Abstract
Microalgae have an enormous ecological relevance as they contribute significantly to global carbon fixation. But also for biotechnology microalgae became increasingly interesting during the last decades as many algae provide valuable natural products. Especially the high lipid content of some species currently attracts much attention in the biodiesel industry. A further application that emerged some years ago is the use of microalgae as expression platform for recombinant proteins. Several projects on the production of therapeutics, vaccines and feed supplements demonstrated the great potential of using microalgae as novel low-cost expression platform. This review provides an overview on the prospects and advantages of microalgal protein expression systems and gives an outlook on potential future applications.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
In 1982 human insulin was the first protein that was produced in a microbial system and approved for pharmaceutical use. Today recombinant proteins are indispensible in daily life, as they became essential instruments in many industrial sectors like food, fuel, textile and pharma industry. Especially the medical sector is a fast growing market and complex eukaryotic proteins like monoclonal antibodies, hormones and growth factors are needed in high quantity and dominated the biotech field for the last years reaching US sales of more than 40 billion dollar in 2011 [1]. Unfortunately, the production costs for most of these proteins are still very high limiting a broad therapeutic use, hence present expression systems need to be improved and novel production platforms should be explored.
Bacteria were the first expression systems for recombinant proteins established already in the 1970s and today still represent the basic workhorse in white biotechnology. They are first choice for production of most industrial enzymes and also 30 % of pharmaceutical proteins are still produced in bacteria as growth rates and expression levels are very high and overall production costs are relatively low [2]. For production of most complex eukaryotic proteins, however, bacterial systems are not feasible as proteins lack eukaryotic post-translational modifications critical for folding, stability and biological activity e.g. disulfide bond formation, phosphorylation and glycosylation being most important modifications [3]. Furthermore, many eukaryotic proteins accumulate in bacteria as insoluble aggregates and need costly downstream processing for purification and refolding. Some of these problems can be overcome by the use of yeasts like S. cerevisiae. These fungi are able to perform eukaryotic post-translational modifications and are as cost-effective as bacteria exhibiting high growth rates, high productivity and easy scalability [4]. However, a major problem concerning production of pharmaceutical proteins in yeast is linked to N-linked glycosylation, which differs from mammalian systems and hinders expression of correctly modified complex human therapeutics. Inefficient secretion and proteolysis are further critical issues for high scale expression of complex therapeutics like monoclonal antibodies [5]. Nevertheless, advances in metabolic engineering e.g. towards humanized glycosylation patterns might make yeast more interesting for pharmaceutical protein production in future [6–9]. Other eukaryotic expression systems like insect cells exist but 50 % of licensed pharmaceutical proteins are currently produced in mammalian cells, i.e. hamster cell lines (CHO), human cell lines or hybridoma cell lines in case of monoclonal antibodies [2, 10]. The major advantage of these expression systems is that recombinant proteins produced in mammalian cell lines exhibit correct post-translational modifications needed for therapeutic applications. On the other side, however, mammalian systems are very limited in scale up options and the production process is very cost-intense due to expensive media and complex cultivation processes representing serious bottlenecks for high scale production pipelines [11]. The contamination with human pathogens is a further critical issue necessitating thorough checks on biosafety [12].
To overcome high production costs and reduce the risk of pathogenic contaminations the idea of using plants as expression platform for recombinant proteins became very popular in the 1990s and is often referred to as molecular farming [13–16]. Plant-based production is fueled by sunlight, thus the production process itself should be very cheap and agricultural cultivation is well established. Ideally, plants might be used as production platform for edible vaccines making such therapeutics available for large parts of the population and especially in developing countries where they are needed most [17, 18]. In the last 25 years a lot of effort was put into that field of research and trials with engineered plant systems producing humanized glycosylation patterns look promising [19, 20]. However, major hurdles for using whole plants as expression system are low production levels and costly purification processes for products with no oral application. Furthermore, ethical concerns as well as the risk of contaminating food crops make the cultivation of transgenic plants a highly controversial issue [21]. Plant cell culture based systems appear more promising as higher production levels and protein secretion can be achieved and contained reactors allow production with good manufacturing practice (GMP) [22, 23]. In 2012 human glucocerebrosidase produced in a carrot cell culture system was the first plant made pharmaceutical approved by the Food and Drug Administration (FDA) [1]. However, cultivation costs in plant cell cultures are still significant.
2 Algae as Bioreactor for Recombinant Protein Production
Algae are solar-powered like plants and especially unicellular microalgae that possess high photosynthesis rates and yield biomass much faster than plants are interesting for diverse biotechnological applications. Many microalgae species provide valuable natural compounds such as vitamins, pigments, proteins and lipids and have been used in cosmetic, food and veterinary industry for many years [24, 25]. Microalgae attract currently much attention in the biofuel sector as many species possess high lipid content and might provide a sustainable and cheap source for biodiesel in future [26–29]. Still underestimated though, is the idea of using microalgae as expression platform for recombinant proteins. Microalgae can be cultivated with low costs needing basically water and sunlight and combine rapid growth rates, easy handling and high scale up capacity with the advantages of eukaryotic expression systems [30–33]. The genome sequence of different microalgae species became available within the last years and basic genetic tools like stable transfection of the nucleus and chloroplast genome and inducible promoter systems were established to express recombinant proteins within different cellular compartments or target proteins for secretion into the culture medium. Compared to complex systems like plants or mammalian cells, microalgae are very robust and easily accessible for genetic manipulation making rapid high-throughput analysis possible. Many microalgae are used as food source and are regarded as safe as they contain no harmful components and are no host for human pathogens. Hence, the expression of therapeutic proteins and also oral application of whole cell extracts represent a promising option. Within the last years different therapeutic proteins like monoclonal antibodies, immunotoxins, subunit vaccines and feed supplements have been expressed in microalgae [31]. Most of these studies focused so far on the green alga Chlamydomonas reinhardtii, which is the model alga in basic research and was the first microalga to be sequenced and accessible for genetic engineering. At present C. reinhardtii is mainly used for recombinant protein expression in the chloroplast, but also other algal systems like Dunaliella and Chlorella species and the diatom Phaeodactylum tricornutum are now explored and especially P. tricornutum reveals great potential in using alga for expressing recombinant proteins from the nucleus genome. This review highlights recent progress in using microalgae as expression system for recombinant proteins and gives an overview on general concepts and practical considerations.
3 Pro- and Eukaryotic Expression Traits Within One Cell
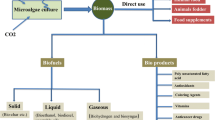
In contrast to most other expression systems microalgae provide the opportunity to express recombinant proteins either from the nucleus genome in a eukaryotic environment or in an “advanced” prokaryotic milieu within the chloroplast. Most research so far focused on expression in the chloroplast as in the model alga C. reinhardtii higher expression levels were observed for the chloroplast than for the nucleus genome ranging from 0.1 to 5 % of total soluble protein and even 21 % in one study (Table 16.1). Another interesting feature for protein expression within the chloroplast is the fact that this originally prokaryotic organelle, unlike bacteria, harbors an advanced set of chaperons and enzymes to form disulfide bonds [73, 74]. Complete IgG antibodies and special variants like dimeric single chain antibodies can be produced in the chloroplast of C. reinhardtii and are fully assembled and able to bind to the target antigens [36, 39]. Recently, it was shown that the chloroplast is also interesting for the production of immunotoxins, chimeric proteins that consist of an antigen-binding domain fused to a eukaryotic toxin like PE40 or gelonin [37, 38]. As these components are toxic for eukaryotes that kind of therapeutics normally have to be produced in bacterial systems with the drawback that protein complexity is very limited. In the chloroplast, however, complex divalent immunotoxins can be produced showing enhanced cytotoxicity compared to the monovalent form [37]. Beside antibodies, further complex structured eukaryotic proteins like Plasmodium falciparum peptides interesting as malaria transmission-blocking vaccine were shown to be expressed as soluble and correctly folded proteins within the chloroplast [43–45]. Most proteins of P. falciparum are not glycosylated and expression in classical eukaryotic expression systems is problematic [75]. As chloroplasts harbor no glycosylation machinery, proteins expressed within the chloroplast remain aglycosylated, which represents a further advantage in this special case preventing allergic reactions to foreign glycoprofiles. Feeding mice with freeze-dried algae expressing P. falciparum surface protein 25 elicited specific IgA and IgG antibodies demonstrating that algae are like plants highly interesting in terms of oral vaccination [44]. Protein storage within the chloroplast might come along with enhanced protein protection as in another study a chloroplast expressed Staphylococcus aureus protein was shown to be potent in lyophilized algae for at least 20 month at room temperature and protected against proteolysis within a stomach-like pepsin environment [41]. In summary, the algal chloroplast is of special interest for the expression of complex aglycosylated or toxic proteins that cannot be produced in bacterial systems. Oral delivery might represent an ideal application form for different subunit vaccines as well as for some feed supplements [31, 76] (Fig. 16.1).
Microalgae as solar powered expression system for recombinant proteins. Microalgae possess rapid growth rates, are very robust as well as easily scalable and might provide low-cost production of different therapeutic proteins as well as feed supplements in future. Proteins can be expressed either from the nucleus genome or within the chloroplast. Both options provide advantages depending on the application and the protein of interest. Most studies concentrated on the green alga C. reinhardtii so far but also other systems like P. tricornutum, Chlorella and Dunaliella species start to get explored and might provide some advantages. ER endoplasmatic reticulum, mt mitochondrion, nu nucleus, pl plastid
Protein expression from the nucleus genome provides the advantage of eukaryotic post-translational modifications, which are important for conformation, stability and activity of most eukaryotic proteins. Nevertheless expression from algal nucleus genomes was so far mostly disregarded as most studies in C. reinhardtii showed rather low expression levels ranging from 0.05 to 0.25 % of total soluble protein (Table 16.1). Research especially from the last years, however, revealed that this topic deserves more attention and that it is worth to test other algal species as well. In 2011 a human IgG antibody against the Hepatitis B Virus Surface protein was expressed in the diatom P. tricornutum and accumulated to ~9 % of total soluble protein—corresponding to about 22 mg antibody per 1 g dry weight [34]. Interestingly, further analyses revealed that the deletion of an initially used ER-retention signal even led to secretion of the fully assembled and functional IgG antibodies into the culture medium [35]. As P. tricornutum does not seem to secrete many proteins by natural means, the antibodies were remarkably pure and accumulated in this assay without further engineering to about 2.5 mg/L medium [35]. The secretion of proteins into the culture medium is of course a great benefit simplifying downstream processes and reducing cost-intense purification steps incredibly. Recent work demonstrated that also engineered C. reinhardtii cells can secrete high amounts of recombinant protein into the medium as shown exemplarily for the reporter protein gLuc (Gaussia princeps luciferase) yielding approximately 10 mg per liter culture medium in case of an engineered cell wall deficient strain [77]. Altogether, research especially from the last years reveals the potential of microalgae as solar-driven expression system capable to produce complex eukaryotic proteins that can be secreted efficiently into the culture media. The chloroplast as an advanced prokaryotic compartment represents a further expression site within the algal cell interesting for special products like complex aglycosylated proteins or eukaryotic toxins (Fig. 16.1).
4 Genetic Engineering for Enhanced Protein Expression in Microalgae
Transformation of the green alga C. reinhardtii was established about 25 years ago [78, 79] and today many other algae, e.g., the green algae species Dunaliella and Chlorella as well as Heterokon-tophytes like P. tricornutum, Thalassiosira pseudonana and Nannochloropsis species can be routinely transformed. Microparticle bombardment is widely used for introducing new genes into the nucleus or chloroplast genome but also electroporation can be applied for most species (Table 16.2) representing a more economical approach for large-scale assays. For detailed reviews on algal transformation techniques and selectable marker genes see Gong et al. [100], Leon-Banares et al. [101], and Potvin and Zhang [102]. In general, chloroplast transformation techniques take advantage of homologous recombination to integrate gene constructs into a specific genomic context [68, 78, 84]. Integration into the nucleus genome occurs in case of most transformation techniques random via non-homologous end joining. Only for N. oceanica it was shown so far that homologous recombination works also for the nucleus genome [86] representing a great benefit as the integration context can influence gene expression considerably. Targeted gene knock out is a further great advantage as it offers many engineering options possible in other algae like C. reinhardtii and P. tricornutum only via RNA interference at the moment [103, 104]. Very recently, however, also in these organisms some progress has been made for targeted insertion and gene knock out regarding the nuclear genome as engineered zinc-finger nucleases have been shown to allow specific DNA insertion in C. reinhardtii [105] and also for P. tricornutum genomic modifications via meganucleases and TALE nucleases have been reported [106, 107].
Like in other expression systems also for microalgae the adaption of DNA-sequences to the host specific codon-usage is beneficial and turned out to be very important at least for C. reinhardtii (with a GC-content of 61 %) in order to obtain detectable protein levels [108–110]. For the diatom P. tricornutum, which possesses a typical eukaryotic GC-content of 48 % this might be less critical. Foreign genes can also be expressed without codon optimization and even bacterial enzymes can be expressed from the nucleus genome of P. tricornutum as shown in a study on bioplastic production [111].
The choice of the promoter is a further critical feature for efficient protein expression and regarding expression from the chloroplast genome 5′UTRs of psbA, psbD, atpA and rbcL are most frequently used (Table 16.2). The psbA promoter is one of the most efficient in C. reinhardtii, at least when the endogenous psbA gene is deleted [51, 55, 112], and expression levels of 0.1–21 % TSP were observed (Table 16.1). Photosynthesis can be restored in these strains by reintroducing an attenuated psbA gene in a different gene context [55]—however production rates decreased and strains were still unviable for commercial scale. Recently, psbA complementation was optimized, though, allowing high phototrophic growth rates while maintaining high production levels [113]. For recombinant protein expression from the nucleus genome of C. reinhardtii the 5′UTRs of hsp70A, psaD, rbcS2 or the fusion hsp70A-rbcS2 have been used in most assays (Table 16.1), however expression levels are very low, which might be a problem of gene silencing. Best expression levels were observed in a genetic screen of a mutant library accumulating recombinant protein to 0.2 % of TSP [114]. For a more detailed review on chloroplast and nuclear promoter studies in C. reinhardtii see Specht et al. [115]. The diatom P. tricornutum came into focus as expression system for recombinant proteins only very recently and therefore less data is available, but much higher nuclear expression levels than in C. reinhardtii were observed in initial tests when using the inducible promoter of the endogenous nitrate reductase (8.7 % of TSP and 0.7 %) [34, 35]. This promoter was established previously in the diatom T. pseudonana and can be tightly controlled via ammonia/nitrate in the culture medium [116]. The light inducible promoters of fcpA and fcpB of P. tricornutum are frequently used in basic research but no quantifications on expression levels are available so far.
Further strategies that have shown to enhance nuclear expression of recombinant proteins in microalgae include the insertion of introns from native genes [56, 117] and the expression as transcriptional fusion to an antibiotic resistance gene [67, 109]. Both strategies were applied in C. reinhardtii and might help to counteract transgene silencing. For the industrial enzyme xylanase it was exemplarily shown that expression levels could increase to 100-fold when expressing this protein in fusion with the coding region for the selection marker. The insertion of a viral self-cleavable peptide guaranteed a discrete protein as final product, which could also be secreted when including an endogenous signal peptide [67].
Altogether, the molecular toolbox for microalgae was more and more extended within the last years and not only the model alga C. reinhardtii but also other species like P. tricornutum and N. oceanica start to get explored revealing beneficial traits like higher expression levels from the nucleus genome or targeted gene insertion, respectively. In future, additional engineering might help to increase expression levels and provide modifications such as humanized glycosylation profiles—a critical quality attribute with the basics just being about to get investigated in microalgae [118–120]. In future, also other algal species like Chlorella might become interesting as expression platform as Chlorella possesses very rapid growth rates and provides valuable natural compounds interesting for food, cosmetic and biodiesel industry (Table 16.2). Compared to other microalgae, however, molecular tools for the expression of recombinant proteins in Chlorella are still very limited and studies on recombinant protein expression are still in a very early stage.
5 Expression of Protein Complexes
Multi-protein complexes are essential for many cellular processes and for their structural and biochemical analyses as well as for therapeutic applications large-scale production is necessary. However, the heterologous expression of protein complexes consisting of multiple protein subunits still represents a great challenge. Historically, protein subunits were initially expressed separately, purified and reconstituted in vitro, but of course this is problematic in many cases as proteins form aggregates, have to be refolded and additional factors that might be needed for the assembly process are not present. Today co-expression and complex formation within the cell is favored and different complexes have been successfully expressed in bacteria, yeast, insect cells, plants as well as some mammalian cell lines. Different strategies are applied like the use of multiple expression vectors, plasmids with multiple cloning sites or polycistronic units in case of bacteria, as well as the expression of fusion proteins (see Kerrigan et al. [121] for a review). Data on the expression of protein complexes in microalgae is so far very limited but as mentioned previously the efficient expression of completely assembled IgG complexes consisting of two heavy and two light chains was shown to be feasible in P. tricornutum [34, 35]. The complex is assembled within the ER and can be secreted into the culture medium. Also in the chloroplast of C. reinhardtii completely assembled IgG complexes can be produced. The expression of other protein complexes has yet not been tested in microalgae; however, the molecular tools for the co-expression of multiple protein subunits are basically available. In P. tricornutum multiple plasmids can be co-transformed [122]; this technique was applied for example to introduce three bacterial enzymes for production of the bioplastic PHB [111]. As different resistance markers are available for many algae sequential transfections can be performed as well. Also plasmids with multiple cloning sites like the plasmid pPha-DUAL-[2xNR], which contains two multiple cloning sites, both under the control of a nitrate-inducible promoter, are available and have been used for the expression of IgG antibodies in P. tricornutum [34]. In C. reinhardtii it was shown very recently that also fusion proteins separated by viral self-cleaving sequences can be expressed from the nucleus genome leading to separate gene products [67, 123]. Hence, basic tools for the expression of multiple protein subunits in microalgae are available and it will be highly interesting to start expression assays of multi-subunit complexes in future.
6 Algal Produced Therapeutics, Feed Supplements and Other Proteins
Within the last 15 years a broad spectra of recombinant proteins has been produced in different microalgae ranging from therapeutic proteins like antibodies, vaccines, hormones to feed supplements and industrial relevant enzymes (Table 16.1). The following sections provide an overview on different studies in the field.
6.1 Antibodies
Therapeutic antibodies currently represent the best-selling class of biologics with US sales reaching 20.3 billion dollar in 2011 [1]. Antibody production is based on mammalian cell culture, which is very expensive and therefore alternative expression systems are highly desirable. In 2003, a large single-chain antibody against Herpes simplex virus glycoprotein D was the first antibody to be expressed in an algal system [39]. This antibody was produced in the chloroplast of C. reinhardtii and was proven to assemble as dimer that binds to its target antigen. Further studies on antibody expression include the production of a complete human IgG antibody against anthrax in the chloroplast of C. reinhardtii [36] and the expression of a human IgG antibody against the Hepatitis B Virus surface protein. In contrast to previous studies, the latter was expressed from the nucleus genome in the diatom P. tricornutum and was produced very efficiently with 9 % of total soluble protein [34]. The deletion of a retention signal led to efficient secretion of the fully assembled and functional antibodies into the culture medium [35]. As rarely other proteins were detected in the media, the antibody was relatively pure without further treatment. In 2013, the production of mono and dimeric single chain immunotoxins was shown to be feasible in the chloroplast of C. reinhardtii. These algal produced antibody variants were able to bind and kill B cell lymphoma cells in vitro and showed anti tumor activity in mice [37, 38]. Very recently, also camelid antitoxins were expressed in the chloroplast of C. reinhardtii. The algal produced nanobodies against botulinum neurotoxin A showed in vitro activity in toxin protection assays and remained intact in the gastrointestinal tract of mice fed with antitoxin-producing microalgae [40]. Altogether, these studies demonstrate the great potential of using microalgae as expression system for antibodies and further engineering concerning productivity and glycopatterns might make microalgae a low-cost production platform with little risk for pathogenic contaminations in future.
6.2 Vaccines
Protein based vaccines represent a further important class of therapeutics and microalgae might be of interest especially for the production of oral subunit vaccines. Many algal species are used as nutritional supply in food industry, hence complete cell extracts could be administered directly thereby bypassing costly purification steps and facilitating production as well as needle-free application [76]. Different reports on the expression of oral vaccines in microalgae have been published within the last years. In 2010, the D2 fibronectin-binding domain of S. aureus was expressed in fusion with the adjuvants CTB (cholera toxin B subunit) in the chloroplast of C. reinhardtii. Orally vaccinated mice showed mucosal as well as systemic immune response resulting in protection from lethal S. aureus infections [41]. The vaccine was stable within lyophilized algae for at least 20 month at room temperature. Furthermore, stability assays demonstrated that the protein was protected against proteolysis within a stomach-like pepsin environment representing a critical point for absorption within the intestine. The study demonstrates that the algal chloroplast could represent an ideal compartment for the expression of oral vaccines resulting in enhanced antigen stability and protection. In another study Plasmodium berghei antigens were targeted to chloroplast starch granules in C. reinhardtii [42]. Oral vaccination of mice led to reduced parasitemia and specific IgG antibodies could be detected inhibiting intra-erythrocytic asexual development of different Plasmodium species in vitro. A further study on the production of a malaria transmission blocking vaccine in the chloroplast of C. reinhardtii, the Plasmodium falciparum surface protein 25 (Psf25-CTB), demonstrated that the protein is correctly folded and elicits transmission blocking antibodies in mice when injected intraperitoneally [43]. Orally vaccinated mice produced specific mucosal IgA antibodies but no systemic IgG antibody production was observed [44]. Of course, not every vaccine is suitable for oral administration to generate a systemic immune response and also the adjuvants used in this study might not be ideal for stimulating IgG production. But even though oral vaccination is still very limited due to the complexity of the mucosal system, ongoing research will certainly help to overcome present challenges with microalgae representing promising edible, low-cost expression systems [75]. Also in veterinary medicine and especially in aqua culture using microalgae as vaccination vehicle might be of great interest. One of the first reports on the production of oral therapeutics in microalgae is the expression of the anti-microbial peptide bovine lactoferricin in Nannochloropsis oculata [46]. Feeding experiments with medaka fish revealed a survival rate of 85 % after Vibrio parahaemolyticus infection. In another study the protein VP28 of the White Spot Syndrome Virus was expressed in the green alga Dunaliella salina. Even though expression levels were only very low, oral vaccination of crayfish resulted in significant survival rates after white spot syndrome virus (WSSV) infection [47].
Other vaccines that were produced in microalgae but not tested for oral application are the hepatitis B virus surface antigen (HBsAg) produced in the chloroplast of D. salina as well as in the endoplasmic reticulum of P. tricornutum [34, 48]. Additionally, different Plasmodium antigens interesting for malaria control [43, 45] and proteins of different viruses, i.e., human papilloma virus 16 [49], white spot syndrome virus [51], foot and mouth disease virus [50] and classical swine fever virus [52], were expressed within the chloroplast of C. reinhardtii with expression levels of 0.12–21 % of total soluble protein (Table 16.1).
6.3 Other Therapeutic Proteins
Human growth hormone (hGH) was one of the first therapeutic proteins that were tested for expression in an algal system. In 1999, when molecular engineering of microalgae was still in the very beginning, Hawkins and colleagues expressed hGH in Chlorella vulgaris as well as in Chlorella sorokiniana, and showed that the hormone is expressed and secreted into the culture medium, even though transfection was only transient in these initial studies [54]. The bovine mammary-associated serum amyloid protein (M-SAA) is an anti-microbial protein that is found in the colostrum (first milk) of mammals and prevents bacterial infections by stimulating mucin synthesis in the small intestines. M-SAA was shown to be expressed efficiently in the chloroplast of C. reinhardtii as bioactive molecule stimulating mucin production in epithelial cell culture [55]. The study demonstrates the great potential of using microalgae for the production of edible gut active therapeutics and only recently, the US company Triton Health and Nutrition started the microalgal production of M-SAA. Further therapeutics that were expressed in algal systems include human erythropoietin, which was produced in the chloroplast of C. reinhardtii [57] as well as shown to be secreted when expressed from the nucleus genome [56]. Furthermore, diverse therapeutics such as interferon-β, proinsulin [57] and a marker for diabetes I diagnostics [61] were expressed in the chloroplast of C. reinhardtii. All proteins were shown to be biologically active and accumulated to up to 3 % of total soluble protein. The production of anti-microbial peptides was also tested in D. salina and C. ellipsoidea [58, 59, 62], which might become interesting as an expression system when more molecular tools become available in future.
6.4 Animal Feed Supplements
Many algae are used as feed additives since many species are rich in valuable natural compounds like long-chain polyunsaturated fatty acids (PUFAs), carotenoids, vitamins and high quality protein and carbohydrate. The opportunity to combine nutritional supply and direct delivery of recombinant feed additives like growth hormones or dietary enzymes for fiber break down makes microalgae an interesting low-cost expression system in that field. In 2002 the flounder growth hormone (fGH) was expressed in C. ellipsoidea and it was shown that flounder fry fed on transformed algae exhibit a 25 % growth increase after 1 month [64]. Promising results were also obtained when feeding tilapia fry with transgenic N. oculata cells expressing yellowfin porgy growth hormone (ypGH) [65]. After 4 weeks, a 212 % increase in weight and 71 % increase in length were observed. In another study, a bacterial phytase was expressed in C. reinhardtii to facilitate phytate digestion in monogastric animals. Feeding experiments on broiler chicks revealed a reduced excretion of fecal phytate [66]. The production of other phytases and further enzymes used as dietary supplements like α-galactohydrolases and a xylanase was also shown to be feasible in C. reinhardtii and D. tertiolecta [26].
6.5 Bioremediation and Environmental Control
Algae are used in wastewater treatment to provide oxygen supply for bacterial biodegradation and remove inorganic nitrogen and phosphorus [124]. In addition, the removal of heavy metals like cadmium, nickel and zinc was shown for some species [125, 126] and might be enhanced by genetic engineering. In two studies the expression of metallothioneins was assayed in C. reinhardtii demonstrating that transgenic cells exhibit higher cadmium binding capacity and can grow to higher densities at toxic cadmium concentrations [70, 127]. Another study presents an algal-based approach on mosquito control. An insecticide against mosquito larvae acting on trypsin biosynthesis in the mosquito gut was produced in Chlorella sp. [71]. Feeding of transgenic algae to mosquito larvae caused larval mortality.
Altogether, a broad repertoire of recombinant proteins like many different therapeutic proteins, feed supplements for animal welfare and proteins interesting for environmental control have been expressed in microalgae (Table 16.1) demonstrating the great potential of microalgal expression systems.
7 Conclusions and Perspectives
The demand for recombinant protein therapeutics and industrial enzymes is enormous nowadays and the market for biologics is constantly growing. In future, it will be essential to improve existing expression systems but also to establish novel low-cost production platforms to guarantee affordable products. Microalgae are powered by sunlight, possess rapid growth rates and are genetically well accessible. In recent years significant progress has been made in recombinant protein expression in microalgae and many different projects on the production of therapeutics, vaccines and feed supplements demonstrate the great potential of using microalgae as novel low-cost expression platform. Microalgae are of special interest for the production of complex eukaryotic proteins that currently have to be produced in mammalian cell lines involving high production costs and the risk of human pathogenic contaminations. Especially the production of IgG antibodies that were shown to be secreted into the algae culture medium could offer an attractive option in future. The expression of oral vaccines and feed supplements represent a further promising approach. As many microalgae are edible and harbor valuable vitamins, proteins and fatty acids the complete cell extract could be administered directly saving expensive purification costs. Furthermore, the algal chloroplast represents an interesting expression platform especially for the production of complex aglycosylated proteins or eukaryotic toxins coupled to complex proteins like bivalent immunotoxins that are difficult to produce in other expression systems.
Concerning commercial applications, the production of recombinant proteins in microalgae is still at an early stage. Comparable to the establishment of previous expression systems it will be necessary to enhance productivity and secretion efficiency and establish glycoengineering approaches to provide therapeutics with humanized glycoprofiles in future. In addition to C. reinhardtii other microalgal strains like P. tricornutum, N. oceanica and Chlorella should be included for detailed expression studies as it was started in some projects only recently. Higher expression levels, exclusive molecular tools and higher growth rates could be some of the advantages. As microalgae biotechnology became very popular within the last years, a lot of effort has also been put into the development of algae photobioreactors. Promising solutions for large-scale cultivation can be provided by now [128] representing a further important aspect concerning technology transfer to industrial scale in future. Triton Health and Nutrition (USA) and Algenics (France) belong to the first companies that started to use microalgae as expression platform for recombinant proteins. But certainly other companies will follow soon considering the great potential of using microalgae as solar fueled, low-cost expression system.
References
Aggarwal SR (2012) What’s fueling the biotech engine-2011 to 2012. Nat Biotechnol 30(12):1191–1197
Ferrer-Miralles N, Domingo-Espin J, Corchero JL, Vazquez E, Villaverde A (2009) Microbial factories for recombinant pharmaceuticals. Microb Cell Factories 8:17
Walsh G, Jefferis R (2006) Post-translational modifications in the context of therapeutic proteins. Nat Biotechnol 24(10):1241–1252
Martinez JL, Liu L, Petranovic D, Nielsen J (2012) Pharmaceutical protein production by yeast: towards production of human blood proteins by microbial fermentation. Curr Opin Biotechnol 23(6):965–971
Frenzel A, Hust M, Schirrmann T (2013) Expression of recombinant antibodies. Front Immunol 4:217
Amano K, Chiba Y, Kasahara Y, Kato Y, Kaneko MK, Kuno A, Ito H, Kobayashi K, Hirabayashi J, Jigami Y, Narimatsu H (2008) Engineering of mucin-type human glycoproteins in yeast cells. Proc Natl Acad Sci U S A 105(9):3232–3237
De Pourcq K, De Schutter K, Callewaert N (2010) Engineering of glycosylation in yeast and other fungi: current state and perspectives. Appl Microbiol Biotechnol 87(5):1617–1631
Wildt S, Gerngross TU (2005) The humanization of N-glycosylation pathways in yeast. Nat Rev Microbiol 3(2):119–128
Ye J, Ly J, Watts K, Hsu A, Walker A, McLaughlin K, Berdichevsky M, Prinz B, Sean Kersey D, d’Anjou M, Pollard D, Potgieter T (2011) Optimization of a glycoengineered Pichia pastoris cultivation process for commercial antibody production. Biotechnol Prog 27(6):1744–1750
el Redwan RM (2007) Cumulative updating of approved biopharmaceuticals. Hum Antibodies 16(3–4):137–158
Dietmair S, Nielsen LK, Timmins LE (2012) Mammalian cells as biopharmaceutical production hosts in the age of omics. Biotechnol J 7(1):75–89
Pauwels K, Herman P, Van Vaerenbergh B, Dai Do thi C, Berghmans L, Waeterloos G, Van Bockstaele D, Dorsch-Häsler K, Sneyers M (2007) Animal cell cultures: risk assessment and biosafety recommendations. Appl Biosaf 12(1):26–38
Daniell H, Singh ND, Mason H, Streatfield SJ (2009) Plant-made vaccine antigens and biopharmaceuticals. Trends Plant Sci 14(12):669–679
Fischer R, Stoger E, Schillberg S, Christou P, Twyman RM (2004) Plant-based production of biopharmaceuticals. Curr Opin Plant Biol 7(2):152–158
Franken E, Teuschel U, Hain R (1997) Recombinant proteins from transgenic plants. Curr Opin Biotechnol 8(4):411–416
Ma JK, Drake PM, Christou P (2003) The production of recombinant pharmaceutical proteins in plants. Nat Rev Genet 4(10):794–805
Fooks AR (2000) Development of oral vaccines for human use. Curr Opin Mol Ther 2(1):80–86
Giddings G, Allison G, Brooks D, Carter A (2000) Transgenic plants as factories for biopharmaceuticals. Nat Biotechnol 18(11):1151–1155
Gomord V, Fitchette AC, Menu-Bouaouiche L, Saint-Jore-Dupas C, Plasson C, Michaud D, Faye L (2010) Plant-specific glycosylation patterns in the context of therapeutic protein production. Plant Biotechnol J 8(5):564–587
Webster DE, Thomas MC (2012) Post-translational modification of plant-made foreign proteins; glycosylation and beyond. Biotechnol Adv 30(2):410–418
Ruybicki EP (2009) Plant-produced vaccines: promise and reality. Drug Discov Today 14(1–2):16–24
Hellwig S, Drossard J, Twyman RM, Fischer R (2004) Plant cell cultures for the production of recombinant proteins. Nat Biotechnol 22(11):1415–1422
Xu J, Ge X, Dolan MC (2011) Towards high-yield production of pharmaceutical proteins with plant cell suspension cultures. Biotechnol Adv 29(3):278–299
Buono S, Langellotti AL, Martello A, Rinna F, Fogliano V (2014) Functional ingredients from microalgae. Food Funct 5(8):1669–1685
Raja R, Hemaiswarya S, Kumar NA, Sridhar S, Rengasamy R (2008) A perspective on the biotechnological potential of microalgae. Crit Rev Microbiol 34(2):77–88
Georgianna DR, Mayfield SP (2012) Exploiting diversity and synthetic biology for the production of algal biofuels. Nature 488(7411):329–335
Mata TM, Martins AA, Caetano NS (2010) Microalgae for biodiesel produciton and other applications: A review. Renew Sust Energ Rev 14(1):217–232
Moody JW, McGinty CM, Quinn JC (2014) Global evaluation of biofuel potential from microalgae. Proc Natl Acad Sci U S A 111(23):8691–8696
Wijffels RH, Barbosa MJ (2010) An outlook on microalgal biofuels. Science 329(5993):796–799
Franklin SE, Mayfield SP (2004) Prospects for molecular farming in the green alga Chlamydomonas. Curr Opin Plant Biol 7(2):159–165
Rasala BA, Mayfield SP (2015) Photosynthetic biomanufacturing in green algae; production of recombinant proteins for industrial, nutritional, and medical uses. Photosynth Res 123(3):227–239
Rosales-Mendoza S, Paz-Maldonado LM, Soria-Guerra RE (2012) Chlamydomonas reinhardtii as a viable platform for the production of recombinant proteins: current status and perspectives. Plant Cell Rep 31(3):479–494
Walker TL, Purton S, Becker DK, Collet C (2005) Microalgae as bioreactors. Plant Cell Rep 24(11):629–641
Hempel F, Lau J, Klingl A, Maier UG (2011) Algae as protein factories: expression of a human antibody and the respective antigen in the diatom Phaeodactylum tricornutum. PLoS One 6(12):e28424
Hempel F, Maier UG (2012) An engineered diatom acting like a plasma cell secreting human IgG antibodies with high efficiency. Microb Cell Factories 11:126
Tran M, Zhou B, Pettersson PL, Gonzalez MJ, Mayfield SP (2009) Synthesis and assembly of a full-length human monoclonal antibody in algal chloroplasts. Biotechnol Bioeng 104(4):663–673
Tran M, Van C, Barrera DJ, Pettersson PL, Peinado CD, Bui J, Mayfield SP (2013) Production of unique immunotoxin cancer therapeutics in algal chloroplasts. Proc Natl Acad Sci U S A 110(1):E15–E22
Tran M, Henry RE, Siefker D, Van C, Newkirk G, Kim J, Bui J, Mayfield SP (2013) Production of anti-cancer immunotoxins in algae: ribosome inactivating proteins as fusion partners. Biotechnol Bioeng 110(11):2826–2835
Mayfield SP, Franklin SE, Lerner RA (2003) Expression and assembly of a fully active antibody in algae. Proc Natl Acad Sci U S A 100(2):438–442
Barrera DJ, Rosenberg JN, Chiu JG, Chang YN, Debatis M, Ngoi SM, Chang JT, Shoemaker CB, Oyler GA, Mayfield SP (2015) Algal chloroplast produced camelid V H antitoxins are capable of neutralizing botulinum neurotoxin. Plant Biotechnol J 13(1):117–124
Dreesen IA, Charpin-El Hamri G, Fussenegger M (2010) Heat-stable oral alga-based vaccine protects mice from Staphylococcus aureus infection. J Biotechnol 145(3):273–280
Dauvillee D, Delhaye S, Gruyer S, Slomianny C, Moretz SE, d’Hulst C, Long CA, Ball SG, Tomavo S (2010) Engineering the chloroplast targeted malarial vaccine antigens in Chlamydomonas starch granules. PLoS One 5(12):e15424
Gregory JA, Li F, Tomosada LM, Cox CJ, Topol AB, Vinetz JM, Mayfield S (2012) Algae-produced Pfs25 elicits antibodies that inhibit malaria transmission. PLoS One 7(5):e37179
Gregory JA, Topol AB, Doerner DZ, Mayfield S (2013) Alga-produced cholera toxin-Pfs25 fusion proteins as oral vaccines. Appl Environ Microbiol 79(13):3917–3925
Jones CS, Luong T, Hannon M, Tran M, Gregory JA, Shen Z, Briggs SP, Mayfield SP (2013) Heterologous expression of the C-terminal antigenic domain of the malaria vaccine candidate Pfs48/45 in the green algae Chlamydomonas reinhardtii. Appl Microbiol Biotechnol 97(5):1987–1995
Li S, Tsai H (2009) Transgenic microalgae as a non-antibiotic bactericide producer to defend against bacterial pathogen infection in the fish digestive tract. Fish Shellfish Immunol 26(2):316–325
Feng S, Feng W, Zhao L, Gu H, Li Q, Shi K, Guo S, Zhang N (2014) Preparation of transgenic Dunaliella salina for immunization against white spot syndrome virus in crayfish. Arch Virol 159(3):519–525
Geng D, Wang Y, Wang P, Li W, Sun Y (2003) Stable expression of hepatitis B surface antigen gene in Dunaliella salina (Chlorophyta). J Appl Phycol 15(6):451–456
Demurtas OC, Massa S, Ferrante P, Venuti A, Franconi R, Giuliano G (2013) A Chlamydomonas-derived Human Papillomavirus 16 E7 vaccine induces specific tumor protection. PLoS One 8(4):e61473
Sun M, Qian K, Su N, Chang H, Liu J, Shen G (2003) Foot-and-mouth disease virus VP1 protein fused with cholera toxin B subunit expressed in Chlamydomonas reinhardtii chloroplast. Biotechnol Lett 25(13):1087–1092
Surzycki R, Greenham K, Kitayama K, Dibal F, Wagner R, Rochaix JD, Ajam T, Surzycki S (2009) Factors effecting expression of vaccines in microalgae. Biologicals 37(3):133–138
He DM, Qian KX, Shen GF, Zhang ZF, Li YN, Su ZL, Shao HB (2007) Recombination and expression of classical swine fever virus (CSFV) structural protein E2 gene in Chlamydomonas reinhardtii chroloplasts. Colloids Surf B: Biointerfaces 55(1):26–30
Soria-Guerra RE, Ramírez-Alonso JI, Ibánez-Salazar A, Govea-Alonso DO, Paz-Maldonado LMT, Banuelos-Hernández B, Korban SS, Rosales-Mendoza S (2014) Expression of an HBcAg-based antigen carrying angiotensin II in Chlamydomonas reinhardtii as a candidate hypertension vaccine. Plant Cell Tissue Organ Cult 116(2):133–139
Hawkins RL, Nakamura M (1999) Expression of human growth hormone by the eukaryotic alga, Chlorella. Curr Microbiol 38(6):335–341
Manuell AL, Beligni MV, Elder JH, Siefker DT, Tran M, Weber A, McDonald TL, Mayfield SP (2007) Robust expression of a bioactive mammalian protein in Chlamydomonas chloroplast. Plant Biotechnol J 5(3):402–412
Eichler-Stahlberg A, Weisheit W, Ruecker O, Heitzer M (2009) Strategies to facilitate transgene expression in Chlamydomonas reinhardtii. Planta 229(4):873–883
Rasala BA, Muto M, Lee PA, Jager M, Cardoso RM, Behnke CA, Kirk P, Hokanson CA, Crea R, Mendez M, Mayfield SP (2010) Production of therapeutic proteins in algae, analysis of expression of seven human proteins in the chloroplast of Chlamydomonas reinhardtii. Plant Biotechnol J 8(6):719–733
Chen Y, Wang Y, Sun Y, Zhang L, Li W (2001) Highly efficient expression of rabbit neutrophil peptide-1 gene in Chlorella ellipsoidea cells. Curr Genet 39(5–6):365–370
Bai LL, Yin WB, Chen YH, Niu LL, Sun YR, Zhao SM, Yang FQ, Wang RR, Wu Q, Zhang XQ, Hu ZM (2013) A new strategy to produce a defensin: stable production of mutated NP-1 in nitrate reductase-deficient Chlorella ellipsoidea. PLoS One 8(1):e54966
Yang Z, Li Y, Chen F, Li D, Zhang Z, Liu Y, Zheng D, Wang Y, Shen G (2006) Expression of human soluble TRAIL in Chlamydomonas reinhardtii chloroplast. Chin Sci Bull 51(14):1703–1709
Wang X, Brandsma M, Tremblay R, Maxwell D, Jevnikar AM, Huner N, Ma S (2008) A novel expression platform for the production of diabetes-associated autoantigen human glutamic acid decarboxylase (hGAD65). BMC Biotechnol 13(4):460–70
Chai X, Chen H, Xu W, Xu Y (2013) Expression of soybean Kunitz trypsin inhibitor gene SKTI in Dunaliella salina. J Appl Phycol 25(1):139–144
Sun Y, Yang Z, Gao X, Li Q, Zhang Q, Xu Z (2005) Expression of foreign genes in Dunaliella by electroporation. Mol Biotechnol 30(3):185–192
Kim DH, Kim YT, Cho JJ, Bae JH, Hur SB, Hwang I, Choi TJ (2002) Stable integration and functional expression of flounder growth hormone gene in transformed microalga, Chlorella ellipsoidea. Mar Biotechnol (NY) 4(1):63–73
Chen HL, Li SS, Huang R, Tsai HJ (2008) Conditional production of a functional fish growth hormone in the transgenic line of nannochloropsis oculata (eustigmatophyceae). J Phycol 44(3):768–776
Yoon SM, Kim SY, Li KF, Yoon BH, Choe S, Kuo MM (2011) Transgenic microalgae expressing Escherichia coli AppA phytase as feed additive to reduce phytate excretion in the manure of young broiler chicks. Appl Microbiol Biotechnol 91(3):553–563
Rasala BA, Lee PA, Shen Z, Briggs SP, Mendez M, Mayfield SP (2012) Robust expression and secretion of Xylanase1 in Chlamydomonas reinhardtii by fusion to a selection gene and processing with the FMDV 2A peptide. PLoS One 7(8):e43349
Georgianna DR, Hannon MJ, Marcuschi M, Shuiqin W, Botsch K, Lewis AJ, Hyun J, Mendez M, Mayfield SP (2013) Production of recombinant enzymes in the marine alga Dunaliella tertiolecta. Algal Res 2(1):2–9
Hou Q, Qiu S, Liu Q, Tian J, Hu Z, Ni J (2013) Selenoprotein-transgenic Chlamydomonas reinhardtii. Nutrients 5(3):624–636
Han S, Hu Z, Lei A (2008) Expression and function analysis of the metallothionein-like (MT-like) gene from Festuca rubra in Chlamydomonas reinhardtii chloroplast. Sci China C Life Sci 51(12):1076–1081
Borovsky D, Powell CR, Dawson WO, Shivprasad S, Lewandowski D, DeBondt HL, DeRanter C, DeLoof A (eds) (1998) Trypsin modulating oostatic factor (TMOF): a new biorational insecticide against mosquitoes. Insects, Chemical Physiological and Environmental Aspects. Wroclaw University Press, Wroclaw
Zhang YK, Shen GF, Ru BG (2006) Survival of human metallothionein-2 transplastomic Chlamydomonas reinhardtii to ultraviolet B exposure. Acta Biochim Biophys Sin (Shanghai) 38(3):187–193
Kim J, Mayfield SP (1997) Protein disulfide isomerase as a regulator of chloroplast translational activation. Science 278(5345):1954–1957
Schroda M (2004) The Chlamydomonas genome reveals its secrets: chaperone genes and the potential roles of their gene products in the chloroplast. Photosynth Res 82(3):221–240
Gregory JA, Mayfield SP (2014) Developing inexpensive malaria vaccines from plants and algae. Appl Microbiol Biotechnol 98(5):1983–1990
Specht EA, Mayfield SP (2014) Algae-based oral recombinant vaccines. Front Microbiol 5:60
Lauersen KJ, Berger H, Mussgnug JH, Kruse O (2013) Efficient recombinant protein production and secretion from nuclear transgenes in Chlamydomonas reinhardtii. J Biotechnol 167(2):101–110
Boynton JE, Gillham NW, Harris EH, Hosler JP, Johnson AM, Jones AR, Randolph-Anderson BL, Robertson D, Klein TM, Shark KB et al (1988) Chloroplast transformation in Chlamydomonas with high velocity microprojectiles. Science 240(4858):1534–1538
Debuchy R, Purton S, Rochaix JD (1989) The argininosuccinate lyase gene of Chlamydomonas reinhardtii: an important tool for nuclear transformation and for correlating the genetic and molecular maps of the ARG7 locus. EMBO J 8(10):2803–2809
Bowler C, Allen AE, Badger JH, Grimwood J, Jabbari K, Kuo A, Maheswari U, Martens C, Maumus F, Otillar RP, Rayko E, Salamov A, Vandepoele K, Beszteri B, Gruber A, Heijde M, Katinka M, Mock T, Valentin K, Verret F, Berges JA, Brownlee C, Cadoret JP, Chiovitti A, Choi CJ, Coesel S, De Martino A, Detter JC, Durkin C, Falciatore A, Fournet J, Haruta M, Huysman MJ, Jenkins BD, Jiroutova K, Jorgensen RE, Joubert Y, Kaplan A, Kroger N, Kroth PG, La Roche J, Lindquist E, Lommer M, Martin-Jezequel V, Lopez PJ, Lucas S, Mangogna M, McGinnis K, Medlin LK, Montsant A, Oudot-Le Secq MP, Napoli C, Obornik M, Parker MS, Petit JL, Porcel BM, Poulsen N, Robison M, Rychlewski L, Rynearson TA, Schmutz J, Shapiro H, Siaut M, Stanley M, Sussman MR, Taylor AR, Vardi A, von Dassow P, Vyverman W, Willis A, Wyrwicz LS, Rokhsar DS, Weissenbach J, Armbrust EV, Green BR, Van de Peer Y, Grigoriev IV (2008) The Phaeodactylum genome reveals the evolutionary history of diatom genomes. Nature 456(7219):239–244
Apt KE, Kroth-Pancic PG, Grossman AR (1996) Stable nuclear transformation of the diatom Phaeodactylum tricornutum. Mol Gen Genet 252(5):572–579
Miyahara M, Aoi M, Inoue-Kashino N, Kashino Y, Ifuku K (2013) Highly efficient transformation of the diatom Phaeodactylum tricornutum by multi-pulse electroporation. Biosci Biotechnol Biochem 77(4):874–876
Zhang C, Hu H (2013) High-efficiency nuclear transformation of the diatom Phaeodactylum tricornutum by electroporation. Mar Genomics 16:63–66
Xie WH, Zhu CC, Zhang NS, Li DW, Yang WD, Liu JS, Sathishkumar R, Li HY (2014) Construction of novel chloroplast expression vector and development of an efficient transformation system for the diatom phaeodactylum tricornutum. Mar Biotechnol (NY) 16(5):538–546
Vieler A, Wu G, Tsai CH, Bullard B, Cornish AJ, Harvey C, Reca IB, Thornburg C, Achawanantakun R, Buehl CJ, Campbell MS, Cavalier D, Childs KL, Clark TJ, Deshpande R, Erickson E, Armenia Ferguson A, Handee W, Kong Q, Li X, Liu B, Lundback S, Peng C, Roston RL, Sanjaya SJP, Terbush A, Warakanont J, Zauner S, Farre EM, Hegg EL, Jiang N, Kuo MH, Lu Y, Niyogi KK, Ohlrogge J, Osteryoung KW, Shachar-Hill Y, Sears BB, Sun Y, Takahashi H, Yandell M, Shiu SH, Benning C (2012) Genome, functional gene annotation, and nuclear transformation of the heterokont oleaginous alga Nannochloropsis oceanica CCMP1779. PLoS Genet 8(11):e1003064
Kilian O, Benemann CS, Niyogi KK, Vick B (2011) High-efficiency homologous recombination in the oil-producing alga Nannochloropsis sp. Proc Natl Acad Sci U S A 108(52):21265–21269
Radakovits R, Jinkerson RE, Fuerstenberg SI, Tae H, Settlage RE, Boore JL, Posewitz MC (2012) Draft genome sequence and genetic transformation of the oleaginous alga Nannochloropis gaditana. Nat Commun 3:686
Armbrust EV, Berges JA, Bowler C, Green BR, Martinez D, Putnam NH, Zhou S, Allen AE, Apt KE, Bechner M, Brzezinski MA, Chaal BK, Chiovitti A, Davis AK, Demarest MS, Detter JC, Glavina T, Goodstein D, Hadi MZ, Hellsten U, Hildebrand M, Jenkins BD, Jurka J, Kapitonov VV, Kroger N, Lau WW, Lane TW, Larimer FW, Lippmeier JC, Lucas S, Medina M, Montsant A, Obornik M, Parker MS, Palenik B, Pazour GJ, Richardson PM, Rynearson TA, Saito MA, Schwartz DC, Thamatrakoln K, Valentin K, Vardi A, Wilkerson FP, Rokhsar DS (2004) The genome of the diatom Thalassiosira pseudonana: ecology, evolution, and metabolism. Science 306(5693):79–86
Poulsen N, Chesley PM, Kröger N (2006) Molecular genetic manipulation of the diatom Thalassiosira pseudonana (Bacillariophyceae). J Phycol 42(5):1059–1065
Merchant SS, Prochnik SE, Vallon O, Harris EH, Karpowicz SJ, Witman GB, Terry A, Salamov A, Fritz-Laylin LK, Marechal-Drouard L, Marshall WF, Qu LH, Nelson DR, Sanderfoot AA, Spalding MH, Kapitonov VV, Ren Q, Ferris P, Lindquist E, Shapiro H, Lucas SM, Grimwood J, Schmutz J, Cardol P, Cerutti H, Chanfreau G, Chen CL, Cognat V, Croft MT, Dent R, Dutcher S, Fernandez E, Fukuzawa H, Gonzalez-Ballester D, Gonzalez-Halphen D, Hallmann A, Hanikenne M, Hippler M, Inwood W, Jabbari K, Kalanon M, Kuras R, Lefebvre PA, Lemaire SD, Lobanov AV, Lohr M, Manuell A, Meier I, Mets L, Mittag M, Mittelmeier T, Moroney JV, Moseley J, Napoli C, Nedelcu AM, Niyogi K, Novoselov SV, Paulsen IT, Pazour G, Purton S, Ral JP, Riano-Pachon DM, Riekhof W, Rymarquis L, Schroda M, Stern D, Umen J, Willows R, Wilson N, Zimmer SL, Allmer J, Balk J, Bisova K, Chen CJ, Elias M, Gendler K, Hauser C, Lamb MR, Ledford H, Long JC, Minagawa J, Page MD, Pan J, Pootakham W, Roje S, Rose A, Stahlberg E, Terauchi AM, Yang P, Ball S, Bowler C, Dieckmann CL, Gladyshev VN, Green P, Jorgensen R, Mayfield S, Mueller-Roeber B, Rajamani S, Sayre RT, Brokstein P, Dubchak I, Goodstein D, Hornick L, Huang YW, Jhaveri J, Luo Y, Martinez D, Ngau WC, Otillar B, Poliakov A, Porter A, Szajkowski L, Werner G, Zhou K, Grigoriev IV, Rokhsar DS, Grossman AR (2007) The Chlamydomonas genome reveals the evolution of key animal and plant functions. Science 318(5848):245–250
Kindle KL (1990) High-frequency nuclear transformation of Chlamydomonas reinhardtii. Proc Natl Acad Sci U S A 87(3):1228–1232
Brown LE, Sprecher SL, Keller LR (1991) Introduction of exogenous DNA into Chlamydomonas reinhardtii by electroporation. Mol Cell Biol 11(4):2328–2332
Kumar SV, Misquitta RW, Reddy VS, Rao BJ, Rajamani MV (2004) Genetic transformation of the green alga—Chlamydomonas reinhardtii by Agrobacterium tumefaciens. Plant Sci 166(3):731–738
Blanc G, Duncan G, Agarkova I, Borodovsky M, Gurnon J, Kuo A, Lindquist E, Lucas S, Pangilinan J, Polle J, Salamov A, Terry A, Yamada T, Dunigan DD, Grigoriev IV, Claverie JM, Van Etten JL (2010) The Chlorella variabilis NC64A genome reveals adaptation to photosymbiosis, coevolution with viruses, and cryptic sex. Plant Cell 22(9):2943–2955
Chow KC, Tung WL (1999) Electrotransformation of Chlorella vulgaris. Plant Cell Rep 18(9):778–780
Dawson HN, Burlingame R, Cannons AC (1997) Stable transformation of chlorella: rescue of nitrate reductase-deficient mutants with the nitrate reductase gene. Curr Microbiol 35(6):356–362
Tan C, Qin S, Zhang Q, Jiang P, Zhao F (2005) Establishment of a micro-particle bombardment transformation system for Dunaliella salina. J Microbiol 43(4):361–365
Feng S, Xue L, Liu H, Lu P (2009) Improvement of efficiency of genetic transformation for Dunaliella salina by glass beads method. Mol Biol Rep 36(6):1433–1439
Walker TL, Becker DK, Dale JL, Collet C (2005) Towards the development of a nuclear transformation system for Dunaliella tertiolecta. J Appl Phycol 17(4):363–368
Gong Y, Hu H, Gao Y, Xu X, Gao H (2011) Microalgae as platforms for production of recombinant proteins and valuable compounds: progress and prospects. J Ind Microbiol Biotechnol 38(12):1879–1890
Leon-Banares R, Gonzalez-Ballester D, Galvan A, Fernandez E (2004) Transgenic microalgae as green cell-factories. Trends Biotechnol 22(1):45–52
Potvin G, Zhang Z (2010) Strategies for high-level recombinant protein expression in transgenic microalgae: a review. Y. Le Gal, H. O. Halvorson, Publisher: Springer US, Biotechnol Adv 28(6):910–918
De Riso V, Raniello R, Maumus F, Rogato A, Bowler C, Falciatore A (2009) Gene silencing in the marine diatom Phaeodactylum tricornutum. Nucleic Acids Res 37(14):e96
Schroda M (2006) RNA silencing in Chlamydomonas: mechanisms and tools. Curr Genet 49(2):69–84
Sizova I, Greiner A, Awasthi M, Kateriya S, Hegemann P (2013) Nuclear gene targeting in Chlamydomonas using engineered zinc-finger nucleases. Plant J 73(5):873–882
Daboussi F, Leduc S, Marechal A, Dubois G, Guyot V, Perez-Michaut C, Amato A, Falciatore A, Juillerat A, Beurdeley M, Voytas DF, Cavarec L, Duchateau P (2014) Genome engineering empowers the diatom Phaeodactylum tricornutum for biotechnology. Nat Commun 5:3831
Weyman PD, Beeri K, Lefebvre SC, Rivera J, McCarthy JK, Heuberger AL, Peers G, Allen AE, Dupont CL (2014) Inactivation of phaeodactylum tricornutum urease gene using transcription activator-like effector nuclease-based targeted mutagenesis. Plant Biotechnol J 13(4):460-70
Fuhrmann M, Hausherr A, Ferbitz L, Schodl T, Heitzer M, Hegemann P (2004) Monitoring dynamic expression of nuclear genes in Chlamydomonas reinhardtii by using a synthetic luciferase reporter gene. Plant Mol Biol 55(6):869–881
Fuhrmann M, Oertel W, Hegemann P (1999) A synthetic gene coding for the green fluorescent protein (GFP) is a versatile reporter in Chlamydomonas reinhardtii. Plant J 19(3):353–361
Shao N, Bock R (2008) A codon-optimized luciferase from Gaussia princeps facilitates the in vivo monitoring of gene expression in the model alga Chlamydomonas reinhardtii. Curr Genet 53(6):381–388
Hempel F, Bozarth AS, Lindenkamp N, Klingl A, Zauner S, Linne U, Steinbuchel A, Maier UG (2011) Microalgae as bioreactors for bioplastic production. Microb Cell Factories 10:81
Mayfield SP, Schultz J (2004) Development of a luciferase reporter gene, luxCt, for Chlamydomonas reinhardtii chloroplast. Plant J 37(3):449–458
Gimpel JA, Hyun JS, Schoepp NG, Mayfield SP (2014) Production of recombinant proteins in microalgae at pilot greenhouse scale. Biotechnol Bioeng. doi:10.1002/bit.25357
Neupert J, Karcher D, Bock R (2009) Generation of Chlamydomonas strains that efficiently express nuclear transgenes. Plant J 57(6):1140–1150
Specht E, Miyake-Stoner S, Mayfield S (2010) Micro-algae come of age as a platform for recombinant protein production. Biotechnol Lett 32(10):1373–1383
Poulsen N, Kroger N (2005) A new molecular tool for transgenic diatoms: control of mRNA and protein biosynthesis by an inducible promoter-terminator cassette. FEBS J 272(13):3413–3423
Lumbreras V, Stevens DR, Purton S (1998) Efficient foreign gene expression in Chlamydomonas reinhardtii mediated by an endogenous intron. Plant J 14(4):441–447
Baiet B, Burel C, Saint-Jean B, Louvet R, Menu-Bouaouiche L, Kiefer-Meyer MC, Mathieu-Rivet E, Lefebvre T, Castel H, Carlier A, Cadoret JP, Lerouge P, Bardor M (2011) N-glycans of Phaeodactylum tricornutum diatom and functional characterization of its N-acetylglucosaminyltransferase I enzyme. J Biol Chem 286(8):6152–6164
Mathieu-Rivet E, Kiefer-Meyer MC, Vanier G, Ovide C, Burel C, Lerouge P, Bardor M (2014) Protein N-glycosylation in eukaryotic microalgae and its impact on the production of nuclear expressed biopharmaceuticals. Front Plant Sci 5:359
Mathieu-Rivet E, Scholz M, Arias C, Dardelle F, Schulze S, Le Mauff F, Teo G, Hochmal AK, Blanco-Rivero A, Loutelier-Bourhis C, Kiefer-Meyer MC, Fufezan C, Burel C, Lerouge P, Martinez F, Bardor M, Hippler M (2013) Exploring the N-glycosylation pathway in Chlamydomonas reinhardtii unravels novel complex structures. Mol Cell Proteomics 12(11):3160–3183
Kerrigan JJ, Xie Q, Ames RS, Lu Q (2011) Production of protein complexes via co-expression. Protein Expr Purif 75(1):1–14
Falciatore A, Casotti R, Leblanc C, Abrescia C, Bowler C (1999) Transformation of nonselectable reporter genes in marine diatoms. Mar Biotechnol (NY) 1(3):239–251
Rasala BA, Chao SS, Pier M, Barrera DJ, Mayfield SP (2014) Enhanced genetic tools for engineering multigene traits into green algae. PLoS One 9(4):e94028
Shi J, Podola B, Melkonian M (2007) Removal of nitrogen and phosphorus from wastewater using microalgae immobilized on twin layers: an experimental study. J Appl Phycol 19(5):417–423
Morris CA, Nicolaus B, Sampson V, Harwood JL, Kille P (1999) Identification and characterization of a recombinant metallothionein protein from a marine alga, Fucus vesiculosus. Biochem J 338(Pt 2):553–560
Rajamani S, Siripornadulsil S, Falcao V, Torres M, Colepicolo P, Sayre R (2007) Phycoremediation of heavy metals using transgenic microalgae. Adv Exp Med Biol 616:99–109
Cai X, Traina S, Sayre RT (1998) Heavy metal binding properties of wild type and transgenic algae (Chlamydomonas sp.). In: New Developments in Marine Biotechnology. Y. Le Gal, H. O. Halvorson: Springer US pp 189–192
Wang B, Lan CQ, Horsman M (2012) Closed photobioreactors for production of microalgal biomasses. Biotechnol Adv 30(4):904–912
Acknowledgments
We are grateful to Dr. Stefan Zauner (Marburg) for comments on the manuscript. This work was supported by the LOEWE program of the state of Hesse, Germany.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Hempel, F., Maier, U.G. (2016). Microalgae as Solar-Powered Protein Factories. In: Vega, M. (eds) Advanced Technologies for Protein Complex Production and Characterization. Advances in Experimental Medicine and Biology, vol 896. Springer, Cham. https://doi.org/10.1007/978-3-319-27216-0_16
Download citation
DOI: https://doi.org/10.1007/978-3-319-27216-0_16
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-27214-6
Online ISBN: 978-3-319-27216-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)