Abstract
Background
The goal of neurosurgery for cerebral intraparenchymal neoplasms of the eloquent areas is maximal resection with the preservation of normal functions, and minimizing operative risk and postoperative morbidity.
Currently, modern technological advances in neuroradiological tools, neuronavigation, and intraoperative magnetic resonance imaging (MRI) have produced great improvements in postoperative morbidity after the surgery of cerebral eloquent areas.
The integration of preoperative functional MRI (fMRI), intraoperative MRI (volumetric and diffusion tensor imaging [DTI]), and neuronavigation, defined as “functional neuronavigation” has improved the intraoperative detection of the eloquent areas.
Methods
We reviewed 142 patients operated between 2004 and 2010 for intraparenchymal neoplasms involving or close to one or more major white matter tracts (corticospinal tract [CST], arcuate fasciculus [AF], optic radiation).
All the patients underwent neurosurgery in a BrainSUITE equipped with a 1.5 T MR scanner and were preoperatively studied with fMRI and DTI for tractography for surgical planning.
The patients underwent MRI and DTI during surgery after dural opening, after the gross total resection close to the white matter tracts, and at the end of the procedure.
We evaluated the impact of fMRI on surgical planning and on the selection of the entry point on the cortical surface.
We also evaluated the impact of preoperative and intraoperative DTI, in order to modify the surgical approach, to define the borders of resection, and to correlate this modality with subcortical neurophysiological monitoring.
We evaluated the impact of the preoperative fMRI by intraoperative neurophysiological monitoring, performing “neuronavigational” brain mapping, following its data to localize the previously elicited areas after brain shift correction by intraoperative MRI.
Results
The mean age of the 142 patients (89 M/53 F) was 59.1 years and the lesion involved the CST in 66 patients (57 %), the language pathways in 24 (21 %), and the optic radiations in 25 (22 %).
The integration of tractographic data into the volumetric dataset for neuronavigation was technically possible in all cases.
In all patients intraoperative DTI demonstrated a shift of the bundle position caused by the surgical procedure; its dislocation was both outward and inward in the range of +6 mm and −2 mm.
Conclusion
We found a high concordance between fMRI/DTI and intraoperative brain mapping; their combination improves the sensitivity of each technique, reducing pitfalls and so defining “functional neuronavigation”, increasing the definition of eloquent areas and also reducing the time of surgery.
Access provided by CONRICYT-eBooks. Download conference paper PDF
Similar content being viewed by others
Keywords
Introduction
The goal of neurosurgery for cerebral intraparenchymal neoplasms of the eloquent areas is maximal resection with the preservation of normal functions, minimizing the operative risk and postoperative morbidity [1, 4, 6, 32].
Neurophysiological monitoring has been traditionally used to define the exact localization of perirolandic gyri and the corticospinal tract (CST), while awake surgery allows the detection of the phasic cortical areas and the arcuate fasciculus (AF) [6].
Currently, modern technological advances in neuroradiological tools, neuronavigation, and intraoperative magnetic resonance imaging (MRI) have produced great improvements in postoperative morbidity after the surgery of cerebral eloquent areas.
Nevertheless, preoperative functional MRI (fMRI) and diffusion tensor imaging (DTI) are insufficient for accurate neuronavigation because of the brain shift during surgery; thus, their best results are achieved when integrated with intraoperative MRI.
The integration of preoperative fMRI, intraoperative MRI (volumetric and DTI), and neuronavigation, defined as “functional neuronavigation”, improves the intraoperative detection of the eloquent areas by comparison of the previously detected functional areas and neurophysiological brain mapping [6].
We analyzed three points: the benefit of preoperative fMRI during surgery with asleep patients; the role of intraoperative DTI in relation to neuronavigation and brain shift; and the relation between intraoperative DTI and neurophysiological recordings.
Methods and Materials
We reviewed 142 patients operated between 2004 and 2010 for intraparenchymal neoplasms involving or close to one or more major white matter tracts (CST, arcuate fasciculus [AF], optic radiation).
This series included cases affecting the sensorimotor area, the optic radiations, and the AF.
All the patients underwent neurosurgery in the BrainSUITE, BrainLAb Germany, equipped with a 1.5 T MR scanner and were preoperatively studied with fMRI and DTI for tractography for surgical planning.
Neurological examination was performed preoperatively, immediately after surgery, at discharge, and 1 month after surgery.
The patients underwent MRI and DTI during surgery after dural opening, after the gross total resection close to the white matter tracts, and at the end of the procedure.
We evaluated the impact of fMRI on surgical planning and on the selection of the entry point on the cortical surface.
We also evaluated the impact of preoperative and intraoperative DTI in order to modify the surgical approach, to define the borders of resection, and to correlate this modality with subcortical neurophysiological monitoring.
We evaluated the impact of the preoperative fMRI by intraoperative neurophysiological monitoring, performing neuronavigational brain mapping, following its data to localize the previously elicited areas after brain shift correction by intraoperative MRI.
We track the mono- or bipolar probe for neuronavigation through a reference star that is recognized and “neuronavigated”; therefore using it for the dual role of pointer and electrical probe (Fig. 1). Motor-evoked potentials were recorded with subdermal platinum/iridium needle electrodes [5] (MRI-compatible), positioned on muscles of the limbs and face (Fig. 2).
Our neurophysiologist prepared the patients some days before the functional MRI to correctly perform the requested movements; the selected muscles were the same as those elicited during preoperative functional MRI (fMRI).
In all the patients, evaluation of the motor area was confirmed by the study of phase reversal and with “neuronavigational” brain mapping.
Preoperative fMRI was performed to localize the Wernicke cortical area in all the neoplasms affecting the cortical and subcortical language pathways.
Before the study was initiated, the hospital ethics committee was consulted and all patients gave their informed written consent to participate.
Preoperative MR Protocol
The MR examinations were performed by using a 1.5-T magnet (Sonata; Siemens, Erlangen, Germany), identical to the one in the operating room. The following sequences were acquired: T2, FLAIR, and isotropic volumetric T1-weighted magnetization-prepared rapid acquisition gradient echo (MPRAGE) before and after the intravenous administration of paramagnetic contrast material and DT sequences. The DTI study was performed with 12 non-collinear directions (b value = 0 and 1,000 s/mm2) and echo planar sequences (TE 86 ms,TR 9,200 ms, matrix 128 × 128, FOV 240 mm, slice thickness 1.9 mm, bandwidth 1,502 Hz/Px, 60 slices, no gap, acquisition time 5 min 31 s).
Tractography post-processing was performed with a method similar to those presented by Basser et al., Mori et al., and Stieltjes et al. [2, 20, 33], using the planning software iPlan 2.6 (BrainLAB AG, Feldkirchen, Germany).
Color maps were used to define an appropriate region of interest (ROI) for the subsequent tractography procedure. The fiber-tracking technique contemplates the three-dimensional (3D) reconstruction of white matter trajectories of the CST by using a fractional anisotropy threshold of 0.17 and a processing angle above 55°. The positioning of the ROI for the fiber tracking changed according to the trajectories of the fibers to be reconstructed (posterior arm of the internal capsule for the pyramidal tract, geniculate ganglion for the optic radiation and, only in the right-handed patients with lesions on the left side, the ROI encompassed the horizontal fibers lateral to the corona radiata and medial to the cortex of the posterior part of the ventrolateral frontal lobe). Tracking was initiated in both the retrograde and orthograde directions according to the direction of the principal eigenvector in each voxel of the ROI. The reconstructed trajectories were transformed into 3D objects.
Mean data processing time of the CST was 2–3 min, while the mean data processing time of the AF was 8-10 min. Tractography results were saved in a file containing the x/y/z coordinates for each fiber. These data were imported together with the b = 0 diffusion images into the navigation software (import module for iPlan 1.0 programmed by U. Mezger [Brain Lab, Heimstetten, Germany]). After rigid registration of the b = 0 images with the anatomical volumetric package, and after having verified that there were no discrepancies between data (differences greater than 3 mm) in the region of the tumor, the white matter tracts could be displayed in standard anatomical images. Fiber margins were then segmented to allow them to be defined as objects in the navigation system and to be depicted intraoperatively. These objects were automatically enlarged by the software 2 mm in every direction. The trajectories were considered suitable for surgical planning if there were no interruptions on any of the layers at the level of the lesion. The boundaries of the tumors were defined, considering the outer rim of enhancement for grade III and IV gliomas and the T2 signal for grade II gliomas. The distance between the CST and tumor margins was calculated. The mean overall time for this data processing, which was generally performed the day prior to surgery, was 30 min. During surgery the position of the CST was used as a reference for tumor resection.
Intraoperative MR Protocol (BrainSUITE)
Currently we do not perform intraoperative presurgical MRI because of infrared matching of head’s patients with previously acquired volumetric exams, but for prone position.
Total acquisition time and time for sending preoperative images to the neuronavigation system for surgical planning was limited and was less than 2 min.
We perform the first intraoperative MRI after the dural opening, acquiring an intraoperative volumetric MRI for navigation and an intraoperative DTI for tractography. This step is fundamental for correcting possible brain shift.
Average total acquisition and processing time was about 15 min.
The same neuroradiologist reconstructed the white matter tracts and uploaded the new tractographic data in the neuronavigation system; these data were subsequently used for further surgery.
When the neurosurgeon was confident that most of the lesion was removed, intraoperative MRI and DTI were again performed and the same neuroradiologist reconstructed the tractographic data with the previously mentioned technique.
The new tractographic data were again uploaded into the neuronavigation system and subsequently used for further surgery and eventual intraoperative subcortical stimulation.
At the end of the procedure, we performed the last MRI to check the amount of resection and the sparing of the white matter tracts.
Brain Shift Evaluation
Pre- and intraoperative DTI were registered with automatic image fusion software (iPlan 2.6; BrainLAB AG), which was used to perform semiautomatic rigid registration. After registration, the images could be displayed side by side or in an overlay mode. The extent of shifting was considered as the maximum distance between the preoperative and intraoperative contours of the trajectories of the evaluated white matter tracts on identical/registered axial slices.
According to the direction of the shift we assigned positive or negative values in relation to the craniotomy opening. A positive value was assigned if movement was outward (toward the surface), and a negative value was assigned if movement was inward.
We measured peritumoral edema and tumor volume using the planning software noted above, and, as well, we calculated the craniotomy size and the distance of the tumor from the cortical surface.
Results
The mean age of the 142 patients (89 M/53 F) was 59.1 years and the lesion involved the CST in 66 patients (57 %), the language pathways in 24 (21 %), and the optic radiations in 25 (22 %).
All the patients were affected by intraparenchymal neoplasms and histological examination revealed low-grade gliomas, oligodendroglioma, metastases, and high-grade gliomas.
Cortical motor-evoked potentials (MEPs) followed preoperative fMRI and were performed after brain shift correction to choose the optimal site for corticectomy and brain retraction.
Preoperative fMRI permitted us to localize the Wernicke cortical area in all the patients with neoplasms affecting the language areas, except for three who had severe preoperative dysphasic disturbances; AF reconstruction was achieved in all the patients.
Less severe dysphasia did not prejudice the possibility of identifying the Wernicke area and, moreover, did not play any role in DTI.
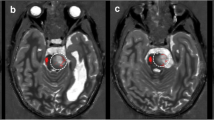
The integration of tractographic data into the volumetric dataset for neuronavigation was technically possible in all cases (Fig. 3).
In all patients intraoperative DTI demonstrated the shift of the bundle position caused by the surgical procedure (Figs. 4 and 5) and its dislocation was both outward and inward, in the range of +6 mm and −2 mm.
We considered that the neuronavigation system had an average error of 0.79 ± 0.25 mm and a maximum error of 2.0 mm..
In 40 % of the cases an outward shift was observed during surgery; an inward shift was observed in 50 %, while in 10 % no intraoperative displacement was detected.
The maximum intraoperative shifting of the CST ranged from an inward value of 9.7 mm to an outward value of 13.8 mm. Only peritumoral edema showed a statistically significant correlation with the amount of shift (P = 0.001), indicating a more pronounced outward shift correlated with larger edema. In a comparison of patients showing inward and outward shifting, statistically significant differences were evident, and peritumoral edema was more pronounced in patients with outward shifting (P =0.001), as was the craniotomy size (P = 0.038). No statistically significant differences were evident when comparing the tumor sizes.
A direct correlation was evident between craniotomy size and shifting after dura mater opening (P = 0.05).
Evaluation of the visualized trajectories related to the lesion produced an a-priori modification of the surgical approach to corticectomy in 21 % of our cases, and in 64 % had an important impact on the definition of the resection margins during surgery. The overall percentage impact on the surgical procedure was 82 %.
In all patients, pre- and intraoperative tractography demonstrated the white matter bundle containing the pyramidal tract, and the motor function was preserved in all but three patients, who showed a transient weakness of the contralateral side, which dramatically improved between 1 month and 3 months after the procedure. In all three cases the lesions were considered to be in contact with the motor cortex and the CST.
During surgery, close to the pyramidal tract, several electrical “neuronavigational” stimulations were repeatedly performed following the neuronavigation after intraoperative volumetric MRI and DTI, demonstrating a deep residual tumor volume and correcting the eventual brain shift. On preoperative tractography, the average overall distance between the lesion and the CST was 2.26 mm and the average distance between the CST and the site of subcortical MEP was 3.5 mm.
We performed subcortical MEPs in 61 % of the cases between 0 and 2 mm from the CST, in 33 % between 4 and 8 mm, and in 6 % between 12 and 15 mm, detecting the CST in all cases by intraoperative neurophysiology, except for two cases, in which the CST was far from the lesion.
Intraoperative postsurgical MRI demonstrated complete tumor removal in all the patients in this series and the postoperative outcome was excellent in all the patients.
The immediate postoperative overall outcome was excellent in 89 % of the patients and we registered a transient worsened motor deficit in only 11 % of the patients.
In 77.7 % of the patients with neoplasms of the language areas a complete resection was performed, while in the remaining patients we performed a subtotal resection.
A subtotal resection was defined in relation to a safe distance of 0–2 mm from the AF or to its neoplastic involvement.
At immediate postoperative examination and at discharge the neurological status was normal in 81.5 % of the patients and preoperative symptoms were improved in 74 % ; the asymptomatic patients maintained their negative neurological status and we did not register any case of deteriorated clinical status, while, in particular, dysphasic disturbances recovered in 81.2 % of the patients.
The outcome was excellent, without deficits affecting a normal quality of life, in 85.1 % of the patients, and good (moderate deficit affecting the normal quality of life) in 14.8 %.
In particular, the patients who underwent complete tumor removal (95.2 %) obtained an excellent outcome despite the aggressive resection (χ 2 p < 0.05).
Discussion
The quality of life in patients affected by malignant neoplasms in eloquent areas must be a priority parameter [8] for choosing surgery rather than biopsy because of their expected short survival.
This goal can be achieved through the exact localization and consequent preservation of the cortical and subcortical functional areas.
It was recently reported that MR tractography may have an impact on surgical planning, leading to changes in the surgical approach and in the limits of resection [31], and in our experience, tractography modified the surgical approach to corticotomy (in 21 % of cases), permitted definition of the resection margins (in 64 %), and resulted in an overall modification of the procedure in 82 % of cases.
The intraoperative DTI for reconstruction of white matter tracts after dural opening and during surgery with subcortical “neuronavigational” neurophysiological monitoring allowed a better resection of these lesions.
We strongly agree with Bello et al. [3], who suggested a strong connection between DTI and subcortical mapping.
Many authors consider resection close to the white matter tracts and, moreover, close to the CST, to be risky and they prefer to maintain a “safe distance” to reduce transient/permanent postoperative morbidity; however, our data disagree with their findings, probably because we had the opportunity of updating DTI data when we were quite close to the tract [6].
DTI and tractography may affect the surgical management of patients with brain tumors [30] and match with electrical mapping in the range of 0–2 cm [1].
Li et al. [18] demonstrated a good correlation between the CST estimated with DTI and the subcortical electrical stimulation; Mikuni et al. [19] also reported this.
Subcortical MEPs were consistently produced at distances of less than 7 mm and were absent at distances of more than 13 mm from the fiber tracking of the pyramidal tracts in the present study.
Gambini et al. [10] reported correspondences between intraoperative subcortical neurostimulation sites and tractography of 84% and 79 % for the motor tract and the speech circuit, respectively.
Intraoperative diffusion tractography solves the problem of ? so well, correlating with subcortical stimulation, as recently demonstrated by Ozawa et al. [28], because intraoperative DTI corrected the brain shift, thus allowing image validation and reporting positive MEPs between 0 and 4.7 mm from the stimulation site to the depicted bundle.
We always updated intraoperative tractography, because, in our experience, brain shift can cause dislocation of the pyramidal tract, in the range of 8 mm; shifting of white matter tracts during neurosurgical procedures has been demonstrated since 2005 by Nimsky et al. [22, 23], ranging from 8 mm inward to 15 mm outward.
In their studies, shifting was observed in 89 % of cases, and shifting was confirmed in our study [1] during tumor removal.
Other authors [16] believe that fiber tracking cannot accurately estimate the size of the white matter tracts using navigation based on preoperative DTI, because it is affected by brain shift both in the deep white matter and the cortex.
Shifting of the deep tumor portions during resection and, consequently, shifting of the white matter tracts, is currently accepted, so that preoperative MRI and DTI should be considered inaccurate [27] because of brain shift.
Standard neuronavigation, without intraoperative upgrade, is inaccurate because of brain shift and because preoperative MRI is a good but insufficient tool for intraoperative “functional” neuronavigation [6].
Brain shift is a serious problem and, even after a simple craniotomy, shifting of up to 0.5-1 cm has been described, and shifting has also been described after the dura was opened [7, 12, 14].
We registered a shift of the white matter bundles of up to 29.7 mm simply after dural opening and Nimsky and colleagues reported a shift of up to 20-24 mm [13, 22, 24].
During surgery the shifting of deep structures, so-called subsurface shifting, seems to be much more relevant [25, 29] than the shifting of cortical structures, which is clearly visible during surgery.
We documented that shift involving the CST can be observed in 90 % of cases during surgery, similarly to other studies [7, 22, 28].
Nimsky et al. [22] described a shift ranging from 28 to +15 mm; for Ozawa et al. [28] the shift ranged from 28 to +8.7 mm, and in our study it ranged from 29.7 to +11 mm.
However, intraoperative tractography has some pitfalls [6], so that we advocate neurophysiological “neuronavigational” monitoring to compensate for these drawbacks.
Duffau et al. [9] stated that direct fiber stimulation was safe, accurate, and reliable, while Kamada et al. [15] observed some technical difficulties, such as the selection of the optimal stimulus point, visually indistinguishable subcortical pathways, continuously interrupted surgery, and long wasted time.
Again, we note that the integration of the neurophysiological techniques with the neuronavigation system, based on the intraoperative DTI, improves the precision of stimulation and reduces the time of the surgical procedure.
These techniques allowed us to reach a verified average distance between the CST and the site of subcortical MEP of 3.93 mm, with an excellent postoperative course.
Similarly to our experience, Nimsky et al. [26] reported mild postoperative deficits, which completely recovered during the postoperative course, and they had only one case of motor deficit, after 3 months.
We believe that the higher incidence of postoperative deficits that other studies report, although this could be due to surgical traction, heat from bipolar coagulation, cytotoxic edema, and/or microvascular reorganization [11], is caused by non-updated and unreliable information regarding the spatial (DTI tractography) and functional (subcortical electric stimulation) anatomy of the CST.
In particular, the intraoperative updating of anatomical and functional data allows us to reverse the surgical strategy, while the use of a stimulator probe “neuronavigated” onto updated tractographic objects guarantees control of the integrity of the tract before removal of the residual lesion volume, in contrast to verifying the integrity of the tract after a potential injury.
The goal is not to remove and to check, but to verify after an intraoperatively guided removal.
In regard to the CST, we recognized parameters—such as the amount of peritumoral edema and the craniotomy size—that predict its shift independent of direction [30], but shifting is not necessarily unidirectional, because the subsurface motion during resection is driven not by external pressure, but by the relief of weight and intraparenchymal pressure [7, 21, 29].
The direction of white matter tract shifting in the outward or inward direction seems to be unpredictable [30].
In our study, an outward shifting of the CST was mostly related to a large amount of edema and to a large craniotomy size, but in patients showing inward shifting, tumor size played the most important role, although we did not find any statistical significance for this parameter [30].
We strongly agree with Nabavi et al. [21], who suggest even more frequent or, if possible, continuous imaging for brain shift tracking, because only serial imaging with high spatial resolution allows the elucidation of deformation patterns with differing reactions to surgical manipulations.
We aim to achieve these results with neuronavigated brain mapping with fMRI and direct cortical stimulation for the surgery of lesions involving the motor and the language cortex.
The identification of the motor cortex is the first step in this surgery, and even if perirolandic gyri have been traditionally identified by phase reversal and by direct motor cortex stimulation, many studies report the limitations and failure of these techniques, especially for perirolandic mass lesions, in which localization was questionable or impossible in 10-18 %, because these tumors produce sensory evoked potential (SEP) latencies and amplitudes of high variability [6].
The neuronavigation with preoperative fMRI helps to better identify the stimulation sites and the precentral gyrus [6, 34].
Electrophysiological difficulties can also be related to neoplastic desynchronization of the afferent electrical impulses and to the mass effect distortion on the cortical electrical dipoles on the brain surface, and these difficulties may eventually lead to the surgeon choosing an inappropriate recording site.
Brain mapping alone allows identification of the primary motor cortex in only 60 % of cases [17], while the combination of neurophysiological monitoring and fMRI improves its accuracy [6], with a high concordance between the procedures.
We absolutely agree with these findings, and we also found a high concordance between fMRI and intraoperative brain mapping; their combination improves the sensitivity of each technique, reducing pitfalls (such as the Blood-oxygen-level dependent (BOLD) effect; motion-related artifacts caused by heartbeat, breathing, or head motion; and a too sensitive signal to large draining veins with poor spatial resolution on fMRI and electrical artifacts on direct cortical stimulation).
This combination (i.e., fMRI and intraoperative brain mapping), which is also defined as “functional neuronavigation”, increases the definition of eloquent areas by comparison of the previously detected functional areas (fMRI) and the brain mapping recordings, and so reduces the time of surgery.
References
Bozzao A, Romano A, Angelini A, D’Andrea G, Calabria LF, Coppola V, Mastronardi L, Fantozzi LM, Ferrante L (2010) Identification of the pyramidal Tract by neuronavigation based on intraoperative magnetic resonance tractography: correlation with subcortical stimulation. Eur Radiol 20:2475–2481
Basser PJ, Pajevic S, Pierpaoli C, Duda J, Aldroubi A (2000) In vivo fiber tractography using DT-MRI data. Magn Reson Med 44(4):625–632
Bello L, Castellano A, Fava E, Casaceli G, Riva M, Scotti G et al (2010) Intraoperative use of diffusion tensor imaging fiber tractography and subcortical mapping for resection of gliomas: technical considerations. Neurosurg Focus 28(2):E6, 1–14
Berger MS, Rostomily RC (1997) Low grade gliomas: functional mapping resection strategies, extent of resection and outcome. J Neuroonc 34:85–101
D’Andrea G, Angelini A, Foresti C, Familiari P, Caroli E, Frati A (2014) Platinum-iridium subdermal magnetic resonance imaging-compatible needle electrodes are suitable for intraoperative neurophysiological monitoring during image-guided surgery with high-field intraoperative magnetic resonance imaging: an experimental study. Neurosurgery 10(3):387–392
D’Andrea G, Angelini A, Romano A, Di Lauro A, Sessa G, Bozzao A, Ferrante L (2012) Intraoperative DTI and brain mapping for surgery of neoplasm of the motor cortex and the corticospinal tract: our protocol and series in BrainSUITE. Neurosurg Rev 35:401–412
Dorward NL, Alberti O, Velani B, Gerritsen FA, Harkness WF, Kitchen ND et al (1998) Postimaging brain distortion: magnitude, correlates, and impact on neuronavigation. J Neurosurg 88(4):656–662
Duffau H (2009) Surgery of low-grade gliomas: towards a ‘functional neurooncology’. Curr Opin Oncol 21(6):543–549
Duffau H, Capelle L, Sichez N, Denvil D, Lopes M, Sichez JP et al (2002) Intraoperative mapping of the subcortical language pathways using direct stimulations. An anatomo-functional study. Brain 125(Pt 1):199–214
Gambini A, Bello L, Falini A (2006) Corrispondenza tra fiber tracking e siti di neurostimolazione sottocorticale intraoperatoria dei circuiti motori e del linguaggio in pazienti affetti da tumori cerebrali. Neuroradiol J 19(Suppl 1):70–71
González-Darder JM, González-López P, Talamantes F, Quilis V, Cortés V, García-March G et al (2010) Multimodal navigation in the functional microsurgical resection of intrinsic brain tumors located in eloquent motor areas: role of tractography. Neurosurg Focus 28(2):E5, 1–10
Hall WA, Liu H, Truwit CL (2005) Functional magnetic resonance imaging-guided resection of low-grade gliomas. Surg Neurol 64(1):20–27
Hastreiter P, Rezk-Salama C, Soza G, Bauer M, Greiner G, Fahlbusch R et al (2004) Strategies for brain shift evaluation. Med Image Anal 8(4):447–464
Hill DL, Maurer CR Jr, Maciunas RJ, Barwise JA, Fitzpatrick JM, Wang MY (1998) Measurement of intraoperative brain surface deformation under a craniotomy. Neurosurgery 43(3):514–528
Kamada K, Todo T, Masutani Y, Aoki S, Ino K, Takano T et al (2005) Combined use of tractography integrated functional neuronavigation and direct fiber stimulation. J Neurosurg 102(4):664–672
Kinoshita M, Yamada K, Hashimoto N, Kato A, Izumoto S, Baba T, Maruno M, Nishimura T, Yoshimine T (2005) Fiber-tracking does not accurately estimate size of fiber bundle in pathological condition: initial neurosurgical experience using neuronavigation and subcortical white matter stimulation. Neuroimage 25(2):424–429
Kombos T, Suess O, Funk T, Kern BC, Brock M (2000) Intraoperative mapping of the motor cortex during surgery in and around the motor cortex. Acta Neurochir (Wien) 142(3):263–268
Li ZX, Jp D, Jiang T et al (2006) Function magnetic resonance imaging and diffusion tensor tractography in patients with brain gliomas involving motor areas: clinical application and outcome. Zhonghua Wai Ke Za Zhi 44(18):1275–1279
Mikuni N, Okada D, Enatsu R et al (2007) Clinical impact of integrated functional neuronavigation and subcortical electrical stimulation to preserve motor function during resection of brain tumours. J Neurosurg 106:593–598
Mori S, VanZijl PC (2002) Fiber tracking: principles and strategies- a technical review. NMR Biomed 15:468–480
Nabavi A, Black PM, Gering DT et al (2001) Serial intraoperative magnetic resonance imaging of brain shift. Neurosurgery 48(4):787–798
Nimsky C, Ganslandt O, Hastreiter P, Wang R, Benner T, Sorensen AG, Fahlbusch R (2005) Intraoperative diffusion-tensor MR imaging: shifting of white matter tracts during neurosurgical procedures – initial experience. Radiology 234(1):218–225
Nimsky C, Ganslandt O, Merhof D, Sorensen AG, Fahlbusch R (2006) Intraoperative visualization of the pyramidal tract by diffusion-tensor-imaging-based fiber tracking. Neuroimage 30(4):1219–1229
Nimsky C, Ganslandt O, Hastreiter P, Wang R, Benner T, Sorensen AG et al (2005) Preoperative and intraoperative diffusion tensor imaging-based fiber tracking in glioma surgery. Neurosurgery 56(1):130–138
Nimsky C, Ganslandt O, Cerny S, Hastreiter P, Greiner G, Fahlbusch R (2000) Quantification of, visualization of, and compensation for brain using intraoperative magnetic resonance imaging. Neurosurgery 47(5):1070–1080
Nimsky C, Ganslandt O, Fahlbusch R (2006) Implementation of fiber tract navigation. Neurosurgery 58(ONS Suppl 2):292–304
O’Shea JP, Whalen S, Branco DM, Petrovich NM, Knierim KE, Golby AJ (2006) Integrated image- and function-guided surgery in eloquent cortex: a technique report. Int J Med Robot 2(1):75–83
Ozawa N, Muragaki Y, Nakamura R et al (2009) Identification of he pyramidal yract by neuronavigation based on intraoperative diffusion weighted imaging combined with subcortical stimulation. Stereotact Funct Neurosurg 87:18–24
Paulsen KD, Miga MI, Kennedy FE, Hoopes PJ, Hartov A, Roberts DW (1999) A computational model for tracking subsurface tissue deformation during stereotactic neurosurgery. IEEE Trans Biomed Eng 46(2):213–225
Romano A, Ferrante M, Cipriani V, Fasoli F, Ferrante L, Fantozzi LM, D’ Andrea G, Bozzao A (2007) Role of magnetic resonance tractography in the preoperative planning and intraoperative assessment of patients with intra-axial brain tumours. Radiol Med 112(6):906–920
Romano A, D’Andrea G, Minniti G et al (2009) Pre-surgical planning and MR-tractography utility in brain tumour resection. Eur Radiol 19(12):2798–2808
Sanai N, Berger MS (2008) Glioma extent of resection and its impact on patient outcome. Neurosurgery 62:753–766
Stieltjes B, Kaufmann WE, van Zijl PC, Fredericksen K, Pearlson GD, Solaiyappan M et al (2001) Diffusion tensor imaging and axonal tracking in the human brainstem. Neuroimage 14(3):723–735
Suess O, Suess S, Brock M, Kombos T (2006) Intraoperative electrocortical stimulation of brodman area 4: a 10-year analysis of 255 cases. Head Face Med 2(20):1–13
Conflict of Interest Statement
We have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing Switzerland
About this paper
Cite this paper
D’Andrea, G., Trillo’, G., Picotti, V., Raco, A. (2017). Functional Magnetic Resonance Imaging (fMRI), Pre-intraoperative Tractography in Neurosurgery: The Experience of Sant’ Andrea Rome University Hospital. In: Visocchi, M., Mehdorn, H.M., Katayama, Y., von Wild, K.R.H. (eds) Trends in Reconstructive Neurosurgery. Acta Neurochirurgica Supplement, vol 124. Springer, Cham. https://doi.org/10.1007/978-3-319-39546-3_36
Download citation
DOI: https://doi.org/10.1007/978-3-319-39546-3_36
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-39545-6
Online ISBN: 978-3-319-39546-3
eBook Packages: MedicineMedicine (R0)