Abstract
The activation of microglia has been recognized for over a century by their morphological changes. Long slender microglia acquire a short sturdy ramified shape when activated. During the past 20 years, microglia have been accepted as an essential cellular component for understanding the pathogenic mechanism of many brain diseases, including neurodegenerative diseases. More recently, functional studies and imaging in mouse models indicate that microglia are active in the healthy central nervous system. It has become evident that microglia release several signal molecules that play key roles in the crosstalk among brain cells, i.e., astrocytes and oligodendrocytes with neurons, as well as with regulatory immune cells. Recent studies also reveal the heterogeneous nature of microglia diverse functions depending on development, previous exposure to stimulation events, brain region of residence, or pathological state. Subjects to approach by future research are still the unresolved questions regarding the conditions and mechanisms that render microglia protective, capable of preventing or reducing damage, or deleterious, capable of inducing or facilitating the progression of neuropathological diseases. This novel knowledge will certainly change our view on microglia as therapeutic target, shifting our goal from their general silencing to the generation of treatments able to change their activation pattern.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
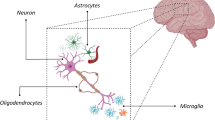
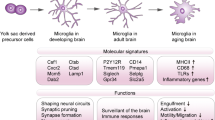
Microglia are the resident immune cells of the central nervous system (CNS), accounting for approximately 10 % of the total cell number in the healthy mammalian brain (Prinz and Priller 2014). They derive from myeloid progenitors, being related to peripheral monocyte-macrophages (Ginhoux et al. 2010). Microglial cell progenitors originating in the yolk sac migrate and colonize the CNS during embryonic development , before the blood–brain barrier is established, differentiating and becoming confined into the CNS. Throughout the life, microglia appear to be capable of local self-renewal. Adult healthy animals show very little exchange between blood and brain parenchyma (Mildner et al. 2007). Thus, maintenance of their population normally does not depend on recruitment of circulating progenitors. However, monocytes invading the brain have been observed under pathological conditions, such as blood–brain barrier damage by trauma or severe inflammation and ischemic vascular damage (Ajami et al. 2007; Casano and Peri 2015; Kierdorf et al. 2013), where they transform into microglia with a ramified phenotype (Mildner et al. 2007). The environment provided by the brain parenchyma appears to be key for microglia phenotype. Astrocytes-conditioned medium induces morphological and functional changes of microglia and blood monocytes in culture (Sievers et al. 1994; Ramirez et al. 2005; Tichauer et al. 2007; von Bernhardi and Ramírez 2001; Orellana et al. 2013), an effect that is at least partly mimicked by adenosine triphosphate (ATP) or adenosine. Other mediators capable of modifying microglia activation are cytokines released from astrocytes, including transforming growth factor β (TGFβ), macrophage colony-stimulating factor (M-CSF), and granulocyte/macrophage colony stimulating factor (GM-CSF) (Schilling et al. 2001; Alarcón et al. 2005; Flores and von Bernhardi 2012; Herrera-Molina et al. 2012; Tichauer et al. 2014; Tichauer and von Bernhardi 2012).
Microglia, as member of the monocyte-macrophage family, function as mononuclear phagocytes, recognizing and scavenging dead cells, pathogens and several endogenous, and exogenous compounds. As mentioned in Chapter “Glial Cells and Integrity of the Nervous System,” under physiological conditions and in the absence of inflammatory stimuli, microglia are found in a “surveillance state,” morphologically defined by having a small soma with long fine-ramified processes. Surveillance microglia are highly dynamic, retracting and extending their processes in response to environmental cues, interacting with blood vessels, neurons, ependymal cells, and other glial cells (Nimmerjahn et al. 2005; Ramirez et al. 2005; Chen and Trapp 2015; Heneka et al. 2015). They express constitutive markers like Iba-1, and several other markers, such as MHC-I, MHC-II, FcR, CD68, depending on the environmental cues they sense (Fig. 1), being involved in antigen presentation, cytotoxic activation, phagocytosis, antibody-associated phagocytosis, etc. An astrocyte labeled with antibody against glial fibrillary acidic protein (GFAP) is also included to compare their morphologies. Microglial cell surveillance is highly relevant for CNS development and function throughout life.
Labeling of glia by activation markers. Immunohistochemical detection of glia activation markers in hipocampal cryosections obtained from unstimulated adult mice counterstaining with Harris Haematoxylin. Iba-1 and GFAP antibodies identified microglia and activated astrocytes, respectively. MHC-I, MHC-II, FcR, and CD68 identify microglia populations that are functionally different, showing differences in their morphology as well as in the labeling pattern. Scale bar = 10 µm
Microglia Motility and Migration
Microglia exhibit two types of motility: the active movement of their processes, sensing the environment, and their translocation in the brain parenchyma. Migration is frequently observed during development, when invading cells migrate into the CNS, and when recruited after an insult and migrate to the site of injury/stimulation. As discussed in Chapters “Glial Cells and Integrity of the Nervous System and Purine Signaling and Microglial Wrapping,” many molecules appear to signal for microglia migration, including ATP, cannabinoids, chemokines, lysophosphatidic acid, bradykinin, ion channels, and transporters (Davalos et al. 2005; Walter et al. 2003; Schwab 2001; Rappert et al. 2002; Schilling et al. 2004; Ifuku et al. 2007).
Although under nonstimulated conditions they do not migrate, real-time imaging reveals that microglial processes are constantly moving (Davalos et al. 2005; Nimmerjahn et al. 2005). Processes move rapidly toward an injury. Time-lapse microscopy of brain slices from adult mice shows extensive migration of microglia 24 h after an injury (Carbonell et al. 2005).
Recruitment of microglia to a lesion involves several factors including chemokines released from both neurons and glial cells, among others. Most of the chemokines are released as soluble factors that form chemotactic gradients for cell migration, although CX3CL1 (fractalkine) occurs also as a surface-bound molecule. Microglial activation following neuropathological challenges affects the expression of chemokine receptors (Kremlev et al. 2004), acting as a source and a target of chemokines in an auto/paracrine fashion. Activation of CXCR3 receptor by chemokine CCL21 is linked to microglial migration (Rappert et al. 2002), and the CCL2/CCR2 system appears to be crucial for the recruitment of peripheral monocytes to the CNS, where they become microglia (Davoust et al. 2008; Mildner et al. 2007; Prinz and Priller 2010). Expression of CCR, receptor for CCL2 ligands, identifies functional subsets of microglia. The CX3CR1, the receptor for fractalkine, is also a key molecule for the CNS-relevant macrophage subclassification (Prinz and Priller 2010).
It is especially interesting that chemokines including CCL2, CCL21, or CX3CL1 also appear to serve as signals from endangered neurons to microglia (Biber et al. 2008). It has been suggested that CX3CL1 expressed by neurons could provide a constitutive calming influence on CX3CR1-expressing microglia, thus representing a neuron-to-microglia signaling system similar to those described for CD200/CD200R or CD47/SIRP-1α. Interruption of this regulatory mechanism could facilitate enhanced responses to activating signals. In fact, deficiency in fractalkine signaling results in enhanced severity of CNS damage in several disease models (Cardona et al. 2006; Prinz and Priller 2010). Similarly, activation of CCR5 by the chemokine CCL5 “regulated on activation, normal T cell expressed and secreted” (RANTES), supresses lipopolysaccharide (LPS)-induced expression of inflammatory cytokines , such as interleukin (IL)1β, IL6 and tumor necrosis factor (TNF)α, and inducible nitric oxide synthase (iNOS) in microglia. In contrast, motor neuron death after nerve injury is accelerated in CCR5 knock-out animals, suggesting that CCR5-mediated suppression of microglia toxicity protects neurons (Gamo et al. 2008).
Microglia-Mediated Phagocytosis
Microglia are the professional phagocytes of the CNS. Phagocytosis is a key function during development as well as in the normal and pathological adult brain (Neumann et al. 2009). During development, microglia remove apoptotic cells, mediated by an “eat me” signal produced by apoptotic cells to microglia (Marin-Teva et al. 2004). They are also involved in synapse removal (Stevens et al. 2007) and in pruning synapses in the developing and postnatal brain (see Chapter “Purine Signaling and Microglial Wrapping” for a complete description on microglial wrapping).
Phagocytosis depends on different mechanisms (Table 1). Pathogens are recognized by Toll-like receptors (TLRs), and apoptotic neurons are recognized by various receptor systems, including asialoglycoprotein-like-, vitronectin-, and phosphatidylserine receptors (Witting et al. 2000). Multiple factors regulate phagocytosis, including ATP, through the metabotropic P2Y6 receptor (Inoue et al. 2009). The P2Y6 receptor is upregulated when neurons are damaged and could be a trigger for phagocytosis (Koizumi et al. 2007). In contrast, activation of P2X7 receptors suppresses phagocytosis, whereas inhibition of P2X7 expression by shRNA or oxATP/BBG restores phagocytosis (Fang et al. 2009). The ciliary neurotrophic factor (CNTF), glia derived neurotrophic factor (GDNF), and M-CSF potentiates phagocytic by microglia (Chang et al. 2006; Lee et al. 2009; Mitrasinovic and Murphy 2003). Substrate-bound complement component C1q enhance both FcR and CR1-mediated phagocytosis (Webster et al. 2000), whereas the prostanoid receptor subtype 2 (EP2), downregulates phagocytosis (Liang et al. 2005; Shie et al. 2005).
As the resident immune cells of the CNS, microglia are the first line of defense against exogenous threats. The pattern recognition receptors (PRRs), abundantly expressed in microglia, detect infectious agents and assist in the control of the adaptive immunity and the cooperative activities of effector cells (Beurel et al. 2010; Hanisch et al. 2008; Padovan et al. 2007). In addition to pathogen detection by pathogen-associated molecular patterns (PAMPs), several PRRs, including TLRs, also bind endogenous molecules that are generated or modified upon tissue injury. These molecules are classified as damage- or danger-associated molecular patterns (DAMPs) (Bianchi 2007; Kono and Rock 2008; Matzinger 2007). The TLRs 1–9 and co-receptors, like CD14 are widely expressed in cells of the innate as well as adaptive immune system, but also in nonimmune cells (Schaffler et al. 2007). In the brain, TLRs are mainly expressed in glia, although some has been detected in neurons (Aravalli et al. 2007b; Carpentier et al. 2008; Hanisch et al. 2008; Okun et al. 2009; Konat et al. 2006).
The stimulation of TLRs triggers various programs of microglial activation and activates secretion of cytokines and chemokines (Aravalli et al. 2007b; Okun et al. 2009). Several reports indicate the importance of TLRs in various CNS diseases including infection, trauma, stroke, neurodegeneration, and autoimmunity (Babcock et al. 2006; Caso et al. 2007; Lehnardt et al. 2002; Nau and Bruck 2002; Nguyen et al. 2004).
Participation of Microglia in Development
Microglia are intimately involved in the development of the nervous system (Table 1). They have roles both in neurogenesis and neuronal death. Microglia appears to have both detrimental and supportive effects on neurogenesis (Ekdahl et al. 2009), which could depend in the activation state of microglia (Schwartz et al. 2006). Differentiation of neural precursors in culture requires the presence of soluble factors secreted by microglia (Nakanishi et al. 2007; Walton et al. 2006). Those factors are also involved in directing migration of newly generated neural cells (Aarum et al. 2003).
The role of microglia for neuronal loss by programmed cell death during development has been described in several brain regions, including the retina, where the pro-apoptotic action of microglia is mediated through nerve growth factor (NGF) (Frade and Barde 1998). Similarly, microglia induce apoptotic death of Purkinje neurons by releasing superoxide ions (Marin-Teva et al. 2004), and motoneurons apoptosis via secretion of TNFα in the embryo (Sedel et al. 2004).
In early postnatal development, elimination of excess synapses—known as synaptic pruning—appears also to be a microglia-mediated mechanism. Mice lacking fractalkine receptor (CX3CR1), have reduced numbers of brain microglia, and show impairment of synaptic pruning, resulting in an abnormally high number of synaptic spines (Paolicelli et al. 2011).
On the other hand, microglia are also involved in the formation of new synapses, especially in the early postnatal brain. Microglia stimulate synaptogenesis by secreting the extracellular matrix proteins thrombospondins (TSPs) (Moller et al. 1996), which are also produced by astrocytes (Christopherson et al. 2005). TSP1 interacts with the integrin-associated protein CD47, which is regulated by signal regulatory protein (SIRP)α, a transmembrane protein expressed by neurons and macrophages (Matozaki et al. 2009). The SIRPα-CD47 complex is involved in the regulation of migration and phagocytosis, immune homeostasis, and neuronal networks, playing homeostatic roles in the immune system, and participating in synaptic patterning (Umemori and Sanes 2008). Microglia also serve roles on the functional maturation of synapses (Paolicelli and Gross 2011). Behavioral abnormalities, including impairment of social interaction and autistic-like behavior (Tang et al. 2014; Zhan et al. 2014) have been reported on several models of microglial cell dysfunction.
Participation of Microglia in Adult Life
As the name implies, surveillance microglia actively survey the parenchyma, to rapidly activate upon appearance of a threat to the CNS. Microglial activation in response to various stimuli correlates with conspicuous morphological changes. Microglia reduce the complexity and shortens their branched processes (Fig. 2). Several stages can be identified, including process withdrawal, and formation of new processes allowing mobility in the tissue (Lynch 2009; Stence et al. 2001; Streit et al. 2005).
Inflammatory activation-dependent morphological changes of microglia. Immunohistochemical labeling of the constitutive identity marker Iba-1 and the phagocytic activation-specific marker CD68, and counterstaining with Harris Haematoxylin, of hipocampal cryosections obtained from inflammatory unstimulated and stimulated young mice. Low (4×) magnification microphotographs of hippocampal section labeled with CD68 show slender-shaped microglia evenly distributed. In contrast, the distribution of microglia in the hippocampus becomes more cluster-like. At high magnification, activated microglia shows shorter sturdier processes. CD68-labeled microglia show an amoeba-like shape with a big cell body and very short processes, whereas Iba-1 shows many cells with long, although studier processes than those observed in unstimulated animales. Scale bar = 100 µm in the right panel and 10 µm in the higher magnification microphotographs at the left
Microglia is a nonhomogeneous population, their activation being a highly regulated process. Thus, activated microglia can acquire distinct functional states (Hanisch and Kettenmann 2007; Perry et al. 2007; Perry and Holmes 2014; Schwartz et al. 2006). Activation is not an all-or-none process, but varies depending on the stimulation context (Hanisch and Kettenmann 2007; von Bernhardi et al. 2015b; Areschoug and Gordon 2009). Multiple signals converge to maintain or change their functional state and to regulate their specific functional repertoire (Table 1). Activation is triggered when microglia detect the appearance, abnormal concentration, or altered format of molecules that serve as signals (Block and Hong 2005; Block et al. 2007; Hanisch and Kettenmann 2007). The involvement of two signaling systems has been proposed, an “on” receptor-mediated signaling corresponding to a novel molecule that is recognized by microglia triggering activation; and an “off” receptor-mediated signaling that persistently signals to maintain microglia in a certain default activation state (Biber et al. 2007; Hanisch and Kettenmann 2007; Kettenmann et al. 2011).
“On” signals include structures associated with bacterial cell walls, viral envelopes, or their DNAs and RNAs, typically identified as signs of infection. Pathogen structures are sensed through PRRs, such as TLRs (Hanisch et al. 2008) and Scavenger Receptors (SRs) (Ozeki et al. 2006; Godoy et al. 2012; Murgas et al. 2014). Molecules released after tissue damage are also signals, and they induce especially robust microglial responses (Nimmerjahn et al. 2005; Lu et al. 2010; Napoli and Neumann 2009). Intracellular proteins or serum factors can activate microglia when they are induced upon stress, appear in new compartments or suffer biochemical modifications (Hanisch et al. 2008; Lehnardt et al. 2008; Rubartelli and Lotze 2007), as well as some neurotransmitters indicating impaired neuronal activity (Boucsein et al. 2003; Haynes et al. 2006). This, both pathogen- and damage-associated molecular patterns (PAMPs/DAMPs, respectively), activate microglia.
The “off” receptor-mediated signaling is due to the loss of constitutive control signaling in the normal CNS, as observed with ligand-receptor systems CD200-CD200R, CX3CL1- CX3CR1, and CD172a-CD47 (Barclay et al. 2002; Brooke et al. 2004; Cardona et al. 2006; Hoek et al. 2000). Thus, the “on signals” are identified as a sign of threat to the CNS homeostasis. Whereas in the “off signal,” the loss of regulation is the signal.
The CNS show regional variations in glial and neuronal cell populations as well as in their environment. For example, the different vulnerability of CA1 versus CA3 neurons depends on the regional microglia response upon stimulation (Hanisch and Kettenmann 2007), with hippocampal neuronal cell death and glial activation depending on the chemokine/receptor system CXCL10/CXCR3 (van Weering et al. 2011).
As discussed in Chapter “Glial Cells and Integrity of the Nervous System,” acute self-limited activation of microglia should be deemed as protective, given microglia primarily support and protect the structural and functional integrity of the CNS. Although research has mostly focused on the detrimental consequences of microglia-mediated neuroinflammation , and their potentiation of neuronal damage, it is now accepted that microglia activation is important for protection and repair of the diseased and injured brain. However, the final outcome will depend on the environmental context and timeframe of action (Hellwig et al. 2013; Kierdorf and Prinz 2013; von Bernhardi et al. 2015b). When encountering a mild injury or impairment, microglia could act immediately to repair and offer trophic support, and even reduce activating synaptic input by remodeling synapses (Trapp et al. 2007; Wake et al. 2009). However, the everyday activity of microglia is very difficult to assess (Hanisch and Kettenmann 2007). Thus, in general there is much more evidence on the failure and harmful contributions of microglia than on their physiological roles.
Microglia serve several functional roles, modulating neuronal activity and viability in culture and in the adult brain through direct contact with neurons (Li et al. 2012; Kohman et al. 2013) and through their release of soluble mediators, including cytokines and reactive species (Herrera-Molina and von Bernhardi 2005; Ramírez et al. 2008; Ramirez et al. 2005; Tichauer et al. 2007; von Bernhardi and Eugenin 2004; Glass et al. 2010; Di Filippo et al. 2010; von Bernhardi and Eugenin 2012).
Microglia play an active role in the functional integrity of the CNS and its normal physiological performance even affecting learning and behavior (Ziv et al. 2006; Ziv and Schwartz 2008), through their effect, together with T cells, at various levels. Both synaptic contacts and neuron trophism could depend on factors produced by activated microglia. Microglia express several neurotrophins (Elkabes et al. 1996; Kim and de Vellis 2005; Ferrini and De Koninck 2013), releasing many factors with powerful neurotrophic actions (Morgan et al. 2004). A number of cytokines appear to have roles in the maturating CNS. In the adult CNS, IL1 drives astrocytes proliferation in response to injury (Giulian et al. 1988).
Microglia also contribute to the plasticity of the CNS through support of neurogenesis in adult individuals (Butovsky et al. 2006; Ziv et al. 2006; McPherson et al. 2011; Ekdahl et al. 2003), which appears to depend on certain subpopulations of microglia (Ribeiro Xavier et al. 2015). Microglia have neurotransmitter receptors and are responsive to serotonin (5-HT) (Pocock and Kettenmann 2007) and cytokine levels, and can influence precursor cells, showing a positive regulation of neurogenesis by 5-HT and a negative regulation by stress and elevated glucocorticoids (Kempermann 2002; Kempermann and Kronenberg 2003). Thus, under certain conditions, microglia can adopt a pro-neurogenic phenotype, which involves the expression of neurotrophins and regulatory cytokines, such as insulin-like growth factor 1 (IGF1), BDNF, and IL4 (Parkhurst et al. 2013; Chen and Trapp 2015; Ribeiro Xavier et al. 2015). However, in inflammatory activation states, microglia consistently appears to inhibit neurogenesis (Monje et al. 2003; Nakanishi et al. 2007).
Similar to the synaptic pruning observed during development , microglia keep a structural role in circuit refinement throughout life. The role of microglia in removing synapses, is known as “synaptic stripping.” It is also observed in response to focal inflammation (Trapp et al. 2007). The “stripping” predominantly removes excitatory glutamatergic synapses, thus limiting neuronal excitability and glutamate excitotoxicity (Linda et al. 2000). Microglia scan synapses, establishing contacts with them that last a few minutes. In ischemia, contacts become longer, lasting for around an hour (Wake et al. 2009). These long lasting interactions often result in the disappearance of that synaptic contact. Any abnormalities in synaptic performance could activate microglia. However, the signal for microglia to remove a synapsis is poorly understood. The specificity of this action is associated with major histocompatibility complex (MHC) class F receptors, which are present in both neurons and microglia (Cullheim and Thams 2007) (see Chapter “Purine Signaling and Microglial Wrapping” for further reading on synaptic stripping).
Participation of Microglia in Pathophysiological Conditions
Both the absence of protective functions served by microglia, or their abnormal or excessive activation (von Bernhardi 2007; von Bernhardi et al. 2015b), could led to functional impairment and eventually to development of a disease of the CNS. The relevance of microglia activation and subsequent proliferation in aging, in which condition they adopt an “activated-like” morphology (Fig. 3; see Chapter “Age-dependent Changes in the Activation and Regulation of Microglia” for further reading on aging) (Conde and Streit 2006; Gavilan et al. 2007; von Bernhardi 2007; von Bernhardi et al. 2015b) as well as in many pathological contexts have been discussed over the past years (von Bernhardi et al. 2010; Heneka et al. 2014; Perry and Holmes 2014; Heneka et al. 2015; Hu et al. 2015; von Bernhardi et al. 2015a; Yirmiya et al. 2015).
Activation of hippocampal microglia with aging. Hippocampal cross section obtained from 9-, 13-, and 16-month old mice were labeled for Iba-1 and counterstaining with Harris Haematoxylin, a monocyte-macrophage identity marker that labels constitutively microglia, to compare the morphological features as the animal ages. At 9-months-old animals, microglia have long and ramified processes, which persisted at 13 months of age. In contrast, microglia from 16-month-old mice begins to shorten their processes, which become sturdier and the cell body increases in size. Scale bar = 10 µm
Any disturbance on brain homeostasis, as observed in infection, trauma, ischemia, altered neuronal activity, and both acute and chronic neurological injuries and diseases, induces profound changes in microglial cell shape, gene expression and functional behavior in which is defined as microglial cell activation (Hanisch and Kettenmann 2007; Block et al. 2007; Colton and Wilcock 2010; Colton 2009; Davoust et al. 2008; Graeber and Streit 2010; Streit et al. 2005; van Rossum and Hanisch 2004). Activated microglia show enlarged cell bodies and short and sturdy processes (Fig. 2). They can become motile and be actively recruited to the injury site following chemotactic gradients, and can also increase their proliferation. This phenotype is also correlated with functional changes occurring in complex and broad spectrum responses (Table 1). The range of microglial cell activities covers induction and release of multiple factors with inflammatory and immunoregulatory effects, phagocytotic activities to clear debris, damaged cells, or pathogens, production of neurotrophins and interaction with damaged neurons. Inflammatory response goes from responses centered around the production and release of inflammatory cytokines , such as TNFα, IL1β, and IL6 to release of factors with an anti-inflammatory effect (Casano and Peri 2015; Hu et al. 2015; Chen and Trapp 2015). Although some authors consider inflammatory microglia as detrimental, and anti-inflammatory regulatory microglia as neuroprotective, this rigid classification fails to recognize the complexity of microglial cell function and regulation (Fenn et al. 2014). Furthermore, regulatory microglia do not show always neuroprotective effects (Cherry et al. 2014).
The role of microglia-mediated phagocytosis in neurodegeneration has been established by several experimental approaches. Microglia are needed for removal of the dendritic trees of interneurons in the dentate gyrus after entorhinal cortex lesions (Rappert et al. 2004). In response to the lesion, microglia accumulate at the molecular layer in the dentate gyrus, mediated by signaling through the chemokine receptor CXCR3. Deletion of CXCR3 results in the failure of microglia recruitment, and the dendritic trees of interneurons are preserved.
Microglia also phagocytose molecules and debris such as myelin or amyloid deposits. Several studies report that Aβ is taken up by microglia in culture through mechanisms depending on Scavenger Receptors (Koenigsknecht and Landreth 2004; Alarcón et al. 2005; Cornejo and von Bernhardi 2013; Murgas et al. 2012), among others.
As discussed in Chapter “Age-dependent Changes in the Activation and Regulation of Microglia,” there is increasing evidence for altered chemokine signaling in diverse CNS diseases such as Alzheimer’s disease (AD) or multiple sclerosis (Gebicke-Haerter et al. 2001; Trebst et al. 2008) which may involve microglia activation (Stewart et al. 2010). Microglial cells from AD brains may have elevated levels of CCR3 and CCR5 receptors (Gebicke-Haerter et al. 2001). CXCL10 and its receptor CXCR3 have been linked to various CNS pathologies (van Weering et al. 2011). Studying the mechanisms by which this system mediates N-methyl-d-aspartate (NMDA)-induced neuronal toxicity in the hippocampus, the authors demonstrated that astrocytes and microglia cooperate to deliver the effect and that the deficiency in either the ligand or the receptor diminished or enhanced cell death depending on the tissue subregion and that microglia was the responsible cellular element by which this difference in neuronal vulnerability is organized.
A mechanism involving microglia, astrocytes, and chemokines has been proposed for glutamate toxicity (Bezzi et al. 2001). Binding of CXCL12 (stromal cell-derived factor, SDF-1α) to its receptor CXCR4 in astrocytes, results in Inositol trisphosphate (InsP3) production, [Ca2+]i increase, and release of TNFα. The binding of TNFα to its receptor triggers signaling, through autocrine a paracrine mechanism that causes prostaglandin E2 (PGE2) production. The PGE2, in turn, induces the release of glutamate, which can participate in glia-glia or glia-neuron communication, but can also initiate neurotoxicity. In the latter situation, SDF-1α would also act on microglia, thus driving enhanced TNFα release from both glial populations and ultimately causing massive glutamate release.
AD is associated with a significant elevation of TLR expression in the brain (Letiembre et al. 2009; Walter et al. 2007). Treatment with Aβ potentiated TLR2 and TLR4-mediated responses, while inhibiting TLR9 in mouse microglia cultures, (Lotz et al. 2005). At the same time, all three receptors (TLR2, TLR4, and TLR9) stimulated the uptake of Aβ by microglia (Tahara et al. 2006). The levels of TLRs in CNS are generally upregulated in many neurodegenerative diseases, including multiple sclerosis, Parkinson’s disease, and amyotrophic lateral sclerosis (ALS) (Okun et al. 2009).
Microglia activation, in turn, upregulates the synthesis of TLRs (Kielian et al. 2005; McKimmie and Fazakerley 2005). Similarly, the levels of TLRs in microglia are increased following hypoxia (Ock et al. 2007) and cerebral ischemia (Ziegler et al. 2007) and by inflammatory processes; for example, TNFα stimulates expression of TLR2 in cultured mouse microglia (Syed et al. 2007).
TLRs also regulate microglial death following pathological activation. TLR4 triggers microglial apoptosis via autocrine production of interferon gamma (IFNγ), whereas TLR2 is coupled to caspase-8-dependent apoptotic pathways (Lehnardt et al. 2007). Similarly, TLR2 participate in microglial apoptosis following human immunodeficiency virus (HIV-1) infection (Aravalli et al. 2007a, 2008).
A link of neurodegenerative processes in AD to microglial TLR4 is suggested because Aβ fibers bind to CD14, the co-receptor for LPS signaling via TLR4 (Fassbender et al. 2004). CD14 and TLR-dependent mechanisms appear to promote Aβ clearance and participate in inflammatory responses of microglia (Fassbender et al. 2004; Landreth and Reed-Geaghan 2009; Reed-Geaghan et al. 2009; Tahara et al. 2006). Pronounced CD14 immunoreactivity is observed in microglia close to AD lesion sites in AD brains (Liu et al. 2005). Importantly, a microglial CD36-TLR4-TLR6 complex appears to promote inflammation in response to Aβ (Stewart et al. 2010).
However, TLR signaling can also be neuroprotective, by both driving clearance of infectious agents, and by organizing CNS-intrinsic as well as immune system-mediated support of neural cell survival, tissue preservation, and CNS functioning (Glezer et al. 2007; Hanisch et al. 2008). Thus, a critical issue is to understand the mechanisms by which TLRs could engage in detrimental or in beneficial programs.
Concluding Remarks
Microglia affects the development , structure, and function of neuronal networks. They constantly monitor the status of synaptic contacts and receive information from neuronal activity. Multiple activation states of microglia may allow for the existence of microglia with different functions, which dynamically interact with neurons and potentiate their plastic capabilities. Furthermore, they appear to be also able to remodel neuronal connectivity and thus participate in physiological processes.
Commitment to distinct reactive phenotypes depending on their activation profile would then have a variable effect on neurons. It will be important to identify the nature of such instructing signals as they govern functional orientations of microglia. Little is also known about the heterogeneity of microglia, i.e., the differences in functional capacities of individual microglial populations within different CNS regions. Finally, in pathological situations with blood-derived monocytes/macrophages infiltrating the CNS, features and functions of resident microglia and the newly invading cells may complement each other, with both detrimental and beneficial consequences (Shechter et al. 2009; Simard et al. 2006). Understanding this various issues will be especially interesting to develop migroglia-based strategies for the management of several impairments of the nervous system.
Abbreviations
- 5-HT:
-
Serotonin
- Aβ:
-
β-amyloid
- AD:
-
Alzheimer’s disease
- ALS:
-
Amyotrophic lateral sclerosis
- ATP:
-
Adenosine triphosphate
- BDNF:
-
Brain derived neurotrophic factor
- CNS:
-
Central nervous system
- CNTF:
-
Ciliary neurotrophic factor
- DAMPs:
-
Damage- or Danger-associated molecular patterns
- EP2:
-
Prostanoid receptor subtype 2
- GABA:
-
Gamma aminobutyric acid
- GDNF:
-
Glia derived neurotrophic factor
- GM-CSF:
-
Granulocyte/macrophage colony stimulating factor
- HIV-1:
-
Human immunodeficiency virus
- IFNγ:
-
Interferon gamma
- IGF1:
-
Insulin-like growth factor 1
- IL1:
-
Interleukin 1
- iNOS:
-
Inducible nitric oxide synthase
- InsP3:
-
Inositol trisphosphate
- LPS:
-
Lipopolysaccharides
- LTP:
-
Long time Potentiation
- M-CSF:
-
Macrophage colony-stimulating factor
- MHC:
-
Class I molecules of histocompatibility major complex
- NGF:
-
Nerve growth factor
- NMDA:
-
N-methyl-d-aspartate
- NO:
-
Nitric Oxide
- NT:
-
Neurotrophin
- PAMPs:
-
Pathogen-associated molecular patterns
- PGE2:
-
Prostaglandin E2
- PRRs:
-
Pattern recognition receptors
- RANTES:
-
Regulated on activation, normal T cell expressed and secreted—chemokine CCL5
- RNS:
-
Reactive nitrogen species
- ROS:
-
Reactive oxygen species
- Rs:
-
Receptors
- SDF-1α:
-
Stromal cell-derived factor
- SIRPα:
-
Signal regulatory protein α
- SRs:
-
Scavenger receptors
- TGFβ:
-
Transforming growth factor-β
- TLRs:
-
Toll-like receptors
- TNFα:
-
Tumor necrosis factor α
- TSPs:
-
Thrombospondins
References
Aarum J, Sandberg K, Haeberlein SL, Persson MA (2003) Migration and differentiation of neural precursor cells can be directed by microglia. Proc Natl Acad Sci USA 100(26):15983–15988. doi:10.1073/pnas.2237050100
Ajami B, Bennett JL, Krieger C, Tetzlaff W, Rossi FM (2007) Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nat Neurosci 10(12):1538–1543. doi:10.1038/nn2014
Alarcón R, Fuenzalida C, Santibanez M, von Bernhardi R (2005) Expression of scavenger receptors in glial cells. Comparing the adhesion of astrocytes and microglia from neonatal rats to surface-bound beta-amyloid. J Biol Chem 280(34):30406–30415. doi:10.1074/jbc.M414686200
Aravalli RN, Hu S, Lokensgard JR (2007a) Toll-like receptor 2 signaling is a mediator of apoptosis in herpes simplex virus-infected microglia. J Neuroinflammation 4:11. doi:10.1186/1742-2094-4-11
Aravalli RN, Hu S, Lokensgard JR (2008) Inhibition of toll-like receptor signaling in primary murine microglia. J Neuroimmu Pharmacol Off J Soc NeuroImmu Pharmacol 3(1):5–11. doi:10.1007/s11481-007-9097-8
Aravalli RN, Peterson PK, Lokensgard JR (2007b) Toll-like receptors in defense and damage of the central nervous system. J Neuroimmu Pharmacol Off J Soc NeuroImmu Pharmacol 2(4):297–312. doi:10.1007/s11481-007-9071-5
Areschoug T, Gordon S (2009) Scavenger receptors: role in innate immunity and microbial pathogenesis. Cell Microbiol 11(8):1160–1169. doi:10.1111/j.1462-5822.2009.01326.x
Avital A, Goshen I, Kamsler A, Segal M, Iverfeldt K, Richter-Levin G, Yirmiya R (2003) Impaired interleukin-1 signaling is associated with deficits in hippocampal memory processes and neural plasticity. Hippocampus 13:826–834
Babcock AA, Wirenfeldt M, Holm T, Nielsen HH, Dissing-Olesen L, Toft-Hansen H, Millward JM, Landmann R, Rivest S, Finsen B, Owens T (2006) Toll-like receptor 2 signaling in response to brain injury: an innate bridge to neuroinflammation. J Neurosci 26(49):12826–12837. doi:10.1523/JNEUROSCI.4937-05.2006
Barclay AN, Wright GJ, Brooke G, Brown MH (2002) CD200 and membrane protein interactions in the control of myeloid cells. Trends Immunol 23(6):285–290
Beattie MS, Malenka RC (2002) Control of synaptic strength by glial TNFalpha. Science 295:2282–2285
Ben-Hur T, Ben-Menachem O, Furer V, Einstein O, Mizrachi-Kol R, Grigoriadis N (2003) Effects of proinflammatory cytokines on the growth, fate, and motility of multipotential neural precursor cells. Mol Cell Neurosci 24:623–631
Beurel E, Michalek SM, Jope RS (2010) Innate and adaptive immune responses regulated by glycogen synthase kinase-3 (GSK3). Trends Immunol 31(1):24–31. doi:10.1016/j.it.2009.09.007
Bezzi P, Domercq M, Brambilla L, Galli R, Schols D, De Clercq E, Vescovi A, Bagetta G, Kollias G, Meldolesi J, Volterra A (2001) CXCR4-activated astrocyte glutamate release via TNFalpha: amplification by microglia triggers neurotoxicity. Nat Neurosci 4(7):702–710. doi:10.1038/89490
Bianchi ME (2007) DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol 81(1):1–5. doi:10.1189/jlb.0306164
Biber K, Neumann H, Inoue K, Boddeke HW (2007) Neuronal ‘On’ and ‘Off’ signals control microglia. Trends Neurosci 30(11):596–602. doi:10.1016/j.tins.2007.08.007
Biber K, Vinet J, Boddeke HW (2008) Neuron-microglia signaling: chemokines as versatile messengers. J Neuroimmunol 198(1–2):69–74. doi:10.1016/j.jneuroim.2008.04.012
Block ML, Hong JS (2005) Microglia and inflammation-mediated neurodegeneration: multiple triggers with a common mechanism. Prog Neurobiol 76(2):77–98. doi:10.1016/j.pneurobio.2005.06.004
Block ML, Li G, Qin L , Wu X, Pei Z, Wang T et al (2006) Potent regulation of microglia-derived oxidative stress and dopaminergic neuron survival: substance P vs. dynorphin. FASEB J 20:251–258
Block ML, Zecca L, Hong JS (2007) Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci 8(1):57–69. doi:10.1038/nrn2038
Boucsein C, Zacharias R, Farber K, Pavlovic S, Hanisch UK, Kettenmann H (2003) Purinergic receptors on microglial cells: functional expression in acute brain slices and modulation of microglial activation in vitro. Eur J Neurosci 17(11):2267–2276
Brooke G, Holbrook JD, Brown MH, Barclay AN (2004) Human lymphocytes interact directly with CD47 through a novel member of the signal regulatory protein (SIRP) family. J Immunol 173(4):2562–2570
Butovsky O, Ziv Y, Schwartz A, Landa G, Talpalar AE, Pluchino S, Martino G, Schwartz M (2006) Microglia activated by IL-4 or IFN-gamma differentially induce neurogenesis and oligodendrogenesis from adult stem/progenitor cells. Mol Cell Neurosci 31(1):149–160. doi:10.1016/j.mcn.2005.10.006
Carbonell WS, Murase S, Horwitz AF, Mandell JW (2005) Migration of perilesional microglia after focal brain injury and modulation by CC chemokine receptor 5: an in situ time-lapse confocal imaging study. J Neurosci 25(30):7040–7047. doi:10.1523/JNEUROSCI.5171-04.2005
Cardona AE, Pioro EP, Sasse ME, Kostenko V, Cardona SM, Dijkstra IM, Huang D, Kidd G, Dombrowski S, Dutta R, Lee JC, Cook DN, Jung S, Lira SA, Littman DR, Ransohoff RM (2006) Control of microglial neurotoxicity by the fractalkine receptor. Nat Neurosci 9(7):917–924. doi:10.1038/nn1715
Carpentier PA, Duncan DS, Miller SD (2008) Glial toll-like receptor signaling in central nervous system infection and autoimmunity. Brain Behav Immun 22(2):140–147. doi:10.1016/j.bbi.2007.08.011
Casano AM, Peri F (2015) Microglia: multitasking specialists of the brain. Dev Cell 32(4):469–477. doi:10.1016/j.devcel.2015.01.018
Caso JR, Pradillo JM, Hurtado O, Lorenzo P, Moro MA, Lizasoain I (2007) Toll-like receptor 4 is involved in brain damage and inflammation after experimental stroke. Circulation 115(12):1599–1608. doi:10.1161/CIRCULATIONAHA.106.603431
Cepko CL, Austin CP, Yang X, Alexiades M, Ezzeddine D (1996) Cell fate determination in the vertebrate retina. Proc Natl Acad Sci USA 93:589–595
Chakrabarty P, Jansen-West K, Beccard A, Ceballos-Diaz C, Levites Y, Verbeeck C (2010) Massive gliosis induced by interleukin-6 suppresses Abeta deposition in vivo: evidence against inflammation as a driving forcé for amyloid deposition. FASEB J 24:548–559
Chamak B, Dobbertin A, Mallat M (1995) Immunohistochemical detection of thrombospondin in microglia in the developing rat brain. Neuroscience 69:177–187
Chang YP, Fang KM, Lee TI, Tzeng SF (2006) Regulation of microglial activities by glial cell line derived neurotrophic factor. J Cell Biochem 97(3):501–511. doi:10.1002/jcb.20646
Chen Z, Trapp BD (2015) Microglia and neuroprotection. J Neurochem. doi:10.1111/jnc.13062
Cherry JD, Olschowka JA, O’Banion MK (2014) Neuroinflammation and M2 microglia: the good, the bad, and the inflamed. J Neuroinflammation 11:98. doi:10.1186/1742-2094-11-98
Christopherson KS, Ullian EM, Stokes CC, Mullowney CE, Hell JW, Agah A, Lawler J, Mosher DF, Bornstein P, Barres BA (2005) Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell 120(3):421–433. doi:10.1016/j.cell.2004.12.020
Colton C, Wilcock DM (2010) Assessing activation states in microglia. CNS Neurol Disord: Drug Targets 9(2):174–191
Colton CA (2009) Heterogeneity of microglial activation in the innate immune response in the brain. J Neuroimmu Pharmacol Off J Soc NeuroImmun Pharmacol 4(4):399–418. doi:10.1007/s11481-009-9164-4
Conde JR, Streit WJ (2006) Microglia in the aging brain. J Neuropathol Exp Neurol 65(3):199–203. doi:10.1097/01.jnen.0000202887.22082.63
Cornejo F, von Bernhardi R (2013) Role of scavenger receptors in glia-mediated neuroinflammatory response associated with Alzheimer’s disease. Mediat Inflamm 2013:895651. doi:10.1155/2013/895651
Corriveau RA, Huh GS, Shatz CJ (1998) Regulation of class I MHC gene expression in the developing and mature CNS by neural activity. Neuron 21:505–520
Cullheim S, Thams S (2007) The microglial networks of the brain and their role in neuronal network plasticity after lesion. Brain Res Rev 55(1):89–96. doi:10.1016/j.brainresrev.2007.03.012
Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, Littman DR, Dustin ML, Gan WB (2005) ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci 8(6):752–758. doi:10.1038/nn1472
Davoust N, Vuaillat C, Androdias G, Nataf S (2008) From bone marrow to microglia: barriers and avenues. Trends Immunol 29(5):227–234. doi:10.1016/j.it.2008.01.010
Di Filippo M, Chiasserini D, Tozzi A, Picconi B, Calabresi P (2010) Mitochondria and the link between neuroinflammation and neurodegeneration. J Alzheimers Dis 20(Suppl 2):S369–S379. doi:10.3233/JAD-2010-100543
Ekdahl CT, Claasen JH, Bonde S, Kokaia Z, Lindvall O (2003) Inflammation is detrimental for neurogenesis in adult brain. Proc Natl Acad Sci USA 100(23):13632–13637. doi:10.1073/pnas.2234031100
Ekdahl CT, Kokaia Z, Lindvall O (2009) Brain inflammation and adult neurogenesis: the dual role of microglia. Neuroscience 158(3):1021–1029. doi:10.1016/j.neuroscience.2008.06.052
Elkabes S, DiCicco-Bloom EM, Black IB (1996) Brain microglia/macrophages express neurotrophins that selectively regulate microglial proliferation and function. J Neurosci 16(8):2508–2521
Fang KM, Yang CS, Sun SH, Tzeng SF (2009) Microglial phagocytosis attenuated by short-term exposure to exogenous ATP through P2X receptor action. J Neurochem 111(5):1225–1237. doi:10.1111/j.1471-4159.2009.06409.x
Fassbender K, Walter S, Kuhl S, Landmann R, Ishii K, Bertsch T, Stalder AK, Muehlhauser F, Liu Y, Ulmer AJ, Rivest S, Lentschat A, Gulbins E, Jucker M, Staufenbiel M, Brechtel K, Walter J, Multhaup G, Penke B, Adachi Y, Hartmann T, Beyreuther K (2004) The LPS receptor (CD14) links innate immunity with Alzheimer’s disease. FASEB J 18(1):203–205. doi:10.1096/fj.03-0364fje
Fenn AM, Hall JC, Gensel JC, Popovich PG, Godbout JP (2014) IL-4 signaling drives a unique arginase+/IL-1beta+microglia phenotype and recruits macrophages to the inflammatory CNS: consequences of age-related deficits in IL-4Ralpha after traumatic spinal cord injury. J Neurosci 34(26):8904–8917. doi:10.1523/JNEUROSCI.1146-14.2014
Ferrini F, De Koninck Y (2013) Microglia control neuronal network excitability via BDNF signalling. Neural Plast 2013:429815. doi:10.1155/2013/429815
Flores B, von Bernhardi R (2012) Transforming growth factor beta1 modulates amyloid beta-induced glial activation through the Smad3-dependent induction of MAPK phosphatase-1. J Alzheimer Dis 32(2):417–429. doi:10.3233/JAD-2012-120721 Y05258137G173L5 V[pii]
Frade JM, Barde YA (1998) Microglia-derived nerve growth factor causes cell death in the developing retina. Neuron 20(1):35–41
Gamo K, Kiryu-Seo S, Konishi H, Aoki S, Matsushima K, Wada K, Kiyama H (2008) G-protein-coupled receptor screen reveals a role for chemokine receptor CCR5 in suppressing microglial neurotoxicity. J Neurosci 28(46):11980–11988. doi:10.1523/JNEUROSCI.2920-08.2008
Gavilan MP, Revilla E, Pintado C, Castano A, Vizuete ML, Moreno-Gonzalez I, Baglietto-Vargas D, Sanchez-Varo R, Vitorica J, Gutierrez A, Ruano D (2007) Molecular and cellular characterization of the age-related neuroinflammatory processes occurring in normal rat hippocampus: potential relation with the loss of somatostatin GABAergic neurons. J Neurochem 103(3):984–996. doi:10.1111/j.1471-4159.2007.04787.x
Gebicke-Haerter PJ, Spleiss O, Ren LQ, Li H, Dichmann S, Norgauer J, Boddeke HW (2001) Microglial chemokines and chemokine receptors. Prog Brain Res 132:525–532. doi:10.1016/S0079-6123(01)32100-3
Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, Mehler MF, Conway SJ, Ng LG, Stanley ER, Samokhvalov IM, Merad M (2010) Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 330(6005):841–845. doi:10.1126/science.1194637
Giulian D, Young DG, Woodward J, Brown DC, Lachman LB (1988) Interleukin-1 is an astroglial growth factor in the developing brain. J Neurosci 8(2):709–714
Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH (2010) Mechanisms underlying inflammation in neurodegeneration. Cell 140(6):918–934. doi:10.1016/j.cell.2010.02.016
Glezer I, Simard AR, Rivest S (2007) Neuroprotective role of the innate immune system by microglia. Neuroscience 147(4):867–883. doi:10.1016/j.neuroscience.2007.02.055
Goddard CA, Butts DA, Shatz CJ (2007) Regulation of CNS synapses by neuronal MHC class I. Proc Natl Acad Sci USA 104:6828–6833
Godoy B, Murgas P, Tichauer J, Von Bernhardi R (2012) Scavenger receptor class A ligands induce secretion of IL1beta and exert a modulatory effect on the inflammatory activation of astrocytes in culture. J Neuroimmunol 251(1–2):6–13. doi:10.1016/j.jneuroim.2012.06.004
Goshen I, Kreisel T, Ounallah-Saad H, Renbaum P, Zalzstein Y, Ben-Hur T, Levy-Lahad E, Yirmiya R (2007) A dual role for interleukin-1 in hippocampal dependent memory processes. Psychoneuroendocrinology 32:1106–1115
Graeber MB, Streit WJ (2010) Microglia: biology and pathology. Acta Neuropathol 119(1):89–105. doi:10.1007/s00401-009-0622-0
Hanisch UK, Johnson TV, Kipnis J (2008) Toll-like receptors: roles in neuroprotection? Trends Neurosci 31(4):176–182. doi:10.1016/j.tins.2008.01.005
Hanisch UK, Kettenmann H (2007) Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci 10(11):1387–1394. doi:10.1038/nn1997
Haynes SE, Hollopeter G, Yang G, Kurpius D, Dailey ME, Gan WB, Julius D (2006) The P2Y12 receptor regulates microglial activation by extracellular nucleotides. Nat Neurosci 9(12):1512–1519. doi:10.1038/nn1805
Hellwig S, Heinrich A, Biber K (2013) The brain’s best friend: microglial neurotoxicity revisited. Front Cell Neurosci 7:71. doi:10.3389/fncel.2013.00071
Heneka MT, Golenbock DT, Latz E (2015) Innate immunity in Alzheimer’s disease. Nat Immunol 16(3):229–236. doi:10.1038/ni.3102
Heneka MT, Kummer MP, Latz E (2014) Innate immune activation in neurodegenerative disease. Nat Rev 14(7):463–477. doi:10.1038/nri3705
Herrera-Molina R, Flores B, Orellana JA, von Bernhardi R (2012) Modulation of interferon-gamma-induced glial cell activation by transforming growth factor beta1: a role for STAT1 and MAPK pathways. J Neurochem 123(1):113–123. doi:10.1111/j.1471-4159.2012.07887.x
Herrera-Molina R, von Bernhardi R (2005) Transforming growth factor-beta 1 produced by hippocampal cells modulates microglial reactivity in culture. Neurobiol Dis 19(1–2):229–236. doi:10.1016/j.nbd.2005.01.003
Hoek RM, Ruuls SR, Murphy CA, Wright GJ, Goddard R, Zurawski SM, Blom B, Homola ME, Streit WJ, Brown MH, Barclay AN, Sedgwick JD (2000) Down-regulation of the macrophage lineage through interaction with OX2 (CD200). Science 290(5497):1768–1771
Hu X, Leak RK, Shi Y, Suenaga J, Gao Y, Zheng P, Chen J (2015) Microglial and macrophage polarization-new prospects for brain repair. Nat Rev Neurol 11(1):56–64. doi:10.1038/nrneurol.2014.207
Huh GS, Boulanger LM, Du H, Riquelme PA, Brotz TM, Shatz CJ (2000) Functional requirement for class I MHC in CNS development and plasticity. Science 290:2155–2159
Ifuku M, Farber K, Okuno Y, Yamakawa Y, Miyamoto T, Nolte C, Merrino VF, Kita S, Iwamoto T, Komuro I, Wang B, Cheung G, Ishikawa E, Ooboshi H, Bader M, Wada K, Kettenmann H, Noda M (2007) Bradykinin-induced microglial migration mediated by B1-bradykinin receptors depends on Ca2+ influx via reverse-mode activity of the Na+/Ca2+ exchanger. J Neurosci 27(48):13065–13073. doi:10.1523/JNEUROSCI.3467-07.2007
Inoue K, Koizumi S, Kataoka A, Tozaki-Saitoh H, Tsuda M (2009) P2Y(6)-evoked microglial phagocytosis. Int Rev Neurobiol 85:159–163. doi:10.1016/S0074-7742(09)85012-5
Kempermann G (2002) Regulation of adult hippocampal neurogenesis—implications for novel theories of major depression. Bipolar Disord 4(1):17–33
Kempermann G, Kronenberg G (2003) Depressed new neurons–adult hippocampal neurogenesis and a cellular plasticity hypothesis of major depression. Biol Psychiatry 54(5):499–503
Kettenmann H, Hanisch UK, Noda M, Verkhratsky A (2011) Physiology of microglia. Physiol Rev 91(2):461–553. doi:10.1152/physrev.00011.2010
Kielian T, Esen N, Bearden ED (2005) Toll-like receptor 2 (TLR2) is pivotal for recognition of S. aureus peptidoglycan but not intact bacteria by microglia. Glia 49(4):567–576. doi:10.1002/glia.20144
Kierdorf K, Katzmarski N, Haas CA, Prinz M (2013) Bone marrow cell recruitment to the brain in the absence of irradiation or parabiosis bias. PLoS One 8(3):e58544. doi:10.1371/journal.pone.0058544
Kierdorf K, Prinz M (2013) Factors regulating microglia activation. Front Cell Neurosci 7:44. doi:10.3389/fncel.2013.00044
Kim SU, de Vellis J (2005) Microglia in health and disease. J Neurosci Res 81(3):302–313
Kirischuk S, Moller T, Voitenko N, Kettenmann H, Verkhratsky A (1995) ATP-induced cytoplasmic calcium mobilization in Bergmann glial cells. J Neurosci 15:7861–7871
Koenigsknecht J, Landreth G (2004) Microglial phagocytosis of fibrillar beta-amyloid through a beta1 integrin-dependent mechanism. J Neurosci 24(44):9838–9846. doi:10.1523/JNEUROSCI.2557-04.2004
Kohman RA, Bhattacharya TK, Kilby C, Bucko P, Rhodes JS (2013) Effects of minocycline on spatial learning, hippocampal neurogenesis and microglia in aged and adult mice. Behav Brain Res 242:17–24. doi:10.1016/j.bbr.2012.12.032
Koizumi S, Shigemoto-Mogami Y, Nasu-Tada K, Shinozaki Y, Ohsawa K, Tsuda M, Joshi BV, Jacobson KA, Kohsaka S, Inoue K (2007) UDP acting at P2Y6 receptors is a mediator of microglial phagocytosis. Nature 446(7139):1091–1095. doi:10.1038/nature05704
Konat GW, Kielian T, Marriott I (2006) The role of Toll-like receptors in CNS response to microbial challenge. J Neurochem 99(1):1–12. doi:10.1111/j.1471-4159.2006.04076.x
Kono H, Rock KL (2008) How dying cells alert the immune system to danger. Nat Rev 8(4):279–289. doi:10.1038/nri2215
Koo JW, Duman RS (2008) IL-1beta is an essential mediator of the antineurogenic and anhedonic effects of stress. Proc Natl Acad Sci USA 105:751–756
Kremlev SG, Roberts RL, Palmer C (2004) Differential expression of chemokines and chemokine receptors during microglial activation and inhibition. J Neuroimmunol 149(1–2):1–9. doi:10.1016/j.jneuroim.2003.11.012
Labrousse VF, Costes L, Aubert A, Darnaudery M, Ferreira G, Amedee T, Laye S (2009) Impaired interleukin-1beta and c-Fos expression in the hippocampus is associated with a spatial memory deficit in P2X(7) receptor-deficient mice. PLoS One 4:e6006
Lalo U, Pankratov Y, Wichert SP, Rossner MJ, North RA, Kirchhoff F, Verkhratsky A (2008) P2X1 and P2X5 subunits form the functional P2X receptor in mouse cortical astrocytes. J Neurosci 28:5473–5480
Landreth GE, Reed-Geaghan EG (2009) Toll-like receptors in Alzheimer’s disease. Curr Top Microbiol Immunol 336:137–153. doi:10.1007/978-3-642-00549-7_8
Lee TI, Yang CS, Fang KM, Tzeng SF (2009) Role of ciliary neurotrophic factor in microglial phagocytosis. Neurochem Res 34(1):109–117. doi:10.1007/s11064-008-9682-0
Lehnardt S, Lachance C, Patrizi S, Lefebvre S, Follett PL, Jensen FE, Rosenberg PA, Volpe JJ, Vartanian T (2002) The toll-like receptor TLR4 is necessary for lipopolysaccharide-induced oligodendrocyte injury in the CNS. J Neurosci 22(7):2478–2486. doi:20026268
Lehnardt S, Schott E, Trimbuch T, Laubisch D, Krueger C, Wulczyn G, Nitsch R, Weber JR (2008) A vicious cycle involving release of heat shock protein 60 from injured cells and activation of toll-like receptor 4 mediates neurodegeneration in the CNS. J Neurosci 28(10):2320–2331. doi:10.1523/JNEUROSCI.4760-07.2008
Lehnardt S, Wennekamp J, Freyer D, Liedtke C, Krueger C, Nitsch R, Bechmann I, Weber JR, Henneke P (2007) TLR2 and caspase-8 are essential for group B Streptococcus-induced apoptosis in microglia. J Immunol 179(9):6134–6143
Letiembre M, Liu Y, Walter S, Hao W, Pfander T, Wrede A, Schulz-Schaeffer W, Fassbender K (2009) Screening of innate immune receptors in neurodegenerative diseases: a similar pattern. Neurobiol Aging 30(5):759–768. doi:10.1016/j.neurobiolaging.2007.08.018
Li Y, Du XF, Liu CS, Wen ZL, Du JL (2012) Reciprocal regulation between resting microglial dynamics and neuronal activity in vivo. Dev Cell 23(6):1189–1202. doi:10.1016/j.devcel.2012.10.027
Liang X, Wang Q, Hand T, Wu L, Breyer RM, Montine TJ, Andreasson K (2005) Deletion of the prostaglandin E2 EP2 receptor reduces oxidative damage and amyloid burden in a model of Alzheimer’s disease. J Neurosci 25(44):10180–10187. doi:10.1523/JNEUROSCI.3591-05.2005
Liang KJ, Lee JE, Wang YD, Ma W, Fontainhas AM, Fariss RN, Wong WT (2009) Regulation of dynamic behavior of retinal microglia by CX3CR1 signaling. Investig Ophthalmol Vis Sci 50:4444–4451
Lim SH, Park E, You B, Jung Y, Park AR, Park SG, Lee JR (2013) Neuronal synapse formation induced by microglia and interleukin 10. PLoS One 8:e81218 astrocytes. J Neurosci 28:5473–5480
Linda H, Shupliakov O, Ornung G, Ottersen OP, Storm-Mathisen J, Risling M, Cullheim S (2000) Ultrastructural evidence for a preferential elimination of glutamate-immunoreactive synaptic terminals from spinal motoneurons after intramedullary axotomy. J Comp Neurol 425(1):10–23
Liu Y, Walter S, Stagi M, Cherny D, Letiembre M, Schulz-Schaeffer W, Heine H, Penke B, Neumann H, Fassbender K (2005) LPS receptor (CD14): a receptor for phagocytosis of Alzheimer’s amyloid peptide. Brain 128(Pt 8):1778–1789. doi:10.1093/brain/awh531
Loscher CE, Mills KH, Lynch MA (2003) Interleukin-1 receptor antagonist exerts agonist activity in the hippocampus independent of the interleukin-1 type I receptor. J Neuroimmunol 137:117–124
Lotz M, Ebert S, Esselmann H, Iliev AI, Prinz M, Wiazewicz N, Wiltfang J, Gerber J, Nau R (2005) Amyloid beta peptide 1-40 enhances the action of Toll-like receptor-2 and -4 agonists but antagonizes Toll-like receptor-9-induced inflammation in primary mouse microglial cell cultures. J Neurochem 94(2):289–298. doi:10.1111/j.1471-4159.2005.03188.x
Lu C, Hua F, Liu L, Ha T, Kalbfleisch J, Schweitzer J, Kelley J, Kao R, Williams D, Li C (2010) Scavenger receptor class-A has a central role in cerebral ischemia-reperfusion injury. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab 30(12):1972–1981. doi:10.1038/jcbfm.2010.59
Lynch MA (2009) The multifaceted profile of activated microglia. Mol Neurobiol 40(2):139–156. doi:10.1007/s12035-009-8077-9
Marin-Teva JL, Dusart I, Colin C, Gervais A, van Rooijen N, Mallat M (2004) Microglia promote the death of developing Purkinje cells. Neuron 41(4):535–547
Matozaki T, Murata Y, Okazawa H, Ohnishi H (2009) Functions and molecular mechanisms of the CD47-SIRPalpha signalling pathway. Trends Cell Biol 19(2):72–80. doi:10.1016/j.tcb.2008.12.001
Matzinger P (2007) Friendly and dangerous signals: is the tissue in control? Nat Immunol 8(1):11–13. doi:10.1038/ni0107-11
McKimmie CS, Fazakerley JK (2005) In response to pathogens, glial cells dynamically and differentially regulate Toll-like receptor gene expression. J Neuroimmunol 169(1–2):116–125. doi:10.1016/j.jneuroim.2005.08.006
McPherson CA, Kraft AD, Harry GJ (2011) Injury-induced neurogenesis: consideration of resident microglia as supportive of neural progenitor cells. Neurotox Res 19(2):341–352. doi:10.1007/s12640-010-9199-6
Mildner A, Schmidt H, Nitsche M, Merkler D, Hanisch UK, Mack M, Heikenwalder M, Bruck W, Priller J, Prinz M (2007) Microglia in the adult brain arise from Ly-6ChiCCR2+ monocytes only under defined host conditions. Nat Neurosci 10(12):1544–1553. doi:10.1038/nn2015
Mitrasinovic OM, Murphy GM Jr (2002) Accelerated phagocytosis of amyloid-B by mouse and human microglia overexpressing the macrophage colony-stimulating factor receptor. J Biol Chem 277:29889–29896
Mitrasinovic OM, Murphy GM Jr (2003) Microglial overexpression of the M-CSF receptor augments phagocytosis of opsonized Abeta. Neurobiol Aging 24(6):807–815
Moller JC, Klein MA, Haas S, Jones LL, Kreutzberg GW, Raivich G (1996) Regulation of thrombospondin in the regenerating mouse facial motor nucleus. Glia 17(2):121–132. doi:10.1002/(SICI)1098-1136(199606)17:2<121:AID-GLIA4>3.0.CO;2-5
Monje ML, Toda H, Palmer TD (2003) Inflammatory blockade restores adult hippocampal neurogenesis. Science 302(5651):1760–1765. doi:10.1126/science.1088417
Morgan SC, Taylor DL, Pocock JM (2004) Microglia release activators of neuronal proliferation mediated by activation of mitogen-activated protein kinase, phosphatidylinositol-3-kinase/Akt and delta-Notch signalling cascades. J Neurochem 90(1):89–101. doi:10.1111/j.1471-4159.2004.02461.x
Mount MP, Lira A, Grimes D, Smith PD, Faucher S, Slack R, et al (2007) Involvement of interferon-gamma in microglial-mediated loss of dopaminergic neurons. J Neurosci 27:3328–3337
Murgas P, Cornejo FA, Merino G, von Bernhardi R (2014) SR-A regulates the inflammatory activation of astrocytes. Neurotox Res 25(1):68–80. doi:10.1007/s12640-013-9432-1
Murgas P, Godoy B, von Bernhardi R (2012) Abeta potentiates inflammatory activation of glial cells induced by scavenger receptor ligands and inflammatory mediators in culture. Neurotox Res 22(1):69–78. doi:10.1007/s12640-011-9306-3
Nakajima Y, Tohyama T, Kurihara S, Kohsaka S (2005) Axotomy-dependent urokinase induction in the rat facial nucleus: possible stimulation of microglia by neurons. Neurochem Int 46:107–116
Nakajima K, Tohyama Y, Maeda S, Kohsaka S, Kurihara T (2007) Neuronal regulation by which microglia enhance the production of neurotrophic factors for GABAergic, catecholaminergic, and cholinergic neurons. Neurochem Int 50:807–820
Nakanishi M, Niidome T, Matsuda S, Akaike A, Kihara T, Sugimoto H (2007) Microglia-derived interleukin-6 and leukaemia inhibitory factor promote astrocytic differentiation of neural stem/progenitor cells. Eur J Neurosci 25(3):649–658. doi:10.1111/j.1460-9568.2007.05309.x
Napoli I, Neumann H (2009) Microglial clearance function in health and disease. Neuroscience 158(3):1030–1038. doi:10.1016/j.neuroscience.2008.06.046
Nau R, Bruck W (2002) Neuronal injury in bacterial meningitis: mechanisms and implications for therapy. Trends Neurosci 25(1):38–45
Neumann H, Kotter MR, Franklin RJ (2009) Debris clearance by microglia: an essential link between degeneration and regeneration. Brain 132(Pt 2):288–295. doi:10.1093/brain/awn109
Nguyen MD, D’Aigle T, Gowing G, Julien JP, Rivest S (2004) Exacerbation of motor neuron disease by chronic stimulation of innate immunity in a mouse model of amyotrophic lateral sclerosis. J Neurosci 24(6):1340–1349. doi:10.1523/JNEUROSCI.4786-03.2004
Nimmerjahn A, Kirchhoff F, Helmchen F (2005) Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 308(5726):1314–1318. doi:10.1126/science.1110647
Ock J, Jeong J, Choi WS, Lee WH, Kim SH, Kim IK, Suk K (2007) Regulation of Toll-like receptor 4 expression and its signaling by hypoxia in cultured microglia. J Neurosci Res 85(9):1989–1995. doi:10.1002/jnr.21322
Okun E, Griffioen KJ, Lathia JD, Tang SC, Mattson MP, Arumugam TV (2009) Toll-like receptors in neurodegeneration. Brain Res Rev 59(2):278–292. doi:10.1016/j.brainresrev.2008.09.001
Oliveira AL, Thams S, Lidman O, Piehl F, Hökfelt T, Kärre K, Lindå H, Cullheim S (2004) A role for MHC class I molecules in synaptic plasticity and regeneration of neurons after axotomy. Proc Natl Acad Sci USA 101(51):17843–17848
Olson JK, Miller SD (2004) Microglia initiate central nervous system innate and adaptive immune responses through multiple TLRs. J Immunol 173:3916–3924
Orellana JA, Montero TD, von Bernhardi R (2013) Astrocytes inhibit nitric oxide-dependent Ca(2+) dynamics in activated microglia: involvement of ATP released via pannexin 1 channels. Glia 61(12):2023–2037. doi:10.1002/glia.22573
Ozeki Y, Tsutsui H, Kawada N, Suzuki H, Kataoka M, Kodama T, Yano I, Kaneda K, Kobayashi K (2006) Macrophage scavenger receptor down-regulates mycobacterial cord factor-induced proinflammatory cytokine production by alveolar and hepatic macrophages. Microb Pathog 40(4):171–176. doi:10.1016/j.micpath.2005.12.006
Padovan E, Landmann RM, De Libero G (2007) How pattern recognition receptor triggering influences T cell responses: a new look into the system. Trends Immunol 28(7):308–314. doi:10.1016/j.it.2007.05.002
Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, Giustetto M, Ferreira TA, Guiducci E, Dumas L, Ragozzino D, Gross CT (2011) Synaptic pruning by microglia is necessary for normal brain development. Science 333(6048):1456–1458. doi:10.1126/science.1202529
Paolicelli RC, Gross CT (2011) Microglia in development: linking brain wiring to brain environment. Neuron Glia Biol 7(1):77–83. doi:10.1017/S1740925X12000105
Parkhurst CN, Yang G, Ninan I, Savas JN, Yates JR 3rd, Lafaille JJ, Hempstead BL, Littman DR, Gan WB (2013) Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell 155(7):1596–1609. doi:10.1016/j.cell.2013.11.030
Perry VH, Cunningham C, Holmes C (2007) Systemic infections and inflammation affect chronic neurodegeneration. Nat Rev 7(2):161–167. doi:10.1038/nri2015 nri2015[pii]
Perry VH, Holmes C (2014) Microglial priming in neurodegenerative disease. Nature reviews Neurology 10(4):217–224. doi:10.1038/nrneurol.2014.38
Pocock JM, Kettenmann H (2007) Neurotransmitter receptors on microglia. Trends Neurosci 30(10):527–535. doi:10.1016/j.tins.2007.07.007
Prinz M, Priller J (2010) Tickets to the brain: role of CCR2 and CX3CR1 in myeloid cell entry in the CNS. J Neuroimmunol 224(1–2):80–84. doi:10.1016/j.jneuroim.2010.05.015
Prinz M, Priller J (2014) Microglia and brain macrophages in the molecular age: from origin to neuropsychiatric disease. Nat Rev Neurosci 15(5):300–312. doi:10.1038/nrn3722
Ramírez G, Rey S, von Bernhardi R (2008) Proinflammatory stimuli are needed for induction of microglial cell-mediated AbetaPP_{244-C} and Abeta-neurotoxicity in hippocampal cultures. J Alzheimer Dis 15(1):45–59
Ramirez G, Toro R, Dobeli H, von Bernhardi R (2005) Protection of rat primary hippocampal cultures from A beta cytotoxicity by pro-inflammatory molecules is mediated by astrocytes. Neurobiol Dis 19(1–2):243–254. doi:10.1016/j.nbd.2005.01.007
Rappert A, Bechmann I, Pivneva T, Mahlo J, Biber K, Nolte C, Kovac AD, Gerard C, Boddeke HW, Nitsch R, Kettenmann H (2004) CXCR3-dependent microglial recruitment is essential for dendrite loss after brain lesion. J Neurosci 24(39):8500–8509. doi:10.1523/JNEUROSCI.2451-04.2004
Rappert A, Biber K, Nolte C, Lipp M, Schubel A, Lu B, Gerard NP, Gerard C, Boddeke HW, Kettenmann H (2002) Secondary lymphoid tissue chemokine (CCL21) activates CXCR3 to trigger a Cl- current and chemotaxis in murine microglia. J Immunol 168(7):3221–3226
Reed-Geaghan EG, Savage JC, Hise AG, Landreth GE (2009) CD14 and toll-like receptors 2 and 4 are required for fibrillar A{beta}-stimulated microglial activation. J Neurosci 29(38):11982–11992. doi:10.1523/JNEUROSCI.3158-09.2009 29/38/11982[pii]
Ribeiro Xavier AL, Kress BT, Goldman SA, Lacerda de Menezes JR, Nedergaard M (2015) A distinct population of microglia supports adult neurogenesis in the subventricular zone. J Neurosci 35(34):11848–11861. doi:10.1523/JNEUROSCI.1217-15.2015
Rogers JT, Morganti JM, Bachstetter AD, Hudson CE, Peters MM, Grimmig BA, Weeber EJ, Bickford PC, Gemma C (2011) CX3CR1 deficiency leads to impairment of hippocampal cognitive function and synaptic plasticity. J Neurosci 31:16241–16250
Rolls A, Shechter R, London A, Ziv Y, Ronen A, Levy R, Schwartz M (2007) Toll-like receptors modulate adult hippocampal neurogenesis. Nat Cell Biol 9:1081–1088
Roumier A, Bechade C, Poncer JC, Smalla KH, Tomasello E, Vivier E, Gundelfinger ED, Triller A, Bessis A (2004) Impaired synaptic function in the microglial KARAP/DAP12-deficient mouse. J Neurosci 24:11421–11428
Rubartelli A, Lotze MT (2007) Inside, outside, upside down: damage-associated molecular-pattern molecules (DAMPs) and redox. Trends Immunol 28(10):429–436. doi:10.1016/j.it.2007.08.004
Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, Yamasaki R, Ransohoff RM, Greenberg ME, Barres BA, Stevens B (2012) Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron 74:691–705
Schaffler A, Scholmerich J, Salzberger B (2007) Adipose tissue as an immunological organ: Toll-like receptors, C1q/TNFs and CTRPs. Trends Immunol 28(9):393–399. doi:10.1016/j.it.2007.07.003
Schilling T, Nitsch R, Heinemann U, Haas D, Eder C (2001) Astrocyte-released cytokines induce ramification and outward K+ channel expression in microglia via distinct signalling pathways. Eur J Neurosci 14(3):463–473
Schilling T, Stock C, Schwab A, Eder C (2004) Functional importance of Ca2+-activated K+ channels for lysophosphatidic acid-induced microglial migration. Eur J Neurosci 19(6):1469–1474. doi:10.1111/j.1460-9568.2004.03265.x
Schmid AW, Lynch MA, Herron CE (2009) The effects of IL-1 receptor antagonist on beta amyloid mediated depression of LTP in the rat CA1 in vivo. Hippocampus 19:670–676
Schwab A (2001) Ion channels and transporters on the move. News Physiol Sci Int J Physiol Produc Join Int Union Physiol Sci Am Physiol Soc 16:29–33
Schwartz M, Butovsky O, Bruck W, Hanisch UK (2006) Microglial phenotype: is the commitment reversible? Trends Neurosci 29(2):68–74. doi:10.1016/j.tins.2005.12.005
Sedel F, Bechade C, Vyas S, Triller A (2004) Macrophage-derived tumor necrosis factor alpha, an early developmental signal for motoneuron death. J Neurosci 24(9):2236–2246. doi:10.1523/JNEUROSCI.4464-03.2004
Shechter R, London A, Varol C, Raposo C, Cusimano M, Yovel G, Rolls A, Mack M, Pluchino S, Martino G, Jung S, Schwartz M (2009) Infiltrating blood-derived macrophages are vital cells playing an anti-inflammatory role in recovery from spinal cord injury in mice. PLoS Med 6(7):e1000113. doi:10.1371/journal.pmed.1000113
Shie FS, Breyer RM, Montine TJ (2005) Microglia lacking E prostanoid receptor subtype 2 have enhanced Abeta phagocytosis yet lack Abeta-activated neurotoxicity. Am J Pathol 166(4):1163–1172
Shigemoto-Mogami Y, Hoshikawa K, Goldman JE, Sekino Y, Sato K (2014) Microglia enhance neurogenesis and oligodendrogenesis in the early postnatal subventricular zone. J Neurosci 34:2231–2243
Sievers J, Schmidtmayer J, Parwaresch R (1994) Blood monocytes and spleen macrophages differentiate into microglia-like cells when cultured on astrocytes. Ann Anat Anatom Anz Off Organ Anatom Ges 176(1):45–51
Simard AR, Soulet D, Gowing G, Julien JP, Rivest S (2006) Bone marrow-derived microglia play a critical role in restricting senile plaque formation in Alzheimer’s disease. Neuron 49(4):489–502. doi:10.1016/j.neuron.2006.01.022
Stence N, Waite M, Dailey ME (2001) Dynamics of microglial activation: a confocal time-lapse analysis in hippocampal slices. Glia 33(3):256–266
Stevens B, Allen NJ, Vazquez LE, Howell GR, Christopherson KS, Nouri N, Micheva KD, Mehalow AK, Huberman AD, Stafford B, Sher A, Litke AM, Lambris JD, Smith SJ, John SW, Barres BA (2007) The classical complement cascade mediates CNS synapse elimination. Cell 131(6):1164–1178. doi:10.1016/j.cell.2007.10.036
Stewart CR, Stuart LM, Wilkinson K, van Gils JM, Deng J, Halle A, Rayner KJ, Boyer L, Zhong R, Frazier WA, Lacy-Hulbert A, El Khoury J, Golenbock DT, Moore KJ (2010) CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nat Immunol 11(2):155–161. doi:10.1038/ni.1836
Streit WJ, Conde JR, Fendrick SE, Flanary BE, Mariani CL (2005) Role of microglia in the central nervous system’s immune response. Neurol Res 27(7):685–691. doi:10.1179/016164105X49463
Syed MM, Phulwani NK, Kielian T (2007) Tumor necrosis factor-alpha (TNF-alpha) regulates Toll-like receptor 2 (TLR2) expression in microglia. J Neurochem 103(4):1461–1471. doi:10.1111/j.1471-4159.2007.04838.x
Tahara K, Kim HD, Jin JJ, Maxwell JA, Li L, Fukuchi K (2006) Role of toll-like receptor signalling in Abeta uptake and clearance. Brain 129(Pt 11):3006–3019. doi:10.1093/brain/awl249
Tang G, Gudsnuk K, Kuo SH, Cotrina ML, Rosoklija G, Sosunov A, Sonders MS, Kanter E, Castagna C, Yamamoto A, Yue Z, Arancio O, Peterson BS, Champagne F, Dwork AJ, Goldman J, Sulzer D (2014) Loss of mTOR-dependent macroautophagy causes autistic-like synaptic pruning deficits. Neuron 83(5):1131–1143. doi:10.1016/j.neuron.2014.07.040
Tichauer J, Saud K, von Bernhardi R (2007) Modulation by astrocytes of microglial cell-mediated neuroinflammation: effect on the activation of microglial signaling pathways. NeuroImmunoModulation 14(3–4):168–174
Tichauer JE, Flores B, Soler B, Eugenin-von Bernhardi L, Ramirez G, von Bernhardi R (2014) Age-dependent changes on TGFbeta1 Smad3 pathway modify the pattern of microglial cell activation. Brain Behav Immun 37:187–196. doi:10.1016/j.bbi.2013.12.018
Tichauer JE, von Bernhardi R (2012) Transforming growth factor-beta stimulates beta amyloid uptake by microglia through Smad3-dependent mechanisms. J Neurosci Res 90(10):1970–1980. doi:10.1002/jnr.23082
Trapp BD, Wujek JR, Criste GA, Jalabi W, Yin X, Kidd GJ, Stohlman S, Ransohoff R (2007) Evidence for synaptic stripping by cortical microglia. Glia 55(4):360–368. doi:10.1002/glia.20462
Trebst C, Konig F, Ransohoff R, Bruck W, Stangel M (2008) CCR5 expression on macrophages/microglia is associated with early remyelination in multiple sclerosis lesions. Mult Scler 14(6):728–733. doi:10.1177/1352458508089359
Umemori H, Sanes JR (2008) Signal regulatory proteins (SIRPS) are secreted presynaptic organizing molecules. J Biol Chem 283(49):34053–34061. doi:10.1074/jbc.M805729200
van Rossum D, Hanisch UK (2004) Microglia. Metab Brain Dis 19(3–4):393–411
van Weering HR, Boddeke HW, Vinet J, Brouwer N, de Haas AH, van Rooijen N, Thomsen AR, Biber KP (2011) CXCL10/CXCR3 signaling in glia cells differentially affects NMDA-induced cell death in CA and DG neurons of the mouse hippocampus. Hippocampus 21(2):220–232. doi:10.1002/hipo.20742
von Bernhardi R (2007) Glial cell dysregulation: a new perspective on Alzheimer disease. Neurotox Res 12(4):215–232
von Bernhardi R, Cornejo F, Parada GE, Eugenin J (2015a) Role of TGFbeta signaling in the pathogenesis of Alzheimer’s disease. Front Cell Neurosci 9:426. doi:10.3389/fncel.2015.00426
von Bernhardi R, Eugenin-von Bernhardi L, Eugenin J (2015b) Microglial cell dysregulation in brain aging and neurodegeneration. Front Aging Neurosci 7:124. doi:10.3389/fnagi.2015.00124
von Bernhardi R, Eugenin J (2004) Microglial reactivity to beta-amyloid is modulated by astrocytes and proinflammatory factors. Brain Res 1–2:186–193. doi:10.1016/j.brainres.2004.07.084 (1025)
von Bernhardi R, Eugenin J (2012) Alzheimer’s disease: redox dysregulation as a common denominator for diverse pathogenic mechanisms. Antioxid Redox Signal 16(9):974–1031. doi:10.1089/ars.2011.4082
von Bernhardi R, Ramírez G (2001) Microglia-astrocyte interaction in Alzheimer’s disease: friends or foes for the nervous system? Biol Res 34(2):123–128
von Bernhardi R, Tichauer JE, Eugenin J (2010) Aging-dependent changes of microglial cells and their relevance for neurodegenerative disorders. J Neurochem 112(5):1099–1114. doi:10.1111/j.1471-4159.2009.06537.x
Wake H, Moorhouse AJ, Jinno S, Kohsaka S, Nabekura J (2009) Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. J Neurosci 29(13):3974–3980. doi:10.1523/JNEUROSCI.4363-08.2009
Walter L, Franklin A, Witting A, Wade C, Xie Y, Kunos G, Mackie K, Stella N (2003) Nonpsychotropic cannabinoid receptors regulate microglial cell migration. J Neurosci 23(4):1398–1405
Walter S, Letiembre M, Liu Y, Heine H, Penke B, Hao W, Bode B, Manietta N, Walter J, Schulz-Schuffer W, Fassbender K (2007) Role of the toll-like receptor 4 in neuroinflammation in Alzheimer’s disease. Cell Physiol Biochem Int J Exp Cell Physiol Biochem Pharmacol 20(6):947–956. doi:10.1159/000110455
Walton NM, Sutter BM, Laywell ED, Levkoff LH, Kearns SM, Marshall GP 2nd, Scheffler B, Steindler DA (2006) Microglia instruct subventricular zone neurogenesis. Glia 54(8):815–825. doi:10.1002/glia.20419
Webster SD, Yang AJ, Margol L, Garzon-Rodriguez W, Glabe CG, Tenner AJ (2000) Complement component C1q modulates the phagocytosis of Abeta by microglia. Exp Neurol 161(1):127–138. doi:10.1006/exnr.1999.7260
Witting A, Muller P, Herrmann A, Kettenmann H, Nolte C (2000) Phagocytic clearance of apoptotic neurons by Microglia/Brain macrophages in vitro: involvement of lectin-, integrin-, and phosphatidylserine-mediated recognition. J Neurochem 75(3):1060–1070
Yirmiya R, Rimmerman N, Reshef R (2015) Depression as a microglial disease. Trends Neurosci 38(10):637–658. doi:10.1016/j.tins.2015.08.001
Zhan Y, Paolicelli RC, Sforazzini F, Weinhard L, Bolasco G, Pagani F, Vyssotski AL, Bifone A, Gozzi A, Ragozzino D, Gross CT (2014) Deficient neuron-microglia signaling results in impaired functional brain connectivity and social behavior. Nat Neurosci 17(3):400–406. doi:10.1038/nn.3641
Ziegler G, Harhausen D, Schepers C, Hoffmann O, Rohr C, Prinz V, Konig J, Lehrach H, Nietfeld W, Trendelenburg G (2007) TLR2 has a detrimental role in mouse transient focal cerebral ischemia. Biochem Biophys Res Commun 359(3):574–579. doi:10.1016/j.bbrc.2007.05.157
Ziv Y, Ron N, Butovsky O, Landa G, Sudai E, Greenberg N, Cohen H, Kipnis J, Schwartz M (2006) Immune cells contribute to the maintenance of neurogenesis and spatial learning abilities in adulthood. Nat Neurosci 9(2):268–275. doi:10.1038/nn1629
Ziv Y, Schwartz M (2008) Immune-based regulation of adult neurogenesis: implications for learning and memory. Brain Behav Immun 22(2):167–176. doi:10.1016/j.bbi.2007.08.006
Acknowledgments
This work was supported by grant FONDECYT 1131025 to RvB.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
von Bernhardi, R., Heredia, F., Salgado, N., Muñoz, P. (2016). Microglia Function in the Normal Brain. In: von Bernhardi, R. (eds) Glial Cells in Health and Disease of the CNS. Advances in Experimental Medicine and Biology, vol 949. Springer, Cham. https://doi.org/10.1007/978-3-319-40764-7_4
Download citation
DOI: https://doi.org/10.1007/978-3-319-40764-7_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-40762-3
Online ISBN: 978-3-319-40764-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)