Abstract
Epithelial ovarian cancer is a lethal gynecological cancer. It is related to high mortality because the majority of the patients present in advanced stage and because of the high recurrence rates of the disease. Recurrent ovarian cancer is classified according to the time interval between the last platinum-based chemotherapy and the occurrence of recurrence, to platinum-sensitive and platinum-resistant. Many theories tried to explain development of resistance to platinum-based therapy. “Cancer stem cells” is one of these theories and is being currently under investigation by many groups. This chapter will demonstrate the suggested contribution of cancer stem cells to the development of recurrent ovarian cancer.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
2.1 Ovarian Cancer, Treatment, and Recurrence
Epithelial ovarian cancer (EOC) accounts for approximately 238,000 new cases annually worldwide and is responsible for at least 150,000 deaths every year [1]. More than 70% of patients present with advanced stages III or IV [2]. The estimated 5-year overall survival (OS) of stages III and IV are 35% and 22%, respectively [3]. The standard treatment of EOC is primary debulking, aiming at complete cytoreduction followed by six cycles of combined carboplatin and paclitaxel and, eventually, bevacizumab [4, 5]. This treatment strategy is based on many studies showing a remarkable benefit for patients who receive a complete macroscopic resection through aggressive cytoreductive surgery [6,7,8]. Thus, patients with tumor residues <1 cm after primary debulking have relatively worse prognoses, while patients with tumor resides >1 cm have prognoses compared with those who do not undergo debulking [9, 10]. Carboplatin and paclitaxel significantly influence OS compared with other regimens [11,12,13,14,15]. Bevacizumab confers survival benefits upon patients with advanced EOC, particularly those with tumor residues after debulking surgery [16, 17].
Accumulating evidence shows that neoadjuvant chemotherapy followed by interval debulking leads to increases in complete tumor resection rates, with OS rates comparable with those of primary debulking followed by adjuvant chemotherapy [18, 19]. Moreover, this regimen achieves lower morbidity, mortality, and better quality of life [20]. These findings led oncologists to consider neoadjuvant chemotherapy followed by interval debulking as a possible therapeutic option in certain clinical situations, where primary debulking surgery is difficult to perform because of patients’ unfavorable general conditions or a very advanced nonresectable tumor stage [4, 21]. Unfortunately, as many as 70% of patients with advanced EOC will experience recurrence after standard treatment [12, 15, 22]. Recurrences are so frequent in such patients that only 10–30% survive long term [23].
EOC recurrences are classified as “platinum-sensitive” and “platinum-resistant,” according to the response to initial platinum-based therapy (see below). This classification determines platinum resistance according to recurrence based on clinical symptoms, clinically detectable disease or radiological evidence of disease recurrence, or both [24,25,26]. A patient is designated “platinum-sensitive” if she initially responds to platinum-based chemotherapy and does not experience a relapse for ≥6 months after initial treatment. Approximately 30–90% of these patients will respond to further platinum-based chemotherapy with a median survival of 2 years, although survival can range from a few months to more than a decade [27,28,29]. Many patients will receive multiple lines of treatment over time, but with few exceptions, will ultimately develop platinum-resistant disease. Patients who relapse within 6 months of completing first-line therapy are classified as “platinum-resistant” and typically have response rates <15% to subsequent chemotherapy, progression-free survival of 3–4 months, and median survival <1 year [30].
2.2 Cytotoxic Effects of Platinum and Platinum Resistance
The cytotoxicity of platinum therapy is mainly caused by DNA damage [31]. Platinum-based drugs react with guanine nucleotides to form platinum–DNA mono-adducts, which often react with a second purine nucleotide to form interstrand and intrastrand crosslinks, leading to increased cytotoxicity. Carboplatin and cisplatin form the same platinum–DNA crosslinks in vivo because of their identical cis-diamine ligands [31]. The formation of intrastrand and interstrand crosslinks leads to cell death through apoptosis or necrosis. These processes are irreversible unless the crosslinks are repaired. Apoptosis is executed by a series of cysteine proteases termed caspases [32]. Caspase activation leads to mitochondrial dysfunction [33] and DNA fragmentation [34].
Carboplatin and cisplatin share similar in vitro chemoresistance spectra and clinical indications, although cisplatin is possibly more effective for certain cancers [31]. Factors associated with resistance to platinum include those that limit the formation of cytotoxic platinum–DNA adducts and those that prevent cell death after platinum-adduct formation [35]. The former may result from reduced uptake of cisplatin into cells, increased efflux via alterations to transport proteins, or through inactivation of intracellular cisplatin by its conversion to cisplatin-thiol conjugates. The latter form of resistance may occur through increased DNA repair after adduct formation. The five major DNA repair mechanisms are as follows: nucleotide excision repair, mismatch repair, homologous recombination, base-excision repair, and translesion synthesis [35].
The cancer stem cell (CSC) model and the environment-mediated drug resistance model (EMDR) were proposed to explain the origin of drug-resistant cells [36]. The CSC model proposes that genetic or epigenetic alterations, or both, which occur in multipotent, tissue-specific adult stem cells, may induce malignant transformation to generate CSCs. CSCs possess stem cell-like properties, including self-renewal and cell division to form tumors that acquire further genetic or epigenetic alterations. Such alterations may contribute to the development of invasive properties that allow the tumor to metastasize to distant sites [30, 37, 38]. CSCs may be intrinsically resistant to chemotherapy through different mechanisms and may represent a major source of chemoresistant cells within tumors [39, 40].
In the EMDR model, resistance emerges as the cancer cells interact with their surrounding microenvironment and enter a quiescent state caused by the complex interplay between the tumor and its microenvironment. Tumors that develop a prominent desmoplastic reaction are associated with poor prognosis as well as with platinum resistance [30, 40].
2.3 CSCs
Stem cells are defined as cells that perpetuate through self-renewal and differentiation to mature cells of a particular tissue [41]. Stem cells must therefore be prospectively identified and carefully purified to study their properties. Unfortunately, isolation of tissue-specific stem cells could not be universally achieved, as somatic stem cells identification and isolation have been achieved only in a few instances. For example, human hematopoietic stem cells [42] generate and reconstitute the hematopoietic and immune (hematolymphoid) systems [41, 42]. Although CSCs were originally described in hematological cancers, they have been isolated from solid tumors [43, 44].
An important issue in stem cell biology is understanding the mechanisms that regulate self-renewal. Self-renewal , which is crucial to stem cell function, is a property of diverse stem cells required for their lifelong persistence. Moreover, whereas stem cells from different organs may vary in their developmental potential, all stem cells must self-renew and regulate the relative balance between self-renewal and differentiation. Understanding the regulation of the self-renewal of normal stem cells is fundamental to the understanding of the regulation of cancer cell proliferation because cancer can be considered a disease of unregulated self-renewal.
Another fundamental attribute of stem cells is their transient or long-term quiescence (also termed dormancy) [45], which is a component of the mechanism of regulated self-renewal. Accordingly, stem cells are often identified by their propensity to retain labeled DNA much longer than their rapidly proliferating offspring. Moreover, dormancy may serve as a crucial mechanism for the resistance of CSCs to chemotherapy. The dormancy of CSCs may explain the appearance of local recurrence or distant metastasis after long delays [45]. Figure 2.1 shows a hypothetical model of the CSC concept and its evolution.
A theoretical synthesis of the clonal Cancer stem cell evolution and CSC concepts. Top to bottom: clonal evolution drives tumor progression [46]. (1) The first oncogenic mutation (lightning arrow) occurs in a stem cell (or, alternatively, benign Stem cell in a progenitor or even a differentiated cell) of lesion (progenitor or a healthy epithelium), resulting in the growth of a genetically homogeneous benign lesion. (2) The second hit targets one of the cells in the benign lesion, which leads to the growth of a more malignant and invasive clone within the primary tumor. (3) A third hit in a cell within the malignant sub clone causes further transformation, visualized as entry into a blood vessel for distant metastasis. Genetically independent sub clones can coexist within the tumor. (4) Final mutational hit leads to tumor being entirely taken over by cells that behave as malignant cancer stem cells. Shown, left to right: at each tumor stage of this clonal evolution process, tumors and sub clones within tumors contain some cells that behave as CSCs
2.4 Identification of CSCs
Many markers define CSC populations , and the most commonly reported for solid tumors are CD24, CD44, and CD133. CSC populations are commonly defined by the presence or absence of various combinations of cell surface proteins. Reacting the cells with antibodies against these markers readily identifies cell populations of interest, which are isolated using fluorescence-activated cell sorting [43]. Figure 2.2 shows the currently available markers used to identify different subsets of CSCs in different tumors.
Currently identified surface markers for CSC [47]
2.5 Ovarian CSCs
CSCs isolated from ovarian cancer are associated with worse prognosis and recurrence [48]. The use of markers of ovarian CSCs, such as CD44, CD24, and CD133, is proposed by recent studies.
2.5.1 CD44
CD44 is a glycoprotein that is widely presented on the outer surface of many mammalian cells such as endothelial cells, epithelial cells, fibroblasts, and leukocytes [46]. CD44 is a surface marker of CSCs in many tissues such as breast, pancreas, gastric, prostate, head, neck, ovarian, and colon [47, 49]. CD44 is associated with diseases such as cancer, arthritis, interstitial lung disease, vascular disease as well as in wound healing and infections. Several studies focus on CD44–HA signaling and its implications in malignancies of solid organs such as breast and ovarian cancer [47, 49, 50].
A single gene encodes CD44, which is located on chromosome 11 in humans and chromosome 2 in mice. There are approximately 20 CD44 isoforms ranging from 80 to 200 kDa. The heterogeneity of this group is generated by post-transcriptional regulation, including alternative splicing and protein modifications. All isoforms are encoded by exons 1–5 and 16–18, whereas exons 6–15 and 19–20 are present in isoforms generated by alternative splicing [50, 51]. Specific to the tissue and isoform, CD44 plays roles in adhesion, motility, proliferation, and cell survival [52]. CD44 contains four major domains, including the conserved extracellular hyaluronan-binding domain and variably spliced regions, the transmembrane sequence, and the intracellular cytoskeletal/signaling domain. Figure 2.3 shows the structure and genomic organization of CD44 [51].
Genetic encoding of CD44 (a) and its basic structure (b) [52]
Interactions between CD44 and the extracellular matrix glycosaminoglycan hyaluronan (HA) are currently under investigation. HA is enriched in the stem cell niche and likely plays an integral role in the function of CD44 in CSCs [53, 54]. CD44 guides the epithelial stromal reaction with the extracellular microenvironment (ECM) to direct intracellular signaling and modifies the ECM. The extracellular domain of CD44 binds ECM components such as collagen, laminin, fibronectin, and HA [55]. CD44 contains binding sites for glycosaminoglycans other than HA, for example, osteopontin [56]. HA is the best-characterized CD44 ligand and possesses an immense repertoire of biological functions. HA, which is a cell-surface-associated glycosaminoglycan that is ubiquitous in extracellular and pericellular matrices, is synthesized and simultaneously secreted by transmembrane HA synthases as a 106–107 kDa polymer [56, 57].
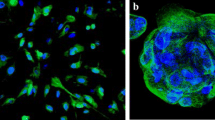
CD44 modulates many signaling activities through interactions with its cytoplasmic tail. Treatment with soluble low or high molecular weight HA induces cell invasion and migration through CD44-mediated activation of Rho family GTPases. Hyaluronan–CD44 interactions initiate recruitment of signaling molecules such as Tiam1, p115, Rac1, Rho Gefs, Rho-associated protein kinase, and cSrc. Interactions with signaling molecules lead to activation of the PI3K signaling pathway and a number of cellular functions such as survival and invasion [57]. Figure 2.4 illustrates CD44-mediated signal transduction [57] and Fig. 2.5 shows an example of immunohistochemical detection of CD44.
Signal transduction of CD44 [58]
2.5.2 CD24
Mouse CD24 was first identified as a heat-stable antigen 30 years ago, and the CD24 gene was molecularly cloned and found to encode a small protein whose mature form comprises 27 amino acid residues [58, 59]. Human CD24 is located on chromosome 6q21, as determined by in situ hybridization [60]. The CD24 isoforms isolated from different tissues or cell types have different molecular masses, ranging from 20 to 70 kDa, depending on cell or tissue type, demonstrating that the glycosylation of CD24 is highly variable and cell-type dependent [61]. CD24 is expressed by hematopoietic cells such as B cells and T cells as well as by nonhematopoietic cells such as neurons, epithelial cells, and epithelial stem cells [61,62,63].
The main role of CD24 in most cell types is unclear; however, certain immune regulatory functions of CD24 are known [64]. CD24 is broadly overexpressed by many types of tumor tissues, particularly those of the breast [65] and ovary [66]. For example, in breast cancer, cell surface and cytoplasmic expression of CD24 is associated with poor prognosis, histological grades, tumor size, and lymph node positivity [65, 67]. CD24 is expressed in epithelial ovarian cancer. Although most published work demonstrates an association of CD24 expression with advanced disease stage and poor prognosis, the association is controversial. CD24+ cells exhibit increased tumor-forming and tumor-initiating capacities. Interestingly, CD24+ or CD24− cells can initiate a tumor. This may be explained by in vitro and in vivo lineage tracking experiments showing the conversion of CD24− to CD24+ cells. Therefore, CD24+ may act as a transition phase between cancer stem cells and tumorigenesis [68].
2.5.3 CD133
CD133 is a surface marker that was identified in epithelial ovarian cancer [69], endometrial cancer, neuronal cancer, and colon cancer [70]. The role of CD133 in tumor progression is unclear. CD133 may serve as a prognostic marker of low-risk endometrial cancer [71]. The expression of CD133 may be associated with enhanced tumorigenesis in animal models of human melanoma and colon cancer [72, 73]. The role of CD133 in disease progression and resistance to chemotherapy is unclear.
2.6 Resistance to Chemotherapy and CSCs
It is often suggested that CSCs are resistant to therapy in the same way that normal stem cells are protected against insult. These protections include the aforementioned quiescence as well as expression of drug pumps, high expression of antiapoptotic proteins, and resistance to DNA damage [74]. Some groups have started to determine if CSCs are more resistant to therapy than their progeny. For example, CD133-expressing glioma cells are more resistant to ionizing radiation compared with CD133negative tumor cells [75]. CD44high/CD24low breast cancer CSCs appear intrinsically resistant to conventional chemotherapy and ionizing radiation [76], and chronic myeloid leukemia is sustained by leukemic stem cells that are relatively resistant to imatinib [77].
In EOC, platinum resistance is a very important issue because of the high recurrence rate of the disease. Several studies attempted to explain the development of platinum resistance in EOC, but there is no consensus regarding its development. Patients with primary “platinum refractory” disease are intrinsically drug resistant and do not respond or progress very early following treatment. Primary platinum-refractory ovarian cancers are uncommon and usually occur with nonserous ovarian cancers such as clear cell carcinoma or mucinous carcinoma vs. the more common high-grade serous carcinoma. It is likely that the mechanisms of resistance among these various histotypes are very different.
Patients who experience an initial response to platinum chemotherapy may have tumors comprising populations of intrinsically platinum-resistant and platinum-sensitive cells. The sensitive cells undergo apoptosis following chemotherapy (tumor response), but the resistant subpopulation persists and expands, leading to early recurrence in platinum-resistant disease. Platinum-sensitive patients may respond repeatedly to platinum, because of the regrowth of the sensitive population. Ultimately however the sensitive cells may alter, rendering them resistant, or the resistant cell population will outgrow the sensitive population [30].
Another important characteristic of EOC is its heterogeneity. Heterogeneity exists spatially within the primary tumor and between the primary tumor and its metastases that are transient, as indicated by biopsies performed at different times [78, 79]. This heterogeneity significantly adds to the complexity of assessing or interpreting the response to treatment and patients’ outcomes. This property is supported by anecdotal clinical observations of patients with differential responses to treatment, with progression at one site and responses at other sites. The mechanisms that explain how frequently this occurs are unknown, and there is no guidance or consensus on the appropriate management of these patients. In future studies, particularly of targeted therapies, repeat biopsies upon recurrence and after further treatment will be essential to gain a better understanding of the mechanisms of resistance. The CSC theory can explain this heterogeneity, where different subsets of CSCs proliferate in the same tumor and during the development of different metastasis, leading to different phenotypes of the same tumor.
Treatment of EOC recurrence is a dilemma, particularly for platinum-resistant patients. Chemotherapeutic agents such as doxorubicin, gemcitabine, topotecan, trabectedin, and paclitaxel as well as targeted therapies such as bevacizumab, olaparib, and niraparib were evaluated in clinical trials designed to develop a strategy to achieve an adequate response. Until recently, phase III trials did not reveal any significant improvement in the progression-free interval (PFI) or OS. Two studies of chemotherapy plus an antiangiogenic agent achieved improved PFI, but not OS, in the platinum-resistant subset. The AURELIA study (involving chemotherapy combined with bevacizumab) achieved an approximate doubling of the PFI (3.4 vs. 6.7 months, HR 0.48, p < 0.001) vs. without bevacizumab, but no improvement in OS [80]. The TRINOVA 1 study (paclitaxel combined with the angiopoietin 1/2 inhibitor trebananib) achieved an improved PFI (5.4 vs. 7.2 months, p < 0.001) [81].
Poly ADP-ribose inhibitors achieved promising results in reducing the recurrence of EOC. Patients with platinum-sensitive recurrence with or without a BRCA mutation experience a slightly better response to olaparib and niraparib [82, 83]. An unanswered question is if the same effect will appear in the platinum-resistant subset of patients.
Recurrent EOC remains a significant treatment dilemma, mainly because of our limited understanding of the development of the resistance to chemotherapy. CSCs may contribute to recurrent EOC, and this will remain a hypothetical possibility until experimentally and clinically verified.
References
Torre, L. A., Bray, F., Siegel, R. L., Ferlay, J., Lortet-Tieulent, J., & Jemal, A. (2015). Global cancer statistics, 2012. CA: A Cancer Journal for Clinicians, 65, 87–108.
Sehouli, J., Senyuva, F., Fotopoulou, C., Neumann, U., Denkert, C., Werner, L., et al. (2009). Intra-abdominal tumor dissemination pattern and surgical outcome in 214 patients with primary ovarian cancer. Journal of Surgical Oncology, 99, 424–427.
Garcia, M., Jemal, A., Ward, E., Center, M., Hao, Y., Siegel, R., et al. (2007). Global cancer facts & figures 2007. American Cancer Society: Atlanta, GA.
National-Cancer-Comprehensive-Network. (2017). NCCN clinical practice guidelines in oncology (NCCN Guidelines®) ovarian cancer. NCCN.
AGO-Leitlinienkommission. (2013). S3-Leitlinie Diagnostik, Therapie und Nachsorge maligner Ovarialtumoren.
du Bois, A., Reuss, A., Pujade-Lauraine, E., Harter, P., Ray-Coquard, I., & Pfisterer, J. (2009). Role of surgical outcome as prognostic factor in advanced epithelial ovarian cancer: A combined exploratory analysis of 3 prospectively randomized phase 3 multicenter trials: By the Arbeitsgemeinschaft Gynaekologische Onkologie Studiengruppe Ovarialkarzinom (AGO-OVAR) and the Groupe d’Investigateurs Nationaux Pour les Etudes des Cancers de l’Ovaire (GINECO). Cancer, 115, 1234–1244.
Trimbos, J. B., Vergote, I., Bolis, G., Vermorken, J. B., Mangioni, C., Madronal, C., et al. (2003). Impact of adjuvant chemotherapy and surgical staging in early-stage ovarian carcinoma: European Organisation for Research and Treatment of Cancer-Adjuvant ChemoTherapy in Ovarian Neoplasm trial. Journal of the National Cancer Institute, 95, 113–125.
Hoskins, W. J., McGuire, W. P., Brady, M. F., Homesley, H. D., Creasman, W. T., Berman, M., et al. (1994). The effect of diameter of largest residual disease on survival after primary cytoreductive surgery in patients with suboptimal residual epithelial ovarian carcinoma. American Journal of Obstetrics and Gynecology, 170, 974–9; discussion 9–80.
Winter, W. E., III, Maxwell, G. L., Tian, C., Carlson, J. W., Ozols, R. F., Rose, P. G., et al. (2007). Prognostic factors for stage III epithelial ovarian cancer: A Gynecologic Oncology Group Study. Journal of Clinical Oncology, 25, 3621–3627.
Ataseven, B., Grimm, C., Harter, P., Heitz, F., Traut, A., Prader, S., et al. (2015). Prognostic impact of debulking surgery and residual tumor in patients with epithelial ovarian cancer FIGO stage IV. Gynecologic Oncology, 140(2), 215–220.
Piccart, M. J., Bertelsen, K., James, K., Cassidy, J., Mangioni, C., Simonsen, E., et al. (2000). Randomized intergroup trial of cisplatin-paclitaxel versus cisplatin-cyclophosphamide in women with advanced epithelial ovarian cancer: Three-year results. Journal of the National Cancer Institute, 92, 699–708.
McGuire, W. P., Hoskins, W. J., Brady, M. F., Kucera, P. R., Partridge, E. E., Look, K. Y., et al. (1996). Cyclophosphamide and cisplatin compared with paclitaxel and cisplatin in patients with stage III and stage IV ovarian cancer. The New England Journal of Medicine, 334, 1–6.
Omura, G., Blessing, J. A., Ehrlich, C. E., Miller, A., Yordan, E., Creasman, W. T., et al. (1986). A randomized trial of cyclophosphamide and doxorubicin with or without cisplatin in advanced ovarian carcinoma. A Gynecologic Oncology Group Study. Cancer, 57, 1725–1730.
du Bois, A., Luck, H. J., Meier, W., Adams, H. P., Mobus, V., Costa, S., et al. (2003). A randomized clinical trial of cisplatin/paclitaxel versus carboplatin/paclitaxel as first-line treatment of ovarian cancer. Journal of the National Cancer Institute, 95, 1320–1329.
Ozols, R. F., Bundy, B. N., Greer, B. E., Fowler, J. M., Clarke-Pearson, D., Burger, R. A., et al. (2003). Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: A Gynecologic Oncology Group study. Journal of Clinical Oncology, 21, 3194–3200.
Burger, R. A., Brady, M. F., Bookman, M. A., Fleming, G. F., Monk, B. J., Huang, H., et al. (2011). Incorporation of bevacizumab in the primary treatment of ovarian cancer. The New England Journal of Medicine, 365, 2473–2483.
Oza, A. M., Cook, A. D., Pfisterer, J., Embleton, A., Ledermann, J. A., Pujade-Lauraine, E., et al. (2015). Standard chemotherapy with or without bevacizumab for women with newly diagnosed ovarian cancer (ICON7): Overall survival results of a phase 3 randomised trial. The Lancet Oncology, 16, 928–936.
Kehoe, S., Hook, J., Nankivell, M., Jayson, G. C., Kitchener, H., Lopes, T., et al. (2015). Primary chemotherapy versus primary surgery for newly diagnosed advanced ovarian cancer (CHORUS): An open-label, randomised, controlled, non-inferiority trial. The Lancet, 386, 249–257.
Vergote, I., Amant, F., Kristensen, G., Ehlen, T., Reed, N. S., & Casado, A. (2011). Primary surgery or neoadjuvant chemotherapy followed by interval debulking surgery in advanced ovarian cancer. European Journal of Cancer, 47, S88–S92.
Fagotti, A., Ferrandina, G., Vizzielli, G., Fanfani, F., Gallotta, V., Chiantera, V., et al. (2016). Phase III randomised clinical trial comparing primary surgery versus neoadjuvant chemotherapy in advanced epithelial ovarian cancer with high tumour load (SCORPION trial): Final analysis of peri-operative outcome. European Journal of Cancer, 59, 22–33.
Wright, A. A., Bohlke, K., Armstrong, D. K., Bookman, M. A., Cliby, W. A., Coleman, R. L., et al. (2016). Neoadjuvant chemotherapy for newly diagnosed, advanced ovarian cancer: Society of Gynecologic Oncology and American Society of Clinical Oncology clinical practice guideline. Journal of Clinical Oncology, 34, 3460–3473.
Armstrong, D. K., Bundy, B., Wenzel, L., Huang, H. Q., Baergen, R., Lele, S., et al. (2006). Intraperitoneal cisplatin and paclitaxel in ovarian cancer. The New England Journal of Medicine, 354, 34–43.
Cannistra, S. A. (2004). Cancer of the ovary. The New England Journal of Medicine, 351, 2519–2529.
Markman, M., Rothman, R., Hakes, T., Reichman, B., Hoskins, W., Rubin, S., et al. (1991). Second-line platinum therapy in patients with ovarian cancer previously treated with cisplatin. Journal of Clinical Oncology, 9, 389–393.
Blackledge, G., Lawton, F., Redman, C., & Kelly, K. (1989). Response of patients in phase II studies of chemotherapy in ovarian cancer: Implications for patient treatment and the design of phase II trials. British Journal of Cancer, 59, 650–653.
Gore, M. E., Fryatt, I., Wiltshaw, E., & Dawson, T. (1990). Treatment of relapsed carcinoma of the ovary with cisplatin or carboplatin following initial treatment with these compounds. Gynecologic Oncology, 36, 207–211.
Balbi, G., Di Prisco, L., Musone, R., Menditto, A., Cassese, E., Balbi, C., et al. (2002). Second-line with paclitaxel and carboplatin for recurrent disease following first paclitaxel and platinum compounds in ovarian carcinoma. European Journal of Gynaecological Oncology, 23, 347–349.
Hoekstra, A. V., Hurteau, J. A., Kirschner, C. V., & Rodriguez, G. C. (2009). The combination of monthly carboplatin and weekly paclitaxel is highly active for the treatment of recurrent ovarian cancer. Gynecologic Oncology, 115, 377–381.
Rose, P. G., Fusco, N., Fluellen, L., & Rodriguez, M. (1998). Second-line therapy with paclitaxel and carboplatin for recurrent disease following first-line therapy with paclitaxel and platinum in ovarian or peritoneal carcinoma. Journal of Clinical Oncology, 16, 1494–1497.
Davis, A., Tinker, A. V., & Friedlander, M. (2014). “Platinum resistant” ovarian cancer: What is it, who to treat and how to measure benefit? Gynecologic Oncology, 133, 624–631.
Goodisman, J., Hagrman, D., Tacka, K. A., & Souid, A. K. (2006). Analysis of cytotoxicities of platinum compounds. Cancer Chemotherapy and Pharmacology, 57, 257–267.
Cohen, G. M. (1997). Caspases: The executioners of apoptosis. The Biochemical Journal, 326(Pt 1), 1–16.
Petit, P. X., Zamzami, N., Vayssiere, J. L., Mignotte, B., Kroemer, G., & Castedo, M. (1997). Implication of mitochondria in apoptosis. Molecular and Cellular Biochemistry, 174, 185–188.
Nagata, S. (2000). Apoptotic DNA fragmentation. Experimental Cell Research, 256, 12–18.
Tapia, G., & Diaz-Padilla, I. (2013). Molecular mechanisms of platinum resistance in ovarian cancer. In I. Diaz-Padilla (Ed.), Ovarian cancer—A clinical and translational update. Rijeka: InTech.
Siddik, Z. H. (2003). Cisplatin: Mode of cytotoxic action and molecular basis of resistance. Oncogene, 22, 7265–7279.
Dean, M., Fojo, T., & Bates, S. (2005). Tumour stem cells and drug resistance. Nature Reviews. Cancer, 5, 275–284.
Massard, C., Deutsch, E., & Soria, J. C. (2006). Tumour stem cell-targeted treatment: Elimination or differentiation. Annals of Oncology, 17, 1620–1624.
Mimeault, M., Hauke, R., Mehta, P. P., & Batra, S. K. (2007). Recent advances in cancer stem/progenitor cell research: Therapeutic implications for overcoming resistance to the most aggressive cancers. Journal of Cellular and Molecular Medicine, 11, 981–1011.
Helleman, J., Jansen, M. P., Burger, C., van der Burg, M. E., & Berns, E. M. (2010). Integrated genomics of chemotherapy resistant ovarian cancer: A role for extracellular matrix, TGFbeta and regulating microRNAs. The International Journal of Biochemistry & Cell Biology, 42, 25–30.
Reya, T., Morrison, S. J., Clarke, M. F., & Weissman, I. L. (2001). Stem cells, cancer, and cancer stem cells. Nature, 414, 105–111.
Baum, C. M., Weissman, I. L., Tsukamoto, A. S., Buckle, A. M., & Peault, B. (1992). Isolation of a candidate human hematopoietic stem-cell population. Proceedings of the National Academy of Sciences of the United States of America, 89, 2804–2808.
Lapidot, T., Sirard, C., Vormoor, J., Murdoch, B., Hoang, T., Caceres-Cortes, J., et al. (1994). A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature, 367, 645–648.
Al-Hajj, M., Wicha, M. S., Benito-Hernandez, A., Morrison, S. J., & Clarke, M. F. (2003). Prospective identification of tumorigenic breast cancer cells. Proceedings of the National Academy of Sciences of the United States of America, 100, 3983–3988.
Ebben, J. D., Treisman, D. M., Zorniak, M., Kutty, R. G., Clark, P. A., & Kuo, J. S. (2010). The cancer stem cell paradigm: A new understanding of tumor development and treatment. Expert Opinion on Therapeutic Targets, 14, 621–632.
Sherman, L., Sleeman, J., Herrlich, P., & Ponta, H. (1994). Hyaluronate receptors: Key players in growth, differentiation, migration and tumor progression. Current Opinion in Cell Biology, 6, 726–733.
Slevin, M., Krupinski, J., Gaffney, J., Matou, S., West, D., Delisser, H., et al. (2007). Hyaluronan-mediated angiogenesis in vascular disease: Uncovering RHAMM and CD44 receptor signaling pathways. Matrix Biology, 26, 58–68.
Elzarkaa, A. A., Sabaa, B. E., Abdelkhalik, D., Mansour, H., Melis, M., Shaalan, W., et al. (2016). Clinical relevance of CD44 surface expression in advanced stage serous epithelial ovarian cancer: A prospective study. Journal of Cancer Research and Clinical Oncology, 142, 949–958.
Misra, S., Hascall, V. C., Berger, F. G., Markwald, R. R., & Ghatak, S. (2008). Hyaluronan, CD44, and cyclooxygenase-2 in colon cancer. Connective Tissue Research, 49, 219–224.
Louderbough, J. M., & Schroeder, J. A. (2011). Understanding the dual nature of CD44 in breast cancer progression. Molecular Cancer Research, 9, 1573–1586.
Screaton, G. R., Bell, M. V., Jackson, D. G., Cornelis, F. B., Gerth, U., & Bell, J. I. (1992). Genomic structure of DNA encoding the lymphocyte homing receptor CD44 reveals at least 12 alternatively spliced exons. Proceedings of the National Academy of Sciences of the United States of America, 89, 12160–12164.
Marhaba, R., & Zoller, M. (2004). CD44 in cancer progression: Adhesion, migration and growth regulation. Journal of Molecular Histology, 35, 211–231.
Tölg, C., Hofmann, M., Herrlich, P., & Ponta, H. (1993). Splicing choice from ten variant exons establishes CD44 variability. Nucleic Acids Research, 21, 1225–1229.
Haylock, D. N., & Nilsson, S. K. (2006). The role of hyaluronic acid in hemopoietic stem cell biology. Regenerative Medicine, 1, 437–445.
Aruffo, A., Stamenkovic, I., Melnick, M., Underhill, C. B., & Seed, B. (1990). CD44 is the principal cell surface receptor for hyaluronate. Cell, 61, 1303–1313.
Weber, G. F., Ashkar, S., Glimcher, M. J., & Cantor, H. (1996). Receptor-ligand interaction between CD44 and osteopontin (Eta-1). Science, 271, 509–512.
Stern, R., Asari, A. A., & Sugahara, K. N. (2006). Hyaluronan fragments: An information-rich system. European Journal of Cell Biology, 85, 699–715.
Springer, T., Galfre, G., Secher, D. S., & Milstein, C. (1978). Monoclonal xenogeneic antibodies to murine cell surface antigens: Identification of novel leukocyte differentiation antigens. European Journal of Immunology, 8, 539–551.
Kay, R., Takei, F., & Humphries, R. K. (1990). Expression cloning of a cDNA encoding M1/69-J11d heat-stable antigens. Journal of Immunology, 145, 1952–1959.
Hough, M. R., Rosten, P. M., Sexton, T. L., Kay, R., & Humphries, R. K. (1994). Mapping of CD24 and homologous sequences to multiple chromosomal loci. Genomics, 22, 154–161.
Rougon, G., Alterman, L. A., Dennis, K., Guo, X. J., & Kinnon, C. (1991). The murine heat-stable antigen: A differentiation antigen expressed in both the hematolymphoid and neural cell lineages. European Journal of Immunology, 21, 1397–1402.
Shackleton, M., Vaillant, F., Simpson, K. J., Stingl, J., Smyth, G. K., Asselin-Labat, M. L., et al. (2006). Generation of a functional mammary gland from a single stem cell. Nature, 439, 84–88.
Lawson, D. A., Xin, L., Lukacs, R. U., Cheng, D., & Witte, O. N. (2007). Isolation and functional characterization of murine prostate stem cells. Proceedings of the National Academy of Sciences of the United States of America, 104, 181–186.
Bai, X. F., Li, O., Zhou, Q., Zhang, H., Joshi, P. S., Zheng, X., et al. (2004). CD24 controls expansion and persistence of autoreactive T cells in the central nervous system during experimental autoimmune encephalomyelitis. The Journal of Experimental Medicine, 200, 447–458.
Kristiansen, G., Winzer, K. J., Mayordomo, E., Bellach, J., Schluns, K., Denkert, C., et al. (2003). CD24 expression is a new prognostic marker in breast cancer. Clinical Cancer Research, 9, 4906–4913.
Kristiansen, G., Denkert, C., Schluns, K., Dahl, E., Pilarsky, C., & Hauptmann, S. (2002). CD24 is expressed in ovarian cancer and is a new independent prognostic marker of patient survival. The American Journal of Pathology, 161, 1215–1221.
Athanassiadou, P., Grapsa, D., Gonidi, M., Athanassiadou, A. M., Tsipis, A., & Patsouris, E. (2009). CD24 expression has a prognostic impact in breast carcinoma. Pathology, Research and Practice, 205, 524–533.
Burgos-Ojeda, D., Wu, R., McLean, K., Chen, Y. C., Talpaz, M., Yoon, E., et al. (2015). CD24+ ovarian cancer cells are enriched for cancer-initiating cells and dependent on JAK2 signaling for growth and metastasis. Molecular Cancer Therapeutics, 14, 1717–1727.
Szotek, P. P., Pieretti-Vanmarcke, R., Masiakos, P. T., Dinulescu, D. M., Connolly, D., Foster, R., et al. (2006). Ovarian cancer side population defines cells with stem cell-like characteristics and Mullerian inhibiting substance responsiveness. Proceedings of the National Academy of Sciences of the United States of America, 103, 11154–11159.
Ricci-Vitiani, L., Lombardi, D. G., Pilozzi, E., Biffoni, M., Todaro, M., Peschle, C., et al. (2007). Identification and expansion of human colon-cancer-initiating cells. Nature, 445, 111–115.
Mancebo, G., Sole-Sedeno, J. M., Pino, O., Miralpeix, E., Mojal, S., Garrigos, L., et al. (2017). Prognostic impact of CD133 expression in endometrial cancer patients. Scientific Reports, 7, 7687.
O’Brien, C. A., Pollett, A., Gallinger, S., & Dick, J. E. (2007). A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature, 445, 106–110.
Monzani, E., Facchetti, F., Galmozzi, E., Corsini, E., Benetti, A., Cavazzin, C., et al. (2007). Melanoma contains CD133 and ABCG2 positive cells with enhanced tumourigenic potential. European Journal of Cancer, 43, 935–946.
Zhou, B. B., Zhang, H., Damelin, M., Geles, K. G., Grindley, J. C., & Dirks, P. B. (2009). Tumour-initiating cells: Challenges and opportunities for anticancer drug discovery. Nature Reviews. Drug Discovery, 8, 806–823.
Bao, S., Wu, Q., McLendon, R. E., Hao, Y., Shi, Q., Hjelmeland, A. B., et al. (2006). Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature, 444, 756–760.
Li, X., Lewis, M. T., Huang, J., Gutierrez, C., Osborne, C. K., Wu, M. F., et al. (2008). Intrinsic resistance of tumorigenic breast cancer cells to chemotherapy. Journal of the National Cancer Institute, 100, 672–679.
O’Hare, T., Corbin, A. S., & Druker, B. J. (2006). Targeted CML therapy: Controlling drug resistance, seeking cure. Current Opinion in Genetics & Development, 16, 92–99.
Gerlinger, M., Rowan, A. J., Horswell, S., Math, M., Larkin, J., Endesfelder, D., et al. (2012). Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. The New England Journal of Medicine, 366, 883–892.
Bashashati, A., Ha, G., Tone, A., Ding, J., Prentice, L. M., Roth, A., et al. (2013). Distinct evolutionary trajectories of primary high-grade serous ovarian cancers revealed through spatial mutational profiling. The Journal of Pathology, 231, 21–34.
Poveda, A. M., Selle, F., Hilpert, F., Reuss, A., Savarese, A., Vergote, I., et al. (2015). Bevacizumab combined with weekly paclitaxel, Pegylated liposomal doxorubicin, or topotecan in platinum-resistant recurrent ovarian cancer: Analysis by chemotherapy cohort of the randomized phase III AURELIA trial. Journal of Clinical Oncology, 33, 3836–3838.
Monk, B. J., Poveda, A., Vergote, I., Raspagliesi, F., Fujiwara, K., Bae, D.-S., et al. (2014). Anti-angiopoietin therapy with trebananib for recurrent ovarian cancer (TRINOVA-1): A randomised, multicentre, double-blind, placebo-controlled phase 3 trial. The Lancet Oncology, 15, 799–808.
Oza, A. M., Cibula, D., Benzaquen, A. O., Poole, C., Mathijssen, R. H. J., Sonke, G. S., et al. (2015). Olaparib combined with chemotherapy for recurrent platinum-sensitive ovarian cancer: A randomised phase 2 trial. The Lancet Oncology, 16, 87–97.
Mirza, M. R., Monk, B. J., Herrstedt, J., Oza, A. M., Mahner, S., Redondo, A., et al. (2016). Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. The New England Journal of Medicine, 375, 2154–2164.
Acknowledgments
We thank Prof Dr. Bassma El-Sabaa for her courtesy in providing material for Fig. 2.5.
We thank Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Soliman, A.A., Elzarkaa, A.A., Malik, E. (2021). Epithelial Ovarian Cancer and Cancer Stem Cells. In: Schatten, H. (eds) Ovarian Cancer: Molecular & Diagnostic Imaging and Treatment Strategies. Advances in Experimental Medicine and Biology, vol 1330. Springer, Cham. https://doi.org/10.1007/978-3-030-73359-9_2
Download citation
DOI: https://doi.org/10.1007/978-3-030-73359-9_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-73358-2
Online ISBN: 978-3-030-73359-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)