Abstract
In this chapter we consider the cycling of Carbon (C), Nitrogen (N) and Phosphorus (P) in green roof ecosystems. The focus is placed primarily on N and P because these are the nutrients most often limiting to plant growth in terrestrial ecosystems, and because leaching of these elements to downstream aquatic ecosystems is a concern due to their potential to contribute to eutrophication. Extensive green roofs are commonly sources of phosphorus and dissolved organic carbon in runoff, while they may be either a source or a sink for nitrogen. Plant communities, substrate characteristics, substrate depth, and roof age all play a role in regulating nutrient export. Seasonal variation in runoff nutrient concentrations suggests the importance of temperature and light-mediated processes. Nitrogen leaching may drop off rapidly with the age of the ecosystem and vary with new inputs (atmospheric deposition of N, new fertilizer additions), while roofs leach out P for years or decades under current construction regimes, likely resulting from mineralization of P-rich organic matter in the roof substrate. Conceptual models of nutrient cycling developed from natural terrestrial ecosystems provide a useful starting point for interpreting the important nutrient cycling processes on green roofs. However, the engineered nature of green roof ecosystems, often with a high-nutrient substrate coupled to plants adapted to low-nutrient, extreme environments, gives rise to unique characteristics. There is still little known of the dynamics of important processes for recycling of nutrients within green roof ecosystems, and more studies which include modeling, full roof-scale experiments, and long-term monitoring are needed for improved understanding of these ecosystems.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
5.1 Organization and Scope of this Chapter
We begin with a general overview of nutrient cycling processes and rationale behind their study in green roof ecosystems and then highlight the unique characteristics of green roofs relevant to nutrient cycling. We review the current state of knowledge with respect to the cycling of C, N and P in green roof ecosystems, most of which is based on observations of dissolved nutrient concentrations in roof runoff. We further examine observations of temporal dynamics in runoff nutrient concentrations, as a window into process understanding. The applicability of a simple terrestrial ecosystems nitrogen cycling model to green roofs is discussed. We highlight gaps in knowledge, and finish with a series of open questions relevant to green roof nutrient cycling, which we hope will provide a springboard for future research studies.
5.2 Rationale for Studying Nutrient Cycling in Green Roof Ecosystems
Our motivation for studying N and P derives from an interest in balancing the healthy functioning of green roof ecosystems (particularly the vegetation) with concerns for pollution and eutrophication of downstream aquatic ecosystems (eg. Carpenter et al. 1998). Carbon (C) is the currency of chemical energy flow within ecosystems, and its cycling couples with the cycling of N and P through biomass production and decomposition. There is also a general interest in the C sequestration potential of different ecosystems, related to efforts to slow atmospheric greenhouse gas accumulation.
Improved runoff water quality, including reduction of nutrients in runoff, has been touted as one of the benefits of green roofs, but it is not clear under what conditions this can be expected. As engineered ecosystems, green roofs are designed to have a sufficient availability of potentially limiting nutrients (especially N and P) to support a healthy, thriving plant community . As a result, there is often an excess of N and P, some of which is leached out during runoff events. This is particularly true for new roofs or roofs which have been newly fertilized. An understanding of the processes underlying C, N and P dynamics in green roof ecosystems would help in predicting the response of these systems to changes in environmental conditions over time, and in predicting and understanding the effects of varying system design.
5.3 Context: Nutrient Cycling in Terrestrial Ecosystems
5.3.1 Overview and Description of Elements
Nutrient cycling and availability to biota are factors of central importance in regulating the structure and function of ecosystems . As defined here, nutrient cycling involves the movement and transformation of bioactive elements into, out of, and within a given ecosystem (Fig. 5.1). Inputs typically involve atmospheric deposition or weathering of minerals from the geosphere, while exports include hydrological leaching losses. Microbially-mediated gas exchange may contribute either inputs to the system from the atmosphere (e.g. photosynthesis, nitrogen fixation) or exports from the system to the atmosphere (e.g. respiration, denitrification) . Internal recycling involves physical, chemical and biochemical transformations and movement within and between different living and non-living components of the ecosystem.
A generalized schematic illustrating major pools and pathways for nutrient storage, transformation, and movement in ecosystems. (From Dahlgren 1998)
Carbon (C) is the most abundant element in living matter, and carbon-carbon bonds in organic matter represent the common currency of chemical energy in ecosystems. Net ecosystem carbon balance (NECB; Chapin et al. 2006), describes one of the most fundamental characteristics of any ecosystem. Major fluxes in and out of terrestrial ecosystems are typically CO2 in (associated with primary production) and CO2 out (associated with autotrophic and heterotrophic respiration), with the balance of these two terms defining Net Ecosystem Production (NEP) . Other fluxes include hydrologic export (runoff) of dissolved organic carbon (DOC) and dissolved inorganic carbon (DIC; dissolved CO2 and bicarbonate) from the leaching and weathering of soils; gaseous fluxes of CH4, CO, and volatile organic compounds (VOC); and soot and CO2 loss in the event of fire (Chapin et al. 2006) .
Nitrogen (N) is a key building block for amino acids, proteins, and the nitrogenous bases of DNA. It is commonly the productivity-limiting nutrient in terrestrial ecosystems (Chapin et al. 2011). In unpolluted terrestrial ecosystems, microbially-mediated fixation of atmospheric N2 provides the main source of reactive nitrogen (Nr, Galloway et al. 2003) while atmospheric deposition of the inorganic forms Ammonium (NH4 +) and primarily Nitrate (NO3 −) provide an additional source (Fig. 5.2). Both NH4 + and NO3 − are accessible to plants and microbes and can be assimilated or immobilized into organic pools of N by plants and microbes, respectively. Microbial communities mineralize these organic forms of N into NH4 +, by decomposing organic matter . The inorganic forms of N have differential mobility in soils: NH4 + binds on cation-exchange soil surfaces, thus experiences slow diffusion; NO3 −, however, diffuses rapidly through soils and can readily leach out if present during periods of hydrologic flushing (Chapin et al. 2011). Importantly, several redox transformations involving N occur in soils. Nitrification is a process in which specialized microbes use NH4 + as an energy source, oxidizing it to NO3 −. In low oxygen environments, denitrifying microbes use NO3 − as a terminal electron acceptor in the process of denitrification , producing N2O and N2 as byproducts.
Phosphorus (P) is a necessary macronutrient, required for biosynthesis of key compounds including ATP, DNA and phospholipids. In natural systems, the major sources of P are from the weathering of rocks and the mineralization of organic material by microbes. Both of these processes release the water-soluble and biologically-accessible form of P, i.e. phosphate (PO4 3−) . Phosphate can either be taken up by plants or microbes, adsorbed to substrate, precipitated out of solution, or lost from the system via runoff. Phosphate is chemically active, commonly forms precipitates in the soil, and thus tends to experience slow diffusion (Chapin et al. 2011). Unlike N, very little P is introduced to systems via atmospheric sources.
Simplified diagram of the terrestrial nitrogen cycle with major pools (boxes) and fluxes (arrows) represented for a model ecosystem. (Modeled after Dahlgren 1998)
5.3.2 Nutrient Cycling Dynamics in Natural Ecosystems
As a consequence of their high concentration in living tissue relative to environmental sources, the availability of Nitrogen (N) and Phosphorus (P) limit primary productivity in many ecosystems, with N limitation particularly common in terrestrial ecosystems (Vitousek and Howarth 1991; Crews 1999). Co-limitation by N and P occurs at a fairly consistent mass ratio of 15:1 for available N:P, with higher ratios leading to P limitation , and lower ratios leading to N limitation (Chapin et al. 2011). Labile organic C supply may also limit secondary production (e.g., microbial decomposition rates)(Marschner and Kalbitz 2003).
In terrestrial ecosystems, nutrient (N and P) cycling involves highly localized exchanges between plants, microbes , and their physical environment (Chapin et al. 2011). Unmanaged ecosystems tend to be nearly closed systems with respect to limiting nutrients, where internal recycling of nutrients is very high relative to inputs and outputs. On an annual basis, more than 90 % of N and P taken up by plants in natural terrestrial ecosystems commonly comes from recycled nutrients, i.e. from soils that store nutrients derived from the previous years’ plant material (Likens et al. 1977). The macronutrients N, P, and K are typically required by plants in excess of that obtained through mass flow (water uptake by roots), thus diffusion and saturated flow in soils are important sources of these nutrients. Plant associations with mycorrhizal fungi are common especially in low-nutrient environments, and enable substantially increased uptake rates by increasing the effective root surface area and capacity to hold and store nutrients (Smith and Read 2008). Mycorrhizal fungi are most helpful in increasing plant access to slowly-diffusing nutrients, i.e. PO4 3− and NH4 +.
Internal nutrient cycling in terrestrial ecosystems consists of negative feedback loops that, over time, can lead to homeostasis within the system, where nutrient availability and plant uptake are balanced so as to maintain steady state . For instance, high nutrient uptake and retention (high nutrient use efficiency) by plants leads to nutrient depleted soils, which leads in turn to slower decomposition, which leads to lower nutrient availability, which slows plant growth. Conversely, high nutrient availability typically leads to high nutrient losses from a system, ultimately decreasing availability (Shaver and Melillo 1984).
5.3.3 Nutrient Cycling in Managed or Otherwise Heavily Impacted Ecosystems
Managed systems with substantial human influence can have considerably different characteristics than unmanaged ecosystems. This distinction is related both to changes (typically increases) in nutrient inputs, as well as changes in internal cycling processes. For instance, a nitrogen budget study of the city of Phoenix, AZ, USA revealed that commercial fertilizer and combustion were two major sources of nitrogen in this urban ecosystem . Atmospheric NOX export, denitrification , and accumulation within the system (in large part as buried rubbish) were the major fates for nitrogen (Baker et al. 2001). This contrasts with unmanaged temperate ecosystems, where N-fixation is typically the major input, and major fates are denitrification, hydrologic runoff export, or accumulation in biomass for aggrading systems (Chapin et al. 2011).
5.4 Distinctive Characteristics of Green Roofs Relevant to Nutrient Cycling
Like many other managed or human-impacted systems, green roofs tend to have high inputs of N and P, in this case mostly due to the integration of nutrient-rich organic and inorganic fertilizers into the substrate. Their location within urban areas also means that atmospheric deposition fluxes will tend to be high, containing NOX from automobile exhaust and other combustion processes, and possibly higher concentrations of P in mobilized dust (Pett-Ridge 2009).
In the context of nutrient cycling, the distinction between intensive and extensive green roofs is important. Intensive green roofs or “roof gardens” have deep substrate (> 20 cm), can be quite heavy, and may have a wide variety of plant types (even shrubs or trees), requiring substantial management. Extensive green roofs are a modern modification of the roof-garden concept with shallower substrates (often < 10 cm), and are more strictly functional in purpose than intensive roofs, requiring less maintenance (Oberndorfer et al. 2007). In this chapter we primarily consider extensive green roofs.
Extensive green roofs are engineered ecosystems with distinctive characteristics that may give rise to unique patterns of nutrient cycling. Although they include interacting biotic and abiotic components like natural ecosystems, the components have been selected/designed for specific purposes rather than co-developing over time. This contrasts with natural ecosystems, in which soil and plants have developed in tandem, and are linked by local climate and geology, such that plant communities and soil types “match” . Thus, while in natural ecosystems high nutrient soils will typically be vegetated with nutrient-loving plants, green roofs commonly contain high-nutrient substrate coupled with plants adapted to low-nutrient environments. The match or mismatch between plant and substrate characteristics is a function of the design and relatively young age of these ecosystems.
Important constraints on green roof system design (e.g., FLL 2008) include climate and exposure conditions of the roof environment; weight limitations due to underlying building structure; cost; and limited body of available knowledge about these ecosystems. These conditions combine to severely restrict the available plant palette for extensive green roofs to drought-tolerant, wind-tolerant, low-profile, typically slow-growing plants, and the succulent CAM-photosynthesizing Sedums have been commonly used (Though, see other chapters in this book for examples of diverse green roof plant communities) .
Green roof substrate is an engineered soil analog that has been designed to provide structure and nutrition to support a healthy, thriving plant community . Substrates have typically not been designed with nutrient retention, or runoff water quality, in mind. In service of the plant community , the substrate is typically nutrient-rich (often containing compost, which tends to be enriched in P), and may be supplemented with slow-release inorganic fertilizers containing N, P, and K. Integration of P-rich material commonly leads to substrate with about equal amounts of N and P, in spite of 15-fold higher plant demand for N (e.g., Chapin et al. 2011). Likely due to cost restrictions and to simplify design , there are no distinct soil horizons in typical extensive green roof substrate. Instead, organic matter is combined with inorganic aggregate material in a homogeneous mixture. This homogeneous mixture contrasts to natural soils , where distinct horizons develop and organic matter content decreases from the surface downward. Notably, some green roofs now contain a dual-layer construction, with a top organic nutrient-rich layer and a mineral sub-layer designed in part to retain leaching nutrients (e.g., Wang et al. 2013), more analogous to the physical arrangement of natural soil profiles. The homogenous and nutrient-rich nature of green roof substrate presumably influences nutrient cycling processes.
Green roofs are catchments, in that there is a fixed, measurable influx and outflow of water with a solvable water balance—but they have unique characteristics relative to most natural watersheds. Green roof substrate and underlying layers are designed to drain freely, in order to avoid extended periods of standing water around plant roots , anoxia and associated difficulties for the plant communities. Designers attempt to balance free drainage with runoff reduction by including substrate materials with high water holding capacity, and in some systems by including drain board to retain standing water below the substrate in a separate layer (e.g., FLL 2008). As a consequence, hydrologic residence times can be expected to vary greatly among roofs depending on roof design and climate conditions, and within a given roof over time due to changes in season, precipitation patterns, and evapotranspiration rates. Green roofs can experience extended periods of complete water retention, when precipitation events are relatively small and evapotranspirative demand is high; on the other hand, during larger storm or melt events, hydrologic residence times shorten once the system is saturated. This unique hydrology has important ramifications for nutrient cycling within green roofs, but at this point there have been few detailed water balance studies that would shed light on the interaction between hydrology and the cycling of C, N and P in these systems.
5.5 Current State of Knowledge on Nutrient Cycling in Green Roofs
5.5.1 Overview
Most of the current knowledge about nutrient cycling in green roofs involves the recording of patterns (mainly, nutrient concentrations in runoff) rather than the direct measurement of processes. Most green roof research, at least that published in the English-language literature involves relatively short-term (< 1 year) monitoring studies, and there is a shortage of experimental and modeling studies. As such, the review of the current state of the knowledge presented here is biased towards relatively young roofs, and towards extensive roofs more common in North America and Northern Europe, and the subject of most of the published research studies. However, enough of a body of research to synthesize information about the general patterns of C, N and P in green roof runoff is available to offer some conjecture about important contributing mechanisms. In the following section we detail the patterns observed in the literature to date with respect to each nutrient in green roofs, with a focus on the role of substrate variation. The reader is also referred to Berndtsson 2010, who provide an excellent review of green roof impacts on runoff quantity and quality including fluxes of metals, and Rowe (2011), who provides an informative overview of environmental impacts of green roofs.
5.5.2 Carbon Cycling in Green Roofs: Patterns Observed, and Implications for Processes
Like any vegetated space, green roofs have the potential for biomass and soil accumulation that can represent a carbon sink if inputs exceed exports. For green roofs, the major fluxes in and out of the ecosystem are presumably atmospheric CO2 in (associated with primary production), atmospheric CO2 out (associated with autotrophic and heterotrophic respiration), and hydrologic runoff of dissolved organic carbon (DOC) from the leaching of soil organic matter.
There is very little known about the dynamics of atmospheric CO2 exchange in green roof ecosystems, which would give insight into short-term changes in carbon stocks. However, Gaumont-Guay and Halsall (2013) carried out detailed, year-long measurements of CO2 exchange for a newly-established extensive green roof in British Columbia, Canada. These measurements were used to model rates of photosynthesis, autotrophic respiration, and heterotrophic respiration, enabling calculations of net ecosystem productivity (NEP). The NEP for the monitored green roof was 2 g C m−2 yr−1, equivalent to only about 1 % of the value for a growing temperate forest (Aber and Melillo 2001). Based on these measurements, this particular roof was near steady state with respect to atmospheric carbon exchange, i.e. not serving as either a strong sink or source.
Although extensive green roofs have the potential to rapidly accumulate carbon over the short-term especially if they are established using seeds or small cuttings, the total biomass plateaus at a low value relative to most natural vegetated ecosystems. Using newly-established extensive green roof plots seeded with Sedum spp., Getter et al. (2009) measured a net carbon storage of 275 g C m−2 in biomass, and 100 g C m−2 in substrate, over the course of 2 years . This net storage rate is on par with storage rates in aggrading temperate forests, which typically have NEP ranging from 200 to 400 g C m−2 yr−1 (Aber and Melillo 2001). However, a survey of 12 existing extensive green roofs in Michigan and Maryland, USA revealed that C storage in aboveground biomass averaged only 162 g C m−2 (Getter et al. 2009), with total biomass estimated at 260 g C m−2. This is slightly lower than average biomass storage in the arctic tundra, desert , or temperate grassland biomes (325–375 g C m−2), and much lower than the temperate forest biome (13,350 g C m− 2) (Saugier et al. 2001).
Green roofs are a source of dissolved organic carbon (DOC), which leaches out in runoff, often reaching concentrations above 50 mg/L. This range of DOC is comparable to that in streams draining peatlands (Mulholland 2003), and gives runoff from many green roofs a brownish tint typical of humic-rich waters. The variation in DOC effluent concentrations is primarily related to organic matter content in the substrate, with higher organic matter content typically giving rise to higher effluent DOC (Berndtsson et al. 2009). Runoff DOC concentrations are also sensitive to vegetation type and cover (Table 5.1) and substrate depth (Seidl et al. 2013). Anecdotally, an older, intensive green roof in Japan had runoff DOC concentrations of < 15 mg C L−1, lower than most reported values for extensive roofs (Berndtsson et al. 2009). Few studies have focused on DOC, but amendment of substrate with biochar was found to decrease runoff DOC levels in one plot-scale study (Beck et al. 2011).
Hydrologic export of DOC can represent a substantial proportion of a green roof’s net annual carbon exchange. Assuming atmospheric deposition of DOC averaging 1–5 mg C L−1, and average runoff concentrations of DOC ranging from 20 to 80 mg C L−1, an extensive green roof receiving of 1000 mm yr−1 of precipitation and exporting 400 mm yr−1 of runoff (i.e., 60 % runoff reduction, Gregoire and Clausen 2011), would have a net DOC export of 3–31 g C m−2 yr−1. This represents a large range of uncertainty, but even the lowest estimate is greater than the annual NEP measured by Gaumont-Guay and Halsall (2013), and the highest estimate is on the same order as the short-term substrate sequestration rate measured by Getter et al. (2009). This implies that hydrologic export is of importance to the net carbon budget of green roofs, and should be included in any study concerned with carbon balance in these systems.
Because of the low potential for long-term plant biomass accumulation on extensive roofs, appreciable long-term carbon storage depends mainly on substrate accumulation of organic matter . Substrate organic matter content on newly constructed extensive roofs can be up to 65 g/L (~ 6.5 % by weight, assuming bulk density ~ 1 g/cm3) based on widely-used guidelines (FLL 2008), but is typically lower. This translates to an initial C stock of up to ~ 3250 g C m−2 for a 10 cm thick substrate layer. Where measurements on older roofs have been made, there is evidence for an increase in substrate organic matter content over time (Schrader and Böning 2006; Köhler and Poll 2010), particularly for shallow, single layer extensive green roofs (Thuring and Dunnett 2014). Organic matter accumulation has been observed in new green roof plots planted from seed, in which substrate organic matter content increased from 2.3 to 4.3 % over 5 years (Getter et al. 2007), and for established green roofs which increased from ~ 4 to ~ 6 % organic matter over the course of about 20 years (Köler and Poll 2010). Each 1 % increase in substrate organic matter content would amount to a net C storage of about 500 g C m−2 for a 10 cm thick substrate layer (again, assuming bulk density ~ 1 g/cm3). Although it is not known how generalizable these observations are, the patterns suggest appreciable rates of potential C storage in substrate, with total storage slightly lower than that in forest soils, on a per area basis (e.g., Lal 2005).
Of note, increased organic matter also brings the practical concern of increased weight to the roof, mainly due to the high water holding capacity of the organic matter (discussed in Thuring and Dunnett 2014). The patterns observed suggest variation in carbon sequestration potential among different extensive green roofs. Some studies have noted that extensive green roofs can be a sink for carbon if they are planted from seed (Getter et al. 2009); however many green roofs are constructed with vegetation in place, sometimes at already high coverage, as in the case of pre-vegetated mats. In that case, there is less potential for C sequestration in plant biomass . Gaumont-Guay and Halsall’s (2013) study showing atmospheric CO2 exchange essentially in balance for a new extensive green roof in British Columbia, coupled with the fact that there is appreciable runoff of DOC from most green roof ecosystems, suggests that some roofs are actually net sources for carbon. However, other studies have indicated that organic matter content in green roof substrate can rise over time, indicating longer-term C storage (Schrader and Böning 2006; Köhler and Poll 2010). The actual dynamic will depend on the initial conditions (substrate organic matter content and type, and vegetation type and coverage), as well as local climate and roof hydrology. The change over time in substrate organic matter content will be a good indication of whether green roof ecosystems are gaining or losing carbon over the longer term.
5.5.3 Nitrogen Cycling in Green Roofs: Patterns Observed, and Implications for Processes
Most research on N in green roofs has focused on the dissolved phase; this includes the concentrations or fluxes of N into the system via atmospheric deposition (primarily NO3 −) and out of the system via roof runoff (total nitrogen (TN), NO3 −, and NH4 +) . In general, most roofs appear to operate as sinks for NH4 + (Berndtsson et al. 2006). Nitrate and total N, however, may be higher or lower in green roof runoff than precipitation (Teemusk and Mander 2007; Hathaway et al. 2008; Mendez et al. 2011). These inconsistent patterns may be attributable to variations in climate, vegetation, substrate type, fertilizer regime, substrate depth, and age of the green roof.
Green roof vegetation may impact N runoff flux by several mechanisms: runoff volume reduction due to evapotranspiration, plant uptake and assimilation of N , and the release of N from litter and root exudates. The presence of typical green roof plants, compared to substrate only green roofs, usually reduces concentrations and fluxes of TN, NO3 −, and NH4 + in green roof runoff (Table 5.1). Other studies have shown NO3 − concentrations and fluxes in green roof runoff may vary with plant species (Table 5.1).
Substrate composition and fertilizer regime impact green roof N dynamics (Tables 5.2 and 5.3). If N levels in the substrate exceed biological (plant and microbe) requirements, either due to the initial substrate conditions or subsequent fertilization, the green roof will likely act as a N source (via runoff). Substrate mixes and fertilizers that include high NO3 − levels will result in particularly high losses of N in runoff, due to the leachability of NO3 − (Tables 5.2 and 5.3). For example, a sod roof in Estonia exposed to a fertilizer amendment resulted in runoff concentrations of over 30 mg/L TN, made up primarily of NO3 − (Teemusk and Mander 2011). Ammonium additions may also result in NO3 − runoff, due to the transformation of NH4 + to NO3 − through the microbially-mediated process of nitrification (Fig. 5.2). This process may be responsible for the high levels of NO3 − observed in runoff from green roof plots amended with NH4 + (Emilsson et al. 2007). A Sedum extensive green roof in Ohio (see Civic Garden Center green roof case study (Chap. 16) exposed to fertilization with corn gluten, resulted in a more than 8-fold increase in NO3 − runoff concentrations (Buffam et al. Submitted). This was presumably due to mineralization of organic N in the corn gluten to NH4 + and subsequent nitrification, but requires further controlled study. Similarly, increasing substrate compost levels resulted in higher concentrations of TN in runoff (Hathaway et al. 2008). Substrates with high organic matter content sometimes, but not always, have increased N leaching (Van Seters et al. 2009). The inconsistencies may be due to different microbial communities or environmental conditions, as well as interactions with other substrate components . For example, the ion exchange capacity of green roof substrates will depend on the concentration and total volume of charged components, such as organic material and/or clay.
Substrate depth may influence the N cycling dynamics of green roofs by altering green roof hydrology, interaction times, substrate moisture and temperature, microbial habitat, binding and exchange sites, and the amount of leachable material. Only a few controlled studies have been performed, and these found that NO3 − leaching increased with substrate thickness (Seidl et al. 2013; Table 5.4). Even with increases in the water holding capacity of the substrate at increased substrate depths, fluxes of NO3 − can be higher (Seidl et al. 2013). In a study employing a dual substrate layer, with a basal layer for adsorbing nutrient runoff and an overlying organic layer to supply nutrients to green roof vegetation, N leaching was reduced when the depth of the adsorption layer was increased (Wang et al. 2013; Table 5.4).
Implications for N Cycling Processes:
Patterns in NO3 − and NH4 + found in runoff suggest that N mineralization and nitrification are likely occurring at appreciable rates in the substrate of many extensive green roofs, but direct process measurements have not been made. Other N transformations (Fig. 5.2) may be important but their rates have not been measured. Changes in the N sources (fertilizer amendments, substrate composition , vegetation), as well as reservoirs (vegetation presence and species, substrate), influence the export of N. Substrate depth may also impact N cycling, but this requires further controlled study.
5.5.4 Phosphorus Cycling in Green Roofs: Patterns Observed, and Implications for Processes
Research has consistently shown that green roofs act as sources of P for the first several years following installation, predominantly in the form of PO4 3− (Monterusso et al. 2004; Berndtsson et al. 2006), with concentrations ranging from very low (ex. 0.025; Gregoire and Claussen 2011) to very high (ex. 29 mg/L; Vijayaraghavan et al. 2012). Concentrations of PO4 3− in green roof runoff may reach and exceed levels comparable to wastewater (3–10 mg/L) (Metcalf and Eddy 1991). As P is typically the limiting nutrient in freshwater ecosystems, and P enrichment can lead to eutrophication (Carpenter et al. 1998), runoff from green roofs may pose a threat to aquatic ecosystems. Understanding how green roofs function as ecosystems may allow for improved green roof designs that limit or at least slow the export of P in runoff from green roofs. The export of P in green roof runoff under different experimental conditions and over time can provide clues as to the dominant processes controlling green roof P cycling.
Green roof vegetation may impact green roof P dynamics in a number of ways including: uptake of PO4 3− and assimilation into organic tissues; reduced erosion of sediments containing bound P; and hydrologic and moisture changes due to evapotranspiration. One might therefore expect a negative relationship between plant presence and P runoff. Some studies do indeed indicate that plant presence reduces both TP and PO4 3− concentrations and fluxes (Table 5.1) and that plant species identity may (Beck et al. 2011) or may not (Monterusso et al. 2004; Aitkenhead-Peterson et al. 2011) be important in altering P in runoff. However, it is still unclear what plant-mediated processes are responsible for observed patterns.
The composition of the green roof substrate and substrate amendments has been implicated as one of the most important determinants of P in green roof runoff. If P levels exceed the binding and uptake capacities of the substrate and biota, then P will be leached from the system. Imbalances are commonly attributed to P from the organic matter component of the substrate (Hathaway et al. 2008; Van Seters et al. 2009), presumably released by microbial mineralization of organic P to produce PO4 3−. Compost, which typically has very high P content, has been suggested as the source of PO4 3− to green roof runoff in several studies (Fig. 5.3; Berndtsson et al. 2009). Likewise, amendments of conventional fertilizers at high levels, in contrast to controlled release fertilizers (CRF), result in higher concentrations of TP and PO4 3− in runoff (Emilsson et al. 2007) (Table 5.2). Conventional fertilizers release nutrients faster than controlled release fertilizers, likely overwhelming binding and exchange sites and exceeding the P requirements of plants and microbes. Substrate amended with Biochar (pyrolyzed biomass, with high binding capacity and surface area), showed a minimal ability to bind PO4 3−, slightly reducing export via runoff (Beck et al. 2011; Table 5.3).
Leachable PO4 3− and Dissolved Inorganic Nitrogen (DIN = NO3-N + NH4-N) for commonly used green roof substrate components. Results are expressed in terms of mg of nutrient released per kg of dry material, for a 1:10 mixture of the respective component and either deionized water (for phosphate) or 2 M KCl solution (for DIN). Error bars are standard error of the mean for 3 replicates. (Buffam and Licardi, unpublished data)
The depth of green roof substrate may play a role in green roof P dynamics by altering green roof hydrology, biology, and the physicochemical processes. In a controlled study, increases in substrate depth for extensive green roofs resulted in higher runoff concentrations of PO4 3− (Seidl et al. 2013). On the other hand, for dual substrate layer green roofs (a nutrient substrate layer over an adsorption layer), increasing the thickness of the adsorption layer reduced TP concentrations in runoff (Wang et al. 2013). In contrast to most extensive green roofs, an older, intensive green roof in Japan was found to release little or no P in runoff (Berndtsson et al. 2009). These mixed results indicate the need for further study of the impacts of substrate depth on P dynamics.
Green roof age may alter green roof P cycling due to changes in the sources of P within the substrate, vegetation establishment and growth, and the water holding capacity of the substrate . Studies of green roof runoff water quality have found decreases in P leaching over time (Berndtsson et al. 2006; Buffam et al. Submitted). Köhler et al. (2002) found that the ability of a green roof to retain P increased from 26.1 % in the first year of installation to 79.9 % retention after 4 years. The authors concluded that this trend was likely due to plant establishment . Similar trends in other studies were attributed to the gradual leaching of available P in the green roof substrate over time (Van Seters et al. 2009; Buffam et al. Submitted).
Implications for P Cycling Processes:
P is exported from green roof systems via runoff, especially for recently installed green roofs. It appears that for many green roofs, the major source of P is the organic component of the substrate, especially compost when present. PO4 3− released by mineralization or substrate weathering may overwhelm the uptake and binding capacities of the biological and physicochemical pools within the system. Amending green roofs with fertilizers, especially conventional as opposed to controlled release fertilizers, either worsens this imbalance or, for older roofs, reestablishes it. However, in the absence of fertilizer amendments, since the primary sources of P are within the substrate, the export of P in runoff should and does appear to decline with the age of the roof.
5.5.5 Temporal Dynamics Observed in Green Roof Nutrient Runoff
Although most available data on nutrient patterns from green roofs is for relatively few points in time for a given system, a small number of studies have examined temporal variation, and these dynamics can be analyzed to reveal information about potentially important nutrient cycling processes. The temporal dynamics can be partitioned into four time scales: within event variation, among-event variation, seasonal variation, and longer-term variation related to aging of the roof over years-decades.
Within-event green roof nutrient dynamics studies have mostly focused on contrasting the first runoff from the green roof with a sample taken later in the precipitation event. The first-flush effect is the term used to describe observations of higher pollutant loads in the initial runoff water due to the flushing of materials from dry and/or wet deposition or otherwise accumulated in the system (Zobrist et al. 2000; Berndtsson et al. 2006). A first flush effect would suggest the importance of new atmospheric deposition or between-event internal nutrient cycling for dynamic runoff concentrations, while a lack of first flush (steady concentrations during a runoff event) would suggest that substrate leachable nutrient concentrations are stable over short (hours-days) timescales. Few measurements of this type have been made for green roofs, but there are examples of a first flush effect with higher concentrations of TP, NH4 +, and NO3 − (Berndtsson et al. 2008) in initial runoff samples compared to samples taken later in the event.
Among event variation in nutrient cycling may be expected due to variations in precipitation event intensity, duration, and antecedent conditions such as roof moisture and temperature. These changes may alter the physicochemical (sorption and weathering) and biological (mineralization and plant or microbial uptake) process rates within green roofs. Additionally, a higher precipitation volume will most often result in more runoff, potentially leading to a dilution of dissolved compounds in the runoff. Studies have found lower specific conductivity in green roof runoff following larger precipitation events (Berghage et al. 2009) and higher concentrations of total P and PO4 3− and lower NO3 − in green roof runoff during large events (Teemusk and Mander 2007). Other studies, however, have observed no effect of event size on total P concentrations (Gregoire and Clausen 2011). These inconsistencies suggest the importance of climatic and roof conditions, which vary between studies and through time.
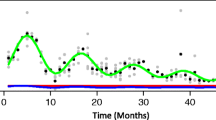
Seasonally-driven green roof nutrient dynamics may be expected due to variation in plant productivity and microbial activity responding to variation in temperature and light within the green roof ecosystem. Researchers have observed increased runoff NO3 − concentrations in the summer months compared to fall and winter (Van Seters et al. 2009; Buffam et al. Submitted), and higher levels of P in green roof snowmelt vs. rain-driven runoff (Gregoire and Clausen 2011). Frequent temporal sampling has revealed contrasting seasonal patterns for different systems (Fig. 5.4). A 2-year study of runoff water quality on a 1–3 year old sloped extensive green roof with a pre-vegetated Sedum mat revealed strong seasonal patterns, with most nutrient and base cation concentrations reaching their maximum concentrations in the warmest months (Buffam et al. Submitted). In contrast, monthly measurements of N and P concentrations and fluxes in runoff from newly-established tray-based extensive green roof plots showed a winter maximum for phosphate, and no clear seasonal pattern in nitrate (Fig. 5.4). Phosphate runoff fluxes were not measurably affected by the presence or absence of plants in this plot-scale study, while nitrate runoff fluxes were decreased in the presence of plants (Fig. 5.4, Johnson 2014). These results suggest that nutrient dynamics in newly-established green roofs are influenced both by plant uptake of N , and by temperature-mediated substrate processes such as weathering or organic matter mineralization.
a, c (leftmost panels): Seasonal dynamics for nitrate (NO3 −) and phosphate (PO4 3−) runoff concentrations from the Civic Garden Center cottage green roof in Cincinnati, OH, for the majority of the runoff events for 2 years (For roof description see Case Study, Chap. 16). Dots indicate concentration; the solid black line indicates weekly average air temperature. Note the increase in NO3 − in the second year likely due to a May fertilization event, and the decline in PO4 3− from 1 year to the next, attributed to aging of the roof (From Buffam et al. submitted). b, d (rightmost panels) Seasonal dynamics for NO3 − and PO4 3 − runoff concentrations from newly established tray-based extensive green roof plots, either with (light green dots) or without (black dots) vegetation. (From Johnson 2014)
Long-term aging of a green roof ecosystem may influence its water holding capacity and runoff chemistry. For example, substrate on a 5 year old roof had more than 3 times the water holding capacity of the original substrate, nearly twice the organic matter and pore space, and increased free airspace (Getter et al. 2007). While it appears that runoff of P, and potentially N, decreases with age (eg., Berndtsson et al. 2006, Köhler et al. 2002), few studies have directly investigated changes in C, N or P cycling in green roofs over longer (decadal) timescales. Changes in the plant community, microbial community, substrate makeup, and water retention with roof age are all likely to impact green roof nutrient dynamics and thus runoff water quality; however these dynamics are not well understood.
Taken together, these observations of temporal variation indicate that green roofs are dynamic systems, which are strongly influenced by environmental conditions (weather, climate) and time. Future controlled studies may help to isolate which variables are most responsible for these patterns and in doing so, uncover the dominant green roof nutrient cycling processes.
5.6 Conceptual Model of Long Term Green Roof Nutrient Cycling Dynamics
The long-term (decadal-scale) changes in nutrient cycling in green roofs are of relevance to managers and researchers alike. Although little is currently known about these long-term dynamics from direct measurements, paradigms and models developed for natural terrestrial ecosystems provide a useful framework for generating hypotheses about green roofs. The “Nutrient Retention Hypothesis” , developed to describe nutrient cycling over successional time (centuries-millennia) in N-limited forest ecosystems (Vitousek and Reiners 1975) offers one such starting point. In this conceptual model, there are several predictable phases during succession, with different nutrient retention characteristics. For primary succession, there is a long time period during which the system is nutrient limited, while vegetation grows and soils build up. During this “aggradation” period, nutrients are held tightly within the system, and export of the limiting nutrient will be essentially zero, while export of other essential nutrients will also be reduced relative to inputs. After vegetation and soils have built up, the system experiences a period of transition where nutrient exports increase, until reaching steady state (inputs = outputs). Secondary succession generally follows the same sequence as primary succession, but begins with a disturbance, resulting in high loss of nutrients during a reorganization period, after which the aggradation period starts again as vegetation regrows. The ecosystem then proceeds as with primary succession, towards steady state (Vitousek and Reiners 1975; Aber and Melillo 2001).
This basic pattern of changing nutrient retention vs. export over successional time can be envisioned with a 3-compartment model (Fig. 5.5; Vitousek et al. 1998), which is a simplified version of a generalized nutrient cycle (Fig. 5.1). In this simplified model, the eventual steady state occurs as a consequence of internal feedbacks resulting in equilibrium between the rate of formation of organic nutrient pools (vegetation, soil) which hold nutrients within the system, and the rate of decomposition of those pools into soluble, bioavailable inorganic nutrients which can be lost through leaching (Vitousek et al. 1998).
Nutrient cycling within a terrestrial ecosystem can be modeled in a simplified way with 3 compartments: available inorganic nutrients, plant nutrients, and soil nutrients (including microbially bound nutrients). (Top) The 3-compartment model, developed to represent changes in N cycling during succession in a N-limited forest ecosystem. (Bottom) Model predictions for variation in outputs (hydrologic runoff), and total system N storage during succession, in the absence of disturbance and with constant, moderate inorganic nitrogen inputs (atmospheric deposition). Under this scenario, a N-limited ecosystem will be a sink for N for a period of time (many centuries, in the model) while vegetation and soil pools grow. Internal feedbacks between the rates of plant uptake, litterfall, and net mineralization result in the system tending towards steady state over time, where N outputs are equal to inputs. (Adapted from Vitousek et al. 1998)
Applying the concepts from this model allows us to construct a basic set of hypotheses for the long-term trajectory of N and P outputs from green roof ecosystems (Fig. 5.6). These hypotheses are informed by observations of nutrient runoff concentrations from green roof ecosystems (Sect. 5.5, and summarized below), and acknowledge the unique characteristics of extensive green roofs as engineered ecosystems (Sect. 5.4).
Summary of five relevant and commonly observed nutrient patterns from extensive green roofs:
-
Organic matter integrated into substrate, and fertilizers either initially integrated or added later, provide a large nutrient supply for green roof ecosystems.
-
Green roofs are a source of high P and N in runoff, for new roofs and following fertilization events.
-
Substrate typically contains very low N:P, i.e. is enriched in P relative to N supply, and relative to plant or microbial demand.
-
After an initial period of leaching, green roofs may be either a source or a sink for N.
-
Green roofs are consistently a source for P in runoff, but runoff concentrations may be low for older roofs.
Different hypothesized trajectories for long-term N (panel a, above) and P (panel b, below) dynamics in an extensive green roof, based on application of a simplified 3-compartment model for nutrient cycling (Vitousek et al. 1998). Both nutrients begin with high rates of leaching outputs (thin, solid line) due to excess initial nutrient stocks in substrate relative to plant demands. Both are also predicted to eventually approach steady state where plant uptake is matched by the substrate mineralization rate, and nutrient outputs equal inputs (thin, dotted line). a Total system N (thick line) may start at levels relatively close to the steady state system N content, and thus may approach steady state fairly rapidly. During the intervening transition period, the roof may serve as a sink or a source for N. The dynamics of this transition depend on the initial nutrient supply, how long plants take to reach their maximum biomass, and the rates of internal processes (plant uptake, net mineralization, and litterfall production). b For P, the system is consistently a source and the approach to steady state is delayed. This is mainly because P is typically present in great excess relative to N (thus non-limiting). P (whether in organic or inorganic form) is also “stickier” than NO3- in terms of binding to soil organic and mineral particles, thus will have a greater tendency to resist being flushed quickly out of the system
There are a few ways in which the development of green roofs over time is clearly different than the development of forest ecosystems as proposed in the 3-compartment model (Vitousek et al. 1998), and in general models of terrestrial nutrient dynamics over successional time (Chapin et al. 2011; Vitousek and Reiners 1975). One difference is that green roofs as currently constructed start with an abundance of nutrients in the system, relative to plant needs. As a result, green roofs can be expected to leach out high levels of nutrients when they are newly constructed (Fig. 5.6), and this has been broadly observed (e.g., Tables 5.1, 5.2, 5.3 and 5.4). This contrasts with the models of primary succession in forested ecosystems (Fig. 5.5), in which nutrients are in short supply, thus tightly held within the system during early succession. The creation of a green roof ecosystem may instead be analogous to a disturbance in a natural ecosystem, which is typically followed by a brief period of high nutrient leaching.
In the absence of disturbance, based on the simple ecosystem model the system is predicted to gradually approach steady state , where nutrient inputs = outputs (e.g., Vitousek and Reiners 1975; Fig. 5.5). The intervening transition phase could take on different characteristics, depending on the initial nutrient stocks and on the balance between inorganic nutrient consumption (plant uptake and soil organic matter formation) and production (net mineralization) over time (e.g., Fig. 5.6). If initial nutrient substrate stocks are not too high, and plants continue to grow over an extended period of time, the ecosystem may experience an “aggradation” phase where outputs < inputs, i.e. the system holds tightly to nutrients, particularly the limiting nutrient. This may be the case with N for some green roofs. However, if initial nutrient stocks in the substrate are very high relative to demand, and the nutrient in question is in organic form or otherwise bound in the substrate, then the system may continue to leach out the nutrient over a long period of time, and never experience a phase where outputs < inputs. This appears to be the case with P for most green roofs as currently constructed.
This model has interesting implications when applied to green roof ecosystems. Assuming that basic models and paradigms from natural forested ecosystems apply, green roofs over time will tend to approach steady state with respect to nutrients—though, it should be noted that even in forested ecosystems the real situation is typically more complex than the model would predict (Vitousek et al. 1998; Aber and Melillo 2001). Even if the systems do generally approach steady state, a key question is how long this takes. Observations to date suggest that this may happen relatively rapidly (within a few years) for N, but may take many years/decades for P.
There are, additionally, several factors that may prevent green roofs from conforming to predictions based on the 3-compartment model. Foremost, the model is an extreme simplification of a complex and dynamic ecosystem, and for many green roofs it may be necessary to include other processes to accurately characterize the system. For example, in extensive green roof ecosystems, productivity may be limited by factors other than nutrient availability, such as moisture availability, wind stress, the low growth potential of the plants, or management choices. Also, the plant species, which by design are typically slow-growing and lack an overstory layer, have lower potential productivity and biomass accumulation than a forest ecosystem. Any subsequent fertilization of the roof would likely lead to additional pulses of nutrient export, slowing the approach to steady state. The lifespan of these ecosystems are also limited, as green roofs are likely to be replaced every 50 years or so. These factors contribute to the potential for a green roof ecosystem to be restricted to the “reorganization” phase of development for much of their lifespan, especially for P.
The simple 3-compartment model allows us to generate hypotheses about potential nutrient behavior of green roofs over a long (decadal) time frame (e.g., Fig. 5.6). However, although this approach does provide a useful starting point, there is not yet enough available field data to determine whether model predictions are borne out. Detailed long-term monitoring of green roofs is needed to fill this knowledge gap. As we continue to learn more about these ecosystems, it will be interesting to see where their long-term trajectories lie relative to those of non-engineered ecosystems.
5.7 Areas of Greatest Uncertainty and Suggestions for Future Research Directions
5.7.1 Overview
We are at an exciting juncture in green roof research, where there is a growing interest by policy-makers and managers in green roof implementation, and by researchers from different disciplines in green roofs as a study system. In spite of the growing interest and body of research, there remain a number of limitations to our understanding of nutrient cycling in green roofs (Table 5.5). These are in part related to the relatively narrow scope of published research studies. The majority of current knowledge of C, N and P cycling in green roofs consists of observations of patterns of concentrations (and occasionally fluxes) of these elements in runoff. Hydrologic fluxes are presumably the largest N and P loss pathway for most green roof systems, and runoff fluxes are of environmental concern because of their potential impact on downstream water quality. However, there are a number of other important transformations and fluxes influencing nutrient cycling and movement through ecosystems (Fig. 5.1), ultimately impacting both plant health within the ecosystem and nutrient runoff. Most of these internal pathways have not been quantified in green roof ecosystems, so their current degree of uncertainty is very high (Table 5.5).
Ecosystem analysis benefits from complementary approaches including the “four legs” supporting ecosystem science: theory, long-term study, cross-ecosystem comparison, and experiments (Carpenter 1998). To date, studies of green roofs in general, and certainly nutrient cycling in green roofs, have suffered from a relative lack of experimental and modeling studies; and most of our knowledge is based on short-term monitoring only, with a few comparative studies examining different types of roofs. Furthermore, full-scale studies are unreplicated, and the wide range of initial roof designs involving different substrate types and depths, different plant palettes, different climates, and different roof ages complicates among-roof comparisons. Controlled experimental treatments have tended to be small scale studies, typically using plots of < 1 m2 and often in a greenhouse setting. It is not clear how the small scale of these studies, and the restricted greenhouse climate (with higher and more consistent humidity than most natural systems), influences the nutrient cycling dynamics relative to full-scale roofs in a real climate setting. Finally, many of the studies to date have only included measurements of nutrient concentrations in runoff, rather than also measuring water balance and calculating nutrient fluxes. To move forward, an expanded scope of approaches is needed.
It is relevant to note that this assessment is based on the English-language scientific literature only. Broad implementation of extensive green roofs in German cities, especially during the mid-late twentieth century, has led to a rich history of green roof design and associated research in that country. Thus, there is an existing deep knowledge base, only some of which has been accessed by the scientific community writing in English. There is good potential for expanding partnerships to build rapidly on this knowledge base—by making existing studies available across different language platforms, by international research collaborations, and by carrying out new studies on older, well-studied roofs in Germany (see for instance Köhler and Poll 2010; Thuring and Dunnett 2014).
5.7.2 Questions for Future Research
As an aid in thinking about the future of nutrient cycling research in green roofs, we present several pressing research questions below , ranging from the theoretical to the practical:
Q1: How can green roof nutrient cycles best be modeled, conceptually and mathematically?
Development of models is important for increased understanding of green roof ecosystems, and for prediction of the impacts of variation in roof design, or in environmental conditions including climate. In this chapter we presented a rough conceptual schematic with associated expectations based on a simple 3-compartment model from N-limited terrestrial ecosystem over successional time (Vitousek et al. 1998). This enabled discussion of similarities and differences compared to the (relatively) well-understood nutrient-cycling processes in natural forested ecosystems, but there are many other types of models that could be useful in describing green roof ecosystem dynamics (e.g., Jorgensen 2011).
Q2: How does varying nutrient stoichiometry impact the nutrient cycling within green roof ecosystems, and specifically the capacity of these systems to support thriving plant communities while minimizing hydrologic loss of dissolved N and P?
Currently, most green roofs have an abundance of P relative to N, based on the very low N:P ratios observed in effluent water. This suggests that P sources could be reduced without stressing plants, and this might result in more efficient nutrient retention within the ecosystem.
Q3: What role do soil microbial communities, including bacteria, saprotrophic fungi, and mycorrhizal associations play in processing C, N and P in green roof substrate, and in regulating the runoff of these nutrients?
Very little is known on this topic, though McGuire et al. (2013) found that green roofs can be home to diverse fungal communities and that these communities differ among roofs. Mycorrhizal associations could be of importance for nutrient delivery to plants, particularly if engineering of green roofs shifts to lower nutrient substrates (See also Chap. 7 in this book).
Q4: Is Nitrogen-fixation an important source of N to green roof ecosystems?
N-fixation can provide an important source of fixed nitrogen to ecosystems, especially in high light, N-limited environments (Chapin et al. 1991). Currently, N-fixation is probably not of major importance in most extensive green roofs, since Sedums are not among the families of plants commonly associated with N-fixing bacteria (Paul and Clark 1996). However, free-living soil microbes can perform N-fixation under appropriate environmental conditions, and there are already examples of plants associated with N-fixation on green roofs (Chap. 16). The use of N-fixing plant associations could provide an important contribution of N to green roof ecosystems under some engineering designs, and would allow for reduced use of N fertilizers.
Q5: What is the role of green roof ecosystems in surface-atmosphere exchange of the trace greenhouse gases (GHG) methane (CH 4 ) and Nitrous Oxide (N2O)?
Although overall carbon exchange over long time frames may be relatively small in green roofs, CH4 and N2O exchange could still be relevant to urban GHG balances. It is not known whether green roofs are a source or a sink for these gases, nor how substrate characteristics affect CH4 or N2O exchange. Green roofs are unlikely to be sources of CH4 because they are designed to be well-drained and thus presumably lack anoxic zones for CH4 genesis. Green roofs could however be a sink for CH4, as has been found for other vegetated ecosystems (Groffman and Pouyat 2009). Green roofs could be a source of N2O, which is a byproduct of both nitrification and denitrification processes (Groffman et al. 1998; Bateman and Baggs 2005). N2O emissions often increase after fertilization events (Aber and Melillo 2001), due to an increase in the rates of those processes. Denitrification is unlikely to be important for extensive green roofs that are well-drained, as this process requires anoxic microsites typical of waterlogged soils. However, denitrification could be important in some roofs that have thicker substrate or natural soils that drain less freely, and nitrification may contribute to N2O emissions.
Q6: How can substrate amendments increase the capacity of green roof substrate to reduce hydrologic losses and bind nutrients?
Early experiments with the integration of biochar, an agricultural soil amendment, have shown promising results in terms of increasing water-holding capacity and reducing leaching of DOC and sometimes N and P (Beck et al. 2011 ).
Q7: How can fertilizer applications best be matched with plant nutrient demands to reduce expensive fertilizer requirements and reduce impacts of pollution downstream?
Unlike agro-ecosystems in which high productivity and harvest are the main goals, green roof ecosystems are designed for stability , sustainability, and minimal management requirements. Inherent slow growth and low nutrient requirements are characteristics of plants like Sedum, which are adapted to low-fertility environments (Chapin et al. 1986). These characteristics may persist even when they are placed in a high-nutrient environment, like a fertilized green roof. Therefore, the nutrient supply could be kept relatively low, with the goal of minimizing fertilizer nutrient inputs and designing a system that holds and recycles nutrients.
5.8 Synthesis and Implications
5.8.1 Summary of Current Knowledge
Most of the current knowledge about green roof nutrient cycling involves observation of patterns of runoff concentrations. As most extensive green roofs are currently constructed, both available N and especially P substrate pools begin high relative to the plant community’s capacity to uptake nutrients. In spite of providing a substantial reduction in runoff volume, the runoff is often enriched in nutrients, particularly in younger roofs. Organic carbon and inorganic phosphorus are consistently high in runoff; Ammonium is typically reduced in runoff relative to the incoming atmospheric deposition flux, while nitrate may be either reduced or enriched. Although phosphorus leaching may be a transient effect, it represents a substantial disservice for at least several years after construction for many green roofs, with P concentrations in effluent water from many green roofs as high as in wastewater (Metcalf and Eddy 1991). It is also largely unnecessary since most of the systems have excess P relative to plant demand, and relative to availability of N and other nutrients.
A number of characteristics of green roof systems influence nutrient cycling and nutrient efflux in runoff. These include plant density or coverage, plant identity and functional characteristics, substrate type, and substrate depth. Substrate characteristics have been found to be a primary control on runoff nutrient fluxes from green roof systems. The organic component of the substrate has been implicated as the primary source for N and P leaching out of some green roof ecosystems (Hathaway et al. 2008; Fig. 5.3), while slow-release mineral fertilizers have been suggested as the main source of leaching N and P in other green roofs (Berndtsson et al. 2006). Plants also play an important role in sequestering N in biomass, reducing N losses in runoff (Table 5.1). Carbon sequestration in substrate or biomass in green roofs may occur but is likely a minor factor relative to the initial energy costs of building the system, and other important functions provided by the system, including long-term energy savings (Getter et al. 2009).
5.8.2 Looking Forward
In any complex ecological system, the observed hydrologic nutrient efflux is the result of the balance between other ecosystem processes (Fig. 5.1), many of which remain poorly studied in green roofs (Table 5.5). An understanding of the internal process dynamics is important for predicting responses to environmental variation (e.g., weather variability and climate shifts) and impacts of varying system characteristics (e.g., substrate organic matter content, substrate N:P ratio, substrate depth, plant assemblage). Studies of temporal dynamics, on time-scales ranging from minutes to years, provide useful information on potentially important processes. Studies taking a mass-balance approach to following C, N, and P dynamics through green roof ecosystems would provide a valuable addition to the knowledge base. Future studies should emphasize controlled experimental manipulations at the full-roof scale, direct process measurements, mathematical process-based models linking green roof hydrology and biogeochemistry, and long-term observations spanning decades, which is a relevant time-scale for these ecosystems.
5.8.3 Management Implications
At this point, there is enough information available to suggest next-generation substrate designs that would minimize nutrient losses while sustaining plants and providing the other services for which green roofs are designed. To reduce the output of nutrients, particularly inorganic N and P, the keys are minimizing inputs and/or retaining nutrients more tightly within the system. Lowering nutrient inputs in precipitation is not under control of local managers, but the initial nutrient pool sizes can be lowered by decreasing the amount of N and (especially) P rich fertilizers and organic matter in the substrate, by encouraging biological N-fixation rather than fertilization as a source of N, and by matching fertilization regime carefully to measured system needs. This may mean elimination of P in added fertilizers, since most systems as currently constructed have an abundance of P relative to N.
Nutrient retention is a challenge because of the high potential for nutrient leaching loss in systems like green roofs that are well-drained and rapidly flushed during large precipitation and melt events. Strategies to increase retention (and decrease runoff) could include increased stability of the substrate nutrient pool, and increased uptake rates by, and storage in, vegetation. Increased retention within the substrate could be achieved by amendments which bind nutrients tightly and thereby lower rates of loss by mineralization, desorption, and weathering, or by any enhancement to water retention within the ecosystem. For example, initial experiments with biochar have shown some promise in both water and nutrient retention (Beck et al. 2011). Increased storage by vegetation could either be sustained by harvesting vegetation periodically, or by using vegetation that would decompose slowly and thus contribute to the long-term accumulation of organic matter in the substrate. Finally, runoff from green roofs can be re-used/re-cycled such that the excess P is used for fertilizing other vegetation, or at least prevented from entering local waterways.
References
Aber J, Melillo J (2001) Terrestrial ecosystems. Harcourt Academic Press, San Diego
Aitkenhead-Peterson J, Dvorak B, Voider A, Stanley N (2011) Chemistry of growth medium and leachate from green roof systems in south-central Texas. Urban Ecosyst 14(1):17–33
Baker L, Hope D, Xu Y, Edmonds J, Lauver L (2001) Nitrogen balance for the Central Arizona-Phoenix (CAP) ecosystem. Ecosystems 4(6):582–602
Bateman E, Baggs E (2005) Contributions of nitrification and denitrification to N2O emissions from soils at different water-filled pore space. Biol Fertil Soils 41(6):379–388
Beck D, Johnson G, Spolek G (2011) Amending greenroof soil with biochar to affect runoff water quantity and quality. Environ Pollut 159(8–9):2111–2118
Berghage R, Beattie D, Jarrett A, Thuring C, Razei F, O’Connor T (2009) Green roofs for stormwater runoff control. Cincinnati, OH. EPA/600/R-09/026
Berndtsson J (2010) Green roof performance towards management of runoff water quantity and quality: A review. Ecological Engineering 36:351–360
Berndtsson J, Emilsson T, Bengtsson L (2006) The influence of extensive vegetated roofs on runoff water quality. Sci Total Environ 355(1–3):48–63
Berndtsson J, Bengtsson L, Jinno K (2008) First flush effect from vegetated roofs during simulated rain events. Hydrol Res 39(3):171–179
Berndtsson J, Bengtsson L, Jinno K (2009) Runoff water quality from intensive and extensive vegetated roofs. Ecol Eng 35(3):369–380
Buffam I, Mitchell M, Durtsche RD (Submitted) Seasonal variation in green roof runoff water quality
Carpenter, S (1998), The need for large-scale experiments to assess and predict the response of ecosystems to perturbation. In: Pace ML, Groffman PM (eds) Successes, Limitations, and Frontiers in Ecosystem Science. Springer, New York, pp 287–312
Carpenter S, Caraco N, Correll D, Howarth R, Sharpley A, Smith V (1998) Nonpoint pollution of surface waters with phosphorus and nitrogen. Ecol Appl 8(3):559–568
Chapin F, Shaver G, Kedrowski R (1986) Environmental controls over carbon, nitrogen and phosphorus fractions in Eriophorum-vaginatum in Alaskan tussock tundra. J Ecol 74(1):167–195
Chapin F, Bliss L, Bledsoe L (1991) Environmental regulation of nitrogen fixation in a high arctic lowland ecosystem. Can J Bot 69:2744–2755
Chapin F, Woodwell G, Randerson J, Rastetter E, Lovett G, Baldocchi D, Clark D, Harmon M, Schimel D, Valentini R, Wirth C, Aber J, Cole J, Goulden M, Harden J, Heimann M, Howarth R, Matson P, McGuire A, Melillo J, H Mooney, Neff J, Houghton R, Pace M, Ryan M, Running S, Sala O, Schlesinger W, Schulze E (2006) Reconciling carbon-cycle concepts, terminology, and methods. Ecosystems 9(7):1041–1050
Chapin F, Matson P, Vitousek P (2011) Fundamentals of terrestrial ecosystem ecology. Springer, New York
Clark M, Zheng Y (2013) Plant nutrition requirements for an installed Sedum−vegetated green roof module system:effects of fertilizer rate and type on plant growth and leachate nutrient content. HortScience 48:1173–1180
Crews T (1999) The presence of nitrogen fixing legumes in terrestrial communities: evolutionary vs ecological considerations. Biogeochemistry 46(1–3):233–246
Dahlgren R (1998) Effects of forest harvest on stream-water quality and nitrogen cycling in the Caspar Creek Watershed. USDA Forest Service Gen Tech Rep PSW-GTR 168:45–53
Emilsson T, Berndtsson J, Mattsson J, Rolf K (2007) Effect of using conventional and controlled release fertiliser on nutrient runoff from various vegetated roof systems. Ecol Eng 29(3):260–271
FLL (2008) Guidelines for the planning, construction, and maintenance of green roofing- green roofing guideline. Forschungsgesellschaft Landschaftsentwicklung Landschaftsbau, Bonn
Galloway J, Aber J, Erisman J, Seitzinger S, Howarth R, Cowling E, Cosby B (2003) The nitrogen cascade. Bioscience 53(4):341–356
Gaumont-Guay D, Halsall R (2013) What’s the carbon sequestration potential in green roofs? 11th Annual Cities Alive Green Roof and Wall Conference, San Francisco, CA
Getter K, Rowe D, Andresen J (2007) Quantifying the effect of slope on extensive green roof stormwater retention. Ecol Eng 31(4):225–231
Getter K, Rowe D, Robertson G, Cregg B, Andresen J (2009) Carbon sequestration potential of extensive green roofs. Environ Sci Technol 43(19):7564–7570
Gregoire B, Clausen J (2011) Effect of a modular extensive green roof on stormwater runoff and water quality. Ecol Eng 37(6):963–969
Groffman P, Pouyat R (2009) Methane uptake in urban forests and lawns. Environ Sci Technol 43(14):5229–5235
Groffman P, Gold A, Jacinthe P (1998) Nitrous oxide production in riparian zones and groundwater. Nutr Cycl Agroecosyst 52(2–3):179–186
Hathaway A, Hunt W, Jennings G (2008) A field study of green roof hydrologic and water quality performance. Trans Asabe 51(1):37–44
Johnson C (2014) Role of plant species richness on the quantity and quality of stormwater runoff in green roof plots. M.S. Thesis, Department of Biological Sciences, University of Cincinnati
Jorgensen S (ed) (2011) Handbook of ecological models used in ecosystem and environmental management. Taylor and Francis, Boca Raton
Köhler M, Poll PH (2010) Long-term performance of selected old Berlin green roofs in comparison to younger extensive green roofs in Berlin. Ecol Eng 36:722–729
Köhler M, Schmidt M, Grimme FW, Laar M, Assunção Paiva VL, Tavares S (2002) Green roofs in temperate climates and in the hot-humid tropics-far beyond the aesthetics. Environ Manage Health 13(4):382–391
Lal R (2005) Forest soils and carbon sequestration. Forest Ecol Manage 220:242–258
Likens G, Bormann F, Pierce R, Eaton J, Johnson N (1977) Biogeochemistry of a forested ecosystem. Springer-Verlag, New York
Marschner B, Kalbitz K (2003) Controls of bioavailability and biodegradability of dissolved organic matter in soils. Geoderma 113:211–235
McGuire K, Payne S, Palmer M, Gillikin C, Keefe D, Kim S, Gedallovich S, Discenza J, Rangamannar R, Koshner J, Massmann A, Orazi G, Essene A, Leff J, Fierer N (2013) Digging the New York City Skyline: soil fungal communities in green roofs and city parks. PLoS One 8(3):e58020
Mendez C, Klenzendorf J, Afshar B, Simmons M, Barrett M, Kinney K, Kirisits M (2011) The effect of roofing material on the quality of harvested rainwater. Water Res 45(5):2049–2059
Metcalf and Eddy (1991) Wastewater engineering: treatment, disposal, and reuse. McGraw-Hill, New York
Monterusso M, Rowe D, Rugh C, Russell D (2004) Runoff water quantity and quality from green roof systems. Expanding roles for horticulture in improving human well-being and life quality. Acta Hortic 639:369–376
Mulholland P (2003) Large-scale patterns in dissolved organic carbon concentration, flux, and sources. In: Findlay S, Sinsabaugh R (eds) Aquatic ecosystems: interactivity of dissolved organic matter. Academic Press/Elsevier Science, San Diego, pp 139–159
Oberndorfer E, Lundholm J, Bass B, Coffman R, Doshi H, Dunnett N, Gaffin S, Koehler M, Liu K, Rowe B (2007) Green roofs as urban ecosystems: ecological structures, functions, and services. Bioscience 57(10):823–833
Paul E, Clark F (1996) Soil microbiology and biochemistry, 2nd edn. Academic Press, San Diego
Pett-Ridge J (2009) Contributions of dust to phosphorus cycling in tropical forests of the Luquillo Mountains, Puerto Rico. Biogeochemistry 94(1):63–80
Rowe D (2011) Green roofs as a means of pollution abatement. Environ Pollut 159(8–9):2100–2110
Saugier B, Roy J, Mooney H (2001) Estimations of global terrestrial productivity: converging toward a single number? In: Roy J, Saugier B, Mooney H (eds) Terrestrial global productivity. Academic Press, San Diego, pp 543–557
Schrader S, Böning M (2006) Soil formation on green roofs and its contribution to urban biodiversity with emphasis on Collembolans. Pedobiologia 50:347–356
Seidl M, Gromaire M, Saad M, De Gouvello B (2013) Effect of substrate depth and rain-event history on the pollutant abatement of green roofs. Environ Pollut 183:195–203
Shaver G, Melillo J (1984) Nutrient budgets of marsh plants- efficiency concepts and relation to availability. Ecology 65(5):1491–1510
Smith S, Read D (2008) Mycorrhizal symbiosis, 3rd edn. Academic Press, London
Teemusk A, Mander U (2007) Rainwater runoff quantity and quality performance from a greenroof: the effects of short-term events. Ecol Eng 30(3):271–277
Teemusk A, Mander U (2011) The influence of green roofs on runoff water quality: a case study from Estonia. Water Res Manage 25(14):3699–3713
Thuring CE, Dunnett N (2014) Vegetation composition of old extensive green roofs (from 1980s Germany). Ecol Processes 3:4
Van Seters T, Rocha L, Smith D, MacMillan G (2009) Evaluation of green roofs for runoff retention, runoff quality, and leachability. Water Qual Res J Can 44(1):33–47
Vijayaraghavan K, Joshi U, Balasubramanian R (2012) A field study to evaluate runoff quality from green roofs. Water Res 46(4):1337–1345
Vitousek P, Howarth R (1991) Nitrogen limitation on land and in the sea-how can it occur. Biogeochemistry 13(2):87–115
Vitousek PM, Reiners WA (1975) Ecosystem succession and nutrient retention: a hypothesis. BioScience 25(6):376–381
Vitousek P, Hedin L, Matson P, Fownes J, Neff J (1998) Within-system element cycles, input-output budgets, and nutrient limitation. In: Pace M, Groffman P (eds) Successes, limitations, and frontiers in ecosystem science. Springer, New York, pp 432–451
Wang X, Zhao X, Peng C, Zhang X, Wang J (2013) A field study to evaluate the impact of different factors on the nutrient pollutant concentrations in green roof runoff. Water Sci Technol 68(12):2691–2697
Zobrist J, Muller S, Ammann A, Buchelli T, Mottier V, Ochs M, Schoenenberger R, Eugster J, Boller M (2000) Quality of roof runoff for groundwater infiltration. Water Res 34(5):1455–1462
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Buffam, I., Mitchell, M. (2015). Nutrient Cycling in Green Roof Ecosystems. In: Sutton, R. (eds) Green Roof Ecosystems. Ecological Studies, vol 223. Springer, Cham. https://doi.org/10.1007/978-3-319-14983-7_5
Download citation
DOI: https://doi.org/10.1007/978-3-319-14983-7_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-14982-0
Online ISBN: 978-3-319-14983-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)