Abstract
Water shortage and pollution are now serious challenges for many countries. Nanomaterials are promising new tools for water quality management due to their unique physicochemical properties, high economic benefit, high removal efficiency and environmental friendliness. Here we present four types of nanomaterials for water and wastewater treatment: nanofiltration membranes, nano-photocatalytic materials, nano-adsorption materials and nano-reducing materials. We discuss their properties, application scope and mechanism of pollutant removal. We also review nanomaterials used for water quality monitoring, especially for the detection of the extremely low concentration organic pollutants, inorganic pollutants and pathogens. Such nanomaterials include carbon nanotubes, magnetic nanoparticles, noble metal nanomaterials and quantum dots.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
6.1 Introduction
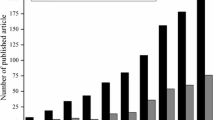
Water is the source of life. Rapid development of economy, heavy application of chemicals and imperfection of water management policy resulted in a series of water problems. Currently, Water shortage and pollution are the two major problems in both developing and developed countries. Water shortage and pollution are influenced by many factors such as human activities, population growth, users demand and global climate change (Savage and Diallo 2005). Polluted waters usually contain suspended matter, heavy metal, organic matter, bacteria, virus and many other complex compounds. Conventional water and wastewater treatment technologies such as adsorption, precipitation, coagulation and activated sludge process have many drawbacks such as low treatment efficiency, high cost and secondary pollution. Due to their unique physicochemical properties, economic benefit, efficiency and environmental friendliness, much attention has been paid to nanomaterials in water quality management recently (Fig. 6.1). Nanomaterials could remove many kinds of pollutants in water and wastewater treatment including refractory matters, organic matters and heavy metals.
Nanotechnology is related to the preparation of materials small than 100 nm in size. Several kinds of nanomaterials, such as iron-based nanomaterials, carbon-based nanomaterials and nTiO2, have been widely studied in water quality management. These nanomaterials possess effective detection, adsorption and removal/degradation capacity to pollutants in water and wastewater. For example, nanoscale zero-valent iron has been proven to remove/degrade pollutants effectively including dyes (Fan et al. 2009), chlorinated solvents (Wang and Zhang 1997), pesticides (Elliott et al. 2009), lead (Ponder et al. 2001; Li and Zhang 2007) and cadmium (Li and Zhang 2007). Nano-Au can sensitively detected chlorpyrifos, malathion (Lisha et al. 2009), Hg2+ and CH3Hg+ (Lin and Tseng 2010).
So far, many relative reviews on nanomaterials in water monitoring and treatment have been reported. Andreescu et al. (2009) reviewed the application of advanced nanomaterials in environmental monitoring; Liu et al. (2014) reviewed the application of nanomaterials for monitoring emerging chemical pollutants; Qu et al. (2013) reviewed the performance of nanomaterials in water and wastewater treatment; Bystrzejewska-Piotrowska et al. (2009) reviewed the application of nanoparticles in environmental management. However, a comprehensive view on nanomaterials in water quality management is still lacking.
In this paper, an overview of recent advances in nanomaterials for water pollution remediation and monitoring were presented. Nanomaterials could be used in the treatment of surface water, ground water and wastewater. Nanofiltration membrane, nano-photocatalytic materials, nano-adsorption materials and nano-reducing materials could remove turbidity, hardness, heavy metal, organic matters and inorganic ions. Besides, nanomaterials including magnetic nanoparticles, carbon nanotubes and noble metal nanomaterials could be used in water quality monitoring. However, cost-effectiveness, technical hurdles and potential risk of nanomaterials are still challenges for their widespread application. Hence, more studies about nanomatrials in water pollution remediation and monitoring need to be done.
6.2 Application of Nanomaterials in Water and Wastewater Treatment
Nanomaterials are the materials which sized below 100 nm at least one dimension. Nanomaterials possess unique properties such as mechanical, electrical, optical, catalytic, magnetic and photonic properties in comparison to common materials. Based on the properties of nanomaterials and the removal principle of pollutants, nanomaterials in water and wastewater treatment can be classified as nanofiltration membrane, nano-photocatalytic materials, nano-adsorption materials and nano-reducing materials. Considering the kinds of pollutants, increasing production and usage of nanomaterials offer opportunities for the removal of various pollutants, which was listed in Table 6.1.
6.2.1 Nanofiltration Membranes
A nanofiltration membrane is a type of semipermeable membrane, which allows solvent molecules or some low molecular weight solutes or low ion permeation. Nanofiltration membranes possess pore size generally of 1–2 nm, molecular weight cut-off of 300–500 Da, water permeability of 5–50 L m−2 h−1 bar−1 and Operating pressure typically of 2–10 bar. Recently, the application of nanofiltration membrane has received wide attention due to its high flux, low investment, low operation pressure and cost. Cellulose acetate, polyamide, polyvinyl alcohol and sulfonated polysulfone can be used to form nanofiltration membrane (Savage and Diallo 2005; Cheng et al. 2011). Nanofiltration membrane could be used in the treatment of surface water, ground water and wastewater. Apart from the purpose to remove turbidity, hardness, fluorides, disinfection by-products and pesticides, recent studies have reported that nanofiltration has also being used for the removal of arsenic and emerging contaminants such as pharmaceuticals, hormones and personal care products (Table 6.2) (Mohammad et al. 2015). However, studies on the removal of the pharmaceutical active compounds from drinking water and surface water by nanofiltration membranes are relatively scarce so far (Radjenović et al. 2008; Verliefde et al. 2007).
The most common application field of nanofiltration membrane is the softening of water. Fang et al. (2013) developed new composite nanofiltration hollow fiber membranes for surface water softening, the results showed that the new hollow fiber membranes rejections for Ca2+ and Mg2+ were 90 % while test for 3000 ppm total dissolved salt feed stream at 2 bar pressure, suggesting that the composite nanofiltration hollow fiber membranes could be effectively applied to surface water softening.
Numerous researches have proved that nanofiltration membrane showed an excellent performance on the remove of pesticides (Van der Bruggen et al. 2001; Van der Bruggen and Vandecasteele 2003). The removal efficiencies of pesticides with nanofiltration membrane were affected by pesticides, types of nanofiltration membrane, transmembrane pressures and solution pH value. Van der Bruggen et al. (2001) found the rejections for simazine, atrazine, diuron and isoproturon were all in the 90–95 % range with nanofiltration membrane. Kiso et al. (2000) studied the removal of 12 pesticides including imidacloprid, simetryn and dichlorvos with four nanofiltration membrane, the results show that the rejections of 12 pesticides decreased in an order from nanofiltration membrane-1 to nanofiltration membrane-4. The removal efficiencies of the pesticides except chlorpyrifos for each nanofiltration membrane were 86.7–99.95 % for nanofiltration membrane-1, 46.2–99.95 % for nanofiltration membrane-2, 3.7–99.32 % for nanofiltration membrane-3, and 2.9–99.51 % for nanofiltration membrane-4, respectively. The rejection of chlorpyrifos was more than 99 % for four types of nanofiltration membrane. Moreover, pesticides might transform and produce new transformation products during transporting to groundwater. The pesticide transformation products (PTPs) have been found in groundwater in recent years, which are different from pesticides (Mohammad et al. 2015). Madsen et al. (Madsen and Søgaard 2014) compared the performance of nanofiltration/low pressure reverse osmosis/reverse osmosis membrane for treatment of pesticides and pesticide transformation products in groundwater, the results show that low pressure reverse osmosis membrane could effectively reject the pesticides and pesticide transformation products, but nanofiltration membrane could not effectively reject pesticide transformation products. Therefore, some measures should be taken to reject pesticide transformation products, such as low pressure reverse osmosis membrane.
Sentana et al. (2010) studied the remove of the disinfection by-products by three commercial nanofiltration (nanofiltration 27, nanofiltration 90 and Desal-HL-51) membranes, the results show that the nanofiltration 90 membrane performed better in reducing in the formation of trihalomethane and haloacetic acid formation potential compared with nanofiltration 90 and Desal-HL-51 membranes.
The problem of arsenic (As) has received wide attention. Nanofiltration membrane was capable of converting As3+ into As5+ and removing As (Sen et al. 2010). However, most studies of the removal of arsenic from groundwater by nanofiltration membrane still at the laboratorial scale.
Hormones stem from agricultural, industrial, medical and domestic activities, which could enter aquatic environment and cause adverse effects to human health. Different studies investigated the feasibility of using nanofiltration to remove hormones, demonstrating that these compounds could be largely rejected according to the adsorption effect (Semião and Schäfer 2011; Schäfer et al. 2011; Sanches et al. 2012).
Although nanofiltration membrane has some great advantages such as high membrane flux, low investment and low operation cost, membrane fouling is still a great limitation. Membrane fouling is occurred when a particle or solute molecule is deposited on a membrane surface or in membrane pores, and then the membrane’s performance is degraded. And it is determined by some factors including concentration polarization, membrane pore blocking and surface deposition. Membrane fouling can lead to some adverse effects such as flux decline, cost increase and membrane degradation. Hence, some measures should be taken to control membrane fouling such as feed pretreatment, membrane surface modification, physical cleaning and chemical cleaning (Mohammad et al. 2015; Hilal et al. 2004). Generally, physical cleaning such as washing, backwashing and immerse, gas-liquid mixing flushing is carried out regularly during the membrane operation. The water permeability of the membrane can achieve a certain degree of recovery in the short time, but the membrane flux will fall again. So physical cleaning can only be used to inhibit the growth of membrane fouling, and can’t make the membrane flux completely recovered. The fouled materials can be washed with chemical agents such as acid, alkali. Acid could make insoluble substances convert into soluble substances. Alkali mainly remove protein, algae and other biological pollutants, colloid pollutants and organic pollutants (Lim and Bai 2003; Madaeni et al. 2001).
6.2.2 Nanophotocatalytic Materials
Photocatalysis is a promising process for the removal of trace contaminants and microbial pathogens (Aarthi and Madras 2007). But the limited photocatalytic activity limits its wide application. Compared to common photocatalytic materials, nano-photocatalytic materials have attracted more attention (Han and Ba 2009; Gupta and Tripathi 2011). Because of their large specific surfaces, nano-photocatalytic materials can enhance photocatalytic activity effectively (Lan et al. 2013). Up to now, nTiO2, nZnO, nWO3, nBiVO4 and nAl2O3 are widely used nano-photocatalytic materials. Among them, nTiO2 is the most commonly used nanomaterial in water and wastewater treatment due to its high reactivity, low toxicity, thermal stability, and abundance as raw material (Table 6.3) (Gupta and Tripathi 2011; Chen and Mao 2007).
In aqueous environment, nTiO2 photocatalyst can generate an electron/hole pair when it is irradiated with energy greater than the band gap. Then, electron/hole pair can migrate to the surface of nTiO2 and form reactive oxygen species (·OH, H2O2, etc.). The positive holes react with H2O and form hydroxyl radical, which promote the oxidation of organics (Kwon et al. 2008). nTiO2 has been successfully applied for the organic wastewater treatment, including dye wastewater (Aarthi and Madras 2007; Nagaveni et al. 2004), chemical industry wastewater, pesticide wastewater, oily wastewater (Yang et al. 2012); inorganic wastewater treatment and microbial control (Kwon et al. 2008; Qu et al. 2013; García et al. 2012). Although nTiO2 has some great advantages in the field of water and wastewater treatment, especially for dye wastewater and paper mill wastewater, it has some drawbacks such as low absorb efficiency of visible light, low recycle rate and high cost. In order to overcome above-mentioned drawbacks, several approaches have been studied including dye sensitization, doping, coupling and capping of nTiO2 (Gupta and Tripathi 2011). In particular, ion doping has received wide attention due to easy operation, high efficiency and more rapid reaction rate. Choi et al. (1994) reported that nTiO2 doped with Fe3+, Mo5+, Ru3+, Os3+, Re5+, V4+ and Rh3+ significantly increased the photochemical reactivity of nTiO2 for the oxidation of trichloromethane and reduction of carbon tetrachloride. The dopant content influences the rate of electron/hole recombination and photocatalytic activity. As the dopant content increases, the electron/hole pairs within the space-charge region are separated by the large electric field before recombination. However, when the dopant content is high, the rate of electron/hole pairs recombination in the nTiO2 increases. Therefore, there is an optimum content of dopant ion. Xin et al. (2007) studied the effect of the different doping ratio Fe3+–nTiO2, the results revealed that the nTiO2 with a low doping concentration of Fe3+ (Fe/Ti lower than 0.03 mol) enhanced the photocatalytic activity of nTiO2. However, the nTiO2 containing a high doping concentration of Fe3+ (Fe/Ti higher than 0.03 mol) is unfavorable to photocatalytic reactions.
6.2.3 Nanoadsorption Materials
Adsorption is commonly employed as pretreatment or advanced treatment to remove organic pollutants, heavy metals and residual chlorine in water and wastewater. The efficiency of nano-adsorption materials is higher than that of conventional absorbents due to high specific surface area, associated sorption sites and surface chemistry.
6.2.3.1 Carbon Based Nanoadsorbents
Carbon nanotubes, a new type of nanomaterials, have received wide attention due to their unique properties, such as large specific surface area, high thermal stability and high chemical stability. Carbon nanotubes can be divided into single-walled carbon nanotubes and multi-walled carbon nanotubes. Carbon nanotubes are good adsorption materials for the remove of organic matter, heavy metal (Table 6.4).
Numerous studies have shown that carbon nanotubes were effective adsorbents and their efficiency was superior to activated carbon on adsorption of organic chemicals in water and wastewater treatment (Pan and Xing 2008; Su and Lu 2007; Wang et al. 2007). Su and Lu (2007) reported that the adsorption capacities of carbon nanotubes on natural dissolved organic matter (11.61 mg g−1) is higher than that of granular activated carbon (3.55 mg g−1), and the average weight losses of the carbon nanotubes (2.65 %) is lower than that of granular activated carbon (6.40 %). El-Sheikh et al. (2008) reported that the absorption capacity of multi-walled carbon nanotubes is three times that of activated carbon towards the pesticides. Long and Yang (2001) found that carbon nanotubes were better than activated carbon for dioxin removal. Its high adsorption capacity is mainly because of the large specific surface area and the pollutants-carbon nanotube interactions. In aqueous environment, carbon nanotubes strongly adsorb low molecular weight polar organic compounds due to the organic compounds-carbon nanotube interactions including hydrophobic effect, π–π interactions, hydrogen bonding, and electrostatic interactions (Pan and Xing 2008; Qu et al. 2013). Different adsorption mechanisms might act simultaneously. The dominant adsorption mechanism might be affected by carbon nanotubes, organic chemicals and environmental conditions (Pan and Xing 2008).
Carbon nanotubes could adsorb heavy metals including Pb2+, Cd2+, Cu2+, Co2+, Ni2+ and Zn2+ effectively (Rao et al. 2007; Pyrzyńska and Bystrzejewski 2010; Li et al. 2005). Pyrzyńska and Bystrzejewski (2010) reported that carbon nanotubes have higher adsorption efficiency towards Co2+ and Cu2+ compared with activated carbons.
Regeneration is a key factor determining the cost-effectiveness of carbon nanotubes. Lu et al. (2006) reported that the adsorption of Zn2+ on single-walled carbon nanotubes and multi-walled carbon nanotubes can be reversed by 0.1 mol L−1 nitric acid solution and the adsorption capacity was maintained after ten cycles of the regeneration and reuse. This suggested that carbon nanotubes could be regenerated by reducing solution pH value. The adsorption capacity of carbon nanotubes was not much fluctuant after several cycles of adsorption/desorption reaction.
6.2.3.2 Metal Based Nanoadsorbents
Iron oxide nanomaterials as adsorbent has received wide attention due to its high surface area, low toxicity and easy synthesis (Deliyanni et al. 2004; Huang et al. 2007; Xu et al. 2012). Iron oxide nanomaterials Iron oxide is a general designation of a large class of substance including many types. nFe3O4, n-γFe2O3 and n-αFe2O3 are the most common three kinds of iron oxides nanomaterials in water and wastewater treatment.
Iron oxide nanomaterials could adsorb a variety of heavy metals including Pb2+, Cd2+, Cu2+, Hg2+, As3+ and Zn2+ (Li and Zhang 2006; Huang and Chen 2009; White et al. 2009); Organic pollutants (e.g., red dye, 1-naphthylamine, polycyclic aromatic hydrocarbons) (Iram et al. 2010; Hu et al. 2011; Zhang et al. 2010a) and radionuclides (Qu et al. 2013) (Table 6.5). Nassar (2010) reported that the maximum adsorption capacity of Pb2+ onto nFe3O4 was much higher than that of reported adsorbents. However, the adsorption of heavy metal onto iron oxide nanomaterials is still at the lab scale (Xu et al. 2012). Practical application is limited.
Other than iron oxide nanoparticle, nTiO2, nZnO and nAl2O3 were also effective adsorbents for the removal of heavy metals, metallic pollutants and radionuclides (Hua et al. 2012). Similar to carbon nanotubes, metal oxide nano-adsorbents could also be regenerated by changing solution pH (Sharma et al. 2009), and then the adsorption capacity remained relatively stable (Hu et al. 2006). However, opposite results were also reported. Deliyanni et al. (2003) reported that adsorption of As5+ on akaganéite-type nanocrystals can be reversed, but the adsorption capacity would reduce about 25–30 % after each cycles of the regeneration and reuse. So akaganéite-type nanocrystals must be replaced after 2–4 regenerations.
6.2.3.3 Polymeric Nanoadsorbents
Recently, polymeric nano-adsorbents have emerged as a novel type of adsorbent materials for the removal of heavy metals and organic pollutants in water and wastewater. Regarding the environmental concerns, these adsorbents are typically made up of polystyrene or polyacrylic ester matrix (Ray and Shipley 2015). Although polymeric nano-adsorbents have excellent properties such as pore size distribution, large surface area, tunable surface chemistry and excellent mechanical rigidity, they have some drawbacks such as low adsorption capacities and high cost (Ray and Shipley 2015; Pan et al. 2009).
6.2.4 Nanoreducing Materials
As a kind of effective reductant for pollutants removal in water, nano zero-valent metals have attracted much attention science 1980s. Iron is a metal with standard redox potential (E0 = −0.44 V). It is thus an effective reductant when reacting with oxidized contaminants in water. Nanoscale zero-valent iron is the particle size of zero-valent iron between 1 and 100 nm. The use of nanoscale zero-valent iron for the removal of contaminants in water and wastewater has received wide attention due to its high reduction performance, large specific surface area and high reactivity. Other types of nano-reducing materials have also been tested for water and wastewater treatment, such as nZn and nNi. In particular, nanoscale zero-valent iron is the most commonly used nanomaterial for groundwater remediation (Mueller et al. 2012). Nanoscale zero-valent iron has been successfully applied for the treatment of water and wastewater contaminated with chlorinated organic compounds (Arnold et al. 2002), arsenic (Kanel et al. 2005), heavy metals, including chromium (Scott et al. 2011), cadmium (Scott et al. 2011), copper (Li and Zhang 2007; Karabelli et al. 2008), silver (Li and Zhang 2007), zinc (Li and Zhang 2007; Klimkova et al. 2011), dyes (Lin et al. 2008; Fan et al. 2009) and phenol (Liu et al. 2005; Elliott et al. 2009; Crane and Scott TB 2012) (Table 6.6). Due to the significant variation in contaminant chemistry, numerous possible contaminant removal pathways have been performed, including sorption, complexation, (co)precipitation and surface mediated chemical reduction.
The pollutants removal by nanoscale zero-valent iron is affected by many factors such as type of pollutants, nanoscale zero-valent iron concentration, temperature and solution pH value. Lin et al. (2008) reported that the removal of AB24 dye by nanoscale zero-valent iron, the results showed that the degradation efficiency of AB24 dye increased with increasing nanoscale zero-valent iron concentration (0–4 g L−1) and temperature (10–50 °C). The reaction rate is highly pH-dependent, the rate constants decreased as the pH increased from 3 to 6 or above 9 and the rate increased as pH increased from 6 to pH 9. The reduction of AB24 dye by ZVI dominated the surface reaction at pH lower than 6; whereas at pH higher than 6, the removal of AB24 dye was mainly due to an adsorption reaction. Li and Zhang (2007) reported that the removal efficiency of eight metal ions including Cd2+, Ni2+, Zn2+, Cr6+, Cu2+, Pb2+ and Ag+ with nanoscale zero-valent iron is 36.5 %, 71.0 %, 92.5 %, 97.5 %, 99.7 %, 99.7 % and 99.8 %. In the study performed by Li and Zhang (2007), as for metals with standard potential E0 very close to or more negative than that of iron (E0, −0.41 V), such as Zn2+ and Cd2+, the removal mechanism is sorption and surface complexation. As for metals with E0 greatly more positive than iron such as Cu2+, Ag+, and Hg2+, the removal mechanism is predominantly reduction. As for metal ions with E0 slightly more positive than iron such as Ni2+ and Pb2+, they can be immobilized at the nanomaterial surface by both sorption and reduction.
6.3 Applications of Nanomaterials for Water Quality Monitoring
Water quality monitoring is of importance to pollution sources control, water quality management and public health. With the development of water quality monitoring technology, some novel technologies were applied in microbial water quality monitoring including phylochips, quantitative real-time polymerase chain reaction and pyrosequencing (Aw and Rose 2012). Besides, previous studies showed that nanomaterials could be used in organic pollutants, inorganic pollutants and pathogen detection, including magnetic nanoparticles, carbon nanotubes, noble metal nanomaterials and quantum dots.
Pathogens detection in water is vital for human health. Many pathogens including bacteria such as Legionella, Escherichia coli and Helicobacter, viruses such as Enteroviruses, Hepatitis viruses and Rotaviruses, and protozoan such as Cryptosporidium and Giardia associated with drinking water are closely related to human diseases. Nanomaterial enabled pathogens sensors are consist of recognition agents, nanomaterials and a signal transduction mechanism. Among three components, nanomaterials are used to improve the detection sensitivity and response of pathogens due to their unique properties such as optical, electrochemical and magnetic properties.
Magnetic nanomaterials and carbon nanotubes have been applied for sample concentration and purification. Magnetic nanocomposite can be used to develop pathogen detection kits. Although carbon nanotubes performed the excellent sensitivity, heterogeneity is a great challenge. The carbon nanotubes production and purification processes often introduce contaminants and impurities, and even the carbon nanotubes structure degradation. Hence, it is necessary to produce homogeneous carbon nanotubes.
Hahn et al. (2005) used functionalized quantum dots to detect single cells of Escherichia coli O157: H7 serotype, the results showed that quantum dots were superior to traditional fluorescent dyes in terms of sensitivity and stability. Quantum dots can overcome the limitations of traditional fluorescence dyes and simultaneously detect multiple emission peaks from a single wavelength of light. For example, quantum dots can detect Escherichia coli O157: H7 and salmonella typhimurium simultaneously and expected to detect 3–4 species simultaneously in the near future (Yang and Li 2006). Taking advantage of the optical properties of quantum dots, they will help in pathogen detection certainly (Yang and Li 2006; Hahn et al. 2005). Quantum dots were capable of differentiating minute amounts of pathogenic bacterial cells among larger populations of innocuous cells due to their sensitivity and larger absorption cross sections (Hahn et al. 2005).
Nanomaterials can also be used in the detection of organic and inorganic pollutants. Nano-Au could detect chlorpyrifos and malathion at per billion levels from surface water (Lisha et al. 2009). Lysozyme Type VI-Stabilized Gold Nanoclusters was used to detect Hg2+ and CH3Hg+ (Lin and Tseng 2010), the limits of detection for Hg2+ and CH3Hg+ were estimated to be 3 pM and 4 nM. Lysozyme Type VI-Stabilized Gold Nanoclusters provided an about 330-fold improvement in the detection of Hg2+ in comparison to bovine serum albumin-stabilized gold Nanoclusters. More immortally, this probe was successfully used in seawater. Vega et al. (2007) reported the use of the carbon nanotube-modified glassy carbon electrode for the detection of phenolic estrogens.
6.4 Challenges of Applying Nanomaterials in Water Quality Management
Although nanomaterials enabled water and wastewater treatment and monitoring have shown great potentials in the future, cost-effectiveness and technical hurdles are still challenges for their development and commercialization. The cost of nanomaterials is relatively high. Studies on some nanomaterials such as nanoscale zero-valent iron are mostly carried out at the lab scale. Many laboratory studies have evaluated the performance of nanoscale zero-valent iron for removing various pollutants. However, the research on the treatment performance and the long-term performance of real water and waste water with nanomaterials is limited due to the short time of laboratory studies and complication of real water and wastewater.
In addition, potential risk of nanomaterials is another challenge for their widespread application. An increasing number of nanomaterials will be released to the environment due to the increasing application of nanomaterials in water and wastewater treatment and monitoring, which have attracted more and more concern. The environmental behavior and possible environmental effects of nanomaterials are still unknown. Human health risk assessment and ecological risk assessment of nanomaterials are limited (Moore 2006). Relevant laws and regulations were still lacking. Hence, more studies about nanotoxicology and nanoecotoxicology need to be done.
6.5 Conclusion
The application of nanomaterials in water quality management has received wide attention due to their unique electrical, mechanical, catalytic, optical, magnetic and photonic properties. Nanofiltration membrane could be used in the production of potable water and the removal of metals, disinfection by-products, pesticides and emerging contaminants from contaminated water. However membrane fouling is still a great limitation. nTiO2 has some excellent performance in the field of water and wastewater treatment, especially for dye wastewater and paper mill wastewater. The surface modification of nTiO2 is being studied for optimization. However, it has some drawbacks including narrow light response range and low recycle rate. Carbon nanotubes, iron oxides nanomaterials, nTiO2 and polymeric nano-adsorbents have shown high adsorption capacities. Enhancing the regeneration of absorbents must be explored to reduce the cost in water quality management. In addition, nanoscale zero-valent iron could be used to remove heavy metals and organic pollutants by reduction or oxidation and degree of removal could be enhanced through functionalization. However, the persistence of activity is limited. Nanomaterials-based sensors have the potential to detect heavy metals, organic pollutants and pathogen in water and wastewater. Nanomaterials are used to improve the detection sensitivity and response speed of pollutions. In a word, nanomaterials have received extensive research in water pollution remediation and monitoring. However, there are many problems for their practical application need to be study and solve including cost-effectiveness, technical hurdles and potential risk of nanomaterials.
References
Aarthi T, Madras G (2007) Photocatalytic degradation of rhodamine dyes with nano-TiO2. Ind Eng Chem Res 46:7–14
Aarthi T, Madras G (2008) Photocatalytic reduction of metals in presence of combustion synthesized nano-TiO2. Catal Commun 9:630–634
Ahmad A, Rafatullah M, Sulaiman O, Ibrahim MK, Chii YY, Siddique BM (2009) Removal of Cu(II) and Pb(II) ions from aqueous solutions by adsorption on sawdust of Meranti wood. Desalination 247:636–646
Andreescu S, Njagi J, Ispas C, Ravalli MT (2009) JEM spotlight: applications of advanced NMs for environmental monitoring. J Environ Monit 11:27–40
Arnold WA, Winget P, Cramer CJ (2002) Reductive dechlorination of 1,1,2,2-tetrachloroethane. Environ Sci Technol 36:3536–3541
Aw TG, Rose JB (2012) Detection of pathogens in water: from phylochips to qPCR to pyrosequencing. Curr Opin Biotechnol 23:422–430
Banerjee SS, Chen DH (2007) Fast removal of copper ions by gum Arabic modified magnetic nano-adsorbent. J Hazard Mater 147:792–799
Bódalo A, Gómez E, Hidalgo AM, Gómez MM, Murcia MD, López I (2009) Nanofiltration membranes to reduce phenol concentration in wastewater. Desalination 245:680–686
Bystrzejewska-Piotrowska G, Golimowski J, Urban PL (2009) Nanoparticles: their potential toxicity, waste and environmental management. Waste Manag 29:2587–2595
Cao J, Elliott D, Zhang W (2005) Perchlorate reduction by nanoscale iron particles. J Nanopart Res 7:499–506
Chakrabortty S, Roy M, Pal P (2013) Removal of fluoride from contaminated groundwater by cross flow nanofiltration: transport modeling and economic evaluation. Desalination 313:115–124
Chang YC, Chang SW, Chen DH (2006) Magnetic chitosan nanoparticles: studies on chitosan binding and adsorption of Co(II) ions. React Funct Polym 66:335–341
Chang F, Liu W, Wang X (2014) Comparison of polyamide nanofiltration and low-pressure reverse osmosis membranes on as(III) rejection under various operational conditions. Desalination 334:10–16
Chen X, Mao SS (2007) Titanium dioxide nanomaterials: synthesis, properties, modifications, and applications. Chem Rev 107:2891–2959
Chen C, Wang X (2006) Adsorption of Ni(II) from aqueous solution using oxidized multiwall carbon nanotubes. Ind Eng Chem Res 45:9144–9149
Chen C, Li X, Zhao D, Tan X, Wang X (2007) Adsorption kinetic, thermodynamic and desorption studies of Th(IV) on oxidized multi-wall carbon nanotubes. Colloid Surf A Physicochem Eng Asp 302:449–454
Chen Z, Jin X, Chen Z, Megharaj M, Naidu R (2011) Removal of methyl orange from aqueous solution using bentonite-supported nanoscale zero-valent iron. J Colloid Interf Sci 363:601–607
Cheng R, Wang J, Zhang W (2007) Comparison of reductive dechlorination of p-chlorophenol using Fe0 and nanosized Fe0. J Hazard Mater 144:334–339
Cheng S, Oatley DL, Williams PM, Wright CJ (2011) Positively charged nanofiltration membranes: review of current fabrication methods and introduction of a novel approach. Adv Colloid Interf 164:12–20
Choe S, Lee SH, Chang YY, Hwang K, Khim J (2001) Rapid reductive destruction of hazardous organic compounds by nanoscale Fe0. Chemosphere 42:367–372
Choi W, Termin A, Hoffmann MR (1994) The role of metal ion dopants in quantum-sized TiO2: correlation between photoreactivity and charge carrier recombination dynamics. J Phys Chem 98:13669–13679
Crane RA, Scott TB (2012) Nanoscale zero-valent iron: future prospects for an emerging water treatment technology. J Hazard Mater 211:112–125
Dai K, Peng T, Chen H, Zhang R, Zhang Y (2008) Photocatalytic degradation and mineralization of commercial methamidophos in aqueous titania suspension. Environ Sci Technol 42:1505–1510
Deliyanni EA, Bakoyannakis DN, Zouboulis AI, Matis KA (2003) Sorption of as (V) ions by akaganeite-type nanocrystals. Chemosphere 50:155–163
Deliyanni EA, Lazaridis NK, Peleka EN, Matis KA (2004) Metals removal from aqueous solution by iron-based bonding agents. Environ Sci Pollut Res 11:18–21
Du Q, Zhang S, Pan B, Lv L, Zhang W, Zhang Q (2013) Bifunctional resin-ZVI composites for effective removal of arsenite through simultaneous adsorption and oxidation. Water Res 47:6064–6074
Ducom G, Cabassud C (1999) Interests and limitations of nanofiltration for the removal of volatile organic compounds in drinking water production. Desalination 124:115–123
Elliott DW, Lien HL, Zhang WX (2009) Degradation of lindane by zero-valent iron nanoparticles. J Environ Eng 135:317–324
El-Sheikh AH, Sweileh JA, Al-Degs YS, Insisi AA, Al-Rabady N (2008) Critical evaluation and comparison of enrichment efficiency of multi-walled carbon nanotubes, C18 silica and activated carbon towards some pesticides from environmental waters. Talanta 74:1675–1680
Fan J, Guo Y, Wang J, Fan M (2009) Rapid decolorization of azo dye methyl orange in aqueous solution by nanoscale zerovalent iron particles. J Hazard Mater 166:904–910
Fan X, Yang H, Tian W, Nie A, Hou T, Qiu F, Zhang X (2011) Catalytic oxidation of chlorobenzene over MnOx/Al2O3-carbon nanotubes composites. Catal Lett 141:158–162
Fang Z, Chen J, Qiu X, Qiu X, Cheng W, Zhu L (2011) Effective removal of antibiotic metronidazole from water by nanoscale zero-valent iron particles. Desalination 268:60–67
Fang W, Shi L, Wang R (2013) Interfacially polymerized composite nanofiltration hollow fiber membranes for low-pressure water softening. J Membr Sci 430:129–139
Frost RL, Xi Y, He H (2010) Synthesis, characterization of palygorskite supported zero-valent iron and its application for methylene blue adsorption. J Colloid Interface Sci 341:153–161
García A, Delgado L, Torà JA, Casals E, González E, Puntes V, Font X, Carrera J, Sánchez A (2012) Effect of cerium dioxide, titanium dioxide, silver, and gold nanoparticles on the activity of microbial communities intended in wastewater treatment. J Hazard Mater 199:64–72
Gupta SM, Tripathi M (2011) A review of TiO2 nanoparticles. Chin Sci Bull 56:1639–1657
Hahn MA, Tabb JS, Krauss TD (2005) Detection of single bacterial pathogens with semiconductor quantum dots. Anal Chem 77:4861–4869
Han H, Ba R (2009) Buoyant photocatalyst with greatly enhanced visible-light activity prepared through a low temperature hydrothermal method. Ind Eng Chem Res 48:2891–2898
Harisha RS, Hosamani KM, Keri RS, Nataraj SK, Aminabhavi TM (2010) Arsenic removal from drinking water using thin film composite nanofiltration membrane. Desalination 252:75–80
Hilal N, Al-Zoubi H, Darwish NA, Mohamma AW, Arabi MA (2004) A comprehensive review of nanofiltration membranes: treatment, pretreatment, modelling, and atomic force microscopy. Desalination 170:281–308
Hu J, Chen G, Lo IMC (2006) Selective removal of heavy metals from industrial wastewater using maghemite nanoparticle: performance and mechanisms. J Environ Eng 132:709–715
Hu J, Lo IMC, Chen G (2007) Performance and mechanism of chromate (VI) adsorption by δ-FeOOH-coated maghemite (γ-Fe2O3) nanoparticles. Sep Purif Technol 58:76–82
Hu J, Shao D, Chen C, Sheng G, Ren X, Wang X (2011) Removal of 1-naphthylamine from aqueous solution by multiwall carbon nanotubes/iron oxides/cyclodextrin composite. J Hazard Mater 185:463–471
Hua M, Zhang S, Pan B, Zhang W, Lv L, Zhang Q (2012) Heavy metal removal from water/wastewater by nanosized metal oxides: a review. J Hazard Mater 211:317–331
Huang SH, Chen DH (2009) Rapid removal of heavy metal cations and anions from aqueous solutions by an amino-functionalized magnetic nano-adsorbent. J Hazard Mater 163:174–179
Huang YH, Hsueh CL, Cheng HP, Su LC, Chen CY (2007) Thermodynamics and kinetics of adsorption of Cu(II) onto waste iron oxide. J Hazard Mater 144:406–411
Iram M, Guo C, Guan Y, Ishfaq A, Liu H (2010) Adsorption and magnetic removal of neutral red dye from aqueous solution using Fe3O4 hollow nanospheres. J Hazard Mater 181:1039–1050
Jiang Z, Lv L, Zhang W, Du Q, Pan B, Yang L, Zhang Q (2011) Nitrate reduction using nanosized zero-valent iron supported by polystyrene resins: role of surface functional groups. Water Res 45:2191–2198
Joo SH, Zhao D (2008) Destruction of lindane and atrazine using stabilized iron nanoparticles under aerobic and anaerobic conditions: effects of catalyst and stabilizer. Chemosphere 70:418–425
Kanel SR, Manning B, Charlet L, Choi H (2005) Removal of arsenic(III) from groundwater by nanoscale zero-valent iron. Environ Sci Technol 39:1291–1298
Kanel SR, Greneche JM, Choi H (2006) Arsenic(V) removal from groundwater using nano scale zero-valent iron as a colloidal reactive barrier material. Environ Sci Technol 40:2045–2050
Karabelli D, Üzüm C, Shahwan T, Eroğlu AE, Scott TB, Keith R, Hallam KR, Lieberwirth L (2008) Batch removal of aqueous Cu2+ ions using nanoparticles of zero-valent iron: a study of the capacity and mechanism of uptake. Ind Eng Chem Res 47:4758–4764
Kim Y, Lee B, Yi J (2003) Preparation of functionalized mesostructured silica containing magnetite(MSM) for the removal of copper ions in aqueous solutions and its magnetic separation. Sep Sci Technol 38:2533–2548
Kim SA, Kamala-Kannan S, Lee KJ, Parkb Y, Shea PJ, Lee W, Kim H, Oh B (2013) Removal of Pb(II) from aqueous solution by a zeolite–nanoscale zero-valent iron composite. Chem Eng J 217:54–60
Kiso Y, Nishimura Y, Kitao T, Nishimura K (2000) Rejection properties of non-phenylic pesticides with nanofiltration membranes. J Membr Sci 171:229–237
Klimkova S, Cernik M, Lacinova L, Filip J, Jancik D, Zboril R (2011) Zero-valent iron nanoparticles in treatment of acid mine water from in situ uranium leaching. Chemosphere 82:1178–1184
Kwon S, Fan M, Cooper AT, Yang H (2008) Photocatalytic applications of micro-and nano-TiO2 in environmental engineering. Crit Rev Env Sci Technol 38:197–226
Lan Y, Lu Y, Ren Z (2013) Mini review on photocatalysis of titanium dioxide nanoparticles and their solar applications. Nano Energy 2:1031–1045
Lee C, Kim JY, Lee WI, Nelson KL, Yoon J, Sedlak DL (2008) Bactericidal effect of zero-valent iron nanoparticles on Escherichia coli. Environ Sci Technol 42:4927–4933
Li X, Zhang W (2006) Iron nanoparticles: the core-shell structure and unique properties for Ni(II) sequestration. Langmuir 22:4638–4642
Li X, Zhang W (2007) Sequestration of metal cations with zerovalent iron nanoparticles a study with high resolution X-ray photoelectron spectroscopy (HR-XPS). J Phys Chem C 111:6939–6946
Li YH, Wang S, Cao A, Zhao D, Zhang X, Xu C, Luan Z, Ruan D, Liang J, Wu D, Wei B (2001) Adsorption of fluoride from water by amorphous alumina supported on carbon nanotubes. Chem Phys Lett 350:412–416
Li YH, Wang S, Wei J, Zhang X, Xu C, Luan Z, Wu D, Wei B (2002) Lead adsorption on carbon nanotubes. Chem Phys Lett 357:263–266
Li YH, Ding J, Luan Z, Di Z, Zhu Y, Xu C, Wu D, Wei B (2003a) Competitive adsorption of Pb2+, Cu2+ and Cd2+ ions from aqueous solutions by multiwalled carbon nanotubes. Carbon 41:2787–2792
Li YH, Wang S, Luan Z, Ding J, Xu C, Wu D (2003b) Adsorption of cadmium(II) from aqueous solution by surface oxidized carbon nanotubes. Carbon 41:1057–1062
Li YH, Di Z, Ding J, Wu D, Luan Z, Zhu Y (2005) Adsorption thermodynamic, kinetic and desorption studies of Pb2+ on carbon nanotubes. Water Res 39:605–609
Lien HL (2005) Application and implication of zero-valent iron for transformation of disinfection by-products: a comparison study between nanoscale palladized iron and microscale iron. J Chin Inst Environ Eng 15:213–221
Lien HL, Zhang W (2001) Nanoscale iron particles for complete reduction of chlorinated ethenes. Colloids Surf A Physicochem Eng Asp 191:97–105
Liga MV, Bryant EL, Colvin VL, Li Q (2011) Virus inactivation by silver doped titanium dioxide nanoparticles for drinking water treatment. Water Res 45:535–544
Lim AL, Bai R (2003) Membrane fouling and cleaning in microfiltration of activated sludge wastewater. J Membr Sci 216:279–290
Lin YH, Tseng WL (2010) Ultrasensitive sensing of Hg2+ and CH3Hg+ based on the fluorescence quenching of lysozyme type VI-stabilized gold nanoclusters. Anal Chem 82:9194–9200
Lin YT, Weng CH, Chen FY (2008) Effective removal of AB24 dye by nano/micro-size zero-valent iron. Sep Purif Technol 64:26–30
Lisha KP, Anshup, Pradeep T (2009) Enhanced visual detection of pesticides using gold nanoparticles. J Environ Sci Health B 44:697–705
Liu Y, Majetich SA, Tilton RD, Sholl DS, Lowry GV (2005) TCE dechlorination rates, pathways, and efficiency of nanoscale iron particles with different properties. Environ Sci Technol 39:1338–1345
Liu T, Wang ZL, Zhao L, Yang X (2012) Enhanced chitosan/Fe0-nanoparticles beads for hexavalent chromium removal from wastewater. Chem Eng J 189:196–202
Liu Q, Zhou Q, Jiang G (2014) Nanomaterials for analysis and monitoring of emerging chemical pollutants. TrAC-Trend Anal Chem 58:10–22
Long RQ, Yang RT (2001) Carbon nanotubes as superior sorbent for dioxin removal. J Am Chem Soc 123:2058–2059
Long F, Gong J, Zeng G, Chen L, Wang X, Deng J, Niu Q, Zhang H, Zhang X (2011) Removal of phosphate from aqueous solution by magnetic Fe–Zr binary oxide. Chem Eng J 171:448–455
Lu C, Chiu H (2006) Adsorption of zinc(II) from water with purified carbon nanotubes. Chem Eng Sci 61:1138–1145
Lu C, Chung YL, Chang KF (2005) Adsorption of trihalomethanes from water with carbon nanotubes. Water Res 39:1183–1189
Lu C, Chiu H, Liu C (2006) Removal of zinc(II) from aqueous solution by purified carbon nanotubes: kinetics and equilibrium studies. Ind Eng Chem Res 45:2850–2855
Luo S, Qin P, Shao J, Peng L, Zeng Q, Gu J (2013) Synthesis of reactive nanoscale zero valent iron using rectorite supports and its application for orange II removal. Chem Eng J 223:1–7
Madaeni SS, Mohamamdi T, Moghadam MK (2001) Chemical cleaning of reverse osmosis membranes. Desalination 134:77–82
Madsen HT, Søgaard EG (2014) Applicability and modelling of nanofiltration and reverse osmosis for remediation of groundwater polluted with pesticides and pesticide transformation products. Sep Purif Technol 125:111–119
Mahdavian AR, Mirrahimi MAS (2010) Efficient separation of heavy metal cations by anchoring polyacrylic acid on superparamagnetic magnetite nanoparticles through surface modification. Chem Eng J 159:264–271
Maher A, Sadeghi M, Moheb A (2014) Heavy metal elimination from drinking water using nanofiltration membrane technology and process optimization using response surface methodology. Desalination 352:166–173
Mohammad AW, Teow YH, Ang WL, Chung YT, Oatley-Radcliffe DL, Hilal N (2015) Nanofiltration membranes review: recent advances and future prospects. Desalination 356:226–254
Moore MN (2006) Do nanoparticles present ecotoxicological risks for the health of the aquatic environment? Environ Int 32:967–976
Mueller NC, Braun J, Bruns J, Černík M, Rissing P, Rickerby D, Nowack B (2012) Application of nanoscale zero valent iron (NZVI) for groundwater remediation in Europe. Environ Sci Pollut Res 19:550–558
Musbah I, Ciceron D, Saboni A, Alexandrova S (2013) Retention of pesticides and metabolites by nanofiltration by effects of size and dipole moment. Desalination 313:51–56
Nagaveni K, Sivalingam G, Hegde MS, Madras G (2004) Solar photocatalytic degradation of dyes: high activity of combustion synthesized nano TiO2. Appl Catal B Environ 48:83–93
Nassar NN (2010) Rapid removal and recovery of Pb(II) from wastewater by magnetic nanoadsorbents. J Hazard Mater 184:538–546
Pan B, Xing B (2008) Adsorption mechanisms of organic chemicals on carbon nanotubes. Environ Sci Technol 42:9005–9013
Pan B, Pan B, Zhang W, Lv L, Zhang Q, Zheng S (2009) Development of polymeric and polymer-based hybrid adsorbents for pollutants removal from waters. Chem Eng J 151:19–29
Parra S, Olivero J, Pulgarin C (2002) Relationships between physicochemical properties and photoreactivity of four biorecalcitrant phenylurea herbicides in aqueous TiO2 suspension. Appl Catal B Environ 36:75–85
Peng X, Li Y, Luan Z, Di Z, Wang H, Tian B, Jia Z (2003) Adsorption of 1, 2-dichlorobenzene from water to carbon nanotubes. Chem Phys Lett 376:154–158
Ponder SM, Darab JG, Bucher J, Caulder D, Craig L, Davis L, Edelstein N, Lukens W, Nitsche H, Rao L, Shuh DK, Mallouk TE (2001) Surface chemistry and electrochemistry of supported zerovalent iron nanoparticles in the remediation of aqueous metal contaminants. Chem Mater 13:479–486
Pyrzyńska K, Bystrzejewski M (2010) Comparative study of heavy metal ions sorption onto activated carbon, carbon nanotubes, and carbon-encapsulated magnetic nanoparticles. Colloid Surf A 362:102–109
Qu X, Alvarez PJJ, Li Q (2013) Applications of nanotechnology in water and wastewater treatment. Water Res 47:3931–3946
Radjenović J, Petrović M, Ventura F, Barceló D (2008) Rejection of pharmaceuticals in nanofiltration and reverse osmosis membrane drinking water treatment. Water Res 42:3601–3610
Rao GP, Lu C, Su F (2007) Sorption of divalent metal ions from aqueous solution by carbon nanotubes: a review. Sep Purif Technol 58:224–231
Ray PZ, Shipley HJ (2015) Inorganic nano-adsorbents for the removal of heavy metals and arsenic: a review. RSC Adv 5:29885–29907
Sanches S, Penetra A, Rodrigues A, Ferreira E, Cardoso VV, Benoliel MJ, Crespo MTB, Pereira VJ, Crespo JG (2012) Nanofiltration of hormones and pesticides in different real drinking water sources. Sep Purif Technol 94:44–53
Savage N, Diallo MS (2005) Nanomaterials and water purification: opportunities and challenges. J Nanopart Res 7:331–342
Schäfer AI, Akanyeti I, Semião AJC (2011) Micropollutant sorption to membrane polymers: a review of mechanisms for estrogens. Adv Colloid Interf 164:100–117
Schierz A, Zänker H (2009) Aqueous suspensions of carbon nanotubes: surface oxidation, colloidal stability and uranium sorption. Environ Pollut 157:1088–1094
Scott TB, Popescu IC, Crane RA, Noubactep C (2011) Nano-scale metallic iron for the treatment of solutions containing multiple inorganic contaminants. J Hazard Mater 186:280–287
Semião AJC, Schäfer AI (2011) Estrogenic micropollutant adsorption dynamics onto nanofiltration membranes. J Membr Sci 381:132–141
Sen M, Manna A, Pal P (2010) Removal of arsenic from contaminated groundwater by membrane-integrated hybrid treatment system. J Membr Sci 354:108–113
Sentana I, Rodríguez M, Sentana E, M’Birek C, Prats D (2010) Reduction of disinfection by-products in natural waters using nanofiltration membranes. Desalination 250:702–706
Sharma YC, Srivastava V, Singh VK, Kaul SN, Weng CH (2009) Nano-adsorbents for the removal of metallic pollutants from water and wastewater. Environ Technol 30:583–609
Shi L, Zhang X, Chen Z (2011) Removal of chromium(VI) from wastewater using bentonite-supported nanoscale zero-valent iron. Water Res 45:886–892
Shih Y, Hsu C, Su Y (2011) Reduction of hexachlorobenzene by nanoscale zero-valent iron: kinetics, pH effect, and degradation mechanism. Sep Purif Technol 76:268–274
Shu HY, Chang MC, Chen CC, Chen P (2010) Using resin supported nano zero-valent iron particles for decoloration of acid blue 113 azo dye solution. J Hazard Mater 184:499–500
Skubal LR, Meshkov NK (2002) Reduction and removal of mercury from water using arginine-modified TiO2. J Photoch Photobio A 148:211–214
Su F, Lu C (2007) Adsorption kinetics, thermodynamics and desorption of natural dissolved organic matter by multiwalled carbon nanotubes. J Environ Sci Health A 42:1543–1552
Tian H, Li J, Mu Z, Li L, Hao Z (2009) Effect of pH on DDT degradation in aqueous solution using bimetallic Ni/Fe nanoparticles. Sep Purif Technol 66:84–89
Üzüm Ç, Shahwan T, Eroğlu AE, Lieberwirth I, Scott TB, Hallam KR (2008) Application of zero-valent iron nanoparticles for the removal of aqueous Co2+ ions under various experimental conditions. Chem Eng J 144:213–220
Van der Bruggen B, Vandecasteele C (2003) Removal of pollutants from surface water and groundwater by nanofiltration: overview of possible applications in the drinking water industry. Environ Pollut 122:435–445
Van der Bruggen B, Everaert K, Wilms D, Vandecasteele C (2001) Application of nanofiltration for removal of pesticides, nitrate and hardness from ground water: rejection properties and economic evaluation. J Membr Sci 193:239–248
Vega D, Agüí L, González-Cortés A, Yáñez-Sedeño P, Pingarrón JM (2007) Electrochemical detection of phenolic estrogenic compounds at carbon nanotube-modified electrodes. Talanta 71:1031–1038
Venkatachalam N, Palanichamy M, Murugesan V (2007) Sol–gel preparation and characterization of alkaline earth metal doped nano TiO2: efficient photocatalytic degradation of 4-chlorophenol. J Mol Catal A Chem 273:177–185
Verliefde A, Cornelissen E, Amy G, Van der Bruggen B, van Dijk H (2007) Priority organic micropollutants in water sources in Flanders and the Netherlands and assessment of removal possibilities with nanofiltration. Environ Pollut 146:281–289
Vinu R, Madras G (2007) Kinetics of simultaneous photocatalytic degradation of phenolic compounds and reduction of metal ions with nano-TiO2. Environ Sci Technol 42:913–919
Vinu R, Madras G (2008) Kinetics of sonophotocatalytic degradation of anionic dyes with nano-TiO2. Environ Sci Technol 43:473–479
Vulliet E, Emmelin C, Chovelon JM, Guillard C, Herrmann J (2002) Photocatalytic degradation of sulfonylurea herbicides in aqueous TiO2. Appl Catal B Environ 38:127–137
Wang CB, Zhang WX (1997) Synthesizing nanoscale iron particles for rapid and complete dechlorination of TCE and PCBs. Environ Sci Technol 31:2154–2156
Wang SG, Liu XW, Gong WX, Nie W, Gao B, Yue Q (2007) Adsorption of fulvic acids from aqueous solutions by carbon nanotubes. J Chem Technol Biotechnol 82:698–704
Wei L, Yang G, Wang R, Ma W (2009) Selective adsorption and separation of chromium(VI) on the magnetic iron–nickel oxide from waste nickel liquid. J Hazard Mater 164:1159–1163
White BR, Stackhouse BT, Holcombe JA (2009) Magnetic γ-Fe2O3 nanoparticles coated with poly-l-cysteine for chelation of as(III), Cu(II), Cd(II), Ni(II), Pb(II) and Zn(II). J Hazard Mater 161:848–853
Xin B, Ren Z, Wang P, Liu J, Jing L, Fu H (2007) Study on the mechanisms of photoinduced carriers separation and recombination for Fe3+–TiO2 photocatalysts. Appl Surf Sci 253:4390–4395
Xu Y, Zhao D (2007) Reductive immobilization of chromate in water and soil using stabilized iron nanoparticles. Water Res 41:2101–2108
Xu P, Zeng GM, Huang DL, Feng CL, Hu S, Zhao MH, Lai C, Wei Z, Huang C, Xie XG, Liu ZF (2012) Use of iron oxide nanomaterials in wastewater treatment: a review. Sci Total Environ 424:1–10
Yang L, Li Y (2006) Simultaneous detection of Escherichia coli O157: H7 and salmonella typhimurium using quantum dots as fluorescence labels. Analyst 131:394–401
Yang K, Wu W, Jing Q, Zhu L (2008) Aqueous adsorption of aniline, phenol, and their substitutes by multi-walled carbon nanotubes. Environ Sci Technol 42:7931–7936
Yang Y, Wang H, Li J, He B, Wang T, Liao S (2012) Novel functionalized nano-TiO2 loading electrocatalytic membrane for oily wastewater treatment. Environ Sci Technol 46:6815–6821
Yu H, Wang X, Sun H, Huo M (2010) Photocatalytic degradation of malathion in aqueous solution using an Au–Pd–TiO2 nanotube film. J Hazard Mater 184:753–758
Yuan P, Fan M, Yang D, He H, Liu D, Yuan A, Zhu J, Chen T (2009) Montmorillonite-supported magnetite nanoparticles for the removal of hexavalent chromium [Cr(VI)] from aqueous solutions. J Hazard Mater 166:821–829
Zhang S, Niu H, Hu Z, Cai Y, Shi Y (2010a) Preparation of carbon coated Fe3O4 nanoparticles and their application for solid-phase extraction of polycyclic aromatic hydrocarbons from environmental water samples. J Chromatogr A 1217:4757–4764
Zhang X, Lin Y, Shan X, Chen Z (2010b) Degradation of 2, 4, 6-trinitrotoluene (TNT) from explosive wastewater using nanoscale zero-valent iron. Chem Eng J 158:566–570
Zhang Y, Li Y, Li J, Hu L, Zheng X (2011) Enhanced removal of nitrate by a novel composite: nanoscale zero valent iron supported on pillared clay. Chem Eng J 171:526–531
Zhao X, Wang J, Wu F, Wang T, Cai Y, Shi Y, Jiang G (2010) Removal of fluoride from aqueous media by Fe3O4@Al(OH)3 magnetic nanoparticles. J Hazard Mater 173:102–109
Zhong PS, Widjojo N, Chung TS, Weber M, Maletzko C (2012) Positively charged nanofiltration (NF) membranes via UV grafting on sulfonated polyphenylenesulfone (sPPSU) for effective removal of textile dyes from wastewater. J Membr Sci 417:52–60
Zhu H, Jia Y, Wu X, Wang H (2009) Removal of arsenic from water by supported nano zero-valent iron on activated carbon. J Hazard Mater 172:1591–1596
Acknowledgement
This work was supported by the Fundamental Research Funds for the Central Universities, and the Research Funds of Renmin University of China (Grant No. 11XNK016), which are greatly acknowledged.
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Xue, Xy., Cheng, R., Shi, L., Ma, Z., Zheng, X. (2016). Nanomaterials for Monitoring and Remediation of Water Pollution. In: Ranjan, S., Dasgupta, N., Lichtfouse, E. (eds) Nanoscience in Food and Agriculture 2. Sustainable Agriculture Reviews, vol 21. Springer, Cham. https://doi.org/10.1007/978-3-319-39306-3_6
Download citation
DOI: https://doi.org/10.1007/978-3-319-39306-3_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-39305-6
Online ISBN: 978-3-319-39306-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)