Abstract
The High Oum-Er-Rbia basin, located in the Moroccan Middle Atlas, is a karstic region with significant water sources that have essential functions regarding agriculture, hydropower production, industrial and drinking water. The region contains abundant wetlands, especially springs, rivers and natural lakes. These systems are highly sensitive to the effects of climate change, experiencing considerable lake level, water chemistry, and biological fluctuations in response to regional hydrological balances. This study focuses on the hydrogeochemical processes and mechanisms that control the chemical composition and variability of Azigza Lake, a typical tectono-karstic lake system of the region. Water monitoring was implemented from July 2013 to October 2014 with a monthly water sampling for physicochemical measurements and major ion concentration analyses of lake water and the surrounding groundwater. Both waters show a relatively low salinity due to the fresh input from the Lower Jurassic karst formation. Lake waters are slightly alkaline and of the calcium-magnesium-bicarbonate type. The geochemistry of the lake waters is mainly controlled by carbonate weathering through water–rock interaction and, to a lesser extent, by cation exchange and precipitation of carbonate minerals. The hydrochemistry of the lake showed clear responses to seasonal changes in precipitation and evaporation, with higher conductivity during the wet period. During the beginning of the wet season, groundwater evolution could be explained by a simple first flush stormwater. The rapid response of lake water to subsurface and underground waters confirms the dominance of an underground conduct flow regime. These changes and behaviors highlight the sensitivity of Azigza system to regional hydrological and climatic changes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Karstic landscapes are mainly dominated by carbonate rock such as limestones and dolomites with complex networks produced by rock dissolution (Kiraly 2003). In view of their high storage capacity, karst aquifers are a very significant water resource often used for irrigation and drinking purposes but generally have highly heterogeneous characteristics, which makes them extremely complex (Hughes et al. 1994; Zielhofer 2004). The High Oum ER-Rbia (OER) basin is typical of karstic hydrological systems in the Moroccan Middle Atlas region. Carbonate rocks are widely distributed as the main abundant geological units in the region. These units satisfy the drinking water needs of the largest cities and villages of the region. The hydrogeochemical evolution of surface and groundwater is controlled by natural processes including the geological structure and lithology of the aquifer, and water–rock interaction along the flow paths (Khalil et al. 2004; Bouchaou et al. 2009). Anthropogenic activities also have a strong influence on the water hydrogeochemical characteristics and evolution (Etabaai et al. 2012; Benamar et al. 2019; Karroum et al. 2019). A sustainable management of the region’s water resources and wetlands in these complex karst settings requires understanding the dominant processes that govern their hydrogeochemical evolution. Previous studies aimed to determine groundwater recharge and flow path, the source of solutes, water/rock interactions, and contaminant transport (Bouchaou et al. 1995, 1997, 2009; Amrani and Hinaje 2014). For example, Miche et al. (2018) used the hydrochemical and isotopic characterization of spring and well water samples to understand the groundwater flows and their renewal in the karstic Tabular Middle Atlas Causses. Other studies investigated the hydrological and hydrochemical behavior of this karst system using discharge and turbidity and showed a variety of spring behaviors related to differences in hydraulic connectivity within each spring basin, evidencing the complex and heterogeneous structure and functioning of the system (Bouchaou et al. 2002; Amraoui et al. 2003; Howell et al. 2019). In such areas where it is difficult to conduct direct investigations, the analysis of major ions of surface water and groundwater can effectively extract key information from water samples, providing explanations about the hydrogeology of the system, the material loads into lakes/streams, as well as the local processes that influence water chemistry.
Few studies have investigated the hydrochemical composition of surface water of the high OER watershed (Khalil et al. 2004; Karroum et al. 2019). Based on these studies, the spatial distribution of some hydrochemical components (EC, HCO3−, Ca2+, and Cl−) demonstrated that the variations in the chemical properties of surface water were mainly related to the characteristics of the geological formations of the area (Fig. 1). These approaches provided an overview of the hydrochemical characteristics of the basin but were restricted to surface reservoirs and did not use a temporal approach. In some cases, hydro-geochemical studies of representative lake systems can provide precise information on the functioning of the karst on which they depend (Xu et al. 2010; Cui and Li 2015; Cui et al. 2016; Cejudo et al. 2020). Previous investigations of the Middle Atlas lakes were based on the physical properties, elementary chemistry and biological pollutants and the lake level variations in response to climate: Dayet Aoua (Abba et al. 2012; Sayad and Chakiri 2010), Aguelmam Sidi Ali (Chillasse and Dakki 2004; Sayad et al. 2011; Menjour et al. 2016; Haddout et al. 2018; Lazhar et al. 2020) and Ifrah (Etebaai et al. 2012). Nevertheless, systematic studies to understand the processes that control the geochemistry of lake waters including regular survey, their hydrogeochemical evolution and the interactions between groundwater and lakes within this region have not yet been performed.
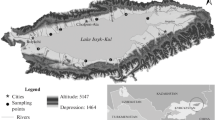
a Study area in the Middle Atlas of Morocco. b Simplified geological map of the high Oum Er-Rbia watershed (modified from El Jihad 2010) with sampling location of surface water from Azigza Lake (this study), Tarhat station (ABHOER) and from previous measurements carried out on the basin of the high OER watershed (Khalil et al. 2004; Krroum et al. 2019), c spatial distribution of hydrochemical components (EC, Cl−, HCO3−, and Ca2+) across the high OER basin
In this context, the hydrogeochemical monitoring of Azigza Lake located in the high OER basin was conducted. Monthly physicochemical measurements and analysis of lacustrine, springs and well waters were carried out between July 2013 and October 2014. The main objectives of this study were to: (1) investigate the hydrochemistry of Azigza Lake water and groundwater, (2) identify the geochemical factors and mechanisms controlling water chemical composition, (3) highlight the impact of the intra-annual climate variability on the geochemistry of the lake, and (4) investigate the groundwater flow from/to the lake in order to provide an understanding of the hydrochemical functioning of the regional karst system.
2 Materials and methods
2.1 Study area
Azigza Lake (32°58' N, 5°26' W, 1550 m a.s.l.) is a natural mountain lake located in the Tabular Moroccan Middle Atlas Causse in the province of Khenifra (Fig. 1a). It is of tectono-karst origin (Hinaje and Ait Brahim 2002) belonging to the high OER watershed. The geology in the area (Fig. 1b) is mainly dominated by Lower Jurassic carbonate rocks (karstified limestones and dolomites) overlying Triassic clays, evaporates and basalts (Lepoutre et al. 1967). The climate is Mediterranean subhumid with cold winters. The lake is located in a relatively well-watered area with mean annual rainfall approaching 900 mm with considerable interannual variation (Martin 1981). The rainy season lasts from October to May. The monthly average temperature is highest in August (22.8 °C) and lowest in January (3.2 °C). Vegetation is dominated by oak associated with cedar at altitude above 1400 masl.
Today, Azigza lake has no outflow streams and occupies a unique depression with an area reaching up 0.55 km2 (Jouve et al. 2019; Adallal et al. 2019). The maximum water depth is about 42 m with a mean depth of about 26 m recorded in April 2013 (Fig. 2). Its watershed is relatively small with an area of about 10.2 km2 (Adallal et al. 2019). The lake is mainly fed by rainwater, snow, surface runoff and through the subsurface inflows (Benkaddour et al. 2008). The level of Azigza Lake has experienced large fluctuations from year to year and seasonally, and it has undergone a severe decline in recent decades with a drastic drop in 2008 as shown on aerial photographs (Jouve et al. 2019). Littoral sediment stratigraphy in the catchment shows that many changes in lake level have occurred during the past few decades (Flower and Foster 1992). Recent hydrological results from Azigza Lake reveal that water levels are mainly driven by precipitation (Vidal et al. 2016; Jouve et al. 2019). This was confirmed using hydrological mass balance modelling during the period 2012 to 2016 (Adallal et al. 2019). The lake has also been the subject of several studies conducted on the physicochemical and limnological properties (Gayral and Panouse 1954; Flower et al. 1989; Flower and Foster 1992; Benkaddour et al. 2008).
2.2 Sampling and analysis
Water samples from lake, well and springs were collected monthly from July 2013 to October 2014. Lake water samples were collected from three different points on the lake (L1, L2 and L3) in order to obtain a representative distribution of the lake water. The water temperature (T °C), pH and electrical conductivity (E.C. µS/cm) were measured in situ using a multiparametric probe. Total alkalinity was measured by titration within a few hours following the sampling, using 0.02 N sulfuric acid (Welch, 1948). Major cations (Ca2+, Mg2+, Na+, K+) and anions (Cl−, NO3−, SO42−) were analyzed by ion chromatography using HPLC-ICS 1100 at the Centre d’Analyse et de Caractérisation (CAC), at Cadi Ayyad University, Marrakech, Morocco. The ionic balance error was estimated to test the accuracy of the analyses. Only samples having an ionic balance not exceeding 10% were selected. Total dissolved solids (TDS) were calculated as the sum of ionic concentrations.
Saturation Index (SI) was calculated through PHREEQC geochemical software (Parkhurst and Apello 1999) for each sample. Results were plotted on a Piper diagram (Piper 1944) to identify the water type, and on a Gibbs diagram (Gibbs 1970) to investigate the natural mechanisms that control the water chemistry. PCA analysis and matrix correlation were performed using R software, to have a general idea of the relationship between elements. Other exploratory analyses of experimental data were carried out by statistical analysis.
3 Results
3.1 Physical properties
Water physical parameters such as pH, electrical conductivity (EC), temperature and major ion compositions are shown in Table 1. The average surface water temperature of Azigza Lake was approximately 16.7 °C (during the year 2013). The lowest temperature (7.5 °C) and the highest temperature (25.7 °C) were recorded in January and July, respectively. During the same year, we had determined the trophic state of the lake as a monomictic one. The thermal stratification of the water body is maintained nearly all the year and single water mixing in January with a temperature of the water masses about 8 °C along the 42 m depth of the water column.
Groundwater (well and spring) temperatures were lower than those of surface lake waters, ranging between 9.2 and 19.7 °C. Temperatures showed clear seasonal variations, indicating that both lake water and groundwater are mainly influenced by hydrometeorological factors.
The EC values of the lake water ranged from 341 to 645 µS/cm (mean value 423 µS/cm). Well and spring waters were more concentrated, with mean EC values of about 422 µS/cm and 659 µS/cm, respectively. The pH of lake water measured at the three lake sampling points (L1, L2 and L3) showed considerable seasonal variation. The recorded pH values indicate that the lake waters are the most alkaline (mean value 8.2) followed by well water (mean value 7.6). Lower pH values were recorded in the intermittent springs (mean value 7).
3.2 Hydrochemical characteristics
The chemical composition of the lake water was similar to that of the groundwater with higher concentrations in the groundwater than in the lake water. The lake water anion concentrations were gradually variable and were in the order HCO3− > CO32− > Cl− > SO42− > NO3−, while the cation concentrations were in the order Ca2+ > Mg2+ > Na+ > K+. Thus, HCO3−, Mg2+ and Ca2+ were the dominant elements (Table 1). Groundwaters showed a strong temporal variation in their chemistry while the lake waters showed slight temporal changes. In general, lake waters were characterized by the relatively high Mg2+, Na+ and K+ concentrations and the relatively low Ca2+, HCO3−, Cl−, SO42−, NO3− concentrations. However, the dominance of HCO3−, together with Ca2+ and Mg2+, and low contents of K+ and Na+ in major ion composition is also typical of many lake waters in karst areas (White 1988).
The geochemical facies in the Piper ternary diagram also supported the dominance of alkaline earth elements over alkali elements in the surface and groundwater waters of Azigza Lake (Fig. 3). Specifically, with respect to cations, most samples were scattered in the zone between the magnesian and calcic poles of the lower-left triangle, indicating that lake waters are of the magnesium-calcium type. Regarding anions, waters were grouped at the carbonate-bicarbonate pole of the lower-right triangle (Fig. 3), which means that bicarbonate-type water is predominant. Thus, as expected in a karst-influenced region, the site samples are clearly of the HCO3−–Mg2+–Ca2+ hydrochemical facies type.
The EC of the Azigza Lake system water is lower than that in the surrounding area (Fig. 1); this can be seen by comparing the results of Azigza lake water and groundwater analyses with the nearby Oum Er-Rbia springs (samples OER11 and OER12). The EC of Oum Er-Rbia springs and of the Srou River are significantly higher than those of Lower Jurassic limestone bedrock water samples from the upstream part of the watershed (Fig. 1). The Oum Er-Rbia springs and the Srou River are located downstream of the study area and are impacted by the contact with Triassic saline clays. These results highlight the influence of geological characteristics on the physicochemical properties of the regional hydrosystems.
3.3 Statistical analysis
Pearson correlations among the main ions and physicochemical parameters of Azigza system water are shown in Table 2. Bicarbonates and Ca2+ are the major ions significantly correlated with TDS (0.97 and 0.87, respectively) and EC (0.72 and 0.85, respectively), indicating that these elements are the principal source of salinity. A significant positive correlation was found between HCO3− and Ca2+ with correlation coefficients of 0.79 indicating strong carbonate weathering, which was also confirmed by the strong negative correlation observed between pH and bicarbonates (− 0.87) and Ca2+ (− 0.79). A good correlation was observed between NO3− and SO42− and EC (0.72 and 0.73, respectively), indicating that these elements come from gypsum and rock salt contained in the Triassic clays. Calcium and sulfate were highly correlated (0.73), which indicates that Azigza site water chemistry is dominated by the dissolution of Lower Jurassic carbonates and, to a lesser extent, by the dissolution of the Triassic evaporitic rock layers located at the base of Lower Jurassic.
A principal component analysis (PCA) was performed to understand the complex hydrogeochemical processes controlling the hydrochemistry in this system. Results (Fig. 4) show three principal components, which explained more than 75% of the total sample variance. The first three factor loadings explained 54.8%, 12% and 8.7% of the total variance, respectively. The first factor (54.8% total variance) has a significant positive correlation with CE, Ca2+, HCO3−, NO3− and SO42− and a negative correlation with the temperature, pH, CO3−, Na+ and K+. This axis represents carbonate dissolution as the primary source of the ions with evaporite rock dissolution. The second and third factors (12% and 8.7% total variance, respectively) were related to Cl− and Mg2+ and represent the contribution of soluble salts and dolomite to the water composition.
3.4 Saturation indices
Saturation indices (SI) with respect to calcite, dolomite, aragonite and gypsum were estimated (Fig. 5). This index is widely used to predict mineral precipitation in a solution with particular physicochemical characteristics (Nordstrom et al. 2015; Lecomte et al. 2016). Calcite, aragonite and dolomite showed positive SI values in lake water samples (Fig. 5), indicating that oversaturation and precipitation of carbonates are possible within the lake. Groundwaters, especially springs, plot well below the equilibrium line for the carbonate saturation index. This can be mainly attributed to their high CO2 pressure as well as to the recent recharge from fresh rainfall water and its rapid circulation within the karst conduits. The saturation conditions of gypsum were not reached in any of the Azigza lacustrine system waters for the data set (Fig. 5).
4 Discussion
The hydrochemical analysis and monitoring of a lake can provide valuable clues for understanding a complex karst hydrogeological system. In this section, we will first discuss the sources of dissolved ions in Azigza lake system waters and the processes that control their composition. Then, the physicochemical variations of Azigza Lake water during the different hydrological periods (dry and wet seasons) will be documented. Lastly, we will evaluate the hydrochemical response of the lake system to underground water circulation from/to the lake in order to understand the hydrodynamic functioning of the water system.
4.1 Geochemical Processes
4.1.1 Water–Rock Interaction
The chemistry of natural waters is mainly affected by rock and soil weathering and anthropogenic activities, and partly by atmospheric inputs. The mechanisms controlling the water composition on solutes are evaluated with the boomerang envelope model developed by Gibbs (1970). It can be interpreted in terms of a simple plot of TDS versus the ratio of Na+/(Na+ + Ca2+) and/or Cl−/(Cl− + HCO3−). Three distinct fields constitute the segments in the Gibbs diagram: precipitation dominance, rock weathering dominance and evaporation–crystallization dominance. All of the samples (lake, well and springs) are within the evolutionary path from ‘‘Rock dominance’’ in the Gibbs diagram (Fig. 6), suggesting that water–rock interaction is the major geochemical process responsible for the chemical composition of the lake water and groundwater. This result is in agreement with the findings of previous studies in the Middle Atlas region. The bedrock is mainly composed by dolomite and limestone, the reason why most of the lakes are alkaline and characterized by magnesium bicarbonate-type waters. For example, hydrochemical studies on Ifrah Lake and Tigalmamine Lake indicated that their water chemistry is mainly controlled by carbonate weathering (Benkaddour et al. 2005; Etebaai et al. 2012). However, unlike groundwater samples, lake samples were located between rock weathering and the evaporation zone, albeit closer to rock weathering. This indicates that the chemical compositions of the lake waters were affected by rock weathering and, to a lesser extent, by evaporation. Adallal et al. (2019) showed that stable isotope values of Azigza Lake were relatively higher than those of groundwater and rainfall, which suggests that lake waters undergo a certain degree of evaporation. Compared to other regional wetlands, evaporation is relatively less pronounced in Azigza Lake as expected from an open system with a short residence time. Generally, closed or small lakes are more affected by evaporation and are enriched in ionic concentrations. The typical example in the Middle Atlas region is Lake Iffer (Benkaddour et al. 2008).
4.1.2 Dissolution of Carbonate and Evaporite Minerals
The origin of dissolved salts and the mechanisms that control the observed mineralization of water can also be revealed by the dissolved ions and their relationships with one another (Hussein 2004; Su et al. 2009). Water chemistry in the Azigza system is dominated by HCO3−, Ca2+, Mg2+ and CO3−. These ions are mostly generated by the dissolution of carbonates (such as calcite and dolomite) in karstic aquifers.
Correlations between different hydrogeochemical parameters show a good correlation between Ca2+ and HCO3− (Fig. 7, Table 2). This indicates that the weathering of carbonates releases ions to the Azigza Lake water and groundwater due to the widespread occurrence of carbonate rocks in the study area. Dissolution of carbonate minerals may take place due to the attack of H2CO3 derived from CO2 dissolution in the water which can be described by the following equations:
According to reactions (1) and (2), the dissolution of calcite and dolomite releases equally charged amounts of Ca2+ and HCO3−, (Ca2+ + Mg2+) and HCO3−, respectively. Crossplots for Azigza Lake water and groundwater samples plot above the 1:1 line of the Mg2+ + Ca2+ versus HCO3− plot (Fig. 7b), indicating that the dissolution of dolomite was not the sole source of Ca2+, Mg2+, and HCO3− in Azigza waters. Considering Ca2+ and HCO3−, most of the samples were distributed between the 1:1 and 1:2 lines (Fig. 7a), implying that the dissolution of calcite and dolomite was the major source of Ca2+ and HCO3− for these waters. Moreover, the SI indicates that lake water samples were mostly saturated to oversaturated with calcite, dolomite and aragonite (Fig. 5), implying that the dissolution of carbonate minerals is the prevailing process affecting the chemical composition of Azigza Lake water. Furthermore, most groundwater samples were undersaturated or nearly saturated with respect to calcite and dolomite (Fig. 5), indicating that these carbonates dissolve into water from the fissured aquifer along the flow path and increase the contents in TDS.
The results of PCA showed that PC2 were of great loading on Cl− (Fig. 4). The dissolution of halite was one of the major sources of Na+ and Cl− in the site waters. The Na+ vs Cl− plots show approximately a 1:1 relationship (Fig. 7c). Since Cl− is generally derived from the dissolution of evaporites, this 1:1 relationship possibly suggests that evaporites are the unique source of Na+ and Cl− in Azigza catchment waters and that they influence the water chemistry to a moderate extent. It is reasonable to consider the dissolution of evaporitic minerals as a source of these elements due to the widespread occurrence of salutiferous clays (Trias strata) in the study area. The human impact on water chemistry in the area is relatively weak, because, if strong, the Na+ versus Cl− line would be expected to deviate from the 1:1 equilibration line. However, the well water deviates slightly from the 1:1 Na+ versus Cl− line, suggesting that other processes may control the chemical composition of the well water such as cation exchange and human activities which could increase or decrease the concentration of Na+ or Cl− in the well water. In addition, all Azigza Lake waters and groundwaters were under-saturated with respect to halite, with halite SI values less than 0 (Fig. 5), indicating that the dissolution of halite may take place in the waters of the study area.
As displayed in the correlation analysis of geochemical parameters, a good correlation between Ca2+ and SO42− indicates a possible dissolution of gypsum and anhydrite that may release ions to the lake water and groundwater, as expressed in Reaction (3):
Generally speaking, the weathering and dissolution of minerals in the host rocks represent the main source of ions in Azigza waters. However, unlike other watershed water samples, Azigza groundwater and lake water are marked by their relatively low salinity and chloride, sodium and sulfate contents. This indicates that the site water arises mainly from water–rock interactions within the Lower Jurassic limestone dolomite aquifer and is weakly affected by Triassic rocks which are not exposed in the lake site. In addition, Azigza site waters (outcropping from the mountain) are close to the recharge area of groundwater, which leads to a shorter residence time of groundwater runoff. Thus, the influence of rock weathering is relatively weak compared to its influence on the waters of downstream areas.
4.1.3 Ionic Exchange
Cation exchange is also an important process that influences the evolution of the hydrochemical characteristics of water (Xiao et al. 2012). Two indices of base exchange, namely the chloro-alkaline indices (CAI-1 and CAI-2), were examined to evaluate ion exchange between Na+ and K+ in Azigza site waters with Ca2+ and Mg2+ in the host environment (Schoeller 1965):
Positive Schoeller indices imply that an exchange of Na+ and K+ from water with Ca2+ and Mg2+ from rocks takes place, representing the reverse ion exchange process. Negative CAI indicate that Ca2+ and Mg2+ from waters are replaced by K+ and Na+, representing ion exchange with the aquifer material. If the CAI value is 0, it indicates no exchange and implies a state of equilibrium between the chemical constituents present in waters and aquifer material. In this study, the CAI-1 and CAI-2 values were negative for most of the lake and spring water samples and some well samples (Fig. 8), indicating that Na+ and K+ were released to the lake and spring water from the aquifer through cation exchange with Na+ previously adsorbed in the aquifer matrix. However, most of the well samples show CAI values greater than zero, indicating a reverse ion exchange process (Fig. 8). These results confirm that dissolution of halite is not the sole source of Na+ in Azigza waters, but that cation exchange also plays an important role and can increase or decrease the concentration of Na+ in conjunction with rock weathering.
4.2 Seasonal Hydrochemical Variations
Monthly geochemical data of Azigza Lake waters were used to explain the temporal variations in water chemistry and illustrate the potential impacts of climate and subsurface features on the geochemical processes operating in the lake. The time-series of major physicochemical components of Azigza Lake water were compared with variations in regional precipitation (Fig. 9). The seasonal precipitation in the study area and at Azigza Lake shows a marked wet season from October to April and a dry season from May to September (Adallal et al. 2019). The salinity of the lake water showed responses to seasonal changes in precipitation with higher conductivity following a wet period while a decline in conductivity was recorded in the dry season (Fig. 9). The dominant ions Ca2+ and HCO3− account for 61–79% of the total dissolved ions in the lake samples. Thus, lower conductivities during the drought season could result from higher carbonate precipitation due to low rainfall and higher evaporation rates resulting from high atmospheric temperatures (Fig. 9). Indeed, an increase in temperature contributes to a decrease in the solubility of calcite which increases when the temperature decreases (Plummer and Busenberg 1982; Cao et al. 2012). Factor 1 in the PCA (Fig. 4) shows this influence, as the temperature exhibits a negative relationship with Ca2+ and HCO3−. Endogenic calcite precipitation caused by ion saturation enhanced by algal blooms and increasing water temperatures is typical in lakes emplaced in carbonate-rich bedrock areas (Kelts and Hsü 1978; Brauer 2004; Romero-Viana et al. 2008). On the other hand, the increased conductivity values recorded in Azigza Lake water for the wet season are related to the dissolution of carbonates by CO2-rich karst groundwaters (produced by root respiration and organic matter decomposition in the soil) which release Ca2+ and HCO3− to the lake water. The other major ions such as Cl− and Na+ show an increase in concentration values during the dry season which is attributed to evaporation (Fig. 9). The decrease in concentration values of these ions during the rainy season may be due to dilution by rainwater. The significantly higher conductivity value recorded for Azigza Lake during the wet season suggests that allochthonous materials brought in by runoff draining the catchment area during precipitation play a minor role in the hydrochemistry of the lake compared to the carbonate precipitation/dissolution process. These results are in agreement with the findings of others in the Middle Atlas region. For example, Etabaai et al. (2012) pointed out that the dry season of Lake Ifrah is reflected by lower levels of alkaline earth elements (Ca2+ and Mg2+) as a result of their precipitation as endogenic carbonates and by an increase in salinity manifested mainly by increased alkali contents (Na+ and K+) and chloride. However, the seasonal patterns of chemical composition of this type of lake are disturbed by the increasing impacts from human activities and climate change that have had a negative effect on the water budget along with a major increase in the lake’s salinity (increased concentrations of alkaline elements and chlorides) and nutrient enrichment (phosphates and nitrates) (Etabaai et al. 2012). At Azigza Lake, it is likely that anthropogenic influence in the form of excess nutrients is negligible, as evidenced by the low concentration of NO3−. Furthermore, as mentioned above, the concentration of Cl− was equal to Na+ concentration in most of the lake samples (Fig. 7d), which indicates that there is no excess Cl− or Na+ in the site waters related to anthropogenic activities. The PCA and correlation results do not support relationships between NO3− and other physicochemical parameters such as Cl− or Na+ that could reflect the impact of anthropogenic activities on water quality in the area (Fig. 4, Tab. 2).
Daily precipitation on the region (Tamchachat station), lake level changes in Azigza Lake (Adallal et al. 2019), water temperature of the lake, pH, electronic conductivity (EC) and time variations for Ca2+ and Cl.− concentrations from July 2013 to October 2014
4.3 Lake/Groundwater Interaction
The Azigza lake level fluctuated, with a clear decreasing trend from July 2013 to October 2014 (Fig. 9). Recent studies have demonstrated a strong link of Azigza Lake level fluctuations with precipitation variability (Vidal et al. 2016) supported by paleohydrological evidence in Jouve et al. (2019). A detailed study of Azigza Lake, based on a 4-year observation period, including lake level measurements, isotope analyses of precipitation, lake and spring waters and local meteorological data showed the dominance of groundwater exchanges in the lake water balance, with significant interannual variations related to annual precipitation (Adallal et al. 2019). Azigza groundwater has a similar stable isotope signature of δ18O and δD to that of rainfall (Adallal et al. 2019), indicating rapid recharge of the groundwater by rainfall via karst features. Therefore, the lake water chemistry variability is mainly related to the degree of interaction between surface and groundwater. Concentrations of major ions indicate that both lake water and groundwater belong to a common water type (Ca2+–Mg2+–HCO3–) with similar ion composition (Fig. 3). However, the TDS values showed that groundwaters are more concentrated compared to lake waters, with average values of 427 and 310 mg/L, respectively (Table 1). The reason for the high TDS of groundwaters is the longer flow path which increases the interaction time between water and rocks and hence mineral weathering and dissolution. Therefore, the much higher TDS of lake waters observed during the wet season can result from the groundwater contribution to the increased concentration of solutes in the lake. This rapid response of the lake hydrochemistry to rain episodes is consistent with that observed in the geochemistry of spring waters in the region and explains the turbidity problems after strong rainfall events (Bouchaou et al. 2002; El Khalki and Hafid 2002). Amraoui et al. (2003) showed a weak memory effect and little inertia of the karstic systems based on the rainfall–turbidity analysis. In contrast, Howell et al. (2019) carried out an investigation of the weak response of three Middle Atlas springs to storm events using autocorrelation lag times and spectral regulation times for water temperature and water level and demonstrated significant inertia in those spring basins, which further indicates the limited karstification of limestone–dolomite formations. Other hydrodynamic and hydrochemical studies suggest that there is a high degree of inertia in the system as a whole although an upper karst is active in high water conditions (Bouchaou et al. 1997; Naoura et al. 2021). Azigza Lake lies in a depression of structural origin along an anomalous contact between crushed and faulted limestone formations (Lepoutre et al. 1967; Hinaje and Ait Brahim 2002), which suggests that this structurally favorable area ensures a fast groundwater flow to the lake (Adallal et al. 2019). Regional hydrogeological studies have demonstrated the role played by fracturing and/or faults in the preferential direction of water flow and infiltration in the Middle Atlas area (Muzirafuti et al. 2005; El Fartati et al. 2021; Tahiri et al. 2020). This underlines the importance of changes in groundwater fluxes on the Azigza Lake water level, probably representative of many other lakes and wetlands belonging to the Middle Atlas karstic system and thus, indicates their sensitivity to short-term hydroclimatological changes. Consequently, prolonged drought may significantly reduce discharges and induce a lake level drop, as observed in Azigza Lake where multiple lake level drops after periods of drought have occurred during the last few decades (Flower and Foster 1992; Jouve et al. 2019). Similarly, a dramatic lake level decrease has been observed in most of the Middle Atlas lakes over recent decades due to recurrent droughts: Dayet Aoua (Abba et al. 2012; Sayad and Chakiri 2010), Aguelmam Sidi Ali (Chillasse and Dakki 2004; Sayad et al. 2011; Haddout et al. 2018) and Ifrah (Etebaai et al. 2012). The groundwater resources of the Tabular Middle Atlas are also under pressure from pumping for municipal use and irrigation due to population growth (Amraoui et al. 2005; Miche et al. 2018; Ahmed et al. 2021) which could amplify the impact of ongoing climate change on the incoming surface water at a regional scale that includes lakes, springs and rivers.
The role of geological characteristics and fracturing in the close hydraulic connection and interaction between Azigza Lake water and groundwater is clearly observed on a regional scale (in the high basin of OER). The spatial distribution of hydrochemical components (Fig. 1c) made it possible to further elucidate this finding and illustrates the strong control that regional geology and fracturing exert on surface water chemistry, giving rise to two distinct hydrogeological systems: (1) fresh waters located in the upstream part of the basin and mainly flowing through the Lower Jurassic limestone, (2) and on the other hand, more concentrated waters in contact with the Triassic saline clays through fracturing and faults, giving rise to spring waters with high salinities contaminating the downstream part of the watershed.
5 Conclusion
The hydrology, hydrochemistry and associated processes in the high OER basin-Middle Atlas were studied through the hydrochemical monitoring of a typical Moroccan karstic lacustrine system. The lake and groundwater were considered to be freshwater due to the low ionic concentrations and were of the HCO3−–Ca2+–Mg2+ type. Statistical analysis suggested that the major geochemical processes responsible for the observed chemical composition in the area are the dissolution/precipitation of carbonates of Lower Jurassic formations in the region, cation exchange and, to a lesser extent, evaporation. The seasonal variations in concentrations of chemical components demonstrate that the Azigza Lake geochemistry is sensitive to climatic changes in both precipitation and temperature. These results indicate that, when high precipitation drives an increase in the lake level through high inflows of mineralized water, then the ion concentration in lake water increases due to the dissolution of carbonate minerals such as calcite and dolomite. In contrast, strong lake water evaporation decreases the lake level and leads to a decrease in ion concentrations by precipitation of these minerals. The lake receives inflow mainly from subsurface water which has a much higher solute concentration. This is likely to contribute to the increased concentration of solutes in the lake during the rainy season. The rapid response of the lake water chemistry to underground flows after rainfall events suggests a strong interaction between groundwater and lake water. The results obtained at Azigza Lake, representative of many karstic lakes in the Middle Atlas region, provide insight into the hydrochemical functioning and could help decision makers to develop water resource management strategies in these lake basins in order to minimize the negative natural and/or anthropogenic impacts on the water system.
References
Abba H, Nassali H, Benabid M, El Ibaoui H, Chillasse L (2012) Approche physicochimique des eaux du lac dayet Aoua (Maroc). J Appl Biosci 58:4262–4270
Adallal R, Vallet-Coulomb C, Vidal L, Benkaddour A, Rhoujjati A, Sonzogni C (2019) Modelling lake water and isotope mass balance variations of Lake Azigza in the Moroccan Middle Atlas under Mediterranean climate. Reg Environ Change 19:2697–2709. https://doi.org/10.1007/s10113-019-01566-9
Ahmed M, Aqnouy M, El Messari JS (2021) Sustainability of Morocco’s groundwater resources in response to natural and anthropogenic forces. J Hydrol 603:126866. https://doi.org/10.1016/j.jhydrol.2021.126866
Amrani S, Hinaje S (2014) Hydrodynamisme et minéralisation des eaux souterraines de la nappe phréatique plio-quaternaire du plateau Timahdite-Almis Guigou (moyen Atlas, Maroc). Eur Sci J 10:174–189
Amraoui F, Razack M, Bouchaou L (2003) Turbidity dynamics in karstic systems. Example of Ribaa and Bittit springs in the Middle Atlas (Morocco). Hydrol Sci J 48(6):971–984. https://doi.org/10.1623/hysj.48.6.971.51418
Amraoui F, Razack M, Bouchaou L (2005) Impact of a long drought period on a large carbonate aquifer: the Liassic aquifer of the Saïs plain and Middle Atlas plateau (Morocco). In: Regional hydrological impacts of climatic change: hydroclimatic variability, IAHS Publ. 296, IAHS, Wallingford, UK, pp 184–193
Benamar A, Mahjoubi FZ, Kzaibe F (2019) Evaluation of water quality of Oum Er Rbia River (Morocco) using water quality index (WQI) method. J Appl Surf Interfaces. https://doi.org/10.48442/IMIST.PRSM/jasi-v5i1-3.15437
Benkaddour A, Lamb H, Leng M, Gasse F (2005) Stable isotope records of Holocene environmental change from Moroccan lakes: an emerging synthesis. In: PAGES 2nd open science meeting: paleoclimate, environmental sustainability and our future, PAGES, p 549
Benkaddour A, Rhoujjati A, Nourelbait M (2008) Hydrologie et sédimentation actuelles au niveau des lacs Iffer et Aguelmam Azigza (Moyen Atlas, Maroc). In: Le Quaternaire Marocain Dans Son Contexte Méditerranéen: Actes de La Quatrième Rencontre Des Quaternaristes Marocains (RQM4). Faculté des Sciences d’Oujda, Oujda, pp 108–118
Bouchaou L, Michelot JL, Chauve P, Mania J, Mudry J (1995) Apports des isotopes stables à l’étude des modalités d’alimentation des aquifères du Tadla (Maroc) sous climat semi-aride. C.R. Acad. Sci Paris, Série II a 320:95–101
Bouchaou L, Chauve P, Mudry J, Mania J, Hsissou Y (1997) Fonctionnement et structure d’un hydrosystème karstique en zone montagneuse sous climat semiaride : cas de l’Atlas de Beni Mellal (Maroc). J Africa Earth Sci 25(2):225–236
Bouchaou L, Mangin A, Chauve P (2002) Turbidity mechanism of water from a karstic spring: example of the Ain Asserdoune spring (Beni Mellal Atlas, Morocco). J Hydrol 265(1–4):34–42. https://doi.org/10.1016/S0022-1694(02)00098-7
Bouchaou L, Michelot JL, Qurtobi M, Zine N, Gaye CB, Aggarwal PK, Marah H, Zerouali A, Taleb H, Vengosh A (2009) Origin and residence time of groundwater in the Tadla basin (Morocco) using multiple isotopic and geochemical tools. J Hydrol 379(3–4):323–338. https://doi.org/10.1016/j.jhydrol.2009.10.019
Brauer A (2004) Annually laminated lake sediments and their palaeoclimatic relevance. In: Fischer H et al (eds) The climate in historical times towards a synthesis of holocene proxy data and climate models. Springer, Berlin, pp 109–128. https://doi.org/10.1007/978-3-662-10313-5_7
Cao J, Yuan D, Groves C, Huang F, Yang H, Lu Q (2012) Carbon fluxes and sinks: the consumption of atmospheric and soil CO2 by carbonate rock dissolution. Acta Geol Sin Engl Ed 86:963–972. https://doi.org/10.1111/j.1755-6724.2012.00720.x
Cejudo E, Acosta-González G, Ortega-Camacho D, Tun-Rosado GE (2020) Changes in the hydrochemistry of a karstic lake in Yucatan, Mexico. Environ Earth Sci 79(5):1–13. https://doi.org/10.1007/s12665-020-8838-3
Chillasse L, Dakki M (2004) Potentialités et statuts de conservation des zones humides du MoyenAtlas (Maroc), avec référence aux influences de la sécheresse. Sécheresse 15:337–345
Cui BL, Li XY (2015) Runoff processes in the Qinghai Lake Basin, Northeast Qinghai-Tibet Plateau, China: insights from stable isotope and hydrochemistry. Quat Int 380:123–132. https://doi.org/10.1016/j.quaint.2015.02.030
Cui BL, Li XY, Wei XH (2016) Isotope and hydrochemistry reveal evolutionary processes of lake water in Qinghai Lake. J Great Lakes Res 42(3):580–587. https://doi.org/10.1016/j.jglr.2016.02.007
El Fartati M, Hinaje S, Chaouni AA, Yaagoub D, Amrani S, Gharmane Y (2021) Hydrogeology of the Tazouta basin and its relationship with the Neogene-Quaternary brittle tectonics (Folded Middle Atlas, Morocco). Arab J Geosci 14(19):1–14. https://doi.org/10.1007/s12517-021-08453-w
El Jihad MD (2010) Les difficultés de gestion des ressources « naturelles » et de développement rural dans un milieu anthropisé : l’expérience du Projet Oued Srou (Maroc central). Norois. Environnement, Aménagement, Société 216:25–45
El Khalki Y, Hafid A (2002) Turbidité, indicateur du fonctionnement perturbé du géosystème karstique de l’Atlas de Beni Mellal (Moyen Atlas méridional, Maroc). Karstologia 40(1):39–44. https://doi.org/10.3406/karst.2002.2506
Etebaai I, Damnati B, Raddad H, Benhardouz H, Benhardouz O, Miche H, Taieb M (2012) Impacts climatiques et anthropiques sur le fonctionnement hydrogéochimique du Lac Ifrah (Moyen Atlas marocain). Hydrol Sci J 57:547–561. https://doi.org/10.1080/02626667.2012.660158
Flower R, Foster IDL (1992) Climatic implications of recent changes in lake level at Lac Azigza (Morocco). Bulletin De La Sociéte Géologique De France 163:91–96
Flower RJ, Stevenson AC, Dearing JA, Foster IDL, Airey A, Rippey B, Wilson JPF, Appleby PG (1989) Catchment disturbance inferred from paleolimnological studies of three contrasted sub-humid environments in Morocco. J Paleolimnol 1:293–322. https://doi.org/10.1007/BF00184003
Gayral P, Panouse JB (1954) L’Aguelmame Azigza : Recherches Physiques et Biologiques. Bulletin De La Société Des Sciences Naturelles Et Physiques Du Maroc 36:135–159
Gibbs RJ (1970) Mechanisms Controlling World Water Chemistry. Science 170:1088–1090. https://doi.org/10.1126/science.170.3962.1088
Haddout S, Jamali A, Rhazi M, Aghfir M (2018) Finite volume coastal ocean model for water-level fluctuation due to climate change in Aguelmam Sidi Ali Lake (Middle Atlas, Morocco). In : Annales de Limnologie-International Journal of Limnology, vol 54, p 5. EDP Sciences. https://doi.org/10.1051/limn/2017033
Hinaje S, Ait Brahim L (2002) Les bassins lacustres du Moyen Atlas (Maroc): un exemple d’activité tectonique polyphasée associée à des structures d’effondrement. Instituto Geológico e Mineiro 89:283–294
Howell BA, Fryar AE, Benaabidate L, Bouchaou L, Farhaoui M (2019) Variable responses of karst springs to recharge in the Middle Atlas region of Morocco. Hydrogeol J 27(5):1693–1710. https://doi.org/10.1007/s10040-019-01945-w
Hughes TH, Memon BA, LaMoreaux PE (1994) Landfills in karst terrains. Bull Assoc Eng Geol 31:203–208
Hussein MT (2004) Hydrochemical evaluation of groundwater in the Blue Nile basin, eastern Sudan, using conventional and multivariate techniques. Hydrogeol J 12:144–158. https://doi.org/10.1007/s10040-003-0265-5
Jouve G, Vidal L, Adallal R, Rhoujjati A, Benkaddour A, Chapron E, Tachikawa K, Bard E, Courp T, Dezileau L, Hebert B, Rapuc W, Simonneau A, Sonzogni C, Sylvestre F (2019) Recent hydrological variability of the Moroccan Middle Atlas Mountains inferred from microscale sedimentological and geochemical analyses of lake sediments. Quat Res 91:414–430. https://doi.org/10.1017/qua.2018.94
Karroum L, El Baghdadi M, Barakat A, Meddah R, Aadraoui M, Oumenskou H, Ennaji W (2019) Hydrochemical characteristics and water quality evaluation of the Srou River and its tributaries (Middle Atlas, Morocco) for drinking and agricultural purposes. Dwt 146:152–164. https://doi.org/10.5004/dwt.2019.23632
Kelts K, Hsü KJ (1978) Freshwater carbonate sedimentation. In: Lakes, pp 295–323. Springer, New York. https://doi.org/10.1007/978-1-4757-1152-3_9
Khalil N, Rouane SE, Mania J, Mudry J (2004) Essai de bilan hydrochimique sur les eaux du Haut Bassin de l’Oum Er Rbia (Moyen Atlas, Maroc). Revue Francaise De Geotechnique 109:75–85. https://doi.org/10.1051/geotech/2004109075
Kiraly L (2003) Karstification and groundwater flow. Speleogenesis Evol Karst Aquifers 1(3):155–192
Lazhar H, Obda K, Amyay M (2020) Hydroclimatic variability of Aguelmam Sidi Ali (Middle Atlas Central, Morocco) over the past four decades. In: Proceedings of the 4th edition of international conference on geo-IT and water resources 2020, Geo-IT and water resources 2020, pp 1–6. https://doi.org/10.1145/3399205.3399215
Lecomte KL, Bicalho CC, Silva-Filho EV (2016) Geochemical characterization in karst basin tributaries of the San Franciscan depression: the Corrente River, western Bahia, NE-Brazil. J S Am Earth Sci 69:119–130. https://doi.org/10.1016/j.jsames.2016.03.011
Lepoutre B, Martin J, Chamayou J (1967) Le causse Moyen atlasique. Les Cahiers De La Recherche Agronomique 24:207–226
Martin J (1981) Le Moyen Atlas Central: Etude géomorphologique. Service Géologique du Maroc
Menjour F, Amraoui F, Remmal T (2016) Assessment of the spatio-temporal evolution of Aguelmam Sidi Ali Lake using multitemporal landsat imagery (Middle Atlas-Morocco). In: 6th International conference on cartography and GIS, p 588
Miche H, Saracco G, Mayer A, Qarqori K, Rouai M, Dekayir A, Chalikakis K, Emblanch C (2018) Hydrochemical constraints between the karst Tabular Middle Atlas Causses and the Saïs basin (Morocco): implications of groundwater circulation. Hydrogeol J 26(1):71–87. https://doi.org/10.1007/s10040-017-1675-0
Muzirafuti A, Boualoul M, Randazzo G, Lanza S, Allaoui A, El Ouardi H, Habibi H, Ouhaddach H (2005) The use of remote sensing for water protection in the karst environment of the Tabular Middle Atlas/the causse of El Hajeb/Morocco. ASTER 2000:16
Naoura J, El Kati I, Benaabidate L (2021) Assessment of Ras El Ma Karst Spring features by structural and functional approaches at the Region of Taza, Morocco. J Ecol Eng. https://doi.org/10.12911/22998993/141477
Nordstrom DK, Blowes DW, Ptacek CJ (2015) Hydrogeochemistry and microbiology of mine drainage: an update. Appl Geochem 57:3–16. https://doi.org/10.1016/j.apgeochem.2015.02.008
Parkhurst DL, Apello C (1999) User’s guide to PHREEQC (V2). US Geol. Surv 312
Piper AM (1944) A graphic procedure in the geochemical interpretation of water-analyses. EOS Trans Am Geophys Union 25(6):914–928. https://doi.org/10.1029/TR025i006p00914
Plummer LN, Busenberg E (1982) The solubilities of calcite, aragonite and vaterite in CO2–H2O solutions between 0 and 90 °C, and an evaluation of the aqueous model for the system CaCO3-CO2-H2O. Geochim Cosmochim Acta 46(6):1011–1040. https://doi.org/10.1016/0016-7037(82)90056-4
Romero-Viana L, Julià R, Camacho A, Vicente E, Miracle M (2008) Climate signal in varve thickness: Lake La Cruz (Spain), a case study. J Paleolimnol 40(2):703–714. https://doi.org/10.1007/s10933-008-9194-6
Sayad A, Chakiri S (2010) Impact de l’évolution du climat sur le niveau de Dayet Aoua dans le Moyen Atlas marocain. Sécheresse 21:245–251. https://doi.org/10.1684/sec.2010.0252
Sayad A, Chakiri S, Martin C, Bejjaji Z, Echarfaoui H (2011) Effet des conditions climatiques sur le niveau du lac Sidi Ali (Moyen Atlas, Maroc). Physio-Géo 5:251–268. https://doi.org/10.4000/physio-geo.2145
Schoeller H (1965) Qualitative evaluation of groundwater resources. In: Methods and techniques of groundwater investigations and development. UNESCO, Paris, pp 54–83
Su Y, Zhu G, Feng Q, Li Z, Zhang F (2009) Environmental isotopic and hydrochemical study of groundwater in the Ejina basin, northwest China. Environ Geol 58:601–614. https://doi.org/10.1007/s00254-008-1534-3
Tahiri A, Amraoui F, Sinan M (2020) Remote sensing, GIS, and Fieldwork Studies to assess and valorize groundwater resources in the springs area of oum-Er-rabiaa (Moroccan Middle Atlas). Groundw Sustain Dev 11:100440. https://doi.org/10.1016/j.gsd.2020.100440
Vidal L, Rhoujjati A, Adallal R, Jouve G, Bard E, Benkaddour A, Chapron E, Courp T, Dezileau L, Garcia M, Hebert B, Simmoneau A, Sonzogni C, Sylvestre F, Tachikawa K, Vallet-Coulomb C, Viry E (2016) Past hydrological variability in the Moroccan Middle Atlas inferred from lakes and lacustrine sediments. In: The Mediterranean Region under climate change, pp 57–69
Welch SP (1948) Limnological methods. McGraw Hill Book Co., New York, p 381
White WB (1988) Geomorphology and hydrology of Karst Terrains. Oxford University Press, Oxford
Xiao J, Jin Z, Zhang F, Wang J (2012) Solute geochemistry and its sources of the groundwaters in the Qinghai Lake catchment, NW China. J Asian Earth Sci 52:21–30. https://doi.org/10.1016/j.jseaes.2012.02.006
Xu H, Hou Z, An Z, Liu X, Dong J (2010) Major ion chemistry of waters in Lake Qinghai catchments, NE Qinghai-Tibet plateau, China. Quat Int 212(1):35–43. https://doi.org/10.1016/j.quaint.2008.11.001
Zielhofer C (2004) Hydrographical and hydrochemical characteristics of karst water components (southern Franconian Jura)—a contribution to fresh water protection and karst morphogenesis. Zeitschrift Für Geomorphologie Suppl 136:113–134
Acknowledgements
This work was supported by FR-ECCOREV, LABEX OT-Med (# ANR-11-LABX-0061) (PHYMOR project) (France), CNRST (Morocco), PHC Toubkal (Project # 16/38) and Campus France. The authors extend their thanks to SETEL- and SIGEO- CEREGE and IRD-Rabat for logistic support during the field trips (2013 and 2015).
Funding
This study was partly funded by FR-ECCOREV, LABEX OT-Med (# ANR-11-LABX-0061) (PHYMOR project) (France), CNRST (Morocco), PHC Toubkal (Project # 16/38). The authors have no relevant financial or non-financial interests to disclose.
Author information
Authors and Affiliations
Contributions
Adallal R and Id Abdellah H processed the experimental data, performed the analysis, drafted the manuscript and designed the figures. Vidal L, Benkaddour A and Rhoujjati A commented on previous versions of the manuscript and supervised the work. Vallet-Coulomb C and Sonzogni C aided in interpreting the results and reviewed the manuscript. All authors provided critical feedback and helped shape the research, analysis and manuscript
Corresponding author
Ethics declarations
Conflict of interest
We confirm that there are no known conflict of interest associated with this publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Adallal, R., Id Abdellah, H., Benkaddour, A. et al. Hydrogeochemical Processes of the Azigza Lake System (Middle Atlas, Morocco) Inferred from Monthly Monitoring. Aquat Geochem 29, 25–47 (2023). https://doi.org/10.1007/s10498-022-09409-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10498-022-09409-6