Abstract
Cnidarians, especially skeleton-bearing anthozoans and hydrocorals, are known to host abundant and diverse symbiotic fauna encompassing members of the majority of metazoan taxa, ranging from sponges and flat worms to fishes. Members of the class Polychaeta are between the most diverse and perhaps the least studied taxa of coral symbionts. The last revision (Martin and Britayev, Oceanogr Mar Biol 36:217–340, 1998) reckoned about 60 species of symbiotic polychaetes associated with more than 100 species of cnidarian hosts. However, this number is considerably underestimated. Some populations of scleractinians, sea fans and black corals show up to 100 % infestation by symbiotic polychaetes. Close association and inter-relation of highly host-specific symbionts and cnidarian hosts often lead to dramatic changes in the host morphology. At the moment, actual mechanisms of most of mutual relations between host and symbiont in such associations are generally unknown. The objective of the present paper is to summarize data on species composition and ecology of polychaetes associated with cnidarians. In our review, we report 281 species of cnidarian hosts involved in 324 relationships with symbiotic polychaetes. Most polychaete-hosting cnidarians belong to skeleton-bearing taxa, particularly Scleractinia (125 species or 44.48 % of the total cnidarian hosts), Alcyonaria (73 species or 25.97 %) and Hydrozoa (60 species or 21.35 %). About 120 species of symbiotic polychaetes of ten families are reported from cnidarian hosts. Polynoidae include the highest number of cnidarian-associated polychaetes (almost one half of the currently known species), followed by Syllidae and Serpulidae. Host symbiont interrelations, host specificity, location, infestation characteristics and adaptive modifications of symbionts, as well as host reaction on symbionts presence, have been considered. Our review highlights that (1) every group of cnidarians seems to have their own assemblage of symbiotic polychaetes, (2) some deep-sea alcyonaceans and black corals have never been reported without their often undetermined polynoid symbionts so that its presence has been considered as a species-specific, robust taxonomic character, and (3) we certainly expect the polychaete symbionts associated with deep-sea corals to be a hidden hot-spot of diversity, with many species still waiting to be described.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction. State of Knowledge
It is now well known and accepted that many marine organisms can harbor rich associated fauna. Cnidarians , and in particular skeleton-bearing corals , are between the most preferred hosts for such associations (see e.g. Buhl-Mortensen and Mortensen 2004; Stella et al. 2011; Watling et al. 2011). The complex three-dimensional network created by the massive skeleton or the characteristic branching pattern of alcyonaceans and black corals may provide food , substrate and shelter for various types of organisms. According to the comprehensive review on symbiotic polychaetes by Martin and Britayev (1998), cnidarians appeared to be the second dominant group harboring symbiotic polychaetes (20 % of the 569 by then known host species) yielding only to Echinodermata (36 % of host species). Buhl-Mortensen and Mortensen (2004) in the review of symbiosis in deep-sea water corals counted 311 and 112 species of obligate symbiotic invertebrates (all taxa) from shallow-water and deep-sea corals , respectively. Seven years later, the number of symbiotic invertebrates recorded in association with scleractinian corals only increased up to 869 species (Stella et al. 2011). Among them, only 29 species of polychaetes were recognized as facultative and obligatory coral symbionts . Little is still known about the biology of deep-sea symbionts , since many species are known only from the type localities, or based on limited number of historical dredge samples when hosts and symbionts were often separated during preliminary on-deck sorting. On the other hand, it is hard to distinguish the organisms inhabiting the coral framework and using it just for shelter from those living in close association with the host coral (i.e. real symbionts ).
The purpose of the present paper is to revise the current data on host -symbiont relationships between cnidarians and their associated polychaetes. Also, we try to define main gaps in the present knowledge on the biology of the polychaete-cnidarian symbiosis.
2 Cnidarian Hosts Involved in Coral-Polychaete Associations
To date, 281 species of cnidarian hosts involved in 324 relationships with symbiotic polychaetes have been reported (Table 25.1). Most polychaete-hosting cnidarians belong to skeleton-bearing taxa (Fig. 25.1). Among the anthozoans , most belong to the Scleractinia (125 species or 44.48 % of the total cnidarian hosts), Alcyonacea (73 species or 25.97 %), Hydrozoa (60 species or 21.35 %), and Antipatharia (19 species or 6.76 %). The hosts lacking hard skeleton include only two species of Actiniaria : Bolocera tuediae associated with Alentiana aurantiaca and Metridium senile with Arctonoe vittata. One non-identified zoanthid hosted Lumbrineris flabellicola and several species of not obligate polychaetes are reported from cerianthid tubes. There are no polychaete symbionts reported in association with Corallimorpharia .
2.1 Ceriantharia
Species associated with tube anemones (Ceriantharia) generally inhabit the surface or are embedded into their thick felt-like tubes consisting of discharged ptychocyst threads incrusted with particles of mud and sand. About 30 species of polychaetes were reported from tubes of Pachycerianthus multiplicatus Carlgren, 1912 (Kilkerrin Bay, Ireland). However, most reported species were in fact associated with polychaetes and sipunculids inhabiting the cerianthid tube or use the cerianthid tube as elevated substrate for settlement. Apparently, Myxicola infundibulum is the only polychaete species that is not reported from the surrounding grounds but from cerianthid tubes (O’Connor et al. 1977) and thus this species is considered in the Table 25.1.
2.2 Scleractinia
Scleractinians are apparently the most attractive group of marine cnidarians for symbiotic organisms. Dead skeletons of reef -building scleractinians provide a substrate that is actively eroded and occupied by numerous excavating taxa, including many species of polychaetes (Hutchings 2008; Glynn and Enoch 2011). However, only 29 polychaetes (including borers and cryptic species ) out of 869 invertebrates have been recently reported as scleractinian symbionts in coral reefs (Stella et al. 2011), while five and nine species have been reported as obligatory coral symbionts from shallow-water and deep-sea habitats , respectively (Buhl-Mortensen and Mortensen 2004).
Despite the relatively low number of symbiotic polychaete species, they are often reported in association with a wide range of scleractinian hosts from several families (Zibrowius et al. 1975; Martin and Britayev 1998). On coral reefs, symbiotic polychaetes more often inhabit massive, slow-growing species of Acroporidae , Poritidae, Faviidae etc. (Rowley 2008). In deep-sea habitats, polychates have been mostly reported during last decades in association with intensively studied frame-building corals such as Lophelia pertusa (Fig. 25.2b), Solenosmilia variabilis and Madrepora oculata (Buhl-Mortensen and Mortensen 2004). However, solitary cup-corals (Fig. 25.2a) may be also involved in symbiotic associations (Zibrowius et al. 1975).
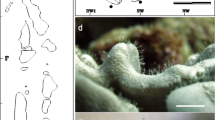
Morphological modifications of cnidarian hosts . (a) Flabellum chunii with scars (arrowhead) from Lumbriconeris flabellicola; (b) tube of Eunice sp. (arrowhead) overgrown by Lophelia pertusa ; (c) syntype of Antipathes cylindrica with eunicid tube (arrowhead) overgrown by coral tissue (Photo courtesy P. Lozouet); (d) Pseudoanthomastus agaricus hosting Neopolynoe acanellae (small arrowheads); (e) mushroom-like colonies of Sphaerasclera flammicerebra, (d) cross section of one specimen of S. flammicerebra showing a groove with symbiotic polychaete (small arrowheads). Scale: (a–c, e) 10 mm, (d, f) – 5 mm
2.3 Antipatharia
Nineteen species of black corals hosting 14 species of symbiotic polychaetes are known to date (Martin and Britayev 1998; Opresko 2006; Molodtsova and Budaeva 2007; Wagner et al. 2012; Britayev et al. 2014), but the number of hosts is clearly underestimated. The genera of black corals infested by polychaetes (i.e., Cupressopathes Opresko, 2001, Tanacetipathes Opresko, 2001 (Fig. 25.3d), Stylopathes Opresko, 2006 (Fig. 25.3c), Asteriopathes Opresko, 2004, Antipathella Brook, 1889) often have bottle-brush colonies that seem to be more favorable for polychaete symbionts . However, the coral growth form may also be somehow influenced by the symbiont presence (Molodtsova and Budaeva 2007). Some species of black corals, like Tanacetipathes spinescens (Britayev et al. 2014), often host polychaete symbionts, while in all species of Stylopathes Opresko, 2006 (Stylopathidae ) a symbiotic polynoid was always present living on the main stem of the monopodial colony.
Morphological modifications of cnidarian hosts. (a) Candidella imbricata with enlarged basal scales forming a tunnel (arrowheads) harboring Gorgoniapolynoe caeciliae; (b) tunnels (arrowheads) in branches of Corallium cf. niobe induced by G. caeciliae; (c) worm-run (arrowheads) in colony of Stylopathes sp. formed by densely anastomosed pinnules; (d) worm-run (arrowheads) along the stem of Tanacetipathes cf. spinescens. Scale: (a, c–d) – 5 mm, (b) – 10 mm
2.4 Octocorallia
Among the 73 octocorals harboring symbiotic polychaetes, only two species of Pennatulacea (Pettibone 1963; Nygren and Pleijel 2010) and one Helioporacea (Martin et al. 2009) were reported. The remaining 70 hosts belong to the Alcyonacea . The shallow water octocorals hosting polychaetes (mainly Nephtheidae , Xeniidae and Melithaeidae ) are relatively scarce and, when reported, they are rarely determined to species level. In turn, the alcyonaceans are the most important deep-sea cnidarians harboring polychaetes (Buhl-Mortensen and Mortensen 2004; Watling et al. 2011), particularly Primnoidae (24 species, mostly belonging to Narella Gray, 1870, Candidella Bayer, 1954 (Fig. 25.3a) and Thouarella, Gray 1870), Coralliidae (8 species), Nephthyidae (8 species), Isidiidae (6 species), Acanthogorgiidae (4 species) and Plexauriidae (3 species). Among the species of Narella , for instance, the presence of a symbiotic polychaete is considered as specific at the species level (Cairns 2012).
2.5 Hydrozoa
More than two thirds of the hydroids reported in association with polychaetes possess massive calcified skeleton and belong to the families Stylasteridae and Milleporidae . Stylasterids or ‘lace corals’ (Anthoathecata: Filifera), are known from all oceans (i.e., from the Arctic circle to the Antarctica and from 0 to 2,789 m depth) but are more common from 200 to 400 m depth (Cairns 2011). Nearly 40 species of Stylasteridae have been reported to harbor polychaete symbionts (mainly the genera Conopora Moseley, 1879, Errina Gray, 1835 and Stylaster Gray, 1831). Particularly, Conopora adeta is known to live exclusively in association with the symbiotic polynoid Benhamipolynoe cairnsi (Cairns 1987).
The species of Milleporidae (Anthoathecata: Capitata) inhabit exclusively tropical shallow waters, being among the most conspicuous skeleton-forming coral reef organisms (Lewis 2006). The Milleporidae comprises only one genus, Millepora Linnaeus, 1758, with 15 valid species, 6 of them being reported as hosts of symbiotic polychaetes (Serpulidae and Spionidae ).
3 Polychaetes Involved in Associations with Cnidarians
About 120 species of symbiotic polychaetes of 10 families are reported from cnidarian hosts (Table 25.1, Fig. 25.4). It is hard to approximate the exact number of species as many host descriptions (e.g. Williams 2003; Cairns and Bayer 2004; Opresko 2006; Cairns 2012) report on symbionts that are not determined, even to the genus level. Apparently, the Polynoidae include the highest number of cnidarian-associated polychaetes: almost one half of the currently known species. They are followed by Syllidae , Serpulidae , Eunicidae and Spionidae . Spionids are generally considered as parasitic, however there are some indications that at least some cnidarian hosts can benefit from presence of these particular symbionts (see the next section). The families Hesionidae , Lumbrineridae , Spintheridae , Spherodoridae and Chaetopteridae comprise each only one or two species reported as symbiotic with cnidarians . The species of Sabellidae are known exclusively from cerianthid tubes (Table 25.1) and it is not clear if they can be considered as symbiotic.
4 Host -Symbiont Interrelations
Symbiosis sensu De Bary (1879) is currently considered as a general term including close long-term associations between organisms of different species (Margolis et al. 1982), which are subsequently characterised according to the cost/benefit for partners (host :symbiont ): commensalism (+:0), parasitism (+:−), and mutualism (+:+). More than 90 % of the relationships between coral hosts and symbiotic polychaetes are commensalisms (Table 25.1). However, the low level of knowledge and scattered available information on the biology of symbionts may artificially exaggerate their relevance (Martin and Britayev 1998). It is evident that coral colonies provide safety shelter for symbiotic polychaetes hidden among their three-dimensional network of branches, inside the skeleton, on the surface of coral branches beneath sclerites , or in tubular galls induced by the polychaetes themselves. So, if there are no clear evidences on negative (parasitism ) or positive (mutualism ) feedbacks for the host, the association is considered as commensalism .
The number of parasitic associates is significantly lower, while mutualists are virtually negligible (7.3 % and 1.8 % respectively). Among symbionts , some species from different families (i.e., spionids , syllids , polynoids ) affect host growth (see section below). For instance, readdressing their energy resources to repair the damages induced by polychaetes instead to somatic growth and reproduction indicates a clear negative effect of the symbionts on their hosts . Moreover, there are indications on polychaetes feeding by stealing host food (Buhl-Mortensen 2001; Mueller et al. 2013) or by consuming coral mucus and tissues (Britayev and San Martín 2001; Britayev et al. 2014). At the same time symbionts may clean coral hosts from detritus, bacteria, fungi and algae, thus increasing their competitiveness, as well as protect them from predators attacks (e.g. Stewart et al. 2006; Bergsma 2009). However, the net outcome of these different processes on the metabolism and survivorship of corals is unknown. Accordingly, the status of some of these associations may need further re-evaluation depending on the appearing of new information on host symbionts relationships .
Nevertheless, there are some well studied associations between polychaetes and coral hosts, which are briefly summarized in the next sections.
4.1 Polynoidae
Polynoids are the most diverse group of polychaetes associated with gorgonian and antipatharian corals, but only one species has been well documented (Table 25.1). The unique species whose relationships with a host coral was studied is the scleractinian associate Hololepidella sp. (Britayev et al. 2015). Most studied specimens of Hololepidella sp. had up to one third of the gut length filled with mucus containing unicellular algae and cnidocysts , and a few of them also had copepod fragments. The algae were very similar in size, shape and color to the zooxantella living in host tissues, indicating that Hololepidella sp. was trophically related to the host coral and, thus, the species can be considered as parasite. In turn, the copepods closely resembled those obligatory associated with the scleractinian corals of the genus Galaxea Oken, 1815. The feeding of Hololepidella sp. on the parasitic siphonostomatoid copepod evidence that one symbiont may control the density of another one living in the same host. The high prevalence, specific location on the host, and feeding strategy clearly suggest that Hololepidella sp. is a specialized scleractinian symbiont, closer to a parasite. In turn, other scleractinians associates (i.e. crabs and shrimps) are known as mutualistic due to their cleaning or guarding activity (Stewart et al. 2006). However, like Hololepidella sp., they also feed on coral mucus and tissues. Therefore, further clarification of the ecological role (parasitism vs. mutualism ) of Hololepidella sp. will require detailed studies including more field observation and experimental approaches.
4.2 Serpulidae
The single well-documented example of coral –polychaete relationship among serpulids is that of the filter-feeding species of Spirobranchus . In this case, the association is considered as mutualism , since the current created by the branchial crown of Spirobranchus spp. draws water up from the coral surface (Strathmann et al. 1984), enhancing the arrival rate of food particles to the coral polyps , improving the water circulation close to coral surface and, consequently, decreasing the susceptibility of the host corals to bleaching (Hunte et al. 1990; Nakamura et al. 2003). An additional advantage for the coral is that the worms may defense the host from the attaks of the carnivorous starfish Acanthaster planci. When contacted by the starfish, the worms hosted by Porites spp. immediately retract and reappear, pushing against the tube feet and arms of the starfish with the ornamented operculum and the branchial crown, forcing the predator to move away (DeVantier et al. 1986).
4.3 Spionidae
Data on the impact of spionids on their respective coral hosts are rare and controversial. In fact, polydorid worms may affect their hosts by weakening their branches and drawing energy to repair the skeletal tissue damaged by polychaete boring activity. For example, the burrow openings of Dipolydora armata on the surface of Millepora complanata develop distinctive, erect spines caused by the combined growth of worm tubes and host tissue. The zooids of Millepora were absent in the vicinity of tube openings and on spines and, thus, the potential feeding surface of the coral is reduced in heavily colonized branches. Burrows and openings were densest at the bases of the branches of Millepora where the skeleton weakening may easily occur (Lewis 1998).
In contrast, indirect evidences prove that the presence of spionids may enhance tissue growth /calcification rate in Astreopora myriophthalma (Wielgus and Levi 2006). In fact, the capture of particulate organic matter from the water column and the adjacent substrate and the production of nitrogen enriched metabolic waste products may affect primary production in coral reefs by influencing the physiology of the coral/zooxanthella association.
Another spionid , Polydora villosa, often inhabits the branched morph of Montipora spp., while is rare in encrusting or columnar morphs. Accordingly, this has been considered as an indirect evidence on the symbiont -mediated modification of Montipora spp. from an encrusting or columnar morph to a branched one (Liu and Hsieh 2000). Interestingly, similar morphological changes induced by a symbiotic amphipod apparently enhanced the resistence of Montipora spp. to predation by pincushion (Culcita novaeguineae) and crown-of-thorns (Acanthaster planci) sea stars. The fingers of the branched colonies of Montipora spp. were both less susceptible to be attacked and more likely able to survive to an attack than the colonies without fingers. Furthermore, the presence of fingers altered the preferences of A. planci prey, as the sea star preferred Montipora spp. without fingers over other common corals , but preferred these other corals when the specimens of Montipora spp. had fingers (Bergsma 2012).
4.4 Chaetopteridae
Finger-like skeletal modifications in the scleractinian coral Montipora spp. induced by the chaetopterid Spiochaetopterus sp. have been recently described by Bergsma (2009). Fingers inhabited by worms were similar in size and shape to those inhabited by amphipods in the same host , while their frequency (29.2 % of colonies and 9.3 % of fingers) and length (up to 122 mm) was lower. The fingers induced by chaetopterids are considerably longer than the 50 mm reported for the otherwise identical structures induced by spionids on Montipora spp. in Taiwan (Liu and Hsieh 2000). These fingers were frequently found detached from their parent colony, which evidences that symbionts may reduce the ability of Montipora spp. to withstand physical disturbances . However, detached coral fingers are able to survive and reattach to form new colonies, which can be helpful for coral dispersal . Morphological changes may also affect corals’ ability to utilize resources and to compete for space. These observations indicate that symbiont -induced growth forms may enhance the reproductive potential and competitive ability of Montipora spp. in Moorea (French Polynesia).
4.5 Host Specificity
The host specificity of symbiotic polychaetes associated with corals is relatively high (Fig. 25.5): 69 out of 107 species determined to a species level (64.48 %) are known from a single cnidarian host , 17 from 2, 8 from 3, and 2 from 4. However, among the “monoxenous” species occurring in only one cnidarian host, some occur also in association with other non-cnidarian taxa, such as echinoderms or mollusks. For instance, Arctonoe vittata, a symbiont of Metridium senile, is also known from at least 30 more hosts including echinoids, asteroids, polychaetes and mollusks. Hololepidella nigropunctata, a symbiont of Lobactis scutaria in the Red sea , commonly occurs in association with at least 20 species, mostly asteroids and brittle-stars. Nevertheless , polyxenous cnidarian-symbiotic polychaetes are relatively scarce: Only 11 species are reported from more than 5 hosts, which usually belong to closely related taxa or inhabit the same ecological niche . For instance, Spirobranchus corniculatus (Serpulidae ) occurs in association with 52 cnidarian hosts , all them belonging to the Scleractinia , except for three Milleporidae . Lumbrineris flabellicola (Lumbrineridae ), reported in 31 associations, lives mostly with ahermatypic scleractinians (29 species), but also with one hydroid and one zoanthid (Zibrowius et al. 1975; Martin and Britayev 1998). Symbiotic polychaetes inhabiting black corals seem to be strict associates of antipatharians (Wagner et al. 2012), except Tottonpolynoe symantipatharia, which was also reported from Sclerisis macquariana (Alcyonacea: Isidiidae). Finally, some cnidarian taxa have no specific symbionts . For instance, all polychaetes reported from Millepora are also known from scleractinian hosts (Lewis 2006).
4.6 Location on the Host
Polychaetes are generally found on the surface of their cnidarian hosts (either on colonies or on individual polyps ). Alternatively, tubes of symbiotic polychaetes can be embedded in the hard skeleton, with only part of the animal appearing at the surface, such as the precious Christmas tree worm Spirobranchus spp. (Serpulidae ), whose calcareous tubes are deeply embedded inside the host (Scleractinia or Milleporidae ) skeleton as they are overgrown by the coral skeleton while being formed.
Some polychaete symbionts live inside tunnels or galleries (formed by modifications of the coenenchyme or the sclerites ) on branches of the host colony as, for example, Gorgoniapolynoe spp. on colonies of Primnoidae (Fig. 25.3a) and Corallidae (Fig. 25.3b) (Eckelbarger et al. 2005; Simpson and Watling 2011; Britayev et al. 2014). Alternatively, galleries may be formed (apparently excavated by the symbionts) inside the host coenenchyme, as in the case of Haplosyllis anthogorgicola (Syllidae ) on Anthogorgia bocki (Martin et al. 2002).
Only few reports of life cycle stages of polychaete symbionts living inside cnidarian hosts are known to date. Among them, the larvae of the syllid Epigamia alexandri (reported as Proceraea rzavskyi) develop inside hydrothecae of Abietinaria turgida. At an early juvenile stage, they apparently begin to feed on the tissues of the hydranth, to the extent that they finally occupied the whole space inside of the zoothecae. When the juveniles reach about 1 mm, they leave the zootheca and start building a mucus tube attached to the main stem of the hydroid colony (Britayev and San Martín 2001). Early larval and juvenile stages of at least some symbiotic polychaetes are planktonic free-living (Eckelbarger et al. 2005; Rowley 2008), whereas in other species eggs are hatched inside the host (Britayev and San Martín 2001).
4.7 Prevalence of Infestations
The relationship between number of infested and total number of hosts, also known as “prevalence of infestation” (Martin and Britayev 1998), is not often discussed in literature. Such information for cnidarian hosts is even more rare and mostly available for easy to spot symbionts . As discussed by Martin and Britayev (1998), this value cannot be considered as characteristic at the species level. Each population of a given commensal species is usually characterized by a different prevalence. Prevalences may range from extremely low to considerable high percentages. For instance, the infestation by Imajimaea draculai reaches 10 % in the population of Funiculina quadrangularis (Pennatulacea ) from the Bratten area (Skagerrak) (Nygren and Pleijel 2010). About 50 % of nephtheids from the Darwin region (Northern Australia) harbor Alcyonosyllis phili, while a 100 % of the colonies of some species of Narella (Primnoidae ) are infested, to the extent that the presence of the symbiotic polynoid is considered a species level indicator (Cairns 2012). Also a 100 % of Conopora adeta (Stylasteridae ) were reported to host the polynoid Benhamipolynoe cairnsi (Cairns 1987), and all hitherto known species of Stylopathes (Antipatharia ) host polynoid polychaetes (Opresko 2006; Molodtsova and Budaeva 2007).
Factors that can be crucial for prevalence of infestation include bathymetry, spatial variability and hydrology (Martin and Britayev 1998). Another factor that can be important is the antropogenic disturbance. For instance, Wielgus et al. (2006) showed that the infestation of reef -building stony corals by spionids was significantly correlated with the total oxydized nytrogen in the water column in the vicinity of organic waste discharges.
If a symbiont occurs on different hosts, the prevalence may vary even within the same locality (Martin and Britayev 1998) depending on how suitable are the different hosts. For instance, Spirobranchus polycerus in Barbados occurs on several species of scleractinians and milleporids, but is most common on Millepora complanata and only occasionally occurs on scleractinian corals (Lewis 2006).
4.8 Intensity of Infestation
The number of symbionts per coral host is also highly variable. Martin and Britayev (1998) reported at least seven species of symbiotic polychaetes associated with cnidarian hosts with known intensities of one symbiont per host . Apparently, the intensity is closely related to a territorial behavior , but no direct evidences have been reported for cnidarian symbionts. There are no references to isolated heterosexual pairs inhabiting the same coral host individual. On the other hand, some coral symbionts show very high intensities. For instance, 18 specimens of Brychinoe karenae were collected from a single relatively small colony of Leiopathes secunda (Hanley and Burke 1991a). About 120 specimens of Gorgoniapolynoe caeciliae were recovered from one-fourth of a single colony of Candidella imbricata (Eckelbarger et al. 2005), while intensities of about 0.2–0.4 of symbionts per 1 cm of the host were reported for the co-generic G. uschacovi (Britayev 1981) and G. guadaloupensis (Pettibone 1991). The intensity of the serpulid Spirobranchus giganteus range from 0.2–12 symbionts per 1 cm2 of living coral surface (Martin and Britayev 1998).
Even closely related species can differ in their intensity. For instance, the maximum intensity reported for H. chamaeleon and H. villogorgicola are about ten symbionts per host colony, whereas H. anthogorgicola can reach up to 15 symbionts per 1 cm of colony (Martin et al. 2002).
5 Host Reactions to Symbiont Presence
The number of studies reporting changes in host morphology caused by the presence of symbiotic polychaetes is very limited. Quite often, these modifications are not attributed to the symbiont presence because the two partners are studied separately. In few cases, cnidarian host did not exhibit any morphological reactions to the presence of symbiotic polychaetes. Haplosyllis chamaeleon inhabiting the surface of the branchlets of Paramuricea clavata did not induce significant changes to the coral host morphology. However the symbiont usually occurred on parts of the colony with high number of living polyps (Martin et al. 2002). No changes in the host morphology were recorded for the Alcyonosyllus phili (Fig. 25.6b, d) (Glasby and Watson 2001; Britayev and Antokhina 2012) or in Funiculina quadrangularis hosting Imajimaea draculai (Nygren and Pleijel 2010).
Scars or grooves induced by Lumbrineris flabellicola were reported on the outer surface of the skeleton in several azooxanthelate scleractinians (Fig. 25.2a) (Zibrowius et al. 1975; Miura and Shirayama 1992; Cairns and Zibrowius 1997). This lumbrinerid polychaete inhabits soft membranous tubes attached to the external surface of the coral skeleton . However, the calcareous skeleton became partly dissolved beneath the tube, giving rise to a grove causing the tube to become partly embedded into the coral skeleton. In turn, the depth of the groove is highly dependent of the host species, being more pronounced in Caryophyllia spp. and Flabellum chunii (Zibrowius et al. 1975).
Frame-building scleractinian corals, such as Lophelia pertusa , Madrepora oculata or Solenosmilia variabilis, often overgrow the tubes of the symbiotic polychaete Eunice spp. (Fig. 25.3b) (Zibrowius 1980; Cairns and Zibrowius 1997). A similar effect was reported for E. norvegica on the stylasterid Errina atlantica (Zibrowius and Cairns 1992). Aquarium experiments with E. norvegica (Buhl-Mortensen 2001; Roberts 2005) showed that their tube-building stimulates the production of coral skeleton. The parchment-like tubes of Eunice spp. are used as cores for calcification and may serve as the main stem for the skeleton, supporting longer branchlets >25 cm long (Buhl-Mortensen 2001). In the presence of eunicid symbionts , calcification rates in Lophelia pertusa increase up to four times (Mueller et al. 2013). Molodtsova and Budaeva (2007) reported overgrowth of eunicid tubes by the chitinous skeleton of the black coral Antipathes cf. cylindrica Brook 1889 (Fig. 25.3c).
Overgrowth of symbiont tubes by host tissues and skeleton leading to changes in the cnidarian host morphology have also been reported from shallow-water reefs . Accordingly, the chaetopterid polychaetes Spiochaetopterus sp. inhabiting colonies of Montipora spp. induce the formation of finger-like branchlets in the host (Bergsma 2009). The spionid Dipolydora sp. from the Gulf of Eilat (Red Sea ) was reported (Wielgus et al. 2002, 2006) to induce skeletal aberrations in 10 % of its host scleractinians , resulting in the formation of 5–25 mm high cones. Dipolydora armata can stimulate formation of distinctive, erect spines at the bases of branches in Millepora complanata on Barbados coral reefs (Lewis 1998).
Surprisingly, symbiotic polychaetes rarely induce gall formation. Apparently, the single case was reported for Proceraea penetrans, which builds calcified blister-like galls on the host Stylaster californicus (Wright and Woodwick 1977, Zibrowius 1981). On the other hand, the formation of tunnels and galleries is characteristic for practically all known taxa of cnidarian hosts harboring symbiotic polychaetes which, to some extent, may be considered as a particular case of gall formation. Symbiotic syllids and polynoids generally produce tunnels or galleries. In soft corals , such galleries can be distinguished by a grove at the edge of capitulum, as that in Pseudoanthomastus spp. induced by Neopolynoe acanellae (Molodtsova 2013), or by a rolled margin, as that in Sphaerasclera flammicerebra induced by an unidentified polychaete (Williams 2003) and giving a distinctive mushroom-shape look to the colony (Fig. 25.2d–f). In both cases, the groove is formed from overgrowths and processes of the soft tissue, and there are no indications in the literature on skeletal elements’ affectation.
Galleries in primnoids are generally attributed to the presence of symbiotic polynoids of the genus Gorgoniapolynoe (Cairns and Bayer 2004; Eckelbarger et al. 2005; Cairns 2012; Britayev et al. 2014). In primnoids , the gallery is formed by highly modified polyp scales: the basal ones of successive adjacent polyps became enormously enlarged and curved to meet together forming a tube that can attain up to 3 mm in diameter (Cairns and Bayer 2004, 2008; Cairns 2012) (Fig. 25.3a).
Galleries induced by symbiotic polynoids were reported on branches of several species of Corallium Cuvier, 1798 (Fig. 25.3b) and Paracorallium Bayer and Cairns, 2003 (Bayer 1964; Britayev 1981; Pettibone 1991a; Simpson and Watling 2011; Britayev et al. 2014). The gallery formation involves not only soft tissues but also the underlying calcareous axis. The mechanism of gallery formation in Corallidae is not really known. Obviously it does not result from any boring activity of the symbiont , but from a gradual formation as a response to its presence. Taking into account that the axial epithelium and free scleroblasts in Corallium have the same cellular origin (Grillo et al. 1993), we can speculate that the free scleroblasts of coenenchymal lobes involved in the gallery formation are induced somehow to form an additional layer of axial epithelium that begins to produce solid axis instead of loose sclerites .
Some symbiotic syllids also produce a kind of galleries in the coenenchyme of cnidarian hosts . For instance, Alcyonosyllis glasbyi is reported to form tubular nests or shelter-like structure on the surface of the host Melithaea flabellifera (San Martín and Nishi 2003). Haplosyllis anthogorgicolla forms galleries inside the coenchym of Anthogorgia bocki , opening near base of polyps as minute tube-like projections. The galleries are located between the surface of the host covered by spicules and inner axis, and appear as well-structured tubes, with tissue-built walls that can be easily distinguished from the remaining unaltered tissue (Martin et al. 2002). The species H. villogorgicola is assumed to induce fusion of two adjacent branchlets in the host Villogorgia bebricoides which forms a cavity inhabited by the symbiotic worm (Martin et al. 2002). The only case of gallery formation in scleractinians was reported for Typosyllis sp. forming so-called groove-and-tube structures in several species of reef -building scleractinian corals in Taiwan (Randall and Eldredge 1976).
Several species of symbiotic polynoids induce malformations in black corals , mainly of the genus Stylopathes (e.g. Bayerpolynoe floridensis inhabiting S. litocrada). These are the so-called “worm runs” (Totton 1923; Opresko 2006; Molodtsova and Budaeva 2007): tubular reticulated structures formed near the base of primary pinnules of numerous short highly anastomosing and fusing secondary and tertiary branchlets and pseudo-lateral pinnules that connect the stem with the worm run, but rarely extend beyond its surface (Fig. 25.3c). Feebly developed worm-runs with few or no anastomoses can be also found in species of the genus Tanacetipathes Opresko, 2001 (e.g. Parahololepidiella greeffi inhabiting T. spinescens) (Molodtsova and Budaeva 2007; Britayev et al. 2014) (Fig. 25.3d) and Asteriopathes Opresko, 2005 (Molodtsova and Budaeva 2007; Molodtsova unpublished data).
There are some other modifications that can affect the length of individual branchlets or the skeletal structures of individual zooids. For instance, the hydrothecae modification by the symbiotic syllid Epigamia alexandri, which caused the formation of an elongated tubular distal part in hydrothecae of Abietinaria turgida (Britayev and San Martín 2001). The spionid Polydora wobberi associated with Lophogorgia sp. apparently affect the length of branches. Worms inhabit narrow U-shaped burrows that open to the exterior at the tips of short stubby 20–30 cm long branches of the gorgonian , whose uninfested branches may reach up to 70 cm long. Molodtsova and Budaeva (2007) reported that presence of symbiotic polychaetes can alternate size and morphology of skeletal spines in black corals .
Changes of the host branching pattern induced by symbiotic polychaetes have also been reported. The presence of the spionid Polydora villosa may result in modifications of the growth form in hosts Montipora spp. from the encrusting or columnar morph to the branched one, but does not affect Porites spp. hosts (Liu and Hsieh 2000). Cairns (2011) described development of the third row of pinnules in a typically uniplanar colony of Thouarella cristata in the presence of undetermined commensal polynoid.
6 Adaptive Modification of Symbiotic Polychaetes
Symbiotic polychaetes have more or less defined morphological features allowing distinguishing them from their free-living relatives (Martin and Britayev 1998), which can be grouped in different categories: coloring, morphology , life cycle and behavioral. Also, these modifications can be adaptive or non-obviously adaptive. ‘Non-adaptive modifications’, either morphological or not, allow to differentiate the symbionts from their free-living relatives, but lack obvious adaptive significance. Cnidarian polychaete symbionts are not an exception. However, only two out of the nine families including cnidarian associates, Syllidae and Polynoidae , are sufficiently represented to evaluate trends in adaptive modifications. Moreover, as very little is currently known on life cycle and behavior adaptations , we have here considered only those affecting morphology and coloration.
6.1 Syllidae
Color adaptive modifications in Syllidae generally imply a correspondence with the host color. Worms inhabiting the surface of corals often have cryptic coloration, mimicking that of the host. Thus, the pale-yellowish Haplosyllis villogorgicola and orange H. anthogorgicola match exactly the color of their hosts; H. chamaeleon can demonstrate a range of colorations from yellow to dark red or violet, with dark violet dorsal marks, but generally matches exactly the color of their hosts Paramuricea clavata and P. grayi, which may exhibit all this color range in the same or in different colonies, respectively (Martin et al. 2002; Lattig and Martin 2009). An alternative color adaptation providing protection when living on the host surface is the disruptive coloration of Alcyonosyllis phili (Fig. 25.6b, d). This cream colored syllid with transverse brown bands can be hardly visible on brightly colored nephtheid or xeniid hosts (Glasby and Watson 2001; Britayev and Antokhina 2012).
The main morphological modifications reported in cnidarian associated Syllidae affect chaetal arrangement and shape (Fig. 25.7). For instance, the number of chaetae per bundle tended to be reduced and their morphology tended to be simplified from the typically articulated type of most free-living forms to either pseudocompound chaeta (H. anthogorgicola), simplified, or hooked forms (H. chamaeleon, Alcyonosyllis spp., symbiotic Autolytinae) (Martin and Britayev 1998; Martin et al. 2002), which probably serve better to allow the worms to remain attached to the host surface.
Hooked chaeta in Haplosyllis spp (Syllidae ) (After Martin et al. 2002). (a, b). H. chamaeleon: (a) Chaetae from the first chaetiger; (b) Tip of a chaeta from posterior-most chaetigers; (c) H. villogorgicola: Tip of ventral mid-body chaetae. (d) H. anthogorgicola: tip of pseudocompound chaeta of the first chaetiger. Scale: (a) – 15 mkm, (b) – 6 mkm, (c) – 4.3, (d) – 5 mkm
6.2 Polynoidae
The adaptive modification in coloration of the Polynoidae strongly depends on the position of the symbiotic polychaete on the host . Polynoids inhabiting the host surface usually have cryptic colorations. For instance, Australaugenira rutilans, was described from Xenia sp. (Octocorallia Xeniidae ) and reported as to be of ‘exactly the same red color as host’ (Wehe 2006) and mimic also the color of another host, Dendronephthya sp. (Britayev and Antokhina 2012). The special case of the almost transparent Uncopolynoe corallicola and Paradyte laevis (Fig. 28.8c) which are hardly visible on the bright surface of their nephtheid hosts Dendronephthya spp. (Britayev and Antokhina 2012), can also be considered as an example of cryptic coloration. Polynoids inhabiting galleries and tunnels formed by skeletal elements or tissues of cnidarian hosts are mostly whitish or fleshy in color as, for instance, the species of Gorgoniapolynoe inhabiting different species of Corallidae and Primnoidae (Eckelbarger et al. 2005; Simpson and Watling 2011; Britayev et al. 2014).
Elytra modifications in Polynoidae (After Britayev et al. 2014). (a–c) Parahololepidella greeffi: elitra of 9th (a), 32nd (b) and 87th (c) segments; (d) Gorgoniapolynoe caeciliae, elytron of the first pair. Scale (a–c) – 2 mm; (d) – 0.1 mm
A true case of mimicry was described for Medioantenna variopinta associated with Solanderia secunda (Hydrozoa , Capitata). The orange body of this symbiotic worm mimics the large orange eumedusoids of the host colony, while the white pigmentation on the cephalic appendages and on dorsal cirri and the finger-like macropapillae of the elytra mimic the coloring of the host polyps (Di Camillo et al. 2011).
Developing hooked chaetae on anterior segments seems also to be a clear adaptive trend in symbiotic polynoids . Uncopolynoe corallicola has stout, strongly bent hooks on segments 2–4, while Australaugeneria rutilans has strongly hooked neurochaetae on the anteriormost segment (Wehe 2006).
While chaetal modifications seems to be an obvious adaption to the symbiotic mode of life, most of morphological modifications of elytra and parapodia in symbiotic polynoids cannot be easily considered as adaptive. The elytra of many commensal scale-worms are small, often leaving much of the dorsal surface uncovered, thin and smooth, and usually lack ornamentations (i.e. papillae and tubercules). Parahololepidella greeffi inhabiting colonies of Tanacetipathes cf. spinescens has soft transparent elytra lacking tubercules and papillae, which are large, covering mid-dorsum on the first 11–12 segments, and then become very small, leaving dorsum and parapodia uncovered (Fig. 25.8a–c) (Britayev et al. 2014). Gorgoniapolynoe spp. inhabiting mostly octocorals of the families Primnoidae , Corallidae and Acanthogorgidae also have relatively small transparent elytra without ornamentation, except on the first pair that is larger and completely cover the prostomium (Pettibone 1991; Britayev et al. 2014). However, these elytra shows an additional adaptive modification: just above the eyes, each elytron of the first pair has a crescent shaped, transparent, chitinized area with scattered microtubercules (Fig. 25.8d) that is apparently connected to the ability of the worm to distinguish between light and dark even from when lied inside the coral gallery. Taking into account the depth range of Gorgoniapolynoe spp. (300–2,000 m), it is hard to expect enough light at these depths. However, there is a number of reports about biolumenescence of corals and polynoids (see e.g. Nicol 1953; Herring 1991; Plyuscheva and Martin 2009; Johnsen et al. 2012) and apparently such adaptation may be connected with bioluminescence ability of host or symbiont . Parapodial modifications are most often reductions in size. Thus P. greeffi has small digitiform notopodia (Britayev et al. 2014) and U. corallicola, associated with undetermined alcyonaceans, completely lacks notopodia (Wehe 2006).
7 Conclusions. Main Gaps in Our Present Knowledge in Biology of Polychaete-Coral Symbiosis
One of the most interesting results revealed by our review is that every group of cnidarians seems to have their own assemblage of symbiotic polychaetes. Accordingly, scleractinian corals more often harbor lumbrinerids, serpulids and spionids , which include a few symbiotic species inhabiting a wide range of hosts. On the other hand, the alcyonaceans and antipatharians are more often associated with polynoids and syllids , which include numerous symbiotic species. It is interesting to notice that the members of these families are among the symbionts inducing the most dramatic changes in host morphology . Also, the species of host genera such as Narella (Primnoidea) or Corallium (Corallidae ), or even whole genera of deep-sea alcyonaceans or black corals such as Stylopathes (Antipatharia, Stylopathidae ) have never been reported without their polynoid symbionts . Despite these hosts are so deeply involved in symbiotic associations that the presence of symbionts has been considered as a species-specific, robust taxonomic character, quite often little is known about their symbiotic partners , which usually remain undetermined even at the genus level. Taking this into account, as well as the high diversity in the host morphology, we certainly expect the fauna associated with deep-sea corals to be a hidden hot-spot of diversity , and many species of polychaete symbionts are waiting to be described in deep sea environments .
Symbiotic associations , particularly parasitic and mutualistic ones, are clearly bidirectional and, thus, a well-known source of co-evolution . This means that not only the symbionts may acquire modifications due to their mode of life, but also the hosts may tend to develop specific adaptations. However, very few studies have been addressed to understand the mechanisms leading the symbionts to influence on the host morphology , which quite often involve particular elements of the host skeleton (e.g., the extra-large sclerites of primnoids or the slow-growing processes of the central axis of corallids, the changes in spine morphology of antipatharians). Also, no studies have been addressed to assess how long it takes to produce the worm tubes or worm runs, neither on the mechanisms and chemical cues that influence skeletogenesis in cnidarian hosts . Implicitly, there are no studies on the extend of the associations or, in other words, whether the symbiont grows in parallel with the host or, if not, when the colonization occurs (i.e., larval settlement , juvenile or adult migration ) and on the mechanisms leading the symbionts to recognize the presence of their hosts, and this is particularly true in deep-sea environments. Last, but not least, there are no studies focusing on how symbiotic worms affect the host fitness , as well as their metabolism, growth rate or reproductive potential.
Despite more than 15 years passed since Martin and Britayev (1998) published their review on the whole symbiotic polychaetes , one of their main conclusive statements still holds for the particular subset of coral associates: more than a closing analyses on a well-developed matter, our review still reveals many gaps in the study of polychaete-coral relationships, meaning that we highly encourage further work to be done on these highly interesting symbiotic associations .
References
Alderslade P (1998) Revisionary systematics in the gorgonian family Isididae, with descriptions of numerous new taxa (Coelenterata: Octocorallia). Rec West Aust Mus S55:1–359
Allen EJ (1915) Polychaeta of Plymouth and the South Devon coast including a list of the Archiannelida. J Mar Biol Assoc 10:592–646
Allen EJ (1921) Regeneration and reproduction of the syllid Procerastea. Philos T R Soc B 211:131–177
Alós C (1988) Anelidospoliquetos de Cabo de Creus (Alt Emporda). Doctoral thesis, Universitat de Barcelona, Barcelona
Alós C (1989) Adiciones a la fauna de Anelidos Poliquetos de la peninsula Iberica: familia Syllidae. Cah Biol Mar 30:329–337
Álvarez-Campos P, San Martín G, Aguado MT (2013) A new species and new records of the commensal genus Alcyonosyllis Glasby & Watson, 2001 and a new species of Parahaplosyllis Hartmann-Schröder, 1990 (Annelida: Syllidae: Syllinae) from Philippine Islands. Zootaxa 3734:156–168
Bailey-Brock JH (1985) Polychaetes from Fijian coral reefs. Pac Sci 39:195–220
Barnich R, Fiege D (2010) On the distinction of Harmothoe globifera (GO Sars, 1873) and some other easily confused polynoids in the NE Atlantic, with the description of a new species of Acanthicolepis Norman in McIntosh, 1900 (Polychaeta, Polynoidae). Zootaxa 2525:1–18
Barnich R, Gambi MC, Fiege D (2012a) Revision of the genus Polyeunoa McIntosh, 1885 (Polychaeta, Polynoidae). Zootaxa 3523:25–38
Barnich R, Orensanz JM, Fiege D (2012b) Remarks on some scale worms (Polychaeta, Polynoidae) from the Southwest Atlantic with notes on the genus Eucranta Malmgren, 1866, and description of a new Harmothoe species. Mar Biodivers 42:395–410
Barnich R, Beuck L, Freiwald A (2013) Scale worms (Polychaeta: Aphroditiformia) associated with cold-water corals in the eastern Gulf of Mexico. J Mar Biol Assoc 93:2129–2143
Bayer FM (1964) The genus Corallium (Gorgonacea: Scleraxonia) in the Western North Atlantic Ocean. Bull Mar Sci 14:465–478
Bellan G (1959) Annélidespolychètes. Campagne de la ‘Calypso’ enMerd’Alboranetdans la BaieIbéro-Marocaine. Ann Inst Oceanogr 37:315–342
Ben-Tzvi O, Einbinder S, Brokovich E (2006) A beneficial association between a polychaete worm and a scleractinian coral? Coral Reefs 25:98–98
Bergsma GS (2009) Tube-dwelling coral symbionts induce significant morphological change in Montipora. Symbiosis 49:143–150
Bergsma GS (2012) Epibiotic mutualists alter coral susceptibility and response to biotic disturbance through cascading trait-mediated indirect interactions. Coral Reefs 31:461–469
Britayev TA (1981) Two new species of commensal polynoids (Polychaeta: Polynoidae) and bibliography on polychaetes, symbionts of Coelenterata. [In Russian]. Zool Zh 60:817–824
Britayev TA (1991) Life cycle of the symbiotic scaleworm Arctonoe vittata (Polychaeta: Polynoidae). Ophelia Suppl 5:305–312
Britayev TA, Antokhina TI (2012) Symbiotic polychaetes from Nhatrang Bay, Vietnam. In: Britayev TA, Pavlov DS (eds) Benthic fauna of the Bay of Nhatrang, Southern Vietnam, vol 2. KMK Scientific Press, Moscow, p 491
Britayev T, San Martín GLA (2001) Description and life-history traits of a new species of Proceraea with larvae infecting Abietinaria turgida (Polychaeta, Syllidae & Hydrozoa, Sertulariidae). Ophelia 54:105–113
Britayev T, Gil J, Altuna A, Calvo M, Martin D (2014) New symbiotic associations involving polynoids (Polychaeta, Polynoidae) from Atlantic waters, with redescriptions of Parahololepidella greeffi (Augener, 1918) and Gorgoniapolynoe caeciliae (Fauvel, 1913). Mem Mus Vic 71:27–43
Britayev TA, Antokhina TI, Marin IN (2015) A scaleworm (Polychaeta: Polynoidae) living with corals. Mar Biodiv 45:627–628. doi:10.1007/s12526-014-0305-5
Buhl-Mortensen P (2001) Aquarium observations on the deep-water coral Lophelia pertusa (L., 1758) (Scleractinia) and selected associated invertebrates. Ophelia 54:83–104
Buhl-Mortensen L, Mortensen PB (2004) Symbiosis in deep-water corals. Symbiosis 37:33–61
Buhl-Mortensen L, Mortensen PB (2005) Distribution and diversity of species associated with deep-sea gorgonian corals off Atlantic Canada. In: Freiwald A, Roberts JM (eds) Cold-water corals and ecosystems. Springer, Berlin, pp 849–879
Cairns SD (1986) Stylasteridae (Hydrozoa: Hydroida) of the Galapagos Islands. Smithson Contrib Zool 426:1–42
Cairns SD (1987) Conopora adeta, new species (Hydrozoa: Stylasteridae) from Australia, the first known unattached stylasterid. Proc Biol Soc Wash 100:141–146
Cairns SD (1991) New records of Stylasteridae (Hydrozoa: Hydroida) from the Galapagos and Cocos Islands. Proc Biol Soc Wash 104:209–228
Cairns SD (2006) Studies on western Atlantic Octocorallia (Coelenterata: Anthozoa). Part 6: The genera Primnoella Gray, 1858, Thouarella Gary, 1870; Dasystenella Versluys, 1906. Proc Biol Soc Wash 119:161–194
Cairns SD (2009) Review of Octocorallia (Cnidaria: Anthozoa) from Hawai’i and adjacent seamounts. Part 2: Genera Paracalyptrophora Kinoshita, 1908; Candidella Bayer, 1954; and Calyptrophora Gray, 1866. Pac Sci 63:413–448
Cairns SD (2011a) Global diversity of the stylasteridae (Cnidaria: Hydrozoa: Athecatae). PLoS ONE 6(7), e21670. doi:10.1371/journal.pone.0021670
Cairns SD (2011b) A revision of the Primnoidae (Octocorallia: Acyonacea) from the Aleutian Islands and Bering Sea. Smithson Contrib Zool 634:1–55
Cairns SD (2012) The Marine Fauna of New Zealand: New Zealand Primnoidae (Anthozoa: Alcyonacea). Part 1. Genera Narella, Narelloides, Metanarella, Calyptrophora, and Helicoprimnoa. NIWA Biodiv Mem 126:1–72
Cairns SD, Bayer FM (2004) Studies on Western Atlantic Octocorallia (Coelenterata: Anthozoa). Part 5: The Genera Plumarella Gray, 1870; Acanthoprimnoa, n. gen.; and Candidella Bayer, 1954. Proc Biol Soc Wash 117:447–487
Cairns SD, Bayer FM (2008) A review of the Octocorallia (Cnidaria: Anthozoa) from Hawai’i and Adjacent Seamounts. The genus Narella Gray, 1870. Pac Sci 62:83–115
Cairns SD, Lindner A (2011) A revision of the Stylasteridae (Cnidaria, Hydrozoa, Filifera) from Alaska and adjacent waters. Zoo Keys 158:1–88
Cairns SD, Zibrowius H (1997) Cnidaria Anthozoa: Azooxanthellate Scleractinia from the Philippine and Indonesian Regions. Mém Mus Natn Hist Nat 172:27–243
Cairns SD, Zibrowius H (2013) Stylasteridae (Cnidaria, Hydrozoa, Filifera) from South Africa. Zootaxa 3691:1–57
Caullery M (1925) Schizogenèse et schizogamie de Procerastea halleziana Malaquin. Parasitism de ce Syllidian sur les Tubularies. Bull Soc Zool Fr 50:204–208
Chisholm JRM, Kelley R (2001) Worms start the reef-building process. Nature (London) 409:152
Day JH (1967) A monograph on the Polychaetes of Southern Africa. Part 1. Errantia. Trust Brit Mus (Nat Hist) 656:1–656
De Bary A (1879) Die Erscheinung der Symbiose. Vortrag auf der Versammlung der Deutschen Naturforscher und Aertze zu Cassel. Verlag von Karl J. Trubner, Strasburg, pp 1–30
DeVantier LL, Reichelt RR, Bradbury RR (1986) Does Spirobranchus giganteus protect host Porites from predation by Acanthaster planci: predator pressure as a mechanism of coevolution? Mar Ecol Prog Ser 32:307–310
Di Camillo CG, Martin D, Britayev TA (2011) Symbiotic association between Solanderia secunda (Cnidaria, Hydrozoa, Solanderiidae) and Medioantenna variopinta sp. nov. (Annelida, Polychaeta, Polynoidae) from North Sulawesi (Indonesia). Helgol Mar Res 65:495–511
Ditlevsen H (1917) Annelids. I. Danish Ingolf-Exped 4(4):1–71
Eckelbarger KJ, Watling L, Fournier H (2005) Reproductive biology of the deep-sea polychaete Gorgoniapolynoe caeciliae (Polynoidae), a commensal species associated with octocorals. J Mar Biol Assoc UK 85:1425–1433
Fage L (1936) Sur l’association d’un annélide polychète Lumbriconereis flabellicola n. sp. et d’un madrépore Flabellum pavoninum distinctum E et H. Congr Int Zool (CR) 1:941–945
Fauchald K (1992) A review of the genus Eunice (Polycaeta: Eunicidae) based upon type material. Smithson Contrib Zool 523:1–422
Fauvel P (1913) Quatrième note préliminare sur les Polychètes provenant des campagnes de l’“Hirondelle” et de la “Princesse-Alice”, ou déposées dans le Musée Océanografique de Monaco. Bull Inst Océanogr Monaco 270:1–80
Fauvel P (1923) Faune de France. Polychètes Errantes Faune de France 5:1–488
Garberoglio RM, Lazo DG (2011) Post-mortem and symbiotic sabellid and serpulid-coral associations from the Lower Cretaceous of Argentina. Rev Bras Paleontolog 14:215–228
Gardiner SL (1976) Errant Polychaete Annelids from North Carolina. J Elisha Mitchell Sci Soc 91:77–220
Genzano GN, San Martín G (2002) Association between the polychaete Procerastea halleziana (Polychaeta: Syllidae: Autolytinae) and the hydroid Tubularia crocea (Cnidaria: Hydrozoa) from the Mar del Plata intertidal zone, Argentina. Cah Biol Mar 43:165–170
George JD, Hartmann-Schröeder G (1985) Polychaetes: British Amphinomida, Spintherida and Eunicida In: Kermack DM, Barnes RSK (eds) Synopses of the British Fauna (New Series) vol 32. Linnean Society, London, Estuarine and Coastal Science association, Hull, pp 1–221
Glasby CJ (1994) A new genus and species of polychaete, Bollandia antipathicola (Nereidoidea, Syllidae), from black coral. Proc Biol Soc Wash 107:615–621
Glasby CJ, Aguado M (2009) A new species and new records of the anthozoan commensal genus Alcyonosyllis (Polychaeta: Syllidae: Syllinae). Beagle Rec N Territ Mus Arts Sci 25:53–61
Glasby CJ, Krell FT (2009) Bollandiella nom. nov. for the polychaete genus Bollandia Glasby, 1994 (Annelida: Polychaeta: Phyllodocida:? Syllidae). Proc Biol Soc Wash 122:355–356
Glasby CJ, Watson C (2001) A new genus and species of Syllidae (Annelida: Polychaeta) commensal with octocorals. Beagle Rec N Territ Mus Arts Sci 17:43–51
Glynn PW, Enoch IC (2011) Invertebrates and their roles in coral reef ecosystems. In: Dubinsky Z, Stambler N (eds) Coral reefs: an ecosystem in transition. Springer, London, pp 273–326
Grillo MC, Goldberg WM, Allemand D (1993) Skeleton and sclerite formation in the precious red coral Corallium rubrum. Mar Biol 117:119–128
Hanley JR (1992) Checklist of scaleworms (Polychaeta: Polynoidae) from Hong Kong. In: Morton B (ed) The marine flora and fauna of Hong Kong and southern China III. Proceedings of the Fourth International Marine Biological Workshop: the Marine Flora and Fauna of Hong Kong and Southern China, Hong Kong, 11–29 April 1989, Hong Kong University Press, Hong Kong, pp 361–369
Hanley JR, Burke M (1991a) A new genus and species of commensal scaleworm (Polychaeta, Polynoidae) from the Cascade Plateau, Tasman Sea. Beagle Rec N Territ Mus Arts Sci 8:97–102
Hanley JR, Burke M (1991b) Polychaeta polynoidae: scaleworms of the Chesterfield Islands and Fairway Reefs, Coral sea. Mém Mus Natn Hist Nat (A) 151:9–82
Hartman O (1954) Marine annelids from the northern Marshall Islands. US Geol Surv Prof Paper 260Q:615–644
Hartmann-Schröder G (1960) Polychaeten aus dem Roten Meer. Kiel Meeresforsch 16:69–125
Hartmann-Schröder G (1985) Polynoe caeciliae Fauvel (Polynoidae), ein mit Korallen assoziierter Polychaet. Mitt Hambg Zool Mus Inst 82:31–35
Hartmann-Schröder G (1989) Polynoe thouarellicola n. sp. aus der Antarktis, assoziiert mit Hornkorallen, und Wiederbeschreibung von Polynoe antarctica Kinberg, 1858 (Polychaeta, Polynoidae). Zool Anz 3(4):205–221
Hartmann-Schröder G (1991) Syllis onkylochaeta sp. n., ein korallenfressender Polychaet (Syllidae) aus dem Korallenaquarium des Löbbecke-Museums. Helgoländer Meeresun 45:59–63
Hartmann-Schröder G (1992) Drei neue Polychaeten-arten der familien Polynoidae und Syllidae von Neu-Kaledonien, assoziiert mit einer verkalten Hydrozoe. Helgoländer Meeresun 46:93–101
Hartmann-Schröder G (1993) Haplosyllys xeniaecola, ein neuer Polychaet (Syllidae) von der Molukken (Indonesien). Helgoländer Meeresun 47:305–310
Hartmann-Schröder G, Zibrowius H (1998) Polychaeta associated with Antipatharia (Cnidaria: Anthozoa): description of Polynoidae and Eunicidae. Mitt Hambg Zool Mus Inst 95:29–44
Herring PJ (1991) Observations on bioluminescence in some deep-water anthozoans. Hydrobiologia 216:573–579
Hoeksema BW, ten Hove HA (2014) First record of a christmas tree worm in a mushroom coral (Loyalty Islands, Southwest Pacific). Coral Reefs 33:717
Horst R (1915) On new and little-known species of Polynoinae from the Netherland’s East-Indies. Zool Meded Leiden 1:2–20
Hove HA ten (1994) Serpulidae (Annelida: Polychaeta) from the Seychelles and Amirante Islands. In: van der Land J (ed) Oceanic reefs of the Seychelles. Cruise Reports of Netherlands Indian Ocean Program, vol 2, Naturalis, Leiden, pp 107–116
Hunte W, Marsden JR, Conlin BE (1990) Habitat selection in the tropical polychaete Spirobranchus giganteus. I: distribution on corals. Mar Biol 104:87–92
Hutchings P (2008) Role of polychaetes in bioerosion of coral substrates. In: Wisshak M, Tapanila L (eds) Current developments in bioerosion. Springer, Heidelberg, pp 249–264
Imajima M (1997) Polychaetous Annelids of Suruga Bay, Central Japan. Nat Sci Mus Monogr 12:149–228
Imajima M (2003) Polychaetous Annelids from Sagami Bay and Sagami Sea collected by the Emperor Showa of Japan and deposited at the Showa Memorial Institute, National Science Museum, Tokyo (II). Orders included within the Phyllodocida, Amphinomida, Spintherida and Eunicida. Nat Sci Mus Monogr 23:1–221
Imajima M, Hartman O (1964) The polychaetous annelids of Japan. Allan Hancock Found Spec Publ 26:1–452
Jensen A, Frederiksen R (1992) The fauna associated with the bank-forming deepwater coral Lophelia pertusa (Scleractinaria) on the Faroe shelf. Sarsia 77:53–69
Johnsen S, Frank TM, Haddock SH, Widder EA, Messing CG (2012) Light and vision in the deep-sea benthos: I. Bioluminescence at 500–1,000 m depth in the Bahamian Islands. J Exp Biol 215:3335–3343
Kumagai NH, Aoki MN (2003) Seasonal changes in the epifaunal community on the shallow-water gorgonian Melithaea flabellifera. J Mar Biol Assoc 83:1221–1222
Lattig P, Martin D (2009) A taxonomic revision of the genus Haplosyllis Langerhans, 1887. Zootaxa 2220:1–40
Laubier L (1960) Une nouvelle sous-espèce de Syllidien: Haplosyllis depressa Augener ssp. nov. chamaeleon, ectoparasite sur l’octocoralliaire Muricea chamaeleon Von Koch. Vie et Milieu 11:75–87
Lewis JB (1998) Reproduction, larval development and functional relationships of the burrowing, spionid polychaete Dipolydora armata with the calcareous hydrozoan Millepora complanata. Mar Biol 130:651–662
Lewis JB (2006) Biology and ecology of the hydrocoral Millepora on coral reefs. Adv Mar Biol 60:1–55
Light WJ (1970a) Polydora alloporis, new species, a commensal spionid (Annelida, Polychaeta) from a hydrocoral off Central California. Proc Calif Acad Sci 37:459–472
Light WJ (1970b) A new spionid (Annelida: Polychaeta) from the Gulf of California. Bull South Calif Acad Sci 69:74–79
Lindner A, Cairns SD, Guzman HM (2004) Distichopora robusta sp. nov., the first shallow-water stylasterid (Cnidaria: Hydrozoa: Stylasteridae) from the tropical eastern Pacific. J Mar Biol Assoc 84:943–947
Liu P-J, Hsieh H-L (2000) Burrow architecture of the spionid polychaete Polydora villosa in the corals Montipora and Porites. Zool Stud 39:47–54
López E, San Martín G, Jiménez M (1996) Syllinae (Syllidae, Annelida, Polichaeta) from Chafarinas Islands (Alborán Sea, W Mediterranean). Misc Zool 19:105–118
Marenzeller E (1904) Steinkorallen. Wiss Ergebn Dt Tiefsee-Exped 7:261–318
Margolis L, Esch GW, Holmes JC, Kuris AM, Schad GA (1982) The use of ecological terms in parasitology (report of a committee of the American Society of Parasitologists). J Parasitol 68:131–133
Marsden JR (1992) Reproductive isolation in two forms of the serpulid polychaete, Spirobranchus polycerus (Schmarda) in Barbados. Bull Mar Sci 51:14–19
Martin D, Britayev TA (1998) Symbiotic polychaetes: review of known species. Oceanogr Mar Biol 36:217–340
Martin D, Núñes J, Riera R, Gil J (2002) On the associations between Haplosyllis (Polychaeta, Syllidae) and gorgonians (Cnidaria, Octocorallaria), with the description of a new species. Biol J Linn Soc 77:455–47
Martin D, Aguado MT, Britayev TA (2009) Review of the symbiotic genus Haplosyllides (Polychaeta: Syllidae), with a description of a new species. Zool Sci 26:646–655
Miranda VDR, Brasil ACDS (2014) Two new species and a new record of scale-worms (Polychaeta) from Southwest Atlantic deep-sea coral mounds. Zootaxa 3856:211–226
Miura T, Shirayama Y (1992) Lumbineris flabellicola (Fage, 1936), a lumbrinerid polychaete associated with a Japanese haermatypic coral. Benthos Res 43:23–27
Molodtsova TN (2006) New species of Hexapathes Kinoshita, 1910 (Anthozoa, Antipatharia, Cladopathidae) from the South-West Pacific. Zoosystema 28:597–606
Molodtsova TN (2013) Deep-sea mushroom soft corals (Octocorallia: Alcyonacea: Alcyoniidae) of the Northern Mid-Atlantic Ridge. Mar Biol Res 9:488–515
Molodtsova T, Budaeva N (2007) Modifications of corallum mophology in black corals as an effect of associated fauna. Bull Mar Sci 81:469–479
Mueller CE, Lundälv T, Middelburg JJ, van Oevelen D (2013) The symbiosis between Lophelia pertusa and Eunice norvegica stimulates coral calcification and worm assimilation. PLoS ONE 8(3), e58660. doi:10.1371/journal.pone.0058660
Nakamura T, Yamasaki H, Van Woesik R (2003) Water flow facilitates recovery from bleaching in the coral Stylophora pistillata. Mar Ecol Prog Ser 256:287–291
Nicol JAC (1953) Luminescence in polynoid worms. J Mar Biol Assoc 32:65–84
Nishi E, Tachikawa H (1999) New record of a commensal scale worm Medioantenna clavata Imajima, 1997 (Polychaeta: Polynoidae), from Ogasawara Islands, Japan. Nat Hist Res 5:107–110
Núñez J, Barnich R, Santos L, Maggio Y (2011) Poliquetos escamosos (Annelida, Polychaeta) colectados en las campañas “Fauna II, III, IV”(Proyecto “Fauna Ibérica”) y catálogo de las especies conocidas para el ámbito íbero-balear. Graellsia 67:187–197
Nutting CC (1908) Descriptions of the Alcyonaria collected by the U.S. Bureau of Fisheries Steamer Albatross in the vicinity of the Hawaiian Islands in 1902. Proc U.S. Nat Mus 34:543–601
Nygren A, Pleijel F (2010) Redescription of Imajimaea draculai—a rare syllid polychaete associated with the sea pen Funiculina quadrangularis. J Mar Biol Assoc UK 90:1441–1448
O’Connor B, Könnecker G, McGrath D, Keegan BF (1977) Pachycerianthus multiplicatus Carlgren, biotope or biocoenosis? In: Keegan BF, Ceidigh PO, Boaden PJS (eds) Biology of benthic organisms. 11th European Symposium on Marine Biology, Galway, October, 1976. Pergamon Press, Oxford, pp 475–482
Okuda S (1937) Spioniform polychaetes from Japan. J Fac Sci Hokk Imp Un Zool 5:217–254
Okuda S (1950) Notes on some commensal polychaetes from Japan. Ann Zool Jpn 24:49–53
Opresko DM (2006) Three new species of the genus Leiopathes (Cnidaria: Anthozoa: Antipatharia) from coastal waters of Australia and Tasmania. Rec South Aust Mus 32:143–154
Pettibone MH (1963) Marine polychaete worms of the New England region. 1. Families Aphroditidae through Trochochaetidae. US Natl Mus Bull 227:1–356
Pettibone MH (1969a) Review of some species referred to Scalisetosus McIntosh (Polychaeta, Polynoidae). Proc Biol Soc Wash 82:1–30
Pettibone MH (1969b) Australaugenira pottsi, new name for Polynoe longicirrus Potts, from the Maldive Islands (Polychaeta: Polynoidae). Proc Biol Soc Wash 82:519–524
Pettibone MH (1969c) The genera Polyeunoa McIntosh, Hololepidella Willei, and three new genera (Polychaeta, Polynoidae). Proc Biol Soc Wash 82:43–62
Pettibone MH (1970) Polychaeta Errantia of the Siboga Expedition. Part IV. Some additional polychaetes of the Polynoidae, Hesionidae, Nereidae, Goniadidae, Eunicidae, and Onuphidae, selected as new species by the late Dr. Hermann Augener with remarks on other related species. Siboga-Exp 24(4):199–270
Pettibone MH (1989) A new species of Benhamipolynoe (Polychaeta: Polynoidae: Lepidasteniinae) from Australia, associated with the unattached stylasterid coral Conopora adeta. Proc Biol Soc Wash 102:300–304
Pettibone MH (1991a) Polynoids commensal with gorgonian and stylasterid corals, with a new genus, new combinations, and new species (Polychaeta: Polynoidae: Polynoinae). Proc Biol Soc Wash 104:688–713
Pettibone MH (1991b) Polynoid polychaetes commensal with antipatharian corals. Proc Biol Soc Wash 104:714–726
Pettibone MH (1993) Scaled polychaetes (Polynoidae) associated with ophiuroids and other invertebrates and review of species referred to Malmgrenia McIntosh and replaced by Malmgeniella Hartman, with descriptions of new taxa. Smithson Contrib Zool 538:1–92
Plyuscheva M, Martin D (2009) On the morphology of elytra as luminescent organs in scale-worms (Polychaeta, Polynoidae). Zoosymposia 2:379–389
Radashevsky VI, Hsieh HL (2000) Polydora (Polychaeta: Spionidae) species from Taiwan. Zool Stud 39:218–235
Randall RH, Eldredge LG (1976) Skeletal modification by a polychaete annelid in some scleractinian corals. In: Mackie GO (ed) Coelenterate ecology and behavior. Plenum Press, New York, pp 453–465
Roberts JM (2005) Reef-aggregating behaviour by symbiotic eunicid polychaetes from cold-water corals: do worms assemble reefs? J Mar Biol Assoc UK 85:813–819
Rowley S (2008) A critical evaluation of the symbiotic association between tropical tube-dwelling polychaetes and their hermatypic coral hosts, with a focus on Spirobranchus giganteus (Pallas, 1766). Plymouth Stud Sci 1:335–353
Rullier F (1974) Quelques annelides polychaetes de Cuba recueillies dans des eponges. Trav Mus Natl Hist Nat Grigore Antipa 14:9–77
Rullier F, Amoureux L (1979) Campagne de la Calypso au large des côtes Atlantiques de l’Amérique du Sud (1961–1962) I 33 Annélides Polychètes. Ann I Oceanogr 55:145–206
San Martín G, Nishi E (2003) A new species of Alcyonosyllis Glasby and Watson, 2001 (Polychaeta: Syllidae: Syllinae) from Shimoda, Japan, commensal with the gorgonian Melithaea flabellifera. Zool Sci 20:371–375
Simpson A, Watling L (2011) Precious corals (Coralliidae) from north-western Atlantic Seamounts. J Mar Biol Assoc UK 91:369–382
Spooner GM, Wilson DP, Trebble N (1957) Phylum Annelida. In: Plymouth Marine Fauna, 3rd edn. Marine Biological Association UK, Plymouth, pp 109–149
Stella JS, Pratchett MS, Hutchings PA, Jones GP (2011) Coral-associated invertebrates: diversity, ecological importance and vulnerability of disturbance. Oceanogr Mar Biol 49:43–104
Stewart HL, Holbrook SJ, Schmitt RJ, Brooks AJ (2006) Symbiotic crabs maintain coral health by clearing sediments. Coral Reefs 25:609–615
Stiller M (1996) Verbreitung und Lebensweise der Aphroditiden und Polynoiden (Polychaeta) im östlichen Weddellmeer und im Lazarevmeer (Antarktis). Ber Polarforsch 185:1–200
Stock JH (1986) Cases of hyperassociation in the Copepoda (Herphyllobiidae and Nereicolidae). Syst Parasitol 8:71–81
Strathmann RR, Cameron RA, Strathmann M (1984) Spirobranchus giganteus (Pallas) breaks the rules for suspension feeders. J Exp Mar Bio Ecol 70:245–249
Sun R, Yang DJ (2004) Invertebrata, vol 33. Annelida, Polychaeta II. Nereidida (= Nereimorpha). Nereididae, Syllidae, Hesionidae, Pilargidae, Nephtydae. Fauna Sinica, China Science Press, Beijing, pp 1–550
Taylor ML, Cairns SD, Agnew DJ, Rogers AD (2013) A revision of the genus Thouarella Gray, 1870 (Octocorallia: Primnoidae), including an illustrated dichotomous key, a new species description, and comments on Plumarella Gray, 1870 and Dasystenella, Versluys, 1906. Zootaxa 3602:1–105
ten Hove HA (1970) Serpulinae (Polychaeta) from the Caribbean. 1. The genus Spirobranchus. Stud Fauna Curaçao Caribb Isl 32:14–19
ten Hove HA (1989) Serpulinae (Polychaeta) from the Caribbean: IV Pseudovermilia madracicola sp.n., a symbiont of corals. Natuurwetenschappelijke Studiekring voor Suriname en de Nederlandse Antillen 123:135–144
Totton AK (1923) Coelenterata. Part III-Antipatharia (and their cirripede commensals). Nat Hist Rep Brit Antarct Exped Zool 5:97–120
Tu T, Dai C, Jeng M (2012) Precious corals (Octocorallia: Coralliidae) from the northern West Pacific region with descriptions of two new species. Zootaxa 3395:1–17
Tu T, Altuna A, Jeng MS (2015) Coralliidae (Anthozoa: Octocorallia) from the INDEMARES 2010 expedition to north and northwest Spain (northeast Atlantic), with delimitation of a new species using both morphological and molecular approaches. Zootaxa 3926:301–328
Uchida H (1978) Serpulid tube worms (Polychaeta, Sedentaria) from Japan with the systematic review of the group. Bull Mar Park Res Stn 2:1–98
Utinomi H (1956) On the so-called ‘Umi-Utiwa’ a peculiar flabellate gorgonacean, with notes on a syllidean polychaete commensal. Publ Seto Mar Biol Lab 5:243–250
Verrill AE (1881) New England Annelida. Part I. Historical sketch, with annotated lists of the species hitherto recorded. Trans Conn Acad Arts Sci 4:285–324
Versluys J (1906) Die Gorgoniden der Siboga-Expedition. II. Die Primnoidae. Siboga- Exped 13a:1–187
Wagner D, Luck DG, Toonen RG (2012) Biology and ecology of black corals (Cnidaria: Anthozoa: Hexacorallia: Antipatharia). Adv Mar Biol 63:67–132
Watling L, France SC, Pante E, Simpson A (2011) Biology of deep-water corals. Adv Mar Biol 60:41–122
Wehe T (2006) Revision of scale worms (Polychaeta: Aphroditoidea) occurring in the seas surrounding the Arabian Peninsula. Part I. Polynoidae. Fauna Arab 22:23–197
Wielgus J, Levy O (2006) Differences in photosynthetic activity between coral sections infested and not infested by boring spionid polychaetes. J Mar Biol Assoc UK 86:727–728
Wielgus J, Glassom D, Chadwick-Furman NE (2002) An aberrant growth form of Red Sea corals caused by polychaete infestations. Coral Reefs 21:315–316
Wielgus J, Glassom D, Chadwick-Furman NE (2006) Patterns of polychaete worm infestation of stony corals in the northern Red Sea and relationships to water chemistry. Bull Mar Sci 78:377–388
Williams GC (2003) Capitate taxa of the soft coral genus Eleutherobia (Octocorallia: Alcyoniidae) from Palau and South Africa; a new species and a new combination. Zool Verh Leiden 345:419–436
Wright JD, Woodwick KH (1977) A new species of Autolytus (Polychaeta: Syllidae) commensal on a Californian hydrocoral. Bull South Calif Acad Sci 76:42–48
Zapata-Guardiola R, López-González PJ (2010) Redescription of Thouarella brucei Thomson and Ritchie, 1906 (Cnidaria: Octocorallia: Primnoidae) and description of two new Antarctic primnoid species. Zootaxa 2616:48–68
Zibrowius H (1980) Les scléractiniaires de la Méditerranée et de l’Atlantique nord-oriental. Mém Inst Océanogr Monaco 11:1–284
Zibrowius H (1981) Associations of Hydrocorallia Stylasterina with gall-inhabiting Copepoda Siphonostomatoidea from the South-West Pacific. Part 1. On the stylasterine hosts, including two new species, Stylaster papuensis and Crypthelia cryptotrema. Bijdr Dierk 51:268–286
Zibrowius H, Cairns SD (1992) Revision of the Northeast Atlantic and Mediterranean Stylasteridae (Cnidaria: Hydrozoa). Mém Mus Natl Hist Nat A Zool 153:1–136
Zibrowius H, Southward EC, Day JH (1975) New observations on a little-known species of Lumbrineris (Polychaeta) living on various Cnidarians, with notes on its recent and fossil Scleractinian hosts. J Mar Biol Assoc UK 55:83–108
Acknowledgements
Authors would like to thank P. Lozouet and O. Savinkin for photos of corals; H. Zibrowius, S. Cairns, D. Opresko, L. van Ofwegen, G. Williams, F. Sinniger, B. Hoeksema and N. Keller, for advises and comments regarding different groups of cnidarian hosts. The research was supported by Russian Foundation of Basic Researches (grant No 13-04-01332) for T.N. Molodzova and Russian Science Foundation (grant No 14-14-01179) for T.A. Britayev. This review is also a contribution of D. Martin to the Research Project MARISYMBIOMICS (CTM2013-43287-P), funded by the Spanish State Research Plan, and to the Consolidated Research Group on Marine Benthic Ecology of the Generalitat de Catalunya (2014SGR120).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Molodtsova, T.N., Britayev, T.A., Martin, D. (2016). Cnidarians and Their Polychaete Symbionts. In: Goffredo, S., Dubinsky, Z. (eds) The Cnidaria, Past, Present and Future. Springer, Cham. https://doi.org/10.1007/978-3-319-31305-4_25
Download citation
DOI: https://doi.org/10.1007/978-3-319-31305-4_25
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-31303-0
Online ISBN: 978-3-319-31305-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)