Abstract
Hyperuricemia, particularly gout, and the immune inflammatory response are highly integrated. Both, long standing hyperuricemia and monosodium urate (MSU) crystal deposition can challenge tendon homeostasis because of their potential to cause inflammation to the host. Knowledge is emerging from clinical imaging research depicting where MSU crystals deposit, including patellar tendon, triceps and quadriceps tendons. Remarkably, subclinical tendon inflammation and damage are also present in asymptomatic hyperuricemia. Monosodium urate crystals act as danger activating molecular patterns (DAMPs), activating the inflammasome and inducing the secretion of IL-1beta, a key mediator of the inflammatory response. The crucial role of IL-1beta in driving the inflammatory events during gout attacks is supported by the clinical efficacy of IL-1beta blockade. Some data implicating IL-1beta as an initiator of tendinopathy exist, but the link between hyperuricemia and the development of tendinopathy remains to be validated. Further knowledge about the interactions of uric acid with both innate immune and tendon cells, and their consequences may help to determine if there is a subclass of hyperuricemic-tendinopathy.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
People with hyperuricemia rela ted tendinopathy represent the intersection between two prevalent entities: millions with hyperuricemia and the millions of people who suffer tendinopathy. The rising frequency of both, hyperuricemia and tendinopathy, is associated to a combination of factors including increased longevity, and shifts in diet and lifestyle.
Hyperuricemia is the gateway for gout, an old disease that concerns almost all civilizations over the ages. The increasing prevalence of hyperuricemia, along with the doubling of the rate of gout in the past decades [56], and its association with important comorbidities such as cardiovascular disease, metabolic syndrome or diabetes among others is creating a heavy burden by driving up medical expenditures.
Gout describes an inflammatory arthritis resulting from monosodium urate (MSU) crystal deposition in specific anatomical sites including joints, and tendons. Currently, medical English has retained old Greek and Latin names such as podagra (from podos, foot and agreos attack), to describe acute gout attacks, more specifically MSU crystal deposition in the first metatarsophalangeal joint. Tophus, (from the Latin tofus meaning porous stone), depicts nodular masses of MSU crystals, most commonly occurring at the base of the great toe and fingers.
Hyperuricemia is defined as a serum urate level higher than 6.8 mg/dL (0.40 μmol/L). Remarkably, patients who overproduce uric acid represent fewer than 20 % of those with gout [11]. As described below, not only gout and associated inflammation, but hyperuricemia can be an intrinsic element that directs immune activities, favoring the development and progression of tendinopathy. Indeed, hyperuricemia can induce cellular and/or metabolic distress [1], thereafter tendon extracellular matrix degeneration as well as sub-clinical inflammation.
In this chapter, we first summarize the main characteristics of hyperuricemia/gout and tendinopathy. Then, we discuss clinical and biological information that can mean an association between both pathologies, and can help us in substantiating a more precise medicine by explaining different subclasses of tendinopathies driven by new diagnostic.
Hyperuricemia and Tendinopathy?
The hypothesis that hyperuricemia can have a role in the development of tendinopathy is based on recent advances of crystal biology and the pathophysiology of tendinopathy.
Hyperuricemia/Gout
Uric acid , (a heterocyclic purine derivative 7,9-dihydro-1H-purine-2, 6, 8 (3H)-trione), is the degradation product of purine metabolism after xanthine oxidase oxidizes purines (Fig. 11.1). In mammals, other than humans and primates, uricase (urate oxidase) forms soluble allantoin, thereby lowering uric acid levels below 2 mg/dL. Instead, human subjects lack uricase, thus reference range of serum urate is higher, i.e. 3.4–7.2 mg/dL for men and 2.4–6.1 mg/dL for women.
Two important physiological mechanisms decide the levels of uric acid in body fluids. On one hand, purine metabolism in the liver, i.e. 2/3 of uric acid is formed naturally and purines in the diet produce 1/3. Thus, excessive intake of food rich in purines can induce overproduction of uric acid, and acute hyperuricemia. On the other hand, renal function is involved in the systemic adjustments of urate levels, i.e. glomerular filtration, tubular reabsorption, secretion and post-secretory reabsorption. Alterations in these mechanisms can cause chronic hyperuricemia.
The fact that native levels of serum urate are very close to saturation point (7.0 mg/dL) suggests that this molecule has important roles in maintaining tissue homeostasis. Indeed, serum urate is a reducing agent that accounts for almost half of the antioxidant potential of blood, influences redox potential and protects against oxidative damage. Uric acid prevents the toxicity by reactive oxygen and nitrogen species with negative influence on critical cell functions. Recent data show that uric acid is protective against Alzheimer, Parkinson, amyotrophic lateral sclerosis, and multiple sclerosis [27, 49]. Instead, the crystalline form that develops after chronic (longstanding) hyperuricemia is well-known because of its inflammatory properties.
Hyperuricemia is the most important risk factor for inflammatory gout, but peculiarly many people with hyperuricemia do not follow crystal deposition and gout attacks. To add complexity, sometimes serum urate concentration is within the normal range during acute gout attacks [36]. In one cohort study with a follow up of 5 years, gout developed in only 22 % of patients with urate levels above 9 mg/dL (535 umol/L) [11]. Figure 11.2a depicts the clinical stages from hyperuricemia to chronic gout.
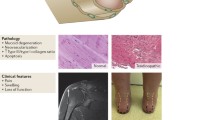
Clinical outcome of hyperuricemia/gout (a), and tendinopathy (b)
(a) Asymptomatic stages, without or with urate deposition, are followed by acute intermittent painful flares that occur most often in lower extremities. Inter-critical periods become shorter as disease progresses. People with advanced chronic gout can have tophi, usually in distal areas of the body.
(b) Tendinopathy, initially characterized by temporary irritation and molecular inflammation, is considered a degenerative disease with changes in extracellular matrix composition and loss of tissue architecture. Ensuing detrimental mechanical properties can result in partial tears or total rupture [42]
Vulnerability to gout is also attributed to genetic influences. The kidneys reabsorb about 90 % of the daily load of filtered urate, and specific anion transporters mediate the process. Actually, genome wide studies have identified polymorphisms, associated with serum urate levels, in various loci codifying glucose and urate protein transporters in the kidney, including GLUT9 (SLC2A9), and ABCG2 [35, 52, 58]. However, data derived from rheumatologic studies in identical twins show a concordance rate of 53 % in monozygotic and 24 % in dizygotic twin pairs, indicating that the genetic susceptibility requires other concomitant factors [25]. Despite increasing knowledge, the factors controlling formation and deposition of MSU crystals are not fully understood.
Inflammatory Cell Reactions to MSU Crystals
Broadly speaking two processes occur after MSU deposition, first cell detection of the crystals, and secondly activation of the inflammatory resp onse driven by chemokines and cytokines (please also see Chap. 20).
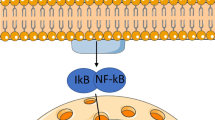
Both immune cells and local cells can detect MSU crystals (Fig. 11.3). As described later, patients with hyperuricemia have elevated levels of circulating MCP-1, a chemokine that mobilizes monocyte/macrophages [21]. Monocyte/macrophages can phagocytose crystals that interact with the inflammasome, of which NLRP3 (NOD-like receptor protein-3) is the best characterized. This interaction triggers activation of caspase 1 in the cytosol, followed by cleavage of pro-IL-1beta into mature IL-1beta. Secreted IL-1beta binds to the IL-1R, present in the membrane of different cell phenotypes. In doing so, IL-1beta activates an inflammatory cascade by promoting the expression and secretion of additional inflammatory molecules, mainly cytokines and chemokines. Other reported inflammatory pathways involve the engagement of TLR2 and TLR4 receptors [31, 32, 53, 54].
Cartoon showing a tentative model that links hyperuricemia with tendinopathy, mainly through activation an inflammatory cascade driven by IL-1beta on immune and tendon cells
MSU crystal detected and phagocyted by macrophages (1), interact with the inflammasome (2) inducing the activation of caspase 1. Ensuing cleavage of pro-IL-1b by caspase 1 (3), generation and secretion of active IL-1beta switch on the inflammatory cascade. IL-1beta binds to IL-1R in macrophages (4) and tendon cells (4) and set in motion gene transcription and production of inflammatory molecules
The crucial role of IL-1beta in initiating the inflammatory cascade during gout attacks is supported by the efficacy of IL-1beta blockade therapy in patients with gout initiating urate-lowering therapy [40]. In fact, several anti-IL-1beta therapies have been tested to prevent gout flares after the sudden urate decline (at the beginning of urate lowering treatment) which induces the release of MSU crystals from tophus.
Supporting the innate immune response to gout, MSU crystals induced cultured monocytes to express the chemokine IL-8, a potent neutrophil chemoattractant [21]. Actually, neutrophil infiltration is a feature of crystal-induced inflammation.
MSU deposition affects not only innate immune cells but also tendon cells. Indeed, the presence of MSU crystals induced a reduction of tendon cell viability and reduced the expression of ECM proteins, specifically type 1 collagen. Besides, MSU crystals up-regulate MMP-2, −3, −13, ADAMTS1, ADAMTS4 and ADAMTS5 [14] (Chhana 2014). Despite up-regulation of ECM detrimental enzymes, tendon rupture in gout is less frequent than cartilage damage and bone erosions. The latter are frequent in gout, as well as decreased viability and function of osteoblast [13]. Of note, altered bone functions may have implication at the enthesis.
Both, MSU and hyperuricemia, are inflammatory adjuvants triggering an adaptive immune response (i.e. antigen-specific response mediated by T, and B cells). Remarkably, it is believed that MSU induced sterile inflammation is different from uric acid mediated adjuvancity, [34] but our understanding of the immunological mechanisms underlying adjuvanticity is still incomplete.
Tendinopathy
The etiology of tendinopathy is multifactorial often triggered by vulnerability factors. Since the myth of the Greek hero Achilles of the Trojan War, telling how vulnerable a tendon is, much has been learned, and current research has produced several biological hypothesis based on histopathological, biochemical and clinical findings that show cell apoptosis, angiofibroblastic features or abnormal biochemical adaptations. These findings suggest that a failed healing response underlies the condition [4].
Here we focus on two mechanisms underlying the loss of tendon homeostasis, i.e. inflammation and cell death, because they are particularly relevant to provide a tentative relationship between hyperuricemia and tendinopathy.
Inflammation
Classical signs of active inflammation , such as histological evidence of leukocyte infiltrates, are scarce in degenerative tendinopathy [3]. For example, the presence of mast cells shows in the patellar tendon of patients with pain and swelling [53] and, in calcaneal overuse tendinopathy [46]. Instead, in acute reactive tendinitis, such as De Quervain disease , inflammatory components, including neutrophil elastase, cyclooxygenase, as well as macrophage infiltration affecting collagen structure is evident [26].
The presence of an inflammatory molecular milieu in degenerative tendinopathy is more obvious, as local tendon cells can synthesize inflammatory molecules in response to tissue stress. This consideration differs from the classical view of inflammation, embodied by the presence of inflammatory cells. Essentially, the biochemical adaptation of prolonged repetitive mechanical loading produces cytokines such as IL-1beta, IL-6, IL-8 and TNF-alpha, prostaglandins such as PGE2, and neuropeptides such as substance P [17, 18, 29, 38]. Some harmful effects of inflammatory cytokines include up-regulated VEGF production along with enhanced production of metalloproteinases, such as MMP-1 MMP-3 and MMP-13 [57] that cause matrix destruction.
In accordance with the concept of intrinsic tendon healing deficits and the weak response to injury, during acute early healing Achilles, tendons did not display detectable levels of pro-inflammatory molecules. Merely, pleiotropic cytokines including IL-6 and IL-8 were elevated [2].
Cell Death and Local Hyperuricemia
Another histopathological feature that emerges in tendinopathy is cell death, suggesting that the problem is the loss of homeostasis, and failed healing response that creates a vicious circle between cell death and progressive matrix disruption leading to chronic degeneration. At least one study has shown many apoptotic cells in ruptured supraspinatus tendon [59], and other studies showed excessive apoptosis in patellar tendinopathic specimens in athletes [30], and noninsertional Achilles tendinopathy [43].
Cell death is associated with sterile inflammation induced by intracellular alarmins released to the extracellular space [39]. Alarmins are danger activating molecular pattern (DAMP) , because they can alert the host by mobilizing innate immune cells, and ensuing inflammatory response through Toll-like receptors (TLR). Uric acid is one of these alarmins. Cells produce even more uric acid when they die and DNA and RNA are metabolized. Actually, large-scale cell death induces robust MSU precipitation caused by intracellular urate released to the extracellular space, creating a supersaturated solution in the high sodium extracellular environment [55].
In what circumstances monosodium urate precipitate, nucleate and form inflammatory crystals have been the focus of recent experimental research. Indeed, the molecular composition of the milieu may influence both the amount and size of crystals, and their inflammatory properties. Besides, crystallization depends on hydration, and extracellular pH, and often occurs in areas of compromised vascularity where the ability to regulate temperature is hampered. Animal studies have proposed that immunoglobulins may be part of a positive feedback loop promoting uric acid crystallization and immunogenicity [23].
Activated tendon cells drive immune cell infiltration [7], the duration and intensity of inflammation; they also control the switch from acute to chronic inflammation. In this context, we have explored whether tendon cells can sense hyperuricemia in their biological milieu, and whether hyperuricemic PRP can incite tendon cells to switch to an inflammatory phenotype. Actually, tenocytes express the main receptors involved in sterile inflammation, TLR2 and TLR4 [16], but it is not clear in what circumstances these receptors are functional. Because serum amyloid protein primed synovial fibroblasts to produce active IL-1beta and IL-1alpha when exposed to high uric acid and MSU crystals, we hypothesized that hyperuricemic PRP with native levels of amyloid protein could trigger a molecular inflammatory response by tendon cells. But, we found that hyperuricemia is a minor stressor for tendon cells as it mitigates the modest inflammatory effect induced by PRP reducing the expression and synthesis of IL-6 and IL8 [5, 6].
Instead, the presence of MSU crystals induce the reduction of tendon cell viability and reduce expression of ECM proteins, specifically type 1 collagen and in parallel up-regulates catabolic ECM proteins [14]. Interestingly, MSU crystals were identified next to and invading the tendon, and at the enthesis [14].
Clinical Observations
The hypothesis that uric acid may play a role in the development, and progression of tendinopathy is predicated on the presence of low-grade inflammation in hyperuricemic patients, and recent findings on tendon imaging.
Low-Grade Inflammation
One potential explanation for how longstanding hyperuricemia can modify tendon homeostasis is the presence of low-grade systemic inflammation. For instance, elevated levels of MCP-1/CCL2, a chemokine involved in leukocyte trafficking, are displayed in patients with acute gout and hyperuricemia [21]. Moreover, in these patients MCP-1/CCL2 concentrations in serum correlates with the increased number of circulating CD14+ monocytes, and the adhesion molecule CD11b. In asymptomatic subjects, there is an association between serum levels of uric acid with inflammatory markers including CRP, IL-6, IL-18 and TNF-a [51].
These findings are consistent with human studies showing enhanced levels of circulating CD14+ monocytes, not only in patients with gout, but also in asymptomatic hyperuricemia compared to normouricemic patients.
Besides, neutrophils from gout patients are primed for enhanced MSU crystal induced superoxide production. Moreover, this neutrophil function persists in asymptomatic hyperuricemi c, and the inflammatory environment likely contributes to higher IL-8 production and neutrophil survival in the absence of direct crystal stimulation [33].
Tendon Imaging
Advances of imaging science permits taking a closer look at soft tissues [12]. A recent ultrasound study has identified not only cartilage, but also tendons as tissues with high frequency deposition of MSU crystals. In particular, the patellar tendon, triceps and quadriceps tendons are often affected [19, 41].
Besides, tophi causing flexor tenosynovitis along with dactylitis is often seen in patients with gout [8, 10]. Gouty tophi causing ruptures of tendons and ligaments are more unusual. Even so, anecdotic tophaceous depositions have shown in the anterior cruciate ligament [37], and distal quadriceps tendon [9]. Moreover, peculiar acute podagra happened in the Achilles tendon of a young long distance runner with normouricemia [22].
Interestingly, subclinical tendon inflammation and subclinical structural damage have been reported in people with asymptomatic hyperuricemia [45, 47]. For example, enthesopathy in the patellar tendon was found in 12 % of hyperuricemic patients and 2 % of normouricemic. Accordingly, Achilles tendon enthesopathy was present in 15 % of hyperuricemic contrasting with 1.9 % of normouricemic subjects. Corroborating these data, Achilles tendon ruptures, characterized as acute trauma of chronically degenerated tendons, occur more often in asymptomatic patients with hyperuricemia than in asymptomatic normouricaemic subjects [45].
Imaging continues to yield information relevant to tendinopathy, and in the past years ultrasound is routinely used by sports physician as a tool for the diagnosis of tendon pathology. When treating tendinopathic symptoms, we shall keep in mind the odds of gouty tophus within patellar tendon [15, 20, 50]. Experience in visualizing the ultrasonographic features of MSU crystals or gouty tophus may accelerate the diagnosis and treatment modality depending on clinical stage.
In the same way, DECT (dual energy computerized tomography) provides correct views in patients with tophaceous go ut and has shown MSU deposition affecting the Achilles tendon and peroneal tendons [15, 24, 28, 48].
Conclusions
Hyperuricemia is an increasingly important metabolic condition. However, its clinical repercussions in tendons are often underestimated, and not clearly understood. While little evidence is available to implicate hyperuricemia in the pathogenesis of tendinopathy, it is more obvious that crystal deposition in and around tendons during gout attacks can trigger cell death, as a consequence of the loss of homeostatic collagen tension owing to microscopic collagen breakdown. Indeed, tendon cells lacking appropriate ECM attachment are rapidly eliminated.
But, not only mechanical interference of crystals with the extracellular matrix, also inflammatory reactions of monocytes/macrophages and tendon cells, ensuing from crystal deposition and IL-1beta induced inflammation, can favor the progression of tendinopathy.
Nevertheless, while the biological mechanisms underlying MSU activation of inflammation are understood, there is little information about hyperuricemia-mediated adjuvancity in tendinopathy. Knowledge about the interactions of urate with both innate immune and local cells, may help the research community to determine if there is a subclass of hyperuricemic-tendinopathy, and set the grounds for clarifying the biology and mechanism behind hyperuricemia linked tendinopathy.
There is, however, much to investigate because most concepts exposed here are still speculative, and future research has to focus on how hyperuricemia-mediated adjuvancity works in tendon inflammation, cell death and extracellular matrix deterioration.
A two-stage approach, firstly urate-lowering therapy designed to dissolve MSU crystals and, secondly keeping uric acid below saturation point for long-life has been recommended [44]. Imaging can be used to evaluate outcomes until MSU crystals are dissolved. Rheumatologists often prescribe prophylactic treatments such as colchicine to minimize inflammatory flares during the initial stages of urate lowering therapy. Investigational prophylactic treatments based on anti-IL-1b blockade are promising in patients who have contraindications for colchicine and NSAIDs [40] and may be beneficial for tendons.
Contrasting with current trends of precision medicine, tendinopathy management is unspecific and merely palliative. Patient heterogeneity hinders advances in novel treatments and clinical trial design claims subpopulations are more clearly understood. Exploring potential connections between tendinopathy and hyperuricemia, and determining whether or not there is a subtype of tendinopathy induced by hyperuricemia may help to tackle part of this important problem. Should the growing evidence that the high urate level is a risk factor for tendinopathy become accepted would have a major impact on the diagnostic and treatment of tendinopathy.
Abbreviations
- ADAMTS1:
-
A Disintegrin-Like And Metalloprotease (Reprolysin Type) With Thrombospondin Type 1 Motif, 1
- ADAMTS4:
-
A Disintegrin-Like And Metalloprotease (Reprolysin Type) With Thrombospondin Type 1 Motif, 4
- ADAMTS5:
-
A Disintegrin-Like And Metalloprotease (Reprolysin Type) With Thrombospondin Type 1 Motif, 5
- CRP:
-
C reactive protein
- DAMP:
-
Danger Associated Molecular patterns
- DECT:
-
dual energy computerized tomography
- DNA:
-
deoxynucleic acid
- IL-1beta, IL-1alpha, IL-6, IL-8, IL-18:
-
Interleukins
- MCP-1/CCL2:
-
macrophage chemotactic protein
- MMP-2, MMP-3, MMP-13:
-
metalloproteinases
- MSU:
-
monosodium urate
- NLRP3:
-
NOD-like receptor protein 3
- NSAIDs:
-
non-steroidal anti-inflammatory drugs
- PGE2:
-
prostaglandin E2
- RNA:
-
ribonucleic acid
- TLR2, TLR4:
-
Toll like receptors
- TNF-alpha:
-
tumor necrosis factor alpha
- VEGF:
-
vascular endothelial growth factor
References
Abate M, Schiavone C, Salini V, Andia I (2013) Occurrence of tendon pathologies in metabolic disorders. Rheumatology (Oxford) 52(4):599–608
Ackermann PW, Domeij-Arverud E, Leclerc P, Amoudrouz P, Nader GA (2013) Anti-inflammatory cytokine profile in early human tendon repair. Knee Surg Sports Traumatol Arthrosc 21(8):1801–1806
Alfredson H (2005) The chronic painful Achilles and patellar tendon: research on basic biology and treatment. Scand J Med Sci Sports 15(4):252–259
Andia I, Sanchez M, Maffulli N (2010) Tendon healing and platelet-rich plasma therapies. Expert Opin Biol Ther 10(10):1415–1426
Andia I, Rubio-Azpeitia E, Maffulli N (2014) Hyperuricemic PRP in tendon cells. Biomed Res Int 2014:926481
Andia I, Rubio-Azpeitia E (2014) Angiogenic and innate immune responses triggered by PRP in tendon cells are not modified by hyperuricemia. Muscles Ligaments Tendons J 4(3):292–297
Andia I, Rubio-Azpeitia E, Maffulli N (2015) Platelet-rich plasma modulates the secretion of inflammatory/angiogenic proteins by inflamed tenocytes. Clin Orthop Relat Res 473(5):1624–1634
Aslam N, Lo S, McNab I (2004) Gouty flexor tenosynovitis mimicking infection: a case report emphasising the value of ultrasound in diagnosis. Acta Orthop Belg 70(4):368–370
Bond JR, Sim FH, Sundaram M (2004) Radiologic case study. Gouty tophus involving the distal quadriceps tendon. Orthopedics 27(1):18, 90–92
Bullocks JM, Downey CR, Gibler DP, Netscher DT (2009) Crystal deposition disease masquerading as proliferative tenosynovitis and its associated sequelae. Ann Plast Surg 62(2):128–133
Campion EW, Glynn RJ, DeLabry LO (1987) Asymptomatic hyperuricemia. Risks and consequences in the normative aging study. Am J Med 82(3):421–426
Chen CK, Chung CB, Yeh L, Pan HB, Yang CF, Lai PH, Liang HL, Resnick D (2000) Carpal tunnel syndrome caused by tophaceous gout: CT and MR imaging features in 20 patients. AJR Am J Roentgenol 175(3):655–659
Chhana A, Callon KE, Pool B, Naot D, Watson M, Gamble GD, McQueen FM, Cornish J, Dalbeth N (2011) Monosodium urate monohydrate crystals inhibit osteoblast viability and function: implications for development of bone erosion in gout. Ann Rheum Dis 70(9):1684–1691
Chhana A, Callon KE, Dray M, Pool B, Naot D, Gamble GD, Coleman B, McCarthy G, McQueen FM, Cornish J, Dalbeth N (2014) Interactions between tenocytes and monosodium urate monohydrate crystals: implications for tendon involvement in gout. Ann Rheum Dis 73(9):1737–1741
Dalbeth N, Kalluru R, Aati O, Horne A, Doyle AJ, McQueen FM (2013) Tendon involvement in the feet of patients with gout: a dual-energy CT study. Ann Rheum Dis 72(9):1545–1548
de Mos M, Joosten LA, Oppers-Walgreen B, van Schie JT, Jahr H, van Osch GJ, Verhaar JA (2009) Tendon degeneration is not mediated by regulation of Toll-like receptors 2 and 4 in human tenocytes. J Orthop Res 27(8):1043–1047
Fredberg U, Stengaard-Pedersen K (2008) Chronic tendinopathy tissue pathology, pain mechanisms, and etiology with a special focus on inflammation. Scand J Med Sci Sports 18(1):3–15
Fedorczyk JM, Barr AE, Rani S, Gao HG, Amin M, Amin S, Litvin J, Barbe MF (2010) Exposure-dependent increases in IL-1beta, substance P, CTGF, and tendinosis in flexor digitorum tendons with upper extremity repetitive strain injury. J Orthop Res 28(3):298–307
Forbess LJ, Fields TR (2012) The broad spectrum of urate crystal deposition: unusual presentations of gouty tophi. Semin Arthritis Rheum 42(2):146–154
Gililland JM, Webber NP, Jones KB, Randall RL, Aoki SK (2011) Intratendinous tophaceous gout imitating patellar tendonitis in an athletic man. Orthopedics 34(3):223
Grainger R, McLaughlin RJ, Harrison AA, Harper JL (2013) Hyperuricaemia elevates circulating CCL2 levels and primes monocyte trafficking in subjects with inter-critical gout. Rheumatology (Oxford) 52(6):1018–1021
Gunawardena H, Churn P, Blake DR (2005) Running for gout research. Rheumatology (Oxford) 44(8):1073–1074
Kanevets U, Sharma K, Dresser K (2009) Shi Y A role of IgM antibodies in monosodium urate crystal formation and associated adjuvanticity. J Immunol 182(4):1912–1918
Kimura-Hayama E, Criales-Vera S, Nicolaou S, Betanzos JL, Rivera Y, Alberú J, Rull-Gabayet M, Hernández-Molina G (2014) A pilot study on dual-energy computed tomography for detection of urate deposits in renal transplant patients with asymptomatic hyperuricemia. J Clin Rheumatol 20(6):306–309
Krishnan E, Lessov-Schlaggar CN, Krasnow RE, Swan GE (2012) Nature versus nurture in gout: a twin study. Am J Med 125(5):499–504
Kuo YL, Hsu CC, Kuo LC, Wu PT, Shao CJ, Wu KC, Wu TT, Jou IM (2015) Inflammation is present in de quervain disease–correlation study between biochemical and histopathological evaluation. Ann Plast Surg 74(Suppl 2):S146–S151
Kutzing MK, Firestein BL (2008) Altered uric acid levels and disease states. J Pharmacol Exp Ther 324(1):1–7
Lagoutaris ED, Adams HB, DiDomenico LA, Rothenberg RJ (2005) Longitudinal tears of both peroneal tendons associated with tophaceous gouty infiltration. A case report. J Foot Ankle Surg 44(3):222–224, Muscles Ligaments Tendons J. 2014 Nov 17;4(3):292–297
Legerlotz K, Jones ER, Screen HR, Riley GP (2012) Increased expression of IL-6 family members in tendon pathology. Rheumatology (Oxford) 51(7):1161–1165
Lian Ø, Scott A, Engebretsen L, Bahr R, Duronio V, Khan K (2007) Excessive apoptosis in patellar tendinopathy in athletes. Am J Sports Med 35(4):605–611
Liu-Bryan R, Pritzker K, Firestein GS, Terkeltaub R (2005) TLR2 signaling in chondrocytes drives calcium pyrophosphate dihydrate and monosodium urate crystal-induced nitric oxide generation. J Immunol 174(8):5016–5023
Liu-Bryan R, Scott P, Sydlaske A, Rose DM, Terkeltaub R (2005) Innate immunity conferred by Toll-like receptors 2 and 4 and myeloid differentiation factor 88 expression is pivotal to monosodium urate monohydrate crystal-induced inflammation. Arthritis Rheum 52(9):2936–2946
Martin WJ, Grainger R, Harrison A, Harper JL (2010) Differences in MSU-induced superoxide responses by neutrophils from gout subjects compared to healthy controls and a role for environmental inflammatory cytokines and hyperuricemia in neutrophil function and survival. J Rheumatol 37(6):1228–1235
Martinon F, Pétrilli V, Mayor A, Tardivel A, Tschopp J (2006) Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 440(7081):237–241
Matsuo H, Yamamoto K, Nakaoka H, Nakayama A, Sakiyama M, Chiba T, Takahashi A, Nakamura T, Nakashima H, Takada Y, Danjoh I, Shimizu S, Abe J, Kawamura Y, Terashige S, Ogata H, Tatsukawa S, Yin G, Okada R, Morita E, Naito M, Tokumasu A, Onoue H, Iwaya K, Ito T, Takada T, Inoue K, Kato Y, Nakamura Y, Sakurai Y, Suzuki H, Kanai Y, Hosoya T, Hamajima N, Inoue I, Kubo M, Ichida K, Ooyama H, Shimizu T, Shinomiya N (2015) Genome-wide association study of clinically defined gout identifies multiple risk loci and its association with clinical subtypes. Ann Rheum Dis 75(4):652–659. pii: annrheumdis-2014-206191
McCarty DJ (1994) Gout without hyperuricemia. JAMA 271(4):302–303
Melloni P, Valls R, Yuguero M, Sáez A (2004) An unusual case of tophaceous gout involving the anterior cruciate ligament. Arthroscopy 20(9):e117–e121
Millar NL, Wei AQ, Molloy TJ, Bonar F, Murrell GA (2009) Cytokines and apoptosis in supraspinatus tendinopathy. J Bone Joint Surg (Br) 91(3):417–424
Millar NL, Murrell GA, McInnes IB (2013) Alarmins in tendinopathy: unravelling new mechanisms in a common disease. Rheumatology (Oxford) 52(5):769–779
Mitha E, Schumacher HR, Fouche L, Luo SF, Weinstein SP, Yancopoulos GD, Wang J, King-Davis S, Evans RR (2013) Rilonacept for gout flare prevention during initiation of uric acid-lowering therapy: results from the PRESURGE-2 international, phase 3, randomized, placebo-controlled trial. Rheumatology (Oxford) 52(7):1285–1292
Naredo E, Uson J, Jiménez-Palop M, Martínez A, Vicente E, Brito E, Rodríguez A, Cornejo FJ, Castañeda S, Martínez MJ, Sanz J, Möller I, Batlle-Gualda E, Garrido J, Pascual E (2014) Ultrasound-detected musculoskeletal urate crystal deposition: which joints and what findings should be assessed for diagnosing gout? Ann Rheum Dis 73(8):1522–1528
Nirschl RP, Ashman ES (2003) Elbow tendinopathy: tennis elbow. Clin Sports Med 22(4):813–836
Pearce CJ, Ismail M, Calder JD (2009) Is apoptosis the cause of noninsertional achilles tendinopathy? Am J Sports Med 37(12):2440–2444
Perez-Ruiz F, Dalbeth N, Bardin T (2015) A review of uric acid, crystal deposition disease, and gout. Adv Ther 32(1):31–41
Pineda C, Amezcua-Guerra LM, Solano C, Rodriguez-Henríquez P, Hernández-Díaz C, Vargas A, Hofmann F, Gutiérrez M (2011) Joint and tendon subclinical involvement suggestive of gouty arthritis in asymptomatic hyperuricemia: an ultrasound controlled study. Arthritis Res Ther 13(1):R4
Pingel J, Wienecke J, Kongsgaard M, Behzad H, Abraham T, Langberg H, Scott A (2013) Increased mast cell numbers in a calcaneal tendon overuse model. Scand J Med Sci Sports 23(6):e353–e360
Puig JG, de Miguel E, Castillo MC, Rocha AL, Martínez MA, Torres RJ (2008) Asymptomatic hyperuricemia: impact of ultrasonography. Nucleosides Nucleotides Nucleic Acids 27(6):592–595
Radice F, Monckeberg JE, Carcuro G (2011) Longitudinal tears of peroneus longus and brevis tendons: a gouty infiltration. J Foot Ankle Surg 50(6):751–753
Rock KL, Kataoka H, Lai JJ (2013) Uric acid as a danger signal in gout and its comorbidities. Nat Rev Rheumatol 9(1):13–23
Rodas G, Pedret C, Català J, Soler R, Orozco L, Cusi M (2013) Intratendinous gouty tophus mimics patellar tendonitis in an athlete. J Clin Ultrasound 41(3):178–182
Ruggiero C, Cherubini A, Ble A, Bos AJ, Maggio M, Dixit VD, Lauretani F, Bandinelli S, Senin U, Ferrucci L (2006) Uric acid and inflammatory markers. Eur Heart J 27(10):1174–1181
Scharpf RB, Mireles L, Yang Q, Köttgen A, Ruczinski I, Susztak K, Halper-Stromberg E, Tin A, Cristiano S, Chakravarti A, Boerwinkle E, Fox CS, Coresh J, Linda Kao WH (2014) Copy number polymorphisms near SLC2A9 are associated with serum uric acid concentrations. BMC Genet 15:81
Scott A, Lian Ø, Bahr R, Hart DA, Duronio V, Khan KM (2008) Increased mast cell numbers in human patellar tendinosis: correlation with symptom duration and vascular hyperplasia. Br J Sports Med 42(9):753–757
Scott P, Ma H, Viriyakosol S, Terkeltaub R, Liu-Bryan R (2006) Engagement of CD14 mediates the inflammatory potential of monosodium urate crystals. J Immunol 177(9):6370–6378
Shi Y, Evans JE, Rock KL (2003) Molecular identification of a danger signal that alerts the immune system to dying cells. Nature 425(6957):516–521
Shields GE, Beard SM (2015) A systematic review of the economic and humanistic burden of gout. Pharmacoeconomics 33(10):1029–1047
Thampatty BP, Li H, Im HJ, Wang JH (2007) EP4 receptor regulates collagen type-I, MMP-1, and MMP-3 gene expression in human tendon fibroblasts in response to IL-1 beta treatment. Gene 386(1–2):154–161
Wen CC, Yee SW, Liang X, Hoffmann TJ, Kvale MN, Banda Y, Jorgenson E, Schaefer C, Risch N, Giacomini KM (2015) Genome-wide association study identifies ABCG2 (BCRP) as an allopurinol transporter and a determinant of drug response. Clin Pharmacol Ther 97(5):518–525
Yuan J, Murrell GA, Wei AQ, Wang MX (2002) Apoptosis in rotator cuff tendonopathy. J Orthop Res 20(6):1372–1379
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Andia, I., Abate, M. (2016). Hyperuricemia in Tendons. In: Ackermann, P., Hart, D. (eds) Metabolic Influences on Risk for Tendon Disorders. Advances in Experimental Medicine and Biology, vol 920. Springer, Cham. https://doi.org/10.1007/978-3-319-33943-6_11
Download citation
DOI: https://doi.org/10.1007/978-3-319-33943-6_11
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-33941-2
Online ISBN: 978-3-319-33943-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)