Abstract
Background
Gout is a chronic and inflammatory form of arthritis that is often overlooked despite the associated pain caused by acute flares and associated joint damage caused by the development of debilitating tophi. The increasing burden of gout, due to an aging population and the increased prevalence of known risk factors for hyperuricaemia, means that there is a continued need for new and effective urate-lowering treatments. The evaluation of these treatments will require a comprehensive and comparative evidence base describing the economic and humanistic burden of gout, taken from the perspective of patients, the healthcare system, and wider society.
Objective
The objective of this study is to review and summarise the current evidence of the disease burden related to chronic gout, assessed in terms of both cost and health-related quality of life (HRQL), and to identify key factors correlated with an increased burden. The overall aim is to support the economic evaluation of new treatments for gout, and to highlight key data gaps that may need further study and exploration.
Methods
Relevant literature dating from January 2000 to July 2014 was sourced through searches of the MEDLINE database via PubMed and The Cochrane Library. Articles published in English and reporting either the economic burden (cost) or the humanistic burden (HRQL/utility) of gout were identified, and key data were extracted and summarised, with key themes and data gaps identified and discussed.
Results
Of the 323 studies identified, 39 met the inclusion criteria, of which 17 and 26 were relevant to the economic and humanistic burden, respectively. The economic burden of gout varied according to numerous factors, most notably serum urate acid levels and number of flares and tophi, resulting in higher healthcare resource use most often attributed to hospitalisation and inpatient stay. The incremental direct cost of gout has been suggested in the range of US$3165 to US$5515 (2004 and 2005 values, respectively) climbing to US$10,222 to US$21,467 (2008 values) per annum where patients are experiencing regular acute flares and have tophi present. The humanistic burden of gout was largely due to physical disability and pain resulting from chronic clinical manifestations. Short Form 6 dimensions (SF-6D) assessed utility weights are estimated at 0.53 for a patient with severe gout (≥3 flares/year and tophi) compared with 0.73 for an asymptomatic gout patient with serum acid levels <6 mg/dl.
Conclusions
The evidence confirms that gout has a growing overall prevalence and represents a significant burden in terms of both direct healthcare cost and HRQL outcomes. In light of this, effective urate-lowering treatments are likely to be valued if they can be clearly demonstrated to be both clinically effective and cost effective. Published data to support healthcare decision making in non-US countries with regards to treatments for gout are currently limited, which is a key limitation of the current evidence base. More research is also required to extend our understanding of the impact of gout on indirect costs, and a need also exists to develop a more comprehensive set of comparative HRQL utility assessments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Gout is associated with a considerable economic and humanistic burden; patients experience a reduced quality of life due to physical disability and pain as well as increased hospital resource utilisation and lost productivity, resulting in higher costs. |
Many of the clinical manifestations of gout are reflected in the costs of care and HRQL, including the presence of tophi, acute flares, serum uric acid levels, number of joints affected and comorbidities. |
The need is clear for further retrospective and prospective studies to explore the economic and humanistic burden of gout. The cost burden has largely only been examined from a US claims database perspective; few studies examined indirect costs, and utility data are lacking. |
1 Background
1.1 Disease Overview
The primary risk factor for the development of gout (an arthritis-related condition) is the build-up to excessive levels of uric acid, or hyperuricaemia that, left untreated, forms needle-like crystals around the joints [1]. The build-up of uric acid crystals leads to acute episodes of gout, which irritates the synovial membrane around joints, causing intense pain [2]. Other symptoms include swelling and red, shiny skin over affected areas [2]. The uric acid crystals can also form masses called tophi, which can become visible under the skin; these indicate that gout has become severe and chronic in nature and are therefore often a reason to begin uric acid-lowering therapy [3]. Without this treatment intervention, tophi can expand and cause severe pain and permanent joint damage. Chronic tophaceous gout develops after several years of persistent gout resulting in the formation of tophi and, in some patients, can lead to bone erosion, chronic pain and joint damage [2]. Other complications of chronic gout include kidney stones, or urolithiasis, caused by excess uric acid crystallising in the kidneys, and psychological effects, such as depression [3].
Hyperuricaemia is most commonly measured directly by physician assessment of serum uric acid (SUA) levels [4]. Many factors contribute to the risk of developing hyperuricaemia, including genetics, diet, hypertension and renal insufficiency. Dietary factors are often described as key determinants of uric acid metabolism, particularly diets high in meat and alcohol [4–6].
1.2 Epidemiology
A review of European epidemiological data recorded the lowest prevalence, 0.3 %, in the Czech Republic and the highest, 1.4 %, in the UK and Germany [7]. Some variation was found between reported rates; another study recorded the overall UK prevalence of gout to be 2.49 % [8]. This variation is likely to be due to different reporting methods and definitions of disease severity across individual studies, as the literature suggests that two-thirds of self-reported gout cannot be verified by a clinician [9].
Epidemiological data also suggest that, in many affluent countries, including the USA and the UK, gout is becoming increasingly prevalent [4]. The UK prevalence of gout was stated to have risen by 63.9 % between 1997 and 2012 [8]. In the USA, from 1978 to 1996, the absolute incidence of gout was reported to have increased from 0.03 to 0.05 %, a similar scale of relative increase as seen for the UK [7]. The increase in the incidence of gout has been partly attributed to an increased prevalence of comorbidities that lead to hyperuricaemia, including hypertension, obesity, metabolic syndrome, diabetes and chronic kidney disease [10]. Changes in diet are also likely to have a role in this increasing prevalence of gout [4]. It seems likely that the observed increased prevalence of gout is due to both the underling increase in incidence and the aging population, as patients with gout are now living with gout for longer [9, 11].
Gout is more prevalent in males; the Framingham Heart Study reported an incidence of gout of 1.4 in women and 4.0 in men per 1000 person-years [4, 11]. The observed difference in prevalence is caused by the generally higher natural SUA levels in males, which increases the underlying risk of developing hyperuricaemia [11]. The prevalence of gout increases with age in both sexes and peaks between the ages of 75 and 84 years for men [12]. Post-menopausal women are also at an increased risk of developing gout, with the incidence of gout in women increasing at approximately 45 years as oestrogen levels decline [11]. From age 60 onwards, the incidence of gout is roughly equal for men and women [11].
1.3 Current Treatments
Two main lines of treatment exist for gout: management of acute episodes and long-term treatment of chronic hyperuricaemia [13].
Non-steroidal anti-inflammatory drugs (NSAIDs), in combination with proton pump inhibitors, are often favoured for treatment of an acute episode of gout, but care needs to be taken with dosing to avoid adverse effects, such as stomach ulcers or bleeding [10, 14, 15]. Colchicine (Colcrys®, URL Pharma, MI, USA) is also a US FDA-approved [16] drug for both the treatment and the prevention of acute gout flares.
Clinical guidelines in the USA and Europe recommend a course of urate-lowering therapy (ULT) drugs for patients presenting with symptoms suggesting long-term chronic gout, such as frequent acute attacks and presence of tophi [15, 17]. The objective of longer-term treatment is to achieve and maintain target SUA levels, reducing symptoms and lowering the risk of developing painful and potentially debilitating tophi. The ULT drugs (e.g. allopurinol, febuxostat and benzbromarone) work by either blocking the production of uric acid or increasing the rate of removal of uric acid by the kidneys, preventing its accumulation in the blood [15, 18]. In clinical practice, allopurinol is generally acknowledged as the preferred first choice of treatment for the management of hyperuricaemia, due to its long-standing use and evidence base; however, issues relating to toxicity and compliance can result in frequent sub-optimal dosing and titration [19]. The American College of Rheumatology (ACR) recommends allopurinol as a long-term uric acid-lowering drug that should be started at a low dose: 100 mg daily, increased by 100 mg every 2–4 weeks if required [10, 17]. It is also recommended that allopurinol dosing is titrated upwards to a maximum of 600–900 mg or until patients reach their target SUA level; however, a substantial number of patients do not reach this level, partly due to concerns regarding the side effects and a lack of monitoring of SUA levels following treatment initiation [20]. Several studies have confirmed that, in clinical practice, adherence to allopurinol treatment is generally poor, with one study finding that patients were compliant with their therapy only 56 % of the time [21]. This may be due to issues in clinical practice, but also in part is often due to poor patient education and understanding of the underlying causes and triggers of gout [13]. Issues of poor adherence and sub-optimal dosing in clinical practice are likely to be reflected either directly or indirectly in any ongoing assessment of the economic and humanistic burden of disease.
A newer wave of ULT drugs are close to gaining, or have recently gained, regulatory approval, with the potential for additional control of SUA levels and acute flares. In particular, these treatments are most relevant for those patients who are currently unable to maintain long-term control of their gout on titrated dosing of oral allopurinol or who remain unresponsive to febuxostat [22, 23]. Pegloticase (Krystexxa®, Savient Pharmaceuticals, NJ, USA), an injected enzyme-based treatment that metabolises uric acid, was approved by the US FDA (September 2010) and European Medicines Agency (EMA) (January 2013) for the treatment of chronic and severe gout in patients whose gout is resistant or intolerant to allopurinol or febuxostat [24, 25]. The potential for serious anaphylaxis and infusion reactions means that pegloticase is likely to remain a treatment administered by clinical specialists under close supervision during the infusion period. Lesinurad (AstraZeneca, London, UK) is a selective uric acid re-absorption inhibitor (SURI) blocking the urate transporter 1 protein that was the subject of three 12-month phase III randomised trials (CLEAR 1, CLEAR 2, and CRYSTAL) as an oral combination therapy (with allopurinol or febuxostat, respectively) for patients whose gout remains uncontrolled on current standard ULT therapy [26]. An EMA market approval application has recently been accepted for lesinurad (January 2015), and it is expected to undergo an FDA new drug application (NDA) process during 2015 [27]. Other novel treatments under current clinical investigation for use in lowering urate levels and controlling acute flares in gout include arhalofenate (phase III), ulodesine (phase II), and levotofisopam [28].
1.4 Study Objectives
Despite the growing prevalence of gout, published data quantifying the economic and humanistic burden of the disease remain limited. As ULTs become more readily available for patients who are sub-optimally treated or non-responsive to conventional therapy with allopurinol, this may go some way in addressing the expected growth in disease burden. However, in continuously improving healthcare services for patients with gout, it is important that we continue to develop as full an understanding as possible of the wider disease burden, particularly for those patients in the more advanced stages of chronic disease. It is also important to find more effective ways to consider health-related quality of life (HRQL) impacts and to assess health state utility for the various stages of symptomatic and chronic gout, beyond a simple assessment of SUA level.
This literature review aims to provide a summary of currently available published evidence on the economic and humanistic burden of gout. The economic burden is summarised by identifying and reviewing key published studies estimating the direct costs and/or indirect costs of gout. Direct costs are payments that can be traced, including direct medical costs, such as drug acquisition costs, and other direct non-medical costs, such as travel expenses to and from hospital [29]. Indirect costs, for which no payments are made but that are a cost to society, are used to quantify potential productivity losses associated with disease, and are increasingly used in economic modelling [29, 30]. Productivity losses are often more difficult to quantify, but include reduced labour market activity, such as days off work, and non-labour market activity [29]. The humanistic burden is summarised by identifying and reviewing key studies that provide estimates based on commonly used generic and disease-specific weights for assessing HRQL in gout. The review particularly focuses on identifying any published HRQL studies that provide utility data linked to commonly used measures of gout severity, as these have greatest relevance for economic evaluations.
The overall aim of the study is to provide an overview of the current published evidence of burden of disease that can be considered when assessing unmet need and the potential for new treatments for gout, and particularly providing an evidence summary for those undertaking economic evaluations in this disease area.
2 Methods
2.1 Literature Search
A structured literature search was conducted based on MEDLINE, the Cochrane Database of Systematic Reviews, the Cochrane Central Register of Controlled Trials, the Database of Abstracts of Reviews of Effects (DARE) and the Health Technology Assessment Database to identify studies relevant to the burden of gout in terms of quality of life and costs, assessed at both the patient and the overall population level. Searches were structured to identify evidence on health status/HRQL and costs (both direct and indirect), published in English between January 2000 and July 2014. No limit was placed upon study design to ensure that all relevant evidence was identified. Across the database searches, common search terms included disease-specific terms, e.g. ‘gouty arthritis’; cost terms, e.g. ‘expenditure’; and quality-of-life terms, including quality-of-life measures, e.g. ‘EQ-5D’, and more general terms, e.g. ‘quality of life’. Search terms and strategies were varied slightly according to the design of the database and search capabilities. An example search term is provided in Appendix A (Electronic Supplementary Material).
All identified citations were manually screened against a set of explicit eligibility criteria to assess their potential relevance to the current literature review. Inclusion criteria were based on studies that both (1) focused on patients with a formal diagnosis of gout and (2) reported quantifying economic or HRQL outcome data. Studies were excluded if they focused on a particular intervention, as the aim of the project was to assess the burden of disease rather than to assess the potential benefits of particular treatments. For citations meeting the eligibility criteria, full-text articles were obtained and studies were re-assessed against inclusion/exclusion criteria. This screening process resulted in a final citation list, within which studies were classified according to whether they focused on the humanistic burden (i.e. quality of life) or the economic burden (i.e. indirect and direct costs), or were relevant to both assessments of burden.
Specific data, including quantitative values for direct and indirect costs and utility outcomes, were extracted where available. Information on study design and patient characteristics were also collected. Studies were not evaluated against any strict criteria for quality assessment; however, the observed variation in quality of included studies is discussed in the results and discussion sections.
2.2 Currency Conversion
To allow for greater comparability between studies, cost data have been converted and presented as 2013/2014 Euro values; this was achieved by applying the latest consumer price index for each country and the year 2010 purchasing power parity conversion factor [31, 32].
3 Results
3.1 Search Results
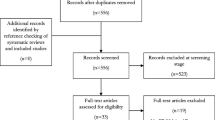
In total, 326 potentially relevant citations were identified through the electronic database searches, once adjusted for duplicates. Primary title and abstract screening resulted in the exclusion of 268 citations on the basis that they clearly did not meet the eligibility criteria of the review. Of the resulting 58 citations accessed in full, 39 were judged as publications of studies meeting the pre-defined inclusion criteria. Of these studies, 17 focused on the economic burden of gout and described relevant indirect and direct costs for the population, and 26 considered the humanistic burden of gout. Some overlap occurred, as several of the studies considered the burden in terms of both cost and quality of life. Of the 39 studies identified, 33 were original (primary) studies, and six were existing reviews. Existing reviews and commentaries were captured and identified in the initial electronic database search so that the authors could judge the overall interest in gout through an assessment of the number of publications (regardless of type); however, the primary studies were the focus of the review and analysis of the burden of gout.
The literature search process flow is depicted in Fig. 1.
PRISMA diagram for inclusion and exclusion. PRISMA preferred reporting items for systematic reviews and meta-analyses. Source: adapted from Moher et al. [33]
3.2 Economic Burden of the Disease
A total of 17 studies included information on the economic burden of gout; 13 were primary studies, and four were reviews/commentaries. Of the 13 original cost studies identified, 11 were based on data from the USA and all except one of these were based on analyses of medical claims data, one was performed using data from Spain, and one was performed using data from Canada (Table 1). This geographic distribution of studies demonstrates that, aside from the USA, there is a substantial paucity of data available that quantifies the overall economic burden of gout. Additionally, only three of the 13 original studies provided any consideration of indirect costs related to the presence of gout and severity of disease, again demonstrating a lack of data in this area. It is also notable that population-wide estimates of cost, which is perhaps one of the easiest ways to judge overall disease burden, were lacking. The identified papers were all published within the last 8 years, with the majority published since 2010 and based on recently collected data. This suggests that the economic burden of gout is becoming a growing field of research. This more recent trend in the publication of burden data is potentially due to healthcare decision makers becoming more aware and concerned about gout due to its increased prevalence, as a result of aging populations and changes in diet/lifestyle in developed countries [34].
Table 1 presents the key characteristics of the 13 primary economic studies. Mean ages of patients in the studies ranged from 45.9 to 77, which is reflective of the burden of gout falling on older individuals. A number of these studies (3 of 13) have considered newly diagnosed patients over the age of 65 years, and therefore, the generalisability of these study results can be questioned if applied to the wider gout population [36, 39, 40]. As expected from the pattern of disease, most studies consider a greater proportion of men, aside from Hanly et al. [39]. Study sizes are generally reasonable, probably due to the reliance on large US claims databases, which makes it easy to access information on a large population. However, the main study on indirect/societal costs is based on a very small sample population, highlighting the need for further research in this area [41].
The advantage of the significant use of US claims data in current studies to describe the burden of gout is that they have adopted a fairly homogenous set of diagnostic criteria (involving clinical modification codes from the International Classification of Diseases, 9th Revision [ICD-9]) and case definitions, which makes the published results more comparable. Again, the large patient numbers seen with claims data studies allow for informative analyses and stratification by disease severity. However, this reliance on claims data for the majority of the information from the included studies (10 of 13) also presents some potential limitations in terms of generalizability across population groups. In particular, the use of administrative data can be of some concern due to accuracy and limitations of reporting complex cases due to restrictions on inputs, hence there may be some increased risk of selection and misclassification bias. It can also present problems when accurately matching patient groups due to limitations placed by privacy and confidentiality guidelines [39]. Finally, only one of the 13 papers considered any stratification of the cost data based on the presence of comorbidities; in an aging population with gout, this would be an interesting aspect to have explored further [43].
In general, the data reported in the cost studies was clear; however, it was frequently found to be limited to the presentation of mean values only (rather than median), and it typically lacked any consistent reporting of underlying distribution or uncertainty (such as reporting of standard deviations). As such, an overall assessment of uncertainty in cost data is not reflected in this review paper, although readers may gain more insight from the original study papers.
3.2.1 Direct Costs
Direct annual costs were reported in eight of the 13 primary studies identified and these data are summarised in Table 2. Not all identified papers reported the direct costs on an annual cost basis, with some studies reporting alternative data based on the cost per flare/episode. The majority of the papers identified (10 of 13) were retrospective cohort studies based on US claims data and provided frequently reported data for direct all-cause healthcare costs and/or gout-related healthcare costs; this is reflected in the table breakdown [35–40, 42–45].
Brook et al. [35] was the first study of this type to identify and analyse data from a large claims database covering employer health plans. The study compared cost data for employees with and without a gout diagnosis, although demographic differences between the groups were significant, which limits comparisons due to confounding variables. Employees with gout had greater utilisation of doctor appointments and inpatient and outpatient visits, which was reflected in a higher economic burden; total annual direct costs were US$3165 higher in the gout group (US$6870 versus US$3705, year 2004 values).
Wu et al. [36] was the first of a number of large retrospective studies focusing on the recently diagnosed over-65 population [39, 40]. Gout patients (n = 11,935) were 1:1 matched by age, sex and geographic region to a gout-free control cohort, and a subgroup analysis was conducted to assess the burden of gout in relation to the presence of tophi and SUA levels [36]. The study concluded that the cost and resource use burden associated with gout is substantial, with gout patients incurring higher all-cause healthcare costs per annum (US$14,734 vs. US$9219; difference of US$5515, year 2005 values) when compared with gout-free control patients, which equated to a US$3038 (33.0 %) difference once adjusted for underlying comorbidities. The majority of gout-related healthcare costs, US$876 on average per patient, were mainly attributed to increased utilisation of hospital inpatient and outpatient services. The study also found that tophi presence was associated with higher annual all-cause healthcare costs and gout-related costs, with an adjusted increase of US$5501 and US$1710, respectively, and was mostly attributed to increased use of inpatient care (unadjusted all-cause direct costs of US$22,562 vs. US$14,574). When patients were divided into SUA level subgroups, outpatient services usage and costs were observed as similar; however, the highest category (SUA ≥9 mg/dl) had far greater utilisation and costs associated with inpatient services, and the lowest group saw the greatest spending on prescription drugs (SUA <6 mg/dl). The patient group with SUA ≥9 mg/dl was associated with an additional US$3103 in total annual all-cause healthcare costs and an increase of US$276 in gout-related costs compared with the lowest SUA group, once adjusted for comorbidities (an unadjusted difference of US$3978; US$18,920 vs. US$14,942).
Hanly et al. [39] and Wu et al. [40] also studied the impact of gout in newly diagnosed older patients (≥ 65 years) in terms of healthcare resource use and cost. Hanly et al. [39] focused on administrative data from Canada, a public insurance scheme, whereas Wu et al. [40] focused on third-party payer claims data from the USA. Hanly et al. [39] noted that an increase in hospitalisations, visits and drug prescriptions resulted in an additional US$134 per month (year 2006 values) spent on healthcare in the gout population. Similar to Wu et al. [36], the greatest driver of this cost increase was hospitalisation. Wu et al. [40] went into further detail by researching the combined impact of SUA and gout flare severity and frequency on the economic burden of gout. Healthcare utilisation and direct costs were studied over a 30-day period following an acute gout episode/flare, defined as a claim for joint pain. The study concluded that patients with high and very high (>6 mg/dl) SUA concentrations were more likely to experience flares than were patients with a normal SUA (<6 mg/dl). Multivariate regressions estimated that each unit increase in SUA above 6 mg/dl resulted in an 11.9 % annual increase in the number of flares experienced by a patient. This was reflected in the costs of treating patients with higher SUA levels; average adjusted total healthcare and gout-related costs per episode were US$2555 and US$356 higher (year 2005 values), respectively, in patients with very high SUA levels compared with matched patients with normal SUA levels.
Many studies have concluded that there is a lack of adherence to treatment in the gout population, which is likely to impact on health and subsequently on the economic burden of the disease [48]. Halpern et al. [38] was the only identified study to assess the relationship between treatment compliance, SUA levels, and subsequent healthcare cost. Fewer than half of the patients adhered to treatment (allopurinol) and, as expected, medication adherence was demonstrated to be associated with improved SUA levels. The study applied generalized linear modelling (GLM) to the cost data to conclude that direct gout-related costs were 58 % higher for patients with SUA 6–9 mg/dl than for patients with SUA <6 mg/dl (with the unadjusted cost for SUA <6 mg/dl reported at US$505, year 2005 values), demonstrating that the economic burden of gout varies according to SUA levels, and replicating the findings of Wu et al. [36, 40] in the elderly gout population [38]. The link between SUA levels and the economic burden of gout was again studied by Park et al. [42] using retrospective claims data. This study confirmed the findings of the previous studies: gout patients with higher SUA levels were less likely to adhere to treatment and had higher rates of hospitalisations, emergency department visits, physician visits and pharmacy claims, which were reflected in higher all-cause direct costs (an unadjusted difference of US$3209; US$14,474 vs. US$11,265, year 2010 values).
The direct cost burden of gout in patients’ refractory to conventional ULT was also studied by Wu et al. [44]; refractory was defined as patients who were experiencing more than three flares per year, despite receiving allopurinol ULT treatment. The paper does not discuss adherence to treatment; however, given the previous studies on adherence, it can be assumed that a proportion of the patients are non-adherent as opposed to being truly refractory. The paper concluded that, compared with the gout-free cohort, patients experiencing three or more gout flares per year had a significantly higher incidence of emergency department visits and urgent care visits, as well as outpatient visits and other medical resource use. This increased resource use was reflected far greater in all-cause healthcare costs; the incremental cost of care for patients experiencing three or more gout flares was US$10,222 (year 2008 values) more (approximately 2.5 times higher) than the costs incurred by the disease-free cohort (adjusted costs of US$17,603 vs. US$6891). In a subgroup analysis of patients experiencing six or more gout flares per year, the burden of care increased again; the incremental cost for patients experiencing six or more flares compared with a matched cohort US$21,467 (adjusted costs of US$25,778 vs. US$4312).
Saseen et al. [43] also studied the impact of frequent flares and contradictions to conventional therapy on healthcare costs. Gout patients experiencing three or more attacks/flares per year were matched to patients with infrequent gout (fewer than three flares per year). In this study, contraindications to gout therapy were defined using comorbidities, which are unlikely to be affected by non-adherence, unlike the definition used by Wu et al. [44]. Patients with frequent gout attacks were more likely to be contraindicated to conventional gout therapies (including allopurinol) and had higher all-cause healthcare costs and gout-related healthcare costs. Mean gout-related costs were US$679 (year 2011 values) higher for patients with frequent gout than for patients with infrequent gout, and all-cause direct costs were US$228 higher. The cost increase was largely due to an increase in outpatient visits. Additionally, the study was the first to consider the impact of comorbidities, noting that gout-related costs were higher among frequent gout patients with comorbidities than among those without comorbidities (US$886 vs. US$513). Although the increase in costs was confirmed with more frequent gout flares, the scale of these differences, as observed by Wu [36], was not reflected in this study, which may be reflective of the patient population differences.
Only one study was identified that quantified the economic burden of gout in a European population; this multicentre observational study was recently conducted and perhaps again is reflective of a growing interest in the burden of gout [46]. This study also focused on the impact of the number of flares on costs, and the results were reflective of the previous US studies, with direct costs increasing with the number of flares experienced over a 2-year period (€2101 for one to two flares and €2517 for three or more flares; year 2007 values). The study did not differentiate between all-cause healthcare costs and gout-related healthcare costs, but the costs appear to be far lower in the European population than in the US populations.
3.2.2 Indirect Costs
Far fewer of the identified studies (3 of 13) focused on the societal and indirect costs of gout, such as lost productivity or work absence, and loss of social activity.
Edwards et al. [41] was the first primary study identified to focus purely on the indirect costs; this US study used 2001 data to examine productivity losses due to gout flares in patients with chronic gout who were refractory to conventional treatment (reflected by a SUA >6 mg/dl) [41]. This small prospective uncontrolled study of 81 patients concluded that the mean annual number of work days lost was 25.1 days. Based on the average 2006 US wage rate of US$19.30 per hour, an approximate productivity cost of US$3900 per patient per year was estimated. Gout flares also resulted in impaired social activities, with an average of 17.1 social days lost each year and 16.9 self-care days impaired each year. The number of activity days lost correlated with the number of flares experienced in a year; especially when considering patients with more than six flares per year, who on average lost 51.9 days of work, 36.1 social days and 31.1 self-care days.
A similar but much larger retrospective US study (n = 3361) was conducted by Lynch et al. [45] using employment claims data in order to calculate the costs of gout according to the number of flares. Patients were split into two groups: less than three and three or more flares per year; employees experiencing more than three gout attacks/flares per year had significantly higher annual short-term disability days and sick leave days. The relationship between the number of flares and indirect costs was shown to be linear once patients were experiencing at least three flares per year. For example, patients with three gout flares had an average of 1.15 sick leave days and US$805 (year 2010 values) in short-term disability costs, and this increased to 1.97 sick leave days for patients experiencing six flares and US$2330 in short-term disability costs.
Finally, the European study by Sicras-Mainar et al. [46] in 3130 patients reported indirect costs in the gout population; non-health costs were estimated using the minimum wage and days of work disability. This 2-year follow–up study determined that non-health costs were €29 (year 2007 values) in the infrequent gout population (one to two flares per year) and €88 in the frequent gout population (three or more flares per year) over the full study period. These low costs are reflected on by the authors as likely due to the use of minimum wage levels and a conservative approach to estimating employment impacts limited to an assessment of sick leave claims only.
3.2.3 Population Costs
Garg et al. [47] looked at emergency department utilisation across the USA to judge the total burden of emergency care in the gout population. The study used the National Emergency Department Sample to conclude that the burden of gout emergency care is growing across the population; in 2006, gout accounted for 168,410 visits, at a cost of US$128 million, which rose to 174,823 visits in 2008, at a cost of US$144 million. Several demographic factors were linked to an increased propensity for emergency department visits, including having an annual income <US$39,000 and hospital locations. Unfortunately, the study only considers one aspect of resource use (emergency visits); however, many of the studies reviewed in Sect. 3.2.1 indicated that hospitalisations were a key contributor to the direct cost burden of gout. Applying the cost results to the estimate gout prevalence of the USA, a recent review estimated that the total population cost would likely be in excess of US$20 billion (in 2006) [36, 49]. However, this is a crude estimation, and as the review of the primary data demonstrates, costs related to treating gout patients can vary substantially according to disease severity, comorbidities and adherence. Therefore, estimating an accurate population cost is likely to be highly complex.
3.3 Humanistic Burden of the Disease
The humanistic burden of gout is typically assessed through the measurement of the impact of disease on patients’ HRQL, which may be adversely affected by the combination of symptoms and complications of the disease, such as pain and discomfort, a loss of independence due to increased disability, and associated co-morbidities. The literature search identified 26 studies that had included relevant information and measures on the humanistic burden of gout; 21 of these were primary studies, and five were reviews/commentaries, including most notably a recent review by Chandratre et al. [50].
The key characteristics of the 21 primary studies focusing on the humanistic burden of gout are presented in Table 3. Similar to the evidence on economic burden, the search identified only one relevant study published before 2008, demonstrating again that gout is an area for which, until recently, focused research has been lacking. The study populations varied, particularly in relation to factors that would be expected to be correlated with the severity of the disease: presence of tophi, active/historical treatment and disease duration. Additionally, a number of the studies did not fully present population information, which also makes it difficult to draw useful comparisons and conclusions across the study results. Sample size varies greatly: from 73 to 1581 gout patients included in HRQL studies. As expected the average age in the study generally covers the older population, with most patients being in their 50 s and 60 s, and the majority of patients in all studies are male, again matching the epidemiology of gout. Many studies failed to report disease duration, but where reported there was a range of between 5.0 years to 13.3 years since disease onset. A large range was also observed in those patients receiving current treatment for gout attacks and/or prevention (28.0–92.6 % at baseline) and for the presence of tophi (7.0–74.0 %). The patient populations vary across the studies and, therefore, as these factors may have a significant clinical impact, the HRQL results may not be easily compared across studies.
3.3.1 Generic and Disease-Specific Measures
The literature review considered evidence collected on generic measures of HRQL as well as disease-specific measures, as they are generally preferred for the derivation of QALY in health technology assessments (HTAs) in many countries, including the UK, Sweden and the Netherlands. Generic measures of HRQL were the most frequently applied instrument in the identified studies, particularly the use of the Health Assessment Questionnaire Disability Index (HAQ-DI) (n = 6), the Health assessment Questionnaire-II (HAQ-II) (n = 3) and the SF-36 (n = 12). These measures were generally found to be valid and reliable measures of HRQL in patients with gout and led to differentiating HRQL assessments, as expected, between patient groups with different clinical symptoms (with and without joint pain, number of flares, swelling and tophi) [53, 57, 58]. Some differences were observed in the sensitivity of instruments to different types of clinical symptoms; for example, the presence of tophi was reflected in worse SF-36 pain and general scores but did not significantly impact the outcomes of the HAQ-DI [58]. Clear evidence of floor and ceiling effects was identified when generic measures were used, with significant proportions achieving the worst or best scores on the selected scale; demonstrating that generic measures may not be as sensitive to the full range of function experienced by patients with gout [53, 54, 57, 66]. Taylor et al. [57] reported 20.5 % of patients reporting the minimum 0 score on the HAQ-DI scale. This floor effect of the HAQ-DI was also observed by Alvarez-Hernandez et al. [53], with 42.2 % of patients reporting the minimum score. This means that generic measures are often seen as limited when considering patient groups with more minor and more severe forms of the disease, as it is likely that the measures will not reflect the full range of impact of disease status on quality of life, which in turn is likely to limit their usefulness in the derivation of meaningful changes in utility and quality-adjusted life-year (QALY) values from significant treatment effects.
The two gout-specific HRQL measures, the Gout Assessment Questionnaire (GAQ)—Gout Impact (GI) section (n = 2) and the Gout Impact Scale (GIS) (n = 3), were established in the published literature to have content validity, reliability and responsiveness and therefore accounted for a wider range of function change than were generic measures [51, 54]. Although not falling within the scope of this review to provide a full assessment of the potential merits of gout-specific patient-reported outcome (PRO) measures, it is useful to highlight recently published studies for further reading. Hirsch et al. [54] found that the GAQ-GI scores correlated more highly with PRO measures of gout severity than the SF-36v2, and demonstrated good test–retest validity (0.77–0.89) for each of the five GAQ-GI scales. This added to the earlier findings of Colwell et al. [51], who had also considered the potential use of the GAQ, comparing it with the SF-36v1 as part of a 4-week phase II trial for a new ULT treatment and a 12-month trial extension, and had concluded that the GAQ demonstrated good internal consistency. Sarkin et al. [61] further demonstrated that the GIS instrument was able to more closely correlate with patient-rated severity of symptoms as opposed to focusing purely on doctor-assessed clinical manifestations, such as tophi, which may make the GIS a more meaningful PRO measure of quality of life. The GIS focuses on key aspects of patient worry and concern regarding their gout (in terms of both current and anticipated disease changes) based on the impact from medication side effects/effectiveness of current treatments, impact of gout attack (work, recreational and self-care activities), and finally the impact from the concern regarding future attacks. Additionally, these gout-specific measures did not demonstrate significant floor and ceiling effects, indicating that they are more sensitive to the full range of changes in the disease status [51, 54].
3.3.2 The Impact of Gout on Health-Related Quality of Life (HRQL)
The identified studies are generally consistent in demonstrating that gout results in a reduced HRQL that worsens with disease severity and is mainly attributable to a worsening in physical functioning and higher pain scores, with a lesser relative impact seen on mental health aspects of the assessment scales [52, 56, 58, 59, 62]. However, some variations were observed between study conclusions, as one study, by Singh and Strand [56], found no significant difference in reported physical HRQL between a gout cohort of veterans and a gout-free cohort after adjusting for sociodemographics and comorbidities.
Hoy et al. [70] compared the disutility of a number of musculoskeletal conditions. Patients with acute gout were estimated to have an overall disability weight of 0.293, similar to the disability weight patients with other moderate skeletal conditions, such as rheumatoid arthritis (with an associated disability weight of 0.292) [70]. This was seen to increase to 0.606 for patients with severe chronic gout. Using the HAQ-DI instrument, van Groen et al. [62] suggested that, in general, patients with gout had a better HRQL (with an average HAQ-DI score of 0.54) than patients with rheumatoid arthritis and osteoporosis (who had an average HAQ-DI of 0.97 and 1.00, respectively). However, this study did identify a difference in HRQL impact from gout when using the SF-36 physical component summary (PCS) and the SF-36 mental component summary (MCS) separately, with mean scores of 38 and 43, respectively. This suggests that the physical aspects of gout represent the greater proportion of the overall HRQL impact [62]. Becker et al. [58] evaluated the relationship between patient-reported HRQL and disease severity in a prospective, 1-year observational study of patients with treatment-failure gout. Compared with a more general healthy population, it was determined that gout patients had a reduced HRQL, as the study demonstrated that a relatively young gout cohort (with an average age of 59 years) had HRQL scores more comparable to those of the general population aged ≥75 years with considerably lower SF-36 scores and a mean HAQ-DI of 1.0 at baseline (indicating a moderate disability) [58]. The study reported HAQ-DI scores that were higher (indicating more disability) with observed increases in frequency of flares, number of painful or swollen joints, baseline number of flares per year and the presence of tophi.
The identified studies also reported conflicting links between HRQL in gout patients and productivity losses. Taylor et al. [57] noted a strong positive relationship between HAQ-DI scores and missed days of work (sick leave), and Edwards et al. [41] noted significantly positive correlations between SF-36 scores and missed days of work (with days lost ranging between 0.28 and 0.56 for each of the SF-36 measures). However, this was countered by another study, Taylor et al. [69], which disagreed with this conclusion, showing no significant association between baseline HAQ-II and missed days of work.
3.3.3 Factors Affecting HRQL
Becker et al. [58] showed the link between gout severity and HRQL to be significantly associated with worse physical functioning due to higher flare rates (p < 0.02). The link between HRQL and the frequency and severity (number of joints affected) of gout flares was again examined by Lee et al. [59], who concluded that mean SF-36 PCS and mean SF-36 MCS were lower for those experiencing a greater number of flares with a greater number of affected joints (p < 0.005 and p < 0.001, respectively). The mean PCS and mean MCS scores for the entire sample of gout patients were 37.9 and 48.5, respectively, dropping to 32.9 and 42.3 for patients experiencing more than ten flares per annum. The study also reported that patients who continued to have joint pain for the majority or all of the time between acute flares had the worst PCS and MCS scores of all gout patients included in the study (with a reported PCS of 28.9–31.8 and MCS of 37.2–39.6). After adjusting for age, sex and comorbidities, the greatest impact on HRQL was attributed to the worst gout flare experienced and the number of joints involved. Similar correlations between the number and intensity of flares and HRQL were demonstrated in further studies [60, 65, 68].
A number of other clinical manifestations of disease activity were also linked to HRQL, including the number of tender/affected joints, whereby a greater number of affected joints resulted in increased physical disability and higher pain scores, which were reflected in reduced HRQL and greater disutility [58–60, 68]; and the presence of tophi, which was demonstrated to be correlated with worse pain scores, reduced general health and physical functioning and subsequently reduced quality of life [58, 60, 68].
In contrast to the assessment of economic burden, in which many studies demonstrated a link between SUA and direct healthcare costs, SUA was generally not demonstrated to have a significant direct impact on any HRQL measurement in the identified studies. Only one study found SUA to be an indicator of HRQL; this was the only study that used a gout-specific scale to link SUA and HRQL, thus demonstrating that the different scales may be more or less sensitive to certain gout symptoms and suggesting that the choice of scale could be influenced by the focus on the study [60].
A study by Dalbeth et al. [63] used a number of patient questionnaires to examine the relationship between illness perception and HRQL. Interestingly, negative perceptions of illness (e.g. patients feeling as though they lack control over their disease) were linked most strongly to higher pain scores, lower adherence, and musculoskeletal disability [63]. However, the study also noted that perceptions were affected by demographics, such as ethnicity; therefore, without matching patient groups, it is difficult to judge the impact of confounders. Becker et al. [58] noted that subject perceptions of gout-functioning and pain were strong indicators of HRQL scores in gout, and Khanna et al. [55] found that patients who rated gout as their top concern had a significantly larger disutility than patients who viewed it as a more minor concern.
The relationship between ULT treatment success and patient quality of life was only reported in one of the identified studies. Khanna et al. [65] conducted an observational study looking at real-world evidence and concluded that the use of ULT (such as allopurinol) and colchicine (used to prevent gout flares) was associated with improvements in HRQL, as measured using the SF-36. This varied according to the subset of the scale used; for example, the effect on the MCS was minor (0.08) and the bodily pain scale was large (1.09); again in line with the previous studies observing that physical symptoms were the main driver of HRQL.
Several studies reviewed the impact of comorbidities on HRQL and concluded that the existence of comorbidities results in a decrease in HRQL as a result of poorer physical activity scores [52, 58, 59, 67]. Becker et al. [58] judged that, while comorbidities resulted in worse physical functioning, reflected in lower HRQL, mental functioning remained unchanged. DiBonaventura et al. [67] examined the impact of gout on the HRQL of patients with hypertension, a common comorbidity of gout. By comparing matched cohorts, the study concluded that, independent of hypertension, gout has a significant impact on HRQL and results in lower health utility scores (0.68 vs. 0.73; p < 0.0001). Coronary artery disease and kidney disease were also shown to significantly negatively impact PCS scores, although the impact was quite minor [59].
3.3.4 Utility Assessment in Gout
A key area for using HRQL-based data in support of economic evaluations comes from the derivation of utility weights to calculate QALY outcomes. Only three of the 21 primary studies provided any level of assessment of utility [55, 67, 68], and the key utility paper was found to be the study report by Khanna et al. [68]. This international study of 620 patients with self-reported gout symptoms was based on the 2010 US and EU National Health and Wellness Surveys. The study assessed utility using a web-based survey based on a generic HRQL instrument, the SF-12v2, which retains both PCS and MCS components within its structure and can be converted to SF-6D scores and utility weights. Patients were categorised by the presence of self-reported tophi and self-reported acute gout flares over the previous 12 months; providing a sense of disease severity using clinical outcomes other than SUA.
SF-6D utility was reported at 0.74 (standard deviation [SD] 0.14) for the 349 patients who reported having no tophi compared with 0.64 (SD 0.16) when tophi was judged to be present, which is a significant difference (p < 0.001) [68]. Similarly, the frequency of acute flares in the previous 12 months was also negatively correlated with SF-6D utility; no flares had a utility of 0.73 (SD 0.12), three flares had a utility of 0.67 (SD 0.15), and six or more flares had a utility of 0.61 (SD 0.13), representing a significant difference (p < 0.0001). The most informative assessment of utility data is the presentation of the combined impact of having both frequent flares and also the presence of tophi which, irrespective of underlying SUA levels, represents a patient group with potentially severely impacting chronic gout. In this case, the study reported an overall SF-6D utility of 0.528 compared with 0.732 in gout patients who are predominantly asymptotic or only experiencing mild symptoms.
Due to the importance of utility scores to support future health economic modelling, and given the possibility that potential sources may remain in the unpublished grey literature, or may have fallen outside the precise search terms of the systematic review, additional targeted desk-based research (non-systematic) was carried out (March 2015) to identify any additional studies presenting utility values for gout. These additional searches identified three further data sources.
Brown et al. [71] studied the time trade-off utility associated with age-related macular degeneration, and included a comparison with other disease groups, including gout, for which an overall utility was stated as 0.86. This was noticeably higher than the estimates given by the studies identified in the systematic review; however, some of the values for other diseases listed in the Brown et al. [71] paper also look high (e.g. 0.91 for osteoporosis and 0.82 for symptomatic HIV). A set of unpublished data from the Institute of Medical Science (IMS) has also been previously quoted and applied in two further cost-utility studies [72, 73]. This data source reported utilities in the range of 0.6435 for treatment non-responders (>10 mg/dl) up to 0.7463 for responders (≤6 mg/dl); in line with the published studies, the data found that utility was correlated to the likelihood of experiencing a flare. Finally, a more recent published utility study was also identified: this Dutch cross-sectional study compared utility in gout patients with that in a gout-free cohort [74]. This study concluded that patients with gout had substantially reduced HRQL; however, it also noted that different assessment measures led to variations seen in the potential impact of gout on HRQL. When considering generic measures using the EQ-5D, the mean utility was reported at 0.74 (SD 0.23), falling to 0.69 (SD 0.13) when the SF-36 instrument was used.
4 Discussion
Gout is an extremely variable form of arthritic disease that undoubtedly presents differing challenges and scales of impact across individual patients and that is influenced in part by general lifestyles, level of adherence to current ULT and overall understanding of their chronic condition and underlying causal triggers of acute flares. Strong evidence also exists that this condition is increasing internationally in terms of overall observed prevalence rates, and that this is not only due to the aging population but also to the increasing influence of the western diet [6–11]. In the UK, only one-quarter of gout patients are currently prescribed ULT within 1 year following their initial diagnosis [8]. It is also recognised that, in current practice, the clinical effectiveness of allopurinol is also limited in terms of both suboptimal dosing and poor adherence, and that this pattern of prescribing did not change dramatically from 1997 to 2012 [8]. This goes to highlight that there remains a significant proportion of patients whose gout remains inadequately treated, which is highly likely to continue to contribute further to the growing economic and humanistic burden of the disease. Indeed, some of the identified published studies have also linked patients’ own negative perspectives of gout to levels of poor adherence, leading to ineffective control and ultimately reduced HRQL [55, 58]. It is therefore important in terms of effective healthcare planning that we continue to investigate the natural progression and impact of chronic gout and look to identify effective ways of managing and treating the condition, with patient education and the development of novel and effective ULT therapies to control the underlying SUA levels.
This review of the published literature confirms that gout has a clear, measurable and significant cost and HRQL impact from both a patient and a healthcare provider perspective. Furthermore, the literature confirms that this overall burden is seen to increase with both disease severity, when considered in terms of clinical manifestations such as the frequency of acute flares, the number of affected joints and the presence of tophi, and also in terms of patient concerns and worries about their underlying condition and treatment.
Direct healthcare costs were found to vary according to a number of factors, with positive correlations between the presence of tophi, increased SUA levels, lower adherence, higher comorbidities, higher flares and the total direct all-cause and gout-related healthcare costs. These factors were shown to largely impact cost through an increased burden on hospitalisation resource use, particularly in terms of inpatient stays and hospitalisation in general. Differences of approximately US$3000–5000 (year 2004–2010 values) per annum were reported in all-cause healthcare costs based on the diagnosis of gout compared with matched controls [35, 36, 42]. However, when considering the more advanced stages of disease, for example three or more flares per annum and/or the presence of tophi, then the overall incremental direct cost compared with patients with milder disease was estimated in the region of US$10,000–20,000 (year 2008 values) per annum [44].
The positive news is that over recent years an evidence base is growing focusing on the direct costs of gout, which can be utilised to populate future economic evaluations and cost models for new treatments for gout. However, this is partially countered, as the review also confirms a number of clear limitations remaining in this evidence base.
First, there remains a lack of studies considering impacts of gout on indirect costs. Some limited data were found suggesting an associated increase in days spent off work and reduced productivity, especially in relation to the frequency of gout flares. However, these data need confirming and further exploration. This may be an important consideration in future economic modelling and evaluations of ULTs as, although gout affects the older population, much of the incidence occurs well before typical retirement age.
A further, and probably more significant, limitation in the existing evidence base is the finding of a paucity of published data on direct healthcare costs for countries outside of the USA, with a heavy reliance on data derived from US claims databases [35–40, 42–45]. Only one EU-based economic study was identified through literature searching, and this reported a much lower impact of gout on direct costs [46]. This lack of multinational study data is despite the finding of a more recent increase in the number of published studies since 2008, reflecting a general overall increase in awareness of gout. Therefore, a clear need exists for larger comparative studies of the economic burden of gout, from both a European and a wider international perspective, ideally based on prospective study design or accessing data from patient registries. Such studies need to continue to explore the severity of disease defined in terms of both the severity and the frequency of flares and also the presence of tophi as key determinants of economic impact, as well as SUA level as a more traditional measure of disease severity. It would also seem prudent to track details on comorbidity and patient baseline characteristics, as the published data suggest that these strongly influence cost of care.
This current lack of published non-US burden studies certainly cannot be explained by any basic underlying country-specific differences in formal reimbursement requirements or processes. Campaigns to further increase the awareness of gout may be helpful in creating the necessary environment for the design of larger and more comprehensive prospective international burden studies. Although cross-country variations in terms of data capture and service provision are likely, these are seldom found to be insurmountable issues and can be typically accommodated in a common overall study design. Indeed, other recent examples exist of successfully implemented international burden-of-illness studies for similar chronic conditions, using consistent study designs, outcome definitions and comparative reporting formats. These include the BOLD (Burden of Obstructive Lung Disease) study, the CODE-2 (Cost of Type-2 Diabetes in Europe) study, and the IBMS (International Burden of Migraine Study) [75–83].
The review also confirms that gout has a clear and measurable adverse impact on HRQL, largely due to physical disability and pain resulting from clinical manifestations such as flares and tophi. The observed variations in study populations, such as presence of tophi, active/historical treatment and disease duration, presents some difficulties in drawing comparisons and conclusions across the results. However, recurring themes can be identified. Despite the chronic pain and comorbidities associated with the disease, the primary impact was seen more in terms of physical HRQL measures than in terms of mental HRQL measures (through the PCS and MCS in the SF-36) [56]. This fits with the typical view of gout as primarily a physical condition. However, this may also be due to the choice of assessment scale and the use of a generic or disease-specific instrument. As such, this finding needs some confirmation using different assessment measures. Some conclusions were also conflicting in the humanistic studies identified, such as whether SUA is reflected in poor HRQL, which may be due to different baseline characteristics, or potentially due to the responsiveness of different HRQL measures to different disease aspects, with the majority of studies using the generic HAQ-DI (n = 6), HAQ-II (n = 3) or SF-36 (n = 12). There is potential to conduct further studies using disease-specific measures, such as the GAQ-GI and GIS, which have been validated in part for use in assessing HRQL in gout, and which seem initially better suited to deal with detecting changes and HRQL impacts in the mild and severe extremes of disease, avoiding the significant floor and ceiling effects seen with the generic measures.
Finally, and of primary relevance to the development of economic evaluations of ULT for gout, a need exists for the development of a set of reliable utility weights differentiating across the full spectrum of disease severity. The review findings confirm that utility data remain only limited, namely the study of Khanna et al. [68], with the SF-6D-assessed utility ranging from 0.73 for asymptomatic patients to 0.61 for patients with six or more flares per annum, and 0.528 in patients experiencing regular flares and having tophi. This is a single well-conducted survey-based study, and ideally would need to be replicated in other settings, using clinically diagnosed patients and alternative generic HRQL assessment instruments/country-specific tariffs. However, it does already provide further support to a very clear message of significant worsening of HRQL with clinical severity of disease, which can be realistically measured and used to produce QALY outcomes in economic assessments.
5 Conclusion
The primary aim of this study was to identify the current landscape in terms of evidence describing and quantifying the humanistic and economic burden of disease for gout, and to reflect on the relative strengths and weaknesses of these data with relevance to economic evaluations. This is particularly important to researchers as the development of newer ULTs are considered for the treatment of gout, and as HTA considerations are given to the ideal placement of ULTs in the pathway for the management of chronic gout. A number of recommendations have been drawn from the review findings. First, it is critical that further economic studies of gout are conducted using large patient datasets based on patient cohorts outside of the USA, as the paucity of published non-US data presents a severe limitation and variations in cost burden between countries are likely. Second, the indirect cost impact of gout needs to be more fully explored and confirmed in further studies, as early data suggest a potential significant impact alongside that seen for direct costs, which will add to the value of effective ULT. Finally, studies are needed to confirm the HRQL impacts of gout using disease-specific measures based on accepted definitions of disease severity that ideally sit outside of definitions based purely on SUA level. Ideally, these studies will involve mapping of results to recognised instruments to assess utility weights, and to provide a range of utility estimates, as current data remain limited.
References
National Health Service. NHS England advice on gout. 2014. http://www.nhs.uk/conditions/Gout/Pages/Introduction.aspx. Accessed 27 Aug 2014.
University of Maryland. Gout. 2014. http://umm.edu/health/medical/reports/articles/gout#ixzz3BzqKxW00. Accessed 1 Sept 2014.
National Health Service. NHS choices. Gout. 2014. http://www.nhs.uk/Conditions/Gout/Pages/Complications.aspx. Accessed 27 Aug 2014.
Roddy E, Doherty M. Epidemiology of gout. Arthritis Res Ther. 2010;12(6):223.
Rho YH, Zhu Y, Choi HK. The epidemiology of uric acid and fructose. Semin Nephrol. 2011;31(5):410–9.
Nuki G, Simkin PA. A concise history of gout and hyperuricemia and their treatment. Arthritis Res Ther. 2006;8(Suppl 1):S1.
Smith EU, Diaz-Torne C, Perez-Ruiz F, March LM. Epidemiology of gout: an update. Best Pract Res Clin Rheumatol. 2010;24(6):811–27.
Kuo CF, Grainge MJ, Mallen C, Zhang W, Doherty M. Rising burden of gout in the UK but continuing suboptimal management: a nationwide population study. Ann Rheum Dis. 2015;74(4):661–7.
Chen LX, Schumacher HR. Gout: an evidence-based review. J Clin Rheumatol. 2008;14(5 Suppl):S55–62.
Khanna D, Fitzgerald JD, Khanna PP, Bae S, Singh MK, Neogi T, et al. 2012 American College of Rheumatology guidelines for management of gout. Part 1: systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care Res. 2012;64(10):1431–46.
Weaver AL. Epidemiology of gout. Cleve Clin J Med. 2008;75(Suppl 5):S9–12.
Doherty M. New insights into the epidemiology of gout. Rheumatology. 2009;48(Suppl 2):ii2–8.
Tausche AK, Jansen TL, Schroder HE, Bornstein SR, Aringer M, Muller-Ladner U. Gout–current diagnosis and treatment. Deutsches Arzteblatt Int. 2009;106(34–35):549–55.
Jordan KM, Cameron JS, Snaith M, Zhang W, Doherty M, Seckl J, et al. British society for rheumatology and british health professionals in rheumatology guideline for the management of gout. Rheumatology. 2007;46(8):1372–4.
Zhang W, Doherty M, Bardin T, Pascual E, Barskova V, Conaghan P, et al. EULAR evidence based recommendations for gout. Part II: management. Report of a task force of the EULAR standing committee for international clinical studies including therapeutics (ESCISIT). Ann Rheum Dis. 2006;65(10):1312–24.
US Food and Drug Administration. FDA Approves Colchicine for Acute Gout, Mediterranean Fever. 2009. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm174620.htm. Accessed 8 Apr 2015.
Khanna D, Khanna PP, Fitzgerald JD, Singh MK, Bae S, Neogi T, et al. 2012 American college of rheumatology guidelines for management of gout. Part 2: therapy and antiinflammatory prophylaxis of acute gouty arthritis. Arthritis Care Res. 2012;64(10):1447–61.
Pacher P, Nivorozhkin A, Szabo C. Therapeutic effects of xanthine oxidase inhibitors: renaissance half a century after the discovery of allopurinol. Pharmacol Rev. 2006;58(1):87–114.
Hande KR, Noone RM, Stone WJ. Severe allopurinol toxicity. Description and guidelines for prevention in patients with renal insufficiency. Am J Med. 1984;76(1):47–56.
Jennings CG, Mackenzie IS, Flynn R, Ford I, Nuki G, De Caterina R, et al. Up-titration of allopurinol in patients with gout. Semin Arthritis Rheum. 2014;44(1):25–30.
Riedel AA, Nelson M, Joseph-Ridge N, Wallace K, MacDonald P, Becker M. Compliance with allopurinol therapy among managed care enrollees with gout: a retrospective analysis of administrative claims. J Rheumatol. 2004;31(8):1575–81.
Robinson PC, Dalbeth N. Advances in pharmacotherapy for the treatment of gout. Expert Opin Pharmacother. 2015;16(4):533–46.
Crittenden DB, Pillinger MH. New therapies for gout. Annu Rev Med. 2013;64:325–37.
European Medicines Agency. Krystexxa, pegloticase. 2013. http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/002208/human_med_001591.jsp&mid=WC0b01ac058001d124. Accessed 8 Apr 2015.
US Food and Drug Administration. FDA approves new drug for gout. 2010. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm225810.htm. Accessed 8 Apr 2015.
AstraZeneca. AstraZeneca announces top-line results from the Phase III programme of lesinurad in combination with xanthine oxidase inhibitors in gout patients. 2014. http://www.astrazeneca.com/Media/Press-releases/Article/13082014–astrazeneca-announces-topline-results-from-the-phase. Accessed 8 Apr 2015.
AstraZeneca. Marketing Authorisation Application for gout treatment lesinurad accepted by European Medicines Agency. 2015. http://www.astrazeneca.com/Media/Press-releases/Article/20150122–marketing-authorisation-application-for-gout. Accessed 8 Apr 2015.
Edwards NL, So A. Emerging therapies for gout. Rheum Dis Clin N Am. 2014;40(2):375–87.
Drummond M, Sculpher M, Torrance G, O’Brien B, Stoddart G. Methods for the economic evaluation of health care programmes. 3rd ed. Oxford University Press; 2005.
Jacobs P, Fassbender K. The measurement of indirect costs in the health economics evaluation literature. A review. Int J Technol Assess Health Care. 1998;14(4):799–808.
Organisation for Economic Co-operation and Development. PPPs and exchange rates. 2013. http://stats.oecd.org/Index.aspx?DataSetCode=SNA_Table4#. Accessed 18 Dec 2014.
Organisation for Economic Co-operation and Development. Consumer prices: consumer prices—annual inflation. 2014. http://stats.oecd.org/index.aspx?queryid=22519#. Accessed 18 Dec 2014.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097.
Brook RA, Forsythe A, Smeeding JE, Lawrence Edwards N. Chronic gout: epidemiology, disease progression, treatment and disease burden. Curr Med Res Opin. 2010;26(12):2813–21.
Brook RA, Kleinman NL, Patel PA, Melkonian AK, Brizee TJ, Smeeding JE, et al. The economic burden of gout on an employed population. Curr Med Res Opin. 2006;22(7):1381–9.
Wu EQ, Patel PA, Yu AP, Mody RR, Cahill KE, Tang J, et al. Disease-related and all-cause health care costs of elderly patients with gout. J Manag Care Pharm. 2008;14(2):164–75.
Halpern R, Fuldeore MJ, Mody RR, Patel PA, Mikuls TR. The effect of serum urate on gout flares and their associated costs: an administrative claims analysis. J Clin Rheumatol. 2009;15(1):3–7.
Halpern R, Mody RR, Fuldeore MJ, Patel PA, Mikuls TR. Impact of noncompliance with urate-lowering drug on serum urate and gout-related healthcare costs: administrative claims analysis. Curr Med Res Opin. 2009;25(7):1711–9.
Hanly JG, Skedgel C, Sketris I, Cooke C, Linehan T, Thompson K, et al. Gout in the elderly–a population health study. J Rheumatol. 2009;36(4):822–30.
Wu EQ, Patel PA, Mody RR, Yu AP, Cahill KE, Tang J, et al. Frequency, risk, and cost of gout-related episodes among the elderly: does serum uric acid level matter? J Rheumatol. 2009;36(5):1032–40.
Edwards NL, Sundy JS, Forsythe A, Blume S, Pan F, Becker MA. Work productivity loss due to flares in patients with chronic gout refractory to conventional therapy. J Med Econ. 2011;14(1):10–5.
Park H, Rascati KL, Prasla K, McBayne T. Evaluation of health care costs and utilization patterns for patients with gout. Clin Ther. 2012;34(3):640–52.
Saseen JJ, Agashivala N, Allen RR, Ghushchyan V, Yadao AM, Nair KV. Comparison of patient characteristics and gout-related health-care resource utilization and costs in patients with frequent versus infrequent gouty arthritis attacks. Rheumatology. 2012;51(11):2004–12.
Wu EQ, Forsythe A, Guerin A, Yu AP, Latremouille-Viau D, Tsaneva M. Comorbidity burden, healthcare resource utilization, and costs in chronic gout patients refractory to conventional urate-lowering therapy. Am J Ther. 2012;19(6):e157–66.
Lynch W, Chan W, Kleinman N, Andrews LM, Yadao AM. Economic burden of gouty arthritis attacks for employees with frequent and infrequent attacks. Popul Health Manag. 2013;16(2):138–45.
Sicras-Mainar A, Navarro-Artieda R, Ibanez-Nolla J. Resource use and economic impact of patients with gout: a multicenter, population-wide study. Reumatol Clin. 2013;9(2):94–100.
Garg R, Sayles HR, Yu F, Michaud K, Singh J, Saag KG, et al. Gout-related health care utilization in US emergency departments, 2006 through 2008. Arthritis Care Res. 2013;65(4):571–7.
De Vera MA, Marcotte G, Rai S, Galo JS, Bhole V. Medication adherence in gout: a systematic review. Arthritis Care Res. 2014;66(10):1551–9.
Wertheimer A, Morlock R, Becker MA. A revised estimate of the burden of illness of gout. Curr Ther Res Clin Exp. 2013;75:1–4.
Chandratre P, Roddy E, Clarson L, Richardson J, Hider SL, Mallen CD. Health-related quality of life in gout: a systematic review. Rheumatology. 2013;52(11):2031–40.
Colwell HH, Hunt BJ, Pasta DJ, Palo WA, Mathias SD, Joseph-Ridge N. Gout assessment questionnaire: initial results of reliability, validity and responsiveness. Int J Clin Pract. 2006;60(10):1210–7.
Roddy E, Zhang W, Doherty M. Is gout associated with reduced quality of life? A case-control study. Rheumatology. 2007;46(9):1441–4.
Alvarez-Hernandez E, Pelaez-Ballestas I, Vazquez-Mellado J, Teran-Estrada L, Bernard-Medina AG, Espinoza J, et al. Validation of the health assessment questionnaire disability index in patients with gout. Arthritis Rheum. 2008;59(5):665–9.
Hirsch JD, Lee SJ, Terkeltaub R, Khanna D, Singh J, Sarkin A, et al. Evaluation of an instrument assessing influence of gout on health-related quality of life. J Rheumatol. 2008;35(12):2406.
Khanna D, Ahmed M, Yontz D, Ginsburg SS, Park GS, Leonard A, et al. The disutility of chronic gout. Qual Life Res. 2008;17(5):815–22.
Singh JA, Strand V. Gout is associated with more comorbidities, poorer health-related quality of life and higher healthcare utilisation in US veterans. Ann Rheum Dis. 2008;67(9):1310–6.
Taylor WJ, Colvine K, Gregory K, Collis J, McQueen FM, Dalbeth N. The health assessment questionnaire disability index is a valid measure of physical function in gout. Clin Exp Rheumatol. 2008;26(4):620–6.
Becker MA, Schumacher HR, Benjamin KL, Gorevic P, Greenwald M, Fessel J, et al. Quality of life and disability in patients with treatment-failure gout. J Rheumatol. 2009;36(5):1041–8.
Lee SJ, Hirsch JD, Terkeltaub R, Khanna D, Singh JA, Sarkin A, et al. Perceptions of disease and health-related quality of life among patients with gout. Rheumatology. 2009;48(5):582–6.
Hirsch JD, Terkeltaub R, Khanna D, Singh J, Sarkin A, Shieh M, et al. Gout disease-specific quality of life and the association with gout characteristics. Patient Relat Outcome Meas. 2010;1(2010):1–8.
Sarkin AJ, Levack AE, Shieh MM, Kavanaugh AF, Khanna D, Singh JA, et al. Predictors of doctor-rated and patient-rated gout severity: gout impact scales improve assessment. J Eval Clin Pract. 2010;16(6):1244–7.
van Groen MM, ten Klooster PM, Taal E, van de Laar MA, Glas CA. Application of the health assessment questionnaire disability index to various rheumatic diseases. Qual Life Res. 2010;19(9):1255–63.
Dalbeth N, Petrie KJ, House M, Chong J, Leung W, Chegudi R, et al. Illness perceptions in patients with gout and the relationship with progression of musculoskeletal disability. Arthritis Care Res. 2011;63(11):1605–12.
Khanna D, Sarkin AJ, Khanna PP, Shieh MM, Kavanaugh AF, Terkeltaub RA, et al. Minimally important differences of the gout impact scale in a randomized controlled trial. Rheumatology. 2011;50(7):1331–6.
Khanna PP, Perez-Ruiz F, Maranian P, Khanna D. Long-term therapy for chronic gout results in clinically important improvements in the health-related quality of life: short form-36 is responsive to change in chronic gout. Rheumatology. 2011;50(4):740–5.
ten Klooster PM, Oude Voshaar MA, Taal E, van de Laar MA. Comparison of measures of functional disability in patients with gout. Rheumatology. 2011;50(4):709–13.
DiBonaventura M, Andrews LM, Yadao AM, Kahler KH. The effect of gout on health-related quality of life, work productivity, resource use and clinical outcomes among patients with hypertension. Exp Rev Pharmacoecon Outcomes Res. 2012;12(6):821–9.
Khanna PP, Nuki G, Bardin T, Tausche AK, Forsythe A, Goren A, et al. Tophi and frequent gout flares are associated with impairments to quality of life, productivity, and increased healthcare resource use: Results from a cross-sectional survey. Health Qual Life Outcomes. 2012;10:117.
Taylor WJ, House M, Horne A, McQueen FM, Dalbeth N. The work instability scale predicts absenteeism in people with gout and suggests a higher risk for those in manual occupations. J Clin Rheumatol. 2012;18(8):405–10.
Hoy DG, Smith E, Cross M, Sanchez-Riera L, Buchbinder R, Blyth FM, et al. The global burden of musculoskeletal conditions for 2010: an overview of methods. Ann Rheum Dis. 2014;73(6):982–9.
Brown GC, Brown MM, Sharma S, Stein JD, Roth Z, Campanella J, et al. The burden of age-related macular degeneration: a value-based medicine analysis. Trans Am Ophthalmol Soc. 2005;103:173–86.
Beard S, von Scheele B, Nuki G, Pearson I. Cost-effectiveness of febuxostat in chronic gout. Eur J Health Econ. 2014;15(5):453–63.
Jutkowitz E, Choi HK, Pizzi LT, Kuntz KM. Cost-effectiveness of allopurinol and febuxostat for the management of gout. Ann Intern Med. 2014;161(9):617–26.
Spaetgens B, Tran-Duy A, Wijnands JM, van der Linden S, Boonen A. Health and utilities in patients with gout under care of a rheumatologist: which factors contribute? Arthritis Care Res. 2015. doi:10.1002/acr.22551
Buist AS, Vollmer WM, Sullivan SD, Weiss KB, Lee TA, Menezes AM, et al. the burden of obstructive lung disease initiative (BOLD): rationale and design. Copd. 2005;2(2):277–83.
Rutten-van Molken M. Raising the awareness: projecting the future burden of COPD with the BOLD model. Eur Respir J. 2009;34(4):787–9.
Buist AS, Vollmer WM, McBurnie MA. Worldwide burden of COPD in high- and low-income countries. Part I. The burden of obstructive lung disease (BOLD) initiative. Int J Tuberc Lung Dis. 2008;12(7):703–8.
Massi-Benedetti M. The cost of diabetes Type II in Europe: the CODE-2 Study. Diabetologia. 2002;45(7):S1–4.
Jonsson B. Revealing the cost of type II diabetes in Europe. Diabetologia. 2002;45(7):S5–12.
Bagust A, Beale S. Modelling EuroQol health-related utility values for diabetic complications from CODE-2 data. Health Econ. 2005;14(3):217–30.
Bloudek LM, Stokes M, Buse DC, Wilcox TK, Lipton RB, Goadsby PJ, et al. Cost of healthcare for patients with migraine in five European countries: results from the International Burden of Migraine Study (IBMS). J Headache Pain. 2012;13(5):361–78.
Stokes M, Becker WJ, Lipton RB, Sullivan SD, Wilcox TK, Wells L, et al. Cost of health care among patients with chronic and episodic migraine in Canada and the USA: results from the International Burden of Migraine Study (IBMS). Headache. 2011;51(7):1058–77.
Payne KA, Varon SF, Kawata AK, Yeomans K, Wilcox TK, Manack A, et al. The International Burden of Migraine Study (IBMS): study design, methodology, and baseline cohort characteristics. Cephalalgia. 2011;31(10):1116–30.
Acknowledgments
Shields GE and Beard SM are invited authors on this review paper and have not received payment, and have no financial relationship with any organization related to this manuscript. Beard SM has previously published work on the cost effectiveness of febuxostat for gout therapy (Beard SM, von Scheele BG, Nuki G, Pearson IV. Cost-effectiveness of febuxostat in chronic gout. Eur J Health Econ. 2014 Jun;15(5):453–63).
Author contributions
Beard SM and Shields GE were involved in the planning, completion and interpretation of the results of the systematic review. Shields GE led the drafting of the paper, with Beard SM providing guidance, reviews and revisions to all drafts. Both authors approved the final submitted version and guaranteed its content.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shields, G.E., Beard, S.M. A Systematic Review of the Economic and Humanistic Burden of Gout. PharmacoEconomics 33, 1029–1047 (2015). https://doi.org/10.1007/s40273-015-0288-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40273-015-0288-5