Abstract
Rapid economic growth, industrialization, urbanization, and improper implementation of environmental regulations have contributed to increased tropospheric O3 levels since preindustrial times, and this increase has produced a serious air pollution problem. Apart from being a hazardous air pollutant, O3 has also been recognized as the third major (carbon dioxide and methane) green house gas in terms of additional radiative forcing and climate change (Forster et al. 2007). Because of its oxidative capacity, high O3 levels in the atmosphere are detrimental to living organisms, including plants. Ozone is among the most damaging air pollutants to which plants are exposed, and produces substantive plant biomass and yield (seed weight) reductions (Thompson 1992; Agrawal et al. 2005; Manning 2005; Hassan 2006; Hassan and Tewfik 2006; Singh et al. 2009a, 2014; Wahid 2006 a, b; Sarkar and Agrawal 2010a, b; Tripathi and Agrawal 2013). The economic loss for 23 horticultural and agricultural crops from O3 exposure was estimated to be approximately $6.7 billion for the year 2000 in Europe (Holland et al. 2006). Wang and Mauzerall (2004) anticipated economic losses of upto 9 % for four important cereal crops (viz., wheat, rice, maize and soybean) grown in China, South Korea and Japan. To minimize such crop losses many potential antioxidants (e.g., fungicides, insecticides, growth regulators and plant extracts) have been evaluated. Among these, the systemic antioxidant, ethylene diurea, –N-[2-(2-oxo-1-imidazolidinyl) ethyl]-N′ phenylurea (popularly known as EDU) was found to be the most effective.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Rapid economic growth, industrialization, urbanization, and improper implementation of environmental regulations have contributed to increased tropospheric O3 levels since preindustrial times, and this increase has produced a serious air pollution problem. Apart from being a hazardous air pollutant, O3 has also been recognized as the third major (carbon dioxide and methane) green house gas in terms of additional radiative forcing and climate change (Forster et al. 2007). Because of its oxidative capacity, high O3 levels in the atmosphere are detrimental to living organisms, including plants. Ozone is among the most damaging air pollutants to which plants are exposed, and produces substantive plant biomass and yield (seed weight) reductions (Thompson 1992; Agrawal et al. 2005; Manning 2005; Hassan 2006; Hassan and Tewfik 2006; Singh et al. 2009a, 2014; Wahid 2006 a, b; Sarkar and Agrawal 2010a, b; Tripathi and Agrawal 2013). The economic loss for 23 horticultural and agricultural crops from O3 exposure was estimated to be approximately $6.7 billion for the year 2000 in Europe (Holland et al. 2006). Wang and Mauzerall (2004) anticipated economic losses of upto 9 % for four important cereal crops (viz., wheat, rice, maize and soybean) grown in China, South Korea and Japan. To minimize such crop losses many potential antioxidants (e.g., fungicides, insecticides, growth regulators and plant extracts) have been evaluated. Among these, the systemic antioxidant, ethylene diurea, –N-[2-(2-oxo-1-imidazolidinyl) ethyl]-N′ phenylurea (popularly known as EDU) was found to be the most effective.
In this review, we address how O3 is formed, and the phytotoxic losses it has produced over the past three decades. Moreover, we address and summarize the literature relating to efforts designed to identify antioxidants that can potentially protect vegetation from O3 damage. We give special emphasis to the most competent and most studied of these synthetic antioxidants viz., ethylene diurea (EDU). We review EDU’s effectiveness at the morphological, physiological and biochemical levels and its role in preventing yield losses in plants.
2 The Chemistry of O3 Formation, and Its Uptake and Fate in Plants
Ozone is a secondary air pollutant that is not directly emitted to the atmosphere. It is formed by the reaction between primary air pollutants (viz., nitrogen oxides, hydrocarbons such as volatile organic carbons, carbon oxides, methane or non-methane volatile organic compounds, etc.) and solar radiation (Finlayson-Pitts and Pitts 1997). Nitrogen oxides (NOx–NO + NO2) and volatile organic carbon (VOCs) emissions result from both natural and anthropogenic sources. Natural sources of NOx are from lightening discharges and from direct emissions from soil, whereas VOCs are released primarily from vegetation. Anthropogenic-sourced emissions of NOx result from combustion processes in motor vehicles, thermal power generation and various industrial activities. Volatile organic compounds are emitted when combustion processes are incomplete, e.g., from motor vehicle engines and from manufacturing and processing in the petrochemical industry, motor fuel production, and distribution and solvent use. In Fig. 1, we depict the steps by which O3 is formed.
The steps by which ozone is formed via photochemical processes in the troposphere (modified after Staehelin and Poberaj 2008)
The emission of nitric oxide (NO2) results from the reaction between nitrogen and oxygen present in atmosphere under high temperatures generally attained in the combustion chambers of engines. NO2 thus produced is easily dissociated by the ultraviolet element of solar radiation into nitrogen monoxide and singlet oxygen (1O2). 1O2 spontaneously reacts with molecular oxygen to give rise to O3. Under these conditions, the life time of O3 is very short, because the O3 produced reacts with NO to produce NO2 and O2 again. Therefore, an equilibrium is established between O3 formation and degradation (Lorenzini and Saitanis 2003). Alternatively, when non-methanic hydrocarbons react with NO, toxic PAN (peroxyacetyl nitrate—CH3C (O) OONO2) and other organic substances are formed. Thus, the ozone level is controlled by a complex set of photochemical reactions. There are also other chemical reactions involved in tropospheric O3 formation that comprise a series of complex cycles, in which carbon-monoxide and VOCs are oxidized to form water vapor and carbon dioxide. The carbon monoxide oxidation results from the presence of the hydroxyl radical (OH·). The resultant hydrogen atom rapidly reacts with oxygen to give a per-oxy radical (HO2 ·). Peroxy radicals then react with NO to give NO2, which is photolyzed to give the atomic oxygen. The atomic oxygen reacts with a molecule of oxygen to form O3. The entire process represents a chain reaction, in which O3 becomes photo dissociated by near ultraviolet radiation to form an excited oxygen atom. Excited oxygen again reacts with water vapor to regenerate .OH radicals, which drives the chain process. O3 destruction also results from photochemical reactions involving NO, HO2 or .OH. Staehelin and Poberaj (2008) reported that the NOx species restrict ozone formation by having their concentration gradually decreased from the formation of HNO3. Ozone concentrations are influenced by precursor availability, meteorological conditions and chemical reactions over local, regional and hemispherical distances (Lenka and Lenka 2012). In low latitude regions, O3 generally exhibits a diurnal bell-shaped pattern, reaching peak concentration during mid day and early afternoon hours and gradually decreasing during late afternoon and evening hours (Lorenzini and Saitanis 2003). Although O3 frequently originates in urban areas, it can be transported long distances to agricultural areas via prevailing winds.
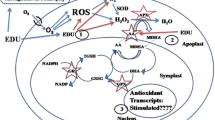
It is known that atmospheric wind turbulence facilitates O3 transport to the plant surface (Bennett and Hill 1973). The cuticle of plant leaves act as an impermeable barrier (Kerstein and Lendzian 1989), which restricts O3 uptake. When O3 enters plants it does so largely via stomata. When plants are exposed to ozone, stomatal conductance is reduced (Mansfield and Pearson 1996; Castagna et al. 2001; Guidi et al. 2001; Pasqualini et al. 2002; Degl’Innocenti et al. 2003; Singh et al. 2009a). How O3 influences stomatal conductance (Paoletti and Grulke 2005) is uncertain, although it has been postulated that O3 may act by altering abscisic acid signaling, or by generating an active oxygen species (AOS) burst directly in guard cells (Kangasjärvi et al. 2005). After entering through stomata, O3 reaches the sub-stomatal cavity and air spaces in the leaf. O3 does not persist in the apoplast for long and rapidly degrades to form various reactive oxygen species (ROS) and/or reacts with biomolecules present in the cell wall, apoplastic fluid or plasma membrane (Laisk et al. 1989; Mishra et al. 2013b). Diara et al. (2005) reported that extracellular H2O2 accumulation is one of the earliest detectable responses to O3 exposure. The appearance of leaf lesions has also been correlated with H2O2 accumulation (Pellinen et al. 1999; Wohlgemuth et al. 2002). H2O2 disrupts photosynthesis and activates NAD(P)H-dependent oxidase (Park et al. 1998; Rao and Davis 1999), which leads to ROS accumulation. Alscher and Hess (1993) suggested that the superoxide radical is formed and produces leaf injury under O3 exposure. Among other ROS, the hydroxyl radical is the most reactive of oxygen species. It reacts rapidly with proteins, lipids and DNA and causes cell damage (Iqbal et al. 1996). ROS leads to a chain of reactions, which cause significant effects on the cellular metabolism of the plants. In Fig. 2, we illustrate the entry of O3 into plant leaves, its mechanism of toxicity and consequent defense responses.
ROS cause membrane damage and deleterious effects on the normal functioning of cells. To protect against the damaging effects of ROS, plants have developed and utilize several non-enzymatic (ascorbic acid, carotenoids, glutathione, α-tocopherol) and enzymatic antioxidants (superoxide dismutase, various peroxidases, catalases, glutathione reductase, etc.) that exist in different cell compartments (Fig. 3). Carotenoids are non-photosynthetic pigments in leaves that help maintain the chlorophyll pool that is used against photooxidative damage by ROS. Glutathione (GSH), is the most abundant thiol in plants, and functions as an antioxidant that scavenges cytotoxic H2O2 and other ROS such as OH·, O2 ·− (Larson 1988). Ascorbic Acid (AA) is the most abundant low molecular weight antioxidant, is synthesized in leaf cells (Castillo and Greppin 1988; Smirnoff 2000) and forms the first line of defense against O3 exposure (Polle et al. 1995). The ability of AA to donate electrons in a wide range of enzymatic and non-enzymatic reactions signifies it as being the main ROS detoxifying compound. In addition alpha-tocopherol is an important antioxidant for its ability to directly scavenge oxidizing radicals and to prevent chain propagation during lipid autoxidation, when plants are exposed to O3 (Serbinova and Packer 1994).
The processes by which ROS are scavenged in the cells. DHA dehydroascorbate; AsA ascorbate; DHAR dehydroascorbate reductase; GR glutathione reductase; GSSG oxidized glutathione; GSH reduced glutathione; NAD(P) Nicotinamide adenine dinucleotide phosphate; NAD(P)H Nicotinamide adenine dinucleotide phosphate reduced MDHAR monodehydroascorbate reductase; MDHA monodehydroascorbate; APX ascorbate peroxidase; SOD superoxide dismutase; GPX glutathione peroxidase; CAT catalase; H 2 O 2 hydrogen peroxide; PSI photosystem I (modified after Dizengremel et al. 2008)
Among enzymatic antioxidants, the primary one is superoxide dismutase (SOD), which represents a family of metalloenzymes that catalyze the dismutation of O2 ·− to H2O2 (Bowler et al. 1992, 1994). Another important enzyme is catalase (CAT), which exists primarily in peroxisomes and catalyzes the degradation of H2O2 (Willekens et al. 1995; Scandalios et al. 1997). Ascorbate peroxidase (APX), glutathione reductase (GR), monodehydroascorbate (MDHAR) and dehydroascorbate reductase (DHAR) are enzymes of the ascorbate-glutathione cycle, and help to regulate ROS formed in the presence of O3 (Biemelt et al. 1998; Jimenez et al. 1998; Noctor and Foyer 1998).
Endogenous production of ROS also activates a MAP kinase cascade, which plays crucial roles in plant signal transduction pathways. MAPKs target various effector proteins, which consist of kinases, enzymes or transcription factors (Rodriguez et al. 2010). The transcription factors participate in the expression of genes that are involved in defense pathways, and in primary or secondary metabolism. Ethylene and salicylic acid are produced in plants and together foster the development of lesions and cause cell death (Castagna and Ranieri 2009). When cell death occurs, certain products of lipid peroxidation serve as substrates for synthesis of jasmonic acid. Jasmonic acid acts antagonistically and reduces ethylene-dependent ROS production and the spread of cell death (Kangasjärvi et al. 2005).
The biochemical and physiological performance of plants subjected to O3-related oxidative stress is greatly affected and such plants display reduced growth and biomass production both of which reduce yield (Morgan et al. 2003; Ambasth and Agrawal 2003; Biswas et al. 2008; Feng et al. 2008). Yield is of foremost concern, because reduced growth of crop plants translates directly to economic losses. Hence, yield response of plants under O3 exposure is a major parameter that is routinely studied by plant scientists. A meta analysis of existing data has indicated that soybeans exposed to an average O3 level of 70 ppb suffer a 24 % reduction in seed yield (Morgan et al. 2003). Moreover, Rai et al. (2007) reported that a wheat cultivar (HUW-234) grown under ambient and elevated O3 in open top chambers suffered reduced yield. Mishra et al. (2013a) experienced the same results with two other cultivars: HUW-37 and K-9107. De Temmerman et al. (2007) found reduced root yield and altered quality of Beta vulgaris (sugar yield, alpha-amino-N, Na and K contents) from O3 exposure. Intraspecific differences were observed in yield responses of rice (Ariyaphanphitak et al. 2005), wheat (Wahid 2006b; Singh and Agrawal 2009) barley (Wahid 2006a), bean (Flowers et al. 2007), potato (Piikki et al. 2004) and cotton (Zouzoulas et al. 2009) plants under different O3 exposure regimes (Table 1). In Table 1, we summarize the results of experiments conducted to assess yield responses of different plants exposed to O3.
3 Protectants Used to Prevent Ozone Toxicity
O3-induced oxidative stress caused by O3 has been identified as the major cause for plant yield losses. Hence, many chemical substances have been applied to protect plants against such losses. In recent decades, several researchers have tested various antioxidants in attempts to understand how O3 injury may be mitigated. The types of chemicals applied have included fungicides, insecticides, growth regulators, natural plant extracts and antioxidants, among others.
Middleton et al. (1953) reported first that O3 induced injury in pinto bean was reduced when aqueous solution of manganese (maneb) or zinc ethylenebis dithiocarbamate (zineb) was sprayed prior to O3 fumigation. When sprayed onto poinsettia plants, the fungicides benomyl and diphenylamine (DPA) reduced O3 injury (Manning et al. 1973a). Similar results were achieved with pinto bean (Pell 1976), potato (Carrasco-Rodriguez et al. 2005) and tobacco (Reinert and Spurr 1972) plants. Gilbert et al. (1975) reported that dust and liquid applications of DPA on apple, and dust application of DPA on bean, muskmelon and petunia also provided protection against O3 injury. DPA was also used to quantify the effects of ambient oxidants on plants during air quality monitoring in Georgia, U.S.A. (Walker and Barlow 1974). A foliar spray of DPA at 1,000 ppm onto apple and at 1 % in bean, melon, petunia and tobacco reduced O3 damage by 50 % or more (Lisk 1975). Carrasco-Rodriguez et al. (2005) recorded an increase in fresh biomass and tuber numbers of potato after DPA was applied.
Foliar application of two modern fungicides (azoxystrobin and epoxiconazole) prior to fumigation with injurious doses of O3 (150–250 ppb; 5days; 7 h/day) reduced visible injury by 50–60 % in spring barley by inducing an antioxidative defense system (Wu and Tiedemann 2002). To prevent O3 injury benomyl is the most studied benzimidazole derivative fungicide (Manning et al. 1972, 1973a, b, c; Manning and Vardaro 1973a, b). In the 1970s, the discovery that benomyl can protect plants against O3 injury opened new investigations because carboxin had similar beneficial effects in reducing O3 injury in plants. However, benomyl was more effective than carboxin, because, to achieve equal protection of benomyl carboxin required a dose that was nearly phytotoxic (Rich et al. 1974; Taylor and Rich 1974; Papple and Ormrod 1977). Hofstra et al. (1978) found that benomyl and carboxin were equally effective in causing yield recovery in navy beans exposed to O3. Recently, the fungicide ‘strobi’ conferred the best protective effects on O3 sensitive clover and tobacco among the studied modern agrochemicals (Blum et al. 2011).
Insecticides have also been applied to achieve protection, although their application has been limited to areas where crop loss does not result from insects. Of ninety chemicals tested, only five showed significant protection from O3 injury (Koiwai et al. 1974). These five were: 3, 4-methylenedioxyphthaldehyde, benzimidazole, safroxane, xanthone and piperonyl butoxide. Phytohormones such as auxins, cytokinins and abscisic acid (ABA) acted like antioxidants in reducing O3 injury in plants (Kurchii 2000; Pauls and Thopson 1982; Verbeke et al. 2000). Abscisic acid (ABA), a chemical that induces stomatal closure reduced the O3 injury in bean (Fletcher et al. 1972). Protective effects from the application of N-6-benzyladenine (BA), gibberellic acid (GA) and indole acetic acid (IAA) against O3 were observed to occur in radish, and BA was the most effective protectant of the three (Adedipe and Ormrod 1972). Runeckles and Resh (1975) reported that the cytokinins in BA and kinetin stimulated leaf growth and reduced chlorophyll loss, which generally is the most susceptible constituent to O3 attack.
Ascorbic acid and its salt are effective in minimizing the detrimental effects of O3 in bean, celery, lettuce, barley, citrus and petunia (Freebairn 1960; Freebairn and Taylor 1960; Dass and Weaver 1968; Lee et al. 1990; Macher and Wasescha 1995). In contrast, Siegel (1962) reported that ascorbic acid failed to provide any appreciable protection to cucumber plants after O3 exposure. Ozoban, an isomer of ascorbic acid reduced the photosynthetic rate in a low-O3 environment, but was harmful to chloroplastic plant pigments that were exposed to elevated levels of O3 (Kuehler and Flagler 1999). Agrawal et al. (2004) studied three wheat cultivars and showed that an ascorbic acid spray improved biochemical parameters and increased biomass and yield, but did not impart any significant change in stomatal conductance. How application of ascorbate induces plant-resistance to O3 stress is still unclear (Didyk and Blum 2011). Among all protectant types, the performance of ethylene diurea (EDU) is the best documented, and has been suggested to be the most efficient research tool for analyzing O3-induced stress in a variety of plants (Manning 1992).
4 Ethylenediurea (EDU) as a Protectant to Prevent Phytotoxicity
The positive effect of ethylenediurea (EDU, N-[2-(2-oxo-1-imidazolidinyl) ethyl]-N′-phenylurea) on plant productivity against ambient O3 was first reported by Carnahan et al. (1978). It is specific in suppressing O3 injury and has no effects on peroxylacetylnitrate (PAN) and sulphur dioxide (SO2)-induced injury (Cathey and Heggestad 1982a; Lee et al. 1992). EDU consists of two urea moieties having an imidazole ring (urea) and a phenylurea ring; these rings are joined by an ethylene group (Fig. 4). An investigation of the relative effectiveness of EDU and constituent amounts of urea and phenylurea in EDU in prevention of O3 injury was performed by Godzik and Manning (1998). They suggested that urea did not impart protection against O3 injury, although phenylurea reduced O3 injury significantly. Urea treatment was clearly not as effective as the treatment with EDU. However, when O3 exposed plants were treated with 300 ppm EDU or phenylurea, both compounds equally prevented O3 injury. No published reports addressed the role that the nitrogen present in EDU had in protecting plants from O3. EDU neither acts as a nutrient, nor shows any pesticidal or plant regulatory effects (Manning 1992). Paoletti et al. (2008) investigated the biochemical leaf responses in EDU infused ash trees and reported that EDU did not contribute nitrogen as a fertilizer (Fraxinum excelsior). Lee and Chen (1982) reported cytokinin-like activity of EDU and indicated that EDU retarded the breakdown of chlorophyll, protein and RNA in O3-sensitive tobacco leaf discs. Whitaker et al. (1990) studied the role of EDU in protecting foliar lipids and concluded that EDU conferred O3 tolerance by inducing enzyme systems involved in the elimination of activated oxygen species and free radicals.
4.1 Methods and Timing of EDU Application
In most experiments, EDU has mainly been applied as a soil drench or foliar spray (Table 2). Other EDU application methods have included stem injection and gravitational infusion (Fig. 5), and these have commonly been used in trees and other woody species (Roberts et al. 1987; Ainsworth and Ashmore 1992; Ainsworth et al. 1996; Bortier et al. 2001; Paoletti et al. 2007). Bortier et al. (2001) found that stem injection of EDU in Populus nigra was effective in preventing O3-induced visible injury, accelerated senescence, and led to significant increases in diameter and height of O3 exposed trees. Ainsworth et al. (1996) reported similar results with two clones of hybrid poplar that showed incremental chlorophyll content increases after EDU treatment. Paoletti et al. (2007) found a significant reduction in O3-induced foliar injury in ash trees when EDU (300 and 450 ppm) was applied as a gravitational infusion into the trunk. Percent O3 injury was reduced to 3 % in EDU-infused trees as compared to control plants that manifested 13 % injury on the leaf surface.
Although EDU is usually applied as a soil drench to protect against O3 injury in plants, the applied EDU may accumulate in soil and produce subsequent toxicity. Some argue therefore that foliar applications are safer than drenching. Moreover, utilizing drenching on a large scale basis, or in large fields, is not always feasible at all stages of plant development, particularly in non-row crops such as hay and broadcasted cereals. In addition, large amounts of EDU are required when used at the field scale.
Surface applications are an alternative application method, but have their own drawbacks, such as being dependent on precipitation to effect the chemical uptake by roots. Feng et al. (2010) performed a meta analysis, which indicated that EDU applied as a soil drench significantly ameliorated plant growth suffering from O3 stress. In contrast, after EDU was applied as a foliar spray, only a few parameters showed significant positive effects of EDU. Alternative application approaches, like stem injections and gravitational infusion of EDU solution are not possible for delicate plants like vegetables and cereal crops.
Application timing of EDU is critical for achieving maximum protection to plants against O3 injury. Weidensaul (1980) found that pinto beans were best protected from O3 injury when EDU was applied 3–7 days prior to O3 exposure, but afforded no protection to leaves that were not formed at the time of chemical application. McClenahen (1979) reported almost complete protection from O3 injury in Fraxinus americana and Prunus serotina that received up to 300 ppb EDU weekly during the seedling stage. Using 14C-EDU (Roberts et al. 1987) and a HPLC technique (Regner-Joosten et al. 1994), Gatta et al. (1997) established that EDU translocation was primarily acropetal, probably via the xylem stream and that EDU remained in the apoplastic region of leaves for more than 10 days, without being redistributed to newly formed leaves. Carnahan et al. (1978) documented that EDU was not translocated to newer or untreated leaves, which suggested that repeated applications were needed to assure continuous protection from O3 injury. EDU is a systemic antioxidant, and is not redistributed to new tissues; hence, repeated applications are indeed needed to maintain continuous levels of protection (Weidensaul 1980; Regner-Joosten et al. 1994). Depending on plant sensitivity to O3, Manning et al. (2011) suggested foliar spraying with EDU at weekly or bi-weekly intervals. Paoletti et al. (2009) reviewed the use of EDU on Italian crops and trees and reported that amelioration of visible O3-induced injury was obtained by regularly applying EDU at 2 or 3 week intervals.
4.2 Application Dose of EDU
To determine the optimal EDU dose that gives the best protection against O3, without producing side effects, the application rates of EDU must be standardized for each species. Carnahan et al. (1978) was first in trying to standardize the EDU application rates (0–500 ppm) adequate to protect pinto beans from O3 exposure effects. EDU at 500 ppm was seen as an application rate that optimally protected plants from acute O3 injury (Carnahan et al. 1978; Weidensaul 1980; Cathey and Heggestad 1982a, b, c). Cathey and Heggestad (1982a, b), in an exposure/response screening trial, confirmed that a foliar spray or soil drench of 500 ppm EDU provided optimal protection for 4 cultivars of petunia and 44 species of herbaceous plants.
EDU has been used as a survey tool for measuring O3 effects under field conditions. Postiglione and Fagnano (1995) studied ambient O3 effects on lettuce, subterranean clover, bean and tomato by using EDU. Fumagalli et al. (1997) used EDU (150 ppm) in an ambient O3 environment, coupled with open-top chambers to study the effects of O3 on white clover in the Milan region of Italy. In both studies, researchers found that EDU had positive effects in reducing O3 injury to the respective tested plants.
Manning (1988, 1992, 1995), Kostka-Rick and Manning (1993b, c), Tiwari et al. (2005) and Singh et al. (2010a) performed studies that yielded appropriate dose-responses. Cathey and Heggestad (1982c) observed that a 500 ppm rate of EDU was most appropriate for woody plant protection. Later studies utilized repeatitive (weekly or biweekly) EDU applications to protect plants against chronic O3 exposures (Clarke et al. 1983, 1990; Hofstra et al. 1983; Bambawale 1986; Heggestad 1988; Brennan et al. 1990; Legassicke and Ormrod 1981; Toivonen et al. 1982). Additional studies utilized variable dosages of EDU (300–500 ppm) to protect plants from acute and chronic O3 levels, viz., 300 ppm (Hassan 2006), 400 ppm (Wahid et al. 2001; Singh and Agrawal 2009; Singh et al. 2009b) and 500 ppm (Agrawal et al. 2004, 2005). Tiwari et al. (2005) and Wang et al. (2007) conducted dose-response studies on wheat. Results were that ambient O3-exposed plants treated with 300 ppm EDU, but not at 200 and 400 ppm, displayed various improved growth characteristics. EDU treatment at 450 ppm caused significant increments in growth parameters of Loblolly pine (Manning et al. 2003). In contrast, Szantoi et al. (2009) reported a significant decrease in root and total biomass of Rudbeckia laciniata as EDU concentrations increased (200, 400 and 600 ppm), although the percentage of leaf injury was reduced. After performing a meta analysis study, Feng et al. (2010) suggested that EDU applied as a soil drench at a concentration range of 200–400 ppm had the most positive effects on field grown crops.
4.3 Effectiveness of EDU and Its Toxicity
Most EDU studies did not show toxic effects from EDU treatment, when applied at optimal concentrations to address O3 stress. No protective effects of EDU were reported when it was applied under the following conditions: (i) applied to a O3-resistant cultivar, (ii) applied on plants grown in O3-free air; however, an EDU application manifested toxic effects on plants when the EDU concentration was very high. Legassicke and Ormrod (1981) and Foster et al. (1983) found that EDU treatment did not increase plant yield in the O3 resistant ‘New Yorker’ tomato cultivar and ‘White Rose’ potato cultivar. Clarke et al. (1983) observed a similar result in the ‘Green Mountain’ potato cultivar. Elagoz and Manning (2002) used one sensitive (S156) and another resistant (R123) variety of bean to validate that EDU caused a side-effect on the resistant variety. A significant increase in the above ground biomass (pod and seed weight) of an EDU-treated sensitive variety was observed. In contrast, significant reductions in the above parameters were observed in R123 (a resistant variety). A similar response trend was observed in sensitive and resistant varieties of tobacco (Godzik and Manning 1998).
No significant yield increases of an O3-sensitive potato grown in O3-free air were recorded. In contrast, increasing application frequency resulted in over-dosing and caused side-effects from EDU treatment on root and shoot biomass (Foster et al. 1983; Bisessar and Palmer 1984). No significant differences in chlorophyll content, foliar injury, plant height, pod number and seed yield of soybean were observed in EDU-treated and untreated plants, when O3 was absent (Greenhalgh et al. 1987). Hassan et al. (1995) also reported insignificant differences in root, shoot, total biomass and the root-shoot ratio in radish and turnip grown in filtered chambers, in the presence or absence of EDU. No significant difference between CF (controlled filtered air)/-EDU and CF (controlled filtered air)/+EDU treatments were observed for the measured parameters (healthy leaf number, injured leaf number, green stem, root and leaf biomass, leaf area index, total nitrogen content in plant parts, total dry biomass, grain yield, seed weight, seed protein, seed oil, etc.) of soybean plants (Ali and Abdel-Fattah 2006). Other literature reports suggested that EDU did not affect plant growth and productivity in the absence of O3, e.g., for soybean (Kostka-Rick and Manning 1993b) and tobacco (Godzik and Manning 1998).
EDU has been reported to be toxic at high concentrations. In Nicotiana tabacum, foliar sprays did not cause visible injury, but soil drench applications at 500, 1,000 and 2,000 ppm caused phytotoxicity in Bel-W3 seedlings (Lee and Chen 1982). Eckardt and Pell (1996) reported complete protection (from a lower EDU dose of 15 ppm) of accelerated foliar senescence that had been induced by O3-exposure in potato. Higher dosages of EDU (45 and 75 ppm) were associated with leaf curling and marginal necrosis. Szantoi et al. (2009) found a linear reduction in root and total biomass of R. laciniata as EDU levels increased. Weidensaul (1980) suggested that higher concentrations of EDU (800–5,000 ppm) applied as foliar sprays are more effective in preventing foliar injury in pinto bean, an O3 sensitive plant. However, when applied in excess, negative effects on plant physiology were reported by Bennett et al. (1978) and Heagle (1989). Heggestad (1988) reported that weekly applications of a 500 ppm EDU spray on four cultivars of field-grown sweet corn produced phytotoxicity, reduced growth and yield. It is therefore evident that the efficacy of EDU is more apparent in O3 sensitive cultivars when applied at optimum doses, but only when O3 stress exists. Resistant varieties gain no benefit from EDU applications. However, low concentrations of EDU that do not affect plant growth can nonetheless provide protection from visible O3 injury.
5 EDU and Its Modes of Action
In our review of the literature, we sought to better understand the biochemical, growth and metabolic roles of EDU’s action and mechanism. In this section we have compiled the results that bear on these points below.
5.1 Effects of EDU on Growth Characteristics and Biomass Accumulation
Plant growth is a complex phenomenon that results from integration and coordination of various physiological and biochemical processes that are genetically controlled and greatly influenced by various environmental factors. Ozone is a strong oxidant that causes many effects in plant species, and in particular cultivars of those species, often at the developmental stage. EDU provides O3 tolerance by modifying several plant processes, and ultimately protects plants from O3 damage. Kostka-Rick and Manning (1992) reported that EDU treatment increased root biomass of Raphanus sativus (radish) during early growth stages, but imparted no protective effects at later stages. However, EDU treatment did not alter shoot or total biomass of radish. Kostka-Rick and Manning (1993a) reported no growth-related changes in EDU-treated plants of Phaseolus vulgaris, whether grown in synthetic substrates or in the field, although symptoms related to EDU toxicity were observed at a high concentration. A general conclusion of all researchers is that EDU treatment reduced or delayed O3 injury symptoms in plant foliage, and also delayed the senescence process.
In a dose–response study conducted in closed chambers on O3-exposed P. vulgaris, increasing EDU treatment levels (150–300 mg L−1) significantly helped plants to accumulate more stem, leaf and total biomass, in comparison to plants not treated with EDU (Astorino et al. 1995). Brunschon-Harti et al. (1995a) reported reduced growth suppression by O3 in the form of higher leaf, root and shoot dry weight of EDU-treated plants of P. vulgaris. However, Ainsworth et al. (1996) did not find a significant difference in growth characteristics of two clones of hybrid poplar, after EDU was injected in stems at rates of 250 ppm and 1,000 ppm. However, a significant reduction in O3 injury, decreasing senescence of leaves and an induction in chlorophyll content were observed.
In Table 2, we have summarized studies conducted to assess the impact of O3 on growth characteristics and biomass accumulation in plants that were subjected to EDU applications. Agrawal et al. (2005) noticed significant increases (viz., 12.3, 16.8, 30 and 24 %, respectively in shoot length, number of leaves, leaf area and total biomass) of EDU-treated (500 ppm) mungbean plants, compared to non-EDU treated plants (Table 2). Singh et al. (2010b) reported that EDU given as soil drench (400 ppm) to mungbean caused significant growth parameter increases, including biomass, but noted a reduction in number of leaves in EDU-treated plants (Table 2).Wang et al. (2007), however, found that EDU concentrations of 150 and 300 ppm had an insignificant effect on the above ground biomass of wheat and rice, although a decline in biomass was observed at the 450 ppm EDU treatment level.
5.2 EDU and Visible Injury
Ozone-induced visible injury on plants includes flecking, the appearance of having a water soaked area, interveinal stippling, chlorosis, bronzing and necrosis, all of which lead to early senescence of leaves. Young leaves are less susceptible to O3 injury than are old leaves, because aging leaves contain lower levels of antioxidants than younger ones (Bisessar 1982). This suggests that EDU is more effective during the later stages of plant life than at early stages. Lee et al. (1981) also showed that senescence of red clover leaf discs was delayed when discs were floated on a EDU solution in the dark or under low intensity light. Kostka-Rick and Manning (1992) noted complete O3 injury protection in EDU-treated radish plants (Table 3).
In Table 3, we provide a summary of experiments conducted to assess the response of O3 on foliar injury when EDU treatments were used. EDU at 400 ppm completely protected against visible injury from O3 exposure in soybean plants in Pakistan (Wahid et al. 2001). EDU, used as a low dose soil drench (15 ppm), provided complete protection from accelerated foliar senescence in potato that had been exposed for 11 day to 0.1 μL L−1 O3 for 5 h day−1 (Eckardt and Pell 1996). Kostka-Rick and Manning (1993b, c) showed similar foliar injury protection at a low EDU dose (100 mg L−1), although toxicity symptoms occurred at higher dosages (300–800 mg L−1). Postiglione and Fagnano (1995) used three O3-sensitive plants (subterraneum clover, bean and tomato) and one O3-tolerant plant (lettuce) to substantiate that EDU protection was not complete, (i.e., visible) injury was observed, though it was delayed and displayed a reduced intensity. Fumagalli et al. (1997) noted that the percent of O3 injured leaves was significantly reduced in potted Trifolium repens plants that had been treated with 150 ppm EDU as a soil drench.
5.3 Role of EDU in Physiology and Photosynthetic Pigments of Plants
The physiological effects of EDU that are linked with its protective properties are still unclear (Blum et al. 2011). Previous studies showed that EDU mitigated O3 effects through biochemical modifications in plants and not by biophysical changes (Bennett et al. 1978; Lee and Bennett 1982; Hassan et al. 1995). Stomatal conductance in EDU-treated plants that were exposed to ambient O3 has been measured in only a few studies. In most such studies, the authors have reported no significant EDU effects on stomatal conductance (Bennett et al. 1978; Ainsworth et al. 1996; Hassan et al. 2007). Agrawal and Agrawal (1999) were first to provide evidence that EDU may act against O3 exposure at the biophysical level by decreasing stomatal conductance. The percent closed stomata were 39.1, 45.7, 55.3 and 66.7, respectively, in control, EDU treated, O3-exposed and EDU + O3-exposed snap bean plants. Later, field study results disclosed the action of EDU on plant physiology (see Table 4). Recently, Wahid et al. (2012) found that sesame plants treated with 500 ppm EDU showed an increase of 52 % in stomatal conductance and a 61 % increase in net-photosynthesis rate, compared to non-EDU treated plants. Ozone is known to damage the thylakoid membrane in chloroplasts, which negatively affects the light quenching capacity of chlorophyll. EDU application improved fluorescence kinetics in O3-exposed plants of Beta vulgaris (Tiwari and Agrawal 2009), Triticum aestivum (Singh and Agrawal 2009) and Trifolium repens (Singh et al. 2010d) (Table 4). A study on the photosynthetic capacity of ash trees revealed a slight effect of EDU in preventing O3-induced inactivation of photosynthetic reaction centers (Contran et al. 2009).
Photosynthetic pigments are the basic entities of the plant photosynthetic machinery, and EDU is effective in maintaining high levels of photosynthetic pigments. Lee and Chen (1982) reported that non-EDU-treated leaf discs of tobacco lost more than half of their original chlorophyll after 10 days, whereas discs treated with 1 × 10−3 M EDU lost only 10 % of the initial chlorophyll. Whitaker et al. (1990), however, found that EDU treatment did not alter leaf chlorophyll or carotenoid contents, but a reduction of 14 % was observed when plants were exposed to O3 alone. Potato plants treated with 45 and 75 mg EDU L−1 had higher chlorophyll content, and the chlorophyll was retained for a longer period than in untreated plants (Eckardt and Pell 1996). One main reason that EDU provides protection was that it enhances retention of chlorophyll for longer time (Lee et al. 1997). In Table 5, we summarized results of investigations that have been conducted to evaluate the effects on photosynthetic pigments from O3 exposure and EDU treatment.
5.4 EDU Protection in Relation to Antioxidants
EDU does not act as an antioxidant itself, but helps to maintain higher levels of cellular antioxidants during O3 stress in Phaseolus vulgaris (Lee et al. 1997). Superoxide dismutase (SOD) activity is normally associated with O3 tolerance. Lee and Bennett (1982) found a significant induction of SOD and catalase (CAT) activities when snap beans were treated with EDU. Pitcher et al. (1992) refuted the hypothesis that EDU’s mode of action works by increasing SOD activity. There was no EDU associated increases in Cu/Zn-SOD or Mn-SOD activities in plants exposed to O3. Lee et al. (1997) did not observe significant differences in activities of ascorbate peroxidase (APX), guaicol peroxidase (GPX) and SOD in O3 exposed snap bean leaves that were treated with EDU as compared to untreated plants. However, EDU-treated plants maintained a higher glutathione reductase (GR) activity. Batini et al. (1995) hypothesized that the protection mechanism against O3 that had been exerted by EDU was caused by the stimulation of only the APX detoxifying system involved in scavenging hydrogen peroxide molecules. Singh et al. (2010b) found more significant reductions in POX and SOD activities in leaves of EDU-treated mungbean plants than in non EDU-treated ones. A compilation of experiments conducted to assess the effects of EDU on antioxidant enzymes under O3 treatment is given in Table 6.
Among non-enzymatic antioxidants, apoplastic ascorbic acid acts as a ‘first line of defense’ against O3 damage (Chameides 1989). Hence, ozone tolerance is directly correlated to increased apoplastic ascorbic acid in P. major population (Zheng et al. 2000), snap bean ecotypes (Burkey 1999; Burkey et al. 2003), soybean cultivars (Robinson and Britz 2000, 2001) and Sedum album (Castillo and Greppin 1988). Various studies have revealed that EDU plays a role in maintenance/ synthesis of the ascorbic acid pool in plants. Significant increase in the ratio of ascorbic acid (AA)/dehydroascorbic acid (DHA) in EDU-treated plants has been shown by Brunschon-Harti et al. (1995b). However, Gillespie et al. (1998) did not find any significant effect of EDU on the AA and DHA contents of snap bean plants. Singh et al. (2010b) reported a significantly higher content of ascorbic acid in mungbean that had been treated with EDU. Higher ascorbic acid content in foliage is commonly observed to occur after EDU treatment (Table 5).
To understand the EDU-induced O3 tolerance and the role of glutathione (GSH) in snap bean, Lee et al. (1997) used an HPLC technique to study the effect of EDU. They found that total glutathione and GSH concentrations decreased significantly in O3 fumigated plants (no EDU + O3), but GSSG concentrations increased vis-a-vis a decrease in the GSH/GSSG ratio, compared to controls (no O3). Pretreatment with EDU significantly increased the levels of total glutathione and GSH, and reduced the level of GSSG, in comparison to control snap bean plants. Higher concentrations of total glutathione and GSH and a lower GSSG reserve were observed in EDU-treated plants after O3 exposure (EDU + O3) vs. control plants (no EDU + O3). This suggested that EDU-treated plants that were under O3 stress showed no reduction in glutathione reductase (GR) activity. Lee et al. (1997) suggested that EDU protection against O3 damage may result from maintenance of GR and GSH levels during O3 exposure. Hassan (2006) used the same technique to determine the glutathione concentration in potato leaves. EDU-treated plants grown under O3 exposure had a higher GSH content, but lower GSSG and total glutathione levels, compared to plants exposed to O3 without EDU treatment. Generally, plants treated with EDU have a higher GSH/GSSG ratio than do the controls.
Polyamines are known as stabilizing factors of biomembranes and macromolecules and are also free radical scavengers (Drolet et al. 1986), either directly or via conjugation with other molecules such as free ferulic and caffeic acids (Didyk and Blum 2011). Bors et al. (1989) suggested that polyamines conjugate with hydroxycinnamic acids and play a role in protecting against the accumulation of O3-triggered reactive oxygen species. Polyamine content in fully expanded leaves of snap bean treated with EDU was compared with the control (-EDU) leaves before and after O3 exposure. It was reported that EDU did not alter the polyamine composition of leaves, although O3 alone (-EDU) induced significant increases in total polyamines (Lee et al. 1992).
5.5 Effects of EDU on Soluble Protein, MDA (Malondialdehyde) Content and Foliar Lipids
Brunschon-Harti et al. (1995b) showed that the soluble protein content increased following EDU treatment in snap bean plants, while increasing O3 dose did not cause any change in total soluble protein in EDU-treated plants. Studies have revealed that an increased O3 dose increased the MDA content in EDU-treated plants, thus reflecting more damage to plasma membranes. Most study results support the view that the MDA content is increased from EDU treatment, although there are exceptions (Table 5). Whitaker et al. (1990) found that pretreatment with EDU conferred protection against O3-induced losses of glycerolipids. Leaves of untreated snap bean plants lost approximately 50 % of both the galactolipids (GL) and phospholipids (PL), while EDU-treated plants showed no significant loss of foliar GL and PL. In controls (no EDU), sterylglycosides (SG) showed an incremental increase, while EDU-treated plants showed a small increase in SG.
5.6 Effects of EDU on Carbohydrates
Increments in sucrose and other soluble sugars in bean leaves, 48 h after EDU treatment was correlated with development of resistance against O3 (Lee et al. 1981). Miller et al. (1994) reported higher starch levels in EDU-treated O3-exposed snap bean plants, whereas foliar starch levels declined under O3 exposure without EDU treatment, compared to plants exposed to charcoal filtered (CF) air. Singh et al. (2010c) observed that all three test cultivars of blackgram (barkha, shekhar and TU-94-2) showed a significant decrease in reducing sugar content, and a respective increase in total soluble sugar content in seeds of plants treated with EDU. In contrast, starch content increased significantly only in seeds of EDU-treated black gram cultivar TU-94-2 as compared to non-EDU treated ones. Al-Qurainy (2008) studied broad bean plants grown without EDU at O3 concentrations of 21.2 and 62.7 ppb in ambient air, respectively at King Saud University and King Fahad Street, displayed reduced soluble, insoluble and total leaf carbohydrates as they aged. In contrast, plants treated with EDU at 250 mg L−1showed increments in all forms of carbohydrates with increasing age, compared to non EDU-treated plants.
6 The Role of EDU in Assessing Yield Losses
O3-related yield losses are a serious and growing concern throughout the world. Different approaches have been utilized to estimate the level of economic losses experienced from O3 exposure. EDU is a widely used biomonitoring tool that is commonly utilized to assess plant yield loss from O3 exposure. EDU successfully protects a wide variety of plants from O3. EDU has also been used to screen for O3 sensitive and tolerant/resistant cultivars, and to estimate the extent of damage caused by O3. Such information is useful for selecting cultivars to grow in areas that experience high O3 levels. Yield loss assessments are performed by comparing the yield estimates of EDU-treated and untreated plants in O3 polluted areas of the USA (Ensing et al. 1985; Smith et al. 1987), Asia (Wahid et al. 2001; Agrawal et al. 2004, 2005; Tiwari et al. 2005; Wang et al. 2007; Singh and Agrawal 2009; Singh et al. 2010b, c), Africa (Hassan et al. 1995; Hassan 2006) and Europe (Ribas and Penuelas 2000; Brunschon-Harti et al. 1995a; Pleijel et al. 1999). Yield increments after the application of EDU onto O3 exposed plants have been reported for onion (Wukasch and Hofstra 1977), navy bean (Hofstra et al. 1978; Temple and Bisessar 1979; Toivonen et al. 1982), tomato (Legassicke and Ormrod 1981), potato (Bisessar 1982; Clarke et al. 1990; Hassan 2006), tobacco (Bisessar and Palmer 1984), watermelon (Fieldhouse 1978), peanut (Ensing et al. 1985), radish (Kostka-Rick and Manning 1992; Hassan et al. 1995), carrot (Tiwari and Agrawal 2010), bush bean (Kostka-Rick and Manning 1993a, c), snap bean (Vandermeiren et al. 1995), mungbean (Agrawal et al. 2005; Singh et al. 2010b), soybean (Wahid et al. 2001), wheat (Agrawal et al. 2004; Tiwari et al. 2005; Wang et al. 2007; Singh and Agrawal 2009) and black gram (Singh et al. 2010c).
Applying EDU has increased the number of seeds per plant and total seed weight per plant for two O3-sensitive cultivars of soybean, while insignificant effects were observed in the O3-tolerant lines for these parameters (Damicone 1985). No significant difference in seed size was observed vs. controls in soybean cultivars that were treated with EDU and then exposed to O3 (Smith et al. 1987). EDU treatment has led to increased seed size in wheat (Agrawal et al. 2004; Singh and Agrawal 2009), and mungbean (Agrawal et al. 2005). Wahid et al. (2001) reported significantly higher seed weight/plant, number of seeds/pod and number of pods/plant for Glycine max under EDU treatment at all experimental sites (suburban, rural and rural roadside) in Pakistan that experienced higher O3 concentrations during pre-monsoon and post-monsoon experiments. Significantly higher tuber weight and number of tubers were reported in EDU-treated potato plants, compared to EDU-untreated plants at both rural and suburban sites in Egypt in areas experiencing high O3 concentrations (Hassan 2006). In Table 7, we have summarized results of experiments, in which the effect of O3 on yield was assessed, along with the mitigating effects of having used EDU.
EDU has been widely and successfully used to protect against the effects of O3, as a research tool to assess plant responses to O3 stress, and to estimate crop/plant yield losses. The advantages and disadvantages of using EDU for these purposes are stated below:
6.1 Advantages
-
1.
No chambers or costly investments like FACE (Free Air Carbon dioxide Enrichment) are required. The use of EDU requires only ambient conditions; no modifications of microclimate are needed.
-
2.
Plant numbers and plot sizes can vary according to the requirements of the experiment, which facilitates having many replications.
-
3.
Low technical input is required to utilize EDU, whose utilization is comparatively simple and easy to execute.
6.2 Disadvantages
-
1.
Ozone dose-response studies cannot be performed, unless coupled with OTCs (open top chambers).
-
2.
Ambient O3 concentrations and environmental variables need careful monitoring.
-
3.
Repeated applications of EDU can cause phytotoxicity, especially under dry soil conditions; hence, detailed plant toxicological studies are needed before starting field experiments.
-
4.
High cost and commercial non availability of EDU has led it to be used mainly in monitoring experiments; the commercial scale use of EDU as a crop protectant has not yet occurred.
-
5.
The use of EDU requires extensive labor and time for application.
7 Summary
Urbanization, industrialization and unsustainable utilization of natural resources have made tropospheric ozone (O3) one of the world’s most significant air pollutants. Past studies reveal that O3 is a phytotoxic air pollutant that causes or enhances food insecurity across the globe. Plant sensitivity, tolerance and resistance to O3 involve a wide array of responses that range from growth to the physiological, biochemical and molecular. Although plants have an array of defense systems to combat oxidative stress from O3 exposure, they still suffer sizable yield reductions. In recent years, the ground-level O3 concentrations to which crop plants have been exposed have caused yield loses that are economically damaging. Several types of chemicals have been applied or used to mitigate the effects produced by O3 on plants. These include agrochemicals (fungicides, insecticides, plant growth regulators), natural antioxidants, and others. Such treatments have been effective to one degree to another, in ameliorating O3-generated stress in plants. Ethylene diurea (EDU) has been the most effective protectant used and has also served as a monitoring agent for assessing plant yield losses from O3 exposure. In this review, we summarize the data on how EDU has been used, the treatment methods tested, and application doses found to be both protective and toxic in plants. We have also summarized data that address the nature and modes of action (biophysical and biochemical) of EDU.
In general, the literature discloses that EDU is effective in reducing ozone damage to plants, and indicates that EDU should be more widely used on O3 sensitive plants as a tool for biomonitoring of O3 concentrations. Biomonitoring studies that utilize EDU are very useful for rural and remote areas and in developing countries where O3 monitoring is constrained from unavailability of electricity. The mechanism(s) by which EDU prevents O3 toxicity in plants is still not completely known. EDU possesses great utility for screening plant sensitivity under field conditions in areas that experience high O3 concentrations, because EDU prevents O3 toxicity only in O3 sensitive plants. Ozone-resistant plants do not respond positively to EDU applications. However, EDU application dose and frequency must be standardized before it can be effectively and widely used for screening O3 sensitivity in plants. EDU acts primarily by enhancing biochemical plant defense and delaying O3-induced senescence, thereby reducing chlorophyll loss, and maintaining physiological efficiency and primary metabolites; these actions enhance growth, biomass and yield of plants.
We believe that future studies are needed to better address the EDU dose-response relationship for many plant species, and to screen for new cultivars that can resist O3 stress. Although some research on the physiological and biochemical mechanisms of action of EDU have been performed, the new ‘omics’ tools have not been utilized to evaluate EDUs mechanism of action. Such data are needed, as is gene expression and proteome profiling studies on EDU-treated and -untreated plants.
References
Adedipe NO, Ormrod DP (1972) Hormonal regulation of ozone phytotoxicity in Raphanus sativus L. Z Fuer Pflanzenphysiologie 68:254–258
Agrawal SB, Agrawal M (1999) Low temperature scanning electron microscope studies of stomatal response in snap bean plants treated with ozone and ethylenediurea. Biotronics 28:45–53
Agrawal M, Singh B, Rajput M, Marshall F, Bell JNB (2003) Effect of air pollution on peri-urban agriculture: a case study. Environ Pollut 126:323–329
Agrawal SB, Singh A, Rathore D (2004) Assessing the effects of ambient air pollution on growth, biochemical and yield characteristics of three cultivars of wheat (Triticum aestivum L.) with ethylenediurea and ascorbic acid. J Plant Biol 31:165–172
Agrawal SB, Singh A, Rathore D (2005) Role of ethylenediurea (EDU) in assessing impact of ozone on Vigna radiata L. plants in a suburban area of Allahabad (India). Chemosphere 61:218–228
Agrawal M, Singh B, Agrawal SB, Bell JNB, Marshall F (2006) The effect of air pollution on yield and quality of mung bean grown in peri-urban areas of Varanasi. Water Air Soil Pollut 169:239–254
Ahmed S (2009) Effects of air pollution on yield of mungbean in Lahore, Pakistan. Pak J Bot 41:1013–1021
Ainsworth EA (2008) Rice production in a changing climate: a meta-analysis of responses to elevated carbon dioxide and elevated ozone concentration. Global Change Biol 14:1642–1650
Ainsworth N, Ashmore MR (1992) Assessment of ozone effects on beech by injection of a protectant chemical. Forest Ecol Manag 51:129–136
Ainsworth N, Fumagalli I, Giorcelli A, Mignanego L, Schenone G, Vieto L (1996) Assessment of EDU stem injections as a technique to investigate the response of trees to ambient ozone in field conditions. Agri Ecosys Environ 59:33–42
Ali AA, Abdel-Fattah RI (2006) Protection of agricultural crops in Egypt against adverse effects of atmospheric pollutants I. By using of ethylene diurea. J Agron 5:158–166
Ali A, Alfarhan A, Robinson E, Bokhari N, Al-Rasheid K, Al-Quraishy S (2008) Tropospheric ozone effects on the productivity of some crops in Central Saudi Arabia. Am J Environ Sci 4:631–637
Al-Qurainy FH (2008) Effect of air pollution and ethylenediurea on broad bean plants grown at two localities in KSA. Int J Botany 4:117–122
Alscher RG, Hess JL (1993) Antioxidants in higher plants. CRC, Boca Raton
Ambasth NK, Agrawal M (2003) Effects of enhance UV-B radiation and tropospheric ozone on physiological and biochemical characteristics of field grown wheat. Biol Plant 47:625–628
Ariyaphanphitak W (2004) Effects of ground-level ozone on crop productivity in Thailand. The Joint international conference on “sustainable energy and environment (SEE)” 1–3 December 2004, Hua Hin, Thailand
Ariyaphanphitak W, Chidthaisong A, Sarobol E, Bashkin VN, Towprayoon S (2005) Effects of elevated ozone concentrations on Thai jasmine rice cultivars (Oryza sativa L.). Water Air Soil Pollut 167:179–200
Astorino G, Margani I, Tripodo P (1995) The response of Phaseolus vulgaris L. cv. Lit. to different dosages of the anti-ozonant ethylenediurea (EDU) in relation to chronic treatment with ozone. Plant Sci 111:237–248
Bambawale OM (1986) Evidence of ozone injury to a crop in India. Atmos Environ 20:1501–1503
Batini P, Ederli L, Pasqualini S, Antonielli M, Valentini V (1995) Effects of ethylenediurea and ozone in detoxificant ascorbic-ascorbate peroxidase system in tobacco plants. Plant Physiol Biochem 33:717–723
Bennett JH, Hill AC (1973) Absorption of gaseous air pollutants by a standardized plant canopy. J Air Pollut Control Assoc 23:203–206
Bennett JH, Lee EH, Heggestad HH (1978) Apparent photosynthesis and leaf stomatal diffusion in EDU treated ozone-sensitive bean plants. In: Proceedings of the 5th Annual Meeting of the Plant Growth Regulator Working Group, pp 242–246
Biemelt S, Keetmsn U, Albrecht G (1998) Re-aeration following hypoxia or anoxia leads to activation of the antioxidative defense systems in roots of wheat seedlings. Plant Physiol 116:651–658
Bisessar S (1982) Effect of ozone, antioxidant protection, and early blight on potato in the field. J Am Soc Hortic Sci 107:597–599
Bisessar S, Palmer KT (1984) Ozone, antioxidant spray and Meloidogyne hapla effects on tobacco. Atmos Environ 18:1025–1027
Biswas DK, Xu H, Li YG, Sun GZ, Wang XZ, Han XG, Jiang GM (2008) Genotypic differences in leaf biochemical, physiological and growth responses to ozone in 20 winter wheat cultivars released over the past 60 years. Global Change Biol 14:46–59
Blum O, Didyk N, Pavluchenko N, Godzik B (2011) Assessment of protective effects of some modern agrochemicals against ozone-induced stress in sensitive clover and tobacco cultivars. J Toxicol 2011:308598. doi:10.1155/2011/308598
Booker FL, Fiscus EL (2005) The role of ozone flux and antioxidants in the suppression of ozone injury by elevated carbon dioxide in soybean. J Exp Bot 56:2139–215
Bors W, Langebartels C, Michel C, Sandermann H (1989) Polyamines as radical scavengers and protectants against ozone damage. Phytochemistry 28:1585–1595
Bortier K, Dekelever G, De Temmerman L, Ceulemans R (2001) Stem injection of Populus nigra with EDU to study ozone effects under field conditions. Environ Pollut 111:199–208
Bou Jaoudé M, Katerji N, Mastrorilli M, Rana G (2008) Analysis of the effect of ozone on soybean in the Mediterranean region II. The consequences on growth, yield and water use efficiency. Eur J Agron 28:519–525
Bowler C, Montagu MV, Inze D (1992) Superoxide dismutase and stress tolerance. Annu Rev Plant Physiol 43:83–116
Bowler C, Van Camp W, Van Montagu M, Inze D (1994) Superoxide dismutase in plants. Crit Rev Plant Sci 13:199–218
Brennan EG, Clarke BB, Greenhalgh-weidman B, Smith G (1990) An assessment of the impact of ambient ozone on field grown crops in New Jersey using the EDU method: Part 2-soybean (Glycine max L.) Merr. Environ Pollut 66:361–73
Brunschon-Harti S, Fangmeier A, Jager HJ (1995a) Influence of ozone and ethylenediurea (EDU) on growth and yield of bean (Phaseolus vulgaris L.) in open-top field chambers. Environ Pollut 90:89–94
Brunschon-Harti S, Fangmeier A, Jager HJ (1995b) Effects of ethylenediurea and ozone on the antioxidative systems in beans (Phaseolus vulgaris L.). Environ Pollut 90:95–103
Burkey KO (1999) Effects of ozone on apoplast/cytoplasm partitioning of ascorbic acid in snap bean. Physiol Plant 107:188–193
Burkey KO, Eason G, Fiscus EL (2003) Factors that affect leaf extracellular ascorbic acid content and redox status. Physiol Plant 117:51–57
Calvo E, Calvo I, Jimenez A, Porcuna JL, Sanz MJ (2009) Using manure to compensate ozone-induced yield loss in potato plants cultivated in the east of Spain. Agric Ecosys Environ 131:185–192
Carnahan JE, Jenner EL, Wat EKW (1978) Prevention of ozone injury in plants by a new protective chemical. Phytopathology 68:1225–1229
Carrasco-Rodriguez JL, Asensi-Fabado A, Del Valle-Tascon S (2005) Effects of tropospheric ozone on potato plants protected by the antioxidant diphenylamine (DPA). Water Air Soil Pollut 161:299–312
Castagna A, Ranieri A (2009) Detoxification and repair process of ozone injury: from ozone uptake to gene expression adjustment. Environ Pollut 157:1461–1469
Castagna A, Nali C, Ciompi S, Lorenzini G, Soldatini GF, Ranieri A (2001) Ozone exposure affects photosynthesis of pumpkin (Cucurbita pepo) plants. New Phytol 152:223–229
Castillo FJ, Greppin H (1988) Extracellular ascorbic acid and enzyme activities related to ascorbic acid metabolism in Sedum album L. leaves after ozone exposure. Environ Exp Bot 28:231–238
Cathey HM, Heggestad HE (1982a) Ozone and sulphur dioxide sensitivity of Petunia: modification by ethylene diurea. J Am Soc Hortic Sci 107:1028–1035
Cathey HM, Heggestad HE (1982b) Ozone sensitivity of herbaceous plants: modification by ethylene diurea. J Am Soc Hortic Sci 107:1035–1042
Cathey HM, Heggestad HE (1982c) Ozone sensitivity of woody plants: modification by ethylenediurea. J Am Soc Hortic Sci 107:1042–1045
Chameides WL (1989) The chemistry of ozone deposition by plant leaves: role of ascorbic acid. Environ Sci Tech 23:595–600
Chaudhary N, Agrawal SB (2013) Intraspecific responses of six Indian clover cultivars under ambient and elevated levels of ozone. Environ Sci Pollut Res 20:5318–5329
Chernikova T, Robinson JM, Lee EH, Mulchi CL (2000) Ozone tolerance and antioxidant enzyme activity in soybean cultivars. Photosyn Res 64:15–26
Clarke BB, Henninger MR, Brennan EG (1983) An assessment of potato losses caused by oxidant air pollution in New Jersey. Phytopathol 73:104–108
Clarke BB, Greenhalgh-weidman B, Brennan EG (1990) An assessment of the impact of ambient ozone on field-grown crops in New Jersey using the EDU method: Part I-white potato (Solanum tuberosum). Environ Pollut 66:351–60
Contran N, Paoletti E, Manning WJ, Tagliaferro F (2009) Ozone sensitivity and ethylenediurea protection in ash trees assessed by JIP chlorophyll a fluorescence transient analysis. Photosynthetica 47:68–78
Damicone JP (1985) Growth, yield and foliar injury response of early maturing soybean genotypes to ozone and Fusarium oxosporum, Ph.D. Thesis, University of Massachusetts, Amherst
Dass HC, Weaver GM (1968) Modification of ozone damage to Phaseolus vulgaris by antioxidants, thiols and sulphydryl reagents. Can J Plant Sci 48:569–574
De Temmerman L, Legrand L, Vandermeiren GK (2007) Effects of ozone on sugar beet grown in open-top chambers. Eur J Agric 26:1–9
Degl’Innocenti E, Vaccà C, Guidi L, Soldatini GF (2003) CO2 photoassimilation and chlorophyll fluorescence in two clover species showing different response to O3. Plant Physiol Biochem 41:485–493
Diara C, Castagna A, Baldan B, Mensuali Sodi A, Sahr T, Langebartels C, Sebastiani L, Ranieri A (2005) Differences in the kinetics and scale of signaling molecule production modulate the ozone sensitivity of hybrid poplar clones: the roles of H2O2, ethylene and salicylic acid. New Phytol 168:351–364
Didyk NP, Blum OB (2011) Natural antioxidants of plant origin against ozone damage of sensitive crop. Acta Physiol Plant 33:25–34
Dizengremel P, Le Thiec D, Bagard M, Jolivet Y (2008) Ozone risk assessment for plants: central role of metabolism-dependant changes in reducing power. Environ Pollut 156:11–15
Drolet G, Dumbroff EB, Legge RL, Thompson JE (1986) Radical scavenging properties of polyamines. Phytochemistry 25:367–371
Eckardt NA, Pell EJ (1996) Effects of ethylenediurea (EDU) on ozone-induced acceleration of foliar senescence in potato (Solanum tuberosum L.). Environ Pollut 92:299–306
Elagoz V, Manning WJ (2002) Ozone and bean plants: morphology matters. Environ Pollut 120:521–524
Ensing J, Hofstra G, Roy RC (1985) The impact of ozone on peanut exposed in the laboratory and field. Phytopathology 75:429–432
Feng Z, Jin M, Zhang F (2003) Effects of ground-level ozone (O3) pollution on the yield of rice and winter wheat in the Yangtze River delta. J Environ Sci 15:360–362
Feng Z, Kobayashi K, Ainsworth EA (2008) Impact of elevated ozone concentration on growth, physiology and yield of wheat (Triticum aestivum L.) a meta-analysis. Global Change Biol 14:2696–2708
Feng Z, Wang S, Szantoi Z, Chen S, Wang X (2010) Protection of plants from ambient ozone by applications of ethylenediurea (EDU): a meta-analytic review. Environ Pollut 158:3236–3242
Fieldhouse DJ (1978) Chemical control of ozone damage on watermelon. HortiScience 13:23–31
Finlayson-Pitts BJ, Pitts JN Jr (1997) Tropospheric air pollution: ozone, air borne toxics, polycyclic aromatic hydrocarbons, and particles. Science 276:1045–1052
Finnan JM, Jones MB, Burke JI (1996) A time-concentration study on the effects of ozone on spring wheat (Triticum aestivum L.) effects on yield. Agric Ecosys Environ 57:159–167
Fletcher RA, Adedipe NO, Ormrod DP (1972) Abscisic acid protects beans leaves from ozone-induced phytotoxity. Can J Bot 50:2389–2391
Flowers MD, Fiscus EL, Burkey KO, Booker FL, Dubois JJB (2007) Photosynthesis, chlorophyll fluorescence, and yield of snap bean (Phaseolus vulgaris L.) genotypes differing in sensitivity to ozone. Environ Exp Bot 61:190–19
Forster P, Ramaswamy V, Artaxo P, Berntsen T, Betts R, Fahey DW, Haywood J, Lean J, Lowe DC, Myhre G, Nganga J, Prinn R, Raga G, Schulz M, Van Dorland R (2007) Changes in atmospheric constituents and in radiative forcing. In: Solomon S, Qin D, Manning M, Chen Z, Marquis M, Averyt KB, Tignor M, Miller HL (eds) Climate change: the physical science basis contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge
Foster KW, Guerard JP, Oshima RJ, Bishop JC, Timm H (1983) Differential ozone susceptibility of Centennial Russet and White Rose potato as determined by fumigation and antioxidant treatments. Am Potato J 60:127–39
Freebairn HT (1960) The prevention of air pollution damage to plants by the use of vitamin C sprays. J Air Pollut Control Assoc 10:314–317
Freebairn HT, Taylor OC (1960) Prevention of plant damage from air-borne oxidizing agents. Proc Am Soc Hortic Sci 76:693–699
Fuhrer J, Grandjean A, Grimm W, Tschannen W, Shariat-Madari H (1992) The response of spring wheat (Triticum aestivum L.) to ozone at higher elevations. II. Changes in yield, yield components, and grain quality in response to ozone flux. New Phytol 121:211–219
Fumagalli I, Mignanego L, Violini G (1997) Effects of tropospheric ozone on white clover plants exposed in open-top chambers or protected by the antioxidant ethylene-diurea (EDU). Agronomie 17:271–281
Fumagalli I, Mignanego L, Mills G (2003) Ozone biomonitoring with clover clones: yield loss and carryover effect under high ambient ozone levels in northern Italy. Agri Ecosys Environ 95:119–128
Gatta L, Mancino L, Federico R (1997) Translocation and persistence of EDU (ethylenediurea) in plants: the relationship with its role in ozone damage. Environ Pollut 96:445–448
Gerosa G, Marzuoli R, Rossini M, Panigada C, Meroni M, Colombo R, Faoro F, Iriti M (2009) A flux-based assessment of the effects of ozone on foliar injury, photosynthesis and yield of bean (Phaseolus vulgaris L. cv. Borlotto Nano Lingua di Fuoco) in open-top chambers. Environ Pollut 157:1727–1736
Gilbert MD, Maylin GA, Elfving DC, Edgerton LJ, Gutenmann WH, Lisk DJ (1975) The use of diphenylamine to protect plants against ozone injury. Hortic Sci 10:228–231
Gillespie C, Bermejo V, Cardoso-Vilhena J, Pearson S, Ollerenshaw J, Barnes J (1998) Mechanism underlying EDU-induced ozone resistance. In: De Kok, L.J., Stulen, I. (eds.), Responses to plants to air pollution. Backhuys, Leiden, pp 309–310.
Godzik B, Manning WJ (1998) Relative effectiveness of ethylenediurea, and constituent amounts of urea and phenylurea in ethylenediurea, in prevention of ozone injury to tobacco. Environ Pollut 103:1–6
Greenhalgh B, Brennan E, Leone I (1987) Evidence in support of the use of EDU (ethylenediurea) to assess ozone-induced plant injury. Phytopathology 77:1761–1772
Guidi L, Nali C, Lorenzini G, Filippi F, Soldatini GF (2001) Effect of chronic ozone fumigation on the photosynthesis process of poplar clones showing different sensitivity. Environ Pollut 113:245–254
Hassan IA (2006) Physiology and biochemical response of potato (Solanum tuberosum L. cv. Kara) to O3 and antioxidant chemicals: possible roles of antioxidant enzymes. Ann Appl Biol 148:197–206
Hassan IA, Tewfik I (2006) CO2 photoassimilation, chlorophyll fluorescence, lipid peroxidation and yield in cotton (Gossypium hirsutum L. cv Giza 65) in response to O3. World Rev Sci Technol Sust Dev 3:70–78
Hassan IA, Ashmore MR, Bell JNB (1995) Effect of ozone on radish and turnip under Egyptian field conditions. Environ Pollut 89:107–114
Hassan IA, Bell JNB, Marshall FM (2007) Effects of air filtration on Egyptian clover (Trifolium alexandrinum L. cv. Messkawy) grown in open-top chambers in a rural site in Egypt. Res J Biol Sci 2:395–402
He X, Ruan Y, Chen W, Lu T (2006) Responses of the anti-oxidative system in leaves of Ginkgo biloba to elevated ozone concentration in an urban area. Bot Stud 4:409–416
Heagle AS (1989) Ozone and crop yield. Annu Rev Phytopathol 27:397–423
Heggestad HE (1988) Reduction in soybean seed yields by ozone air pollution. J Air Pollut Control Assoc 38:1040–1041
Hofstra G, Littlejohns DA, Wukasch RT (1978) The efficacy of the antioxidant Ethylenediurea (EDU) compared to carboxin and benomyl in reducing yield losses from ozone in navy bean. Plant Dis Rep 62:350–352
Hofstra G, Wukasch RT, Drexier DM (1983) Ozone injury on potato foliage as influenced by the antioxidant EDU and sulphur dioxide. Can J Plant Pathol 5:115–119
Holland M, Kinghorn S, Emberson L, Cinderby S, Ashmore M, Mills G, Harmens H, (2006) Development of a framework for probabilistic assessment of the economic losses caused by ozone damage to crops in Europe. CES Project No. C02309NEW. Report to UK Department of Environment, Food and Rural Affairs under contract 1/2/170 1/3/205
Iqbal M, Abdin M, Mahmooduzzafar Z, Yunus M, Agrawal M (1996) Resistance mechanisms in plants against air pollution. In: Iqbal M, Yunus M (eds) Plant response to air pollution. Wiley, New York, pp 195–240
Ishii S, Marshall FM, Bell JNB, Abdullah AM (2004) Impact of ambient air pollution on locally grown rice cultivars (Oryza sativa L.) in Malaysia. Water Air Soil Pollut 154:187–201
Jimenez A, Hernandez JA, Pastori G, Del Rio LA, Sevilla F (1998) Role of the ascorbate-glutathione cycle of mitochondria and peroxisomes in the senescence of pea leaves. Plant Physiol 118:1327–1335
Kangasjärvi J, Jaspers P, Kollist H (2005) Signalling and cell death in ozone-exposed plants. Plant Cell Environ 28:1021–1036
Kerstein G, Lendzian KJ (1989) Interaction between ozone and plant cuticles. I. Ozone deposition and permeability. New Phytol 112:1989–2004
Koiwai A, Kitano H, Fukuda M, Kisaki T (1974) Methylenedioxyphenyl and its related compounds as protectants against ozone injury to plants. Agric Biol Chem 38:301–307
Kollner B, Krause GHM (2000) Changes in carbohydrates, leaf pigments and yield in potatoes induced by different ozone exposure regimes. Agric Ecosys Environ 78:149–158
Kostka-Rick R, Manning WJ (1992) Effects and interactions of ozone and the anti-ozonant EDU at different stages of radish (Raphanus sativus L.) development. J Exp Bot 43:1621–1631
Kostka-Rick R, Manning WJ (1993a) Dynamics of growth and biomass partitioning in field grown bush bean (Phaseolus vulgaris L.) treated with the antiozonant Ethylenediurea (EDU). Agric Ecosys Environ 47:195–214
Kostka-Rick R, Manning WJ (1993b) Dose-response studies with ethylenediurea (EDU) and radish. Environ Pollut 79:249–260
Kostka Rick R, Manning WJ (1993c) Dose response studies with the antizonant etylenedurea (EDU) applied as a soil drench to two growth substrates, on greenhouse-grown varieties of Phaseolus vulgaris L. Environ Pollut 82:63–72
Kuehler EA, Flagler RB (1999) The effects of sodium erythorbate and ethylenediurea on photosynthetic function of ozone-exposed loblolly pine seedlings. Environ Pollut 105:25–35
Kurchii BA (2000) Possible free radical mechanism of action of auxin and kinetin In: 12th congress of the Federation of European Societies of Plant Physiology, 21–25 Aug 2000, Budapest. Plant Physiology and Biochemistry 38, Abstract S08-39, p 91
Laisk A, Kull O, Moldau H (1989) Ozone concentration in leaf intercellular air spaces is close to zero. Plant Physiol 90:1163–1167
Larson RA (1988) The antioxidants of higher plants. Phytochemistry 27:969–978
Lee EH, Bennett JH (1982) Superoxide dismutase: a possible protective enzyme against O3 injury in snap beans (Phaseolus vulgaris L.). Plant Physiol 69:1444–1449
Lee EH, Chen CM (1982) Studies on the mechanisms of ozone tolerance. Cytokinin like activity of N [2-(2-oxo-1-imidazolidinyl) ethyl]-N-phenylurea, a compound protecting against O3 injury. Physiol Plant 56:486–491
Lee EH, Bennett JH, Heggestad HE (1981) Retardation of senescence in red clover leaf discs by a new antiozonant EDU, N-[2-(2-oxo-1-imidazolidinyl) ethyl]-N′-phenylurea. Plant Physiol 67:347–350
Lee EH, Rowland RA, Mulchi CL (1990) Growth regulators serve as a research tool to study the mechanism of plant response to air pollution stimuli. Br Soc Plant Growth Regul Monogr 20:127–137
Lee EH, Kramer GF, Rowland RA, Agrawal M (1992) Antioxidants and growth regulators counter the effects of O3 and SO2 in crop plants. Agric Ecosys Environ 38:99–106
Lee EH, Upadhyay A, Agrawal M, Rowland RA (1997) Mechanism of ethylenediurea (EDU) induced ozone protection: Re-examination of free radical scavenger systems in snap bean exposed to O3. Environ Exp Bot 38:199–209
Legassicke BC, Ormrod DP (1981) Suppression of ozone-injury on tomatoes by ethylenediurea in controlled environments and in the field. Hortic Sci 16:183–184
Lenka S, Lenka NK (2012) Impact of tropospheric ozone on agroecosystem: an assessment. J Agric Phys 12:1–11
Lisk DJ (1975) Protecting plants against injury from air pollution. N Y Food Life Sci 8:3–5
Lorenzini G, Saitanis C (2003) Ozone: a novel plant “pathogen”. In Toppi LSD, Pawlik-Skowronska B (Eds.), Abiotic stress in plants, Springer Science + Business Media, Dordrecht pp 205-229.
Macher F, Wasescha M (1995) Damage by ozone and protection by ascorbic acid in barley leaves. J Plant Physiol 147:469–473
Manning WJ (1988) EDU: A research tool for assessment of the effects of ozone on vegetation. In: Proceedings of the 81st Air pollution control association annual meeting paper No. 88–92, 2–8.
Manning WJ (1992) Assessing the effects of ozone on plants: Use and misuse of ethylenediurea (EDU). In: Proceedings of the 85th Annual meeting and exhibition on air and waste management association 11 pp.
Manning WJ (1995) Use of protective chemicals to assess the effects of ambient ozone on vegetation. Proceedings of the 88th Annual meeting of the air & waste management association 12 pp.
Manning WJ (2005) Establishing a cause and effect relationship for ambient ozone exposure and tree growth in the forest: progress and an experimental approach. Environ Pollut 137:443–454
Manning WJ, Vardaro PM (1973a) Suppression of oxidant injury on beans by systemic fungicides. Phytopathology 63:1415–1416
Manning WJ, Vardaro PM (1973b) Suppression of oxidant air pollution injury on bean plants by systemic fungicides under field conditions. Phytopathology 63:204
Manning WJ, Feder WA, Papia PM (1972) Influence of long-term low levels of ozone and benomyl on growth and nodulation of pinto bean plants. Phytopathology 62:497
Manning WJ, Feder WA, Vardaro PM (1973a) Reduction of chronic ozone injury on poinsettia by benomyl. Can J Plant Sci 53:833–835
Manning WJ, Feder WA, Vardaro PM (1973b) Benomyl in soil and response of pinto bean plants to repeated exposures to a low level of ozone. Phytopathology 63:1539–1540
Manning WJ, Feder WA, Vardaro PM (1973c) Suppression of oxidant injury by benomyl: Effects on yields of bean cultivars in the field. J Environ Qual 3:1–3
Manning WJ, Flagler RB, Frenkel MA (2003) Assessing plant response to ambient ozone: growth of ozone-sensitive loblolly pine seedlings treated with ethylene diurea or sodium erythorbate. Environ Pollut 126:73–81
Manning WJ, Paoletti E, Sandermann H Jr, Ernst D (2011) Ethylenediurea (EDU): a research too, for assessment and verification of the effects of ground level ozone on plants under natural condition. Environ Pollut 159:3283–3293
Mansfield AT, Pearson M (1996) Disturbance in stomatal behaviour in plants exposed to air pollution. In: Iqbal M, Yunus M (eds) Plant response to air pollution. Wiley, Chichester, pp 178–193
McClenahen JR (1979) Effects of ethylenediurea and ozone on the growth of tree seedlings. Plant Dis Rep 63:320–323
Meyer U, Kollner B, Willenbrink J, Krause GHM (2000) Effects of different ozone exposure regimes on photosynthesis, assimilates and thousand grain weight in spring wheat. Agric Ecosys Environ 78:49–55
Middleton JT, Kendrick JB, Darley EF (1953) Olefinic peroxide injury to bean as influenced by age, variety, chemical additions and toxicant dosage. Phytopathology 43:588
Miller JE, Pursley WA, Heagle AS (1994) Effects of ethylenediurea on snap bean at a range of ozone concentrations. J Environ Qual 23:1082–1089
Mishra AK, Rai R, Agrawal SB (2013a) Differential response of dwarf and tall tropical wheat cultivars to elevated ozone with and without carbon dioxide enrichment: growth, yield and grain quality. Field Crops Res 145:21–32
Mishra AK, Rai R, Agrawal SB (2013b) Individual and interactive effects of elevated carbon dioxide and ozone on tropical wheat (Triticum aestivum L.) cultivars with special emphasis on ROS generation and activation of antioxidant defence system. Ind J Biochem Bio 50:139–149
Morgan PB, Ainsworth EA, Long SP (2003) How does elevated ozone impact soybean? A meta-analysis of photosynthesis, growth and yield. Plant Cell Environ 26:1317–1328
Noctor G, Foyer CH (1998) Ascorbate and glutathione: Keeping active oxygen under control. Annu Rev Plant Physiol Plant Mol Biol 49:249–279
Ollerenshaw JH, Lyons T (1999) Impacts of ozone on the growth and yield of field grown winter wheat. Environ Pollut 106:67–72
Ollerenshaw JH, Lyons T, Barnes JD (1999) Impacts of ozone on growth and yield of field grown oilseed rape. Environ Pollut 104:53–59
Pang J, Kobayashi K, Zhu J (2009) Yield and photosynthetic characteristics of flag leaves in Chinese rice (Oryza sativa L.) varieties subjected to free-air release of ozone. Agric Ecosys Environ 132:203–211
Paoletti E, Grulke NE (2005) Does living in elevated CO2 ameliorate tree response to ozone? A review on stomatal responses. Environ Pollut 137:483–493
Paoletti E, Contran N, Manning WJ (2007) Ethylenediurea (EDU) affects the growth of ozone-sensitive and tolerant Ash (Fraxinus excelsior) trees under ambient O3 conditions. Scientific World J 7:128–133
Paoletti E, Contran N, Manning WJ, Castagna A, Ranieri A, Tagliaferro F (2008) Protection of ash (Fraxinus excelsior) trees from ozone injury by ethylenediurea (EDU): roles of biochemical changes and decreased stomatal conductance in enhancement of growth. Environ Pollut 155:464–472
Paoletti E, Contran N, Manning WJ, Ferrara AM (2009) Use of antiozonant ethylenediurea (EDU) in Italy: verification of the effects of ambient ozone on crop plants and trees and investigation of EDU’s mode of action. Environ Pollut 157:1453–1460
Papple DJ, Ormrod DP (1977) Comparative efficacy of ozone-injury suppression by benomyl and carboxin on turfgrass. J Am Soc Hortic Sci 102:792–796
Park JI, Grant CM, Davies MJ, Dawes IW (1998) The cytoplasmic Cu, Zn superoxide dismutase of Saccharomyces cerevisiae is required for resistance to freeze-thaw stress Generation of free radicals during freezing and thawing. J Biol Chem 273:22921–22928
Pasqualini S, Antonielli H, Ederli L, Piccioni C, Loreto F (2002) Ozone uptake and its effect on photosynthetic parameters of two tobacco cultivars with contrasting ozone sensitivity. Plant Physiol Biochem 40:599–603
Pauls KP, Thopson JE (1982) Effects of cytokinins and antioxidants on the susceptibility of membranes to ozone damage. Plant Cell Physiol 23:821–832
Pell EJ (1976) Influence of benomyl soil treatment on pinto bean plants exposed to peroxyacetyl nitrate and ozone. Phytopathology 66:6
Pellinen R, Palva T, Kangasjarvi J (1999) Subcellular localization of ozone-induced hydrogen peroxide production in birch (Betula pendula) leaf cells. Plant J 20:349–356
Persson K, Danielsson H, Sellden G, Pleijel H (2003) The effects of tropospheric ozone and elevated carbon dioxide on potato (Solanum tuberosum L.cv Bintje) growth and yield. Sci Total Environ 310:191–201
Piikki K, Sellden G, Pleijel H (2004) The impact of tropospheric O3 on leaf number duration and tuber yield of the potato (Solanum tuberosum L.) cultivars Bintje and Kardal. Agric Ecosys Environ 104:483–492
Pitcher LH, Brennan E, Zilinskas BA (1992) The antiozonant ethylenediurea does not act via superoxide dismutase induction in bean. Plant Physiol 99:1388–1392
Pleijel H, Norberg A, Sellden G, Skarby L (1999) Tropospheric ozone decreases biomass production in radish plants (Raphanus sativus) grown in rural south-west Sweden. Environ Pollut 106:143–147
Polle A, Wieser G, Havranek WM (1995) Quantification of ozone influx and apoplastic ascorbate content in needles of Norway spruce trees (Picea abies L., Karst.) at high altitude. Plant. Cell Environ 18:681–688
Postiglione L, Fagnano M (1995) Ozone injury and ethylenediurea: first results on different species in the Campania region. Agric Medit Sp Vol. (Proc) 109-118
Rai R, Agrawal M (2008) Evaluation of physiology and biochemical responses of two rice (Oryza sativa L.) cultivars to ambient air pollution using open top chambers at a rural site in India. Sci Total Environ 407:679–691
Rai R, Agrawal M, Agrawal SB (2007) Assessment of yield losses in tropical wheat using open top chambers. Atmos Environ 41:9543–9554
Rao MV, Davis KR (1999) Ozone-induced cell death occurs via two distinct mechanisms in Arabidopsis: the role of salicylic acid. Plant J 17:603–614
Regner-Joosten K, Manderscheid R, Bergmann E, Bahadir M, Weigel HJ (1994) An HPLC method to study the uptake and partitioning of the antiozonant EDU in bean plants. Angew Botanik 68:151–155
Reid CD, Fiscus EL (2008) Ozone and density affect the response of biomass and seed yield to elevated CO2 in rice. Global Change Biol 14:60–76
Reinert RA, Spurr HW (1972) Differential effect of fungicides on ozone injury and brown spot disease of tobacco. J Environ Qual 1:450–452
Ribas A, Penuelas J (2000) Effects of ethylenediurea as a protective antiozonant on beans (Phaseolus vulgaris cv Lit) exposed to different tropospheric ozone doses in Catalonia (NE Spain). Water Air Soil Pollut 117:263–271
Rich S, Ames R, Zukel JW (1974) 1,4-Oxathiin derivatives protect plants against ozone. Plant Dis Rep 58:162–164
Roberts BR, Wilson LR, Cascino JJ, Smith GP (1987) Autoradiographic studies of ethylenediurea distribution in woody plants. Environ Pollut 45:81–86
Robinson JM, Britz SJ (2000) Tolerance of a field grown cultivar to ozone level is concurrent with higher leaflet ascorbic acid level, higher ascorbate-dehydroascorbate redox status, and long term photosynthetic productivity. Photosyn Res 64:77–87
Robinson JM, Britz SJ (2001) Ascorbate-dehydroascorbate level and redox status in leaflets of field-grown soybeans exposed to elevated ozone. Int J Plant Sci 162:119–125
Rodriguez MCS, Petersen M, Mundy J (2010) Mitogen-activated protein kinase signaling in plants. Annu Rev Plant Biol 61:621–649
Runeckles VC, Resh HM (1975) Effects of cytokinins on responses of bean leaves to chronic ozone treatment. Atmos Environ 9:749–753
Sarkar A, Agrawal SB (2010a) Identification of ozone stress in Indian rice through foliar injury and differential protein profile. Environ Monit Assess 161:205–215
Sarkar A, Agrawal SB (2010b) Elevated ozone and two modern wheat cultivars: an assessment of dose dependent sensitivity with respect to growth, reproductive and yield parameters. Environ Exp Bot 69:328–337
Scandalios JG, Guan L, Polidoros AN (1997) Catalase in plants: gene structure, properties, regulation and expression. In: Scandalios JG (ed) Oxidative stress and molecular biology of antioxidant defenses. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, pp 343–406
Schenone G, Lorenzini G (1992) Effects of regional air pollution on crops in Italy. Agric Ecosys Environ 38:51–59
Serbinova EA, Packer L (1994) Antioxidant properties of α-tocopherol and α tocotrienol. Methods Enzymol 234:354–366
Shi G, Yang L, Wang Y, Kobayashi K, Zhu J (2009) Impact of elevated ozone concentration on yield of four Chinese rice cultivars under fully open-air field conditions. Agric Ecosys Environ 131:178–184
Siegel SM (1962) Protection of plants against airborne oxidants: cucumber seedlings at extreme ozone levels. Plant Physiol 37:261–266
Singh S, Agrawal SB (2009) Use of ethylenediurea (EDU) in assessing the impact of ozone on growth and productivity of five cultivars of Indian wheat (Triticum aestivum L.). Environ Monit Assess 159:125–141
Singh S, Agrawal SB (2010) Impact of tropospheric ozone on wheat (Triticum aestivum L.) in the eastern Gangetic plains of India as assessed by ethylenediurea (EDU) application during different developmental stages. Agric Ecosys Environ 138:214–221
Singh S, Agrawal SB (2011) Cultivar specific response of soybean (Glycine max. L) to ambient and elevated concentrations of ozone under open top chamber. Water Air Soil Pollut 217:283–302
Singh P, Agrawal M, Agrawal SB (2009a) Evaluation of physiological, growth and yield responses of a tropical oil crop (Brassica campestris L. var. Kranti) under ambient ozone pollution at varying NPK levels. Environ Pollut 157:871–880
Singh S, Agrawal SB, Agrawal M (2009b) Differential protection of ethylenediurea against ambient ozone for five cultivars of tropical wheat. Environ Pollut 157:2359–2367
Singh E, Tiwari S, Agrawal M (2010a) Variability in antioxidant and metabolite levels, growth and yield of two soybean varieties: an assessment of anticipated yield losses under projected elevation of ozone. Agric Ecosys Environ 135:168–177
Singh S, Agrawal M, Agrawal SB, Emberson L, Büker P (2010b) Use of ethylenediurea for assessing the impact of ozone on mungbean plants at a rural site in a dry tropical region of India. Int J Environ Waste Manage 5:125–139
Singh S, Agrawal SB, Singh P, Agrawal M (2010c) Screening three cultivars of Vigna mungo L. against ozone by application of ethylenediurea (EDU). Ecotoxicol Environ Saf 73:1765–1775
Singh S, Kaur D, Agrawal SB, Agrawal M (2010d) Responses of two cultivars of Trifolium repens L. to ethylenediurea (EDU) in relation to ambient ozone. J Environ Sci 22:1096–1103
Singh AA, Agrawal SB, Shahi JP, Agrawal M (2014) Assessment of growth and yield losses in two Zea mays L. cultivars (quality protein maize and non quality protein maize) under projected levels of ozone. Environ Sci Pollut Res 21(4):2628–41. doi:10.1007/S11356-013-2188-6
Smirnoff N (2000) Ascorbate biosynthesis and function in photoprotection. Philos Trans R Soc Lond B Biol Sci 355:1455–1464
Smith G, Greenhalgh B, Brennan E, Justin J (1987) Soybean yield in New Jersey relative to ozone pollution and antioxidant application. Plant Dis Rep 71:121–125
Staehelin J, Poberaj CS (2008) Long term tropospheric ozone trends: a critical review. Adv Global Change Res 33:271–282
Szantoi Z, Chappelka AH, Muntifering RB, Somers GL (2009) Cutleaf coneflower (Rubeckia laciniata L.) response to ozone and ethylenediurea (EDU). Environ Pollut 157:840–846
Taylor GS, Rich S (1974) Ozone injury to tobacco in the field influenced by soil treatment with benomyl and carboxin. Phytopathology 64:814–817
Temple PJ, Bisessar S (1979) Response of white bean to bacterial blight, ozone, and antioxidant protection in the field. Phytopathology 69:101–103
Thompson AM (1992) The oxidizing capacity of the Earth’s atmosphere. Probable past and future changes. Science 256:1157–1165
Tiwari S, Agrawal M (2009) Protection of palak (Beta vulgaris L. var. All green) plants from ozone injury by ethylenediurea (EDU): Roles of biochemical and physiological variations in alleviating the adverse impacts. Chemosphere 75:1492–1499
Tiwari S, Agrawal M (2010) Effectiveness of different EDU concentrations in ameliorating ozone stress in carrot plants. Ecotoxicol Environ Saf 73:1018–1027
Tiwari S, Agrawal M, Manning WJ (2005) Assessing the effects of ambient ozone on growth and productivity of two cultivars of wheat in India using three rates of application of ethylenediurea (EDU). Environ Pollut 138:153–160
Tiwari S, Agrawal M, Marshall FM (2006) Evaluation of air pollution impact on carrot plants at a suburban site using open top chambers. Environ Monit Assess 119:15–30
Toivonen PMA, Hofstra G, Wukasch RY (1982) Assessment of yield losses in white bean due to ozone using antioxidant EDU. Can J Plant Pathol 4:381–386
Tripathi R, Agrawal SB (2012) Effects of ambient and elevated level of ozone on Brassica campestris L. with special reference to yield and oil quality parameters. Ecotoxicol Environ Saf 85:1–12
Tripathi R, Agrawal SB (2013) Interactive effect of supplemental ultraviolet-B and elevated ozone on seed yield and oil quality of two cultivars of linseed (Linum usitatissimum L.) carried out in open top chambers. J Sci Food Agric 93:1016–1025
Unsworth MH, Lesser VM, Heagle AS (1984) Radiation interception and the growth of soybeans exposed to ozone in open-top field chambers. J Appl Ecol 21:1059–1077
Vandermeiren K, De Temmerman L, Hookham N (1995) Ozone sensitivity of Phaseolus vulgaris in relation to cultivar differences, growth stage and growing conditions. Water Air Soil Pollut 85:1455–1460
Varshney CK, Rout C (1998) Ethylenediurea (EDU) protection against ozone injury in Tomato plants in Delhi. Bull Environ Contam Toxicol 61:188–193
Verbeke P, Siboska GE, Clark BFC, Rattan SIS (2000) Kinetin inhibits protein oxidation and glycoxidation in vitro. Biochem Biophys Res Commun 276:1265–1270
Wahid A (2006a) Productivity losses in barley attributable to ambient atmospheric pollutants in Pakistan. Atmos Environ 40:5342–5354
Wahid A (2006b) Influence of atmospheric pollutants on agriculture in developing countries: a case study with three new wheat varieties in Pakistan. Sci Total Environ 371:304–313
Wahid A, Maggs R, Shamshi SRA, Bell JNB, Ashmore MR (1995) Air pollution and its impact on rice yield in Pakistan Punjab. Environ Pollut 90:323–329
Wahid A, Milne E, Shamshi SR, Ashmore MR, Marshall FM (2001) Effects of oxidants on soybean growth and yield in the Pakistan Punjab. Environ Pollut 113:271–280
Wahid A, Sheikh SA, Zhao Y, Bell JNB (2012) Evaluation of ambient air pollution effects on three cultivars of seasame (Sesamum indicum L.) by using ethlylenediurea. Pak J Bot 44:99–110
Walker JT, Barlow JC (1974) Response of indicator plants to ozone levels in Georgia. Phytopathol 64:1122–1127
Wang X, Mauzerall D (2004) Characterizing distributions of surface ozone and its impact on grain production in China, Japan and South Korea: 1990 and 2020. Atmos Environ 38:4383–4402
Wang X, Zheng Q, Yao F, Chen Z, Feng Z, Manning WJ (2007) Assessing the impact of ambient ozone on growth and yield of rice (Oryza sativa L.) and wheat (Triticum aestivum L.) cultivar grown in the Yangtze delta, China, using three rates of application of ethylenediurea (EDU). Environ Pollut 148:390–395
Weidensaul TC (1980) N-[2-(2-oxo-1-imidazolidinyl) ethyl-]-N′-phenylyurea as a protectant against ozone injury to laboratory fumigated pinto bean plants. Phytopathology 70:42–45
Whitaker BD, Lee EH, Rowland RA (1990) EDU and O3 production: foliar glycerolipids and steryl lipids in snap bean exposed to O3. Physiol Plant 80:286–293
Willekens H, Inzé D, Van Montagu M, Van Camp W (1995) Catalases in plants. Mol Breed 1:207–228
Wohlgemuth H, Mittelstrass K, Kschieschan S, Bender J, Weigel HJ, Overmyer K, Kangasjarvi J, Sandermann H, Langebartels C (2002) Activation of an oxidative burst is a general feature of sensitive plants exposed to the air pollutant ozone. Plant Cell Environ 25:717–726
Wu Y, Tiedemann AV (2002) Impact of fungicides on active oxygen species and antioxidant enzymes in spring barley (Hordeum vulgare L.) exposed to ozone. Environ Pollut 116:37–47
Wukasch RT, Hofstra G (1977) Ozone and Botrytis interactions in onion-leaf dieback: open-top chamber studies. Phytopathology 67(9):1080–1084
Zheng Y, Lyons T, Ollerenshaw JH, Barnes JD (2000) Ascorbate in the leaf apoplast is a factor mediating ozone resistance in Plantago major. Plant Physiol Biochem 38:403–411
Zouzoulas D, Koutroubas SD, Vassiliou G, Vardavakis E (2009) Effects of ozone fumigation on cotton (Gossypium hirsutum L.) morphology, anatomy, physiology, yield and qualitative characteristics of fibers. Environ Exp Bot 67:293–303
Acknowledgement
Authors are thankful to the Head, Department of Botany for facilities and funding agencies Council of Scientific and Industrial Research (CSIR), University Grants Commission (UGC), Department of Science and Technology (DST) and Ministry of Environment and Forests (MOEF), Government of India for providing the financial help. Authors would also like to thank the researchers who helped us indirectly by providing their significant research work on ozone and EDU.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Singh, A.A., Singh, S., Agrawal, M., Agrawal, S.B. (2015). Assessment of Ethylene Diurea-Induced Protection in Plants Against Ozone Phytotoxicity. In: Whitacre, D. (eds) Reviews of Environmental Contamination and Toxicology Volume 233. Reviews of Environmental Contamination and Toxicology, vol 233. Springer, Cham. https://doi.org/10.1007/978-3-319-10479-9_4
Download citation
DOI: https://doi.org/10.1007/978-3-319-10479-9_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-10478-2
Online ISBN: 978-3-319-10479-9
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)