Abstract
The use of plants for remediation of soils and waters contaminated with heavy metals has gained acceptance in the past two decades as a cost-effective and noninvasive technique. In this study, the effectiveness of Common Reed for phytoremediation of heavy metals from municipal waste leachate was investigated. The plants were transplanted into pots containing 10 L of mixed urban waste leachate and water (mixed 80 percentages of waste leachate with 20 % of water; V:V) and aerated during experiments. Central composite design (CCD) and response surface methodology (RSM) were used in order to clarify the nature of the response surface in the experimental design and explain the optimal conditions of the independent variables. In the optimum conditions, the amount of removed Fe, Mn, Cu, and Ni were 25.049, 9.623, 6.112, and 0.900 mg/kg, and Translocation Factor (TF) in 24, 48, and 72 h experiment were 0.47, 0.45, 0.34, 0.38, 1.17, 0.89, 0.69, 0.42, 1.30, 1.12, 1.10, and 1.01 for each heavy metal (Fe, Mn, Cu, and Ni), respectively. The findings showed that Phragmites australis is an effective accumulator plant for phytoremediation of these metals.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
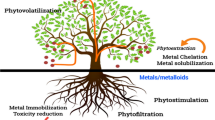
The use of plants for remediation of soils and waters contaminated with heavy metals, has gained acceptance in the past two decades as a cost-effective and noninvasive technique (Mojiri 2012). This approach is emerging as an innovative tool with great potential that is most useful when contaminants are within the root zone of the plants (top 3–6 ft). Furthermore, phytoremediation is energy efficient, cost-effective, aesthetically pleasing technique of remediation sites with low to moderate levels of pollution. The technique of phytoremediation exploits the use of either naturally occurring metal hyperaccumulator plants or genetically engineered plants (Setia et al. 2008). A variety of contaminated waters can be phytoremediated, counting sewage and municipal wastewater, agricultural runoff/drainage water, industrial wastewater, coal pile runoff, landfill leachate, mine drainage, and groundwater plumes (Olguin and Galvan 2010).
A rising method for polluted area remediation is phytoextraction (Ok and Kim 2007). Phytoextraction is the uptake of contaminants by plant roots and translocation within the plants. Contaminants are generally removed by harvesting the plants, and it has been recognized as an appropriated approach to remove pollutants from soil, sediment, and sludge (Singh et al. 2011). Plants may play a vital role in metal removal through absorption, cation exchange, filtration, and chemical changes through the root. There is evidence that wetland plants such as Typha latifolia, Cyperus malaccensis, and Phragmites australis can accumulate heavy metals in their tissues (Mojiri et al. 2013a; Yadav and Chandra 2011).
Introduced Phragmites is a vigorous plant that, once established, rapidly takes over, creating dense patches that consume available growing space and push out other plants, including the native subspecies. It also alters wetland hydrology, increases the potential for fire, and may reduce and degrade wetland wildlife habitat due, in part, to its dense growth habit (Swearingen and Saltonstall 2010).
Phragmites australis (Fig. 7.1), or Common Reed, is a large perennial rhizomatous grass that grows 5–20 ft (1.5–3 m) tall. Its leaves are broad and sheath like, 0.4–1.6 in. (1–4 cm) wide at their base. Phragmites has gray-green foliage during the growing season. New stems grow in the spring, and its rhizomes spread horizontally during the growing season. It flowers in late June, with bushy panicles and seeds forming by August to early fall. During this time, energy stores are translocated from the leaves and stems to the rhizomes of the plant. Phragmites australis is a strong colonizer, producing an abundance of wind-dispersed seeds, though its seed viability is typically low and it exhibits an interannual variation in fecundity (URI CELS Outreach Center 2012).
Burkea et al. (2000) studied release of metals by the leaves of the Salt marsh grasses Spartina alterniflora and Phragmites australis.
The aims of the study were to investigate the heavy metals removal from urban waste leachate by Common Reed and optimization of process parameters using the response surface methodology (RSM).
2 Materials and Methods
2.1 Sample Preparation
The plants were transplanted into pots containing 10 L of mixed urban waste leachate and water (mixed 80 percentages of waste leachate with 20 % of water; V:V), and aeration was done in 2011. Central composite design and response surface methodology were used in order to clarify the nature of the response surface in the experimental design and explain the optimal conditions of the independent variables. Different number of Phragmites australis transplanting in each pot (2–4) and different lengths of time for taking samples (24–72 h) were used.
2.2 Laboratory Analysis
The plant tissues were prepared for laboratory analysis by Wet Digestion method (Campbell and Plank 1998). Iron (Fe), manganese (Mn), and cadmium (Cu), and nickel (Ni) in waste leachate and plant tissues were carried out using a flame atomic absorption spectrometer (Varian Spectra 20 Plus, Mulgrave, Australia) in accordance to the Standard Methods (APHA 2005). Waste leachate and water properties are shown in Table 7.1.
2.3 Statistical Analysis
Central composite design (CCD) and Response surface methodology (RSM) were employed in order to clarify the nature of the response surface in the experimental design and elucidate the optimal conditions of the independent variables. CCD was established through Design Expert Software (6.0.7). The behavior of the system is described through equation 1 an empirical second-order polynomial model:
where Y is the response; X i and X j are the variables; β 0 is a constant coefficient; β j , β jj , and β ij are the interaction coefficients of linear, quadratic, and second-order terms, respectively; k is the number of study factors; and e is the error (Mojiri et al. 2013b).
The results were completely analyzed by analysis of variance (ANOVA) in the Design Expert Software. Number of Phragmites australis transplanting (2, 3, and 4) and times for taking samples (24, 48, and 72 h) were used. To carry out an adequate analysis, three dependent parameters (reducing Fe, Mn, Cu, and Ni concentration in leachate) were measured as responses (Table 7.2).
Descriptive statistical analysis including mean comparison of Fe, Mn, Cu, and Ni accumulation in the roots and shoots of the plants using Duncan’s Multiple Range Test (DMRT) was conducted using the SPSS software.
3 Results and Discussions
Waste leachate properties before the experiment, the results of the experiments, ANOVA results for response parameter, and comparing the means of Fe, Mn, Cu, and Ni accumulation in Phragmites australis roots and shoots are shown in Tables 7.2.
In this work, the RSM was used for analyzing the correlation between the variables (number of Phragmites australis transplanting and the lengths of time for taking samples) and the important process response (the amount of removed Fe, Mn, Cu, and Ni). Predicted vs. actual values plot for metal removals are shown in Figs. 7.2 and 7.3. Considerable model terms were preferred to achieve the best fit in a particular model. CCD permitted the development of mathematical equations where predicted results (Y) were evaluated as a function of the number of Phragmites australis transplanting (A) and the lengths of time for taking samples (B). The results were computed as the sum of a constant, two first order effects (terms in A and B), one interaction effect (AB), and two second-order effects (A 2 and B 2), as shown in the equation (Table 7.3). The results were analyzed by ANOVA to determine the accuracy of fit.
The model was significant at the 5 % confidence level because probability values were less than 0.05. The lack of fit (LOF) F-test explains variation of the data around the modified model. LOF would be significant, if the model did not fit the data well. Generally, large probability values for LOF (>0.05) explained that the F-statistic was insignificant, implying a significant model relationship between variables and process responses.
3.1 Iron (Fe) Removed
Iron is a natural constituent of the Earth’s crust and is present in varying concentrations in all ecosystems. They are stable and persistent environmental contaminants since they cannot be degraded or destroyed. Human activity has drastically changed the biogeochemical cycles and balance of some metals (Anusha 2011). Iron (II) ions have a high solubility in the aquatic environment and can be absorbed by plants and living organisms (Bulai and Cioanca 2011).
The amount of removed Fe ranged from 11.67 mg/kg (two plants transplanting, and 24 h of time for taking samples) to 25.01 mg/kg (four plants transplanting, and 72 h of time for taking samples). The phytoremediation of Fe increased when the number of plants transplanting and time for taking samples were increased.
3.2 Manganese (Mn) Removed
Manganese ions exist in wastewaters from numerous industries, chiefly pyrolusite (MnO2) treatment, ink and dyes, glass and ceramics, paint and varnish, steel alloy dry cell batteries, firework and match, and in metal galvanization plant waste matters (Taffarel and Rubio 2009).
The amount of removed Mn ranged from 3.08 mg/kg (two plants transplanting, and 24 h of time for taking samples) to 9.81 mg/kg (four plants transplanting, and 72 h of time for taking samples). The phytoremediation of Mn increased when the number of plants transplanting and time for taking samples were increased.
3.3 Copper (Cu) Removed
Copper can be found in many wastewater sources including printed circuit board manufacturing, electronics plating, painting manufacturing, and printing operations. This compound can be removed from wastewater by some methods (Yahyaa and Rosebi 2010).
The amount of removed Cu ranged from 2.24 mg/kg (two plants transplanting, and 24 h of time for taking samples) to 6.31 mg/kg (four plants transplanting and 72 h of time for taking samples). The phytoremediation of Cu increased when the number of plants transplanting and time for taking samples were increased.
3.4 Nickel (Ni) Removed
In the environment, Ni is found primarily combined with oxygen (oxides) or sulfur (sulfides) (Ministry of the Environment 2001). Elevated levels of Ni (Ni++) can pose a major threat to both human health and the environment (Hussain et al. 2010).
The amount of removed Ni ranged from 0.31 mg/kg (two plants transplanting, and 24 h of time for taking samples) to 0.91 mg/kg (four plants transplanting and 72 h of time for taking samples). The phytoremediation of Ni increased when the number of plants transplanting and time for taking samples were increased.
3.5 Uptake of Heavy Metals by Common Reed
Metal accumulating plant species can concentrate heavy metals like Cd, Zn, Co, Mn, Ni, and Pb up to 100 or 1,000 times more than those taken up by non-accumulator (excluder) plants. The uptake performance by plant can be greatly improved (Tangahu et al. 2011).
The concentrations of Fe (ppm) in the roots of Phragmites australis were 2.40, 3.98, and 6.10, and in the shoots of Phragmites australis were 1.13, 4.67, and 7.98, after 24, 48, and 72 h, respectively.
The concentrations of Mn (ppm) in the roots of Phragmites australis were 1.10, 1.10, and 4.71, and in the shoots of Phragmites australis were 0.50, 1.89, and 5.29, after 24, 48, and 72 h, respectively.
The concentrations of Cu (ppm) in the roots of Phragmites australis were 0.96, 1.89, and 4.09 and in shoots of Phragmites australis were 0.33, 1.32, and 4.50, after 24, 48, and 72 h, respectively.
The concentrations of Ni (ppm) in the roots of Phragmites australis were 0.10, 0.31, and 0.60, and in the shoots of Phragmites australis were 0.03, 0.13, and 0.61, after 24, 48, and 72 h, respectively.
3.6 Translocation Factor (TF)
The efficiency of phytoremediation can be quantified by calculating translocation factor. The TF expresses the capacity of a plant to store the MTE in its upper part. This is defined as the ratio of metal concentration in the upper part to that in the roots (Chakroun et al. 2010). The translocation factor indicates the efficiency of the plant in translocating the accumulated metal from its roots to shoots. It is calculated as follows (Padmavathiamma and Li 2007).
where C shoot is the concentration of the metal in plant shoots and C root is the concentration of the metal in plant roots.
Based on Table 7.4, translocation factors (TF) were more than 1 in treatment number 3, and in treatment number 2 just for Fe. A translocation factor value greater than 1 indicates the translocation of the metal from root to above-ground part (Jamil et al. 2009). According to Yoon et al. (2006), only plant species with TF greater than 1 have the potential to be used for phytoextraction.
4 Conclusions
Phytoremediation of heavy metals from urban waste leachate by Phragmites australis was studied. CCD and RSM were used in the design of experiments, statistical analysis and optimization of the parameters. The factors were number of Phragmites australis transplanting (2, 3, and 4) and time for taking samples (24, 48, and 72 h); while the responses were removals of Fe, Mn, Cu, and Ni. The findings clarified that the Phragmites australis is an effective accumulator plant for phytoremediation of Fe, Mn, Cu, and Ni. Statistical analysis via Design Expert Software (6.0.7) showed that the optimum conditions for the number of Phragmites australis is transplanting and the time for taking samples were 4.00 and 72.00 h, respectively. For the optimized factors, the amount of removed pollutants Fe, Mn, Cu, and Ni (ppm) were 25.04, 9.62, 6.11, and 0.90 mg/kg, respectively.
References
Anusha G (2011) Removal of iron from wastewater using bael fruit shell as adsorbent. 2011 2nd international conference on environmental science and technology IPCBEE, vol.6© IACSIT Press, Singapore
APHA (2005) Standard methods for examination of water and wastewater, 20th edn. American Public Health Association, Washington, DC
Bulai P, Cioanca ER (2011) Iron removal from wastewater using chelating resin purolite S930. TEHNOMUS—new technologies and products in machine manufacturing technologies
Burkea DJ, Weisa JS, Weisb P (2000) Release of metals by the leaves of the salt marsh grasses Spartina alterniflora and Phragmites australis. Estuar Coast Shelf Sci 51(2):153–159
Chakroun HK, Souissi F, Bouchardon JL, Souissi R, Moutte J, Faure O, Remon E, Abdeljaoused S (2010) Transfer and accumulation of lead, zinc, cadmium, and copper in plants growing in abandoned mining-district area. Afr J Environ Sci Technol 4(10):651–659
Campbell CR, Plank CO (1998) Preparation of plant tissue for laboratory analysis. In: Kalra YP (ed) Handbook of reference method for plant analysis. CRC, Boca Raton, FL, pp 37–49
Hussain ST, Mahmood T, Malik SA (2010) Phytoremediation technologies for Ni++ by water hyacinth. Afr J Biotechnol 9(50):8648–8660
Jamil S, Abhilash PC, Singh N, Sharma PN (2009) Jatropha curcas: a potential crop for phytoremediation of coal fly ash. J Hazard Mater 172:269–275
Ministry of the Environment (2001) Nickel in the environment. Ontario, fact sheet, March 2001
Mojiri A (2012) Phytoremediation of heavy metals from municipal wastewater by Typha domingensis. Afr J Microbiol Res 6(3):643–647
Mojiri A, Aziz HA, Aziz SQ, Selamat MRB, Gholami A, Aboutorab M (2013a) Phytoremediation of soil contaminated with nickel by Lepidium sativum; optimization by response surface methodology. Global NEST Journal 15(1):69–75
Mojiri A, Aziz HA, Zahed MA, Aziz SQ, Selamat MRB (2013b) Phytoremediation of heavy metals from urban waste leachate by southern cattail. Int J Sci Res Environ Sci 1(4):63–70
Ok YS, Kim JG (2007) Enhancement of cadmium phytoextraction from contaminated soils with Artemisia princeps var. orientalis. J Appl Sci 7(2):263–268
Olguin EJ, Galvan GS (2010) Aquatic phytoremediation: novel insights in tropical and subtropical regions. Pure Appl Chem 82(1):27–38
Padmavathiamma PK, Li LY (2007) Phytoremediation technology: hyper-accumulation metals in plants. Water Air Soil Pollut 184:105–126
Setia RC, Kuar N, Setia N, Nayyar H (2008) Heavy metal toxicity in plants and phytoremediation. Crop improvement: strategies and applications. I.K. International Publishing House Pvt. Ltd., New Delhi, pp 206–218
Singh D, Gupta R, Tiwari A (2011) Phytoremediation of lead from wastewater using aquatic plants. Int J Biomed Res 7:411–421
Swearingen J, Saltonstall K (2010) Phragmites field guide: distinguishing native and exotic forms of common reed (Phragmites australis) in the United States. Plant conservation alliance: weeds gone wild. http://www.nps.gov/plants/alien/pubs/index.htm
Taffarel SR, Rubio J (2009) On the removal of Mn2+ ions by adsorption onto natural and activated Chilean zeolites. Miner Eng 22:336–343
Tangahu BV, Abdullah SRS, Basir H, Idris M, Anuar N, Mukhlisin M (2011) A review on heavy metals (As, Pb, and Hg) uptake by plants through phytoremediation. Int J Chem Eng. Article ID 939161, 31p
URI CELS Outreach Center (2012) Common reed (Phragmites australis) control fact sheet. Accessed [http://www.uri.edu/cels/ceoc/documents/commonReed.pdf]
Yadav S, Chandra R (2011) Heavy metals accumulation and ecophysiological effect on Typha angustifolia L. and Cyperus esculentus L. growing in distillery and tannery effluent polluted natural wetland site, Unnao, India. Environ Earth Sci 62:1235–1243
Yahyaa N, Rosebi AF (2010) Copper removal from hazardous waste landfill leachate using peat as an adsorbent. Health Environ J 1(2):51–53
Yoon J, Cao X, Zhou Q, Ma LQ (2006) Accumulation of Pb, Cu, and Zn in native plants growing on a contaminated Florida site. Sci Total Environ 368:456–464
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Mojiri, A., Aziz, H.A., Tajuddin, R.B.M., Gavanji, S., Gholami, A. (2015). Heavy Metals Phytoremediation from Urban Waste Leachate by the Common Reed (Phragmites australis). In: Ansari, A., Gill, S., Gill, R., Lanza, G., Newman, L. (eds) Phytoremediation. Springer, Cham. https://doi.org/10.1007/978-3-319-10969-5_7
Download citation
DOI: https://doi.org/10.1007/978-3-319-10969-5_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-10968-8
Online ISBN: 978-3-319-10969-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)