Abstract
With the growth in civilization and industrialization, there is a rise in the release of organic dyes into water systems, which is causing serious public concern. Although adsorption using biopolymer-based hydrogels has proven to be an ideal technique for treating these dye contaminants from aqueous solutions, these hydrogels suffer from a lack of mechanical stability and recoverability compared to synthetic polymers. Herein, we review the low-cost synthesis of hybrid hydrogel nanocomposites to improve the mechanical stability and separation of the hydrogel in removing dyes from an aqueous solution. The literature reports hydrogels and their nanocomposites as noble adsorbents well-known for addressing water pollution issues. In adsorption technology, hydrogel nanocomposites act as absorbents, prominent to improve the performance of removal efficiency. This current chapter pays particular attention to some recent breakthrough development in water remediation based on hydrogels as efficient adsorbents. In-depth discussions on adsorption and various methods for the synthesis of hydrogels have been devoted to applications of these nanocomposites and are compared in this contribution to the removal efficiency of organic dyes from wastewater.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
7.1 Introduction
Water is a vital resource for the survival of living things on earth [40]. Despite the need for this resource, water pollution continues to be a problem in most countries, including South Africa, where the mainstream water supplies are underground and surface water [72]. Water pollution may be defined as any water that is unsafe for drinking by humans and animals [72]. There are two classes of water contaminants, namely, point sources and non-point sources in which they are defined as a source of pollution at a fixed location (mines, industries, power stations, water treatment station, etc. and pollution from moving sources (cars, buses, and trains, respectively [79, 93]. In point source, water pollutants may be classified as either inorganic (fertilizers and toxic metals), organic (dyes), or microbial (viruses and bacteria) [12]. For example, dyes are organic complexes mostly used by textile industries to colour fabrics and contribute mainly to pollution [110]. Other applications may include medical, pharmaceutical, paper, rubber, plastics, leather, food, and cosmetics industries [56]. Dyes contain aromatic rings in their structure and can be either chromophores or auxochromes [23, 56]. Chromophores are responsible for the production of colour (OH, NH2, NHR, NR2, Cl, COOH), and auxochromes (NO2, NO, and N = N) improve chromophores, make molecules soluble in water and improve their affinity to bind materials [23]. The discharging of dye effluents into either surface and/or groundwater sources leads to contamination, resulting in various health and environmental problems [23, 104]. Consumption of contaminated water by humans can lead to vomiting, mutation, cancer, breathing, difficulties, diarrhoea, eyes burn, nausea, shock, cyanosis, jaundice, and tissue necrosis [23, 56, 61, 62, 104]. The environmental issues include the death of aquatic organisms, leading to the development of foul smell [5]. Hence, the need to eliminate dyes from waste effluents before discharging them into rivers and other water streams.
Due to the above-mentioned health and ecological problems, various techniques have been employed for eliminating dyes from wastewater [89]. However, because of the chemical stability and non-biodegradable nature of the dyes, most of these methods are less effective [56]. Additionally, each method has its major disadvantage, as shown in Table 7.1.
The adsorption technique is most favoured owing to its cheap synthesis and operation costs, easy design, and fast removal of dye [48, 66, 88]. Although the adsorption technique is effective for dye removal. Its efficiency is limited by the type of adsorbent used (Gόmez et al. 20,107). Various materials have been used for organic dyes removal, which includes; activated carbon, fly ash, graphene, clay, carbon nanotubes, and hydrogels [35, 39, 52, 63, 71, 99, 100, 106, 116]. Among these adsorbents, hydrogels are reported as promising adsorbents for organic dyes removal from aqueous solutions owing to their cheap synthesis, tunable properties, and high removal capacity. This chapter summarises the recent advances and developments of hydrogel adsorbents for wastewater treatment. This is realized by doing a detailed review of various hydrogel adsorbents and their modified systems with great emphasis on the structure and properties of the hybrid hydrogels.
7.2 Hydrogels
7.2.1 Background
Hydrogels are crosslinked gel structured polymers that swell in a liquid medium and can trap liquids for a long time without losing their structural integrity [39, 99]. Hydrogels’ swelling capacity and hydrophilicity arise from the presence of hydroxyl, sulfonyl, amide, imide, and carboxylic groups in their 3D backbone [92]. Hydrogels are produced from non-toxic and highly hydrophilic natural polymers called Polysaccharides. Polysaccharides are made by connecting glycosidic bonds between the smaller saccharide units [39, 99, 116]. Polysaccharides provide energy storage and structural support in animals and plants [100]. Examples of these polysaccharides include, alginate, guar gum, locust bean gum, starch, chitosan, carboxymethyl cellulose, carrageenan, and starch [100, 116].
7.2.1.1 Classification of Hydrogels
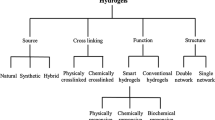
Hydrogels may well be categorized depending on various characteristics depending on their nature. Hydrogels are classified based on whether they are natural (produced from biological monomers), synthetic (made from artificial monomers), or a combination of natural and synthetic monomers forming a hybrid gel [62] (Table 7.2). The method used for the synthesis of a polymeric composite can also be used to classify hydrogels [62]. These include but are not limited to;
-
i.
Homopolymeric hydrogels: they are hydrogels comprising of a similar kind of monomer.
-
ii.
Copolymeric hydrogels: the polymeric gels contain two or more distinct varieties of monomers resulting in a hydrogel with at least one hydrophilic part.
-
iii.
Multipolymer Interpenetrating polymeric hydrogel (IPN): the polymer chain contains two independently cross-linked synthetic and/or natural polymer portions.
Classifications of hydrogels could also be categorized based on the following properties [33],
-
i.
Amorphous,
-
ii.
Crystalline or,
-
iii.
Semicrystallinity: displaying characteristics of both amorphous and crystalline phases.
Moreover, hydrogels can be classified based on whether they are chemically or physically crosslinked. Briefly;
-
i.
The crosslinking of the hydrogel can be achieved physically (1) between oppositely charged groups, (2) by establishing a hydrogen bond, (3) by subjecting gels to freeze and thaw procedure during crystallization using PVA/PVP solution at controlled conditions. Lastly, (4) through hydrophobic connections between polymer chains, the hydrogel strength is improved, and the dissipation energy prevents breakage of bonds. [23].
-
ii.
The crosslinking of hydrogels can be realized chemically through (1) aldehydes, namely; glutaraldehyde, formaldehyde, and acetaldehyde, (2) radiation through ultraviolet rays, gamma rays, an electron beam, or at ambient temperatures to form free radicals where monomers can be added to form or grow the hydrogel chain. Lastly, (3) using MBA crosslinker for free radical polymerization. In this method, an initiator (e.g., potassium persulfate) generates free radicals to interact with monomers in the presence of MBA crosslinks to form the hydrogel [16, 39].
Additionally, the classification of hydrogels could be established according to the charge of the hydrogel polymer chain [21, 55]. Whether the charge is,
-
i.
Non-ionic (neutral).
-
ii.
Ionic (anionic/ cationic)
-
iii.
Zwitterionic (each recurring structural unit has cationic and anionic parts). The overall charge of the polymer chain is zero.
-
iv.
Amphoteric (contains both acidic and basic groups).
Lastly, hydrogels can be categorized depending on whether a chemical or physical stimulus (Table 7.2) encourages their reaction. These hydrogels are sometimes called smart hydrogels [16, 62]. Sometimes environmental conditions can affect the swelling or de-swelling of hydrogels and therefore result in a volume collapse (phase change).
7.2.2 Synthesis of Hydrogels
For application in water remediation processes, the low stability and solubility of hydrogels are improved by developing hybrid hydrogel systems that consist of nanofillers and/or synthetic polymers [21]. Most researches have reported that crosslinking, grafting, and free radical polymerization. Therefore, the type of method used to prepare the hydrogel affects its structural makeup or physical properties. Below are brief descriptions of the methods as mentioned above.
7.2.2.1 Grafting
Grafting uses artificial polymers, namely; methacrylamide, acrylic acid, vinyl alcohol, and acrylamide, to improve the hydrogel backbone [55]. An initiator generates free radical sites to which monomer units are attached [94]. Grafting can initiate through either radiation or chemical stimulus. Chemical grafting makes use of chemicals such as potassium persulfate (KPS), ammonium persulfate (APS) as initiators [68]. Radiation grafting initiates free radicals through microwave or UV visible energy [20].
It was reported in the literature that using a microwave radiation method for synthesizing hydrogels produces sterilized hydrogels [20]. Naturally, polysaccharides have poor mechanical and chemical stability [21]. Grafting solves these problems and improves the biopolymers efficiency by establishing new functional groups from grafted monomers.
7.2.2.2 Crosslinking
Crosslinking process can occur through chemical or physical interaction. During chemical crosslinking, complementary groups within the polymer chain react to form irreversible covalent bonds [91]. In contrast, physical crosslinking occurs through reversible van der Waals forces, hydrogen bonding, and electrostatic reactions [14, 44]. Hydrogels that are crosslinked chemically are mainly applied in the medical field during tissue engineering, wound dressing, wastewater treatment, and drug delivery [57, 105]. Several chemical crosslinkers exist that may be used for hydrogel synthesis. For example, in a study by Mittal et al. hydrogels was produced using a mixture of ascorbic acid and KPS as a redox initiator and MBA as a cross linking agent in the microwave-assisted graft co-polymerization method (Scheme 7.1) [69]. According to the group, this using MBA as the cross linker to establish links between different polymeric chains. [69].
Mechanism for the graft co-polymerization of Gg with P(AAm-co-MAA). Reproduced with permission from [69].
Chemicals such as tri-propyleneglycol diacrylate (TPGDA) tetra-ethylene glycol dimethyl-acrylate (EGDMA), N, N-methylene-bis-acrylamide (MBA), and ethylene glycol dimethyl-acrylate are the most commonly used as crosslinkers in hydrogel synthesis [96]. However, these crosslinkers produce non-biodegradable hydrogels and are generally toxic [118]. Moreover, the resulting hydrogels are very brittle because of the lack of an effective strategy for energy dissipation and inner structural uniformity [108, 109]. As a solution, hybrid hydrogel systems comprising both physical and chemical interactions have been established by researchers to aid in energy dissipation and improve structural properties, respectively [19, 83]. For example, researchers constructed hybrid polymeric gels using poly(N-isopropyl acrylamide-co-itaconic acid) and non-poisonous octa-vinyl polyhedral oligomeric silsesquioxane (OV-POSS) crosslinkers. The analysis of the SEM and TGA (Fig. 7.1) results demonstrated that the surface of the non-hybridized NIPAM-co-IA (SEM image Fig. 7.1a) was not smooth. However, after crossing with various POSS amounts ((a) 8%, (b), 10%, and (c), 12%), the surface texture changed to a honeycomb-like structure with similar pores of different pore sizes as the POSS amount was raised. The group observed disruption of the honeycomb pattern at higher POSS content (12%), demonstrating that the level of homogeneity in the hybridized gel structure could be manipulated by varying the degree of crosslinking [27]. Their TGA thermogram (Fig. 7.1e) indicated that the thermal stability was enhanced through hybridization with POSS, in which the weight loss obtained for poly(NIPAM-co-IA) from 340 to 500 °C was higher compared to the weight loss achieved for the hybridized hydrogel at the same temperature [27].
SEM images of poly (NIPAM-co-IA) hydrogel (a) and hybrid poly (NIPAM-co-IA)/OV-POSS (8%, 10% and 12% POSS) (b–d), e TGA thermogram. Reproduced with permission from [27].
7.2.2.3 Free Radical-Polymerization
Free radical polymerization is when free radicals are generated to which monomers bind progressively, and the polymer chain grows [13]. This technique combines crosslinking and grafting methods. In this process, an initiator breaks down by light, photon, or temperature to form a free radical [18]. The advantages of generating free radicals by photon are (1) cost-efficiency, (2) no chemical solvent is required, and (3) it offers improved time-based and spatial control of the reaction procedure [46]. Scheme 7.2 illustrates the synthesis of hydrogels through the free radical polymerization process. Firstly, free radicals are produced through an initiator (initiation). Next, the monomer interacts with the free radicals to create unoccupied functional sites (propagation), and lastly, crosslinking occurs to form a hydrogel (termination) [65].
An overview of thermal free-radical polymerization and crosslinking. Reproduced with permission from [65].
The most commonly used technique in the polymer industry is free radical polymerization. This technique offers the advantage of easy operation, convenience, and the ability to design and prepare polymers for different uses. Impurities do not easily influence it. Free radical polymerization allows the achievement of in situ properties and well-characterized reaction kinetics [84].
7.2.3 Hybrid Hydrogels
It has been reported that hydrogel properties can be improved through generating hybrid hydrogel systems. Recently, many studies have incorporated nanofillers such as metal oxides [63], carbon-based materials [61, 62], and clay-based materials [65] into the hydrogel matrix during the polymerization process to produce a hydrogel nanocomposite with enhanced recovery and stability.
7.2.3.1 Modification of Hydrogels with Inorganic Materials
Depending on the intended application of the hydrogels, their physical properties can be enhanced through the incorporation of inorganic materials. For example, for use in removing contaminants from aqueous solutions, the hydrogel must be mechanically and thermally stable, especially for removing effluents from industries that utilize water for cooling reactions [22, 38]. Wherein the contaminants may be introduced at that point. Additionally, the hydrogels must be easy to recover.
7.2.3.2 Carbon-Based Hydrogels
Carbon-based hydrogel nanocomposites are hydrogels synthesized by incorporating nanofillers such as graphene oxide (GO), biochar, activated carbon, and carbon nanotubes (CNTs) [11, 64, 95]. GO is a carbon material prepared by oxidizing graphene through chemical or thermal reduction processes. It contains highly hydrophilic groups such as hydroxyl, carboxylic, and epoxy groups that are essential for adsorbing dyes and toxic metals [8]. GO may interact with contaminants through pi-to-pi interactions, electrostatic interactions, or hydrogen bonding [107]. Owing to its outstanding mechanical, electrical and thermal properties, GO has attracted its use in the biomedical, energy and environmental field [31]. An example of a study is by [99]. The group prepared starch hydrogel infused with reduced graphene oxide and reported a high removal capacity of 1106.960 µg g−1 for cationic dyes. The group also reported an improvement in the hydrogel pore size. In another study by [61, 62], xanthan gum-polyacrylic acid hydrogel was modified with reduced GO (XG-cl-pAA/rGO for the removal of MB and MV [28]. The group reported an impressive removal capacity of 1052.63 mg/g and 793.65 mg/g at 25 °C for MB and MV, respectively. Additionally, the XG-cl-pAA/rGO hydrogel nanocomposite was reported to have easy recovery and recyclable.
CNTs are simply graphene sheets rolled up in cylinders of 1 nm in diameter [29]. As a result of their porous structure, large surface area, high tensile strength (0.15 TPa) and elastic modulus (0.91 TPa), CNTs have attracted interest in use for adsorption of pollutants such as dyes, dichlorobenzene, ethylbenzene, and some heavy metals [24, 117]. CNTs can be categorized into two forms, namely, single-walled CNTs (SWCNTs, which are made up of single layers of graphene sheets and multiwalled CNTs (MWCNTs), which are made up of multiple layers of concentric cylinders [4, 29]. Among recent studies that have used carbon-based materials to modify the properties of hydrogels, [62], reported that the incorporation of MWCNT’s onto XG/PAA hydrogel improved the surface hydrophilicity and specific area of xanthan gum. The literature reports that although the incorporation of CNTs in gels improves the mechanical properties, the rate of degradation decreases, which is because carbon-based materials have high hydrothermal stability, which makes them resistant to harsh environments [60]. Another widely used carbon-based adsorbent and nanofiller are activated carbon/activated charcoal (AC). This material is reported to improve the surface properties, adsorption capacity, and porosity of adsorbent materials. A study by [45] reported a removal capacity of 60.9 mg/g for TH dye from wastewater using chitosan (CS) hydrogel modified with activated charcoal (CS/AC). Various carbon-based nanocomposite hydrogels and their adsorption properties for the removal of organic dye contaminants from aqueous solutions are listed in Table 7.3.
7.2.3.3 Clay-Based Hydrogels
Natural clays and their improved forms have recently been used for removing pollutants from water [15]. The most commonly used clays, especially for treating toxic metals and dyes, are modified kaolinite and montmorillonite [36]. However, these clays are difficult to regenerate and reuse because of their colloidal dimensions [113]. This prompted the functionalization of these clays—for example, the modification of montmorillonite (MMT) to form Cloisite 30B. In a study where C30B clay was incorporated onto polypropylene (PP) grafted with maleic anhydride (PP-g-MA) and thermoplastic starch (TPS), it was reported that biodegradation studies performed in compost revealed that the presence of C30B improved the matrix biodegradability [1]. Modification of adsorbents by introducing functionalized clay components may improve both the physical and chemical properties of adsorbents [1, 113]. Other modifications with clay results improve the number of adsorbing active sites, enhanced porosity, and low levels of mineral impurities [1]. In a recent study, C30B was mixed with a culture obtained from an anaerobic sludge to remove hexavalent chromium, where the removal capacity of nearly 100% was obtained [58]. In our recent study, we reported the incorporation of C30B into magnetic carboxymethyl cellulose/poly(acrylic acid) to synthesize the hydrogel nanocomposite [65]. Our findings reported an increased crosslinking density and easy dispersion of magnetite (Fe3O4) nanoparticles into the hydrogel matrix due to the presence of the C30B component in the hydrogel. Another study by [81], prepared cellulose-MMT hydrogels for removing MB. The hydrogels had a maximum removal capacity of 1065 mg/g (Table 7.4). Their viscoelasticity studies from the sweep measurements (Fig. 7.2a) showed that when the storage modulus (G’) < loss modulus (G”), the cellulose-MMT systems were in a viscous liquid form, however with increasing time when G’ > G”, the materials were in a gel form. Figure 7.2b in this case revealed that increasing clay content decreased the gel formation. Figure 7.2c revealed that the storage moduli (G’) of hydrogels containing clay was higher than that of unmodified hydrogel and was proportional to the clay content from 10 to 15 wt.%. However, the incorporation of clay increased the gel strength from 0.3 kPa to 4.7 kPa, as shown in Fig. 7.2d and e, which was approximately 16 times higher. Furthermore, the study reported that an increase in clay content from 10 to 15 wt.% reduced the swelling capacity of the hydrogel (Fig. 7.2f), reportedly due to a high degree of crosslinking. Furthermore, it was reported that the addition of modified clay containing 2,3-epoxypropyltrimethylammonium chloride increased the crosslinking density of the hydrogel networks, eventually resulting in enhanced storage modulus [81].
a Time dependence of storage modulus (G′) and loss modulus (G″), b Gelation time c G′ at 60 min d Compressive-strain curves e Photographs of M-5 f The swelling ratio performed using distilled water. Reproduced with permission from [81].
7.2.3.4 Metal Oxide-Based Hydrogels
Metal oxide-based nanoparticles have been reported to have a high density and restricted size, which are responsible for their fascinating and unique chemical and physical properties [7, 80]. Examples of metal oxides include titanium dioxide (TiO2), iron oxide (Fe3O4), magnesium oxide (MgO), aluminium oxide (Al2O3). Owing to their nontoxic nature, high surface area, high chemical stability, and economical friendliness, Fe3O4 nanoparticles are widely used for the removal of toxic metals and organic pollutants from water [7, 80, 85, 97, 119]. Ion-oxide and zinc oxide nanoparticles are some of the most frequently used metal oxides in treating dyes from aqueous solutions [67].
Amongst the most promising metal-oxides are magnetite (Fe3O4) nanoparticles. Magnetite nanoparticles also called black iron oxide amongst other transition metals, have the strongest magnetism and are stable at ambient temperatures [59]. Magnetite is prepared from the coprecipitation of iron oxide salts that result in an inverse spinel crystal structure consisting of half of the Fe3+ in tetrahedral coordination and the other half Fe2+ ions in octahedral coordination (Fig. 7.3a) [32, 59, 86]. The coprecipitation method is the most useful and suitable technique in preparing magnetite at both lab-scale and industrial scales [53]. Due to their cheap synthesis costs, susceptibility, stability, high porosity, magnetic properties, biocompatibility, and easy chemical modification, MNP’s have attracted much use in the wastewater treatment field [32, 59, 67, 85, 86]). The introduction of MNPs into polymer structures to produce nanocomposite hydrogels leads to a hybrid hydrogel with advantages of both components, thus improved chemical and physical properties. An example of such hybridized hydrogel is Fe3O4-g-pAA hydrogel synthesized via radical polymerization in the presence of MBA crosslinker, which resulted in a highly adsorptive hydrogel of 507.7 mg/g removal capacity for MB [82], as shown in Table 7.5. However, according to Flory’s theory, the swelling degree of a hydrogel depends on the density of crosslinking, the ionic osmotic pressure, and the attraction of the gel for the liquid [50].
When the crosslinking density is high, the space between the polymer chains decreases, making the gel to be stiff due to tiny nonexpendable pores [50]. Additionally, an increase in the amount of MNPs increases the thermal properties but consequently reduces the swelling capacity as the Fe3+ from the incorporated MNPs may act as a physical crosslinking agent [82, 87]. Additionally, in a study by [65] in the removal of MB using CMC-cl-pAA/Fe3O4-C30B, the group reported a decreased removal capacity with improved stability for modified HNC compared to non-modified CMC-cl-pAA hydrogel as a consequence of incorporating metal oxide nanoparticles. The most significant advantage of incorporation MNPs into the hydrogel matrix is the improved stability and easy recovery of the material after application [10]. Another prevalent metal oxide is zinc oxide (ZnO) which can be found in nature as a zincite mineral; however, the majority of it is prepared synthetically. Its crystal structure can be found in a hexagonal wurtzite form of cubic zinc blend form (Fig. 7.3b). The form that is most stable and commonly found at ambient temperature is the wurtzite structure Fig. 7.3b [98]. ZnO is commonly used for treating skin-related problems such as nappy rash, dandruff, and incorporation in ointments used in wound dressing [98]. Other applications of ZnO include the use in catalysis, batteries, sensors, and adsorption of contaminants [3, 30, 73, 112]. Their application as adsorbent material was reported in a study by [49], where a guar gum adsorbent hydrogel incorporated with ZnO nanoparticles was used for removing chromium (VI) from water. The group reported that incorporating ZnO nanoparticles improved the recovery of the adsorbent from the aqueous solution after the removal of Cr (VI) [49]. In another study, CMC hydrogel was modified with ZnO for antimicrobial activity, which was influenced by their inexpensiveness and lack of colour [54].
Another widely used metal-oxide is TiO2, a semiconductor [25, 75]. There exist three forms of TiO2, namely, anatase, rutile, and brookite. Amongst the three forms, anatase has been reported to be more photoreactive thus its wide use in photocatalytic degradation during water treatment studies [34]. This is because TiO2 as a photocatalyst is chemically stable, non-toxic, cost-effective, and remains stable during irradiation [9, 47, 74]. Various techniques have been used to synthesize titanium dioxide, namely, low-temperature dissolution-reprecipitation, gas-phase pyrolysis, ultrasonic spray pyrolysis, sol–gel, ultrasonic spray pyrolysis, and combustion synthesis [76]. TiO2-based nanomaterials have been used in fields such as drug delivery, medical research, self-cleaning, producing antibacterial materials, and energy storage [51, 9]. As nanoparticles for water treatment, TiO2 offers a high surface area, which is very important in the sorption of contaminants from aqueous solutions [37]. A study by [102] reported that an increase in the TiO2 content from 0.05 g to 0.2 g in SA-cl-poly(AA)-TiO2 O/I hydrogel nanocomposite increased anionic centres and the intrinsic charge repulsion within the hydrogel matrix, consequently enhancing the removal capacity for MB.
7.3 Conclusions
In conclusion, this chapter discusses recent studies applied for removing different types of dyes from an aqueous solution using biopolymer-based HNCs. Owing to various advantages such as low cost and easy design, the adsorption method is recognized as the most promising treatment technique for the removal of dyes. This work briefly discussed hydrogels, their classification, methods of synthesis and modifications with inorganic components to improve on their drawbacks. This contribution emphasized the importance of incorporating carbon compounds, clay content, and metal oxide nanoparticles in hydrogels for the removal of dyes. Additionally, various modified hydrogel nanocomposites are summarised in table form for easy study and comparisons depending on which content was used to modify them.
References
A.S. Abreu, M. Oliveira, A.V. Machado, Effect of clay mineral addition on properties of bio-based polymer blends. Appl. Clay Sci. 104, 277–285 (2015)
K.A. Adegoke, O.S. Bello, Dye sequestration using agricultural wastes as adsorbents. Water Resour. Ind. 12, 8–24 (2015)
M. Ahmad, Y. Shi, A. Nisar, H. Sun, W. Shen, M. Wei, J. Zhu, Synthesis of hierarchical flower-ZnO nanostructures and their functionalization by Au nanoparticles for improved photocatalytic and high-performance Li-ion battery anodes. J. Meter. Chem. 21, 7723–7729 (2011)
A. Aqel, K.M. Abou El-Nour, R.A. Ammar, A. Al-Warthan, Carbon nanotubes, science and technology part (I) structure, synthesis and characterisation. Arab. J. Chem. 5(1), 1–23 (2012)
M. Arami, N.Y. Limaee, N.M. Mahmoodi, N.S. Tabrizi, Equilibrium and kinetics studies for the adsorption of direct and acid dyes from aqueous solution by soy meal hull. J. Hazard. Mater. 135(1–3), 171–179 (2006)
S. Asadi, S. Eris, S. Azizian, alginate-based hydrogel beads as a biocompatible and efficient adsorbent for dye removal from aqueous solutions. ACS Omega 3(11), 15140–15148 (2018)
A. Atta, M.A. Akl, A.M. Youssef, M.A. Ibraheim, Superparamagnetic core-shell polymeric nanocomposites for efficient removal of methylene blue from aqueous solutions. Adsorp. Sci. Technol. 31(5), 397–419 (2013)
H. Bai, C. Li, G. Shi, A pH-sensitive graphene oxide composite hydrogel. Commun. Chem. 46, 2376–2378 (2010)
P.S. Basavarajappa, S.B. Patil, N. Ganganagappa, K.R. Reddy, A.V. Raghu, C.V. Reddy, Recent progress in metal-doped TiO2, non-metal doped/codoped TiO2 and TiO2 nanostructured hybrids for enhanced photocatalysis. Int. J. Hydrog. Energy. 45(13), 7764–7778 (2020)
A. Bée, L. Obeid, R. Mbolantenaina, M. Welschbillig, D. Talbot, Magnetic chitosan/clay beads: a magsorbent for the removal of cationic dye from water. J. Magn. Magn. 421, 59–64 (2017)
S. Biswas, T.K. Sen, A.M. Yeneneh, B.C. Meikap, Synthesis and characterization of a novel Ca-alginate-biochar composite as efficient zinc (Zn2+) adsorbent: Thermodynamics, process design, mass transfer and isotherm modelling. Sep. Sci. Technol. 54(7), 1106–1124 (2019)
M. Bodzek, Membrane technologies for the removal of micropollutants in water treatment, in Advances in Membrane Technologies for Water Treatment. Woodhead Publishing (2015), pp. 465–517
Braun D (2009) Origins and development of initiation of free radical polymerization processes. Int J Polym Sci. 2009: 893234.
S.K. Burley, G.A. Petsko, Weakly polar interactions in proteins. Adv. Protein Chem. 39, 125–189 (1988)
F. Cadena, R. Rizvi, R.W. Peters, Feasibility studies for the removal of heavy metal from solution using tailored bentonite (Hazard. Ind. Waste), in Proceedings of the 22nd Mid-Atlantic Industrial Waste Conference (1990), pp. 77–94
N. Çankaya, Synthesis of graft copolymers onto starch and its semiconducting properties. Results Phys. 6, 538–542 (2016)
S. Chatterjee, M.W. Lee, S.H. Woo, Adsorption of Congo red by chitosan hydrogel beads impregnated with carbon nanotubes. Bioresour. Technol. 101(6), 1800–1806 (2010)
M. Chen, M. Zhong, J.A. Johnson, Light-controlled radical polymerization: mechanisms, methods, and applications. Chem. Rev. 116, 10167–11021 (2016)
Q. Chen, L. Zhu, L. Huang, H. Chen, K. Xu, Y. Tan, P. Wang, J. Zheng, Fracture of the physically crosslinked first network in hybrid double network hydrogels. Macromolecules 47(6), 2140–2148 (2014)
J.P. Cook, G.W. Goodall, O.V. Khutoryanskaya, V.V. Khutoryanskiy, Microwave-assisted hydrogel synthesis: a new method for crosslinking polymers in aqueous solutions. Macromol. Rapid Commun. 33(4), 332–336 (2012)
T. Coradin, K. Wang, T. Law, L. Trichet, Type I collagen-fibrin mixed hydrogels: preparation, properties and biomedical applications. Gels 6, 36 (2020)
C. Dannert, B.T. Stokke, R.S. Dias, Nanoparticle-hydrogel composites: from molecular interactions to macroscopic behavior. Polymers 11(2), 275 (2019)
S. Dawood, T.K. Sen, Review on dye removal from its aqueous solution into alternative cost effective and non-conventional adsorbents. J. Chem. Process. Eng. 1(1), 1–11 (2014)
B.G. Demczyk, Y.M. Wang, J. Cumings, M. Hetman, W. Han, A. Zettl, R.O. Ritchie, Direct mechanical measurement of the tensile strength and elastic modulus of multiwalled carbon nanotubes. Mater. Sci. Eng. 334, 173–178 (2002)
M.I. Din, R. Khalid, Z. Hussain, Recent research on development and modification of nontoxic semiconductor for environmental application. Sep. Purif. Rev. 1–18 (2020)
O. Duman, T.G. Polat, C.Ö. Diker, S. Tunç, Agar/κ-carrageenan composite hydrogel adsorbent for the removal of methylene blue from water. Int. J. Biol. Macromol. 160, 823–835 (2020)
B. Eftekhari-sis, V. Rahimkhoei, A. Akbari, H.Y. Araghi, Cubic polyhedral oligomeric silsesquioxane nano-cross-linked hybrid hydrogels: Synthesis, characterization, swelling and dye adsorption properties. React. Funct. Polym. 128, 47–57 (2018)
E.A. Kamoun, X. Chen, M.S.M. Eldin, E.R.S. Kenawy, Crosslinked poly(vinyl alcohol) hydrogels for wound dressing applications: a review of remarkably blended polymers. Arab. J. Chem. 8(1), 1–14 (2015)
A.M.A. Elhissi, W. Ahmed, I.U. Hassan, V.R. Dhanak, A.D. Emanuele, Carbon nanotubes in cancer therapy and drug delivery carbon nanotubes in cancer therapy and drug delivery. J. Drug Deliv. (2012)
M. Farrokhi, S.C. Hosseini, J.K. Yang, M. Shirzad-Siboni, Application of ZnO–Fe3O4 nanocomposite on the removal of azo dye from aqueous solutions: kinetics and equilibrium studies. Water Air Soil Pollut. 225, 1–12 (2014)
S. Fazil, M. Bangesh, W. Rehman, K. Liaqat, S. Saeed, M. Sajid, I. Bibi, Mechanical, thermal, and dielectric properties of functionalized graphene oxide/polyimide nanocomposite films anomater. Nanotechnology 9, 1–8 (2019)
R.A. Frimpong, J.Z. Hilt, Poly (n-isopropylacrylamide)-based hydrogel coatings on magnetite nanoparticles via atom transfer radical polymerization. Nanotechnology. 19(17), 175101 (2008)
S. Garg, A. Garg, Hydrogel: Classification, properties, preparation and technical features. Asian J. Biomater. Res. 2(6), 163–170 (2016)
S. Glass, B. Trinklein, B. Abel, A. Schulze, TiO2 as photosensitizer and photoinitiator for synthesis of photoactive TiO2-PEGDA hydrogel without organic photoinitiator. Front. Chem. 6, 340 (2018)
V. Gómez, M.S. Larrechi, M.P. Callao, Kinetic and adsorption study of acid dye removal using activated carbon. Chemosphere 69, 1151–1158 (2007)
K. Gopal, S. Sen, Adsorption of a few heavy metals on natural and modified kaolinite and montmorillonite: A review. Adv. Colloid Interface Sci. 140, 114–131 (2008)
I.S. Grover, S. Singh, B. Pal, The preparation, surface structure, zeta potential, surface charge density and photocatalytic activity of TiO2 nanostructures of different shapes. Appl. Surf. Sci. 280, 366–372 (2013)
J. Guanghui, L. Wang, H. Yu, W.A. Amer, L. Zhang, Recent progress on study of hybrid hydrogels for water treatment. Colloids Surf. A: Physicochem. Eng. Asp. 416, 86–94 (2013)
M.R. Guilherme, F.A. Aouada, A.R. Fajardo, A.F. Martins, A.T. Paulino, M.F.T. Davi, A.F. Rubira, E.C. Muniz, Superabsorbent hydrogels based on polysaccharides for application in agriculture as soil conditioner and nutrient carrier: a review. Eur. Polym. J. 72, 365–385 (2015)
V.K. Gupta, I. Ali, T.A. Saleh, A. Nayak, S. Agarwal, Chemical treatment technologies for wastewater recycling—an overview. Rsc Adv. 2(16), 6380–6388 (2012)
H. Hosseini, M. Mashaykhi, New chitosan/silica/zinc oxide nanocomposite as adsorbent for dye removal. Int. J. Biol. Macromol. 131, 520–526 (2019)
H. Hosseinzadeh, N. Khoshnood, Removal of cationic dyes by poly (AA-co-AMPS)/montmorillonite nanocomposite hydrogel. Desalin. Water Treat. 57(14), 6372–6383 (2016)
H. Hosseinzadeh, Synthesis of carrageenan/multiwalled carbon nanotube hybrid hydrogel nanocomposite for adsorption of crystal violet from aqueous solution. Pol. J. Chem. Technol. 17(2), 70–76 (2015)
H. Ismail, M. Irani, Z. Ahmad, Starch-based hydrogels: present status and applications. Int J Polym Mater. 62(7), 411–420 (2013)
A.H. Jawad, A. Abdulhameed, M.S. Mastuli, Mesoporous crosslinked chitosan-activated charcoal composite for the removal of thionine cationic dye: Comprehensive adsorption and mechanism study. J. Polym. Environ. 28, 1095–1105 (2020)
E.A. Kamoun, A.M. Omer, S.N. Khattab, H.M. Ahmed, A.A. Elbardan, In-situ UV-photopolymerized PVA-g-GMA hydrogels for biomedical applications: I. Synthesis, characterizations and grafting optimization. J. Appl. Pharm. Sci. 8(1), 034–042 (2018)
W. Kangwansupamonkon, N. Klaikaew, S. Kiatkamjornwong, Green synthesis of titanium dioxide/acrylamide-based hydrogel composite, self -degradation and environmental applications. Eur. Polym. J. 107, 118–131 (2018)
V. Katheresan, J. Kansedo, S.Y. Lau, Efficiency of various recent wastewater dye removal methods: a review. J. Environ. Chem. Eng. 6(4), 4676–4697 (2018)
T.A. Khan, M. Nazir, I. Ali, A. Kumar, Removal of Chromium (VI) from aqueous solution using guar gum–nano zinc oxide biocomposite adsorbent. Arab. J. Chem. 10(2), S2388–S2398 (2017)
M.C. Koetting, J.T. Peters, S.D. Steichen, N.A. Peppas, Stimulus-responsive hydrogels: Theory, modern advances, and applications. Mater. Sci. Eng. R Rep. 93, 1–49 (2016)
T. Kumar, A. Thakur, A. Alexander, H. Badwaik, D.K. Tripathi, Modified chitosan hydrogels as drug delivery and tissue engineering systems: present status and applications. Acta Pharm. Sin. B. 2(5), 439–449 (2012)
L. Largitte, R. Pasquier, A review of the kinetics adsorption models and their application to the adsorption of lead by an activated carbon. Chem. Eng. Res. Des. 109, 495–504 (2016)
D.L. Leslie-pelecky, R.D. Rieke, Magnetic properties of nanostructured materials. Chem. Mater. 8(8), 1770–1783 (1996)
J. Li, L. Fang, W.R. Tait, L. Sun, Preparation of conductive composite hydrogels from carboxymethyl cellulose and polyaniline with a nontoxic crosslinking agent. RSC Adv. 7(86), 54823–54828 (2017)
Y. Liang, X. Zhao, P.X. Ma, B. Guo, Y. Du, X. Han, pH-responsive injectable hydrogels with mucosal adhesiveness based on chitosan-grafted-dihydrocaffeic acid and oxidized pullulan for localized drug delivery. J. Colloid Interface Sci. 536, 224–234 (2019)
C. Liu, A.M. Omer, X. Ouyang, Adsorptive removal of cationic methylene blue dye using carboxymethyl cellulose/k-carrageenan/activated montmorillonite composite beads: Isotherm and kinetic studies. Int. J. Biol. Macromol. 106, 823–833 (2018)
L. Liu, Q. Gao, X. Lu, H. Zhou, In situ forming hydrogels based on chitosan for drug delivery and tissue regeneration. Asian J. Pharm. Sci. 11(6), 673–683 (2016)
G. Lytras, C. Lytras, D. Argyropoulou, N. Dimopoulos, G. Malavetas, A novel two-phase bioreactor for microbial hexavalent chromium removal from wastewater. J. Hazard. Mater. 336, 41–51 (2017)
P. Majewski, B. Thierry, Functionalized magnetite nanoparticles—Synthesis, properties, and bio-applications. Crit. Rev. Solid State Mater. Sci. 32(3–4), 203–215 (2007)
E. Makhado, M.J. Hato, Preparation and characterization of sodium alginate-based oxidized multi-walled carbon nanotubes hydrogel nanocomposite and its adsorption behaviour for methylene blue dye. Front Chem, 9, 576913 (2021)
E. Makhado, S. Pandey, J. Ramontja, Microwave-assisted synthesis of xanthan gum-cl-poly (acrylic acid) based-reduced graphene oxide hydrogel composite for adsorption of methylene blue and methyl violet from aqueous solution. Int. J. Biol. Macromol. 119, 255–269 (2018)
E. Makhado, S. Pandey, P.N. Nomngongo, J. Ramontja, Preparation and characterization of xanthan gum-cl-poly (acrylic acid)/ o-MWCNTs hydrogel nanocomposite as highly effective re-usable adsorbent for removal of methylene blue from aqueous solutions. J. Colloid Interface Sci. 513, 700–714 (2018)
E. Makhado, S. Pandey, K.D. Modibane, M. Kang, M.J. Hato, Sequestration of methylene blue dye using sodium alginate poly (acrylic acid)@ ZnO hydrogel nanocomposite: kinetic, isotherm, and thermodynamic investigations. Int. J. Biol. Macromol. 162, 60–73 (2020)
E. Makhado, S. Pandey, P.N. Nomngongo, J. Ramontja, ‘Xanthan gum-cl-poly (acrylic acid)/reduced graphene oxide hydrogel nanocomposite as adsorbent for dye removal’, in International Conference on Advances in Science, Engineering and Waste Management (Asetwm-171 ) (2017), pp.59–164
N. Malatji, E. Makhado, K.E. Ramohlola, K.D. Modibane, T.C. Maponya, G.R. Monama, M.J. Hato, Synthesis and characterisation of magnetic clay-based carboxymethyl cellulose-acrylic acid hydrogel nanocomposite for methylene blue dye removal from aqueous solution. Environ. Sci. Pollut. Res. 27(35), 44089–44105 (2020)
N.M. Malatji, E, Modibane KD, Ramohlola KE, Maponya TC, Monama GR, Hato MJ, Removal of methylene blue from wastewater using hydrogel nanocomposites: a review. Nanomater. Nanotechnol. 11, 18479804211039424 (2021)
H. Markides, M. Rotherham, A.J. El Haj, Biocompatibility and toxicity of magnetic nanoparticles in regenerative medicine. J. Nanomater. 13, 13 (2012)
S. Mishra, G.U. Rani, G. Sen, Microwave initiated synthesis and application of polyacrylic acid grafted carboxymethyl cellulose. Carbohydr. Polym. 87, 2255–2262 (2012)
H. Mittal, R. Jindal, B. Kaith, A. Maity, S.S. Ray, Flocculation and adsorption properties of biodegradable gum-ghatti-grafted poly (acrylamide-co-methacrylic acid) hydrogels. Carbohydr. Polym. 115, 617–628 (2015)
H. Mittal, S.S. Ray, A study on the adsorption of methylene blue onto gum ghatti/TiO2 nanoparticles-based hydrogel nanocomposite. Int. J. Biol. Macromol. 88, 66–80 (2016)
A.Y. Moreno-lópez, M.E. González-lópez, R. Manríquez-gonzález, J.R. Robledo-ortíz, Evaluation of the Cr (VI) adsorption performance of xanthate polysaccharides supported onto agave fiber-LDPE foamed composites. Water Air Soil Pollut. 230(6), 1–21 (2019)
M. Muller, Understanding the origins of Cape Town’s water crisis, (June 2017) (2018)
M.Y. Nassar, A.A. Ali, A.S. Amin, A facile Pechini sol–el synthesis of TiO2/Zn2TiO2/ZnO/C nanocomposite: an efficient catalyst for the photocatalytic degradation of orange G textile dye. RSC Adv. 7(48), 30411–30421 (2017)
R. Nekooie, T. Shamspur, A. Mostafavi, Novel CuO/TiO2/PANI nanocomposite: preparation and photocatalytic investigation for chlorpyrifos degradation in water under visible light irradiation. J. Photochem. Photobiol. 407, 113038 (2020)
M.N. Ngoepe, M.J. Hato, K.D. Modibane, N.C. Hintsho-Mbita, Biogenic Synthesis of Metal Oxide Nanoparticle Semiconductors for Wastewater Treatment (Scrivener Publishing LLC, 2020), pp. 1–31
P. Nyamukamba, O. Okoh, L. Tichagwa, C. Greyling, Preparation of titanium dioxide nanoparticles immobilized on polyacrylonitrile nanofibres for the photodegradation of methyl orange. Int. J. Photoenergy. 2, 1–9 (2016)
G. Ohemeng-Boahen, D.D. Sewu, S.H. Woo, Preparation and characterization of alginate-kelp biochar composite hydrogel bead for dye removal. Environ. Sci. Pollut. Res. 26(32), 33030–33042 (2019)
P.M. Pakdel, S.J. Peighambardoust, A review on acrylic based hydrogels and their applications in wastewater treatment. J. Environ. Manag. 217, 123–143 (2018)
Y. Park, G.A. Ayoko, R.L. Frost, Application of organoclays for the adsorption of recalcitrant organic molecules from aqueous media. J. Colloid Interface Sci. 354(1), 292–305 (2011)
D. Pasqui, A. Atrei, G. Giani, M. De Cagna, R. Barbucci, Metal oxide nanoparticles as cross-linkers in polymeric hybrid hydrogels. Mater. Lett. 65(2), 392–395 (2011)
N. Peng, D. Hu, J. Zeng, Y. Li, L. Liang, C. Chang, Superabsorbent Cellulose−clay nanocomposite hydrogels for highly efficient removal of dye in water. ACS Sustain. Chem. Eng. 4, 7217–7224 (2016)
M. Pooresmaeil, Y. Mansoori, M. Mirzaeinejad, A.L.I. Khodayari, Efficient removal of methylene blue by novel magnetic hydrogel nanocomposites of poly (acrylic acid). Adv. Polym. Technol. 37(1), 262–274 (2015)
Z. Rahmani, R. Sahraei, M. Ghaemy, Preparation of spherical porous hydrogel beads based on ion-crosslinked gum tragacanth and graphene oxide: study of drug delivery behavior. Adv. Polym. Technol. 194, 34–42 (2018)
N. Ranganathan, J.R. Bensingh, A.M. Kader, S.K. Nayak, Synthesis and Properties of Hydrogels Prepared by Various Polymerization Reaction Systems (Springer International Publishing, Cham, Switzerland, 2018), pp. 1–25
K.G. Rao, C.H. Ashok, K.V. Rao, C.H.S. Chakra, Structural properties of MgO nanoparticles: synthesized by coprecipitation technique. Int. J. Sci. Res. 8–9 (2014)
D.A. Rivani, I. Retnosari, T.E. Saraswati, Influence of TiO2 addition on the magnetic properties of carbon-based iron oxide nanocomposites synthesized using submerged arc-discharge. IOP Conf. Ser.: Mater. Sci. Eng. 509, 012034 (2019)
C. Saikia, A. Hussain, A. Ramteke, H.K. Sharma, T.K. Maji, Crosslinked thiolated starch coated Fe3O4 magnetic nanoparticles: effect of montmorillonite and crosslinking density on drug delivery properties. Starke 66(7–8), 760–771 (2014)
M.A.M. Salleh, D.K. Mahmoud, W.A. Karim, A. Idris, Cationic and anionic dye adsorption by agricultural solid wastes: a comprehensive review. Desalination 280(1–3), 1–13 (2011)
A. Science, Adsorption of cationic dyes on activated carbon obtained from waste Elaeagnus stone. Adsorp. Sci. Technol. 34(9–10), 512–525 (2016)
F. Shakib, A.D. Koohi, A.K. Pirzaman, Adsorption of methylene blue by using novel chitosan-g-itaconic acid/bentonite nanocomposite – equilibrium and kinetic study. Water Sci. Technol. 75(8), 1932–1943 (2017)
C. Shen, Y. Shen, Y. Wen, H. Wang, W. Liu, Fast and highly efficient removal of dyes under alkaline conditions using magnetic chitosan-Fe(III) hydrogel. Water Res. 45(16), 5200–5210 (2011)
S.R. Shirsath, A.P. Patil, R. Patil, J.B. Naik, P.R. Gogate, S.H. Sonawane, Removal of brilliant green from wastewater using conventional and ultrasonically prepared poly (acrylic acid hydrogel loaded with kaolin clay: a comparative study. Ultrason Sonochem. 20(3), 914–923 (2014)
R.A. Shmeis, water chemistry and microbiology. Compr. Anal. Chem. 81, 1–56 (2018)
V. Singh, P. Kumar, R. Sanghi, Progress in polymer science use of microwave irradiation in the grafting modification of the polysaccharides – A review. Prog. Polym. Sci. 37(2), 340–364 (2012)
S.C. Smith, D.F. Rodrigues, Carbon-based nanomaterials for removal of chemical and biological contaminants from water: a review of mechanisms and applications. Carbon 91, 122–143 (2015)
L. Song, M. Zhu, Y. Chen, K. Haraguchi, Temperature-and pH-sensitive nanocomposite gels with semi-interpenetrating organic/inorganic networks. Macromol Chem Phys. 209(15), 1564–1575 (2008)
S. Stankic, S. Suman, F. Haque, J. Vidic, Pure and multi-metal oxide nanoparticles: synthesis, antibacterial and cytotoxic properties. J. Nanobiotechnology. 14(1), 1–20 (2016)
I. Stefaniuk, B. Cieniek, I. Rogalska, I.S. Virt, A. Kosciak, Magnetic properties of ZnO: Co layers obtained by pulsed laser deposition method. Mater. Sci.-Pol. 36(3), 439–444 (2017)
A.A. Subhi, V.M. Kiamahalleh, M. Firouzi, F. Yousefi, H.H. Kyaw, A.M. Abri, A. Firouzi, V.M. Kiamahalleh, Self-assembled graphene hydrogel composites for selective dye removal. Adv. Sustain. Syst. 4(9), 2000055 (2020)
M. Tally, Y. Atassi, Optimized synthesis and swelling properties of a pH-sensitive semi-IPN superabsorbent polymer-based on sodium alginate-g-poly (acrylic acid-co-acrylamide) and polyvinylpyrrolidone and obtained via microwave irradiation. J. Polym. Res. 22(9), 181 (2015)
S. Thakur, O. Arotiba, Synthesis, characterization and adsorption studies of an acrylic acid-grafted sodium alginate-based TiO2 hydrogel nanocomposite. Adsorp. Sci. Technol. 36(1–2), 458–477 (2018)
S. Thakur, S. Pandey, O.A. Arotiba, Development of a sodium alginate-based organic/inorganic superabsorbent composite hydrogel for adsorption of methylene blue. Carbohydr. Polym. 153, 34–46 (2016)
S. Vahidhabanu, D. Karuppasamy, I. Adeogun, Impregnation of zinc oxide modified clay over alginate beads: a novel material for the effective removal of Congo red from wastewater. RSC Adv. 7(10), 5669–5678 (2017)
K. Varaprasad, T. Jayaramudu, E. Rotimi, Removal of dye by carboxymethyl cellulose, acrylamide and graphene oxide via a free radical polymerization process. Carbohydr. Polym. 164, 186–194 (2017)
C. Vasile, D. Pamfil, E. Stoleru, M. Baican, New developments in medical applications of hybrid hydrogels containing natural polymers. Molecules 25(7), 1539 (2020)
S. Wang, Y. Boyjoo, A. Choueib, A comparative study of dye removal using fly ash treated by different methods. Chemosphere 60(10), 1401–1407 (2005)
X. Wang, X. Liu, H. Yuan, H. Liu, C. Liu, T. Li, Z. Guo, Non-covalently functionalized graphene strengthened poly (vinyl alcohol). Mater. Des. 139, 372–379 (2018)
H. Xu, F.K. Shi, X.Y. Liu, M. Zhong, X.M. Xie, How can multi-bond network hydrogels dissipate energy more effectively: an investigation on the relationship between network structure and properties. Soft Matter 16(18), 4407–4413 (2020)
J. Xu, X. Liu, X. Ren, G. Gao, The role of chemical and physical crosslinking in different deformation stages of hybrid hydrogels. Eur. Polym. J. 100, 86–95 (2018)
M.T. Yagub, T.K. Sen, S. Afroze, H.M. Ang, Dye and its removal from aqueous solution by adsorption: A review. Adv. Colloid Interface Sci. 209, 172–184 (2014)
J.Z. Yi, L.M. Zhang, Removal of methylene blue dye from aqueous solution by adsorption onto sodium humate/polyacrylamide/clay hybrid hydrogels. Bioresour. Technol. 99(7), 2182–2186 (2008)
S.J. Young, W.L. Tang, Wireless zinc oxide based pH sensor system. J. Electrochem. Soc. 166(9), B3047 (2019)
G. Yuan, B.K.G. Theng, P. North, N. Zealand, J. Churchman, W.P. Gates, Clays and clay minerals for pollution control. Dev. Clay Sci. 5, 587–644 (2013)
M. Zhang, L. Chang, Y. Zhao, Z. Yu, Fabrication of zinc oxide / polypyrrole nanocomposites for brilliant green removal from aqueous phase. Arab. J. Sci. Eng. 44(1), 111–121 (2019)
Q. Zhang, T. Zhang, T. He, L. Chen, Removal of crystal violet by clay/PNIPAm nanocomposite hydrogels with various clay contents. Appl. Clay Sci. 90, 1–5 (2014)
Y. Zheng, J. Monty, R.J. Linhardt, Polysaccharide-based nanocomposites and their applications. Carbohydr. Res. 405, 23–32 (2015)
Y. Zhou, L. Tang, G. Zeng, J. Chen, Y. Cai, Y. Zhang, G. Yang, Y. Liu, C. Zhang, W. Tang, Mesoporous carbon nitride-based biosensor for highly sensitive and selective analysis of phenol and catechol in compost bioremediation. Biosens. Bioelectron. 61, 519–525 (2014)
M. Zhu, L. Xiong, T. Wang, X. Liu, C. Wang, Z. Tong, High tensibility and pH-responsive swelling of nanocomposite hydrogels containing the positively chargeable 2-(dimethylamino) ethyl methacrylate monomer. React. Funct. Polym. 70(5), 267–271 (2010)
M. Zia, A.R. Phull, J.S. Ali, Challenges of iron oxide nanoparticles. Nanotechnol. Sci. Appl. 9, 49–67 (2016)
M. Zirak, A. Abdollahiyan, B. Eftekhari-sis, M. Saraei, Carboxymethyl cellulose coated Fe3O4@SiO2 core-shell magnetic nanoparticles for methylene blue removal: equilibrium, kinetic, and thermodynamic studies. Cellulose 25(1), 503–515 (2017)
Acknowledgements
This research was supported by the National Research Foundation (NRF) under the Thuthuka programme (UID. 117727), and the University of Limpopo (R202, R232, R355), South Africa.
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Ethics declarations
There are no conflicts of interest to declare.
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Malatji, N., Makhado, E., Modibane, K.D., Pandey, S., Hato, M.J. (2022). Sequestration of Organic Dyes from Wastewater Using Hydrogel Nanocomposites. In: Hato, M.J., Sinha Ray, S. (eds) Functional Polymer Nanocomposites for Wastewater Treatment. Springer Series in Materials Science, vol 323. Springer, Cham. https://doi.org/10.1007/978-3-030-94995-2_7
Download citation
DOI: https://doi.org/10.1007/978-3-030-94995-2_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-94994-5
Online ISBN: 978-3-030-94995-2
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)