Abstract
Wheat is a major staple food and its sensitivity to the gas pollutant ozone (O3) depends on the cultivar. However, few chamber-less studies assessed current ambient O3 effects on a large number of wheat cultivars. In this study, we used ethylenediurea (EDU), an O3 protectant whose protection mechanisms are still unclear, to test photosynthetic pigments, gas exchange, antioxidants, and yield of 15 cultivars exposed to 17.4 ppm h AOT40 (accumulated O3 over an hourly concentration threshold of 40 ppb) over the growing season at Beijing suburb, China. EDU significantly increased light-saturated photosynthesis rate (Asat), photosynthetic pigments (i.e., chlorophyll and carotenoid), and total antioxidant capacity, while reduced malondialdehyde and reduced ascorbate contents. In comparison with EDU-treated plants (control), plants treated with water (no protection from ambient O3) significantly decreased yield, weight of 1000 grains, and harvest index by 20.3%, 15.1%, and 14.2%, respectively, across all cultivars. There was a significant interaction between EDU and cultivars in all tested variables with exception of Asat, chlorophyll, and carotenoid. The cultivar-specific sensitivity to O3 was ranked from highly sensitive (> 25% change) to less sensitive (< 10% change) by comparing the difference of the average grain yield of plants applied with and without EDU. Neither stomatal conductance nor antioxidant capacity contributed to the different response of the cultivars to EDU, suggesting that another mechanism contributes to the large variation in response to O3 among cultivars. Generally, the results indicate that present O3 concentration is threatening wheat production in Northern China, highlighting the urgent need for policy-making actions to protect this critical staple food.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ground-level ozone (O3) is one of the most phytotoxic air pollutants at regional and global level, which has been rising rapidly because of anthropogenic activity since the industrial revolution (Cooper et al. 2014). Ozone is the third greenhouse gas in terms of radiative forcing and has received more and more attention in the past three decades due to its importance to air quality and climate change (Monks et al. 2015). In China, serious O3 pollution occurs at present, with annual averages of peak concentration reaching even 60 ppb (Li et al. 2018) and an increase of about 7% in daily mean concentrations from 2005 to 2010 (Verstraeten et al. 2015). Such O3 concentrations pose serious threats for China’s food security, given that O3 is one of the most detrimental air pollutants for crops and natural ecosystems (Ainsworth et al. 2012; Feng et al. 2015).
Wheat (Triticum aestivum L.) is one of the top staple crops in the world, and the second most-produced cereal after maize (FAO 2016). In 2016, world wheat production was 749 M tons (FAO 2016). Winter wheat, i.e., planted in the fall and matured in the next summer, accounts for about 94% of Chinese total wheat yield (Zhou et al. 2014). The top wheat-producing provinces in China are located in the North China Plain, Middle China, and East China, i.e., areas subject to severe O3 pollution (Tai et al. 2014). A meta-analysis indicated that elevated O3 concentration (50–75 ppb) reduced wheat yield by 24% compared with plants grown in charcoal-filtered air (Feng et al. 2008), and thus wheat is considered an O3-sensitive crop (Mills et al. 2007; Feng and Kobayashi 2009; Pleijel 2011). Elevated O3 accelerates wheat leaf senescence, as indicated by increased lipid oxidation as well as fast decline in pigment amounts and photosynthetic rates (Feng et al. 2016), and reduces 1000 grain weight and yield (Broberg et al. 2015). Estimates suggest that wheat yield would globally decline by 4–17% due to O3 exposure in 2030 relative to 2000 (Avnery et al. 2011). Depending on the climate scenario, O3 either exacerbated or offset a substantial fraction of climate impacts on wheat yield in a simulation of effects in 2050 relative to 2000 (Tai et al. 2014). In contrast, a British study showed that a 10% increase in AOT40 would decrease wheat yield by only 0.23% (Shankar and Neeliah 2005). These conflicting results may be due to different types (O3-tolerant or O3-sensitive) of wheat cultivar (Kaliakatsou et al. 2010). The effects of O3 on wheat, in fact, are cultivar-specific (Biswas et al. 2008; Feng et al. 2016). In an experiment conducted in walk-in chambers, Saitanis et al. (2014) distinguished the O3 sensitivity of ten cultivars of Bangladeshi winter wheat (Triticum aestivum L.) rooted in pots using visible injury and chlorophyll b/a ratio as the response variables.
In the last decades, research aimed at assessing the impacts of O3 on plants has been carried out using different exposure systems, each of them with some advantages and disadvantages (Kobayashi 2015). Plant growth chambers with controlled O3 concentrations are adequate for the study of the mechanisms of O3 damage, but in these facilities plants usually grow in pots and their environmental conditions differ from field conditions (Kobayashi 2015). Growth conditions inside open-top chambers are closer to field conditions and plants can be rooted in the ground, but there is an undesirable “chamber effect” which alters the microclimate inside them (Piikki et al. 2008; Feng et al. 2018). In free-air concentration enrichment (FACE) facilities, plants were grown under actual field conditions and without a “chamber effect.” In this context, the use of the so-called antiozonant chemicals has been proposed as an alternative to evaluate the effects of O3 on plants under real field conditions (Manning et al. 2011; Paoletti et al. 2009). Among them, EDU (N-[2-(2-oxo-1-imidazolidinyl)ethyl]-N′-phenylurea, with formula C4H10N4O2) has been used in many studies, which showed that it can effectively protect crops and trees from the detrimental effects of O3 (Feng et al. 2010; Manning et al. 2011; Agathokleous 2017; Tiwari 2017).
In the case of wheat, EDU has been used to quantify the impacts of ambient O3 in India (Singh and Agrawal 2010; Rai and Agrawal 2014; Tiwari et al. 2005). In addition, intraspecific sensitivity to O3 was assessed by foliar spraying or soil drenching with different concentrations of EDU. For example, Tiwari et al. (2005) used different EDU concentrations, i.e., 0, 150, 300, and 450 ppm, and Singh and Agrawal (2009) used EDU at 400 ppm and found that EDU-treated wheat cultivars showed different seed yield enhancement values compared with those without EDU treatments, indicating different O3 sensitivity among the wheat cultivars. EDU application at 400 ppm significantly increased stomatal conductance, photosynthetic rate, and protective enzyme activities of five wheat cultivars, and the increase magnitude of these measured parameters between EDU and non-EDU treatment reflected the different resistance to O3 (Singh et al. 2009). Notably, only a few wheat cultivars rooted in the field were assessed for their ozone sensitivity (Singh and Agrawal 2009; Feng et al. 2016).
However, the mechanism of EDU protection against O3 is not well known so far (Agathokleous 2017; Tiwari 2017). This fact may raise the question whether the observed EDU protection against O3 is actually due to an O3-specific property or to another mechanism, i.e., an increase of N supply to the plant, given that EDU contains around 22% of N (Godzik and Manning 1998). In order to rule out possible EDU effects in the absence of O3, studies with wheat and rice have applied EDU under low-O3 filtered air conditions and concluded that no significant effects occurred in EDU-treated plants in comparison with not EDU-treated ones (Singh and Agrawal 2010; Ashrafuzzaman et al. 2017). A recent study with rice also concluded that the amount of N supplied by the EDU treatment was negligible in comparison with the fertilization levels applied (Ashrafuzzaman et al. 2017). Therefore, even though the EDU mode of action still needs to be elucidated, these results suggest a specific protective effect of EDU against O3 rather than a fertilization effect (Agathokleous 2017). Therefore, the use of this chemical has been proposed as a suitable method to diagnose O3 damage in the field and for distinguishing tolerant and sensitive genotypes in cereals including wheat (Singh and Agrawal 2010; Ashrafuzzaman et al. 2017; Tiwari 2017).
In the present study, we applied EDU along the entire growing season to 15 Chinese cultivars of winter wheat, with the main objectives of (1) estimating the impact of ambient O3 on the main wheat cultivars widely cultivated in China and ranking them in order of O3 sensitivity based on yield response, (2) determining the factors which contribute to the different response to O3 among cultivars, and establishing cultivar-specific EDU protective mechanisms. We hypothesized that ambient O3 pollution in China is inducing cultivar-specific damage to wheat yield as assessed by EDU, and stomatal conductance or antioxidant capacity contribute to the large variation among cultivars in response to ambient O3. This research will provide useful information for cultivating the most tolerant cultivars in areas at high O3 risk and clarifying the mechanisms of tolerance.
Materials and methods
Experimental conditions
The experimental site was located at XiaoTangShan town (40° 19′ N, 116° 13′ E), Changping District, Northwest of Beijing, China, about 38 km from Beijing city center. Mean monthly minimum and maximum temperatures were − 3.1 °C (January) and 26.7 °C (July), respectively. The annual average precipitation was 600 mm, and 60% of the precipitation was in July and August.
Seeds of 15 winter wheat cultivars planted widely in North China (Table S1), which were obtained from YiXian county Seed Company in Hebei province, were sowed on 3 October 2015, greened up on 28 February 2016, and were harvested on 28 June 2016 (a total of 241 days). Two treatments (EDU and water) and five replications for each treatment per cultivar were carried out. Each 18-m2 (6 m × 3 m) plot was divided into 15 lines, one per cultivar (randomly assigned).
An ultraviolet (UV) absorption O3 analyzer (Model 49i, Thermo Scientific, USA) was used to continuously monitor the concentration of O3 at 1.5 m a.g.l. in the field from 25 days after the greening-up phase (DAG), and calibrated by a 49i-PS calibrator (49i-PS, Thermo Scientific, USA) before the experiment and once a month during the experiment. During the growth period, the 24-h mean O3 concentration was 37.7 ppb and the average ambient O3 concentration in the daily 8-h (M8, between 9:00 and 17:00) was 56.6 ppb, with a minimum value of 16 ppb and a maximum value of 103 ppb (Fig. 1). The 24-h and 8-h mean concentrations of March, April, May, and June were 24.1 and 38.5 ppb, 30.1 and 44.5 ppb, 39.8 and 59.0 ppb, and 46.3 and 70.5 ppb, respectively. Accumulated O3 exposure above an hourly threshold of 40 ppb (AOT40) was 17.4 ppm h at the end of the experiment (Fig. 1). High ambient O3 concentration was observed in May and June, i.e., at the time of anthesis and grain filling, respectively (Fig. 1).
EDU application
Previous experiments showed that EDU concentrations higher than 600 ppm were toxic to plants while 200–450 ppm EDU protected plants against O3 injury (Agathokleous 2017). Four hundred fifty parts per million concentration of EDU was selected in our experiment according to its successful application on many crop species (e.g., Paoletti et al. 2009; Feng et al. 2010; Manning et al. 2011; Yuan et al. 2015; Jiang et al. 2018). One hundred percent EDU powder was dissolved in warm water. The plants in five plots were sprayed with 450 ppm EDU solution and the other five plots were sprayed with water as control. We sprayed the entire foliage of each plant until drip point before sunrise every time in order to avoid the light decomposition of EDU. The EDU treatments started at 25 DAG. Repeated applications are needed to ensure the efficacy of EDU considering that EDU is easily degraded over time (Gatta et al. 1997; Pasqualini et al. 2016). EDU treatments were thus carried out at bi-weekly intervals until the end of the experiment. In case of rain within the 5 days after each application, the treatment was applied again. Total application of EDU was nine times in our experiment.
Measurements
Gas exchange, photosynthetic pigments, and biochemical parameter measurements and samplings were carried out on flag leaves at mid-grain filling stage (105 DAG) because this stage shows the highest sensitivity to O3 for wheat (Pleijel et al. 1998; Feng et al. 2008). The final harvest was carried out at 121 DAG.
Gas exchange
Two fully expanded flag leaves from two plants per cultivar in each plot were selected to measure gas exchange using three inter-calibrated photosynthesis systems (LI-6400, LI-COR Inc., Lincoln, NE, USA). Light-saturated photosynthetic rate (Asat) and stomatal conductance (gs) were measured at saturating light (1500 μmol m−2 s−1), 400 ppm CO2, and 50–70% relative humidity (RH). All measurements were conducted during 08:30–11:30 a.m. on sunny days.
Photosynthetic pigments
After gas exchange measurement, the two flag leaves per cultivar in each plot were sampled for photosynthetic pigment and biochemical parameters. Two milliliters of 95% ethanol was used for analysis of photosynthetic pigments in the dark for 48 h at 4 °C, and a UV spectrophotometer (Alpha-1506, Lab-Spectrum Instruments Co., Ltd., China) was used to assay chlorophyll (Chl) a and b and carotenoid (Car) content according to the specific absorption coefficients described by Lichtenthaler (1987).
Biochemical parameters
Total antioxidant capacity (TAC) was assessed by ferric reducing antioxidant power (FRAP) assay according to Benzie and Strain (1996). Samples were added to 2 ml of cold 70% (v/v) ethanol and homogenized. The mixture was incubated in the dark at 4 °C for 20 min, and then centrifuged at 3000 rpm for 20 min. One milliliters of supernatant was taken for the FRAP assay to express the TAC as Fe3+ equivalents (mmol Fe2+/g fresh mass). Total ascorbate (AsA) was measured by an α-α′-bipyridyl-based method (Gillespie and Ainsworth 2007). Malondialdehyde (MDA) was determined according to the method of Heath and Packer (1968) to evaluate lipid peroxidation by 2-thiobarbituric acid-reactive metabolite (TBA). The detail was described in Jiang et al. (2018).
Yield
The plants over a fixed area (1 m × 0.3 m) were harvested for yield at each line in each plot. All grains per plant were dried in the sun to constant weight. Harvest index (HI, %) was calculated as the ratio of grain weight and total aboveground dry weight, the latter obtained in an oven at 80 °C until constant weight. Number of ears per plant, number of grains per plant, and weight of 1000 grains were recorded from five plants for any cultivar at each plot.
Definition of O3 impact
Similar to Jiang et al. (2018), the cultivar-specific sensitivity to O3 (YEFF) was estimated by comparing the average yield (Y) with and without EDU protection, i.e., YEFF = (YWater − YEDU) × 100/YEDU. The same approach was used to estimate the effect of O3 on each variable.
Statistical analyses
The statistical unit was the single plot, with five plots for EDU and five plots for water treatment per wheat cultivar. Data were checked for normal distribution and homogeneity of variance. Not normally distributed data, i.e., grain number, AsA, and biomass, were log transformed prior to analysis. Data in the tables are not transformed but original data means ± standard deviation (SD). Data were subject to a two-way analysis of variance (ANOVA) including the effects of EDU, cultivar, and their interaction using Statistica 10.0 software (StatSoft, Italy). If those variables showed no significant interaction between EDU and cultivars, student t test was applied for each cultivar.
Results
Gas exchange
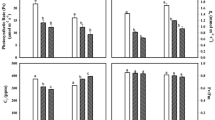
EDU significantly increased Asat and gs by 37.9% and 46.1%, respectively, across all cultivars at grain filling stage (Table 1). There were significant differences between cultivars in both Asat and gs. No significant interaction between EDU treatment and cultivar was found in Asat although EDU significantly increased Asat in most cultivars with exception of JM22, SX616, and ZM411. On the other hand, gs showed a significant interaction between EDU and cultivars, as indicated by the significant increase by EDU in cultivars HN6425, JD22, JM58, SX733, and SX828, but no change or decrease in cultivars SX616, SY20, and ZM411.
Photosynthetic pigments
Across all cultivars, EDU significantly increased Chl and Car contents by 10.3% and 13.0%, respectively (Table 2). Differences between cultivars were found, while interactions between EDU and cultivar were not significant.
Biochemical parameters
MDA was lower (− 17.6%) in all cultivars treated with EDU than in those treated with water (Table 3). The EDU and cultivar interaction was significant, as indicated by the fact that MDA contents in EDU-treated plants were significantly lower by 32.2% for JM58. EDU significantly reduced AsA by 9.8% across all cultivars. A significant interaction between EDU and cultivar was found, as indicated by lower AsA contents in EDU-treated plants for cultivars HN6049, SX828, and TM8 by 31.2%, 38.4%, and 38.5%, respectively. However, EDU significantly increased TAC content across all cultivars by 19.6%. The cultivar with the highest TAC content was JD22 and the lowest was SN22. There were significant interactions between EDU and cultivar, as showed by the significant increase in cultivars JD22 (91.8%), JM58 (43.6%), SX616 (40.9%), and SX733 (45.3%).
Yield parameters
Both HI and grain weight showed a significant variation between cultivars, EDU treatments, and their interaction (Table 4). EDU significantly increased HI and weight of 1000 grains by 16.7% and 17.7% across all cultivars, respectively, although significant increases were only observed for cultivars TM8 for HI and TM8, ZM411, and JM58 for grain weight. EDU did not affect the ears per plant and grains per plant across all cultivars although it significantly reduced the grains per plant in cultivars JM22, TM8, and ZM411.
On average, grain yield of EDU-treated cultivars was 25.4% higher than that of water-treated ones (Fig. 2a). In other words, ambient O3 reduced the yield by 20.3% using EDU plants as control. The yield in 7 out of 15 cultivars was significantly increased by EDU, and consistently the interaction between EDU and cultivars was significant. The cultivar-specific O3 sensitivity (YEFF) showed a significant variation, with the most O3-tolerant cultivars SY20, SX633, and L639 showing insignificant changes (< 10%) and the most O3-sensitive cultivars JM58, SN22, SX616, SX733, and HN6049 showing large reduction (> 25%) (Fig. 2b).
Yield (mean + SD) of 15 wheat cultivars exposed to ambient O3 concentrations (water) or impacts of ambient ozone on grain yield assessed by ethylenediurea (EDU). Cultivars are sorted according to decreasing yield (a) and according to increasing ozone sensitivity, expressed as percent variation when exposed to ambient O3 with water-treated plants as control (b). Two-way ANOVA is shown. An asterisk indicates significant differences between treatments (P < 0.05)
Discussion
One of the main objectives of the present paper was to estimate the impact of ambient O3 on the main wheat cultivars used in Northern China, and classify them in order of O3 sensitivity by using yield as the response variable. The mean O3 concentrations measured in the survey area during the experimental period (56.6 ppb M8 and 17.8 ppm h AOT40) can be considered representative of the O3 levels in spring throughout the North Plain and Central/Eastern regions of China (Li et al. 2018). In our experiment, yield was reduced by 20.3% in control plants compared to the plants protected by EDU. This decrease was similar to the highest decrease reported by Singh et al. (2009) in five varieties of wheat exposed to O3 of a variation of 34.2 ppb during the vegetative phase to 54.2 ppb at reproductive stage in India, i.e., between 1.9 and 20.5% depending on the varieties. Also Singh and Agrawal (2010) found that ambient O3 (50 ppb of M12) induced reductions between 16.2 and 26.7% (depending on the concentration of EDU applied) in grain weight per plant of an O3-sensitive wheat variety in India. In another study in the Yangtze Delta, China, decreases in wheat yield (g plant−1) by ambient O3 were only 6.6% relative to 450 ppm EDU in the cultivar of Jiaxing 002 (Wang et al. 2007). The observed differences in yield loss between regions can be attributed to differences in O3 sensitivity among cultivars or the O3 concentrations (and therefore the degree of potential damage) to which the plants were exposed in the ambient air. In comparison with the studies carried out in India and in the Yangtze Delta (Feng et al. 2012; Singh and Agrawal 2017), the area of Changping in the current study showed somewhat higher O3 levels, with 8-h monthly mean values up to 70.5 ppb. On the other hand, the relative yield reduction (20.3%) observed in our experiment was similar to the 19.5% reported by Feng et al. (2010) for wheat in a meta-analytic review on the protection of EDU from ambient O3. Also, the reduction was little less than the 24.4% depression in yield caused by O3 concentrations (69 ppb) estimated in another meta-analysis by Feng et al. (2008). Considering the European dose-response function for wheat (y = − 0.0161x + 0.99; Mills et al. 2007), the expected losses in yield based on grain weight at an AOT40 of 17.4 ppm h would be 29%. If the equation for Chinese wheat proposed by Wang et al. (2012) based on one cultivar for 4 years is used (y = − 0.0228x+ 1), a 40% reduction would be expected. In both cases, but especially in the second one, the predicted effects on yield overestimated the observed one. It is worth mentioning that in comparison with previous studies based on few cultivars, the present study is remarkable by the use of 15 cultivars, so that differences in responses among cultivars are effectively considered, and it is also representative of the O3 concentrations of a large area of China experiencing high O3 concentrations (Li et al. 2018). Therefore, it provides relevant information on the risk that current ambient O3 levels pose for wheat over a large productive region of China.
As for other parameters related with yield, EDU also significantly increased HI and weight of 1000 grains by 16.7% and 17.7% across all cultivars in comparison to the water treatment in the present experiment. Wang et al. (2007) found significant increases of HI at 300 ppm EDU but not significant differences for the 1000 grain weight index in a single wheat cultivar. Also in the present experiment, the ears per plant did not vary significantly. This variable was also measured by Singh and Agrawal (2009) in an EDU experiment with wheat, in which it was significantly increased in two of the five studied cultivars. Our finding was also supported from OTC and O3-FACE results, in which elevated O3 reduced wheat yield due to smaller grain weight rather than to smaller numbers of grains/ears (Feng et al. 2008; Zhu et al. 2011). Therefore, EDU effectively protected wheat not only from total yield losses but also from declines in other relevant parameters such as HI and the weight of 1000 grains.
The mechanisms through which EDU produced positive effects on yield may involve several processes that are not well known so far (Agathokleous 2017; Agathokleous et al. 2015; Tiwari 2017). As shown by a MDA increase in the water-treated plants in comparison with the EDU-treated ones, EDU prevented leaf lipid peroxidation, helping in membrane protection (Singh and Agrawal 2009; Tiwari 2017). Chlorophyll and carotenoid contents were higher in the EDU treatment than in the water treatment. The same response was observed in other studies with wheat (Singh et al. 2009; Singh and Agrawal 2010), snap bean (Yuan et al. 2015), and other plants (Feng et al. 2010). All in all, 25% of the published studies with EDU includes photosynthetic pigments which are utilized as simple and effective biomarkers of plant stress (Agathokleous and Kitao 2018). Therefore, EDU allows the plants to maintain the membrane integrity and keep an efficient photosynthetic capacity, finally contributing to increasing plant yield. Brunschön-Harti et al. (1995) and Jiang et al. (2018) observed an increase in AsA in EDU-treated plants, which may suggest that EDU may provide plants with a potent antioxidant to cope with oxidative stress. However, an increase in AsA may also result from a lower AsA oxidation rather than from an increase in its synthesis, so that the mechanism behind such an increase is uncertain (Brunschön-Harti et al. 1995). On the other hand, our results do not support a mode of action of EDU through an increase in AsA, since a reduction of AsA was observed in EDU-treated plants. This disparity of results concerning EDU effects on the AsA pool was already pointed out by Manning et al. (2011), underscoring our still-scarce knowledge on the mode of action of this antiozonant molecule at biochemical level (Tiwari 2017). A significant increase in TAC in EDU-treated plants might suggest a possible role of antioxidant molecules other than AsA in preventing O3 damage in EDU-treated wheat plants. On the other hand, since EDU contains urea, it has also been suggested that a foliar fertilizer effect could explain increases in yield and improvements in photosynthesis performance in EDU-treated plants (Manning et al. 2011). However, it is interesting to note that Singh and Agrawal (2010) tested the effect of EDU in wheat under filtered air and find that EDU itself did not affect any of the yield parameters. These results further suggest that the observed reductions in yield are primarily caused by O3 and not by a fertilization effect due to the N content of EDU (Singh and Agrawal 2010; Agathokleous 2017; Tiwari 2017).
Finally, it is worth to note that the differences among cultivars are important, given that some of the cultivars showed significant responses to EDU while others did not. These results highlight that EDU interaction with the plants is not species-specific but cultivar-specific, and that these responses are related with the O3 sensitivity of the plants (Yuan et al. 2015; Agathokleous 2017). In the present experiment, those cultivars for which the responses of yield to EDU differed significantly from the responses to water (> 25% change in JM58, SN22, SX616, SX733, and HN6049) should be regarded as most O3-sensitive. On the contrary, eight of the cultivars showed no significant responses to the EDU treatment and should be considered intermediate sensitivity to current ambient O3 levels. The less-sensitive cultivars SY20, SX633, and L639 showed a < 10% change of yield in response to EDU. Actually, a 10% reduction in the field could induce a large yield loss across China or the world. However, our category is a relative comparison of O3 sensitivity among these cultivars. From a practical point of view, these less-sensitive cultivars may represent an advantage for cultivation in O3-polluted areas and suggest that O3 sensitivity needs to be considered in the crop breeding programs. The ranking from lower to higher sensitivity of winter wheat cultivars (Fig. 2) provides useful information for supporting the selection of the best-suited cultivars for different areas taking into account their ambient O3 levels, as previously reported for soybean cultivars (Jiang et al. 2018). Several studies have estimated yield losses caused by O3 in China and other areas of Asia (e.g., Tang et al. 2013). However, these results should be regarded as unproven hypotheses that should be tested against actual experimental results under current ambient O3 levels. In this sense, EDU experiments can provide estimations of the effects on crop yield and other parameters at a low cost and without problems associated with the modification of the environment of the plants as it is the case of enclosure techniques (Paoletti et al. 2009; Manning et al. 2011; Tiwari 2017). Therefore, experiments with EDU using different varieties of plants can account for the intraspecific variability of O3 sensitivity and represent a practical tool to assess O3 risk to vegetation and to validate model estimations in different areas of the world. However, in order to increase the reliability of this type of studies, it is urgent that the mechanism of antiozonant activity should be better elucidated, which could be helped by the gene expression and protein profiling techniques (Tiwari 2017).
References
Agathokleous E (2017) Perspectives for elucidating the ethylenediurea (EDU) mode of action for protection against O3 phytotoxicity. Ecotox Environ Safe 142:530–537
Agathokleous E, Kitao M (2018) Ethylenediurea (EDU) induces hormesis in plants. Dose-Response 16(2):155932581876528
Agathokleous E, Koike T, Watanabe M, Hoshika Y, Saitanis CJ (2015) Ethylene-di-urea (EDU), an effective phytoproctectant against O3 deleterious effects and a valuable research tool. J Agric Meteorol 71(3):185–195
Ainsworth EA, Yendrek CR, Sitch S, Collins WJ, Emberson LD (2012) The effects of tropospheric ozone on net primary productivity and implications for climate change. Annu Rev Plant Biol 63:637–661
Ashrafuzzaman M, Lubna FA, Holtkamp F, Manning WJ, Kraska T, Frei M (2017) Diagnosing ozone stress and differential tolerance in rice (Oryza sativa L.) with ethylenediurea (EDU). Environ Pollut 230:339–350
Avnery S, Mauzerall DL, Liu J, Horowitz LW (2011) Global crop yield reductions due to surface ozone exposure: 2. Year 2030 potential crop production losses and economic damage under two scenarios of O3 pollution. Atmos Environ 45:2297–2309
Benzie IFF, Strain JJ (1996) The ferric reducing ability of plasma (FRAP) as a measure of ‘antioxidant power’: the FRAP assay. Anal Biochem 239:70–76
Biswas DK, Xu H, Li YG, Sun JZ, Wang XZ, Han XG, Jiang GM (2008) Genotypic differences in leaf biochemical, physiological and growth responses to ozone in 20 winter wheat cultivars released over the past 60 years. Glob Chang Biol 14:46–59
Broberg MC, Feng ZZ, Xin Y, Pleijel H (2015) Ozone effects on wheat grain quality - a summary. Environ Pollut 197:203–213
Brunschön-Harti S, Fangmeier A, Jäger H-J (1995) Effects of ethylenediurea and ozone on the antioxidative systems in beans (Phaseolus vulgaris L.). Environ Pollut 90(1):95–103
Cooper OR, Parrish D, Ziemke J et al. (2014) Global distribution and trends of tropospheric ozone: an observation-based review. Elementa: Science of the Anthropocene 2, 000029, https://doi.org/10.12952/journal.elementa.000029
FAO (2016) World Food Situation. http://www.fao.org/worldfoodsituation/csdb/en/
Feng ZZ, Kobayashi K (2009) Assessing the impacts of current and future concentrations of surface ozone on crop yield with meta-analysis. Atmos Environ 43:1510–1519
Feng ZZ, Kobayashi K, Ainsworth EA (2008) Impact of elevated ozone concentration on growth, physiology, and yield of wheat (Triticum aestivum L.): a meta-analysis. Glob Chang Biol 14:2696–2708
Feng ZZ, Wang SG, Szantoi Z, Chen S, Wang XK (2010) Protection of plants from ambient ozone by applications of ethylenediurea (EDU): a meta-analytic review. Environ Pollut 158:3236–3242
Feng ZZ, Tang HY, Uddling J, Pleijel H, Kobayashi K, Zhu JG, Oue H, Guo WS (2012) A stomatal ozone flux-response relationship to assess ozone-induced yield loss of winter wheat in subtropical China. Environ Pollut 164:16–23
Feng ZZ, Hu EZ, Wang XK, Jiang LJ, Liu XJ (2015) Ground-level O3 pollution and its impacts on food crops in China: a review. Environ Pollut 199:42–48
Feng ZZ, Wang L, Pleijel H, Zhu JG, Kobayashi K (2016) Differential effects of ozone on photosynthesis of winter wheat among cultivars depend on antioxidative enzymes rather than stomatal conductance. Sci Total Environ 572:404–411
Feng ZZ, Uddling J, Tang HY, Zhu JG, Kobayashi K (2018) Comparison of crop yield sensitivity to ozone between open-top chamber and free-air experiments. Glob Chang Biol 24:2231–2238.
Gatta L, Mancino L, Federico R (1997) Translocation and persistence of EDU (ethylenediurea) in plants: the relationship with its role in ozone damage. Environ Pollut 96:445–448
Gillespie KM, Ainsworth EA (2007) Measurement of reduced, oxidized and total ascorbate content in plants. Nat Protoc 2:871–874
Godzik B, Manning WJ (1998) Relative effectiveness of ethylenediurea, and constitutent amounts of urea and phenylurea in ethylenediurea, in prevention of ozone injury to tobacco. Environ Pollut 103:1–6
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts. I Kineticsand stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Jiang LJ, Feng ZZ, Dai LL, Shang B, Paoletti E (2018) Large variability in ambient ozone sensitivity across 19 ethylenediurea-treated Chinese cultivars of soybean is driven by total ascorbate. J Environ Sci 64:10–22
Kaliakatsou E, Bell NJ, Thirtle C, Rose D, Power SA (2010) The impact of tropospheric ozone pollution on trial plot winter wheat yields in Great Britain - an econometric approach. Environ Pollut 158:1948–1954
Kobayashi K (2015) FACE-ing the challenges of increasing surface ozone concentration in Asia. J Agric Meteor 71(3):161–166
Li P, De Marco A, Feng ZZ, Anav A, Zhou DJ, Paoletti E (2018) Large-scale ground-level ozone measurements in China suggest serious risks to forest health. Environ Pollut 237:803–813
Lichtenthaler HK (1987) Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods Enzym 148:350–382
Manning WJ, Paoletti E, Jr HS, Ernst D (2011) Ethylenediurea (EDU): a research tool for assessment and verification of the effects of ground level ozone on plants under natural conditions. Environ Pollut 159:3283–3293
Mills G, Buse A, Gimeno B, Bermejo V, Holland M, Emberson L, Pleijel H (2007) Asynthesis of AOT40-based response functions and critical levels of ozone for agricultural and horticultural crops. Atmos Environ 41:2630–2643
Monks PS, Archibald AT, Colette A, Cooper O, Coyle M, Derwent R, Fowler D, Granier C, Law KS, Mills GE, Stevenson DS, Tarasova O, Thouret V, von Schneidemesser E, Sommariva R, Wild O, Williams ML (2015) Tropospheric ozone and its precursors from the urban to the global scale from air quality to short-lived climate forcer. Atmos Chem Phys 14(23):32709–32933
Paoletti E, Contran N, Manning WJ, Ferrara AM (2009) Use of the antioxidant ethylenediurea (EDU) in Italy: verification of the effects of ambient ozone on crop plants and trees and investigation of EDU's mode of action. Environ Pollut 157:1453–1460
Pasqualini S, Paoletti E, Cruciani G, Pellegrino R, Ederli L (2016) Effects of different routes of application on ethylenediurea persistence in tobacco leaves. Environ Pollut 212:559–564
Piikki K, De Temmerman L, Högy P, Pleijel H (2008) The open-top chamber impact on vapour pressure deficit and its consequences for stomatal ozone uptake. Atmos Environ 42(26):6513–6522
Pleijel H (2011) Reduced ozone by air filtration consistently improved grain yield in wheat. Environ Pollut 159:897–902
Pleijel H, Danielsson H, Gelang J, Sild E, Selldén G (1998) Growth stage dependence of the grain yield response to ozone in spring wheat (Triticum aestivum L.). Agric Ecosyst Environ 70:61–68
Rai R, Agrawal M (2014) Assessment of competitive ability of two Indian wheat cultivars under ambient O3 at different developmental stages. Environ Sci Pollut Res 21(2):1039–1053
Saitanis CJ, Bari SM, Burkey KO, Stamatelopoulos D, Agathokleous E (2014) Screening of Bangladeshi winter wheat (Triticum aestivum L.) cultivars for sensitivity to ozone. Environ Sci Pollut Res 21:13560–13571
Shankar B, Neeliah H (2005) Tropospheric ozone and winter wheat production in England and Wales: a note. J Agr Econ 56:145–151
Singh S, Agrawal SB (2009) Use of ethylene diurea (EDU) in assessing the impact of ozone on growth and productivity of five cultivars of indian wheat (Triticum aestivum L). Environ Monit Assess 159(1–4):125–141
Singh S, Agrawal SB (2010) Impact of tropospheric ozone on wheat (Triticum aestivum L.) in the eastern Gangetic plains of India as assessed by ethylenediurea (EDU) application during different developmental stages. Agric Ecosyst Environ 138:214–221
Singh AA, Agrawal SB (2017) Tropospheric ozone pollution in India: effects on crop yield and product quality. Environ Sci Pollut Res 24:4367–4382
Singh S, Agrawal SB, Agrawal M (2009) Differential protection of ethylenediurea (EDU) against ambient ozone for five cultivars of tropical wheat. Environ Pollut 157:2359–2367
Tai APK, Val Martin M, Heald CL (2014) Threat to future global food security from climate change and ozone air pollution. Nat Clim Chang 4:817–821
Tang HY, Takigawa M, Liu G, Zhu JG, Kobayashi K (2013) A projection of ozone-induced wheat production loss in China and India for the years 2000 and 2020 with exposure-based and flux-based approaches. Glob Chang Biol 19:2739–2752
Tiwari S (2017) Ethylenediurea as a potential tool in evaluating ozone phytotoxicity: a review study on physiological, biochemical and morphological responses of plants. Environ Sci Pollut Res 24:14019–14039
Tiwari S, Agrawal M, Manning WJ (2005) Assessing the impact of ambient ozone on growth and productivity of two cultivars of wheat in India using three rates of application of ethylenediurea (EDU). Environ Pollut 138:153–160
Verstraeten WW, Neu JL, Williams JE, Bowman KW, Worden JR, Boersma KF (2015) Rapid increases in tropospheric ozone production and export from China. Nat Geosci 8:690–695
Wang XK, Zheng QW, Yao FF, Chen Z, Feng ZZ, Manning WJ (2007) Assessing the impact of ambient ozone on growth and yield of a rice (Oryza sativa L.) and a wheat (Triticum aestivum L.) cultivar grown in the Yangtze Delta, China, using three rates of application of ethylenediurea (EDU). Environ Pollut 148:390–395
Wang XK, Zhang QQ, Zheng FX, Zheng QW, Yao FF, Chen Z, Zhang WW, Hou PQ, Feng ZZ, Song WZ, Feng ZW, Lu F (2012) Effects of elevated O3 concentration on winter wheat and rice yields in the Yangtze River Delta, China. Environ Pollut 171(1):118–125
Yuan XY, Calatayud V, Jiang LJ, Manning WJ, Hayes F, Tian Y, Feng ZZ (2015) Assessing the effects of ambient ozone in China on snap bean genotypes by using ethylenediurea (EDU). Environ Pollut 205:199–208
Zhou BW, Sanzsáez Á, Elazab A, Shen TM, Sánchezbragado R, Bort J, Serret MD, Araus JL (2014) Physiological traits contributed to the recent increase in yield potential of winter wheat from Henan province, China. J Integr Plant Biol 56:492–504
Zhu XK, Feng ZZ, Sun TF, Liu XC, Tang HY, Zhu JG, Guo WS, Kobayashi K (2011) Effects of elevated ozone concentration on yield of four Chinese cultivars of winter wheat under fully open-air field conditions. Glob Chang Biol 17:2697–2706
Funding
This work was supported by the State Key Laboratory of Soil and Sustainable Agriculture (No. Y20160030), the Chinese Academy of Sciences President’s International Fellowship Initiative (PIFI) for Senior Scientists (Grant Number 2016VBA057 and 2013T2Z0009), and the CNR-CAS bilateral agreement 2017–2019 (Ozone impacts on plant ecosystems in China and Italy).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Electronic supplementary material
Table S1
(DOCX 24 kb)
Rights and permissions
About this article
Cite this article
Feng, Z., Jiang, L., Calatayud, V. et al. Intraspecific variation in sensitivity of winter wheat (Triticum aestivum L.) to ambient ozone in northern China as assessed by ethylenediurea (EDU). Environ Sci Pollut Res 25, 29208–29218 (2018). https://doi.org/10.1007/s11356-018-2782-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-2782-8