Abstract
The source Ain Moulay Yacoub Hamma located upstream of Oued Hamma (Outita: pre-rifaine ride) 18 km southeast of the city Sidi Slimane, presents non-permanent flows during the three months February, March and April of the year 2017.
The objective of this study is to evaluate the quality of the natural waters of Ain Moulay Yacoub Hamma, the hydro-chemical facies and the origin of their mineralization.
Sampling is carried out at the source of these natural waters in order to carry out physico-chemical analyses in our specialised laboratory.
As a result, the study showed that the waters of the Ain Moulay Yacoub Hamma spring are meso-thermal waters (42 °C), with a poor quality sulphurous odour loaded with mineral salts (chlorides, sodium and sulphates). In the results presented in the Piper, Schoeller and Stiff diagrams, show that these waters are characterized by a chloride sodium chemical facies.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
The river Hamma near the city Sidi Slimane, tributary of the river R'dom, is a non-permanent stream. It is fed by a set of freshwater sources upstream in the Outita pre-Riparian ride.
The discharge of thermal waters from the Ain Hamma Moulay Yacoub spring in the city of Sidi Slimane into the river could modify the quality of the latter's waters. The physico-chemical characteristics of a thermal water are linked to its underground course, its depth (temperature) and the mineral constitution of the rocks. At depth, water can also be enriched with gas (CO2, H2S) depending on the nature of the rock [1].
Thermal waters have been the subject of several scientific studies [2,3,4,5,6,7].
They have particular physico-chemical characteristics that may undoubtedly modify the quality of the receiving environment.
In this context, the study of the quality of natural resources Ain Hamma Moulay Yacoub of Sidi Slimane is included. A follow-up of the physico-chemical parameters is done during the three months February, March and April in 2017. The main objective is on the one hand to study the quality of the spring water and to determine the hydro-chemical facies and the origin of the mineralization of these waters.
2 Materials and Methods
2.1 Presentation of the Study Area
The city of Sidi Slimane is located on the Gharb plain, in north-west Morocco, and belongs to the Rabat-Salé-Kénitra region.
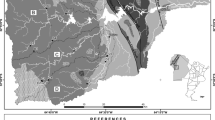
The thermal spring of El Hamma-Outita emerges on the outskirts of the pre-rifain mountain range, 12 km from the town of Sidi Slimane, on the road that leads to Meknes, and is very close to the marabout of Sidi Moulay Yacoub, at the bottom of the N-S cluse of Oued El Hamma, and its coordinates are Lambert, X = 459,60, Y = 392,7 and Z = 120 m, with a scale of 1/50000 from the map of El Kansera.
2.2 Geological Description
2.2.1 Lithostratigraphic Section of the El-Hamma Wrinkle
The section of the El-Hamma wrinkle shows that the two valley wrinkles start with limestones listed as the first (S1) oldest sequence followed by other sequences of number five deposited one on top of the other in a successive manner: S2, S3, S4, S5 and S6 (with; - S2: marl-limestone; - S3: grey marls; - S4: marls and bioclastic limestone; - S5: calcareous marls and sandstones; - S6: molasses) (Fig. 2, 3).
The six sequences are present on the left bank, but on the right bank there is an absence of S4 and S6.
2.2.2 Interpretation of the Lithostratigraphic Section
The two banks of the Hamma wadi in the area of emergence of the My Yacoube Hamma spring, are formed of two lithostratigraphic formations, one limestone-limestone of Lower Domérian age and the other of marl-limestone of Upper Domérian age (FAUGERES 1978, BOUTAKIOUT 1990) (Fig. 6, 7). In the form of a small El Hamma wrinkle also called My Yacoube Hamma wrinkle.
-
A
-The Jurassic
The Jurassic series composed of five formations from Domérien to Bajocien (FAUGERES 1978, BOUTAKIOUT 1990) can be seen from bottom to top:
-
The limestones
-
The marly limestones
-
Grey marls
-
Bioclastic marls and limestones
-
Limestone marl and sandstone.
-
-
B
- The Miocene
The distinct Miocene formations are four in number, from bottom to top: lower molasses, red clays, upper molasses, and white marls. This Miocene series is transgressive on the upper Jurassic formations.
-
Lower molasses
-
Brecciated red clays
-
Upper molasses
-
The White Marls
-
2.3 Sampling Et Analysis
2.3.1 Sample Collection
The sampling campaign was carried out monthly over the period from February to May 2017, because of the flow of the source that is very important during this period on the other hand the other months of the year or this flow is very low to nil. Concerning the sampling method, plastic bottles were used which were rinsed several times with tap water beforehand, then allowed to dry, and before filling the bottles they were rinsed three times with water to be analysed, filled until overflowing to avoid any water–air reaction. Each bottle was labelled with the number, location and date of sampling.
2.3.2 Physico-Chimical Analyses
The analyses concerned the major elements expressed in cations (Ca2+, Mg2+, Na+ and K+) and anions (Cl–, HCO3–, SO42–), overall hardness, full alkali content, dry residue and heavy metals (Pb, Cd, and Ni).
The analyses were carried out in the laboratory of the Institut Nationale de Recherche Agronomique (Rabat). The pH and temperature measurements were made in situ by a portable instrument.
2.3.3 Evaluation of Analyses
The results of the physico-chemical analyses were used in the Avignon Hydrochemistry (L.H.A.) software version 4.2008, which enabled us to classify the waters into chemical facies and drinking and irrigation water classes, and in particular to construct the Piper, Schoeller-Berkaloff and Stiff diagrams [12].
3 Results and Discussion
3.1 Organoleptic Characteristics of the Waters of Hamma Outita Spring
-
Colour: Water is clear, i.e. colourless.
-
Odour: Water has a sulphurous odour.
3.2 The Physico-Chemical Characteristics of the Water Studied
-
a
-Physical parameters
-
Temperature:
The water temperature studied varies between 41 °C and 43 °C, so we can say that this water is ortho-thermal.
-
Hydrogen potential (pH):
-
-
The pH varies according to the concentration of HCO3− or H3O+ ions, the pH of water measured at Hamma Outita source is neutral, varies between 6.89 and 7.06.
-
Electrical conductivity:
The electrical conductivity values are very high for the natural waters studied, varying between 8000 and 10,000 µS/cm. The latter result from the high mineralization of the waters of the Ain Moulay Yacoub Hamma spring.
-
b
- Chemical parameters
-
b-1. The major elements
-
-
The cations
-
The calcium ion Ca2+:
Calcium is introduced into the water system by the weathering of rocks, particularly limestone rocks, and by leaching and runoff from the ground into infiltrating waters. The concentration of calcium in water depends on the residence time of the water in calcium-rich geological formations [8].
The waters of the Hamma Outita spring have an average calcium concentration of around 150 mg/l. We estimate that this is linked to the importance of the liasic formations (limestone and dolomite) of the main karstic aquifer in the supply of this spring.
Moroccan standards recommend a concentration between 75 mg/l and 200 mg/l, which explains why these waters comply with national standards.
-
The Magnesium ion Mg2+:
The magnesium content of the water is extremely varied and mainly related to the nature of the terrain crossed. In areas rich in magnesic rocks, water can contain concentrations of 10 to 50 mg/l of this element [4, 8].
The water from the Hamma Outita spring has a magnesium concentration significantly lower than that of calcium, with an average of 49.34 mg/l. Therefore these waters are of good quality in comparison with the Moroccan standard requiring a maximum concentration of 150 mg/l in Mg2+, The hardness of the water is due to dissolved polyvalent metal ions. Mainly calcium and magnesium ions. In the water samples from the Hamma Outita spring the hardness reaches a value of 57.05 °F, this would be related to the lithological nature of the aquifer formation and in particular to its composition in calcium and magnesium.
Good quality drinking water has a hydrotimetric degree of less than 15 °F, they are acceptable up to 50 °F, but if they exceed 60 °F their use will cause problems either with consumption or with certain domestic uses according to the W.H.O [13].
-
The sodium ion Na+:
The sodium is a so-called conservative element because once in solution, no reaction makes it possible to extract it from groundwater. Precipitation brings a minimal amount of sodium to groundwater, abnormally high levels can come from salt leaching, or percolation through saline soils or brackish water infiltration [4, 8]. In unpolluted groundwater without contact with evaporates, the sodium content is between 1 and 20 mg/l [3].
Analysis of the data showed that the average sodium value of 713.02 mg/l, in the waters of the Hamma Outita spring, is very high, exceeding the Moroccan standard of 200 mg/l.
-
The potassium ion K+:
Potassium is generally the least abundant major element in water after sodium, calcium and magnesium; it only exceptionally ranks third among cations [7]. Potassium occurs as double chlorides in many minerals such as corrollite and sylvinite. It is also found in plant ashes as carbonate. Potassium is an essential element for life and especially for plant growth.
Potassium content is almost constant in natural waters. This usually does not exceed 10 to 15 mg/l [3]. Its concentration in the Hamma Outita spring is high and reaches an average value of around 39.31 mg/l.
-
The anions:
-
Chlorides Cl–:
Chlorides are widely distributed in nature, usually in the form of calcium and potassium salts; they represent about 0.05 of the lithosphere, they are naturally present in groundwater due to weathering and leaching of sedimentary rocks and soils, and dissolution of salt deposits [7].
The average chloride value for the water source studied (Hamma Outita) is very high, reaching 1120.23 mg/l. This high value could be due to water contact with triasic saline deposits. The admissible concentration set by the Moroccan standard is 200 mg/l.
-
Sulphates SO42−:
Under natural conditions, sulphates, the most responsive form of dissolved sulphur in natural waters, have essentially two origins: geochemical and atmospheric [1].
Due to the high solubility of sulphates, groundwater under normal conditions can contain up to 1.5 g/l [3]. Oxidation of sulphides and degradation of biomass in soil are other possible sources. Many human and natural activities can generate sulphate inputs to groundwater: application of sulphate fertilisers, precipitation loaded with sulphur dioxide, etc.
The average value of sulphates in the waters studied (Hamma Outita) is about 655.88 mg/l. This high content seems to be linked to the triasic saline formation brought into contact with the aquifer reservoir through major faults that dominate the Outita wrinkle structure (Fig. 1), [2]. The results obtained exceed the Moroccan standard of between 200 mg/l and 400 mg/l in sulphates.
-
Complete alkalimetric title:
The complete alkalimetric titre (TAC) in the water samples analysed is mainly due to the presence of bicarbonates (HCO3-) is the Water Buffer Index, it is closely related to hardness, although many species of solutes can contribute to it. Alkalinity is expressed in equivalent amount of carbonate or in French degrees °F.
The bicarbonate content of groundwater not subject to human influences varies between 50 and 400 mg/l [3]. The median values of bicarbonate contents are around 302 mg/l in the usual range of unpolluted groundwater [3]. The high average bicarbonate value of the waters studied is of the order of 467.8 mg/l, seems to be due to the circulation of these waters in the aquifer of calcaro-dolomitic nature.
-
b-2. Trace elements
The presence of minor elements or trace elements in thermo-mineral waters is of great interest, because it makes it possible to specify the characteristics of the waters and the mineral deposits through which they pass.
For the thermo-mineral waters of our study site, we performed atomic absorption analyses to determine the concentrations in (mg/l) of certain trace elements which are: Lead (Pb), Cadmium (Cd) and Nickel (Ni).
-
Lead (Pb)
According to the results obtained, during this campaign and by comparison with the maximum admissible values (MAV) (25 μg/l) for drinking water according to the Moroccan standard [9].
The results obtained do not exceed the MAV in the waters of the Hamma spring, which is in the order of 0.0015 mg/l.
-
Cadmium (Cd)
The water from the source analysed has an average value of about 0.015 mg/l, higher than the MAV (3 μg/l) according to the standard for drinking water supply [9].
-
Nickel (Ni)
The average Nickel value of 0.02 mg/l recorded in natural waters from the Hamma source actually exceeds the MAV of drinking water, which is in the order of 500 μg/l [9].
3.3 Study of Relative Values
3.3.1 Basic Exchange Index (B.E.I.)
During its underground journey, water comes into contact with different substances that have the property of exchanging their ions against those contained in the water, among these substances, we have clay minerals: ferric hydroxide and organic substances [11].
Schoeller in 1934 specified that the basic exchange index (B.E.I.) as being the ratio between the exchanged ions and ions of the same nature primitively existing, when there is exchange of Na+ and K+ of water, against the then calcarino-earthly clays:
-
If I.E.B < 0: The water is of crystalline origin (Ca2+ and Mg2+ are exchanged by Na + and K + );
-
If I.E.B > 0: The water is of sedimentary origin (Na+ and K+ are exchanged by Ca2+ and Mg2+);
-
If I.E.B = 0: No ionic exchange between the water and the surrounding soil.
With;
In our case, this index is in the order of –0.005, so we can see that the Ca2+ and Mg2+ ions of the water are exchanged slightly against the K+ and Na+ ions of the surrounding formations.
3.3.2 Characteristic Reports
The characteristic ratio is defined as the ratio of certain chemical elements expressed in milliequivalents per litre (meq/l). The reports studied are:
The study of variations in these ratios provides valuable information on groundwater recharge and circulation and sometimes allows the detection of other non outcropping deep formations.
-
a.
Report r Mg2+/r Ca2+
When this ratio is higher than 1, it reflects the predominance of Magnesium, and when it is lower than 1 Calcium predominates and this is the case of our study (the ratio Mg2+/r Ca2+ is equal to 0.54), this can be explained by the solubility of limestones richer in Calcium than in Magnesium [10].
-
b.
Report r SO42–/r Cl–
-
When this ratio is greater than 1, Sulphates predominate, which are essentially linked to leaching of gypsum soils and oxidation of sulphides (pyrites) [10].
-
When the ratio r SO4 2–/r Cl– is less than 1 there is predominance of Chlorides, this is the case of our study (r SO4 2–/r Cl– equal to 0.43).
-
-
C.
Report r (Na + K+)/ r Cl–+
-
When the ratio r(Na+ + K+)/r Cl– is less than 1, it reflects the predominance of Chlorides which are linked to saline soils.
-
When this ratio is greater than 1, it reflects the predominance of Sodium [11].
-
In our case, this ratio is equal to 1, which explains the existence of a balance between chlorides, potassium and sodium.
3.4 Chemical Classification of Spring Water
The values of the chemical analyses can be plotted on diagrams to classify the waters into chemical families in order to determine the facies of these waters from the Hamma spring.
To determine the different facies crossed by water (source Hamma Outita) and classify them into chemical families, we used the Piper, Stiff and Schoeller Berkaloff diagrams that will be reviewed.
-
Piper Diagram
The results were represented on the Piper diagram, with the position of the representative points of the anions and cations that characterize the chemical composition of the water from the Hamma Outita thermal spring.
According to the Piper diagram (Fig. 3), the hydro-chemical facies of these waters is: Chloro-sodium.
-
Schoeller Diagram
The graphical appearance obtained (Fig. 4) shows the facies of the mineral water concerned. The analysis of Schoeller Berkaloff's diagram allows us to conclude that the waters of the spring Hamma Outita rich in chlorides, sodium and sulphates. They are due to the existence of a source of saline sediments.
The important values of chlorides, sodium and Sulphates indicate a relationship of evaporite rocks formed mainly by chlorinated and sulphated minerals.
-
Stiff Diagram
The representation of the analysis results on the Stiff diagram (Fig. 5), clearly shows the hydro-chemical facies mentioned above which is: Chloride-sodium, with the following chemical formula:

4 Conclusions
The study of the chemistry of the water of the Hamma Moulay Yacoube thermal spring using hydro-chemical and hydrogeochemical tools made it possible to determine its typological characteristics:
-
The waters of the spring are classified in ortho-thermal family because they have a temperature of order 42 °C (37 °C < T = 42 °C < 45 °C).
-
The chemical facies is chloride-sodium, which shows that the waters of the source having circulated through saliferous formations of the Triassic.
-
A study of the relative values of the characteristic ratios of the mineral elements in these waters shows that:
-
The Ca2+ and Mg2+ ions are changed against the Na+ and K+ ions of the surrounding formations;
-
The predominance of calcium in relation to magnesium, this can be explained by the solubility of limestones richer in calcium than in magnesium.
-
The predominance of chloride in relation to sulphate may be due to the solubility of evaporites rich in chloride rather than sulphates.
-
The existence of a balance between chloride, potassium and sodium.
-
-
The physico-chemical quality of the source is poor, because its chemical composition in different elements does not respect the maximum admissible values for the Moroccan standards of drinking water.
References
A. Ezzaïdi, M. Khaloufi, M.A. Bouagou, M. El Youssi, A health tourism site to promote: abaynou resort (Guelmim province). In: The First International Workshop on Geotourism and Ecotourism in the Souss-Massa-Draâ and Guelmim-EsSmara regions. Mirleft-Guelmim and Assa. (2006)
O.K. Hakam, A. Choukri, J.L. Reyss, M. Lferde, Comparison of uranium and radium isotopes activities in some well and thermal springs samples in Morocco. Rev. Sci. 13/2, 185–192 (2000)
A. Duriez, Origin and mineralization process of thermal waters in the Mediterranean continental environment: case of the Thermopylae geothermal system (Greece). Thesis, N° d'ordre: 85 47 Université Paris Sud 11, Faculté des Sciences d’Orsay, p. 292 (2006)
A. Lakhdar, A. Ntarmouchant, M.L. Ribeiro, M. Beqqali, K. El Ouadeihe, L. Benaabidate, M. Dahire, Y. Driouche, A. Benslimane. A., Nouvelle approche géologique et géochimique du complexe hydrothermal de Moulay Yacoub (frontière nord du sillon du Rifain Sud). Comunicacoes Geologicas 93, pp 185–204 (2006)
A. Lakhdar, A. Ntarmouchant, M.L. Ribeiro, M. Beqqali, K. El Ouadeihe, L. Benaabidate, M. Dahire, Y. Driouche, A. Benslimane, Determination of the origin of the mineralization of the thermal waters of Moulay Yacoub by geological and geochemical approaches. Revue des Énergies Renouvelables. CER’07, Oujda, pp. 81–84 (2007)
Y. Zarhloule, A. Rimi, M. Boughriba, M. Verdoya, A. Correia, J. Carneiro, A. Lahrach, The geothermal province of North Eastern Morocco. Revue des Énergies Renouvelables. CER’07, Oujda, pp. 89–94 (2007)
A. Fekraoui, Geochemical characteristics of the geothermal waters of the Oran region. Revue des Énergies Renouvelables CER’07, Oujda, pp. 75–80 (2007)
J. Rodier, B. Legube, N. Merlrt, Water Analysis. 9th edn. (Dunod, Paris, 2009), p. 1579
MOROCCAN STANDARD (2006): Moroccan standard approved by joint order of the Minister of Industry, Trade and Economic Upgrading and the Minister of Equipment and Transport and the Minister of Health No. 221–06 of 2 February 2006, published in the Official Gazette. No. 5404, of 16 March 2006.
M. Benhamza, Contribution of geophysics to the hydrogeological study of the Mercurielle Nord Numidique zone (Azzaba). North-East Algeria, Consequences of the exploitation of mercury deposits on the environment, PhD thesis, UBM Annaba, p. 147 (2007)
H. Dib, The thermalism of the far east of Algeria: Guelma, Souk Ahras, Skikda and Tarf. Supervision of a thesis in Hydrogeology. Faculty of Earth Sciences and Town and Country Planning. Constantine University (2004)
MIOURIGH: Evaluation of the hydrochemical quality of groundwater in the M'Zab valley: Case of Oued N'Tissa. Thesis of State Engineer in Agronomy, option Agricultural Hydraulics.
O.M.S: Drinking Water Quality Guidelines; Hygiene Criteria and Supporting Documentation WHO, Geneva, 2nd edn, vol. 2 (2002), p. 1050
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this paper
Cite this paper
Aitsi, S., Ettaki, J., Doumi, K., Chabli, A., Belghyti, D. (2022). Hydrogeochemical Study of the Hamma My Yacoube, Sidi Slimane – Morocco. In: Ben Ahmed, M., Boudhir, A.A., Karaș, İ.R., Jain, V., Mellouli, S. (eds) Innovations in Smart Cities Applications Volume 5. SCA 2021. Lecture Notes in Networks and Systems, vol 393. Springer, Cham. https://doi.org/10.1007/978-3-030-94191-8_53
Download citation
DOI: https://doi.org/10.1007/978-3-030-94191-8_53
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-94190-1
Online ISBN: 978-3-030-94191-8
eBook Packages: EngineeringEngineering (R0)