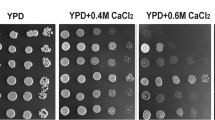
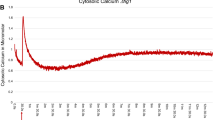
Abstract
Calcium is an essential cation for a cell. This cation participates in the regulation of numerous processes in either prokaryotes or eukaryotes, from bacteria to humans. Saccharomyces cerevisiae has served as a model organism to understand calcium homeostasis and calcium-dependent signaling in fungi. In this chapter it will be reviewed known and predicted transport mechanisms that mediate calcium homeostasis in the yeast. How and when calcium enters the cell, how and where it is stored, when is reutilized, and finally secreted to the environment to close the cycle. As a second messenger, maintenance of a controlled free intracellular calcium concentration is important for mediating transcriptional regulation. Many environmental stimuli modify the concentration of cytoplasmic free calcium generating the “calcium signal”. This is sensed and transduced through the calmodulin/calcineurin pathway to a transcription factor, named calcineurin-responsive zinc finger, CRZ, also known as “crazy”, to mediate transcriptional regulation of a large number of genes of diverse pathways including a negative feedback regulation of the calcium homeostasis system.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
7.1 A Model of Calcium Regulation in Yeasts
In higher eukaryotes entry of calcium in the cell starts concatenated signaling events some of them are of enormous importance in animals such as initiation of the heartbeat or the synapses between neurons. In the budding yeast calcium mediates adaptation to a variety of stimuli such as the presence of mating pheromones (Iida et al. 1990), a damage to endoplasmic reticulum (Bonilla and Cunningham 2003), and different ambient stresses like salinity, alkaline pH or high osmolarity [reviewed in Cunningham 2005]. A general model for calcium homeostasis is depicted in Fig. 7.1 and the elements participating in this process will be reviewed in this chapter.
Essentially, calcium enters the cell through different transport mechanisms generating an increase of cytosolic free calcium concentration. A feedback control system enables an stable concentration of calcium in cytoplasm of 50–200 nM (Aiello et al. 2002; Dunn et al. 1994; Miseta et al. 1999). The elevation of calcium levels in the cytoplasm sequentially activates the calcium binding proteins calmodulin (CaM) and the serine/threonine phosphatase calciuneurin, which is composed of the Cna1/Cnb1 or Cmp2/Cnb1 heterodimer. An important final effector for regulation of transcription is the transcription factor Crz1 a target of calcineurin. Dephosphorylation of Crz1 by calcineurin activity causes its immediate entry in the nucleus. Nuclear Crz1 regulates the transcription of a range of genes. Among these Crz1-dependent genes are those encoding for calcium pumps Pmr1 and Pmc1. These two calcium transporters play a key role in regulating cytoplasmic calcium by either pumping calcium to the ER and Golgi, PMR1, or to the vacuole, PMC1. In addition to the previous transport mechanism, calcium is stored in the vacuole through the activity of the Ca2+/H+ exchanger Vcx1. In the vacuole, calcium associates with polyphosphates constituting the “non-reusable” stock of calcium. However a low level of vacuolar calcium remains free and it can be transported back to the cytoplasm via the specific channel Yvc1. Release of calcium from the internal stores it is tightly regulated and occurs in response a number of stimuli. Calcium in the ER and Golgi may preferentially follow a secretion process depleting the cell of this cation. In a way, calcium exocytosis closes the cycle of calcium in the yeast.
7.2 How Calcium Enters the Cell?
Since calcium is a basic element of cell signaling it is expected that specific transport mechanisms functionally located at the plasma membrane (PM) mediate the influx of this cation. Three different mechanisms of transport have been postulated for Ca2+ entry (Fig. 7.1). To date, the only identified transport mechanism is a calcium channel based on two components shown to be responsible of calcium entry in the cell (Courchesne and Ozturk 2003; Locke et al. 2000). Mid1p and Cch1p are proteins of the PM that are thought to assemble into a calcium channel (Paidhungat and Garrett 1997), however evidence also suggests that they may function independently (Locke et al. 2000). Cch1, for calcium channel homologue, was primarily identified as a Ca2+ channel homologue due to its sequence similarity to the pore-forming subunit (α1) of a plasma membrane, voltage-gated, calcium channel from higher eukaryotes (Paidhungat and Garrett 1997). Cch1p is a large protein of 2039 amino acids with 22–24 predicted transmembrane domains (TMHMM, http://www.yeastgenome.org/locus/S000003449/protein) organized into four hydrophobic repeats (Paidhungat and Garrett 1997).
Mid1, from Mating pheromone-Induced Death, was identified in a screen for mutants deficient in survival after mating differentiation and in calcium uptake (Iida et al. 1994). Later, Mid1 was classified as a stretch-activated channel, with similarities to higher eukaryotes SA-Cat channels (Kanzaki et al. 1999). Mid1 is a 548 amino acid protein with four hydrophobic regions (named H1 to H4) and two cysteine rich regions (C1 and C2) (see PFAM entry PF12929). In addition to a plasma membrane localization, Mid1 was found to be also present in ER as a 200-kDa oligomer by covalent cystein bounding (Yoshimura et al. 2004), probably through the cysteine rich regions. The role of hydrophobic regions in cellular distribution of Mid1 was established, being H1 to H3 required for PM and ER localization and H1 alone for PM localization in response to mating pheromone (Ozeki-Miyawaki et al. 2005).
Importantly, mid1Δ cch1Δ double mutants are indistinguishable of single mutants, this and physiological data early suggested that Mid1 and Cch1 might be actually components of a single yeast Ca2+ channel (Paidhungat and Garrett 1997). Both proteins together could act as a voltage-gated Ca2+ channel (VGCC) becoming activated in response to depolarization (Catterall 2000; Cui et al. 2009a). The presence of mating pheromone or depletion of manganese from the medium (Paidhungat and Garrett 1997), depletion of calcium from the ER (Bonilla et al. 2002), medium alkalinisation (Viladevall et al. 2004), and cold, osmotic or saline stresses (Matsumoto et al. 2002; Peiter et al. 2005; Viladevall et al. 2004), are among others, signals that trigger calcium entry in the cell through the Mid1/Cch1 VGCC. Of importance to understand how calcium entry is regulated, is the fact that absence of either or both components of yeast VGCC does not completely perturb calcium homeostasis. Based on calcium resistance/tolerance experiments and mathematical models another two transport systems have been postulated (Cui and Kaandorp 2006). These have not been identified so far in S. cerevisiae, these transporters or pumps have been termed as transporter X and transporter M. Activities of these Ca2+ influx transporters are modulated by extracellular Mg2+ and the possible identity of these transporters is speculated below.
Finally, it is interesting to note that as important as those mechanisms present in the yeast are those mechanisms which are absent. Different studies have shown the absence of calcium ATPases of the SERCA family at the plasma membrane (reviewed in Cunningham 2005), as well as the mechanism that directly couples entry of calcium through the PM towards the ER. In animal cells, the store-operated calcium channels (SOCs) allows replenishment of the ER when becomes depleted of calcium by the action of resident calcium ATPases (Zhou et al. 2010a, b). This is a mechanism that ensures the adequate level of exchangeable pool of calcium in animal cells, but lacking these systems in S. cerevisiae entry of calcium through the PM is the major bottle neck for appropriate storage of intracellular calcium. Thus it is important to understand how calcium is kept into the yeast cell.
7.3 How Calcium is Stored in a Fungal Cell?
Under certain ambient conditions, a massive entry of calcium in the cell occurs and this represents a major stress for the yeast. Excessive free calcium is toxic because it may interact with numerous proteins or oligomolecules (ie. polyphosphate-derived compounds such as NTPs) in the cytoplasm. To prevent deleterious effects, the excess of calcium is rapidly eliminated by the activity of different Ca2+ pumps and exchangers and cytosolic Ca2+ is maintained at very low concentration, ranging 50–200 nM (see Cui et al. 2009b and references therein). In fact, there are two major mechanisms in this process of calcium sequestration (Fig. 7.1). Calcium can be either stored in the vacuoles or in secretory compartments of ER and Golgi. Most researchers identify the vacuole as the main organelle for storage and sink, and the compartmentalization of Ca2+ in the ER/Golgi revealed a pathway for depleting and recycling intracellular calcium (reviewed in Cui et al. 2009a).
For vacuolar storage of Ca2+ two principal transporters have been identified; the P-type ATPase Pmc1 pump (Cunningham and Fink 1994a, b) and the calcium/hydrogen exchanger, Vcx1 (Cunningham and Fink 1996; Miseta et al. 1999).
Pmc1 is a 1173 amino acid calcium ATPase. Three highly conserved domains among fungal and higher eukaryote Pmc1 homologues, the E1-E2 ATPase (PF00122), the haloacid dehalogenase-like hydrolase motif (PF00702) and the C-terminal ATPase domain (PF00689). Notably, these motifs are shared with other P-type ATPases, such as the Na+/Li+ pumps Ena (Ena1, 2 and 5), the plasma membrane proton pump (H+-ATPase), the proton-potassium pump (H+,K+-ATPase), and the calcium ATPase PMR1.
Since absence of Pmc1 activity is required for tolerance to elevated extracellular calcium levels, it is not surprising that expression of PMC1 is up-regulated when intracellular calcium levels elevate (Marchi et al. 1999). Loss of Pmc1 function reduces the amount of non-exchangeable calcium in vacuole, however inactivation of calcineurin restores calcium sequestration in the vacuole of a null pmc1 mutant (Cunningham and Fink 1994b). The explanation for this phenotype is that tolerance of null pmc1 mutants to calcium is dependent on the activity of Vcx1 which, on the other hand, it is dependent on calcineurin activity (Cunningham and Fink 1996).
Vcx1, named as vacuolar H+/Ca2+ exchanger (Cunningham and Fink 1996), is also known as HUM1, for “high copy number undoes manganese”. Mutations in HUM1 were identified because conferred sensitivity to Mn2+ and this phenotype was exacerbated in hypofunctional calcineurin mutants (Pozos et al. 1996). Vcx1 shares similarities to other cation exchangers, among them are those involved in the antiport of Na+, K+ with H+ (Nhx1 and Vnx1) located the vacuole or prevacuolar compartment/vesicles (Nhx1) (Cagnac et al. 2010). Despite the similarity of Vnx1p to other members of the CAX (calcium exchanger) family of transporters, Vnx1p is unable to mediate Ca2+ transport but is a low affinity Na+/H+ and K+/H+ antiporter (Cagnac et al. 2007).
Biochemical data using purified vacuoles and vacuole membrane vesicles have evidenced that Ca2+ transport activity of Pmc1 and Vcx1, more dramatically for the latter, depends on the pH gradient. Calcium uptake is promoted when the interior of these compartments is acid and lost when alkaline. This optimal acidification of vacuoles is maintained by the vacuolar H+ V-ATPase activity (Dunn et al. 1994) and mutations in subunits of this ATPase strongly reduced tolerance to calcium (see below).
In 1994 it was discovered that the main reservoirs of intracellular calcium were vacuoles and ER/Golgi (Fig. 7.1). Pmc1 in the vacuole and a second calcium P-type ATPAse at the ER/Golgi, Pmr1, were essential for viability of S. cerevisiae (Cunningham and Fink 1994a). Pmr1 is a high affinity calcium pump. Initially, it was located to the vacuoles but subcellular fractionation studies located to ER and Golgi vesicles (Cunningham and Fink 1994a; Sorin et al. 1997; Strayle et al. 1999). In fact, Pmr1 is a P-type Ca2+/Mn2+ ATPase (Antebi and Fink 1992; Rudolph et al. 1989) and the transport of either cation can be specifically modified by affecting distinct amino acids, D778A and Q783A, both locating in transmembrane segment M6 (Mandal et al. 2000). Important for the Pmr1 functionality is the presence of a EF hand like motif at the N-terminal region of this pump (Wei et al. 1999). Mutations in this domain change the affinity of the protein for Ca2+, Mn2+, or both.
Pmr1 activity is essential for growth of a double pmc1 vcx1 mutant. The activity of both P-type Ca2+ ATPases Pmr1 and Pmc1, and the antiporter Vcx1 are key in maintaining the low cytosolic free calcium concentration which is needed to avoid inappropriate calcineurin activation and other effects due to the presence of this cation (Cunningham and Fink 1994a). Deletion of PMR1 causes the elevation of cytoplasmic levels of free calcium and a massive accumulation within vacuoles (Halachmi and Eilam 1996). In fact, a deletion of PMC1 and PMR1 is a lethal genetic combination, leading to elevation of calcium levels, hyper-activation of calcineurin, and subsequent inactivation of Vcx1 antiporter. Transport activity of Vcx1 is inhibited by calcineurin, possibly in a post-translational mechanism (Cunningham and Fink 1996). Since Rcn2 is a negative regulator of calcineurin (see below), it participates in the regulation of Vcx1 (Kingsbury and Cunningham 2000), which in turn involves the activity of Crz1, that modulates transcription of this calcineurin regulator (Mehta et al. 2009). Expression of Pmc1 is dependent on the transcription factor Crz1 (Matheos et al. 1997; Stathopoulos and Cyert 1997), but also activity of Pmc1 is regulated. In a screen for negative regulators of Pmc1, the Nyv1 protein, a vacuolar v-SNARE, was found to inhibit the calcium transport activity of Pmc1 in the vacuole membrane, without affecting its expression levels (Takita et al. 2001).
In contrast to Vcx1 and Pmc1, Pmr1p activity is not postranscriptionally modulated by calcium binding proteins. Pmr1 apparently lacks the calmodulin binding domain at the C-terminus present in other plasma membrane Ca2+ ATPases (PMCAs). However, importance of transport of calcium and manganese to ER extends beyond being an storage for these cations and different studies evidence the role of Pmr1 in the proper functioning of ER, as for the normal secretion of proteins, protein maturation and/or degradation (Durr et al. 1998).
7.4 Efflux of Calcium from Internal Stores and Its Recycling
In addition to calcium entry in the cell, a second source for this cation is the return to cytoplasm from the internal stores. Only part of this intracellular calcium, about 10 % of the total, can be released back to the cytoplasm and it is designated as the “exchangeable” pool of calcium (reviewed in Cunningham and Fink 1994a).
7.4.1 Release of Calcium from Vacuoles
In vacuoles calcium is present in two forms: a free and a non-usable pool. The latter is designated as “non-exchangeable” since calcium is associated to polyphosphates. The concentration of free calcium in vacuoles is at the micromolar range (30 μM) meanwhile the total calcium was estimated at the millimolar range (2 mM) (Dunn et al. 1994). However, this reduced free-calcium pool can be returned to the cytoplasm when required. In the yeast vacuole it was identified a ion channel responsible for efflux of vacuolar calcium to the cytoplasm. This activity was designated as the yeast vacuolar conductance and the YVC1 gene (yeast vacuolar channel 1) was identified (Palmer et al. 2001). Yvc1 is a 675 amino acid protein containing six transmembrane domains and is solely detected in vacuolar membranes. This specific localization in S. cerevisiae contrasts with that shown by members of the same family of transporters in higher eukaryotes which locate at the plasma membrane (reviewed in Cunningham 2005). Yvc1, is a calcium-activated cation channel of the transient receptor protein family (Denis and Cyert 2002; Palmer et al. 2001). When yeast cells need the activity of this mechano-sensitive Ca2+ channel? In the case of a severe hypertonic shock, the vacuolar free Ca2+ can be released into the cytosol through the activity of the Yvc1. This calcium release serves to stimulate the calmodulin/calcineurin network and activates Crz1, thus enabling the transcriptional response to this stress.
7.4.2 Calcium Release from ER and Golgi; the Exocytic Pathway
The absence genes coding for inositol tri-phosphate (IP3) receptors and ryanodine receptors, RyR, in the yeast genome, and in general all fungal genomes (reviewed in Cunningham 2005), clearly indicates that the role of ER and Golgi compartments in calcium homeostasis is different in fungal cells. Since calcium is stored in the ER and Golgi by the activity of PMR1 ATPases, and release seems not to be immediate because other calcium transporters for this activity are unknown a mayor role of exocytosis is proposed to liberate this compartment of any excess in calcium. In this way, it has been measured that when the calcium concentrations in Golgi and ER exceed their resting levels of 300 μM (Pinton et al. 1998) and 10 μM (Aiello et al. 2002; Strayle et al. 1999), respectively, the calcium in ER and Golgi will be secreted along with the canonical secretory pathways. Most importantly, calcium and manganese, both transported by the Pmr1 ATPase, are necessary for the proper processing and trafficking of peptides and proteins through the secretory pathway. While Mn2+ has a role in protein glycosylation, Ca2+ is required for normal protein sorting (Durr et al. 1998). As for calcium, the excess of manganese is eliminated via Golgi and the secretory vesicles (Culotta et al. 2005). Hence, exocytosis is a major mechanism to largely reduce the intracellular, compartmentalized, pool of calcium, and other cations, but increasing the extracellular content of these ions, becoming available for a re-start of calcium transport and signaling.
7.5 Calcium Signaling
S. cerevisiae grows in a wide range of extracellular concentrations of calcium. Yeast cells are able to adapt to large and rapid modifications in environmental calcium, ranging from a low concentration of 1 μM to more than 100 mM (Anraku et al. 1991). Part of this adaptation process relies on the activation of a signaling cascade leading to the modification of the gene expression pattern (Fig. 7.2).
Calcium signaling cascade in S. cerevisiae. (a) the calmodulin/calcineurin cascade towards activation of the transcription factor Crz1. The protein phosphatase calcineurin dephosphorylates this transcription factor leading to its transcriptional activation. Among Crz1 targets are the genes PMR1 and PMC1, coding for the P-type ATPases, and (b) calcineurin regulators RCN2 and RCN1. Modulation of calcineurin activity by Rcn2 and Rcn1 alters signaling of Crz1 and the negative effect of this phosphatase on Vcx1 activity
Upon elevation of extracellular levels of calcium a massive influx of this cation occurs in the yeast. For the free calcium now existing in the cytoplasm, one of the targets is the small and essential protein calmodulin, Cmd1 (reviewed in Cyert 2001; see Fig. 7.2a). Calmodulin has 4 EF-hand moieties (Davis et al. 1986). Each EF-hand is able to bind a Ca2+ atom, but in the case of S. cerevisiae Cmd1 the fourth EF-hand is divergent and most likely is not able to bind calcium (Starovasnik et al. 1993). Calmodulin has many roles in the yeast cell which can be classified into calcium independent and dependent functions (Davis et al. 1986). Among the calcium-dependent roles is to activate the protein phosphatase 2B calcineurin (see Fig. 7.2a). In S. cerevisiae calcineurin is composed of a catalytic subunit, the A subunit encoded by either CMP2 or CNA1 isoforms (Cyert et al. 1991), and the regulatory, B, subunit encoded by CNB1 (Cyert and Thorner 1992). Activity of Cnb1, and subsequently of the calcineurin, is modulated by the α-arrestin Aly1 (O’Donnell et al. 2013) and through myristoylation (Connolly and Kingsbury 2012) in response to a reduction in calcium signal.
The calcineurin phosphatase may have numerous targets. However, these are directly recognized through a short motif namely the calcineurin docking domain (Rodriguez et al. 2009; Roy et al. 2007). An important target of calcineurin is the Crz1 transcription factor (Stathopoulos and Cyert 1997). Crz1 is a three zinc-finger, two classical Cys2His2 fingers and a non-canonical CysCysHisCys finger, transcription factor that is inactivated and activated in a cyclic, pulsatile dynamic, phosphorylation/dephosphorylation process in the cell (Dalal et al. 2014). Crz1 mediates in yeast tolerance to high concentrations of different cations, alkalinity and other types of stresses (reviewed in Cyert 2003).
Calcineurin modulates the activity of Crz1, at least, regulating its nuclei-cytoplasmic trafficking (Stathopoulos-Gerontides et al. 1999). Crz1 is mainly cytoplasmic when in a phosphorylated state. A nuclear export signal (NES) becomes activated and recognized by the exportin Msn5p (Boustany and Cyert 2002). After calcineurin dependent dephosphorylation, in response to an elevation of the cytoplasmic concentration of calcium, the nuclear import signal is dephosphorylated as is the NES. A nuclear transporter Nmd5 (karyopherin Kap119) recognizes and translocates this dephosphorylated form of Crz1 in nucleus following a general nuclear transport process through the pores located at the nuclear envelope (Polizotto and Cyert 2001). A recent work has shown that Crz1 may enter nucleus in a stochastic mode. This is a response to a brief calcium dependent stimulus that, in a non coordinated mode among cells, promote temporal accumulation of Crz1 and other transcription factors into the nucleus (Dalal et al. 2014). This mechanism is proposed as the basis to provide a fast response to ambient stress signals. However, following our case of an elevation of cytosolic calcium levels, this network of calcium binding proteins and de-phosphorylation process activates the nuclear entry of Crz1 in all cells in a culture.
In the nucleus Crz1 will bind to precise DNA sequences known as CDREs, calcineurin-dependent regulatory elements, present in the promoter of genes under its regulation (Mendizabal et al. 2001; Stathopoulos and Cyert 1997). Among these genes are PMR1 and PMC1 (for review, see Cyert 2001) and Crz1 has a positive role on their expression levels (Fig. 7.2a). Thus, Crz1 is responsible for a rise in the levels of Pmr1 and Pmc1 pumps that would locate at the ER/Golgi and vacuoles, respectively. This effect was early noted by (Beeler et al. 1994; Dunn et al. 1994) when found that a rise in the cytosolic calcium concentration increased the non-exchangeable pool of Ca2+. Mutants lacking PMC1 grow poorly in calcium stress conditions, although growth can be restored by overexpression of PMR1 or VCX1. This reflects the importance of calcium sequestration in tolerance to elevated concentrations of extracellular of calcium (Cunningham 2005; Cunningham and Fink 1994b). Therefore, the activity and an adequate level of expression of these pumps have a direct effect on depleting cytosol of calcium causing the attenuation of calcineurin-dependent signaling.
Crz1 activity also influences calcineurin function. Expression of calcineurin regulators RCN1 (regulator of calcineurin) and RCN2 are positively modulated by Crz1 (Fig. 7.2b). Rcn2 is a negative regulator but Rcn1 could act as a positive and negative modulator of calcineurin (Kingsbury and Cunningham 2000; Mehta et al. 2009). Modulation of the phosphorylation levels of Rcn proteins by homologues the glycogen synthase 3 kinase, Gsk3, is the mechanism to activate or inactivate these calcineurin regulators (Hilioti et al. 2004).
A reduction of calcineurin activity has two major consequences in calcium homeostasis. Firstly, the negative effect on Vcx1 activity is reduced and activity of this antiporter is restored, with an immediate consequence in lowering calcium levels in the cytoplasm (Fig. 7.2b). Secondly, a low calcineurin activity will consequently reduce the dephosphorylation process of Crz1, and the kinase activities acting on this TF will restore the pool of inactive Crz1 in the cell. HRR25 encodes for a casein kinase I with multiple roles in the cell (Hoekstra et al. 1991; Mehlgarten and Schaffrath 2003), among them Hrr25 opposes to the activity of calcineurin on the posttranslational modification of Crz1 (Kafadar et al. 2003). Other kinases (see below) may act on restoring phosphorylated levels of Crz1, rendering a cytoplasmic and, thus, inactive form of this TF.
In summary, calcium homeostasis relies on a meticulous regulatory system that senses, transduces and transcriptionally responds to provide with tools (transporters) to “clean” the excess of calcium, and with regulators to modulate and attenuate signal transduction. Survival of cells to calcium stress not only depends on these specific elements but other players are accessory in some ambient conditions and mutant backgrounds of need to sustain yeast’s health.
7.6 Other Protein Activities Involved in Calcium Homeostasis
In 1986, Ohya and collaborators published their work on characterizing recessive mutations affecting growth in the presence of high calcium concentration in the media. Eighteen genes, designated as cls (calcium sensitive mutants) were identified and many of them showed to be necessary for maintaining the structure of function of the vacuole (Ohya et al. 1986). Among these are genes coding for subunits of the vacuolar proton ATPase. cls mutations were isolated in different subunits of the heterocomplex VMA, the vacuolar proton ATPase, constituting the type IV cls mutants. Among these are Vma3, Vma1, Vma11, Vma13 as parts of the ATPase, and Vma12 the assembly factor (Ohya et al. 1986; Tanida et al. 1996 and references therein). The role of Vma complex is to provide the correct amount of protons at the vacuole to allow exchange with Ca2+ via Vcx1 activity. In this screen it was found mutations in other two genes of interest, csg1 and csg2. CSG2 encodes a endoplasmic reticulum membrane protein; required for mannosylation of inositolphosphorylceramide and for growth at high calcium concentrations; protein abundance increases in response to DNA replication stress (Tanida et al. 1996). Csg2 protein is a transmembrane protein, located in the ER. Biochemical and functional studies indicated that Cls2/Csg2 is necessary for mobilization of non-exchangeable pool of calcium distinct from that of the vacuole and plays an important role in calcium tolerance in the yeast, and would cooperate in stimulating the activity of calcineurin (Tanida et al. 1996). It is interesting to note that Csg2 could fulfill the role of ER-calcium efflux transporters from higher eukaryotes (IP3 receptors and RyR), however this might be an specific mechanism of S. cerevisiae since orthologues for Csg2 are not found in other fungi. CSG1 (also known as SUR1, suppressor of r vs161 and rvs167 mutations, (Beeler et al. 1997)) encodes for a mannosylinositol phosphorylceramide synthase catalytic subunit, and forms a complex with regulatory subunit Csg2 at the ER (Desfarges et al. 1993). Notably, the study of membrane composition, specially the synthesis of sphingolipids and related molecules has discovered a novel role in calcium homeostasis via influx of calcium from the ER to the cytoplasm (Birchwood et al. 2001; Desfarges et al. 1993; Dickson and Lester 2002). Also related with the composition of membranes and specifically in aminophospholipids organisation are the type IV of P-type ATPases, constituted by aminophospholipid translocases APTs or flippases. Of importance in maintaining calcium homeostasis are Drs2 (Ripmaster et al. 1993) and Neo1 (Prezant et al. 1996). These flippases are located in late Golgi and are important for proper constitution of plasma membrane and exocytosis. Also the P-type ATPase, type V, is SPF1(YEL031), located to the ER and required for calcium homeostasis. Absence of both Spf1 and Pmr1 function greatly elevate cytoplasmic calcium levels (Cronin et al. 2002). Reinforcing the importance of ER, Golgi and vacuoles in the overall calcium managing in the yeast cell is the participation of proteins located in these compartments, for example, Gdt1, is a transmembrane protein involved in calcium and pH homeostasis in yeast and higher eukaryotes. It localizes to the cis- and medial-Golgi apparatus, but the GFP-fusion protein localizes to the vacuole. The exact role of this protein is unknown, but possibly related to glycosylation since deficiency in its human homologue TMEM165, a human gene which causes congenital disorders of glycosylation (Demaegd et al. 2013). At the vacuole also locate Ccc1 (Cross-Complements Ca2+ phenotype of csg1), this is a vacuolar Fe2+/Mn2+ transporter that also may participate in the respiration process (Fu et al. 1994; Lapinskas et al. 1996), and ECM27 and YDL206W genes coding for two members of the CAX (cation exchangers) family of vacuolar transporters, closely related to Vcx1 and Vnx1,
Finally, it is worth to mention the only mutation found causing the need of large amounts of extracellular calcium for survival, the cal1-1 mutation (Ohya et al. 1984). This mutation locates in CDC43 gene, that encodes for a β-subunit of geranylgeranyltransferase type I, which catalyzes geranylgeranylation to the cysteine residue in proteins containing a C-terminal CaaX sequence ending in Leu or Phe. In addition to this novel role in calcium homeostasis, the substrates under its regulation are important for morphogenesis (Adams et al. 1990).
As for the latter, the precise role in calcium homeostasis of many of these proteins remains obscure and needs clarification. Some of them open new venues for understanding how calcium is regulated and associated factors and mechanisms that modulate calcium storage and managing. However proteins are not the only new regulatory elements found, but other cations and macromolecules are known to participate in calcium homeostasis.
7.7 The Role of Magnesium in Regulating the Calcium Response
Cui and collaborators (2009a) discovered that the calcium sensitivity displayed by a pmc1 mutant was dependent not only on the concentration of extracellular calcium but on the composition of medium. Further analyses revealed that this suppressing factor was the presence of variable concentrations of magnesium in media. In this way, increasing concentrations of Mg2+ elevated the IC50 to calcium toxicity of a pmc1 mutant but also modified sensitivity to calcium caused by the combination of double or triple pmr1, vcx1 and/or calcineurin (cnb1) mutations.
Furthermore, this study indicated the existence of a Mg2+-inhibited calcium transport system located at the PM. Calcium toxicity displayed by a yvc1 cch1 pmc1 vcx1 quadruple mutant was suppressed by addition of magnesium to the medium. In fact, a mathematical modeling of calcium transport across the PM predicted the presence of at least two transport systems, in addition to that integrated by Mid1 and Cch1, in the plasma membrane. Only in this way it could be accommodated the experimental data of calcium sensitivities in a variety of single and double mutants involving pumps and channels, and measurements intracellular levels of calcium. Magnesium will have a major role in regulating, at least one of these calcium transport systems. These authors conclude that transporter M is regulated by Mg2+ and transporter X respond to a hypertonic calcium shock (see also Fig. 7.1).
One of the most abundant divalent cations in cells is magnesium. Mg2+ participates acting a counterion in stabilizing many macromolecules such as RNA and DNA or single nucleotides (i.e. ATP). It also mediates in important catalytic processes and in stabilizing large molecules or membranes (see Wiesenberger et al. 2007 and references there in). Cellular concentrations of Mg2+ are in the millimolar range (from 15 to 20 mM), about three orders of magnitude lower than those of Ca2+ (100–200 nM). In mammals, entry of Mg2+ in the cell is an electrogenic process requiring a negative charge at the inner side of the PM and the activity of two transporters (TRPM6 and TRPM7) (Schlingmann and Gudermann 2005; Schmitz et al. 2003), or by members of a heterogeneous protein family, designated as CorA homologues, and found in lower and high eukaryotes (plants and animals), allowing grow or development even in the presence of very low concentrations of magnesium. In yeast, Mrs2 and Alr1 are orthologues of CorA.
Mg2+ enters the cell through the activity of two members of the CorA family of transporters, Alr1 and Alr2 (Graschopf et al. 2001; Macdiarmid and Gardner 1998). These are the metal ion transporter superfamily, MIT. Alr1 and Alr2 may form oligomeric transporter at the plasma membrane constituting a high affinity Mg2+ uptake system (Wachek et al. 2006). Mg2+ can be stored in two subcellular compartments, the mitochondria, vacuole and ER/Golgi. For the first store the activity of two transporters located in the inner membrane of mitochondria are needed, Mrs2 and Lpe10. Mg2+ is stored in the trans-golgi, and possibly in the vacuole by the activity of a Mg2+/H+ antiporters (Pisat et al. 2009); (Borrelly et al. 2001). Storage of magnesium in the vacuole requires the activity of the vacuolar proton-ATPase, and for Mg2+ efflux it is required the Mrs2 transporter, also belonging to the MIT superfamily (Pisat et al. 2009).
Important for understanding calcium homeostasis were the results of a RNA profile analysis of changes in expression dependent on Mg2+ depletion. These showed the intimate relationship between magnesium and calcium homeostasis (Wiesenberger et al. 2007). A reduction in external Mg2+ upregulated the ENA1, encoding the P-type ATPase sodium pump, and PHO89, encoding a sodium/phosphate cotransporter, which are also upregulated under calcium and alkaline pH stress. In that work they demonstrated that Mg2+ starvation caused an increase in cytoplasmic calcium. A rise in cytosolic free calcium activated the calmodulin/calcineurin network, which led to the activation of Crz1 TF. ENA1 and PHO89 are among the genes regulated by Crz1 (Hu et al. 2007; Mendizabal et al. 2001).
How Mg2+ also influences calcium homeostasis? Immediately from what has been exposed before, a negative role in calcium efflux through the PM is expected, causing a reduction in the intracellular pool of Ca2+. To this effect, an elevation of intracellular Mg2+ will reduce the release of calcium from the internal stores. But a third effect is also predictable, based on the capacity of EF-hands to bind Mg2+ in addition to Ca2+ (see review Grabarek 2011). Calmodulin and other EF-hand containing proteins, such as the regulatory subunit of calcineurin, may bind Mg2+ rendering an alternative conformation to that originated by calcium, in fact magnesium helps to release calcium from these proteins allowing the pass from the holo-enzyme to the apo-enzyme state (Grabarek 2011). Thus, Mg2+ will directly affect the functionality of the calmodulin/calcineurin network, attenuating calcium signaling. Altogether, an excess of Mg2+ will cause a reduction on calcium signaling and probably increase tolerance to this cation, meanwhile low extracellular levels of Mg2+ increase calcium signaling (Wiesenberger et al. 2007).
7.8 Role of Inorganic Phosphate in Calcium Homeostasis
The property of inorganic phosphate or polyphosphates in chelating cations is fundamental to understand calcium and magnesium homeostasis. Actually calcium and phosphate homeostasis have major importance in vertebrates since it is crucial in bone formation, among other cellular and tissue specific processes (Shaker and Deftos 2000).
In the yeast, the presence of polyphosphates in vacuoles is the basis for the immobilization of calcium. In this way, the capacity to maintain the vacuolar Ca2+ concentration up to 2 mM is a result of Ca2+ binding to vacuolar polyphosphate (Dunn et al. 1994). However, Ca2+ can be completely released from isolated vacuoles or from whole cells using the ionophores A23187 or ionomycin, suggesting that the non-exchangeable pool of Ca2+ is soluble (Cunningham and Fink 1994a).
The conserved pathway governing phosphate homeostasis in yeast is composed of the PHO genes (reviewed in Tomar and Sinha 2014). Interestingly there is well established interconnection between calcium- and phosphate-dependent regulation, for example at the level of the Crz1 transcription factor. As cited before, Crz1 up-regulates PHO89 in response to calcium stress or alkalinization, and, in the absence of calcium stress, among the cyclin-dependent kinase Pho85 targets is the transcription factor Crz1p (Sopko et al. 2006).
7.9 Concluding Remarks and Future Prospects
Numerous elements have been described in this chapter participating in transport, signaling and storage of a principal messenger in cells, the calcium ion. Along years, S. cerevisiae has served as a model to understand how cells deal with a messenger that is essential but lethal at the same time. Most of the principal elements are well known, and many functional analyses have been performed to understand the molecular and biochemical mechanisms involved, e.g. the calmodulin/calcineurin pathway and its fungal effector Crz1. S. cerevisiae has served as a model to study calcium homeostasis in other fungi, and in some cases particular variations are found, specially in how Crz homologues are signalized and the transcriptional function of this TF. But a general mechanism underlies in almost all cellular systems under study, from bacteria to human cells, and for that the budding yeast has largely provided with basic and elemental findings to understand this complex homeostatic system. Future research lines will provide with a more detailed view of the exocytic process of calcium and the interrelationships among different ions and ambient stress signals to modulate and generate a coordinated response.
Bibliography
Adams AE, Johnson DI, Longnecker RM, Sloat BF, Pringle JR (1990) CDC42 and CDC43, two additional genes involved in budding and the establishment of cell polarity in the yeast Saccharomyces cerevisiae. J Cell Biol 111:131–142
Aiello DP, Fu L, Miseta A, Bedwell DM (2002) Intracellular glucose 1-phosphate and glucose 6-phosphate levels modulate Ca2+ homeostasis in Saccharomyces cerevisiae. J Biol Chem 277:45751–45758
Anraku Y, Ohya Y, Iida H (1991) Cell cycle control by calcium and calmodulin in Saccharomyces cerevisiae. Biochim Biophys Acta 1093:169–177
Antebi A, Fink GR (1992) The yeast Ca(2+)-ATPase homologue, PMR1, is required for normal Golgi function and localizes in a novel Golgi-like distribution. Mol Biol Cell 3:633–654
Beeler T, Gable K, Zhao C, Dunn T (1994) A novel protein, CSG2p, is required for Ca2+ regulation in Saccharomyces cerevisiae. J Biol Chem 269:7279–7284
Beeler TJ, Fu D, Rivera J, Monaghan E, Gable K, Dunn TM (1997) SUR1 (CSG1/BCL21), a gene necessary for growth of Saccharomyces cerevisiae in the presence of high Ca2+ concentrations at 37 degrees C, is required for mannosylation of inositolphosphorylceramide. Mol Gen Genet 255:570–579
Birchwood CJ, Saba JD, Dickson RC, Cunningham KW (2001) Calcium influx and signaling in yeast stimulated by intracellular sphingosine 1-phosphate accumulation. J Biol Chem 276:11712–11718
Bonilla M, Cunningham KW (2003) Mitogen-activated protein kinase stimulation of Ca(2+) signaling is required for survival of endoplasmic reticulum stress in yeast. Mol Biol Cell 14:4296–4305
Bonilla M, Nastase KK, Cunningham KW (2002) Essential role of calcineurin in response to endoplasmic reticulum stress. EMBO J 21:2343–2353
Borrelly G, Boyer JC, Touraine B, Szponarski W, Rambier M, Gibrat R (2001) The yeast mutant vps5Delta affected in the recycling of Golgi membrane proteins displays an enhanced vacuolar Mg2+/H+ exchange activity. Proc Natl Acad Sci U S A 98:9660–9665
Boustany LM, Cyert MS (2002) Calcineurin-dependent regulation of Crz1p nuclear export requires Msn5p and a conserved calcineurin docking site. Genes Dev 16:608–619
Cagnac O, Leterrier M, Yeager M, Blumwald E (2007) Identification and characterization of Vnx1p, a novel type of vacuolar monovalent cation/H+ antiporter of Saccharomyces cerevisiae. J Biol Chem 282:24284–24293
Cagnac O, Aranda-Sicilia MN, Leterrier M, Rodriguez-Rosales MP, Venema K (2010) Vacuolar cation/H+ antiporters of Saccharomyces cerevisiae. J Biol Chem 285:33914–33922
Catterall WA (2000) Structure and regulation of voltage-gated Ca2+ channels. Annu Rev Cell Dev Biol 16:521–555
Connolly S, Kingsbury T (2012) Regulatory subunit myristoylation antagonizes calcineurin phosphatase activation in yeast. J Biol Chem 287:39361–39368
Courchesne WE, Ozturk S (2003) Amiodarone induces a caffeine-inhibited, MID1-dependent rise in free cytoplasmic calcium in Saccharomyces cerevisiae. Mol Microbiol 47:223–234
Cronin SR, Rao R, Hampton RY (2002) Cod1p/Spf1p is a P-type ATPase involved in ER function and Ca2+ homeostasis. J Cell Biol 157:1017–1028
Cui J, Kaandorp JA (2006) Mathematical modeling of calcium homeostasis in yeast cells. Cell Calcium 39:337–348
Cui J, Kaandorp JA, Ositelu OO, Beaudry V, Knight A, Nanfack YF, Cunningham KW (2009a) Simulating calcium influx and free calcium concentrations in yeast. Cell Calcium 45:123–132
Cui J, Kaandorp JA, Sloot PM, Lloyd CM, Filatov MV (2009b) Calcium homeostasis and signaling in yeast cells and cardiac myocytes. FEMS Yeast Res 9:1137–1147
Culotta VC, Yang M, Hall MD (2005) Manganese transport and trafficking: lessons learned from Saccharomyces cerevisiae. Eukaryot Cell 4:1159–1165
Cunningham KW (2005) Calcium signaling networks in yeast. In: Putney JW (ed) Calcium signaling, 2nd edn. Taylor & Francis Group/CRC Press, Florida, pp 107–201
Cunningham KW, Fink GR (1994a) Ca2+ transport in Saccharomyces cerevisiae. J Exp Biol 196:157–166
Cunningham KW, Fink GR (1994b) Calcineurin-dependent growth control in Saccharomyces cerevisiae mutants lacking PMC1, a homolog of plasma membrane Ca2+ ATPases. J Cell Biol 124:351–363
Cunningham KW, Fink GR (1996) Calcineurin inhibits VCX1-dependent H+/Ca2+ exchange and induces Ca2+ ATPases in Saccharomyces cerevisiae. Mol Cell Biol 16:2226–2237
Cyert MS (2001) Genetic analysis of calmodulin and its targets in Saccharomyces cerevisiae. Annu Rev Genet 35:647–672
Cyert MS (2003) Calcineurin signaling in Saccharomyces cerevisiae: how yeast go crazy in response to stress. Biochem Biophys Res Commun 311:1143–1150
Cyert MS, Thorner J (1992) Regulatory subunit (CNB1 gene product) of yeast Ca2+/calmodulin-dependent phosphoprotein phosphatases is required for adaptation to pheromone. Mol Cell Biol 12:3460–3469
Cyert MS, Kunisawa R, Kaim D, Thorner J (1991) Yeast has homologs (CNA1 and CNA2 gene products) of mammalian calcineurin, a calmodulin-regulated phosphoprotein phosphatase. Proc Natl Acad Sci U S A 88:7376–7380
Dalal CK, Cai L, Lin Y, Rahbar K, Elowitz MB (2014) Pulsatile dynamics in the yeast proteome. Curr Biol 24:2189–2194
Davis TN, Urdea MS, Masiarz FR, Thorner J (1986) Isolation of the yeast calmodulin gene: calmodulin is an essential protein. Cell 47:423–431
Demaegd D, Foulquier F, Colinet AS, Gremillon L, Legrand D, Mariot P, Peiter E, Van SE, Matthijs G, Morsomme P (2013) Newly characterized Golgi-localized family of proteins is involved in calcium and pH homeostasis in yeast and human cells. Proc Natl Acad Sci U S A 110:6859–6864
Denis V, Cyert MS (2002) Internal Ca(2+) release in yeast is triggered by hypertonic shock and mediated by a TRP channel homologue. J Cell Biol 156:29–34
Desfarges L, Durrens P, Juguelin H, Cassagne C, Bonneu M, Aigle M (1993) Yeast mutants affected in viability upon starvation have a modified phospholipid composition. Yeast 9:267–277
Dickson RC, Lester RL (2002) Sphingolipid functions in Saccharomyces cerevisiae. Biochim Biophys Acta 1583:13–25
Dunn T, Gable K, Beeler T (1994) Regulation of cellular Ca2+ by yeast vacuoles. J Biol Chem 269:7273–7278
Durr G, Strayle J, Plemper R, Elbs S, Klee SK, Catty P, Wolf DH, Rudolph HK (1998) The medial-Golgi ion pump Pmr1 supplies the yeast secretory pathway with Ca2+ and Mn2+ required for glycosylation, sorting, and endoplasmic reticulum-associated protein degradation. Mol Biol Cell 9:1149–1162
Fu D, Beeler T, Dunn T (1994) Sequence, mapping and disruption of CCC1, a gene that cross-complements the Ca(2+)-sensitive phenotype of csg1 mutants. Yeast 10:515–521
Grabarek Z (2011) Insights into modulation of calcium signaling by magnesium in calmodulin, troponin C and related EF-hand proteins. Biochim Biophys Acta 1813:913–921
Graschopf A, Stadler JA, Hoellerer MK, Eder S, Sieghardt M, Kohlwein SD, Schweyen RJ (2001) The yeast plasma membrane protein Alr1 controls Mg2+ homeostasis and is subject to Mg2+-dependent control of its synthesis and degradation. J Biol Chem 276:16216–16222
Halachmi D, Eilam Y (1996) Elevated cytosolic free Ca2+ concentrations and massive Ca2+ accumulation within vacuoles, in yeast mutant lacking PMR1, a homolog of Ca2+-ATPase. FEBS Lett 392:194–200
Hilioti Z, Gallagher DA, Low-Nam ST, Ramaswamy P, Gajer P, Kingsbury TJ, Birchwood CJ, Levchenko A, Cunningham KW (2004) GSK-3 kinases enhance calcineurin signaling by phosphorylation of RCNs. Genes Dev 18:35–47
Hoekstra MF, Liskay RM, Ou AC, DeMaggio AJ, Burbee DG, Heffron F (1991) HRR25, a putative protein kinase from budding yeast: association with repair of damaged DNA. Science 253:1031–1034
Hu Z, Killion PJ, Iyer VR (2007) Genetic reconstruction of a functional transcriptional regulatory network. Nat Genet 39:683–687
Iida H, Yagawa Y, Anraku Y (1990) Essential role for induced Ca2+ influx followed by [Ca2+]i rise in maintaining viability of yeast cells late in the mating pheromone response pathway. A study of [Ca2+]i in single Saccharomyces cerevisiae cells with imaging of fura-2. J Biol Chem 265:13391–13399
Iida H, Nakamura H, Ono T, Okumura MS, Anraku Y (1994) MID1, a novel Saccharomyces cerevisiae gene encoding a plasma membrane protein, is required for Ca2+ influx and mating. Mol Cell Biol 14:8259–8271
Kafadar KA, Zhu H, Snyder M, Cyert MS (2003) Negative regulation of calcineurin signaling by Hrr25p, a yeast homolog of casein kinase I. Genes Dev 17:2698–2708
Kanzaki M, Nagasawa M, Kojima I, Sato C, Naruse K, Sokabe M, Iida H (1999) Molecular identification of a eukaryotic, stretch-activated nonselective cation channel. Science 285:882–886
Kingsbury TJ, Cunningham KW (2000) A conserved family of calcineurin regulators. Genes Dev 14:1595–1604
Lapinskas PJ, Lin SJ, Culotta VC (1996) The role of the Saccharomyces cerevisiae CCC1 gene in the homeostasis of manganese ions. Mol Microbiol 21:519–528
Locke EG, Bonilla M, Liang L, Takita Y, Cunningham KW (2000) A homolog of voltage-gated Ca(2+) channels stimulated by depletion of secretory Ca(2+) in yeast. Mol Cell Biol 20:6686–6694
Macdiarmid CW, Gardner RC (1998) Overexpression of the Saccharomyces cerevisiae magnesium transport system confers resistance to aluminum ion. J Biol Chem 273:1727–1732
Mandal D, Woolf TB, Rao R (2000) Manganese selectivity of pmr1, the yeast secretory pathway ion pump, is defined by residue gln783 in transmembrane segment 6. Residue Asp778 is essential for cation transport. J Biol Chem 275:23933–23938
Marchi V, Sorin A, Wei Y, Rao R (1999) Induction of vacuolar Ca2+-ATPase and H+/Ca2+ exchange activity in yeast mutants lacking Pmr1, the Golgi Ca2+-ATPase. FEBS Lett 454:181–186
Matheos DP, Kingsbury TJ, Ahsan US, Cunningham KW (1997) Tcn1p/Crz1p, a calcineurin-dependent transcription factor that differentially regulates gene expression in Saccharomyces cerevisiae. Genes Dev 11:3445–3458
Matsumoto TK, Ellsmore AJ, Cessna SG, Low PS, Pardo JM, Bressan RA, Hasegawa PM (2002) An osmotically induced cytosolic Ca2+ transient activates calcineurin signaling to mediate ion homeostasis and salt tolerance of Saccharomyces cerevisiae. J Biol Chem 277:33075–33080
Mehlgarten C, Schaffrath R (2003) Mutant casein kinase I (Hrr25p/Kti14p) abrogates the G1 cell cycle arrest induced by Kluyveromyces lactiszymocin in budding yeast. Mol Genet Genomics 269:188–196
Mehta S, Li H, Hogan PG, Cunningham KW (2009) Domain architecture of the regulators of calcineurin (RCANs) and identification of a divergent RCAN in yeast. Mol Cell Biol 29:2777–2793
Mendizabal I, Pascual-Ahuir A, Serrano R, de Larrinoa IF (2001) Promoter sequences regulated by the calcineurin-activated transcription factor Crz1 in the yeast ENA1 gene. Mol Genet Genomics 265:801–811
Miseta A, Kellermayer R, Aiello DP, Fu L, Bedwell DM (1999) The vacuolar Ca2+/H+ exchanger Vcx1p/Hum1p tightly controls cytosolic Ca2+ levels in S. cerevisiae. FEBS Lett 451:132–136
O’Donnell AF, Huang L, Thorner J, Cyert MS (2013) A calcineurin-dependent switch controls the trafficking function of alpha-arrestin Aly1/Art6. J Biol Chem 288:24063–24080
Ohya Y, Ohsumi Y, Anraku Y (1984) Genetic study of the role of calcium ions in the cell division cycle of Saccharomyces cerevisiae: a calcium-dependent mutant and its trifluoperazine-dependent pseudorevertants. Mol Gen Genet 193:389–394
Ohya Y, Ohsumi Y, Anraku Y (1986) Isolation and characterization of Ca2+-sensitive mutants of Saccharomyces cerevisiae. J Gen Microbiol 132:979–988
Ozeki-Miyawaki C, Moriya Y, Tatsumi H, Iida H, Sokabe M (2005) Identification of functional domains of Mid1, a stretch-activated channel component, necessary for localization to the plasma membrane and Ca2+ permeation. Exp Cell Res 311:84–95
Paidhungat M, Garrett S (1997) A homolog of mammalian, voltage-gated calcium channels mediates yeast pheromone-stimulated Ca2+ uptake and exacerbates the cdc1(Ts) growth defect. Mol Cell Biol 17:6339–6347
Palmer CP, Zhou XL, Lin J, Loukin SH, Kung C, Saimi Y (2001) A TRP homolog in Saccharomyces cerevisiae forms an intracellular Ca(2+)-permeable channel in the yeast vacuolar membrane. Proc Natl Acad Sci U S A 98:7801–7805
Peiter E, Fischer M, Sidaway K, Roberts SK, Sanders D (2005) The Saccharomyces cerevisiae Ca2+ channel Cch1pMid1p is essential for tolerance to cold stress and iron toxicity. FEBS Lett 579:5697–5703
Pinton P, Pozzan T, Rizzuto R (1998) The Golgi apparatus is an inositol 1,4,5-trisphosphate-sensitive Ca2+ store, with functional properties distinct from those of the endoplasmic reticulum. EMBO J 17:5298–5308
Pisat NP, Pandey A, Macdiarmid CW (2009) MNR2 regulates intracellular magnesium storage in Saccharomyces cerevisiae. Genetics 183:873–884
Polizotto RS, Cyert MS (2001) Calcineurin-dependent nuclear import of the transcription factor Crz1p requires Nmd5p. J Cell Biol 154:951–960
Pozos TC, Sekler I, Cyert MS (1996) The product of HUM1, a novel yeast gene, is required for vacuolar Ca2+/H+ exchange and is related to mammalian Na+/Ca2+ exchangers. Mol Cell Biol 16:3730–3741
Prezant TR, Chaltraw WE Jr, Fischel-Ghodsian N (1996) Identification of an overexpressed yeast gene which prevents aminoglycoside toxicity. Microbiology 142(Pt 12):3407–3414
Ripmaster TL, Vaughn GP, Woolford JL Jr (1993) DRS1 to DRS7, novel genes required for ribosome assembly and function in Saccharomyces cerevisiae. Mol Cell Biol 13:7901–7912
Rodriguez A, Roy J, Martinez-Martinez S, Lopez-Maderuelo MD, Nino-Moreno P, Orti L, Pantoja-Uceda D, Pineda-Lucena A, Cyert MS, Redondo JM (2009) A conserved docking surface on calcineurin mediates interaction with substrates and immunosuppressants. Mol Cell 33:616–626
Roy J, Li H, Hogan PG, Cyert MS (2007) A conserved docking site modulates substrate affinity for calcineurin, signaling output, and in vivo function. Mol Cell 25:889–901
Rudolph HK, Antebi A, Fink GR, Buckley CM, Dorman TE, LeVitre J, Davidow LS, Mao JI, Moir DT (1989) The yeast secretory pathway is perturbed by mutations in PMR1, a member of a Ca2+ ATPase family. Cell 58:133–145
Schlingmann KP, Gudermann T (2005) A critical role of TRPM channel-kinase for human magnesium transport. J Physiol 566:301–308
Schmitz C, Perraud AL, Johnson CO, Inabe K, Smith MK, Penner R, Kurosaki T, Fleig A, Scharenberg AM (2003) Regulation of vertebrate cellular Mg2+ homeostasis by TRPM7. Cell 114:191–200
Shaker JL, Deftos L (2000) Calcium and phosphate homeostasis (De Groot LJ, Beck-Peccoz P, Chrousos G, Dungan K, Grossman A, Hershman JM, Koch C, McLachlan R, New M, Rebar R, Singer F, Vinik A, Weickert MO, eds). MDText.com, Inc., South Dartmouth. PMID: 25905252
Sopko R, Huang D, Preston N, Chua G, Papp B, Kafadar K, Snyder M, Oliver SG, Cyert M, Hughes TR, Boone C, Andrews B (2006) Mapping pathways and phenotypes by systematic gene overexpression. Mol Cell 21:319–330
Sorin A, Rosas G, Rao R (1997) PMR1, a Ca2+-ATPase in yeast Golgi, has properties distinct from sarco/endoplasmic reticulum and plasma membrane calcium pumps. J Biol Chem 272:9895–9901
Starovasnik MA, Davis TN, Klevit RE (1993) Similarities and differences between yeast and vertebrate calmodulin: an examination of the calcium-binding and structural properties of calmodulin from the yeast Saccharomyces cerevisiae. Biochemistry 32:3261–3270
Stathopoulos AM, Cyert MS (1997) Calcineurin acts through the CRZ1/TCN1-encoded transcription factor to regulate gene expression in yeast. Genes Dev 11:3432–3444
Stathopoulos-Gerontides A, Guo JJ, Cyert MS (1999) Yeast calcineurin regulates nuclear localization of the Crz1p transcription factor through dephosphorylation. Genes Dev 13:798–803
Strayle J, Pozzan T, Rudolph HK (1999) Steady-state free Ca(2+) in the yeast endoplasmic reticulum reaches only 10 microM and is mainly controlled by the secretory pathway pump pmr1. EMBO J 18:4733–4743
Takita Y, Engstrom L, Ungermann C, Cunningham KW (2001) Inhibition of the Ca(2+)-ATPase Pmc1p by the v-SNARE protein Nyv1p. J Biol Chem 276:6200–6206
Tanida I, Takita Y, Hasegawa A, Ohya Y, Anraku Y (1996) Yeast Cls2p/Csg2p localized on the endoplasmic reticulum membrane regulates a non-exchangeable intracellular Ca2+ pool cooperatively with calcineurin. FEBS Lett 379:38–42
Tomar P, Sinha H (2014) Conservation of PHO pathway in ascomycetes and the role of Pho84. J Biosci 39:525–536
Viladevall L, Serrano R, Ruiz A, Domenech G, Giraldo J, Barcelo A, Arino J (2004) Characterization of the calcium-mediated response to alkaline stress in Saccharomyces cerevisiae. J Biol Chem 279:43614–43624
Wachek M, Aichinger MC, Stadler JA, Schweyen RJ, Graschopf A (2006) Oligomerization of the Mg2+-transport proteins Alr1p and Alr2p in yeast plasma membrane. FEBS J 273:4236–4249
Wei Y, Marchi V, Wang R, Rao R (1999) An N-terminal EF hand-like motif modulates ion transport by Pmr1, the yeast Golgi Ca(2+)/Mn(2+)-ATPase. Biochemistry 38:14534–14541
Wiesenberger G, Steinleitner K, Malli R, Graier WF, Vormann J, Schweyen RJ, Stadler JA (2007) Mg2+ deprivation elicits rapid Ca2+ uptake and activates Ca2+/calcineurin signaling in Saccharomyces cerevisiae. Eukaryot Cell 6:592–599
Yoshimura H, Tada T, Iida H (2004) Subcellular localization and oligomeric structure of the yeast putative stretch-activated Ca2+ channel component Mid1. Exp Cell Res 293:185–195
Zhou Y, Meraner P, Kwon HT, Machnes D, Oh-hora M, Zimmer J, Huang Y, Stura A, Rao A, Hogan PG (2010a) STIM1 gates the store-operated calcium channel ORAI1 in vitro. Nat Struct Mol Biol 17:112–116
Zhou Y, Ramachandran S, Oh-hora M, Rao A, Hogan PG (2010b) Pore architecture of the ORAI1 store-operated calcium channel. Proc Natl Acad Sci U S A 107:4896–4901
Acknowledgements
Work at the laboratory of Dr. Espeso at the Centro de Investigaciones Biológicas (CIB), from the Spanish Research Council, CSIC, is supported by the Spanish Ministerio de Economía y Competitividad through grant BFU2012-33142.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Espeso, E.A. (2016). The CRaZy Calcium Cycle. In: Ramos, J., Sychrová, H., Kschischo, M. (eds) Yeast Membrane Transport. Advances in Experimental Medicine and Biology, vol 892. Springer, Cham. https://doi.org/10.1007/978-3-319-25304-6_7
Download citation
DOI: https://doi.org/10.1007/978-3-319-25304-6_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-25302-2
Online ISBN: 978-3-319-25304-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)