Abstract
Ca2+ entry pathways play important roles in control of many cellular functions, including long-term proliferation, migration and cell death. In recent years, it is becoming increasingly clear that, in some types of tumors, remodeling of Ca2+ entry pathways could contribute to cancer hallmarks such as excessive proliferation, cell migration and invasion as well as resistance to cell death or survival. In this chapter we briefly review findings related to remodeling of Ca2+ entry pathways in cancer with emphasis on the mechanisms that contribute to increased store-operated Ca2+ entry (SOCE) and store-operated currents (SOCs) in colorectal cancer cells. Finally, since SOCE appears critically involved in colon tumorogenesis, the inhibition of SOCE by aspirin and other NSAIDs and its possible contribution to colon cancer chemoprevention is reviewed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Intracellular Ca2+ Homeostasis and Ca2+ Entry Pathways
Intracellular Ca2+ is a very versatile second messenger involved in the control of many different physiological and cellular processes in the short and the long-term. Unlike other messengers, Ca2+ is not created or destroyed by cells, but transported down electrochemical gradients through specific channels, or transported back against gradients at the expense of the energy stored as ATP (Ca2+ pumps) or coupled with transport of other ions (Ca2+ exchangers). Ca2+ channels, pumps and exchangers are located in the plasma membrane and endomembranes of the endoplasmic reticulum (ER), mitochondria and other cell organelles. Unlike other ions, the cytosolic Ca2+ concentration ([Ca2+]cyt]) is so little (100 nM) that even minimal changes in channel activity may induce large increases in [Ca2+]cyt. Thus, this cation is unique in the sense that has been selected by nature to carry signals inside cells and organelles. Changes in [Ca2+]cyt may be restricted in time and space resulting in elementary events or Ca2+ microdomains that can regulate specifically located cellular functions such as exocytosis in plasma membrane, cell respiration and ATP synthesis in mitochondria or gene transcription in the nucleus. Alternatively, elementary events may give rise to regenerative waves leading to sustained, global changes associated to long-term events like cell growth, differentiation or death. Thus, the study of the intracellular Ca2+ homeostasis is a matter of ion transport across boundaries that requires the use of sophisticated methodologies for recording Ca2+-driven currents (patch-clamp) or measuring the tiny concentrations of Ca2+ in the cytosol or the very variable Ca2+ concentrations in organelles and/or subcellular environments using live cell imaging and targeted calcium probes with different affinities for Ca2+.
Ca2+ pumps and transporters contribute significantly to the maintenance of resting [Ca2+]cyt and to the recovery of basal [Ca2+]cyt after stimulation. However, most increases in [Ca2+]cyt, are rather due to activation of Ca2+ entry pathways at the plasma membrane and Ca2+ release channels at the ER. In the RE, IP3 receptors and ryanodine receptors are ligand-gated Ca2+ channels that mediate Ca2+ release from stores. Ca2+ release channels induce transient increases in [Ca2+]cyt but their activity may secondarily activate Ca2+ channels in plasma membrane that are gated by the filling state of Ca2+ stores. In mitochondria, the main Ca2+ channel is the so-called mitochondrial Ca2+ uniporter (MCU), a Ca2+-activated Ca2+ channel recently characterized at the molecular level [1, 2]. Activation of this channel in physiological conditions leads to mitochondrial Ca2+ uptake and removal of Ca2+ from cytosol. This is due to the fact that mitochondria inner membrane shows a strong mitochondrial potential, negative inside the mitochondrial matrix, thus favoring Ca2+ influx into mitochondria provided that cytosolic Ca2+ is large enough to activate the MCU [3, 4]. At the plasma membrane there are many different types of Ca2+ channels, including receptor-operated and voltage-operated Ca2+ channels that are widely expressed in excitable cells (ROCCs and VOCCs) together with voltage-independent channels that are particularly relevant in non-excitable cells. The most important Ca2+ entry pathway in non-excitable cells is the store-operated Ca2+ entry (SOCE), a Ca2+ entry pathway ubiquitous and responsible for the entry of Ca2+ after agonist-induced activation of phospholipase C and emptying of intracellular Ca2+ stores.
SOCE is activated physiologically after the emptying of the intracellular Ca2+ stores induced by physiological agonists producing IP3 or, pharmacologically, after inhibition of the sarcoplasmic and endoplasmic reticulum Ca2+ ATPase (SERCA) pump with thapsigargin or similar SERCA antagonists. SOCE usually remains active until Ca2+ stores become filled again. Interestingly, SOCE is also regulated by mitochondria. Mitochondrial control of SOCE is due to the ability of these organelles to take up Ca2+, thus preventing refilling of Ca2+ stores and preventing also the Ca2+-dependent inactivation of IP3 receptors and the own Ca2+ release activated channels responsible for SOCE. This mechanism ensures the efficient emptying of Ca2+ stores and SOCE activation and is believed to be critical to maintain Ca2+ entry in those signaling pathways in which a sustained activation is required. This is the case, for instance, of the activation of the nuclear factor of activated T (NFAT) cells during the immunological synapsis. In this case, a sustained entry of Ca2+ through SOCE is needed to promote IL2 gene expression and the clonal expansion of the activated T cell [5].
For years, the molecular basis of SOCE had remained elusive. However, the molecular players involved in SOCE began to be crack after the discovery of the TRP superfamily of ion channels. Some TRP channels were held during quite some time responsible for SOCE. However, more recently, the protein families STIM and ORAI were described to be the cornerstone of SOCE becoming now fully accepted as responsible for Icrac and SOCE in multiple cell types. At the molecular level, SOCE begins with the emptying of intracellular Ca2+ stores. Emptying, in this case, means that Ca2+ concentration inside the ER decreases from around 700 μM before stimulation to about 200 μM after agonist-induced Ca2+ release, as revealed by ER targeted probes with very low affinity for Ca2+ [6]. This “emptying” is detected by a sensor named Stromal Interaction Molecule 1 (STIM1) which, upon dissociation of Ca2+ ions from Ca2+ binding sites, undergoes oligomerization and its interaction with ORAI1, a Ca2+ channel located in specific places of the plasma membrane. This interaction opens Ca2+ specific CRAC channels, thus enabling the entry of Ca2+ into the cytosol. The whole mechanism is reversed when Ca2+ stores become filled again [7–9]. There is another Ca2+ sensor at the ER called STIM2, which has a lower affinity for Ca2+ and is believed to be activated only after moderate depletion of Ca2+ stores [10]. However, its role in SOCE remains controversial. In addition, two other ORAI family members (ORAI2 and ORAI3) may also be involved in SOCE but their role is also poorly known [11]. Finally, other types of channels, including some of the TRP superfamily, particularly TRPC channels may contribute to SOCE as well by forming channel complexes with STIM1 and ORAI1 or simply forming alternative store-operated channels less selective for Ca2+ [12, 13].
Other Ca2+ entry pathways also widely expressed are now collectively termed as store-independent Ca2+ entry (SICE) pathways. They are less known and most of them are characterized only at the functional level but not at the molecular level. They include channels gated by araquidonic acid, diacylglycerol (DAG) and stretch activated channels. Most of these channels may well be mediated by TRP channels and/or channel complexes made thereof. Other chapters in this issue describe in more detail characteristics and molecular basis related to store-independent Ca2+ entry pathways.
2 Ca2+ Entry Remodeling in Cancer
A series of recent reports suggest that changes in intracellular Ca2+ homeostasis (remodeling) may be critically involved in various forms of cancer. This is not surprising if we consider that the so many cellular processes exacerbated in the transformed cell such as excessive cell proliferation, migration and invasion capabilities as well as apoptosis resistance and cell survival are regulated by intracellular Ca2+ [14–16]. A Ca2+ entry pathway that has been implicated in cancer is SOCE [17, 18] which could offer new therapeutic possibilities against cancer as suggested earlier [19]. However, some other Ca2+ entry pathways and channels have been also related to tumorogenesis. For example, it has been reported that voltage-gated Ca2+ channels (VOCCs) are overexpressed in various types of tumors (Table 19.1).
Thus, epithelial tumors express L-type [21] and T type VOCCs and their inhibition may prevent cell proliferation [24]. It is not clear the role of VOCCs in epithelial cells or even if they may work as channels. For example, plasma depolarization with medium containing a high concentration of K+, a typical experimental maneuver intended to activate voltage-gated Ca2+ entry in Ca2+ imaging experiments in excitable cells [25], has no effect in epithelial cells, either normal or tumoral. Accordingly, increased expression of VOCCs in cancer cells may not be related to intracellular Ca2+ homeostasis. Expression of other Ca2+ channels of the TRP superfamily, particularly canonical TRP channels not so selective for Ca2+, has been reported as well to be altered in different tumors (Table 19.2). Other channels of the same superfamily have been also reported to be either overexpressed or downregulated in different tumors, particularly TRPV4, TRPV6 and TRPM8 in carcinomas and other tumors [34, 37, 38]. The role of these channels in cancer remains unclear but recent evidence suggests a role for some of these channels in cancer hallmarks. For instance, it has been shown that the calcium selective channel TRPV6 is able to translocate to the plasma membrane via Orai1-mediated mechanism controlling cancer cell survival [39]. This expanding issue is being covered by excellent recent reviews [40]. See Table 19.3 for further details on changes in the expression of these channels in different tumors.
More recently, it has proposed an essential role for SOCE and/or its molecular players in tumorigenesis. For example, STIM1 and Orai1 may be critical for migration of breast cancer cells and metastasis [27]. STIM1 plays an important role in cell growth and migration in cervical cancer [54]. Indeed STIM1 is overexpressed in 70 % of cervical cancers, which has been associated with an increased risk of metastasis. As a matter of fact, the suppression of STIM1 inhibits human glioblastoma cell proliferation and induces G0/G1 phase arrest [55]. Moreoever, STIM1 and Orai1 mediate CRAC channel activity and are essential for human glioblastoma invasion [56]. Orai1 could be also overexpressed in breast cancer [57]. The entry of Ca2+ mediated by Orai1 regulates also proliferation and survival in glioblastoma cells and hepatoma [34].
The role of other molecular players involved in SOCE in tumorogenesis is unknown except for the case of Orai3 that has been proposed to be an estrogen receptor α-regulated Ca2+ channel that promotes tumorigenesis [58]. Likewise, Orai3 has been also reported to constitute a native SOCE regulating non-small cell lung adenocarcinoma cell proliferation [59]. Therefore, multiple recent evidence suggests an unexpected role for intracellular Ca2+ remodeling, particularly SOCE in cancer [15, 16]. See Table 19.4 for further details on changes in expression of molecular players involved in SOCE in different forms of cancer.
3 Ca2+ Entry Pathways in Normal and Colon Cancer Cells
We have recently reported a deep remodeling of SOCE in colorectal cancer [69]. For investigating Ca2+ remodeling in colon cancer we have compared Ca2+ entry pathways in a series of normal human colonic mucosa cell lines and human colon adenocarcinoma cells. We found that SOCE is significantly larger in colorectal cancer cells than in normal cells [69]. Both normal and tumor cells differed also in their rate of cell proliferation with tumor cells showing always larger rates of cell proliferation. Interestingly, there was a clear correlation between SOCE and the rate of cell proliferation suggesting that the larger rate of cell proliferation of tumor cells follows changes in SOCE [69]. Consistently, SOCE inhibition with antagonists prevents not only tumor cell proliferation but also cell invasion as tested by Matrigel invasion assays. Therefore, enhancement of SOCE in colon cancer cells may contribute not only to increased cell proliferation characteristic of tumor cells but also to cell invasion, both being critical hallmarks of cancer [69].
Differences between normal and tumor cells regarding Ca2+ entry and Ca2+ release induced by physiological agonists were also studied in detail. ATP and Carbachol, two physiological agonists that activate G protein-coupled receptors and phospholipase C, increase [Ca2+]cyt in both normal and tumor cells. However, the rises in [Ca2+]cyt are much larger in tumor cells compared with normal colonic epithelium cells. Analysis in Ca2+ free medium revealed that the two agonists released more Ca2+ in tumor cells than in normal cells. Results on agonist-induced Ca2+ entry were surprising: In fact, both agonists induced Ca2+ entry only in tumor cells but not in normal cells, despite that both released Ca2+ from intracellular stores. To avoid contribution of possible differences in expression of GPCRs in normal and tumor cells, experiments were carried out using caged IP3. Again, the IP3-induced increase of [Ca2+]cyt was larger in tumor cells than normal cells [69]. However, these results could be due to either differences in the level of expression and/or activity of IP3 receptors and/or to differences in the extent of Ca2+ store content. Experiments using ionomycin or cyclopiazonic acid in Ca2+ free medium revealed that Ca2+ stores were paradoxically larger in normal cells than in tumor cells. In other words, Ca2+ stores in tumor cells are partially empty. These data together with the data derived form Ca2+ release experiments, always much larger in tumor cells, suggest that in tumor cells Ca2+ stores are nearly depleted and any minimal stimulation is sufficient to reach the threshold for SOCE activation. However, in normal cells, where Ca2+ stores are full and physiological stimulation releases only a limited amount of Ca2+, SOCE threshold is beyond reach leading to no Ca2+ entry. These data may explain why agonists do activate Ca2+ entry only in tumor cells but not in normal mucosa cells [69]. Of course, we have to take into account that these data derive from the analysis of a few, non isogenic cell lines serving as models of normal and tumor colon cells. Results must be confirmed in additional normal and tumor samples derived from the same specimen.
4 Store-Operated Currents in Normal and Colon Cancer Cells
To identify functional and pharmacologically SOCs in normal and tumor cells we have used planar patch-clamp in the voltage clamp configuration [69]. The depletion of Ca2+ stores with thapsigargin in normal colonocytes induces a small, voltage-independent, inward rectifying current that is highly selective for Ca2+ and sensitive to La3+ and to low concentrations of 2-APB [69]. Therefore, this current is very similar, if not identical, to the Icrac current originally described as responsible for SOCE in mast and T cells. In tumor cells, however, SOCs are very different. Emptying of Ca2+ stores induces two types of currents: First, a current similar to Icrac recorded in normal cells with the only exception that current density is larger in tumor cells. Second, we observe a different Isoc, absent in normal cells, with a large outward component, not selective for Ca2+ and sensitive to high concentrations of 2-APB [69]. The data suggest that SOCE in normal cells is mediated by Orai1 and STIM1, the molecular players previously reported to be involved in Icrac in mast and T cells. However, tumor cells would have two streams, one should be Icrac but with larger current density probably mediated by changes in expression of Orai1 and Stim1. The other current could be mediated by a TRP channel. Accordingly, data suggest that store-operated currents may be mediated by different channels in normal and tumor cells.
5 Molecular Basis of Remodeling of Ca2+ Entry Pathways in Colorectal Cancer
Molecular candidates involved in SOCE and SOCs in normal and colon cancer cells have been investigated using conventional and quantitative RT-PCR. All members of the STIM and Orai families (Orai1, 2, 3 and STIM1 and 2) are expressed in normal and tumor cells. Other TRPC channels including TRPC1 and TRPC4 channels are expressed as well in both normal and tumor cells. However, other genes that have been related to SOCE are expressed only in normal cells but not in tumor cells including TRPV6 and TRPM8 suggesting a loss of function during tumorogenesis. Finally, some other related channels are missing in both cell types. Quantitative RT-PCR and Western blotting show significant increases in the expression of many of the above genes. Interestingly, the expression pattern of the different molecular players involved in SOCE and SOCs is roughly similar in normal and tumor cells except that most genes are rather increased in tumor cells relative to normal cells. At the protein level, Orai1, Orai2, and Orai3 proteins are increased significantly in tumor cells. Similar results are obtained with TRPC1 and STIM1. Surprisingly, although STIM2 gene expression is increased in tumor cells, STIM2 protein is nearly lost in tumor cells [69].
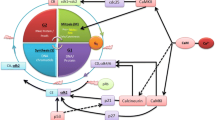
Using siRNA against each of these specific molecular players, we have established that SOCE and SOCs in normal cells are mediated by the interactions between ORAI1, STIM1 and STIM2. However, in tumor cells, SOCs and SOCE are more complex and include not only STIM1 and Orai1 but also TRPC1. The increased expression of both may contribute to explain the increase in SOCE and agonist-induced Ca2+ observed in tumor cells relative to normal cells (Fig. 19.1).
Ca 2+ entry remodeling in colorectal cancer. Normal human colon cells (above) show a small SOCE mediated Orai1 and STIM1 and a large Ca2+ store content associated with the large expression of STIM2. The cells also express TRPC1, TRPC4, TRPC7, TRPV6 and TRPM8. Cells of human colon adenocarcinoma (below) present large and modified SOCE mediated by increases in expression of Orai1, TRPC1 and STIM1, and partial depletion tank contents mediated decreased expression of STIM2. These cells lack TRPC7, TRPV6 and TRPM8. Remodeling may contribute to increased proliferative capacity, invasion and survival of tumor cells [69]
Therefore, SOCs in colon carcinoma cells are made of different molecular players than in colon normal mucosa cells. Differences involve likely changes in channel complexes including the appearance of TRPC1 and the disappearance of STIM2 from those complexes. This view is supported by silencing experiments. TRPC1 silencing has no influence on Icrac in normal cells but it decreases not only the outward component associated to the non-selective current present only in tumor cells but also the inward component. Moreover, ORAI1 silencing decreases Icrac in normal cells and both components inward and outward in tumor cells. Thus, these data suggest strongly that ORAI1 and TRPC1 form a channel complex in tumor cells but not in normal cells. The data invite speculation as to what is the role played by TRPC1 in SOCE. Silencing experiments reveal that TRPC1 knockdown does not decrease SOCE induced by thapsigargin in tumor cells. Therefore, TRPC1 might play roles different from supporting Ca2+ entry in tumor cells. Since this channel permeates mostly Na+, a possible role could be to depolarize plasma membrane and limit Ca2+ entry. Alternatively, TRPC1 may be involved in changes in cell volume related to cell cycle.
What is the biological significance of STIM2 loss in tumor cells? STIM2 is a Ca2+ sensor inside the ER with low affinity for Ca2+. This means that STIM2 should sense Ca2+ concentrations inside the ER when Ca2+ stores are filled at around >500 μM. A little decrease below this value should activate STIM2 and likely SOCE to keep the store filled. Consistently with this view, STIM2 knockout in normal cells decreases SOCE. In tumor cells, the loss of STIM2, leaves STIM1 in charge of sensing and refilling the stores. However, STIM1 is a different sensor with lower affinity for Ca2+ than STIM2 (Kd values around 300 μM). In this scenario, in which STIM1 would became activated only if stores are substantially depleted, the loss of STIM2 should lead to a partial depletion of Ca2+ stores (Fig. 19.2).
Loss of Stim2 in tumor cells decreases Ca 2+ store content in tumor cells. Normal cells express both Stim1 and Stim2, ER Ca2+ sensors with different affinities for Ca2+. Loss of Stim2 in colon cancer cells leaves Stim1 as the only sensor available that refills Ca2+ stores poorly leading to partially depleted stores in tumor cells
The above possibility was tested experimentally using siRNA against STIM2 in normal cells [69]. The decrease in STIM2 expression leads to the partial depletion of Ca2+ stores suggesting that STIM2 loss in tumor cells may contribute to Ca2+ remodeling by modifying Ca2+ store content. This partial depletion may have two relevant functional consequences. First, it may move Ca2+ store content close to the threshold for SOCE activation. Second, as Ca2+ store content has been related to the intrinsic pathway for cell death, loss of STIM2 in tumor cells may favor resistance to cell death and cell survival. Consistently, STIM2 silencing in normal cells decreases Ca2+ store content and increases resistance to apoptosis induced by H2O2 [69]. Therefore, the reciprocal shift in STIM1/STIM2 observed in colon cancer cells may be critical in colorectal tumorogenesis. Consistently with this view, it has been recently reported that STIM1 overexpression promotes colorectal cancer progression, cell motility and COX-2 expression [36].
In summary, colon cancer cells display enhanced and modified store and agonist-induced Ca2+ entry together with enhanced agonist-induced Ca2+ release and decreased Ca2+ store content. These differences are likely mediated by reciprocal changes in the expression of ORAI1, STIM1, TRPC1 and STIM2. These changes contribute to increased cell proliferation, invasion and survival. Therefore, although the above results must be confirmed in samples from colorectal cancer patients, the data strongly suggest a critical role for changes in SOCE and SOCs in colon cancer tumorigenesis. In fact, with the logical limitations on the role of SOCE in the immune response against tumors, SOCE antagonists could be considered for colon cancer. Previous results are consistent with this view. For example, it has been extensively documented in in vitro assays, animal testing and even clinical trials with high risk patients that aspirin may efficiently prevent colon cancer. Interestingly, our previous results clearly showed that the main aspirin metabolite salicylate inhibits SOCE off site and colon cancer cell growth in a mitochondria-dependent manner suggesting that aspirin may prevent colon cancer acting on SOCE [70].
6 Aspirin Prevents Cancer
A large series of epidemiological evidences suggest that aspirin and other non-steroidal anti-inflammatory drugs (NSAIDs) prevent colorectal cancer and other forms of cancer including breast cancer [71]. A recent meta-analysis showed that NSAIDs decreased the frequency of adenomas, colorectal cancer and deaths related to colorectal cancer in 57 out of the 59 studies carried out between 1988 and 2006 [72]. Yet, the lack of clinical trials and the risk of secondary effects associated to chronic NSAID use prevented recommendation of aspirin. Recently, a few clinical trials have been completed in high risk patients including Lynch syndrome patients, a type familial colorectal cancer with 100 % chances of developing colorectal cancer. Aspirin protected largely (63 %) against cancer in these high risk patients which has resulted in recommendation of aspirin for high risk patients of colorectal cancer [73]. Ongoing clinical trials suggest that combinations of aspirin or other NSAIDs with additional chemopreventive compounds may be highly efficient in preventing polyp formation and cancer death in patients that had undergone surgery for tumor removal. These are high risk patients with a 50 % chance of recurrence and death. Similar trials are underway in other forms of familial cancer including breast cancer associated to driving mutations in BRCA1 and 2.
Basic studies carried out in cell lines and animal models of cancer indicate that aspirin and other NSAIDs inhibit tumor cell proliferation and growth, cell migration and invasion and tumor growth in animal models of cancer. Interest on the action mechanism is growing since the realization that a large part of the effects are largely independent of the anti-inflammatory activity of these drugs. This view is based in that antitumor activity remains in tumor cells lacking expression of COX, the classic target of anti-inflammatory compounds. Moreover, structural analogues like R-flurbiprofen that lack ant-inflammatory activity are also efficient in preventing tumor cell growth. Therefore, even though COX-mediated synthesis or prostanoids may contribute to inflammation and colon tumorogenesis and NSAIDs may act partially by preventing inflammation, other targets of aspirin and other NSAIDs are likely involved in the antitumor actions of these drugs. We and others have shown previously that the aspirin metabolite and other NSAIDs may inhibit SOCs and SOCE in colon cancer cells likely providing a candidate mechanism for cancer chemoprevention by these compounds and a novel target for cancer chemoprevention.
7 Aspirin and Other NSAIDs Inhibit SOCE
NSAIDs inhibit SOCE and colon cancer cell proliferation [74]. However, no mechanism of action was provided. A few years ago, our group proposed that the main metabolite of aspirin, salicylate, could prevent tumor cell growth by inhibiting SOCE off site in a mitochondria-dependent manner [70, 75]. Salicylate is a mild mitochondrial uncoupler. This is due to the fact that the negative charge of the carboxylic residue is delocalized throughout the aromatic ring of salicylate. Thus, salicylic acid is neutral and capable of entering the cell and the mitochondrial matrix down a chemical gradient without restriction. However, once inside mitochondria, the matrix pH favors salicylic acid dissociation leading to salicylate formation that can exit the matrix favored by the negative mitochondrial potential. The net result is the release of protons inside mitochondria and mitochondrial uncoupling, partially depolarizing mitochondria and limiting the electromotive force for mitochondrial Ca2+ uptake [70]. Inasmuch as SOCE is strongly regulated by mitochondria, salicylate effect promotes the Ca2+ -dependent inactivation of Icrac and inhibition of SOCE. Since a sustained SOCE pathway is required for cell proliferation, the result is that salicylate inhibits cell proliferation via inactivation of a cancer-relevant Ca2+ channel. This mechanism may contribute to explain the antiproliferative effects of aspirin on T lymphocytes and vascular smooth muscle cells [70, 76, 77]. Interestingly, it has been recently reported that STIM1 overexpression promotes colorectal cancer progression, cell motility and COX-2 expression [36]. Accordingly, the effects of NSAIDs on SOCE may inhibit also colorectal cancer progression by preventing SOCE-mediated expression of COX-2.
References
Baughman JM, Perocchi F, Girgis HS, Plovanich M, Belcher-Timme CA, Sancak Y, Bao XR, Strittmatter L, Goldberger O, Bogorad RL, Koteliansky V, Mootha VK (2011) Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature 476:341–345
De Stefani D, Raffaello A, Teardo E, Szabò I, Rizzuto R (2011) A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature 476:336–340
Montero M, Alonso MT, Carnicero E, Cuchillo-Ibáñez I, Albillos A, García AG, García-Sancho J, Alvarez J (2000) Chromaffin-cell stimulation triggers fast millimolar mitochondrial Ca2+ transients that modulate secretion. Nat Cell Biol 2(2):57–61
Villalobos C, Núñez L, Montero M, García AG, Alonso MT, Chamero P, Alvarez J, García-Sancho J (2002) Redistribution of Ca2+ among cytosol and organella during stimulation of bovine chromaffin cells. FASEB J 16(3):343–353
Quintana A, Pasche M, Junker C, Al-Ansary D, Rieger H, Kummerow C, Nuñez L, Villalobos C, Meraner P, Becherer U, Rettig J, Niemeyer BA, Hoth M (2011) Calcium microdomains at the immunological synapse: how ORAI channels, mitochondria and calcium pumps generate local calcium signals for efficient T-cell activation. EMBO J 30:3895–3912
Alvarez J, Montero M (2002) Measuring [Ca2+] in the endoplasmic reticulum with aequorin. Cell Calcium 32(5–6):251–260
Hewavitharana T, Deng X, Soboloff J, Gill DL (2007) Role of STIM and Orai proteins in the store-operated calcium signaling pathway. Cell Calcium 42:173–182
Putney JW (2009) Capacitative calcium entry: from concept to molecules. Immunol Rev 231:10–22
Muik M, Schindl R, Fahrner M, Romanin C (2012) Ca2+ release-activated Ca2+ (CRAC) current, structure, and function. Cell Mol Life Sci 69:4163–4176
Brandman O, Liou J, Park WS, Meyer T (2007) STIM2 is a feedback regulator that stabilizes basal cytosolic and endoplasmic reticulum Ca2+ levels. Cell 131:1327–1339
Hoth M, Niemeyer BA (2013) The neglected CRAC proteins: Orai2, Orai3, and STIM2. Curr Top Membr 1:237–271
Ma X, Nilius B, Wong JW, Huang Y, Yao X (2011) Electrophysiological properties of heteromeric TRPV4-C1 channels. Biochim Biophys Acta 1808:2789–2797
Cheng KT, Ong HL, Liu X, Ambudkar IS (2013) Contribution and regulation of TRPC channels in store-operated Ca2+ entry. Curr Top Membr 71:149–179
Monteith GR, Davis FM, Roberts-Thomson SJ (2012) Calcium channels and pumps in cancer: changes and consequences. J Biol Chem 287:31666–31673
Prevarskaya N, Ouadid-Ahidouch H, Skryma R, Shuba Y (2014) Remodelling of Ca2+ transport in cancer: how it contributes to cancer hallmarks? Philos Trans R Soc Lond B Biol Sci 369:20130097
Prevarskaya N, Skryma R, Shuba Y (2011) Calcium in tumor metastasis: new roles for known actors. Nat Rev Cancer 11:609–618
Bergmeier W, Weidinger C, Zee I, Feske S (2013) Emerging roles of store-operated Ca2+ entry through STIM and ORAI proteins in immunity, hemostasis and cancer. Channels 7:379–391
Stewart TA, Yapa KT, Monteith GR (2015) Altered calcium signaling in cancer cells. Biochim Biophys Acta 1848 (10PtB):2502–11
Roderick HL, Cook SJ (2008) Ca2+ signalling checkpoints in cancer: remodelling Ca2+ for cell proliferation and survival. Nat Cancer Rev 8:361–375
Zhang L, Zhou W, Velculescu VE, Kern SE, Hruban RH, Hamilton SR, Vogelstein B, Kinzler KW (1997) Gene expression profiles in normal and cancer cells. Science 276:1268–1272
Wang JY, Sun J, Huang MY, Wang YS, Hou MF, Sun Y, He H, Krishna N, Chiu SJ, Lin S, Yang S, Chang WC. STIM1 overexpression promotes colorectal cancer progression, cell motility and COX-2 expression. Oncogene Nov 10. Wang XT, Nagaba Y, Cross HS, Wrba F, Zhang L, Guggino SE (2000) The mRNA of L-type calcium channel elevated in colon cancer: protein distribution in normal and cancerous colon. Am J Pathol 157:1549–1562
Toyota M, Ho C, Ohe-Toyota M, Baylin SB, Issa JP (1999) Inactivation of CACNA1G, a T-type calcium channel gene, by aberrant methylation of its 5′ CpG island in human tumors. Cancer Res 59:4535–4541
Gackiere F, Bidaux G, Delcourt P, Van Coppenolle F, Katsogiannou M, Dewailly E, Bavencoffe A, Van Chuoi-Mariot MT, Mauroy B, Prevarskaya N, Mariot P (2008) Cav3.2 T-type calcium channels are involved in calcium-dependent secretion of neuroendocrine prostate cancer cells. J Biol Chem 283:10162–10173
Taylor JT, Zeng XB, Pottle JE, Lee K, Wang AR, Yi SG, Scruggs JA, Sikka SS, Li M (2008) Calcium signaling and T-type calcium channels in cancer cell cycling. World J Gastroenterol 14:4984–4991
Villalobos C, Fonteriz R, López MG, García AG, García-Sancho J (1992) Inhibition of voltage-gated Ca2+ entry into GH3 and chromaffin cells by imidazole antimycotics and other cytochrome P450 blockers. FASEB J 6(9):2742–2747
Dhennin-Duthille I, Gautier M, Faouzi M, Guilbert A, Brevet M, Vaudry D, Ahidouch A, Sevestre H, Ouadid-Ahidouch H (2011) High expression of transient receptor potential channels in human breast cancer epithelial cells and tissues: correlation with pathological parameters. Cell Physiol Biochem 28:813–822
Yang S, Zhang JJ, Huang XY (2009) Orai1 and STIM1 are critical for breast tumor cell migration and metastasis. Cancer Cell 5:124–134
Aydar E, Yeo S, Djamgoz M, Palmer C (2009) Abnormal expression, localization and interaction of canonical transient receptor potential ion channels in human breast cancer cell lines and tissues: a potential target for breast cancer diagnosis and therapy. Cancer Cell Int 9:23
Veliceasa D, Ivanovic M, Hoepfner FT, Thumbikat P, Volpert OV, Smith ND (2007) Transient potential receptor channel 4 controls thrombospondin-1 secretion and angiogenesis in renal cell carcinoma. FEBS J 274:6365–6377
Shi Y, Ding X, He ZH, Zhou KC, Wang Q, Wang YZ (2009) Critical role of TRPC6 channels in G2 phase transition and the development of human oesophageal cancer. Gut 58:1443–1450
Zhang SS, Wen J, Yang F, Cai XL, Yang H, Luo KJ, Liu QW, Hu RG, Xie X, Huang QY, Chen JY, Fu JH, Hu Y (2013) High expression of transient potential receptor C6 correlated with poor prognosis in patients with esophageal squamous cell carcinoma. Med Oncol 30:607
Cai R, Ding X, Zhou K, Shi Y, Ge R, Ren G, Jin Y, Wang Y (2009) Blockade of TRPC6 channels induced G2/M phase arrest and suppressed growth in human gastric cancer cells. Int J Cancer 125:2281–2287
Ding X, He Z, Zhou K, Cheng J, Yao H, Lu D, Cai R, Jin Y, Dong B, Xu Y, Wang Y (2010) Essential role of TRPC6 channels in G2/M phase transition and development of human glioma. J Natl Cancer Inst 102:1052–1068
El Boustany C, Bidaux G, Enfissi A, Delcourt P, Prevarskaya N, Capiod T (2008) Capacitative calcium entry and transient receptor potential canonical 6 expression control human hepatoma cell proliferation. Hepatology 47:2068–2077
Guilbert A, Dhennin-Duthille I, Hiani YE, Haren N, Khorsi H, Sevestre H, Ahidouch A, Ouadid-Ahidouch H (2008) Expression of TRPC6 channels in human epithelial breast cancer cells. BMC Cancer 8:125
Wang JY, Sun J, Huang MY, Wang YS, Hou MF, Sun Y, He H, Krishna N, Chiu SJ, Lin S, Yang S, Chang WC (2015) STIM1 overexpression promotes colorectal cancer progression, cell motility and COX-2 expression. Oncogene 34(33):4358–4367
Zhuang L, Peng JB, Tou L, Takanaga H, Adam RM, Hediger MA, Freeman MR (2002) Calcium-selective ion channel, CaT1, is apically localized in gastrointestinal tract epithelia and is aberrantly expressed in human malignancies. Lab Invest 82:1755–1764
Capiod T, Shuba Y, Skryma R, Prevarskaya N (2007) Calcium signaling and cancer cell growth. Subcell Biochem 45:405–427
Raphaël M, Lehen’kyi V, Vandenberghe M, Beck B, Khalimonchyk S, Vanden Abeele F, Farsetti L, Germain E, Bokhobza A, Mihalache A, Gosset P, Romanin C, Clézardin P, Skryma R, Prevarskaya N (2014) TRPV6 calcium channel translocates to the plasma membrane via Orai1-mediated mechanism and controls cancer cell survival. Proc Natl Acad Sci U S A 111(37):E3870–E3879
Déliot N, Constantin B (2015) Plasma membrane calcium channels in cancer: alterations and consequences for cell proliferation and migration. Biochim Biophys Acta 1848:2512–2522
Fusi C, Materazzi S, Minocci D, Maio V, Oranges T, Massi D, Nassini R (2014) Transient receptor potential vanilloid 4 (TRPV4) is downregulated in keratinocytes in human non-melanoma skin cancer. J Invest Dermatol 134:2408–2417
Mizuno H, Suzuki Y, Watanabe M, Sokabe T, Yamamoto T, Hattori R, Gotoh M, Tominaga M (2014) Potential role of transient receptor potential (TRP) channels in bladder cancer cells. J Physiol Sci 64(4):305–314
Bolanz KA, Hediger MA, Landowski CP (2008) The role of TRPV6 in breast carcinogenesis. Mol Cancer Ther 7:271–279
Peng JB, Zhuang L, Berger UV, Adam RM, Williams BJ, Brown EM, Hediger MA, Freeman MR (2001) CaT1 expression correlates with tumor grade in prostate cancer. Biochem Biophys Res Commun 282:729–734
Wissenbach U, Niemeyer BA, Fixemer T, Schneidewind A, Trost C, Cavalie A, Reus K, Meese E, Bonkhoff H, Flockerzi V (2001) Expression of CaT-like, a novel calcium-selective channel, correlates with the malignancy of prostate cancer. J Biol Chem 276(22):19461–19468
Fan H, Shen YX, Yuan YF (2014) Expression and prognostic roles of TRPV5 and TRPV6 in non-small cell lung cancer after curative resection. Asian Pac J Cancer Prev 15:2559–2563
Yee NS, Zhou W, Lee M (2010) Transient receptor potential channel TRPM8 is over-expressed and required for cellular proliferation in pancreatic adenocarcinoma. Cancer Lett 297:49–55
Tsavaler L, Shapero MH, Morkowski S, Laus R (2001) Trp-p8, a novel prostate-specific gene, is up-regulated in prostate cancer and other malignancies and shares high homology with transient receptor potential calcium channel proteins. Cancer Res 61:3760–3769
Fuessel S, Sickert D, Meye A, Klenk U, Schmidt U, Schmitz M, Rost AK, Weigle B, Kiessling A, Wirth MP (2003) Multiple tumor marker analyses (PSA, hK2, PSCA, trp-p8) in primary prostate cancers using quantitative RT-PCR. Int J Oncol 23:221–228
Schmidt U, Fuessel S, Koch R, Baretton GB, Lohse A, Tomasetti S, Unversucht S, Froehner M, Wirth MP, Meye A (2006) Quantitative multi-gene expression profiling of primary prostate cancer. Prostate 66(14):1521–1534
Prevarskaya N, Flourakis M, Bidaux G, Thebault S, Skryma R (2007) Differential role of TRP channels in prostate cancer. Biochem Soc Trans 35(Pt 1):133–135
Chodon D, Guilbert A, Dhennin-Duthille I, Gautier M, Telliez MS, Sevestre H, Ouadid-Ahidouch H (2010) Estrogen regulation of TRPM8 expression in breast cancer cells. BMC Cancer 10:212
Liu J, Chen Y, Shuai S, Ding D, Li R, Luo R (2014) TRPM8 promotes aggressiveness of breast cancer cells by regulating EMT via activating AKT/GSK-3β pathway. Tumour Biol 35(9):8969–8977
Chen YF, Chiu WT, Chen YT, Lin PY, Huang HJ, Chou CY, Chang HC, Tang MJ, Shen MR (2011) Calcium store sensor stromal-interaction molecule1-dependent signaling plays an important role in cervical cancer growth, migration and angiogenesis. Proc Natl Acad Sci U S A 108:15225–15230
Li G, Zhang Z, Wang R, Ma W, Yang Y, Wei J, Wei Y (2013) Suppression of STIM1 inhibits human glioblastoma cell proliferation and induces G0/G1 phase arrest. J Exp Clin Cancer Res 32:20
Motiani RK, Hyzinski-García MC, Zhang X, Henkel MM, Abdullaev IF, Kuo YH, Matrougui K, Mongin AA, Trebak M (2013) STIM1 and Orai1 mediate CRAC channel activity and are essential for human glioblastoma invasion. Pflugers Arch 465(9):1249–1260
McAndrew D, Grice DM, Peters AA, Davis FM, Stewart T, Rice M, Smart CE, Brown MA, Kenny PA, Roberts-Thomson SJ, Monteith GR (2011) ORAI1-mediated calcium influx in lactation and in breast cancer. Mol Cancer Ther 10:448–460
Motiani RK, Zhang X, Harmon KE, Keller RS, Matrougui K, Bennett JA, Trebak M (2013) Orai3 is an estrogen receptor α-regulated Ca2+ channel that promotes tumorigenesis. FASEB J 27(1):63–75
Ay AS, Benzerdjeb N, Sevestre H, Ahidouch A, Ouadid-Ahidouch H (2013) Orai3 constitutes a native store-operated calcium entry that regulates non small cell lung adenocarcinoma cell proliferation. PLoS One 8(9):e72889
Zhu M, Chen L, Zhao P, Zhou H, Zhang C, Yu S, Lin Y, Yang X (2014) Store-operated Ca2+ entry regulates glioma cell migration and invasion via modulation of Pyk2 phosphorylation. J Exp Clin Cancer Res 33:98
Umemura M, Baljinnyam E, Feske S, De Lorenzo MS, Xie LH, Feng X, Oda K, Makino A, Fujita T, Yokoyama U, Iwatsubo M, Chen S, Goydos JS, Ishikawa Y, Iwatsubo K (2014) Store-operated Ca2+ entry (SOCE) regulates melanoma proliferation and cell migration. PLoS One 9:e89292
Kim JH, Lkhagvadorj S, Lee MR, Hwang KH, Chung HC, Jung JH, Cha SK, Eom M (2014) Orai1 and STIM1 are critical for cell migration and proliferation of clear cell renal cell carcinoma. Biochem Biophys Res Commun 448:76–82
Faouzi M, Hague F, Potier M, Ahidouch A, Sevestre H, Ouadid-Ahidouch H (2011) Down-regulation of Orai3 arrests cell-cycle progression and induces apoptosis in breast cancer cells but not in normal breast epithelial cells. J Cell Physiol 226:542–551
Holzmann C, Kilch T, Kappel S, Armbruster A, Jung V, Stockle M, Bogeski I, Schwarz EC, Peinelt C (2013) ICRAC controls the rapid androgen response in human primary prostate epithelial cells and is altered in prostate cancer. Oncotarget 4:2096–2107
Dubois C, Vanden Abeele F, Lehen’kyi V, Gkika D, Guarmit B, Lepage G, Slomianny C, Borowiec AS, Bidaux G, Benahmed M, Shuba Y, Prevarskaya N (2014) Remodeling of channel-forming ORAI proteins determines an oncogenic switch in prostate cancer. Cancer Cell 26:19–32
Scrideli CA, Carlotti CG Jr, Okamoto OK, Andrade VS, Cortez MA, Motta FJ, Lucio-Eterovic AK, Neder L, Rosemberg S, Oba-Shinjo SM, Marie SK, Tone LG (2008) Gene expression profile analysis of primary glioblastomas and non-neoplastic brain tissue: identification of potential target genes by oligonucleotide microarray and real-time quantitative PCR. J Neurooncol 88:281–291
Ruano Y, Mollejo M, Ribalta T, Fiano C, Camacho FI, Gomez E, de Lope AR, Hernandez-Moneo JL, Martinez P, Melendez B (2006) Identification of novel candidate target genes in amplicons of glioblastoma multiforme tumors detected by expression and CGH microarray profiling. Mol Cancer 5:39
Aytes A, Mollevi DG, Martinez-Iniesta M, Nadal M, Vidal A, Morales A, Salazar R, Capella G, Villanueva A (2012) Stromal interaction molecule 2 (STIM2) is frequently overexpressed in colorectal tumors and confers a tumor cell growth suppressor phenotype. Mol Carcinog 51:746–753
Sobradillo D, Hernández-Morales M, Ubierna D, Moyer MP, Núñez L, Villalobos C (2014) A reciprocal shift in transient receptor potential channel 1 (TRPC1) and stromal interaction molecule 2 (STIM2) contributes to Ca2+ remodeling and cancer hallmarks in colorectal carcinoma cells. J Biol Chem 289:28765–28782
Núñez L, Valero RA, Senovilla L, Sanz-Blasco S, García-Sancho J, Villalobos C (2006) Cell proliferation depends on mitochondrial Ca2+ uptake: inhibition by salicylate. J Physiol (Lond) 571:57–73
Rothwell PM, Fowkes FG, Belch JF, Ogawa H, Warlow CP, Meade TW (2011) Effect of daily aspirin on long-term risk of death due to cancer: analysis of individual patient data from randomized trials. Lancet 377:31–41
Arber N, Levin B (2008) Chemoprevention of colorectal neoplasia: the potential for personalized medicine. Gastroenterology 134(4):1224–1237
Burn J, Mathers JC, Bishop DT (2013) Chemoprevention in Lynch syndrome. Fam Cancer 12(4):707–718
Weiss H, Amberger A, Widschwendter M, Margreiter R, Ofner D, Dietl P (2001) Inhibition of store-operated calcium entry contributes to the anti-proliferative effect of non-steroidal anti-inflammatory drugs in human colon cancer cells. Int J Cancer 92(6):877–882
Valero RA, Senovilla L, Núñez L, Villalobos C (2008) The role of mitochondrial potential in control of calcium signals involved in cell proliferation. Cell Calcium 44:259–269
Muñoz E, Valero RA, Quintana A, Hoth M, Núñez L, Villalobos C (2011) Nonsteroidal anti-inflammatory drugs inhibit vascular smooth muscle cell proliferation by enabling the Ca2+-dependent inactivation of calcium release-activated calcium/Orai channels normally prevented by mitochondria. J Biol Chem 286:16186–16196
Muñoz E, Hernández-Morales M, Sobradillo D, Rocher A, Núñez L, Villalobos C (2013) Intracellular Ca2+ remodeling during the phenotypic journey of human coronary smooth muscle cells. Cell Calcium 54:375–385
Acknowledgements
This work has been funded by grants from Ministerio de Economía y competitividad, Spain (BFU2012-37146) and Junta de Castilla y León, Spain [BIO/VA46/14]. DS was supported by a predoctoral fellowship from the JAE program, National Research Council (CSIC), Spain.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Villalobos, C., Sobradillo, D., Hernández-Morales, M., Núñez, L. (2016). Remodeling of Calcium Entry Pathways in Cancer. In: Rosado, J. (eds) Calcium Entry Pathways in Non-excitable Cells. Advances in Experimental Medicine and Biology, vol 898. Springer, Cham. https://doi.org/10.1007/978-3-319-26974-0_19
Download citation
DOI: https://doi.org/10.1007/978-3-319-26974-0_19
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-26972-6
Online ISBN: 978-3-319-26974-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)