Abstract
Neurovascular coupling is the relationship between the activity of the brain and the subsequent change in blood flow to the active region. The most common methods of detecting neurovascular coupling are cumbersome and noncontinuous. However, the integration of intracranial pressure (ICP) and electroencephalography (EEG) may serve as an indirect measure of neurovascular coupling.
This study used data collected from burst-suppressed patients who received both ICP and depth EEG monitoring. An adaptive thresholding algorithm was used to detect the start and end of each EEG burst. The morphological clustering and analysis of ICP and pulse morphological template-matching algorithms were then applied to derive several metrics describing the shape of the ICP pulse waveform and track how it changed following an EEG burst. These changes were compared using a template obtained from patients undergoing CO2-induced vasodilation.
All segments exhibited a significant period of vasodilation within 1–2 s after burst, and 4 of 5 had a significant period of vasoconstriction within 4–11 s of the EEG burst, suggesting that there might be a characteristic response of vasodilation and subsequent vasoconstriction after a spontaneous EEG burst. Furthermore, these findings demonstrate the potential of integrated EEG and ICP as an indirect measure of neurovascular coupling.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Traumatic Brain Injury
- Traumatic Brain Injury Patient
- Cerebral Blood Flow Velocity
- Neurovascular Coupling
- aSAH Patient
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
After a traumatic insult, the brain is susceptible to secondary complications that can cause further injury. One such complication is elevated intracranial pressure (ICP), which can lead to ischemia and herniation [3, 8]. One of the tools used to detect increases in ICP is continuous monitoring with a transducer placed within the brain ventricles, parenchyma, or subdural space [11]. After injury, the brain is also at risk for epileptic activity and spreading cortical depressions (CSDs), which can be detected using continuous electroencephalography (EEG). While these two signals are commonly used as part of routine care in a neurocritical care unit, there is no a priori physiological relationship upon which to integrate them.
Neurovascular coupling – the change in blood flow in response to neural activity – is one potential mechanism that can relate continuous ICP and EEG waveforms. EEG is a spatial–temporal measure of neural activity and, based on the Monroe–Kellie hypothesis, changes in cerebral blood volume are reflected in the ICP. Understanding the relationship between these two signals within the context of neurovascular coupling is the first step toward integrating them to extract additional information.
To investigate this relationship, we focused on patients with continuous ICP and depth EEG monitoring who had been burst-suppressed for the treatment of refractory intracranial hypertension, and we observed how the ICP changes during and immediately after a burst of neural activity, as detected on depth EEG.
Important to this hypothesis is determining whether the changes in ICP are caused by vasodilation and vasoconstriction. To do this, we utilized the recently developed morphological clustering and analysis of ICP [5] (MOCAIP) and pulse morphological template-matching (PMTM) algorithms [1, 2]. These algorithms calculate a variety of pulse–waveform metrics describing the shape of a pulsatile signal, such as ICP, and track how these metrics change in response to various stimuli. In this study we characterize the changes that occur in ICP after an EEG burst and assess whether they are caused by vasodilation, by comparing the pulse–waveform trends after an EEG burst using a known vasodilation template.
Materials and Methods
Data segments were collected from patients admitted to the neurocritical care unit at UCLA Ronald Reagan Hospital. Segments were obtained from when the patients were receiving continuous ICP and depth EEG monitoring, and were under high levels of burst suppression. ICP and depth EEG data were upsampled to 2,000 Hz. The onset and cessation of the bursts recorded in the depth EEG were segmented using an adaptive thresholding algorithm.
The pulse waveform metrics of each data segment were computed using the MOCAIP algorithm. MOCAIP is a recently developed tool that takes a segment of a pulsatile signal, such as ICP or cerebral blood flow velocity, and identifies each pulse corresponding to a cardiac cycle. The pulses are then clustered based on their similarities, and from the average of the largest cluster, a dominant pulse is produced. The three sub-peaks of the dominant pulse are found, and from their heights and latencies 128 pulse–waveform metrics are calculated. In this study, our period of interest was in the 20 s after an EEG burst; thus, the pulse–waveform metrics were found for each individual pulse.
The PMTM algorithm was then applied on a 2-s sliding window starting at the onset of the burst to determine which time points were consistent with vasodilation or vasoconstriction. The PMTM algorithm works by fitting sequential pulse–waveform metrics to a line using robust least squares and determining whether the slope of the line is positive, negative, or not significantly nonzero. Previous studies on patients undergoing hypercapnic vasodilation found that 50 of the 128 pulse–waveform metrics increase and 22 decrease during vasodilation, and have the opposite trend during the return to normocapnia.
The pulse–waveform metric changes between each two pulses after an EEG burst were calculated and the proportion that matched those observed in patients, or the vasodilation index (VDI), was found. Pulse–waveform metric changes with a higher VDI were more characteristic of vasodilation, while pulse–waveform metric changes with a lower VDI were more characteristic of vasoconstriction. VDI values were also calculated for many two-pulse intervals randomly selected from each of the segments. The VDI values of each 2-s window for all EEG bursts (e.g., all the VDI values for the ICP pulses between 3 and 4 s post-EEG burst) were combined and compared with the random sample using a t test and Mann–Whitney U test. The t test is reported for simplicity.
Results
Data were collected for two female patients, aged 38 and 53, who received ICP and depth EEG monitoring as part of their treatment for a traumatic brain injury (TBI) and an aneurysmal subarachnoid hemorrhage (aSAH) respectively. Both patients were burst-suppressed with pentobarbital for the treatment of refractory intracranial hypertension. The data set includes ICP and depth EEG monitoring from 3 separate days for the TBI patient and 2 separate days for the aSAH patient. Total data length was 64 min (TBI 27 min, aSAH 37 min) and included 323 bursts (TBI 126 bursts, aSAH 197 bursts). Samples were collected during periods of maximal burst suppression.
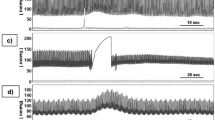
The time-aligned VDI in the 20-s window following the onset of a depth EEG burst exhibited a characteristic pattern consisting of three phases (Fig. 1, bottom right). In the first phase immediately following the onset of the depth EEG burst, there was a brief period lasting 1–3 s, where the mean VDI was significantly higher than random (p <0.05). This was followed by a transition of 1–5 s, where the mean VDI of the burst-aligned ICP was neither significantly higher nor significantly lower than random. A second phase was observed in 4 of the 5 data segments (TBI days 6, 7, and 14; aSAH day 11), where the VDI of the burst-aligned ICP became significantly less than random for 4–7 s. The VDI for the segment obtained form aSAH day 12 was never significantly less than random and remained either significantly higher than random or nonsignificant for the rest of the 20-s window. These four data samples then transitioned to where the mean VDI was not significantly higher or lower than random. After the transition, data segments for TBI days 6 and 14 and aSAH day 11 had a third phase where the mean VDI was again significantly higher than random (Fig. 1).
The mean vasodilation index (VDI) value for each of the 2-s sliding windows after EEG burst onset (solid line). The dashed line shows the boundaries for the two-sided statistical significance, with an alpha of 0.05. Points above the top line are significantly more characteristic of vasodilation than random, and points below the bottom line are significantly more characteristic of vasoconstriction than random. Points between the two lines represent times that are not significantly characteristic of vasodilation or vasoconstriction. The graph bottom right shows the results for all 323 points combined with the three phases of the ICP response in detail
Discussion
The brain has little energy reserve and requires a constant supply of blood containing O2 and glucose to sustain neural function. As neural activity of the brain varies, a tight regulation of CBF through neurovascular mechanisms is necessary to accommodate the metabolic demands of its cellular constituents. However, injury to the brain, such as trauma or hemorrhage, can result in a disruption of neurovascular coupling.
Pathological neurovascular coupling has typically been observed during epileptic activity and CSDs. This pattern is characterized by profound hyperemia followed by persistent oligemia [7]. In the presence of erythrocyte products, as in the case of aSAH or hemorrhagic stroke, the increased depolarization of CSD results in a significant decrease in CBF [6]. While these pathological conditions have been observed in humans, the majority of studies have utilized animal models [10, 12]. Studying neurovascular coupling in humans with acute brain injuries has relied on unconventional monitoring modalities, inhibiting large-scale investigations.
The traditional techniques for measuring neurovascular coupling include functional magnetic resonance imaging, positron emission tomography, and single-photon emission computed tomography. While these modalities provide a high degree of spatial and temporal resolution, they are cumbersome, require patients to be transported out of the ICU, and can only be used intermittently. Other modalities, such as laser Doppler flowmetry [4] and near infrared spectroscopy [9], have been used to some success in the ICU.
This study used our previously developed algorithms [1] for detecting vasodilation based on ICP pulse waveform changes. These techniques demonstrated in the period following an EEG burst that the ICP pulse waveform was characterized by vasodilation for approximately 3 s and subsequent vasoconstriction 8 s. This time scale agrees with the previous average 9-s CBF response in burst-suppressed rats reported by Golanov et al. [4]. However, since the pulse waveform analysis measures trends toward vasodilation or vasoconstriction, and not cerebral blood flow, they may not be directly comparable. This relationship was more predominant in the TBI patient, possibly suggesting either a diminished or pathological neurovascular coupling in the aSAH patient, or other confounding factors related to the patient’s ICU care. These results demonstrate a distinct relationship between ICP and EEG caused by vasodilation and vasoconstriction, and provides a foundation upon which to base further investigations of these two signals.
Because of the specific monitoring requirements, this study was limited by the small cohort of patients. However, the techniques reported here can be applied to surface EEG recordings, allowing a much larger patient cohort in future studies.
References
Asgari S, Bergsneider M, Hamilton R, Vespa P, Hu X (2011) Consistent changes in intracranial pressure waveform morphology induced by acute hypercapnic cerebral vasodilatation. Neurocrit Care 15(1):55–62
Asgari S, Gonzalez N, Subudhi AW et al (2012) Continuous detection of cerebral vasodilatation and vasoconstriction using intracranial pulse morphological template matching. PLoS One 7(11), e50795, Available at: http://dx.doi.org/10.1371/journal.pone.0050795
Bouma GJ, Muizelaar JP, Choi SC, Newlon PG, Young HF (1991) Cerebral circulation and metabolism after severe traumatic brain injury: the elusive role of ischemia. J Neurosurg 75(5):685–693. doi:10.3171/jns.1991.75.5.0685
Golanov EV, Yamamoto S, Reis DJ (1994) Spontaneous waves of cerebral blood flow associated with a pattern of electrocortical activity. Am Physiol Soc Regul Integr Comp Physiol 266(1):R204–R214, Available at: http://ajpregu.physiology.org/content/266/1/R204.abstract
Hu X, Xu P, Scalzo F, Vespa P, Bergsneider M (2009) Morphological clustering and analysis of continuous intracranial pressure. IEEE Trans Biomed Eng 56(3):696–705
Koide M, Sukhotinsky I, Ayata C, Wellman GC (2013) Subarachnoid hemorrhage, spreading depolarizations and impaired neurovascular coupling. Stroke Res Treat 2013:819340. doi:10.1155/2013/819340
Mayevsky A, Doron A, Manor T, Meilin S, Zarchin N, Ouaknine GE (1996) Cortical spreading depression recorded from the human brain using a multiparametric monitoring system. Brain Res 740(1):268–274, Available at: http://www.sciencedirect.com/science/article/pii/S0006899396008748. Accessed 3 Oct 2013
Miller JD, Becker DP, Ward JD, Sullivan HG, Adams WE, Rosner MJ (1977) Significance of intracranial hypertension in severe head injury. J Neurosurg 47(4):503–516. doi:10.3171/jns.1977.47.4.0503
Roche-Labarbe N, Wallois F, Ponchel E, Kongolo G, Grebe R (2007) Coupled oxygenation oscillation measured by NIRS and intermittent cerebral activation on EEG in premature infants. Neuroimage 36(3):718–727, Available at: http://www.sciencedirect.com/science/article/pii/S1053811907003059
Shin HK, Dunn AK, Jones PB, Boas DA, Moskowitz MA, Ayata C (2006) Vasoconstrictive neurovascular coupling during focal ischemic depolarizations. J Cereb Blood Flow Metab 26(8):1018–1030. doi:10.1038/sj.jcbfm.9600252
Steiner LA, Andrews PJD (2006) Monitoring the injured brain: ICP and CBF. Br J Anaesth 97(1):26–38. doi:10.1093/bja/ael110
Strong AJ, Anderson PJ, Watts HR et al (2007) Peri-infarct depolarizations lead to loss of perfusion in ischaemic gyrencephalic cerebral cortex. Brain 130(Pt 4):995–1008. doi:10.1093/brain/awl392
Acknowledgments
The present work is partially supported by NS066008, NS076738, and the UCLA Brain Injury Research Center.
Conflict of Interest
There are no conflicts of interest to declare
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Connolly, M., Vespa, P., Hu, X. (2016). Characterization of Cerebral Vascular Response to EEG Bursts Using ICP Pulse Waveform Template Matching. In: Ang, BT. (eds) Intracranial Pressure and Brain Monitoring XV. Acta Neurochirurgica Supplement, vol 122. Springer, Cham. https://doi.org/10.1007/978-3-319-22533-3_58
Download citation
DOI: https://doi.org/10.1007/978-3-319-22533-3_58
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-22532-6
Online ISBN: 978-3-319-22533-3
eBook Packages: MedicineMedicine (R0)