular asymmetry: asymmetric cell division and its implications for stem cells and cancer. Genes Dev. 2009;23:2675–99.
Sugiarto S, Persson AI, Munoz EG, Waldhuber M, Lamagna C, Andor N, et al. Asymmetry-defective oligodendrocyte progenitors are glioma precursors. Cancer Cell. 2011;20:328–40.
Clevers H. The cancer stem cell: premises, promises and challenges. Nat Med. 2011;17:313–9.
Bu P, Chen KY, Chen JH, Wang L, Walters J, Shin YJ, et al. A microRNA miR-34a-regulated bimodal switch targets Notch in colon cancer stem cells. Cell Stem Cell. 2013;12:602–15.
Valeri N, Braconi C, Gasparini P, Murgia C, Lampis A, Paulus-Hock V, et al. MicroRNA-135b promotes cancer progression by acting as a downstream effector of oncogenic pathways in colon cancer. Cancer Cell. 2014;25:469–83.
Nagel R, le Sage C, Diosdado Bvan der Waal M, Oude Vrielink JA, Bolijn A, et al. Regulation of the adenomatous polyposis coli gene by the miR-135 family in colorectal cancer. Cancer Res. 2008; 68:5795–802.
Michael MZ, O’ Connor SM, van Holst Pellekaan NG, Young GP, James RJ. Reduced accumulation of specific microRNAs in colorectal neoplasia. Mol Cancer Res. 2003;1:882–91.
Drebber U, Lay M, Wedemeyer I, Vallbohmer D, Bollschweiler E, Brabender J, et al. Altered levels of the onco-microRNA 21 and the tumor-supressor microRNAs 143 and 145 in advanced rectal cancer indicate successful neoadjuvant chemoradiotherapy. Int J Oncol. 2011;39:409–15.
Akao Y, Nakagawa Y, Hirata I, Iio A, Itoh T, Kojima K, et al. Role of anti-oncomirs miR-143 and -145 in human colorectal tumors. Cancer Gene Ther. 2010;17:398–408.
Schetter AJ, Okayama H, Harris CC. The role of microRNAs in colorectal cancer. Cancer J. 2012;18:244–52.
Strillacci A, Valerii MC, Sansone P, Caggiano C, Sgromo A, Vittori L, et al. Loss of miR-101 expression promotes Wnt/beta-catenin signalling pathway activation and malignancy in colon cancer cells. J Pathol. 2013;229:379–89.
Kanaan Z, Rai SN, Eichenberger MR, Roberts H, Keskey B, Pan J, et al. Plasma miR-21: a potential diagnostic marker of colorectal cancer. Ann Surg. 2012;256:544–51.
Pan X, Wang ZX, Wang R. MicroRNA-21. A novel therapeutic target in human cancer. Cancer Biol Ther. 2010;10:1224–32.
Xu F, Xu L, Wang M, An G, Feng G. The accuracy of circulating microRNA-21 in the diagnosis of colorectal cancer: a systematic review and meta-analysis. Color Dis. 2015;17:O100–7.
Yamada A, Horimatsu T, Okugawa Y, Nishida N, Honjo H, Ida H, et al. Serum miR-21, miR-29a, and miR-125b are promising biomarkers for the early detection of colorectal neoplasia. Clin Cancer Res. 2015;21:4234–42.
Toiyama Y, Takahashi M, Hur K, Nagasaka T, Tanaka K, Inoue Y, et al. Serum miR-21 as a diagnostic and prognostic biomarker in colorectal cancer. J Natl Cancer Inst. 2013;105:849–59.
Asangani IA, Rasheed SA, Nikolova DA, Leupold JH, Colburn NH, Post S, et al. MicroRNA-21 (miR-21) post-transcriptionally downregulates tumor suppressor Pdcd4 and stimulates invasion, intravasation and metastasis in colorectal cancer. Oncogene. 2008;27:2128–36.
Lin PL, Wu DW, Huang CC, He TY, Chou MC, Sheu GT, et al. MicroRNA-21 promotes tumour malignancy via increased nuclear translocation of beta-catenin and predicts poor outcome in APC-mutated but not in APC-wild-type colorectal cancer. Carcinogenesis. 2014;35:2175–82.
Valeri N, Gasparini P, Braconi C, Paone A, Lovat F, Fabbri M, et al. MicroRNA-21 induces resistance to 5-fluorouracil by down-regulating human DNA MutS homolog 2 (hMSH2). Proc Natl Acad Sci U S A. 2010;107:21098–103.
Wagenaar TR, Zabludoff S, Ahn SM, Allerson C, Arlt H, Baffa R, et al. Anti-miR-21 suppresses hepatocellular carcinoma growth via broad transcriptional network deregulation. Mol Cancer Res. 2015;13:1009–21.
Huang L, Wang X, Wen C, Yang X, Song M, Chen J, et al. Hsa-miR-19a is associated with lymph metastasis and mediates the TNF-alpha induced epithelial-to-mesenchymal transition in colorectal cancer. Sci Rep. 2015;5:13350.
Ciardiello F, Tortora G. EGFR antagonists in cancer treatment. N Engl J Med. 2008;358:1160–74.
Douillard JY, Oliner KS, Siena S, Tabernero J, Burkes R, Barugel M, et al. Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med. 2013;369:1023–34.
Schwartzberg LS, Rivera F, Karthaus MF, Asola G, Canon JL, Hecht JR, et al. PEAK: a randomized, multicenter phase II study of panitumumab plus modified fluorouracil, leucovorin, and oxaliplatin (mFOLFOX6) or bevacizumab plus mFOLFOX6 in patients with previously untreated, unresectable, wild-type KRAS exon 2 metastatic colorectal cancer. J Clin Oncol. 2014;32:2240–7.
Sorich MJ, Wiese MD, Rowland A, Kichenadasse G, McKinnon RA, Karapetis CS. Extended RAS mutations and anti-EGFR monoclonal antibody survival benefit in metastatic colorectal cancer: a meta-analysis of randomized, controlled trials. Ann Oncol. 2015;26:13–21.
Johnson SM, Grosshans H, Shingara J, Byrom M, Jarvis R, Cheng A, et al. RAS is regulated by the let-7 microRNA family. Cell. 2005;120:635–47.
Ruzzo A, Graziano F, Vincenzi B, Canestrari E, Perrone G, Galluccio N, et al. High let-7a microRNA levels in KRAS-mutated colorectal carcinomas may rescue anti-EGFR therapy effects in patients with chemotherapy-refractory metastatic disease. Oncologist. 2012;17:823–9.
Liao WT, Ye YP, Zhang NJ, Li TT, Wang SY, Cui YM, et al. MicroRNA-30b functions as a tumour suppressor in human colorectal cancer by targeting KRAS, PIK3CD and BCL2. J Pathol. 2014;232:415–27.
Guo C, Sah JF, Beard L, Willson JK, Markowitz SD, Guda K. The noncoding RNA, miR-126, suppresses the growth of neoplastic cells by targeting phosphatidylinositol 3-kinase signaling and is frequently lost in colon cancers. Genes Chromosomes Cancer. 2008;47:939–46.
Sheng L, He P, Yang X, Zhou M, Feng Q. miR-612 negatively regulates colorectal cancer growth and metastasis by targeting AKT2. Cell Death Dis. 2015;6:e1808.
Hiraki M, Nishimura J, Takahashi H, Wu X, Takahashi Y, Miyo M, et al. Concurrent targeting of KRAS and AKT by MiR-4689 is a novel treatment against mutant KRAS colorectal cancer. Mol Ther Nucleic Acids. 2015;4:e231.
Fang L, Li H, Wang L, Hu J, Jin T, Wang J, et al. MicroRNA-17-5p promotes chemotherapeutic drug resistance and tumour metastasis of colorectal cancer by repressing PTEN expression. Oncotarget. 2014;5:2974–87.
Wu W, Yang J, Feng X, Wang H, Ye S, Yang P, et al. MicroRNA-32 (miR-32) regulates phosphatase and tensin homologue (PTEN) expression and promotes growth, migration, and invasion in colorectal carcinoma cells. Mol Cancer. 2013;12:30.
Zaravinos A. The regulatory role of MicroRNAs in EMT and cancer. J Oncol. 2015;2015:865816.
Hur K, Toiyama Y, Takahashi M, Balaguer F, Nagasaka T, Koike J, et al. MicroRNA-200c modulates epithelial-to-mesenchymal transition (EMT) in human colorectal cancer metastasis. Gut. 2013;62:1315–26.
Bu P, Wang L, Chen KY, Rakhilin N, Sun J, Closa A, et al. miR-1269 promotes metastasis and forms a positive feedback loop with TGF-beta. Nat Commun. 2015;6:6879.
Wang J, Du Y, Liu X, Cho WC, Yang Y. MicroRNAs as regulator of signaling networks in metastatic colon cancer. Biomed Res Int. 2015;2015:823620.
Yu Y, Kanwar SS, Patel BB, Oh PS, Nautiyal J, Sarkar FH, et al. MicroRNA-21 induces stemness by downregulating transforming growth factor beta receptor 2 (TGFbetaR2) in colon cancer cells. Carcinogenesis. 2012;33:68–76.
Cheng CJ, Bahal R, Babar IA, Pincus Z, Barrera F, Liu C, et al. MicroRNA silencing for cancer therapy targeted to the tumour microenvironment. Nature. 2015;518(7537):107–10.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Yamamoto, H., Mori, M. (2016). MicroRNAs as Therapeutic Targets and Colorectal Cancer Therapeutics. In: Slaby, O., Calin, G. (eds) Non-coding RNAs in Colorectal Cancer. Advances in Experimental Medicine and Biology, vol 937. Springer, Cham. https://doi.org/10.1007/978-3-319-42059-2_13
Download citation
DOI: https://doi.org/10.1007/978-3-319-42059-2_13
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-42057-8
Online ISBN: 978-3-319-42059-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)