Abstract
Marine fungi have been a rich source of bioactive natural products with interesting pharmaceutical activities and potential therapeutic applications. This chapter reviews the recent analytical techniques for discovery and the characterization of bioactive compounds derived from marine fungi, which are highly diversified and are less explored. An overview about bioprospecting, collection, preparation, and preservation of fungi samples are also presented, as well as different methods and strategies used for extraction, fractionation, and structural characterization of the bioactive compounds are discussed, including their advantages and the disadvantages. Possible roles of these natural compounds in several interesting biological activities are also covered in this chapter.
Conflict of Interest
The authors report no declarations of interest.
Access provided by CONRICYT-eBooks. Download reference work entry PDF
Similar content being viewed by others
Keywords
- Analytical methodologies
- Fungus
- Bioprospection
- Preservation
- Collection
- Extraction
- Fractionation
- Chromatography
- Bioactivity
- Bioassay
- Structural characterization
- Online combination
1 Introduction
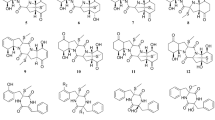
The oceans dominate the surface of the Earth and contain the greatest known biodiversity of life [1]. With the increase of oceans exploration, a growing number of bioactive natural products are being isolated from several marine organisms [2, 3]. The marine environment represents a rich source of both biological and chemical diversity [1]. Recent reports estimate hundreds of millions of marine species depicting over 90 % of total marine biomass containing unique molecules. For marine fungi, only about 465 species are referenced; however, it is estimated that there are 1.5 million species [4, 5]. Marine fungi provide a diverse and remarkable supply of promising bioactive molecules, often with interesting applications in medicine, such as penicillin, caspofungin, mevinolin, and fingolimod [6–8]. These compounds showed several biological properties, including antibacterial, antifungal, and immunomodulatory activities, as well as cholesterol synthesis inhibition [9–11]. Furthermore, in the health care area, studies revealed promising bioactive compounds (BC) isolated from marine fungi sources, with proven anticancer activity: penicisteroid A (1) is a new polyoxygenated steroid isolated from the Penicillium chrysogenum QEN-24S, obtained from a marine red algae. It showed a distinctive chemical structure with tetrahydroxy and C-16-acetoxy groups and exhibited potent cytotoxic activity against the tumor cell lines HeLa, SW1990, and NCI-H460 [12]. From fungus strain KT29 isolated from the red seaweed Kappaphycus alvarezii, one compound, named 2-carboxy-8-methoxy-naphthalene-1-ol (2), was obtained and showed in vitro cytotoxicity against the human bladder carcinoma cell line 5637 [13]. Two other new alkaloids, 2-(3,3-dimethylprop-1-ene)-costaclavine (3) and 2-(3,3-dimethylprop-1-ene)-epicostaclavine (4), were isolated from the marine-derived fungus Aspergillus fumigatus. Both compounds showed cytotoxicity against a mouse leukemia cell line (P388) [14].
The BCs are considered chemical compounds derived and isolated from biological sources. Lately, the characterization of the compositional, structural, and sequential features of BC has been the main focus. Structural information can be used to organize these compounds according to Schmitz’s chemical classification into six major chemical classes, namely, alkaloids, peptides, polyketides, shikimates, sugars, and terpenes [1]. However, marine fungi have not been given the attention they deserve, and a very limited insight into the capabilities and bioactive potential of marine microorganisms is yet available in the scientific literature. There is still scope for more research to explore the potential of marine microorganisms as producers of novel drugs, which are naturally accepted by consumers unlike chemically synthesized drugs [15].
This chapter summarizes different methodologies used to isolate pure bioactive compounds . Different approaches for the collection and preservation of marine fungi samples are presented, as well as some possible techniques for extraction, fractionation, and structural characterization of bioactive compounds.
2 Prospection, Collection, and Preservation of Marine Fungi
2.1 Bioprospecting in Marine Fungi
Bioprospecting is the process of discovery and commercialization of new products based on biological resources [16]. It is the systematic search for and development of new sources of chemical compounds, genes, microorganisms, macroorganisms, and other valuable products from nature. Bioprospecting involves the incessant research for biochemical and genetic sources with high commercial value from nature resource and that have never been used in traditional medicine before [17, 18]. Thus, bioprospecting means looking for ways to commercialize biodiversity . Currently, the development on indigenous knowledge associated to the exploitation and administration of biological resources has also been incorporated into the concept of bioprospecting. Consequently, bioprospecting comprises the conservation and sustainable use of biological resources and the rights of indigenous and local populations [19, 20]. Bioprospecting, when well-managed, can be beneficial, leading to the development of new BC. In contrast, bioprospecting also can lead to environmental problems relating to unauthorized overexploration, as well as social and economic complications [20, 21]. Bioprospecting generates environmental disruption problems during the extraction procedure. Special attention must be given to ethical questions and conservation policies, i.e., research must honor host organizations regarding new discoveries, including new habitats of rare and endangered species [21, 22].
Marine bioprospecting has mainly focused on macroorganisms because of their easy availability, ease of capture, rich biodiversity, and a variety of unique molecules that they developed in response to hostile habitats and environmental conditions. However, a growing effort has increased the research and exploitation of the deep ocean, mostly around hydrothermal vents, due to unexplored biodiversity developed in extreme conditions [23, 24].
Extreme environments such as Antarctica or the high seas, particularly the deep seabed, provide locales for “extremophiles,” which are organisms with unique metabolic properties, which makes them promising and interesting sources of bioactive compounds [25–27]. The biological circumstances which enable these extremophiles to survive in extreme pressures, temperatures, pH, light, salinity, and other exceptional conditions are sources of new prospective for scientific exploration and commercial utilization [28–30]. However, tropical environments and shallow temperate have been the most explored and studied so far [24, 31]. Another challenge to bioprospecting in these habitats, as in all marine ecosystems, is to have access to a sufficient quantity of biological substances in order to get pure bioactive compounds [32, 33]. Bioprospecting of marine compounds commonly depends on collecting wild specimens [34]. Novel and less troublesome solutions can be found through bioprospecting the oceans , especially in mostly unexplored microscopic marine organisms. The combination of modern advances in DNA technologies and increased consciousness of environmental problems, such as global warming, have stimulated the science of marine microbiology [23, 35].
2.2 Collection of Marine Fungi
In order to have a full and undisputable natural compound assessment and to assure the properties discovered, different sampling strategies, depending on the type of habitat and the species ecology, have to be taken into account to ensure the appropriate collection of the mixed cultures from natural environments. Since the collection of microorganisms from the marine environment is not always easy, it is often necessary to also harvest the supporting materials in order to keep the fungi viable until arriving to the laboratory [36].
The biodiversity preservation should be taken into account, reducing the impacts as much as possible, in order to protect marine species from environment disorders during collection. Harvesting of organisms must be restricted to minimum quantities and attention with rare or endangered species must also be taken into account [37].
Distinct microorganisms can be found in different areas of the marine environment, such as (1) in free suspension in the water column; (2) in flocculated particulates in the water column; (3) in the sediment; (4) on surfaces of both living and nonliving bodies; and (5) in endophytic/symbiotic associations [36, 38]. The biggest risks concerning the collection of these organisms are contamination and cross-contamination. Thus, the most appropriate techniques rely on sampling the material together with supporting materials, which include water, sediment, portions of plants and other macroorganisms, or other subtracts [36]. In the case of water samples, in order to avoid contamination and proliferation of cultures, they must be collected with sterilized glass containers. For sediments from intertidal areas, sampling can be performed by removing core samples, while in subtidal regions, sediment grabs, such as Ponar and Van Veen grabs, can be used. Nevertheless, in more hostile environments such as the deep sea, more refined procedures are essential, such as the system developed by Parkes et al. [39]. DeepIsoBUG system was developed for high-pressure systems, allowing the collection of cores and slices of the sediment, with each slice being moved to a low pressure container, thus minimizing the chance of contamination and keeping temperature and pressure [37, 39]. After the collection, preservation must be performed as quickly as possible to avoid genetic and phenotypic modifications of the cultures [36, 37]. It is also important to consider that wild harvest only partially satisfies the demand and is an unsuitable production way [34]. Using big quantities of biomass may have an impact on the number of specimens, and for rare species, it can be impossible to collect enough organisms for the research. A solution to these problems can be the aquaculture of target species, allowing the continuous production of biomass using standardized conditions [40]. When it comes to microorganisms and if the compounds are needed on a commercial scale, the fermentation also is a suitable process for bioactive compounds production [41]. Thus, bioactive compounds can only be obtained by collecting from natural sources, aquaculture, or synthesis [42].
2.3 Preservation of Marine Fungi
Conservation of marine specimens is a prerequisite for field studies in faraway zones or when there are restrictions to return the samples to the lab in due time. The harvesting of marine organisms for the study of BCs is frequently performed in open sea, where assays are often difficult or even impossible [43]. In the case of microorganisms , such as fungi, there often is a macro-host which must also be collected during sampling , avoiding sample degradation until arrival at the laboratory [44]. As most marine organisms are quite vulnerable to fast degradation, the samples should be quickly frozen with dry ice and stored at −20 °C as soon as possible until the next step in processing [45]. Once in the lab, the collected fungi need to be preserved pure and viable for additional studies. Usually, they are brought into pure culture and stored in liquid nitrogen in order to maintain the viability of these cultures, preventing genetic and phenotypic changes induced by repeated passages of the cultures [36, 38].
3 Preparation, Extraction, and Fractionation of Marine Fungi
3.1 Preparation of Bioactive Compounds from Fungi Samples
The bioactive compounds isolated from marine fungi samples must follow a multistep process. One of the first steps to be considered is the culture conditions, since marine fungi samples collected will be cultured in nutritional media at the laboratory. Therefore, special attention must be taken into account for the temperature, incubation time, aeration, media composition, and pH, since less favorable conditions can affect the output and yield of the wanted bioactive compound [46]. The next step includes the biological activity screening . The bioactivity assay is an essential parameter in the development of new drugs, usually conducted to measure the effects of a biopharmaceutical drug on a living organism. Extraction of active samples is the next step, followed by the fractionation, separation, and purification of pure compounds. Finally, it follows the structural characterization of the bioactive compounds [47].
Due to the existence of symbiotic relationships between organisms, some marine fungi may be isolated from other marine organisms, such as algae. In order to get BC, including 1-O-(α -d-mannopyranosyl)chlorogentisyl alcohol (5), Yun et al. [48] isolated the fungi Chrysosporium synchronum from a brown algae Sargassum ringgoldium, which showed a radical scavenging activity against 1,1-diphenyl-2-picrylhydrazyl radical (DPPH) [48].
Before delineating the steps of the isolation methodology, the characteristics of the target compound, i.e., molecular size, charge, stability, solubility, and acid–base properties, should be regarded, since the selection of the best procedure for additional separation allows a faster isolation protocol [47, 49].
3.2 Extraction of Bioactive Compounds from Fungi Samples
The bioactive metabolites extracted from marine fungi can be divided into alkaloids, amino acids, polyketides, sugars, sterols, saponins, peptides, terpenoids, hydrocarbons, and fatty acids [50]. Since the chemical nature of bioactive substances in a mixture is unknown, it is not possible to delineate any specific technique for the separation of these components from the complex mixture. However, a wide separation of the mixture can be obtained by extraction with organic solvents . Other methodologies have also been developed to improve the isolation of BCs which include several extraction techniques . The most common extraction methodologies and the main BCs isolated reported in recent literature are discussed in the ensuing sections.
3.2.1 Extraction by Solvents
Bioactive compounds are generally extracted from mycelium and/or culture medium using a variety of aqueous or organic solvents. After sampling from marine habitat, fungi cultures are submitted to extraction using solvents with different polarities [36]. Examples of bioactive molecules and related solvents usually used for extraction are shown in Table 1.
The extracts of marine fungi showing biological activities could be a mixture of different molecules. Most marine fungi yield hydrophobic compounds, when extracted with organic solvent, such as ethanol (EtOH), methanol (MeOH), chloroform (CHCl3), acetone, and ethyl acetate (EtOAc) [2, 51]. However, bioactive hydrophilic compounds can also be extracted from marine fungi using solvents, such as hexane and carbon tetrachloride [52]. New marine-derived compounds, named hypochromins A and B, were obtained from the Hypocrea vinosa, showing great tyrosine kinase inhibitory activity, when isolated from the ethanol extract [53]. Fractions of different polarities are then submitted to biological assays . Sometimes, biological activity is spread across multiple fractions. However, if the isolation is good, the biological activity may be condensed on a single fraction, thus maximizing time and resources. In contrast, when ideal conditions are not respected and biochemical characteristics not investigated, low recoveries are obtained and additional extraction must be made in order to obtain the best association of extraction solvents to obtain better extraction purity [54]. Low processing cost and ease of operation are some of the advantages of using solvent extraction. In turn, the disadvantages are low extraction efficiency, low selectivity, and production of solvent residues [47, 54].
3.2.2 Extraction by Other Modern Methodologies
Due to the limitations presented by extraction with organic solvents, other methods are also applied in the separation of BC. Among them stand out microwave-assisted extraction (MAE); ultrasound-assisted extraction (UAE); supercritical fluid extraction (SFE); subcritical water extraction (SWE); and pressurized liquid extraction (PLE) which are fast and efficient unconventional extraction methods developed for extracting bioactive compounds from microorganisms [55].
MAE is based on the direct effect of microwaves on molecules of the extracted system caused by two mechanisms: ionic conduction and dipole rotation [56]. MAE heats the extracted system directly by friction between polar molecules, leading to very short extraction times. Intracellular heating of the matrix induces pressurized effects that damage cell walls and membranes, as well as cause electroporation effects. Consequently, a quicker transfer of the molecules from the cells into the extracting solvent is observed [56–58]. Polar solvents are better MAE extracts than nonpolar in the following order: water > methanol > ethanol > acetone > ethyl acetate > hexane [57].
UAE notably decreases isolation time and increases extraction efficiency of several natural compounds, due to the formation of cavitation bubbles in the solvent [55, 59]. This ability is influenced by the properties of ultrasound wave, the solvent characteristics, and the ambient conditions. After a cavitation bubble is formed, it collapses throughout the compression cycle, which pushes the liquid molecules together, and a high-speed micro-jet is created towards the matrix particle, promoting the mixture of the solvent with the matrix. Temperature and high pressure involved in this procedure breaks membranes and cell walls. After cell damage, the solvent can easily penetrate into cells, releasing the intracellular contents [55].
SFE is another extraction method that yields extracts with none or less polar impurities than the traditional organic liquid extracts [60]. It is based on the use of a gas compressed at a pressure and temperature above a critical point, comprises a dense gas state in which the fluid combines hybrid properties of liquid and gas. The supercritical CO2 has properties, such as high diffusivity to extract organic compounds, low viscosity, nonflammable, low cost, easily accessible, critical point conditions, decompression directly to the atmosphere, and harmless to the environment. In order to overcome limitations in the extraction of polar compounds, the addition of an organic modifier, such as ethanol, is recommended [61]. The greatest limitation of supercritical CO2 is to not be adequate to extract polar compounds. Nevertheless, the addition of an organic solvent, such as EtOH or MeOH, can largely improve extraction yield [55].
SWE , also known as pressurized hot water extraction, besides using an environmentally friendly solvent also allows the adjustment of the dielectric constant of the water, and thus the solubility of organic substances, which allows the extraction of polar and medium-polar compounds [62]. Polar molecules with high solubility in water are extracted with more efficiency at lower temperatures, while medium-polar and nonpolar compounds need a less polar medium induced by higher temperature [63]. Based on the scientific results published in the last years, it has been demonstrated that the SWE is quicker, cheaper, and cleaner than the traditional extraction techniques [64].
PLE , also named accelerated solvent extraction, is a developing technology that uses very low volumes of liquid solvents such as acetone, ethanol, and hexane to retrieve target analytes in a short extraction time. This emerging technology combines both high pressures and temperatures to improve the solubility in the pressurized liquids and increase the desorption kinetics of compounds from the matrices [65].
3.3 Fraction of Bioactive Compounds from Fungi Samples
In order to obtain fractions of increasingly pure bioactive substances from a mixture of extract, the fractionation technique is used. The two main approaches to screening BCs from the extracts are the bioassay-guided fractionation and pure compound screening [66]. In the bioassay-guided fractionation procedure , it is possible to exclude the extracts and fractions that do not show bioactivity. In turn, pure compound screening is used less frequently than bioassay-guided fractionation, and it is necessary to select extracts containing compounds, which are not present in the available libraries of pure compounds, since bioactivity is only checked after isolation and structural elucidation [36, 66]. Table 2 shows the extraction and fractionation methods used for the isolation of bioactive compounds from fungi organisms.
3.3.1 Fractionation by Solvent Partition
The fractionation by solvent partition of active extracts is currently performed using the bioassay-guided fractionation procedure [12, 67–69]. This type of approach is the most used standard procedure and is characterized by several steps: (1) assessment of the potential bioactivity of the sample using a bioassay; (2) extraction using different solvents followed by assessment of bioactivity; (3) repeated fractionation of bioactive extracts and fractions in order to obtain the successful isolation of the bioactive compounds; and (4) structural characterization of the bioactive compounds by spectroscopic techniques, followed by pharmacological and toxicological assays [36, 47, 66].
Selected marine extracts contain compounds of different polarities , thus the fractionation by solvent partition separates the active compound from the inactive according to the different partition coefficients of analytes, resulting in full recovery of target compounds [70]. Compounds, such as alkaloid, shikimates, polyketides, sugars, amino acids, polyhydroxysteroids, and saponins are generally obtained in water soluble fractions; peptides are extracted in medium-polarity fractions, and substances like terpenes, hydrocarbons, and fatty acids are found in low-polarity fractions [36]. Each of the obtained fractions is then subjected to purification.
3.3.2 Separation and Purification by Chromatography
After fractionation by solvent partition, the active fractions are separated and purified by chromatography, in order to find pure bioactive molecules. The active fractions can be subjected to fractionation by column chromatography of several types, such as adsorption on silica gel and gel permeation, applying a range of solvents suitable to the polarity of the active fraction [54]. Silica gel column is the most common stationary phase used in the chromatography technique, and gel permeation chromatography (GPC) is a type of size exclusion chromatography (SEC), which separates compounds on the basis of size. In both techniques, for the successful separation, large amounts of organic solvents are needed [71]. In the final step of separation of pure compounds, other methods, such as thin-layer chromatography (TLC) and high-performance liquid chromatography (HPLC), should be used. Thin-layer chromatography (TLC) is a simple, fast, and cheap procedure, capable of processing large amounts of samples in one chromatography run. TLC has the advantage that it can be used after the column chromatography and before the HPLC technique usually for obtaining phenolic compounds and steroids [13, 72, 73]. Smetanina et al. [72] used the thin-layer chromatography to fractionate two new secondary metabolites (isoacremine D (6) and acremine A (7)) from fungus Myceliophthora lutea [72]. Reversed-phase high-performance liquid chromatography (RT-HPLC) is a technique, also commonly used to separate molecules according to their hydrophobicity. The analytes in a mixture are eluted with a pressurized liquid solvent through a column filled with an adsorbent stationary phase containing hydrophobic groups. As reported in many studies, the HPLC technique has been used as a final purification step to obtain pure bioactive compounds (Table 2) [69, 73, 74].
4 Bioassays for Bioactivity Screening
Bioactivity is the ultimate goal desired throughout the extraction, separation, and purification process of marine fungi organisms. Thus, the designed bioassay is crucial for the detection of potential therapeutic applications . Throughout the isolation process, extracts, fractions, and end products are subjected to bioactivity assays in vitro and/or in vivo. Therefore, screening systems must include a broad range of biological assays in order to unravel potential substance-related activities [75].
Commonly, the bioactivity assays can be categorized into primary and secondary bioassay screens. The primary bioassay screens can be applied to a large number of samples in order to evaluate their bioactivities. The general requirements of these bioassays comprise high capacity, providing quick results, being cheap and not quantitative. During the selection of bioactivity assay, other basic qualities, such as validity, reproducibility, sensitivity, accuracy, cost effectiveness, simplicity, lack of ambiguity, and selectivity (in order to narrow the number of substances for secondary bioassay and reject false positives), should be taken into consideration. If a positive result is detected in the primary screening, a secondary screen, which is more accurate and precise, is executed. However, the secondary screening, having low capacity, is time consuming and expensive. In this bioassay, the pure compounds are assessed in various models and test circumstances in order to choose potential candidates for clinical trials [75, 76]. Whenever possible, available information on the target marine organism should be consulted in order to help in selection of the bioactivity screening assay.
The bioactivity screening of extracts of marine sources is an important and indispensable part of any pharmaceutical agent discovery platform.
5 Tools for Structural Characterization and Determination of Bioactive Compounds
Structural elucidation of active molecules from marine fungi sample is not an easy task, especially when considered the diversity of chemical structures comprised in each mixture. Thus, the chemical characterization of a molecule can be easier using literature reports.
Mass spectrometry (MS) and nuclear magnetic resonance (NMR) spectroscopy are among the most promising methods for thoroughly characterizing the structure and composition of marine bioactive compounds, such as amino acids, fatty acids, phenols, sterols, or sugars [77, 78]. However, the inherent complexity of these mixtures hinders the structural determination by means of those high-resolution techniques. Therefore, it is very useful to perform any separation steps before structure analysis in order to decrease the complexity of the marine extracts and in turn provide additional information about the existing active components in the extract [79].
MS has been demonstrated its power and utility to elucidate unknown molecules in several marine organisms, as well as in marine fungi samples. Shushni et al. [73] identified a new 12-membered macrolide, named balticolid (8), using MS spectra. Diverse methods based on high-resolution 1D and 2D NMR spectroscopy are used for the structural characterization of the bioactive compounds [68, 80].
In search of better characterization of BCs, multidimensional separation systems have become visibly interesting techniques for the analysis of complex mixtures [81, 82]. Multidimensional chromatography combines two or more separation techniques that fractionate complex mixtures based on different and independent properties. The major advantage of combining two separation techniques with different selectivity is the reduction of analytes overlap. Two-dimensional (2D) chromatography is the simplest example of a multidimensional separation scheme. Two-dimensional nuclear magnetic resonance spectroscopy (2D NMR) provides more information about a molecule than one-dimensional NMR spectra and is particularly useful in determining their chemical structure, particularly for analytes that are too complex using one-dimensional NMR. Types of 2D proton NMR include correlation spectroscopy (COSY), total correlation spectroscopy (TOCSY), exchange spectroscopy (EXSY), and nuclear overhauser effect spectroscopy (NOESY), which allows the determination of the conformation of the molecule or the relative location of the protons [83].
The complexity of the samples often exceeds the separation capacity of chromatographic systems. This challenge drives researchers all over the world to develop more sophisticated chromatographic methods that enable a greater resolution and peak capacity [79]. The progresses in the improvement of analytical methods linked to fast access and reliable data bases can be used as tools for rapid discovery of known bioactive molecules, thus needing less amounts of sample and simplifying sample preparation [84–86].
6 Online Combination of Bioassays for Detection of Bioactive Compounds
The analysis and isolation of bioactive molecules from complex mixtures without requiring cumbersome purification steps is hard and demanding [87]. The traditional analytical procedure consists in research with laborious bioassay-guided fractionation to isolate an individual bioactive analyte. On the other hand, the online combination or concurrent surveying of bioassays with chemical and structural characterization enables quick analysis and identification of single bioactive agents with various biological activities without needing previous purification procedures [88].
A number of approaches have been tried in analyzing bioactive molecules in marine extracts, containing total or partial online screening. These procedures combine separation techniques, chemical detection techniques, such as mass spectrometry, nuclear magnetic resonance, and biochemical assays [4]. Two predominant approaches, high-throughput screening (HTS) and high-resolution screening (HRS), have been employed by researchers, both with advantages and disadvantages. The strategies used for HTS can normally be classified into precolumn and postcolumn methods. Precolumn techniques have been undertaken based on the fact that a bioactive analyte in a complex mixture is required to interact first with a target protein prior to separation, followed by chemical detection and identification [89, 90]. HTS postcolumn approaches consist of fractionating complex mixtures, recovering the fractions and their evaporation, and detecting bioactive fractions with parallel chemical detection and identification by microplate-based bioassays. HRS usually includes the online coupling of a bioassay of the chromatographic separation [91]. The high resolution achieved with the chromatographic separation stages in HTS postcolumn screening is frequently lost in lower resolution fraction obtained for the bioactivity screen [88]. Obtaining high resolution and sensitivity in HRS requires the integration of fast and simple online biochemical detection assays (BCD), such as enzymes, antioxidant screening assays, and receptor-based assays. The basis of BCD assays is the detection of bioactive molecules in simulated and nonsimulated biochemical reactions [91, 92]. This analysis approach is a mean to overcome preisolation limitations since it directly evaluates the effects of bioactive molecules after separation (postcolumn) and reduces in vitro assays, since only fractions with specific activities need to be isolated and tested. Some development time and effort in order to improve and implement more sensitive and faster novel methodologies is required to reduce the amounts of solvents used.
7 Conclusion
Obtaining a pure compound is a difficult process, requiring long periods of time, significant amounts of work, and large numbers of solvent-consuming steps. The isolation of marine fungi compounds depends on the quality and quantity of the sample, collection, preservation of samples, preparation of fungal cultures, extraction, fractionation, separation, purification, and bioactivity assays screening. There is no specific methodology that can be followed for the separation of BCs in a mixture of marine fungi. However, the marine bioactive compounds are mainly obtained by solvent extraction with different polarities. Fraction of bioactive compounds from fungi samples can be achieved by solvent partition or combining chromatographic techniques. If the purification was effective, the biological activity may be concentrated in a particular fraction; however, sometimes, the compounds may be already known or not show activity. Therefore, the bioactivity assays screening is an important step along the entire separation process. The structural characterization of BCs is also an important step in which MS and NMR play an important role in their determination. An effort to apply quicker and more sensitive techniques in structural analysis will accelerate the discovery of new bioactive compounds. The use of online screening approaches can rapidly provide a great deal of information about the nature of compounds, which is very useful when large numbers of samples need to be processed avoiding unnecessary isolation of certain compounds. The successful investigation reports using marine organisms as potential sources of bioactive compounds encourage the incessant research of new molecules with interesting pharmaceutical applications. However, there is still much to research and explore the potential of marine fungi as source of novel agents, as well as develop strategies based on green analytical chemistry in order to reduce the quantity of solvents used.
References
Gomes AR, Freitas AC, Rocha-Santos TAP, Duarte AC (2014) Bioactive compounds derived from echinoderms. RSC Adv 4:29365–29382
Blunt JW, Copp BR, Keyzers RA, Munro MHG, Prinsep MR (2015) Marine natural products. Nat Prod Rep 32(2):116–211
Martins A, Vieira H, Gaspar H, Santos S (2014) Marketed marine natural products in the pharmaceutical and cosmeceutical industries: tips for success. Mar Drugs 12(2):1066–1101
Krause J, Tobin G (2013) Discovery, development, and regulation of natural products. In: Marianna K (ed) Using old solutions to new problems – natural drug discovery in the 21st century. InTech, Rijeka, Croatia, pp 3–36
Mora C, Tittensor DP, Adl S, Simpson AGB, Worm B (2011) How many species are there on Earth and in the ocean? PLoS Biol 9:1–8
Keating G, Figgitt D (2003) Caspofungin: a review of its use in oesophageal candidiasis, invasive candidiasis and invasive aspergillosis. Drugs 63(20):2235–2263
Gloer JB (2007) Applications of fungal ecology in the search for new bioactive natural products. In: Kubicek CP, Druzhinina IS (eds) Environmental and microbial relationships. Springer, New York/Berlin/Heidelberg, pp 257–83
Strader CR, Pearce CJ, Oberlies NH (2011) Fingolimod (FTY720): a recently approved multiple sclerosis drug based on a fungal secondary metabolite. J Nat Prod 74(4):900–907
Bose U, Hodson MP, Shaw PN, Fuerst JA, Hewavitharana AK (2014) Bacterial production of the fungus-derived cholesterol-lowering agent mevinolin. Biomed chromatog 28(9):1163–1166
Chun J, Hartung H-P (2010) Mechanism of action of oral fingolimod (FTY720) in multiple sclerosis. Clin Neuropharmacol 33(2):91–101
McCormack PL, Perry CM (2005) Caspofungin: a review of its use in the treatment of fungal infections. Drugs 65(14):2049–2068
Gao S-S, Li X-M, Li C-S, Proksch P, Wang B-G (2011) Penicisteroids A and B, antifungal and cytotoxic polyoxygenated steroids from the marine alga-derived endophytic fungus Penicillium chrysogenum QEN-24S. Bioorg Med Chem Lett 21(10):2894–2897
Tarman K, Lindequist U, Wende K, Porzel A, Arnold N, Wessjohann LA (2011) Isolation of a new natural product and cytotoxic and antimicrobial activities of extracts from fungi of indonesian marine habitats. Mar Drugs 9(3):294–306
Zhang D, Satake M, Fukuzawa S, Sugahara K, Niitsu A, Shirai T et al (2012) Two new indole alkaloids, 2-(3,3-dimethylprop-1-ene)-costaclavine and 2-(3,3-dimethylprop-1-ene)-epicostaclavine, from the marine-derived fungus Aspergillus fumigatus. J Nat Med 66(1):222–226
Bhatnagar I, Kim S-K (2010) Immense essence of excellence: marine microbial bioactive compounds. Mar Drugs 8(10):2673–2701
Saslis-Lagoudakis CH, Savolainen V, Williamson EM, Forest F, Wagstaff SJ, Baral SR et al (2012) Phylogenies reveal predictive power of traditional medicine in bioprospecting. Proc Natl Acad Sci U S A 109(39):15835–15840
Leary D, Vierros M, Hamon G, Arico S, Monagle C (2009) Marine genetic resources: a review of scientific and commercial interest. Mar Policy 33(2):183–194
Liu X, Ashforth E, Ren B, Song F, Dai H, Liu M et al (2010) Bioprospecting microbial natural product libraries from the marine environment for drug discovery. J Antibiot 63:415–422
Demunshi Y, Chugh A (2010) Role of traditional knowledge in marine bioprospecting. Biodivers Conserv 19(11):3015–3033
Wildman HG (1999) Pharmaceutical bioprospecting and its relationship to the conservation and utilization of bioresources. Phuket, Thailand, IUPAC
Slobodian L, Kinna R, Kambu A, Ognibene L (2015) Bioprospecting in the global commons: legal issues brief. United Nations Environment Programme – Division of Environmental Law and Conventions. Available at: https://www.unep.org/delc/Portals/119/Biosprecting-Issuepaper.pdf
Beattie AJ, Hay M, Magnusson B, de Nys R, Smeathers J, Vincent JFV (2011) Ecology and bioprospecting. Austral Ecol 36(3):341–356
Abida H, Ruchaud S, Rios L, Humeau A, Probert I, De Vargas C et al (2013) Bioprospecting marine plankton. Mar Drugs 11(11):4594–4611
Postec A, Lesongeur F, Pignet P, Ollivier B, Querellou J, Godfroy A (2007) Continuous enrichment cultures: insights into prokaryotic diversity and metabolic interactions in deep-sea vent chimneys. Extremophiles 11(6):747–757
McMinn A, Müller MN, Martin A, Ryan KG (2014) The response of Antarctic sea ice algae to changes in pH and CO2. PLoS One 9(1):e86984
McMinn A, Pankowski A, Delfatti T (2005) Effect of hyperoxia on the growth and photosynthesis of polar sea ice microalgae. J Phycol 41(4):732–741
Synnes M (2007) Bioprospecting of organisms from the deep sea: scientific and environmental aspects. Clean Techn Environ Policy 9(1):53–59
Mayer AMS, Glaser KB, Cuevas C, Jacobs RS, Kem W, Little RD et al (2010) The odyssey of marine pharmaceuticals: a current pipeline perspective. Trends Pharmacol Sci 31(6):255–265
Jobstvogt N, Hanley N, Hynes S, Kenter J, Witte U (2014) Twenty thousand sterling under the sea: estimating the value of protecting deep-sea biodiversity. Ecol Econ 97:10–19
Matz C, Webb JS, Schupp PJ, Phang SY, Penesyan A, Egan S et al (2008) Marine biofilm bacteria evade eukaryotic predation by targeted chemical defense. PLoS One 3:e2744
Leal MC, Puga J, Serôdio J, Gomes NCM, Calado R (2012) Trends in the discovery of new marine natural products from invertebrates over the last two decades – where and what are we bioprospecting? PLoS One 7:e30580
Molinski TF, Dalisay DS, Lievens SL, Saludes JP (2009) Drug development from marine natural products. Nat Rev 8(1):69–85
Proksch P, Edrada RA, Ebel R (2002) Drugs from the seas – current status and microbiological implications. Appl Microbiol Biotechnol 59(2–3):125–134
Montaser R, Luesch H (2011) Marine natural products: a new wave of drugs? Future Med Chem 3(12):1475–1489
Bowler C, Karl DM, Colwell RR (2009) Microbial oceanography in a sea of opportunity. Nature 459:180–184
Duarte K, Rocha-Santos TAP, Freitas AC, Duarte AC (2012) Analytical techniques for discovery of bioactive compounds from marine fungi. TrAC Trends Anal Chem 34:97–110
Leston S, Nunes M, Rosa J, Lemos MFL, Ramos F, Pardal MA (2014) Chapter 2 – prospection, collection, and preservation of marine samples. Compr Anal Chem 65:15–33
Zhang Y, Arends JBA, Van de Wiele T, Boon N (2011) Bioreactor technology in marine microbiology: from design to future application. Biotechnol Adv 29(3):312–321
Parkes RJ, Sellek G, Webster G, Martin D, Anders E, Weightman AJ et al (2009) Culturable prokaryotic diversity of deep, gas hydrate sediments: first use of a continuous high-pressure, anaerobic, enrichment and isolation system for subseafloor sediments (DeepIsoBUG). Environ Microbiol 11(12):3140–3153
Leal MC, Calado R, Sheridan C, Alimonti A, Osinga R (2013) Coral aquaculture to support drug discovery. Trends Biotechnol 31(10):555–561
Demain AL (2000) Small bugs, big business: the economic power of the microbe. Biotechnol Adv 18(6):499–514
Munro MHG, Blunt JW, Dumdei EJ, Hickford SJH, Lill RE, Li S et al (1999) The discovery and development of marine compounds with pharmaceutical potential. J Biotechnol 70(1–3):15–25
Dawson MN, Raskoff KA, Jacobs DK (1998) Field preservation of marine invertebrate tissue for DNA analyses. Mol Mar Biol Biotechnol 7(2):145–152
Siebert K, Busl M, Asmus I, Freund J, Muscholl-Silberhorn A, Wirth R (2004) Evaluation of methods for storage of marine macroorganisms with optimal recovery of bacteria. Appl Environ Microbiol 70(10):5912–5915
Dhorajiya B, Malani M, Dholakiya B (2012) Extraction and preservation protocol of anti-cancer agents from marine world. Chem Sci J 2012(CSJ-38):1–12
Penesyan A, Kjelleberg S, Egan S (2010) Development of novel drugs from marine surface associated microorganisms. Mar Drugs 8(3):438–459
Justino CIL, Duarte K, Freitas AC, Duarte AC, Rocha-santos T (2014) Chapter 3 – classical methodologies for preparation of extracts and fractions. Compr Anal Chem 65:35–57
Yun K, Kondempudi CM, Choi HD, Kang JS, Son BW (2011) Microbial mannosidation of bioactive chlorogentisyl alcohol by the marine-derived fungus Chrysosporium synchronum. Chem Pharm Bull 59(4):499–501
Sarker SD, Latif Z, Gray AI (2005) Natural products isolation. In: Sarker SD, Latif Z, Gray AI (eds) Natural products isolation, vol 20, 2nd edn. Humana Press, Totowa, pp 1–25
Mayer AMS, Rodríguez AD, Berlinck RGS, Fusetani N (2011) Marine pharmacology in 2007–8: marine compounds with antibacterial, anticoagulant, antifungal, anti-inflammatory, antimalarial, antiprotozoal, antituberculosis, and antiviral activities; affecting the immune and nervous system, and other miscellaneous mechanisms of action. Comp Biochem Physiol 153:191–222
Blunt JW, Copp BR, Hu W-P, Munro MHG, Northcote PT, Prinsep MR (2009) Marine natural products. Nat Prod Rep 26(2):170–244
He J-Z, Ru Q-M, Dong D-D, Sun P-L (2012) Chemical characteristics and antioxidant properties of crude water soluble polysaccharides from four common edible mushrooms. Molecules 17(4):4373–4387
Ohkawa Y, Miki K, Suzuki T, Nishio K, Sugita T, Kinoshita K et al (2010) Antiangiogenic metabolites from a marine-derived fungus. Hypocrea vinosa. J Nat Prod 73(4):579–582
Bhakuni DS, Rawat DS (2005) Separation and isolation techniques. In: Bhakuni DS, Rawat DS (eds) Bioactive marine natural products. Springer Netherlands, New Delhi, pp 64–79
Grosso C, Valentão P, Ferreres F, Andrade PB (2015) Alternative and efficient extraction methods for marine-derived compounds. Mar Drugs 13(5):3182–3230
Kaufmann B, Christen P (2002) Recent extraction techniques for natural products: microwave-assisted extraction and pressurised solvent extraction. Phytochem Anal 13(2):105–113
Veggi PC, Martinez J, Meireles MAA (2013) Fundamentals of microwave extraction. In: Chemat F, Cravotto G (eds) Microwave-assisted extraction for bioactive compounds. Springer US, New York, pp 15–52
Madej K (2009) Microwave-assisted and cloud-point extraction in determination of drugs and other bioactive compounds. TrAC Trends Anal Chem 28(4):436–446
Zou T-B, Jia Q, Li H-W, Wang C-X, Wu H-F (2013) Response surface methodology for ultrasound-assisted extraction of astaxanthin from Haematococcus pluvialis. Mar Drugs 11(5):1644–1655
Joana Gil-Chávez G, Villa JA, Fernando Ayala-Zavala J, Basilio Heredia J, Sepulveda D, Yahia EM et al (2013) Technologies for extraction and production of bioactive compounds to be used as nutraceuticals and food ingredients: an overview. Compr Rev Food Sci Food Saf 12:5–23
Farré M, Pérez S, Gonçalves C, Alpendurada MF, Barceló D (2010) Green analytical chemistry in the determination of organic pollutants in the aquatic environment. TrAC Trends Anal Chem 29(11):1347–1362
Duarte K, Justino CIL, Pereira R, Freitas AC, Gomes AM, Duarte AC et al (2014) Green analytical methodologies for the discovery of bioactive compounds from marine sources. Trends Environ Anal Chem 3–4:43–52
Smith RM (2006) Superheated water: the ultimate green solvent for separation science. Anal Bioanal Chem 385(3):419–421
Asl AH, Khajenoori M (2013) Subcritical water extraction. In: Nakajima H (ed) Mass transfer – advances in sustainable energy and environment oriented numerical modeling. InTech, Rijeka, Croatia, pp 459–487
Duarte K, Justino CIL, Gomes AM, Rocha-Santos T, Duarte AC (2014) Chapter 4 – green analytical methodologies for preparation of extracts and analysis of bioactive compounds. Compr Anal Chem 65:59–78
Wolf D, Siems K (2007) Burning the hay to find the needle – data mining strategies in natural product dereplication. CHIMIA Int J Chem 61(6):339–345
Wang X, Mao Z-G, Song B-B, Chen C-H, Xiao W-W, Hu B et al (2013) Advances in the study of the structures and bioactivities of metabolites isolated from mangrove-derived fungi in the South China Sea. Mar Drugs 11(10):3601–3616
Smetanina OF, Yurchenko AN, Pivkin MV, Yurchenko EA, Afiyatullov SS (2011) Isochromene metabolite from the facultative marine fungus Penicillium citrinum. Chem Natu Compd 47(1):118–119
Lee YM, Li H, Hong J, Cho HY, Bae KS, Kim MA et al (2010) Bioactive metabolites from the sponge-derived fungus Aspergillus versicolor. Arch Pharm Res 33(2):231–235
Otsuka H (2005) Purification by solvent extraction using partition coefficient. In: Sarker SD, Latif Z, Gray AI (eds) Natural products isolation, vol 20, 2nd edn. Humana Press, Totowa, pp 269–73
Chen Y, Mao W, Gao Y, Teng X, Zhu W, Chen Y et al (2013) Structural elucidation of an extracellular polysaccharide produced by the marine fungus Aspergillus versicolor. Carbohydr Polym 93(2):478–483
Smetanina OF, Yurchenko AN, Kalinovskii AI, Berdyshev DV, Gerasimenko AV, Pivkin MV et al (2011) Biologically active metabolites from the marine isolate of the fungus Myceliophthora lutea. Chem Natu Compd 47(3):385–390
Shushni MAM, Singh R, Mentel R, Lindequist U (2011) Balticolid: a new 12-membered macrolide with antiviral activity from an ascomycetous fungus of marine origin. Mar Drugs 9(5):844–851
Song F, Dai H, Tong Y, Ren B, Chen C, Sun N et al (2010) Trichodermaketones A-D and 7-O-methylkoninginin D from the marine fungus Trichoderma koningii. J Nat Prod 73(5):806–810
Martis EA, Radhakrishnan R, Badve RR (2011) High-throughput screening: the hits and leads of drug discovery- an overview. J Appl Pharm Sci 1(1):02–10
Glaser KB, Mayer AMS (2009) A renaissance in marine pharmacology: from preclinical curiosity to clinical reality. Biochem Pharmacol 78(5):440–448
Santos CMM, Silva AMS (2014) Chapter 7 – nuclear magnetic resonance spectroscopy for structural characterization of bioactive compounds. Compr Anal Chem 65:149–191
Tilvi S, Majik MS, Singh KS (2014) Chapter 8 – mass spectrometry for determination of bioactive compounds. Compr Anal Chem 65:193–218
Regina MBO, Duarte ACD (2014) Chapter 9 – chromatography coupled to various detectors as a tool for separation and determination of bioactive compounds. Compr Anal Chem 65:219–252
Paasch S, Brunner E (2010) Trends in solid-state NMR spectroscopy and their relevance for bioanalytics. Anal Bioanal Chem 398(6):2351–2362
Malerod H, Lundanes E, Greibrokk T (2010) Recent advances in on-line multidimensional liquid chromatography. Anal Methods 2:110–122
Meinert C, Meierhenrich UJ (2012) A new dimension in separation science: comprehensive two-dimensional gas chromatography. Angew Chem 51(42):10460–10470
Chen Y, Cai X, Pan J, Gao J, Li J, Yuan J et al (2009) Structure elucidation and NMR assignments for three anthraquinone derivatives from the marine fungus Fusarium sp. (No. ZH-210). Magn Reson Chem 47(4):362–365
Ito T, Odake T, Katoh H, Yamaguchi Y, Aoki M (2011) High-throughput profiling of microbial extracts. J Nat Prod 74(5):983–988
Koehn FE (2008) High impact technologies for natural products screening. Prog Drug Res 65(175):177–210
Mitova MI, Murphy AC, Lang G, Blunt JW, Cole ALJ, Ellis G et al (2008) Evolving trends in the dereplication of natural product extracts. 2. The isolation of chrysaibol, an antibiotic peptaibol from a New Zealand sample of the mycoparasitic fungus Sepedonium chrysospermum. J Nat Prod 71(9):1600–1603
Shi S-Y, Zhang Y-P, Jiang X-Y, Chen X-Q, Huang K-L, Zhou H-H et al (2009) Coupling HPLC to on-line, post-column (bio)chemical assays for high-resolution screening of bioactive compounds from complex mixtures. TrAC Trends Anal Chem 28(7):865–877
Freitas AC, Montalvão SIGHM, Duarte AC, Rocha-Santos T (2014) Chapter 10 – Online combination of bioassays with chemical and structural characterization for detection of bioactive compounds. Compr Anal Chem 65:253–278
Giera M, Irth H (2011) Simultaneous screening and chemical characterization of bioactive compounds using LC-MS-based technologies (Affinity chromatography). In: Brack W (ed) Effect-directed analysis of complex environmental contamination, vol 15. Springer, Berlin/Heidelberg, pp 119–41
Potterat O, Hamburger M (2013) Concepts and technologies for tracking bioactive compounds in natural product extracts: generation of libraries, and hyphenation of analytical processes with bioassays. Nat Prod Rep 30(4):546–564
Kool J, Giera M, Irth H, Niessen WMA (2011) Advances in mass spectrometry-based post-column bioaffinity profiling of mixtures. Anal Bioanal Chem 399(8):2655–2668
Malherbe CJ, de Beer D, Joubert E (2012) Development of on-line high performance liquid chromatography (HPLC)-biochemical detection methods as tools in the identification of bioactives. Int J Mol Sci 13(3):3101–3133
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing Switzerland
About this entry
Cite this entry
Gomes, A.R., Duarte, A.C., Rocha-Santos, T.A.P. (2017). Analytical Techniques for Discovery of Bioactive Compounds from Marine Fungi. In: Mérillon, JM., Ramawat, K. (eds) Fungal Metabolites. Reference Series in Phytochemistry. Springer, Cham. https://doi.org/10.1007/978-3-319-25001-4_9
Download citation
DOI: https://doi.org/10.1007/978-3-319-25001-4_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-25000-7
Online ISBN: 978-3-319-25001-4
eBook Packages: Chemistry and Materials ScienceReference Module Physical and Materials ScienceReference Module Chemistry, Materials and Physics