Abstract
The story of the cell commonder, calcium, reaches into all corners of the cell and controls cell proliferation, differentiation, function, and even death. The calcium-driven eukaryotic revolution is one of the great turning points in the life history, happened about two billion years later when it was converted from a dangerous killer that had to be kept out of cell into the cell master which drives the cell. This review article will take the readers to a tour of tissues chosen to best show the calcium’s many faces (proliferator, differentiator, and killer). The reader will first see calcium and its many helpers, such as the calcium-binding signaler protein calmodulin, directing the key events of the cell cycle. Then the tour will move onto the colon to show calcium driving the proliferation of progenitor cells, then the differentiation and ultimately the programmed death of their progeny. Moreover, the reader will learn of the striking disabling and bypassing of calcium-dependent control mechanisms during carcinogenesis. Finally, recommendations should be taken from the underlying mechanisms through which calcium masters the presistance, progression, and even apoptosis of colorectal cancer cells. Thus, this could be of great interest for designing of chemoprevention protocols.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colorectal cancer progression from normal to malignant phenotype is linked with various epigenetic modifications [1, 2]. Many studies have been performed to indicate that diet components are important factors in the modulation of this cancer type [1, 3, 4]. Calcium is already known as a promising chemopreventive agent [3–5].
Authors suggested that decreased calcium-containing diet is associated with increased risk of pre-neoplastic intestinal lesions in vivo models. In addition, Wild-type mice got colon hyperplasia when fed with that diet for short periods of time without carcinogen administration [6]; however, no malignancies developed in the colon [7]. Accordingly, calcium-enriced diet significantly suppressed these changes in normal and diseased mice [6].
Together, these observations showed the importance of the underlying mechanistic pathways through which calcium drives the presistance and progression of colon cancer. Thus, this could be of great interest for designing of chemoprevention protocols. This review article focuses on the possible strategies of calcium at genomic and non-genomic levels, orcostrating the colorectal tumorigenesis.
Calcium and Cell Cycle
Calcium Drives Cell Cycle
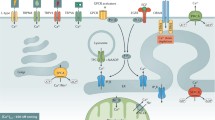
Signaling pathways in cell biology are mainly regulated by internal calcium ions. However, the know-how of this calcium-regulatory system remains obscure. Therefore, we will go to illustrate the cell cycle regulatory pathway by calcium ions as shown in (Fig. 1). First, the calcium sensor calmodulin (CaM) is essential for driving cell cycle [8, 9]; however, it is scarcely mentioned in literature of the cell cycle events checkpoints. Then, the impact of those calcium ions on DNA replication, mitosis, and cytokinesis will be mentioned.
Calcium ions ride the cell cycle waves of cyclin-dependent kinases (CDKs) in the G0/G1, S, and G2/M phases, transitioining of the cell into two daughters. However, calcium wave’s shifing will light up different cellular targets through the different stages of the cycle [10–12]. Intriguingly, scientistis on the verge of being able to perform 4D calcium mapping during the cell cycle is the first step for grasping the cycle checkpoints [9].
Calcium Starts Cell Cycle
Cell cyle starts with a G0 reversible snoozing, due to an absence of the suitable growth factors; however, the cycle will be started upon those presences. Dissimilar to the differentiated cells, those quiescent G0 cells are prepared to react with a mitogen by keeping a pool of untranslated messages starting with cycle started genes [13]. Those quiescent cells need triggering its cycle-shutdown system. It needs to be planted over its Gas-1 protein that in some way or another contributes the concealment for burgeoning [14–16]. This will upregulate the p27Kip1, which might piece any low-level surge from claiming Cdk4/6 cyclin D (CD) action.
The silencing for development variable cell receptors will influence RNA, protein, and also those mitochondrial vitality supply. The calcium ions signs should trigger the major phases of the cell cycle. A calcium surge is included for beginning the G1 development. In a percentage of cells, this calcium goes mostly from the inside, while in others a fundamental piece of the calcium comes through membrane channels [17].
The shutdown mechanism stops the processing of the proliferation driving gene transcripts, demobilizes the messages for growth-promoting peptides, reduces the total mRNA content, decreases ribosomal RNA synthesis, increases ribosomal RNA degradation, increases protein degradation by lysosomes, reduces the flow into the nucleus of short-lived non-histone proteins such as transcription factors, and lowers mitochondrial activity [17].
Signals Orchestrate Cell Cycle
A signal to a quiescent, cultured cell from a growth factor such as platelet derived growth factor (PDGF), to a hepatocyte in the adult rat liver from an endogenous growth factor such as the hepatocyte growth factor released in response to partial hepatectomy, to start the final run-up of DNA replication. This first wake-up signal triggers a veritable explosion of events. The signal-aroused cell will need ATP energy from glucose, the uptake of which is enhanced by c-Myc. The energy is supplied by mitochondria [18].
Calcium signal also activates the cytoskeleton, a striking effect of which is cell surface ruffling, and the nuclear actomyosin motor is switched on to reposition the chromatin to receive and respond to incoming transcription factors and protein kinase and phosphatase modulators of nuclear transcription factors [19, 20]. The c-Myc complexe stimulates the genetic expression of BN51, eIF-4E, nucleolin, ornithine decarboxylase (ODC) and the cycle-starter transcripts such as CAK, Cdk4, cyclin D2, c-Fos, and c-Fra [17].
Growth Factor Velcroceptors Alarm Cell Cycle
Among the most common cellular alarm clocks are the growth factor velcroceptors. Ligand binding to the extracellular parts of the monomeric velcroceptors (e.g., EGF, PDGF), heterodimeric velcroceptors (e.g., IGF-I, insulin), or multi-component or modular velcroceptors (e.g., the T cell receptor, or the IL-6 receptor with its associate JAK protein tyrosine kinase) causes the receptor molecules to activate their cytoplasmic protein tyrosine kinase (PYK) domains, and cross-phosphorylate each other tyrosine residues [21, 22].
Velcroization is a wonderful way of recruiting signal enzymes to a place where they can be coordinately phosphorylated by the receptor protein tyrosine kinase domains, and interact with each other and other membrane-associated molecules to switch on several differently targeted signal mechanisms. To awaken a quiescent cell, it may be induce to express the first set of proliferogenes by activating appropriate mitogenic velcroceptors on its surface. The signals from such receptors will activate the transcription factors that will turn these genes on [22, 23].
The activated MAP kinase can enter the nucleus to phosphorylate and thus activate a variety of transcription factors such as the multipurpose c-Jun and the exclusively proliferation-related c-Myc whose targets are key proliferogenes such as the Cdk4cyclin D-activating CAK [24]. Another key target of the MAP kinases are the S6 Ser/Thr protein kinases, which are activated and phosphorylate five serine residues of the S6 subunits of 40-S ribosomes [17]. These five domaions are on the platform of the 40-S ribosome, which is involved in codon-anticodon tRNA binding. This phosphorylation triggers a configurational change, which increases the 40-S subunit message-binding ability [25].
The first wave of diacylglycerols crashes within a few seconds as the membrane-associated protein kinase-Cs (PKCs) phosphorylate and silence phospholipase-C. However, while they have shut down phospholipase-C, they have stimulated another one, phospholipase-D, which breaks phosphatidyl choline down into phosphatidic acid and choline. The phosphatidic acid is then dephosphorylated to diacylglycerol, which re-activates PKCs, thus setting up a positive-feedback stimulation that produces a second surge of membrane-associated PKCs activity [17].
Calmodulin Activates Cyclins and Kinases
The presence of calcium forms complexes with a variety of cytoskeletal and other proteins, perhaps the most important of which are the complexes with calmodulin (CaM). The calcium surge also facilitates the movement of intermediately sized molecules into the nucleus by removing a central plug or gate in the nuclear membrane pores [26, 27].
CaM waves activate a formidable array of enzymes such as calcineurin (a protein Ser/Thr phosphatase), CaMKII (calmodulin-dependent Ser/Thr protein kinase), CaMKIV, and MLCK (myosin light chain kinase). The nuclear calcium content shoots upwards and the resulting CaM complexes do two important things; they activate MLCK and thus stimulate the nuclear actomyosin machinery to move the chromatin into a responsive configuration [19, 20], and stimulate the expression in the quiescent cell of at least one key proliferogene, c-Myc, by binding to and displacing the transcriptionally inactive Max/Max homodimers from the gene promoter, which enables their replacement by transcriptionally active Myc/Max heterodimers [28].
The Myc/Max transactivates the genes for two very important things needed to turn on Cdk4/6 cyclin D, the G1-starting CDK. First are the components of Cdk7 cyclin H, CAK, which besides being involved in activating Cdk4/6 cyclin D, is part of the RNA polymerase II transcriber pre-initiation complex when associated with the MAT1(p36) protein [29]. Second is the early G1-specific, CDKs-activating STY phosphatase Cdc25A, which like its G2-specific isoform, Cdc25C, might be activated by being phosphorylated by calcium CaM-stimulated CaMKII [30]. Indeed, specifically blocking CaMKII activity with KN-93 greatly reduces the level of cyclin D1 and blocks the G1 buildup in NIH3T3 mouse cells [31].
Starting a cycle can be lethal for a normal cell if there is only an incomplete, thus unbalanced, G1 buildup [32]; for example, hyperexpressing the c-myc proliferation-starter gene in normal fibroblasts without the support of protector signals from serum factors leads to the overexpression of E2F1, which stimulates the accumulation of the apoptogenic p53 and also the expression of cycle-driving components such as cyclin A and Cdk1 cyclin B1, which are apoptogenic when expressed out of context and without protectors such as Bcl-2 and the anti-p53 Mdm2 [24, 32–35].
The signal must trigger in the proper order the first wave of cyclins, the cyclin Ds, and the assembly of active Cdk4 cyclin D protein kinases that start the G1 buildup. However, it must also supply the protectors and downregulate the promiscuous CDK inhibitor p21Cip1/WAF1 that is stimulated to increase by the mitogenic signal. To do this it must receive converging streams of signals from serum factors and a subset of integrin receptors that are activated by binding to substrate proteins such as fibronectin and vitronectin [32, 36].
However, CaM is a part of the DNA-replicating factories, tumor cells simply could not be expected to be able to do without them and they need for CaMKII to start replicating DNA as studied on HeLa cells [37]. They are also needed by fibroblasts transformed by the constitutive hyperactivity of the avian sarcoma virus 60-kDa v-Src protein tyrosine kinase. This wild hyperactive enzyme eliminates the need for adhesion-triggered integrin signals to restrain apoptogenesis and express CDKs such as Cdk2 cyclin A needed to trigger replication [17].
When calcium-binding parts of the replication machines have done their jobs, phospho-Cdk2-cyclin A accumulates in replication factories. When the chromosomes are all replicated and there is no red light and wailing siren from an alerted G2 checkpoint mechanism, the cell shreds the G1/S-specific Cdk2 and starts expressing and using the mitotic Cdk1 which has been suppressed by Cdk2 cyclin A while the chromosomes were being replicated. It also starts expressing the last of the cyclins, the mitotic cyclin Bs [35–37].
Calcium Regulats Mitosis
The Cdk1 cyclin-driven breakdown of the nuclear membrane and all that goes with it are triggered by the strictly localized release of calcium from thousands of endoplasmic reticular vesicles crowding around the nucleus [8, 38]. Preventing the calcium surge prevents prophase, while the calcium-mobilizing caffeine or microinjected Ins(1,4,5)P3 triggers premature prophase [38, 39]. However, Ins(1,4,5)P3 may not be the real physiological calcium liberator because it is freely diffusible and the PLC that would be needed to generate it is not in the membranes of the calcium-storage, regulating vesicles in the prophase mitotic apparatus. Instead, it could be leukotriene B4, which is confined to the vesicles along with the PLA2 and the other enzymes needed to make it [40].
Calcium signal triggers wave of CaMKII activity, but it turns on the late-G1-specific Cdc25C, which activates the Cdk1cyclins A/B that in turn activate the chromosome-condensing condensins and phosphorylate H1 and H3 histones to reconfigure the chromosomes to receive the phospho-condensins that will condense them [30].
The calcium barrage may trigger nuclear envelope breakdown at least in part by directly stimulating the protease calpain [8, 20]. In fact, inhibiting calpain blocks the G2/M transition at least in some cells [41]. This is supported by some evidence that, as mitosis approaches, calpain II relocates from the plasma membrane to the nucleus and its entourage of calcium-loaded vesicles [42].
During the G2 buildup, PKC-II is translocated to the nucleus and plugged into the nuclear membrane alongside its target lamin B. Then, the membrane-tethered kinase is selectively activated when a product of nuclear membrane lipid breakdown, a specific type of nuclear phosphatidylglycerol, binds to its C-terminal region [43]. The activated PKC-II then triggers the disassembly of the lamin network and the release of chromosomes from the nuclear envelope by phosphorylating lamin B [44].
When the chromosomes have all assembled at the mitotic spindle mid-zone and the last of the cycle checkpoint mechanisms that monitors and responds to signals from unattached kinetochores has been switched off, and the tension on the straining sister chromatids from the spindle fibers has reached a critical threshold, the Anaphase signals start to be activated [45].
Cdk1 cylin B induces the spike(s) and the calcium spills out of storage vesicles linked with the spindle [8, 17]. Once again, a calcium wave activates CaMKII, which in turn, activates the spindle-restricted anaphase-promoting complex (APC) that contains a ubiquitin-conjugating E2 enzyme specific for mitotic cyclins [46, 47]. One result of this is the polyubiquitination of the mitotic cyclins which are marked for this and consequently the proteasome shredding by a conserved N-terminal motif known as the destruction box [17, 47].
The loss of the cyclin exposes the naked Cdk1 catalytic subunit to further inactivation by having its Thr161 residue dephosphorylated by type 2A protein phosphatase, which prevents the subunit from grabbing another cyclin. This allow the chromosomes to decondense at telophase, but they are not needed for chromatid separation [47]. This requires the APC-driven destruction of the cohesins that have been tying the sister chromatids together [46], as well as the removal of residual DNA tangles by calcium-stimulated, and centromere-associated topoisomerase II [48, 49].
In addition, calcium may be involved in forming the new nuclear envelope during telophase [20, 50]. While all of these events have been going on, the cell has been putting a belt of cortical actomyosin filaments around its middle. This belt has been kept from tightening prematurely by having its myosin motors turned off by the phosphorylation of certain residues in their light chains by Cdk1cyclin B [17]. The motors are activated by the destruction of Cdk1 cyclin B and then started by the stimulation of their MLC (myosin light chain kinase) by the CaM complexes generated by a final prolonged calcium surge [17, 47].
The belt tightens and pinches the elongating cell into two daughters, each with its own chromosomes awaiting instructions from their surroundings to cycle or differentiate normally or to start extensively cycling if it is going to be malignant cell.
Calcium and Colon Cell Diffpoptosis
The cellular production in a colon crypt is so enormous that the crypt populations are turned over completely every 3 days in the human beings and mice. Each crypt in a mouse’s proximal colon contains about 100 proliferating cells, which generate about 6 new cells per hour, while in the distal colon there are about 200 proliferating cells per crypt, which generate about 21 new cells every hour [51, 52].
Each of the millions of crypts in the human transverse colon produces up to 8.8 cells per hour. Therefore, there has to be a mechanism to keep the cell population at a constant size to avoid overgrowth. Accordingly, it appears that colon cells are eliminated by apoptosis and then by engulfment [53].
Freshly isolated normal human colon cells need about 0.1 mM external calcium to proliferate optimally, while their proliferation is stopped and the colon cells are induced to differentiate by higher calcium concentrations (0.8–2.0 mM) that can be found in the fecal water [54–57]. Moreover, calcium controls proliferation in the crypt where the cells need some of it to proliferate maximally but cannot do so with smaller or larger amounts [55]. Thus, the proliferative activity of cells in crypts from normal rats given a diet containing either 0.5 or 15 g of calcium/kg was significantly lower than the optimal activity in the crypts from rats given a diet containing 5 g of calcium/kg [58].
Brenner group [59] have reported a striking bottom-to-top intracellular calcium gradient in mouse colon crypts and a crypt level-dependent responsiveness to a change in the extracellular calcium concentration and have mentioned that the same gradient has been found in human crypts. Thus, for example, the concentration of calcium in the cells of isolated whole murine crypts rose from 70 to 83 nM in the lower crypt to 157 nM at the crypt mouth when the crypts were in calcium-free buffer. When the crypts were suspended in buffer containing 1 mM calcium, the intracellular calcium rose from 262 to 297 in the lower crypt to 463 nM at the crypt mouth. The maintenance of this gradient required vitamin D3 because it was lost in animals fed a low-vitamin D3 diet. This indicates the involvement of the transcellular transport of calcium by the vitamin D3-dependent protein calbindin from the lumen to the blood. Also, it may indicate the involvement of signals from the CaRs on the cells’ apical surfaces [60–64], because expression of parathyroid gland’s CaR is promoted by the seco-steroid [65].
Calcium and Colon Cancer Diffpoptosis
Deleted in Colon Cancer (DCC) Protein Activates Apoptosis
An important key player in colon cell maturation is a cell–cell adhesion protein belonging to the Neural Cell Adhesion Molecule (N-CAM) family called Deleted in colon cancer (DCC), which is lost during the later stages of the progression to malignant colon cancer [66–68]. It is a receptor activated by contacting the laminin-like netrin-1 with its Ig-like and fibronectin-type III extracellular motifs, and then sends calcium and G-protein signals into the colon cell through its cytoplasmic domain, which suppress anoikis and somehow promote differentiation by inhibiting CDK activity and guide the differentiating cell’s migration up the crypt wall just as neurons use their DCC receptors to steer growing neurites toward their target [66–70].
Furthermore, DCC is also part of fail-safe mechanism that guards against a cell being able to detach from the basal lamina with impunity and metastasize to distant places [71]. Thus, the apoptogenic mechanism is silent when the cell is attached to the basal lamina and DCC is attached to its ligand and presumably steering the cell along the trackway and emitting an anoikissuppressing signal. The colon cell try to detach itself from the trackway by increasing caspase-3 activity and cleaving it at Asp1290, converts DCC into a potent apoptosis driver [71].
These several regulators and adhesion complexes trigger different space–time–frequency-modulated calcium transients of various shapes and sizes in different parts of the cell. These specifically patterned streams of transients are the words and phrases of the complex calcium language that different agents use to tell cells how to reconfigure themselves into mature absorptive or goblet cells [12].
As the DCC directed colon cell proliferation shutdown by the appearance of TGF-βs and fading of proliferation-related integrins and cadherin- mediated adhesion to its neighbors. This in turn shuts off c-Myc and c-Myb genes [72]. It might be expected that as it moves up the crypt and stops cycling, the differentiating colon cell would also stop making its principal autocrine proliferogen, TGF-α, but it does not. It keeps making TGF-α and the EGF/TGF-α receptors [73]. However, these receptors are probably now activated at least in part by the EGF-related amphiregulin, which is expressed only by differentiated columnar epithelial and goblet cells [74]. Because the receptor signals may be different and the signals’ old targets have been replaced in the functionally reconfigured cell by new diffpoptosis-related ones, neither the emerging amphiregulin nor the TGF-α made at the top of the crypt can make the maturing cell start cycling [73].
Eventually, the aged cell starts accumulating DNase I142 and transglutaminase, which will be used to convert it into a hardened corneocyte-like squame that is either swiftly engulfed by its neighbors [75, 76]. However, we must not forget the fibroblasts that have faithfully escorted the epithelial cells and helped nourish and drive their growth and differentiation during the journey up the crypt [53].
Starting the Road of Colon Cancer
TP53 genetic mutation-mediated apoptosis may occurs in both colon and small intestine [77]. It happens that the most apoptosis-prone cells in the mouse small intestine crypts are the clonogenic stem cells in position 4 where they do not contain the anti-apoptogenic Bcl-2 protein, but in the colon the most apoptosis-prone cells are only the non-clonogenic transit amplifying cells in the mid-crypt region (cell positions 11–12) far from the well-protected, Bcl-2-loaded clonogenic stem cells in positions 1 and 2 [78]. The importance of Bcl-2 is demonstrated by the fact that in Bcl-2-null mice the level of spontaneous apoptosis rises significantly in colon, while there is no increased apoptosis in small intestine crypts [79].
According to conventional wisdom, the mutated and epimutated human colorectal cancer clones appear in hyperplastic adenomatous polyps [80, 81]. However, a large fraction of early lesions as well as advanced colorectal cancers have arisen from flat mucosa rather than polyps. In fact, the smallest detectable precursor lesions are clusters or foci of hyperplastic, distended, flask-shaped, branching, dividing aberrant crypts [79]. These Aberrant Crypt Foci (ACFs) are the products an abnormal crypt cycle and stem cell expansion. An ACF starts with excessive clonogenic cell proliferation at the base of a crypt, which causes a branching or fission that moves up until there are two crypts, which in turn branch and divide to produce a crypt cluster [82, 83].
Some hyperplastic ACFs turn into the dangerous dysplastic adenomatous ACFs. The post-hyperplasia-generating mutations that start the carcinogenic process disrupt the differentiation program and extend the proliferative zone. In normal human colon crypts, the nuclear, proliferation-marking Ki67 and the hMSH2 mismatch-repair proteins are intensely expressed in the lower third of the crypt, but they are sharply downregulated and the p21Cip1/WAF1 CKI upregulated when the tethered cells glide out of the proliferative zone. However, in the carcinoma-hatching dysplastic ACFs, the mechanism that shuts off Ki67 and hMSH2 expression and turns on the cycle-stopping p21Cip1/WAF1 at the border of the diffpoptosis zone has been disabled [84].
This extension of the proliferative zone into the differentiation and killing zones is the result of the initiated cells persistently producing autocrine/paracrine growth factors such as platelet-derived growth factor-B (PDGF-B), IGF-I, IGF-I variants, TGF-α, and the EGF-like Cripto, which is not expressed by normal colon cells [85, 86]. Because they can stimulate themselves with their hyperexpressed growth factors, the proliferation of the activated cells is not limited by the need for signals from adhesion-assembled complexes of β1 integrins and expogenous growth factors and their receptors. An example of the importance of a TGF-α- autocrine/paracrine loop for the proliferation of at least some colon carcinoma cells is the ability of a 23-base antisense oligonucleotide that recognizes TGF-α mRNA to reduce its secretion and proliferation of LIM1215 colon carcinoma cells [87].
There is likely to be another serious drawback to loading colons containing initiated cells with calcium above a certain concentration. Cells in the early post-initiation stages are probably proliferatively restrained by things flowing into them from their normal neighbors through their still functional gap junctions. However, gap junctions are designed to be slammed shut by calcium. This is meant to prevent the draining of components from the cellular network through a damaged dying cell [88]. Calcium was chosen as the damage detector and drain plugger simply because its accumulation is a universal consequence of cell injury. The closing of gap junctions by supra-optimal calcium loading would release the initiated colon cells from the restraining influences of their normal neighbors. However, the importance of natural and essential dietry supplemts in cancer chemoprevention [89], it was reported that, in some cases, calcium from food sources and dietary supplements increases the risk of advanced prostate cancer [90].
Ending the Road of Colon Cancer
As the tumor promoters in the fecal stream and the increasingly endogenously unrestrained CDKs relentlessly drive the proliferation of mutating clones, a lucky winner of the mutation lottery will emerge with all the mutations [91]. It needs to escape from advanced adenoma to the ultimate freedom of carcinoma and be resistant to apoptosis [92]. Adenoma cells had increased their expression of the Bcl-2 protector protein, but during the transition to carcinoma, Bcl-2 is downregulated and its antiapoptogenic relative Bcl-w is upregulated to maintain the protection [93].
Another antiapoptosis change, which occurs in about 50–75 % of large adenomas on the threshold of adenocarcinoma, is a loss-of-function mutation of the p53 gene on the short (p) arm of human chromosome 17 [94]. The disabled p53 mutant protein has a prion-like “infectiousness”; it can associate with the product of the normal allele and cause the normal protein to reconfigure itself to the inactive mutant form [95]. In other words, the G1/S checkpoint of a p53 heterozygote is as useless as that of a p53 homozygote. But eventually cells appear which have also lost their normal allele. The loss of p53 function coincides with the appearance of carcinoma in the polyp or adenoma. It has eliminated a major safety checkpoint guardian, which when activated by DNA damage would normally stop cells proliferating until the damage is repaired, or failing this, trigger apoptosis [96].
The ultimate appearance of malignant cells is tightly coupled to the loss of TGF-β signaling, which is most often due to the disabling mutation of the Type II receptor, but can also be due to the loss of functional Smad proteins (Smad 2 or Smad 4) that mediate the proliferation-stopping response of normal colon cells to TGF-βs [97]. Moreover, in about 70–90 % of colon carcinoma cells, the DCC gene is disabled [69].
With these several mutations and epimutations and their strong Bcl-w shield, the carcinoma cells have completed the divorce from control by external calcium that began when their carcinogen-damaged ancestor lost its functional APC. The extent of this divorce is indicated by the fact that while the cells in the early hyperplastic stage up to the well-differentiated adenocarcinoma stage still express CaRs, these receptors, and with them the cells’ responsiveness to proliferation-restraining signals from external calcium, vanish in the later stages [98, 99].
The loss of functional APC results in the over-expression and a buildup in the nucleus of the cycle-driving β-catenin. However, it seems that β-catenin is most strongly expressed by the cells on the tumor’s invasion front, while cells in the core of the tumor have little or no β -catenin in their nuclei, but have it on their cell membranes as do normal colon cells. This raises the possibility that normal cells somehow tell the cells in the oncoming tumor invasion front to load their nuclei with β-catenin [100].
Summary and Prospectives
Our brief tour of the highlights of the parts of the calcium surges concerned with cell cycle, differentiation, and death has ended. We have seen the calcium oscillations that mediates protein synthesis and transcription factors translocation from the cell membrane and cytoplasm to the nucleus. The products of these genes started the first wave of proliferogene activities that, in turn, started the cascade of cyclin-dependent protein kinases, which inactivated the gene transcription suppressing protein (Rp), initiated the buildup of the channels, receptors, and the autocrine/paracrine second signaler, such as IGF-I or IL-2. Moreover, we have explained the emergence of Cdk4 cyclin D’s successor, Cdk2 cyclin E, that in turn continued the inactivation of Rb protein to enable E2F DP-1 transcription factor to stimulate the expression of key DNA replication enzymes. Then there was the downregulation of Cdk2 cyclin E and the cyclic AMP-dependent emergence of Cdk2 cyclin A, which crosstalks with another calcium oscillations triggered by autocrine IGF-I and enhanced by c-Myb to activate the huge DNA replication factories attached to the chromosomal replication origins on the nuclear matrix.
When all of the chromosomes were replicated and after the G2 delay, there was the calcium spike at the nuclear envelope that activated the first of the two emerging mitotic cylins, Cdk1 cyclin A, which triggered the chromosome condensation and nuclear envelope breakdown of mitotic prophase and then crashed to leave the rest of the job to Cdk1 cyclin B. This was followed by another calcium spike when the chromosomes had lined up on the mitotic spindle, by activating the anaphase-promoting polyubiquitination-proteolysis mechanism that destroyed Cdk1 cyclin B, enabled the licensing of the sister chromatids for future replication, the removal of the residual DNA strand tangles holding the sister chromatids together, the destruction of the protein glues that also held the sisters together, and started the motors that moved the sister chromosomes to opposite spindle poles. Calcium surge drove the contraction of an equatorial belt of actomyosin filaments that pinched it into two daughters.
Moreover, we found an early loss of responsiveness to the calcium signals commanding the cells to stop cycling, differentiate, and then die by apoptosis of the malignanant cells. We then saw how calcium might operate the brakes and accelerator of the engine that drives the axon growth cone as it advances toward its target.
Overall, calcium is a very busy ion with lots of different targets and mediators scattered throughout the animal and human beings, especially its role in cell cycle and diffpoptosis. Clearly, further studies on the role of calcium in colon cancer cell diffpoptosis will give us more insights about the awesome networks used by calcium in its roles of cell cycle driver, differentiator, and killer.
References
Potter JD. Colorectal cancer: molecules and populations. J Natl Cancer Inst. 1999;91:916–32.
KInzler KW, Vogelstein B. In: Vogelstein B, Kinzler KW, editors. The genetic basis of human cancer. New York: McGraw-Hill; 2001. p. 583–612.
Lipkin M, Reddy B, Newmark H, Lamprecht SA. Dietary factors in human colorectal cancer. Annu Rev Nutr. 1999;19:545–86.
Milner JA, McDonald SS, Anderson DE, Greenwald P. Molecular targets for nutrients involved with cancer prevention. Nutr Cancer. 2001;41:1–16.
Holt PR, Wolper C, Moss SF, Yang K, Lipkin M. Comparison of calcium supplementation or low-fat dairy foods on epithelial-cell proliferation and differentiation. Nutr Cancer. 2001;41:150–5.
Lipkin M. Preclinical mouse models for cancer chemoprevention studies. Ann NY Acad Sci. 1999;889:14–9.
Risio M. Apoptosis, cell replication, and Western-style diet-induced tumorigenesis in mouse colon. Cancer Res. 1996;56:4910–6.
Santella L. The roles of calcium in the cell cycle: facts and hypotheses. Biochem Biophys Res Commun. 1998;244:317–24.
Whitfield JF. Calcium: cell cycle driver, differentiator and killer. Austin/New York: Landes Bioscience/Chapman & Hall; 1997.
Allbritton NL, Meyer T. Localized calcium spikes and propagating calcium waves. Cell Calcium. 1993;14:691–7.
Allbritton NL, Meyer T, Stryer L. Range of messenger action of calcium ions and inositol 1,4,5-trisphosphate. Science. 1992;258:1812–5.
Berridge MJ, editor. Microdomains and elemental events in calcium signalling. Cell Calcium 1996;20:95–226.
Taub R. Transcriptional control of liver regeneration. FASEB J. 1996;10:413–27.
Del Sal G, Ruaro EM, Philipson L, Schneider C. The growth arrest-specific gene, gas1, is involved in growth suppression. Cell. 1992;70:595–607.
Del Sal G, Ruaro EM, Utrera R, Cole CN, Levine AJ, Schneider C. Gas1-induced growth suppression requires a transactivation-independent p53 function. Mol Cell Biol. 1995;15:7152–60.
Schneider C, King RM, Philipson L. Genes specifically expressed at growth arrest of mammalian cells. Cell. 1988;54:789–93.
Whitfield JF. Calcium in cell cycles and cancer. Boca Raton: CRC Press; 1995. p. 9–58.
Brini M, Carafoli E. Calcium signaling: a historical account, recent developments and future perspectives. Cell Mol Life Sci. 2000;57:354–70.
De Boni U. The interphase nucleus as a dynamic structure. Int Rev Cytol. 1994;150:149–71.
Santella L, Bolshover S. Calcium in the nucleus. In: Carafoli E, Klee C, editors. Calcium as a cellular regulator. New York: Oxford University Press; 1999. p. 487–511.
Fante WJ, Johnson DE, Williams LT. Signalling by tyrosine kinases. Annu Rev Biochem. 1993;62:453–86.
Malarkey K, Belham CM, Paul A, Graham A, McLees A, Scott PH, et al. The regulation of tyrosine kinase signaling pathways by growth factor and G-protein-coupled receptors. Biochem J. 1995;309:361–75.
Buday L. Membrane-targeting of signaling molecules by SH2/SH3 domaincontaining adaptor proteins. Biochim Biophys Acta. 1999;1422:187–204.
Barrett J, Lewis BC, Hoang AT. Cyclin A links c-Myc to adhesion-independent proliferation. J Biol Chem. 1995;270:15923–5.
Morley SJ, Thomas G. Intracellular messengers and the control of protein synthesis. In: Taylor CW, editor. Intracellular messengers. Oxford: Pergamon Press; 1993. p. 447–83.
Perez-Terzic C, Pyle J, Jacomi M, Jaconi L, Stehno-Bittel, Clapham, DE. Conformational states of the nuclear pore complex induced by depletion of nuclear Ca2+ stores. Science. 1996;273:1875–7.
Stehno-Bittel L, Perez-Terzic C, Clapham DE. Diffusion across the nuclear envelope inhibited by depletion of the nuclear Ca2+ store. Science. 1995;270:1835–8.
Latchman DS. Eukaryotic transcription factors. 3rd ed. London: Academic Press; 1998. p. 1–374.
Kaldis P. The cdk-activating kinase (CAK): from yeast to mammals. Cell Mol Life Sci (CMLS). 1999;55:284–96.
Holt PR, Philipova R, Moss S, Schulman H, Hidaka H, Whitaker M. et al. Calcium/calmodulin-dependent phosphorylation and activation of human Cdc25-C at the end of G2/M phase transition in HeLa cells. J Biol Chem. 1999;274:7958–68.
Morris TA, De Lorenzo RJ, Tombes RM. CaMK-II inhibition reduces cyclin D1 levels and enhances the association of p27Kip1 with Cdk2 to cause G1 arrest in NIH 3T3 cells. Exp Cell Res. 1998;240:218–27.
Bellamy COC, Malcomson RDG, Harrison DJ, Wyllie AH. Cell death in health and disease: the biology and regulation of apoptosis. Semin Cancer Biol. 1995;6:3–16.
Bowen ID, Bowen SM, Jones AH. Mitosis and apoptosis. London: Chapman & Hall; 1998.
Kowalik TF, DeGregori J, Leone G, Jakoi L, Nevins JR. E2F1-specific induction of apoptosis and p53 accumulation which is blocked by Mdm2. Cell Growth Differ. 1998;9:113–8.
Kowalik TF, DeGregori J, Schwarz JK. E2F1 overexpression in quiescent fibroblasts leads to induction of cellular DNA synthesis and apoptosis. J Virology. 1995;69:2491–500.
Wary KK, Mainiero F, Isakoff SJ, Marcantonio EE, Giancotti FG. The adaptor protein Shc couples a class of integrins to the control of cell cycle progression. Cell. 1996;87:733–43.
Rasmussen G, Rasmussen C. Calmodulin-dependent protein kinase II is required for G1/S progression in HeLa cells. Biochem Cell Biol. 1995;73:201–7.
Baitinger C, Alderton J, Poenie M, Schulman H, Steinhardt R. Multifunctional Ca2+/calmodulindependent protein kinase is necessary for nuclear envelope breakdown. J Cell Biol. 1990;111:1763–73.
Twigg J, Patel R, Whitaker M. Translational control of InsP3-induced chromatin condensation during the early cell cycles of sea urchin embryos. Nature. 1988;332:366–9.
Silver RB. Imaging structured space–time patterns of Ca2+ signals: essential information for decisions in cell division. FASEB J. 1999;13:S209–S215.98.
March KL, Wilensky RL, Roeske RW, Hathaway DR. Effects of thiol protease inhibitors on cell cycle and proliferation of vascular smooth muscle cells in culture. Circ Res. 1993;72:413–23.
Schollmeyer JE. Calpain II involvement in mitosis. Science. 1988;240:911–3.
Fields AP, Thompson LJ. The regulation of mitotic nuclear envelope breakdown: a role for multiple lamin kinases. Prog Cell Cycle Res. 1995;1:271–86.
Murray NR, Fields AP. Phosphatidylglycerol is a physiologic activator of nuclear protein kinase C. J Biol Chem. 1998;273:11514–20.
Elledge SJ. Cell cycle checkpoints: preventing an identity crisis. Science. 1996;274:1664–72.
Clarke DJ, Giménez-Abián JF. Checkpoints controlling mitosis. BioEssays. 2000;22:351–63.
Lorca T, Abrieu A, Means A, Doree M. Ca2+ is involved through type II calmodulindependent protein kinase in cyclin degradation and exit from metaphase. Biochim Biophys Acta. 1994;1223:325–32.
Osheroff N, Zechiedrich EL. Calcium-promoted DNA cleavage by eukaryotic topoisomerase II: trapping the covalent enzyme-DNA complex in an active form. Biochemistry. 1987;26:4303–9.
Warburton PE, Earnshaw WC. Untangling the role of DNA topoisomerse II in mitotic chromosome structure and function. BioEssays. 1997;19:97–9.
Sullivan KMC, Busa WB, Wilson KL. Calcium mobilization is required for nuclear vesicle fusion in vitro: implications for membrane traffic and IP3 receptor function. Cell. 1993;73:1411–22.
Bach S, Renehan AG, Potten S. Stem cells: the intestinal stem cell as a paradigm. Carcinogenesis. 2000;21:469–76.
Bjerkness M, Cheng H. Clonal analysis of mouse intestinal epithelial progenitors. Gastroenterology. 1999;116:7–14.
Hall PA, Coates PJ, Ansari B, Hopwood D. Regulation of cell number in the mammalian gastrointestinal tract: the importance of apoptosis. J Cell Sci. 1994;107:3569–77.
Buset M, Lipkin M, Winawer S, Swaroop S, Friedman E. Inhibition of human colonic epithelial cell proliferation in vivo and in vitro by calcium. Cancer Res. 1986;46:5426–30.
Friedman EA. A multistage model of human colon carcinoma development integrating cell culture studies with pathology. Cancer Invest. 1985;3:453–61.
Friedman EA. Use of tissue culture of human colonic epithelial cells to study mechanisms of calcium protection. In: Lipkin M, Newmark HL, Kelloff G, editors. Calcium, vitamin D, and the prevention of colon cancer. Boca Raton: CRC Press; 1991. p. 159–68.
Lipkin M, Friedman E, Winawer SJ, Newmark H. Colonic epithelial cell proliferation in responders and non-responders to supplemental dietary calcium. Cancer Res. 1989;49:248–54.
Li H, Kramer PM, Lubet RA, Steele VE, Kelloff GJ, Pereira MA. Effect of calcium on azoxymethane-induced aberrant crypt foci and cell proliferation in the colon of rats. Cancer Lett. 1998;124:39–46.
Brenner BM, Russell N, Albrecht S, Davies RJ. The effect of dietary vitamin D3 on the intracellular calcium gradient in mammalian colonic crypts. Cancer Lett. 1998;127:43–53.
Brown EM, MacLeod RJ. Extracellular calcium sensing and extracellular calcium signaling. Physiol Revs. 2001;81:240–97.
Butters RR, Chattopadhyay N, Nielsen F, Smith CP, Mithal A, Kifor O, et al. Cloning and characterization of a calcium-sensing receptor from the hypercalcemic New Zealand white rabbit reveals unaltered responsiveness to extracellular calcium. J Bone Miner Res. 1997;12:568–79.
Chattopadhyay N, Cheng I, Rogers K, Riccardi D, Hall A, Diaz R, et al. Identification and localization of extracellular Ca2+-sensing receptor in the rat intestine. Am J Physiol. 1998;274:G22–130.
Gama L, Baxendale-Cox LM, Breitwieser GE. Ca2+-sensing receptors in intestinal epithelium. Am J Physiol. 1997;273:C1168–75.
Kállay E, Kifor O, Chattopadhyay N, Brown EM, Bischof MG, Peterlik M, et al. Calcium-dependent c-myc protooncogene expression and proliferation of CACO-2 cells: a role for a luminal extracellular calcium-sensing receptor. Biochem Biophys Res Commun. 1997;232:80–3.
Bikle D, Vitamin D. A calciotropic hormone regulating calcium-induced keratinocyte differentiation. J Am Acad Dermatol. 1997;37:S42–55.
Fearon ER. DCC; Is there a connection between tumorigenesis and cell guidance? Biochim Biophys Acta. 1996;1288:M17–23.
Fearon ER, Piercall WE. The deleted in colorectal cancer (DCC) gene: a candidate tumor suppressor gene encoding a cell surface protein with similarity to neural cell adhesion molecules. Cancer Surv. 1995;24:3–17.
Livesey FJ. Netrins and netrin receptors. Cell Mol Life Sci (CMLS). 1999;56:62–8.
Chen YQ, Hsieh JT, Yao F, Fang B, Pong R, Cipriano SC, et al. Induction of apoptosis and G2/M cell cycle arrest by DCC. Oncogene. 1999;18:2747–54.
Song H, Poo M. Signal transduction underlying growth cone guidance by diffusible factors. Curr Opin Neurobiol. 1999;9:355–63.
Mehlen P, Rabizadeh S, Snipas SJ, Assa-Munt N, Salvesen GS, Bredesen DE. The DCC gene product induces apoptosis by a mechanism requiring receptor proteolysis. Nature. 1998;395:801–4.
Torelli G, Venturelli D, Colo A, Zanni C, Selleri L, Moretti L, et al. Expression of c-myb protooncogene and other cell cycle-related genes in normal and neoplastic human colonic mucosa. Cancer Res. 1987;47:5266–9.
Huang F, Sauma S, Yan Z, Friedman E. Colon absorptive epithelial cells lose their proliferative response to TGF-_ as they differentiate. Exp Cell Res. 1995;219:8–14.
Saeki T, Stronberg K, Qi CF, Gullick WJ, Tahara E, Normanno N, et al. Differential immunohistochemical detection of amphiregulin and cripto in human normal colon and colorectal tumors. Cancer Res. 1992;52:3467–73.
Albaugh GP, Iyengar V, Lohani A, Malayeri M, Bala S, Nair PP. Isolation of exfoliated colonic epithelial cells, a novel, non-invasive approach to the study of cellular markers. Int J Cancer. 1992;52:347–50.
Iyengar V, Albaugh GP, Lohani A, Nair PP. Human stools as a source of viable colonic epithelial cells. FASEB J. 1991;5:2856–9.
Oren M, Prives C. p53: upstream, downstream and offstream. Review of the 8th p53 workshop (Dundee, July 5–9, 1996). Biochim Biophys Acta. 1996;1288:R13–9.
Potten CS. Significance of spontaneous and induced apoptosis in gastrointestinal tract of mice. Cancer Metastasis Rev. 1992;11:179–95.
Merritt AJ, Potten CS, Watson AJ, Loh DY, Nakayama K, Nakayama K, et al. Differential expression of bcl-2 in intestinal epithelia. Correlation with attenuation of apoptosis in colonic crypts and the incidence of colonic neoplasia. J Cell Sci. 1995;108:2261–71.
Fearon ER. Molecular abnormalities in colon and rectal cancer. In: Mendelsohn J, Howley PM, Israel MA, et al., editors. The Molecular Basis of Cancer. Philadelphia: W B Saunders; 1995. p. 340–57.
Shamsuddin AM. Diagnostic Assays for Colon Cancer. Boca Raton: CRC Press; 1992.
Bjerknes M. Expansion of mutant stem cell populations in the human colon. J Theor Biol. 1996;178:381–5.
Fujimitsu Y, Nakanishi H, Inada K, Yamachika T, Ichinose M, Fukami H, et al. Development of aberrant crypt foci involves a fission mechanism as revealed by isolation of aberrant crypts. Jpn J Cancer Res. 1996;87:1199–203.
Polyak K, Hamilton SR, Vogelstein B, Kinzler KW. Early alteration of cell cycle regulated gene expression in colorectal neoplasia. Am J Pathol. 1996;149:381–7.
Anzano MA, Rieman D, Prichett W, Bowen-Pope DF, Greig R. Growth factor production by human colon carcinoma cell lines. Cancer Res. 1989;49:2898–904.
Shirai H, Ueno E, Osaki M, Tatebe S, Ito H, Kaibara N. Expression of growth factors and their receptors in human early colorectal carcinogenesis: immunohistochemical studies. Anticancer Res. 1995;15:2889–94.
Sizeland AM, Burgess AW. Anti-sense transforming growth factor oligonucleotides inhibit autocrine stimulated proliferation of a colon carcinoma cell line. Mol Biol Cell. 1992;3:1235–43.
Loewenstein W. The touchstone of life. New York: Oxford University Press; 1999.
Abd-Rabou AA, Zoheir K, Ahmed HH. Potential impact of curcumin and taurine on human hepatoma cells using huh-7 cell line. Clin Biochem. 2012;45:1519–21.
Giovannucci E, Rimm EB, Wolk A, Ascherio A, Stampfer MJ, Colditz GA, et al. Calcium and fructose intake in relation to risk of prostate cancer. Cancer Res. 1998;58:442–7.
Greaves M. Cancer. The Evolutionary Legacy. Oxford: Oxford University Press; 2000.
Lifshitz S, Lamprecht S, Benharroch D, Schwartz B. Apoptosis (programmed cell death) in colonic cells: from normal to transformed stage. Cancer Lett. 2001;163:229–38.
Wilson JW, Nostro MC, Balzi M, Faraoni P, Cianchi F, Becciolini A, et al. Bcl-w expression in colorectal adenocarcinoma. Br J Cancer. 2000;82:178–85.
Peifer M. β-Catenin as oncogene: the smoking gun. Science. 1997;275:1752–3.
Prion Diseases. Available at: http://www-micro.msb.le.ac.uk/335/Prions.html. Accessed 1999.
Meek DW. Multisite phosphorylation and integration of stress signals at p53. Cell Signal. 1998;10:159–66.
Markovitz S. TGF-_ receptors and DNA repair genes, coupled targets in a pathway of human colon carcinogenesis. Biochim Biophys Acta. 2000;1470:M13–20.
Kállay E, Bajna E, Wrba F, Kriwanek S, Peterlik M, Cross HS. Dietary calcium and growth modulation of human colon cancer cells: role of the extracellular calcium-sensing receptor. Cancer Detect Prev. 2000;24:127–36.
Scheinin Y, Kállay E, Wrba F. Immunocytochemical localization of the extracellular calcium-sensing receptor in normal and malignant large intestinal mucosa. J Histochem Cytochem. 2000;48:595–601.
Brabletz T, Jung A, Hermann K, Günther K, Hohenberger W, Kirchner T. Nuclear overexpression of the oncoprotein β-catenin in colorectal cancer is localized predominantly at the invasion front. Pathol Res Pract. 1998;194:701–4.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abd-Rabou, A.A. Calcium, a Cell Cycle Commander, Drives Colon Cancer Cell Diffpoptosis. Ind J Clin Biochem 32, 9–18 (2017). https://doi.org/10.1007/s12291-016-0562-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12291-016-0562-0