Abstract
The increasing levels of nitrogen oxyanion pollution especially nitrate in water environments have become a critical issues of concern because of the potential risk on ecology and human health. Owing to its distinctive merits of sustainability, lesser operational and maintenance expenditure, the utilization of constructed wetland systems for the treatment of wastewater has turned out to be predominant worldwide. Its nitrogen oxyanion removal performance has received significant attention in the last two decades. This chapter presents a comprehensive outline of the application of constructed wetlands (CW) for nitrogen oxyanion removal from water and wastewater. The removal mechanisms and transformations of nitrogen are also discussed. In addition, the major factors that influence the removal performances in CWs are elucidated, especially the types of carbon sources commonly used, and how it affects the denitrification process. This chapter would be useful to engineers and researchers in the field of water and wastewater engineering.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others

Keywords
12.1 Background
12.1.1 Nitrate in the Environment
Nitrate \({(\mathrm{NO}}_{3}^{-})\) is one of the major generic forms of nitrogen oxyanions that exist naturally in moderate concentrations in different environmental media. The oxidation of nitrites \({(\mathrm{NO}}_{2}^{-})\) majorly generates nitrates during nitrification process of the nitrogen cycle. The nitrogen cycle is the biogeochemical cycle by which organic protein from animals and plants origin is converted into ammonia (\({\mathrm{NH}}_{3})\) and then \({\mathrm{NO}}_{2}^{-},\) and \({\mathrm{NO}}_{3}^{-}\) in the environment. The transformations of the different nitrogen forms are carried out via physicochemical and biological processes (Fig. 12.1). Owing to its high solubility in water, the presence of \({\mathrm{NO}}_{3}^{-}\) has adverse effects on the environment, as it greatly accounts for the pollution of soil, surface water and the groundwater [1]. Several wastewater types such as urban drainage, landfill leachate, industrial and agricultural wastewater that contain nitrogenous compounds initiate undesirable phenomena (e.g. eutrophication and methemoglobinemia (i.e. blue baby syndrome)) when they are released into water bodies [2,3,4]. The concentrations of \({\mathrm{NO}}_{3}^{-}\) in these wastewaters vary from low to high, and thus, demand an appropriate technique for the removal. According to Rajmohan et al. [5], the usual \({\mathrm{NO}}_{3}^{-}\) level in polluted water ranges from 200 to 500 mg/L, based on the nature of the source (Table 12.1), but wastewater from nuclear industries contain up to 50,000 mg/L of \({\mathrm{NO}}_{3}^{-}\).
Excess \({\mathrm{NO}}_{3}^{-},\) discharged from the large-scale utilization of agricultural fertilizers, concentrated livestock feeding operations and disposal of partially treated sewage, that enters the groundwater, is among the priority pollutants of the groundwater system. Over 10,000 public water supply wells are estimated to have high levels of nitrate in the USA and thousands of wells were also ascertained with nitrate concentrations at or above the established health standards, across Western Europe and Asia [14]. Consequently, the maximum permissible concentration limit of \({\mathrm{NO}}_{3}^{-}\) in drinking water was set at 10 mg/L as nitrate-nitrogen (NO3–N) by the US Environmental Protection Agency, while 50 mg/L \({\mathrm{NO}}_{3}^{-}\) was set by World Health Organization to address the concerns of methemoglobinemia in infants [14, 15]. High levels of \({\mathrm{NO}}_{3}^{-}\) is recognized to cause environmental and public health issues. The presence of this nitrogen oxyanion in water environments is a global challenge that needs urgent attention. To this end, various technological solutions, including electrodialysis, chemical reduction, membrane separation, adsorption, sequencing batch reactor, moving bed bioreactors, electrochemical denitrification, reverse osmosis; ion exchange, photocatalytic degradation and membrane bioreactors have been developed to solve this menace [3, 16,17,18,19,20,21,22,23,24,25,26,27]. However, these technologies are always limited by their costly installation and high operational cost, secondary pollution, sludge production that need disposal, incomplete removal efficiency [28, 29]. In this chapter, constructed wetland (CW) systems, which are generally cost effective, simple, environmentally non-disruptive, ecologically sound, with relatively low maintenance cost, will be expounded in relation to \({\mathrm{NO}}_{3}^{-}\) removal.
12.1.2 Constructed Wetlands
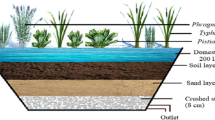
Constructed wetlands, also referred to as treatment wetlands, are engineered systems that are designed and fabricated to treat several kinds of wastewater with relatively low external energy requirements and operationally simple technology and maintenance (Fig. 12.2) [30,31,32,33,34,35]. Milani et al. [36] defined CW as a “sustainable and efficient solutions used around the world to treat wastewater as an alternative or a supplement to intensively engineered treatment plants”. They are complex, integrated systems that involve the interaction of soil, water, plants, animals, microbes and the environment. The CWs have become an essential alternative wastewater treatment system since the method combines relatively high performance of pollutant removal with low maintenance and simple operation [37]. The CWs are planned methods designed and constructed to apply the natural procedures involving wetland vegetation, soils and the associated microbial assemblages to assist in wastewater treatment. It can effectively remove suspended solids, organic pollutants and nutrients from wastewater [38,39,40]. The CWs provide an inexpensive and reliable method for treating a variety of wastewaters such as sewage, landfill leachate, mine leachate, urban storm-water and agricultural run-off. This system of treatment is very efficient for nutrient removal and comparatively simple to construct, operate, maintain and suitable for advanced and polishing treatment if water reuse is an option [41]. The main \({\mathrm{NO}}_{3}^{-}\) removal mechanisms in wetlands are seepage loss, plant uptake and denitrification [42] which are further expatiated in Sect. 12.2. Table 12.2 summarizes research studies on \({\mathrm{NO}}_{3}^{-}\) removal using CWs.
12.2 Nitrogen Transformation in Constructed Wetlands
As an ecological treatment technology, CWs have been largely utilized in recent decades in wastewater treatment plants. Before the arrival of CWs technology, conventional activated sludge-type wastewater treatment plants have been used for nitrogen removal but only minimal quantity is removed, via the consumption of the organic matter fraction of the wastewater.
In wastewater treatment operations, nitrate is removed through a process known as denitrification. This is a process, where organic and ammonia nitrogen is converted, through a process known as nitrification, to \({\mathrm{NO}}_{3}^{-}\) in the absence of oxygen (an anaerobic environment). The \({\mathrm{NO}}_{3}^{-}\) produced through nitrification is further reduced to nitrogen gas in this same anoxic environment, thus completing the denitrification process [65, 66]. This process is carried out by several range of autotrophic and heterotrophic facultative anaerobic bacteria, which are capable of utilizing \({\mathrm{NO}}_{3}^{-}\) (and \({\mathrm{NO}}_{2}^{-}\)), under anoxic conditions, as an electron acceptor [67]. Some of these bacteria include Pseudomonas, Micrococcus, Bacillus, Paracoccus denitrificans and Achromobacter. For better nitrogen removal, an external organic carbon source is needed to act as an electron donor in the respiratory chain [38], and CWs are a better option to achieving this.
Although the eutrophication and the toxic effects of \({\mathrm{NO}}_{3}^{-}\) on aquatic organisms of both vertebrate and invertebrate species are sources of concerns [68], it also boosts plants’ growth, which sequentially promotes the environmental biogeochemistry in the wetlands. The circulation of nitrogen in wetlands involves composite processes, while very straightforward chemical conversion of this element still poses a great task in environmental engineering. Such processes, which include bacterial actions, plant/microbial uptake, adsorption (interaction between ionized NH3 and the media in sub-surface horizontal flow, (SSHF) CWs), and volatilization (i.e. transformation of aquatic \({\mathrm{NH}}_{4}^{+}\) to gaseous NH3, within the operating pH regime of the surface flow CW), mostly achieved nitrogen removals in wetlands [68,69,70].
Nitrogen transformation involves some processes and mechanisms, which lead to the transference of wetland nitrogen from one point to the other without any consequential molecular alteration [69]. As earlier noted, the physical processes of management of nitrogen oxyanion in CW include, settling of particles and re-suspension, dissolution and diffusion, plant translocation, litterfall, volatilization and sorption [68, 69]. Generally, nitrogen oxyanion removal in CW occurs through two processes that include biological and physicochemical treatment processes. The five major biological treatment process include denitrification, nitrification, ammonification (mineralization), assimilation and decomposition [69, 71, 72]. The physicochemical processes include, sedimentation, NH3 stripping, breakpoint chlorination and ion exchange [70, 73]. It was suggested that low oxygen and organic matter contents in the root zone offers restriction to nitrification and denitrification processes [69]. However, an integration of partial nitrification and anaerobic \({\mathrm{NH}}_{4}^{+}\) oxidation has equally been recommended to be resourceful in removing nitrogen from constructed wetlands. This is largely due to the autotrophic nature of anaerobic ammonia oxidation (Anammox) process, in which \({\mathrm{NH}}_{4}^{+}\) is completely converted into nitrogen gas in the presence of \({\mathrm{NO}}_{2}^{-}\) and without the addition of organic matter [69].
12.2.1 Ammonification
The ammonification refers to the process by which the organic nitrogen fraction is transformed to NH3, through a biological process [71]. The first stage of nitrification in sub-surface flow CW (SSFCW) systems is initiated by ammonification, if the inbound wastewater is highly loaded with organic nitrogen [74]. This biochemical process, where the amino acids fractions are exposed to oxidative deamination yielding NH3 is acomplex and exergonic process, (Eq. 12.2.1) [74, 75].
Since the process of occurrence decreases with depth, it shows that ammonification is quickest within the upper zone of the wetlands, where the aerobic condition is prominent. It is time-consuming within the lower zone, where the environment moves from facultative anaerobic condition to obligate anaerobic condition [71, 76]. In CWs, the inorganic ammoniacal-nitrogen is mostly removed by nitrification–denitrification processes, but ammonification kinetically progresses faster than nitrification [71]. Kadlec and Knight [70] suggested that the ammonification process progresses quicker in higher temperature, doubling the rate with a temperature rise of 10 ℃. The pH range observed to be ideal for ammonification is 6.5–8.5 [74, 77, 78]. The ammonification process is therefore generally affected by pH, temperature, carbon-to-nitrogen (C/N) ratio, soil structure and available nutrient [76]. Furthermore, processes such as adsorption, plant uptake and volatilization are suggested to be resourceful in ammonia–nitrogen removal [38], though the effectiveness of nitrification–denitrification processes is, in general, suggested to be the most resourceful in \({\mathrm{NH}}_{4}^{+}\) removal [71].
12.2.2 Nitrification
Nitrification is the major transformation mechanism by which the level of ammonia nitrogen is reduced. This reduction is achieved through the conversion of the ammonia nitrogen into oxidized form of nitrogen (i.e.\({\mathrm{NO}}_{2}^{-}\mathrm{and }{\mathrm{NO}}_{3}^{-}\)). Graaf et al. [79] defined nitrification as the biological formation of nitrate or nitrite from compounds containing reduced nitrogen with oxygen (O2) as their terminal electron receptor. Lee et al. [71] defined it as the chemolithoautotrophic oxidation of NH3 to \({\mathrm{NO}}_{3}^{-}\) in the presence of adequate O2, occurring in two successive oxidative steps, namely ammonia oxidation (NH3 to\({\mathrm{NO}}_{2}^{-}\)) and nitrite oxidation (\({\mathrm{NO}}_{2}^{-}\mathrm{to} {\mathrm{NO}}_{3}^{-}\)), carried out by nitrifying bacteria. These bacteria use NH3 or \({\mathrm{NO}}_{2}^{-}\) as an energy source, O2 as the terminal electron recipient and carbon dioxide as the carbon source [71]. The first stage is the oxidation of NH3 to \({\mathrm{NO}}_{2}^{-}\), by ammonium oxidizing bacteria such as Nitrosomonas or Nitrospira or Nitrosococcus (Eq. 12.2.2) [71, 74].
The above first stage is succeeded by the second stage which is the oxidation of \({\text{NO}}_{2}^{ - }\) by nitrite-oxidizing bacteria such as Nitrobacter or Nitrospira. The second stage is described by Eq. 12.2.3 [71, 74].
The oxygen consumption of nitrification process is estimated to be 3.16 mg O2 per mg \({\text{NH}}_{4} - {\text{N}}\) oxidized, and 1.11 mg O2 per mg \({\text{NO}}_{2} - {\text{N}}\) oxidized, while Nitrosomonas and Nitrobacter produce 0.15 mg cells per mg \({\text{NH}}_{4} - {\text{N}}\) oxidized and 0.02 mg cells per mg \({\text{NO}}_{2} - {\text{N}}\) respectively [71]. Furthermore, alkalinity is necessary as 7.07 mg CaCO3 per mg \({\mathrm{NH}}_{4}-\mathrm{N}\) oxidized [80]. The acid formation (i.e. low pH value) during nitrification process causes alkalinity reduction and a deep reduction in pH [68, 80,81,82], and a swift decline in the nitrification rate below the neutral pH value [81]. Hence, it is important to replenish the alkaline level with lime during the process, when there is a drop in alkalinity [80]. Though nitrification is basically attributed to chemoautotrophic bacteria, it is suggested that heterotrophic nitrification takes place, which can be significant [68]. Aside from autotrophic nitrification, heterotrophic nitrifying bacteria are also capable of producing \({\mathrm{NO}}_{3}-\mathrm{N}\). Some of these species (in bacteria, algae and fungi) are Actinomycetes, Arthrobacter globiformis, Aerobacter aerogenes, Bacillus, Mycobacterium phlei, Streptomyces griseus, Theosphaera and Pseudomonas [38, 74, 83]. Gerardi [83] affirmed that although these heterotrophic nitrifiers are resourceful, the nitrification rates achieved by Nitrosomonas and Nitrobacter groups are significantly greater (relatively greater by 1000 to 10,000 times) [74]. However, owing to constraints against nitrification and denitrification processes, offered by low oxygen and organic matter concentration in SSF, it has been affirmed that a combination of partial nitrification and Anammox is a resourceful means of removing nitrogen from CWs [69]. Moreover, since the Anammox process is autotrophic, the transformation of \({\mathrm{NH}}_{4}^{+}\) to nitrogen could be possible without adding organic matter [69].
12.3 Denitrification
Kadlec and Wallace [68] defined denitrification as the process by which \({\mathrm{NO}}_{3}^{-}\) is transformed to dinitrogen (\({\mathrm{N}}_{2}\)) via intermediates such as \({\mathrm{NO}}_{2}^{-}\), nitric oxide, and nitrous oxide, and finally nitrogen (Eq. 12.2.4). The denitrification process is also called \({\mathrm{NO}}_{3}^{-}\) dissimilation, and it is accomplished by facultative heterotrophic organisms that can use \({\mathrm{NO}}_{3}^{-}\) as the terminal electron receptor, and organic carbon as an electron donor under anoxic condition [71]. During the transformation, inorganic nitrogens such as \({\mathrm{NO}}_{2}^{-}\) and \({\mathrm{NO}}_{3}^{-}\) are usually reduced to harmless nitrogen gas by denitrifying bacteria [71, 84, 85]. Some denitrifiers require organic substrates to get their carbon source for growth and evolution, whereas others use inorganic substances as their energy sources and CO2 as their carbon source [86]. Therefore, denitrifying bacteria are categorized into two main species, namely autotrophs and heterotrophs [71]. However, earlier studies have focussed on the heterotrophic denitrification process, due to its frequency in conventional wastewater treatment plants [71, 87], while the autotrophic denitrification process started gaining attention in recent studies [88,89,90,91,92,93,94].
Moreover, denitrification is led by some heterotrophic microorganisms like Pseudomonas, Micrococcus, Achromobacter and Bacillus, under anaerobic or low-oxygen conditions. Denitrificating microbes can be grouped as: organotrophs (e.g. Pseudomonas, Alcaligenes, Bacillus, Agrobacterium, Flavobacterium, Propionibacterium and Vibrio), chemolithotrophs (e.g. Thiobacillus, Thiomicrospira, Nitrosomonas), photolithotrophs (e.g. Rhodopsuedomonas), diazotrophs (e.g. Rhizobium, Azospirillum), archaea (e.g. Halobacterium) and other microorganisms such as Paracoccus or Neisseria [68]. The fraction of total nitrogen removal through denitrification is normally 60–95%.
12.3.1 Assimilation Process of Nitrogen
The uptake of nitrogen by plants or microbes is regarded as the assimilation process. Masclaux-Daubresse et al. [95] and Xu et al. [96] asserted that the usage of nitrogen by plants encompasses numerous stages, including uptake, assimilation, translocation and, when the plant is ageing, recycling and remobilization. The assimilation process occurs via the formation of organic nitrogen compounds such as amino acids from inorganic nitrogen compounds available in the environment. Organisms like plants, fungi and specific bacteria that cannot fix nitrogen gas (\({\mathrm{N}}_{2}\)) rely on the ability to assimilate \({\mathrm{NO}}_{3}^{-}\) or \({\mathrm{NH}}_{3}\) for their needs. Animals also depend fully on the organic nitrogen form for their food. Several studies have affirmed the significance of the removal of NH3 from water by wetland plants [97,98,99,100,101,102,103,104]. However, many of these studies are commonly seen to portray the measurement of gross nitrogen uptake, without deduction for consequential losses due to plant death and decomposition, with associated leaching as well as re-solubilization of nitrogen [68].
For nitrogen removal from the wetland water, the attention is usually on the net influence of the macrophytes (macroflora) on the water phase concentrations [68]. When discussing plant uptake as a process of nitrogen removal from wetland water, terms such as phytomass (the totality of vegetative materials, living and dead), biomass (all living vegetative materials) and necromass (all dead vegetative materials) are often used [68]. Macrophytes are vital in enhancing nitrogen removal from wetlands due to their functions such as providing surfaces and O2 for the growth of microbes within the rhizosphere, thus improving nitrification [99, 105,106,107], and providing carbon from root secretions (due to photosynthetically fixed carbon, within a range of 5–25% C), enhancing organics removal and denitrification process [97, 108,109,110,111]. Various relative researches between unplanted and planted wetlands indicated good nitrogen and organics removal, with the latter yielding more significant results, hence indicating the necessity of macroflora for enhancing nitrogen removal operations in CWs [74].
Inorganic nitrogen forms are usually transformed into organic compounds through the uptake of NH3 and \({\mathrm{NO}}_{3}^{-}\) by macrophytes. This serves as the building blocks for cells and tissues [78]. The ability of rooted plants to utilize sediment nutrients partly describes their massive yield in comparison with planktonic algae in many systems [112]. Different plant species have varying ability in their ideal nitrogen forms absorbed, and the nutrient concentration of plants tissues also influences the uptake and storage rate of nutrient [71]. However, \({\mathrm{NH}}_{4}^{+}\) preference is conventional in macroflora within \({\mathrm{NH}}_{4}^{+}\) -rich environments where restricted nitrification occurs [113]. In general, the uptake of nitrogen by plants varies along with system configurations, loading ranges, type of wastewater and environmental conditions [74]. In nitrogen removal, plants contribution is affirmed to be about 0.5–40.0% of the total nitrogen removal [74, 103, 104]. Plant biomass accumulates 60% of total nitrogen thus, enhancing nitrogen removal significantly [103]. For efficient nutrient assimilation and storage, plants with features such as high tissue nutrient content, rapid growth and ability to achieve high-standing crops are preferably desired. On the contrary, plants with immense biomass accumulation during autumn and winter have a likelihood of releasing a considerable amount of their stored nitrogen back into the water during the winter season [38]. Brodrick et al. [114] equally suggested that decaying plant materials could also raise the concentration of nutrients in the effluent through leaching [74].
Some selected plants have been employed in constructed wetlands; however, Phragmites australis remains the most typical plant used in SSFCW due to its capability to pass O2 from its leaves through the stems and rhizomes and out of from its fine hair roots into the rhizosphere [115]. Reports from literature about the ability of the plant to convey oxygen (thereby fostering microbial conversion and nitrification) express various illustrations [74, 116,117,118]. Armstrong et al. [116] noted O2 release (per unit wetland area) by phragmites species to be in the range of 5–12 g O2 per square metre per day, while the O2 release by phragmites in a study by Brix and Schierup [117] gives a record of only 0.02 g O2 per square meter in soil substrate. The oxygen released by phragmites species recorded by Bavor et al. [118] is about 0.8 g O2 per square meter in gravel substrate [74]. Figure 12.3 represents the major typical routes for nitrogen removal in SSFCWs.
Key classical nitrogen removal routes in sub-surface flow wetlands [74]
12.4 Factors Affecting Nitrogen Removal Efficiency in CWs
Nitrogen oxyanion removal efficiency, especially \({\mathrm{NO}}_{3}^{-}\), in CWs has been discussed to involve various biological and physicochemical processes. Therefore, various environmental factors are bound to affect the efficiencies of these processes, thereby limiting the oxyanion removal efficiency. Some of such factors include pH, temperature, hydraulic residence time (HRT), \({\mathrm{NO}}_{2}^{-}\) concentration, oxygen concentration, vegetation type (wetland plant species) and density, activity of microorganism, distribution of wastewater, climate, and attributes of influent[71, 74, 119,120,121,122]. It should be noted that most of these factors are interdependent; hence, a variation of one factor often leads to a consequent change in other factors [121]. Furthermore, Kuschk et al. [123] stressed that the two major factors affecting the nitrogen removal from CWs are temperature and HRT [71]. The following subsections give a concise analysis of the key factors influencing nitrogen removal efficiency in CWs.
12.4.1 Carbon Source
Amidst all the aforementioned factors, the carbon source is one of the known dominant external factors which has disreputed the nitrogen oxyanion removal efficiency of CWs [56, 124]. Other factors to be considered in the choice of the carbon source include cost, handling and storage safety/stability, denitrification rate, degree of utilization, kinetics, sludge production, the content of unfavourable/toxic compounds. A commonly used carbon source, which is readily available, and with a high denitrification rate is methanol. Other closely related examples are ethanol and acetic acid. Although the nitrogen removal efficiency, using the methanol carbon source is desirable, the existence of \({\mathrm{NO}}_{2}^{-}\) accumulation in wastewater with high \({\mathrm{NO}}_{3}^{-}\) concentration often results in bacteria growth suppression [125]. Furthermore, the danger of overapplication of easily biodegradable materials of these liquid carbon sources through aerobic degradation can adversely impact nitrogen oxyanion removal [126]. Considering the aforementioned shortcomings, plant-based carbon sources have been considered [124, 127], and used to treat different wastewater, such as domestic sewage, agricultural run-off and industrial effluent. At present, most of the denitrifying bacteria in CWs are heterotrophic which require organic carbon sources for the substantive effect on denitrification process for nourishment and \({\mathrm{NO}}_{3}^{-}\) reduction [128]. In a study by Zhao and Chen [129], it was discovered that ammonia nitrogen, nitrous nitrogen and total nitrogen (when alkali-treated corn stover was used as additional carbon source material) were removed in the upper and middle layers, while nitrate is removed mainly at the bottom layer. Xiao et al. [130] showed the addition of solid carbon source to the vertical flow CW. In the system, there was almost 100% nitrification reaction, when the addition position of the carbon source was at the lower layer, thus giving the total nitrogen removal rate at the highest.
The type of exogenous carbon sources that are widely used for CWs are of three classes, including natural organic matters, low molecular carbohydrates and biodegradable macromolecule polymers.
12.4.1.1 Natural Organic Matters
Plant is a naturally degradable material that is rich in lignin, cellulose, hemicellulose and many more. In CWs, it is the most vital composition because of its ability to absorb nitrogen as nutrient and also to provide suitable environment for nitrification and denitrification [131]. In recent years, the application of natural material (especially plant biomass) as a carbon source for maximum nitrogen removal efficiency has gained substantial ground in CWs, because of the economic viability and practicability [132]. Their effects in nitrogen oxyanion removal vary, due to the diversity in the composition of lignin, cellulose, hemicellulose and other components in plants. Some natural organic matters (mostly plant biomass) have been studied to assess the effectiveness and efficiency of plant carbon source in CW denitrification rate [133] (Table 12.3). Conversely, these natural materials (especially for plant biomass) have some demerits, which include unstable carbon supply and discharge of coloured matter [134], which sometimes affect their applications.
12.4.1.2 Low Molecular Carbohydrate
Low molecular organic carbon sources have some desirable properties, which have also gained them recognition as an external carbon source. They are rich in carbon, which can easily be used up during decomposition. If classified in terms of physical form, they are liquid organic substances, which are termed liquid carbon sources. Examples are glucose [142,143,144], fructose [44], ethanol, methanol [145,146,147] and acetic acid [148].
12.4.1.3 Biodegradable Macromolecule Polymers
In recent time, a wide range of external carbon sources, which by the physical classification are solid organic substances, were checked in some laboratory studies, to function as physical support for biofilm formation in solid-phase denitrification system [149]. Some of the polymers were even blended. Examples of these polymer/polymer blends used so far are polybutylene succinate [150], polycaprolactone [151, 152], polyhydroxyalkanoates [153], polyvinyl alcohol [154], starch [155], starch/polyvinyl alcohol [156], poly(3-hydroxybutyrate-co-3-hydroxyvalerate)/poly(lactic acid) [157] and PHBV/starch [149].
Hitherto, there have been some investigations of denitrification performance and microbial community structure in both liquid and solid carbon sources supported denitrification systems but their differences are scarcely studied. Srinandan et al. [158] reported that both liquid and solid organic carbon sources influence the nitrate removal activity, biofilm architecture and community structure although molecular weight and chemical structure of the biodegradable polymers were generally higher and more complicated when compared with the liquid carbon sources. Furthermore, denitrification performance and microbial diversity using starch/PCL and ethanol as an electron donor for nitrate removal were also investigated through comparison. The outcome revealed that the ethanol system displayed a higher denitrification rate while the blended starch/PCL system had richer microbial diversity [159].
Generally, when blended polymers and other external carbon sources were utilized, they yielded a good denitrification effect [160, 161] but the water of the solution of the blended polymer carbon source took some ample of time, causing some lag period. In addition, the morphology of the blended materials, surface properties and particle size posed great influence on the denitrification rate, with its biodegradability and denitrification performance decreasing with increasing molecular weight [162]. Thus, polymer/polymer blended carbon sources are not commonly used because of some factors such as high market price and slow release of carbon sources which requires a lot of time.
12.4.2 Selected Operating Parameters
12.4.2.1 pH
It has been established that nitrification process consumes alkalinity. Vymazal [38] affirmed that a pH >8.0 is capable of decreasing nitrification and denitrification processes to an insignificant level, with denitrification process occurring slowly at pH 5. Moreover, previous studies have suggested that high pH leads to a decline in dissolved oxygen (DO) in substrate [163], thus influencing nitrification and denitrification processes [32]. Also, some studies suggested that pH <6.0 and >8.0 hinder denitrification [32, 38, 81, 163], while the peak rate is observed at a pH range 7.0–7.5 [74, 164].
12.4.2.2 Temperature
Temperature is a significant environmental factor that controls the solid-phase denitrification process by hindering the activity of the associated enzymes in both hydrolysis of the solid substrate and reduction of \({\mathrm{NO}}_{3}^{-}\) [165]. In other words, temperature affects both microbial activities and diffusion rate of O2 in constructed wetlands [166]. A temperature range between 16.5 and 32 ºC is favourable for nitrification in CWs [74, 167], while the most efficient removal occurs at temperature that ranged between 20 and 25 ℃ [164, 166]. The nitrification- and denitrification-associated microbial activities decreased significantly at temperatures below 15 ℃ and above 30 ℃ [123].
Many studies have investigated the activities of denitrifiers in CW sediments during various climatic conditions and found that their activities are generally more robust in spring and summer than in autumn and winter [74, 168,169,170,171]. Oostrom and Russell [172] affirmed that, in general, the degree of removal of \({\mathrm{NO}}_{3}^{-}\) is greater around summer than during winter [71]. Denitrification is usually believed to terminate at temperatures below 5 ℃ [71]. In soils, the optimal temperature limits for nitrification and ammonification are 30–40 ℃ and 40–60 ℃, respectively [38].
12.4.2.3 Hydraulic Residence Time and Hydraulic Loading
Hydraulic residence time (HRT) is an important factor in nitrogen removal. The nitrogen removal efficiency is highly influenced by the flow condition and the residence time [4]. An increase in wastewater residence time leads to an intense decrease of ammonium and total Kjeldahl nitrogen concentrations in treated effluent [71]. This is because of the lengthier time of contact of nitrogen pollutant with microorganisms that gives advantage to the microbe to play a significant catabolic activity [74]. Lee et al. [71] also stated that lengthier HRT is necessary in nitrogen removal from wetlands than for BOD and COD removal. An eight-day HRT at a temperature above 15 ℃ is needed in SSFCWs [173]. However, if anaerobic conditions dominate in the wetlands, there is likelihood that an increase in HRT will not facilitate \({\mathrm{NO}}_{3}^{-}\) removal [174]. About 3–4 h HRT is required when \({\mathrm{NO}}_{3}^{-}\) concentration is not more than 40 mg \({\mathrm{L}}^{-1}\) and a minimum HRT of 6 h is necessary when \({\mathrm{NO}}_{3}^{-}\) concentration is more than 70 mg \({\mathrm{L}}^{-1}\) [175]. Hydraulic loading is also important in this regard, especially in SSFWs. Saeed and Sun [74] affirmed that the greater the hydraulic loading the faster the passage of wastewater through the media.
12.4.2.4 Dissolved Oxygen (DO)
As earlier discussed, most denitrifiers are facultative anaerobic organisms that use nitrate as a terminal electron recipient in the absence of oxygen or under anoxic condition. DO is a great and energetic electron recipient, and for that reason, it exhibits direct competition or inhibition of enzymes, which consequently results to suppression of the denitrification process [165]. Denitrification could happen at DO concentration to the level of 4.0–5.0 mg \({\mathrm{L}}^{-1}\) [165], though the denitrification rate declined with increase in DO levels [153]. Furthermore, it is vital to note that the presence of DO promotes upsurge in carbon source consumption as a portion of predisposed organic carbon is used up by aerobic respiration instead of denitrification [153, 176]. Since enzymatic actions in reducing nitrate can be inhibited by DO, there may occur nitrate accumulation [165]. The lower the oxygen concentration, the higher the denitrification becomes [177].
Denitrification rate of Diaphorobacter nitroreducens strain NA10B decreased as the DO concentration increased, when using poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) powders as carbon substrate, even when more than 3 mg \({\mathrm{NO}}_{3}-\mathrm{N}\) \({\mathrm{g}}^{-1}\) \({\mathrm{h}}^{-1}\) is maintained under complete aerobic conditions [178]. Gutierrez-Wing et al. [153] reported that the denitrification rate decreased from 5.5 to 0.5 g \({\mathrm{NO}}_{3}-\mathrm{N}\) \({\mathrm{L}}^{-1}\) \({\mathrm{d}}^{-1}\) when the DO concentration increased from 0.5 to 4.0 mg \({\mathrm{L}}^{-1}\) in a circulating aquaculture water system filled with polyhydroxybutyrate (PHB). At the DO levels of 4–5 mg \({\mathrm{L}}^{-1}\), a least denitrification rate of 0.18 g \({\mathrm{NO}}_{3}-\mathrm{N}\) \({\mathrm{L}}^{-1}\) \({\mathrm{d}}^{-1}\) was noticed for 6 days and thereafter declined to zero. Xu et al. [179] also reported that the nitrate removal increased to more than 85% with increasing DO levels in the influent from 1.5 to 4.0 mg \({L}^{-1}\), and decreased to 50% at DO levels > 4.0 mg \({\mathrm{L}}^{-1}\) in a solid-phase denitrification system, using corncobs as carbon source. Wang and Chu [165] thus suggested that controlling the DO levels in the denitrification reactor appeared to be needless, but then it could promote the efficiency of the process.
12.4.3 Vegetation Type
Macrophytes (also known as macroflora, phytoremediators, hydrophytes, wetland plants and aquatic plants) are those plant species naturally found thriving in wetlands of all sorts, either in or on the water. They play a significant role in CWs and have been extensively used for decontamination of water bodies. For instance, their roots provide surface areas for microbial activities and aerobic zones in the wetlands. The rhizosphere is the most active reaction zone in a CW as it promotes the relationship benefits that exit amongst plants, microbes, soil and contaminants, thereby enhancing physical and biochemical processes [71]. Studies revealed that parts (the above-ground and below-ground) of the macrophytes enhanced microbial diversity and offer enormous surface areas for biofilm development which is accountable for the majority of the microbial activities occurring in the CWs [180, 181]. The categories of macrophytes commonly used in CW are emergent plants (Arundo donax L., Juncus spp., Phragmites spp., Typha spp., Iris spp., and Eleocharis spp), submerged plants (Myriophyllum verticillatum, Hydrilla verticillata, Ceratophyllum demersum, and Vallisneria natans), floating leaved plants (water spinach (Ipomoea aquatica), water lettuce (Pistia stratiotes) Nymphaea tetragona, Nymphoides peltata, Trapa bispinosa and Marsilea quadrifolia), free-floating plants (Water hyacinth (Eichhornia crassipes), Lemna minor, Hydrocharis dubia and Salvinia natans) and other large wetland grass-like plants like Bulrushes (e.g. Scirpus luviatilis, Scirpus validus, Scirpus cyperinus). It has been substantiated that planting of more than one species of macrophytes enhances the removal performance of CWs because the presence of diverse kind of plant species offers a more favourable microbial activities and longer retention time [182, 183]. For optimum treatment efficiency and favourable CW design, a detailed understanding of plant species, uniqueness of microorganism groups, and the associations between biogenic matters and particular components in contaminants are required.
12.5 Conclusion
Nitrate pollution remains a vital problem in the pursuit of environmental sustainability in water environments. This chapter has shed light on the viable means of treating nitrate contaminated water using an ecologically based technology called constructed wetland. CWs have been proved to be a beneficial and promising technique in wastewater treatment because of their low-cost, environmental quality preservation and easy maintenance. This chapter also summarizes several factors responsible for nitrogen removal in CW treatment systems from water and wastewater, including the various transformations of nitrogen with a focus on nitrogen oxyanion (nitrate).
References
Patel RK (2016) Nitrates—its generation and impact on environment from mines: a review. In: National conference on sustainable mining practice. India. pp 2–3
Rossi F, Motta O, Matrella S, Proto A, Vigliotta G (2015) Nitrate removal from wastewater through biological denitrification with OGA 24 in a batch reactor. Water 7:51–62. https://doi.org/10.3390/w7010051
Ghafari S, Hasan M, Aroua MK (2008) Bio-electrochemical removal of nitrate from water and wastewater—a review. Biores Technol 99:3965–3974
Taylor GD, Fletcher TD, Wong THF, Breen PF (2005) Nitrogen composition in urban runoff—implications for stormwater management. Water Res 39:1982–1989
Rajmohan KS, Gopinath M, Chetty R (2018) Bioremediation of nitrate-contaminated wastewater and soil. In: Varjani S, Agarwal A, Gnansounou E, Gurunathan B (eds) Bioremediation: applications for environmental protection and management. energy, environment, and sustainability. Springer, Singapore, pp 387–409. https://doi.org/10.1007/978-981-10-7485-1_19
Oladoja NA, Ademoroti CMA (2006) The use of fortified soil-clay as on-site system for domestic wastewater purification. Water Res 40:613–620
Wu C, Chen Z, Liu X, Peng Y (2007) Nitrification–denitrification via nitrite in SBR using real-time control strategy when treating domestic wastewater. Biochem Eng J 36:87–92
Peyton BM, Mormile MR, Petersen JN (2001) Nitrate reduction with halomonas campisalis: kinetics of denitrification at pH 9 and 12.5% NaCl. Water Res 35:4237–4242
Munz G, Gori R, Cammilli L, Lubello C (2008) Characterization of tannery wastewater and biomass in a membrane bioreactor using respirometric analysis. Biores Technol 99:8612–8618
Park JBK, Craggs RJ, Sukias JPS (2009) Removal of nitrate and phosphorus from hydroponic wastewater using a hybrid denitrification filter (HDF). Biores Technol 100:3175–3179
Dorante T, Lammel J, Kuhlmann H, Witzke T, Olfs HW (2008) Capacity, selectivity, and reversibility for nitrate exchange of a layered double-hydroxide (LDH) mineral in simulated soil solutions and in soil. J Plant Nutr Soil Sci 171:777–784
Shen J, He R, Han W, Sun X, Li J, Wang L (2009) Biological denitrification of high-nitrate wastewater in a modified anoxic/oxic-membrane bioreactor (A/O-MBR). J Hazard Mater 172:595–600
Francis CW, Hatcher CW (1980) Biological denitrification of high nitrate wastes generated in the nuclear industry. Bio Fluid Bed Treat Water Waste 1:235–250
Reinsel M (n.d) Nitrate removal technologies: new solutions to an old problem. Guest column on December 10, 2014. https://www.wateronline.com/doc/nitrate-removal-technologies-new-solutions-to-an-old-problem-0001. Accessed 21 April 2020
Tsai HH, Ravindran V, Williams MD, Pirbazari M (2004) Forecasting the performance of membrane bioreactor process for groundwater denitrification. J Environ Eng Sci 3:507–521
Nujić M, Milinković D, Habuda-Stanić M (2017) Nitrate removal from water by ion exchange. Croatian J Food Sci Technol 9:182–186. https://doi.org/10.17508/CJFST.2017.9.2.15
Du R, Peng Y, Cao S, Wu C, Weng D, Wang S, He J (2014) Advanced nitrogen removal with simultaneous Anammox and denitrification in sequencing batch reactor. Biores Technol 162:316–322
Fu F, Dionysiou DD, Liu H (2014) The use of zero-valent iron for groundwater remediation and wastewater treatment: a review. J Hazard Mater 267:194–205
Anderson JA (2011) Photocatalytic nitrate reduction over Au/TiO2. Catal Today 175:316–321
Bhatnagar A, Sillanpaa MA (2011) Review of emerging adsorbents for nitrate removal from water. Chem Eng J 168:493–504
Li M, Feng C, Zhang Z, Yang S, Sugiura N (2010) Treatment of nitrate contaminated water using an electrochemical method. Biores Technol 101:6553–6557
Samatya S, Kabay N, Yüksel Ü, Arda M, Yüksel M (2006) Removal of nitrate from aqueous solution by nitrate selective ion exchange resins. React Funct Polym 66:1206–1214
Wang JL, Kang J (2005) The characteristics of anaerobic ammonium oxidation (ANAMMOX) by granular sludge from an EGSB reactor. Process Biochem 40:1973–1978
Aslan S, Turkman A (2003) Biological denitrification of drinking water using various natural organic solid substrates. Water Sci Technol 48:489–495
Schoeman JJ, Steyn A (2003) Nitrate removal with reverse osmosis in a rural area in South Africa. Desalination 155:15–26
Elmidaoui A, Elhannouni F, Menkouchi Sahli AM, Chay L, Elabbassi H, Hafsi M, Largeteau D (2001) Pollution of nitrate in Moroccan ground water: removal by electrodialysis. Desalination 136:325–332
Kapoor A, Viraraghavan T (1997) Nitrate removal from drinking water—review. J Environ Eng 123:371–380
Della Rocca C, Belgiorno V, Meriç S (2007) Overview of in-situ applicable nitrate removal processes. Desalination 204:46–62
He Q, Feng C, Chen N, Zhang D, Hou T, Dai J, Hao C, Mao B (2019) Characterizations of dissolved organic matter and bacterial community structures in rice washing drainage (RWD)-based synthetic groundwater denitrification. Chemosphere 215:142–152
Ajibade FO, Adewumi JR (2017) Performance evaluation of aquatic macrophytes as a constructed wetland for municipal wastewater treatment. FUTA J Eng Eng Technol (FUTAJEET) 11:1–11
Guo L, Lv T, He K, Wu S, Dong X, Dong R (2017) Removal of organic matter, nitrogen and faecal indicators from diluted anaerobically digested slurry using tidal flow constructed wetlands. Environ Sci Pollut Res 24:5486–5496. https://doi.org/10.1007/s11356-016-8297-2
He K, Lv T, Wu S, Guo L, Ajmal Z, Luo H, Dong R (2016) Treatment of alkaline stripped effluent in aerated constructed wetlands: feasibility evaluation and performance enhancement. Water 8:386
Kizito S, Lv T, Wu S, Ajmal Z, Luo H, Dong R (2017) Treatment of anaerobic digested effluent in biochar-packed vertical flow constructed wetland columns: role of media and tidal operation. Sci Total Environ 592:197–205
Wu S, Lv T, Lu Q, Ajmala Z, Dong R (2016) Treatment of anaerobic digestate supernatant in microbial fuel cell coupled constructed wetlands: evaluation of nitrogen removal, electricity generation, and bacterial community response. Sci Total Environ 580:339–346
Ajibade FO, Wang H, Guadie AA, Ajibade TF, Fang Y, Sharif HMA, Liu W, Wang A (2021) Total nitrogen removal in biochar amended non-aerated vertical flow constructed wetlands for secondary wastewater effluent with low C/N ratio: Microbial community structure and dissolved organic carbon release conditions. Biores Technol 322:124430. https://doi.org/10.1016/j.biortech.2020.124430
Milani M, Marzo A, Toscano A, Consoli S, Cirelli GL, Ventura D, Barbagallo S (2019) Evapotranspiration from horizontal subsurface flow constructed wetlands planted with different perennial plant species. Water 11:2159. https://doi.org/10.3390/w11102159
Mena J, Rodriguez L, Nunez J, Fernández FJ, Villasenor J (2008) Design of horizontal and vertical subsurface flow constructed wetlands treating industrial wastewater. WIT Trans Ecol Environ 111:555–563
Vymazal J (2007) Removal of nutrients in various types of constructed wetlands. Sci Total Environ 380:48–65
Kadlec RH (2008) The effects of wetland vegetation and morphology on nitrogen processing. Ecol Eng 33(1):26–141
Peng L, Hua Y, Cai J, Zhao J, Zhou W, Zhu D (2014) Effects of plants and temperature on nitrogen removal and microbiology in a pilot-scale integrated vertical-flow wetland treating primary domestic wastewater. Ecol Eng 64:285–290
Białowiec A, Albuquerque A, Randerson PF (2014) The influence of evapotranspiration on vertical flow subsurface constructed wetland performance. Ecol Eng 67:89–94
International Water Association (2000) Constructed wetlands for pollution control. Processes, performance, design and operation. IWA Publishing, London, p 156
Omotade IF, Alatise MO, Olanrewaju OO (2019) Recycling of aquaculture wastewater using charcoal based constructed wetlands. Int J Phytorem 21:399–404. https://doi.org/10.1080/15226514.2018.1537247
Lin YF, Jing SR, Wang TW, Lee DY (2002) Effects of macrophytes and external carbon sources on nitrate removal from groundwater in constructed wetlands. Environ Pollut 119:413–420. https://doi.org/10.1016/S0269-7491(01)00299-8
Lin YF, Jing SR, Lee DY, Chang YF, Shih KC (2007) Nitrate removal and denitrification affected by soil characteristics in nitrate treatment wetlands. J Environ Sci Health, Part A: Toxic/Hazard Subst Environ Eng 42:471–479. https://doi.org/10.1080/10934520601187690
Domingos S, Germain M, Dallas S, Ho G (n.d) Nitrogen removal from industrial wastewater by hybrid constructed wetland systems. In: 2nd IWA-ASPIRE conference and exhibition, 28 October–31 November, 2007. Perth, Western Australia. https://researchrepository.murdoch.edu.au/4088/
Xiong J, Guo G, Mahmood Q, Yue M (2011) Nitrogen removal from secondary effluent by using integrated constructed wetland system. Ecol Eng 37:659–662
Aguilar L, Gallegos A, Arias CA, Ferrera I, Sánchez O, Rubio R, Saad MB, Missagia B, Caro P, Sahuquillo S, Pérez C, Morató J (2019) Microbial nitrate removal efficiency in groundwater polluted from agricultural activities with hybrid cork treatment wetlands. Sci Total Environ 653:723–734
Jia L, Liu H, Kong Q, Li M, Wu S, Wu H (2020) Interactions of high-rate nitrate reduction and heavy metal mitigation in iron-carbon-based constructed wetlands for purifying contaminated groundwater. Water Res 169:115285
Wang W, Song X, Li F, Jia X, Hou M (2020) Intensified nitrogen removal in constructed wetlands by novel spray aeration system and different influent COD/N ratios. Bioresources Technol 306:123008. https://doi.org/10.1016/j.biortech.2020.123008
Hang Q, Wang H, He Z, Dong W, Chu Z, Ling Y, Yan G, Chang Y, Li C (2020) Hydrilla verticillata–sulfur-based heterotrophic and autotrophic denitrification process for nitrate-rich agricultural runoff treatment. Int J Environ Res Public Health 17:1574. https://doi.org/10.3390/ijerph17051574
Hang Q, Wang H, Chu Z, Hou Z, Zhou Y, Li C (2017) Nitrate-rich agricultural runoff treatment by vallisneria-sulfur based mixotrophic denitrification process. Sci Total Environ 587–588:108–117. https://doi.org/10.1016/j.scitotenv.2017.02.069
Zhao Y, Song X, Cao X, Wang Y, Zhao Z, Si Z, Yuan S (2019) Modified solid carbon sources with nitrate adsorption capability combined with nZVI improve the denitrification performance of constructed wetlands. Biores Technol 294:122189. https://doi.org/10.1016/j.biortech.2019.122189
Kleimeier C, Liu H, Rezanezhad F, Lennartz B (2018) Nitrate attenuation in degraded peat soil-based constructed wetlands. Water 10:355. https://doi.org/10.3390/w10040355
Ajibade FO, Adeniran KA, Egbuna CK (2013) Phytoremediation efficiencies of water hyacinth in removing heavy metals in domestic sewage (a case study of university of Ilorin, Nigeria). Int J Eng Sci 2:16–27
Chang JJ, Wu SQ, Dai YR, Liang W, Wu ZB (2013) Nitrogen removal from nitrate-laden wastewater by integrated vertical-flow constructed wetland systems. Ecol Eng 58:192–201
Dires S, Birhanu T, Ambelu A (2019) Use of broken brick to enhance the removal of nutrients in subsurface flow constructed wetlands receiving hospital wastewater. Water Sci Technol 79:156–164. https://doi.org/10.2166/wst.2019.037
Zamora S, Marín-Muñíz JL, Nakase-Rodríguez C, Fernández-Lambert G, Sandoval L (2019) Wastewater treatment by constructed wetland eco-technology: influence of mineral and plastic materials as filter media and tropical ornamental plants. Water 11:2344. https://doi.org/10.3390/w11112344
Si Z, Song X, Wang Y, Cao X, Wang Y, Zhao Y, Ge X, Sand W (2020) Untangling the nitrate removal pathways for a constructed wetland sponge iron coupled system and the impacts of sponge iron on a wetland ecosystem. J Hazard Mater 393:122407. https://doi.org/10.1016/j.jhazmat.2020.122407
Si Z, Song X, Cao X, Wang Y, Wang Y, Zhao Y, Ge X, Tesfahunegn AA (2020) Nitrate removal to its fate in wetland mesocosm filled with sponge iron: impact of influent COD/N ratio. Frontiers Environ Sci Eng 2020(14):4. https://doi.org/10.1007/s11783-019-1183-7
Yu G, Peng H, Fu Y, Yan X, Du C, Chen H (2019) Enhanced nitrogen removal of low C/N wastewater in constructed wetlands with co-immobilizing solid carbon source and denitrifying bacteria. Biores Technol 280:337–344. https://doi.org/10.1016/j.biortech.2019.02.043
Sun H, Yang Z, Wei C, Wu W (2018) Nitrogen removal performance and functional genes distribution patterns in solid-phase denitrification sub-surface constructed wetland with micro aeration. Biores Technol 263:223–231
Zhang L, Sun Z, Xie J, Wu J, Cheng S (2018) Nutrient removal, biomass accumulation and nitrogen-transformation functional gene response to different nitrogen forms in enhanced floating treatment wetlands. Ecol Eng 112:21–25
Nakase C, Zurita F, Nani G, Reyes G, Fernández-Lambert G, Cabrera-Hernández A, Sandoval L (2019) Nitrogen removal from domestic wastewater and the development of tropical ornamental plants in partially saturated mesocosm-scale constructed wetlands. Int J Environ Res Public Health 16:4800. https://doi.org/10.3390/ijerph16234800
Tchobanoglous G, Burton FL, Stensel HD (2003) Wastewater engineering. McGraw-Hill, New York, p 56
Li B, Irvin S, Baker K (2007) The variation of nitrifying bacterial population sizes in a sequencing batch reactor (SBR) treating low, mid, high concentrated synthetic wastewater. J Environ Eng Sci 6:651–663
Harold L, Haunschild LK, Hopes G, Tchobanoglous G, Darbya JL (2010) Anoxic treatment wetlands for denitrification. Ecol Eng 36:1544–1551
Kadlec RH, Wallace S (2009) Treatment wetlands, 2nd edn. CRC Press, Boca Raton
Gajewska, M., Skrzypiec, K (2018) Kinetics of nitrogen removal processes in constructed wetlands. E3S Web Conf 26:1–4. https://doi.org/10.1051/e3sconf/20182600001
Kadlec RH, Knight RL (1996) Treatment wetlands. Boca Raton, FL 33431. CRC Press LLC, USA
Lee C, Fletcher TD, Sun G (2009) Nitrogen removal in constructed wetland systems. Eng Life Sci 9:11–22. https://doi.org/10.1002/elsc.200800049
Sonavane PG, Munavalli GR (2009) Modeling nitrogen removal in a constructed wetland treatment system. Water Sci Technol 60:301–309. https://doi.org/10.2166/wst.2009.319
US EPA (1993) Subsurface flow constructed wetlands for wastewater treatment: a technology assessment. Office of Water, Washington, D.C. EPA 832-R-93-008
Saeed T, Sun G (2012) A review on nitrogen and organics removal mechanisms in subsurface flow constructed wetlands: dependency on environmental parameters, operating conditions and supporting media. J Environ Manage 112:429–448
Savant NK, DeDatta SK (1982) Nitrogen transformations in wetland rice soils. Adv Agron 35:241–302
Reddy KR, Patrick WH Jr (1984) Nitrogen transformations and loss in flooded soils and sediments. CRC Crit Rev Environ Control 13:273
Patrick WH Jr, Wyatt R (1964) Soil nitrogen loss as a result of alternate submergence and dying. Proc—Soil Sci Soc Am 28:647–653
Vymazal J (1995) Algae and element cycling in wetlands. CRC Press Inc
Graaf AA, Bruijn P, Robertson LA, Jetten MS, Kuenen JG (1996) Autotrophic growth of anaerobic ammonium oxidizing micro-organisms in a fluidized bed reactor. Microbiology 142:2187–2196
Ahn YH (2006) Sustainable nitrogen elimination biotechnologies: a review. Process Biochem 41:1709–1721
Guadie A, Xia S, Zhang Z, Zeleke J, Guo W, Ngo HH, Hermanowicz SW (2014) Effect of intermittent aeration cycle on nutrient removal and microbial community in a fluidized bed reactor-membrane bioreactor combo system. Biores Technol 156:195–205
Guadie A, Xia S, Zhang Z, Guo W, Ngo HH, Hermanowicz SW (2013) Simultaneous removal of phosphorus and nitrogen from sewageusing a novel combo system of fluidized bed reactor–membranebioreactor (FBR–MBR). Biores Technol 149:276–285
Gerardi MH (2002) Nitrification and denitrification in the activated sludge process. Wiley Inc., New York
Prosnansky M, Sakakibarab Y, Kuroda M (2002) High-rate denitrification and SS rejection by biofilm-electrode reactor (BER) combined with microfiltration. Water Res 36:4801–4810
Szekeres S, Kiss I, Kalman M, Soares MI (2002) Microbial population in a hydrogen-dependent denitrification reactor. Water Res 36:4088–4094
Rijn JV, Tal Y, Schreier HJ (2006) Denitrification in recirculating systems: theory and applications. Aquacult Eng 34:364–376
Breisha GZ, Winter J (2010) Bio-removal of nitrogen from wastewaters—a review. J Am Sci 6:508–528
Chen D, Dai T, Wang H, Yang K (2015) Nitrate removal by a combined bioelectrochemical and sulfur autotrophic denitrification (CBSAD) system at low temperatures. Desalin Water Treat 57:1–7. https://doi.org/10.1080/19443994.2015.1101024
Chen D, Yang K, Wang H (2016) Effects of important factors on hydrogen-based autotrophic denitrification in a bioreactor. Desalin Water Treat 57:3482–3488. https://doi.org/10.1080/19443994.2014.986533
Chen D, Yang K, Wang H, Lv B (2014) Nitrate removal from groundwater by hydrogen-fed autotrophic denitrification in a bio-ceramsite reactor. Water Sci Technol 69:2417–2422. https://doi.org/10.2166/wst.2014.167
Chung J, Amin K, Kim S, Yoon S, Kwon K, Bae W (2014) Autotrophic denitrification of nitrate and nitrite using thiosulfate as an electron donor. Water Res 58:169–178. https://doi.org/10.1016/j.watres.2014.03.071
Wang X, Xing L, Qiu T, Han M (2013) Simultaneous removal of nitrate and pentachlorophenol from simulated groundwater using a biodenitrification reactor packed with corncob. Environ Sci Pollut Res 20:2236–2243. https://doi.org/10.1007/s11356-012-1092-9
Kim J, Park K, Cho K, Nam S, Park T, Bajpai R (2005) Aerobic nitrification–denitrification by heterotrophic Bacillus strains. Biores Technol 96:1897–1906
Kim S, Jung H, Kim KS, Kim IS (2004) Treatment of high nitrate containing wastewaters by sequential heterotrophic and autotrophic denitrification. J Environ Eng 130:1475–1480
Masclaux-Daubresse C, Daniel-Vedele F, Dechorgnat J, Chardon F, Gaufichon L, Suzuki A (2010) Nitrogen uptake, assimilation and remobilization in plants: challenges for sustainable and productive agriculture. Ann Bot 105:1141–1157. https://doi.org/10.1093/aob/mcq028
Xu G, Fan X, Miller AJ (2012) Plant nitrogen assimilation and use efficiency. Annu Rev Plant Biol 63:153–182. https://doi.org/10.1146/annurev-arplant-042811-105532
Bialowiec A, Janczukowicz W, Randerson PF (2011) Nitrogen removal from wastewater in vertical flow constructed wetlands containing LWA/gravel layers and reed vegetation. Ecol Eng 37:897–902
Dan TH, Quang LN, Chiem NH, Brix H (2011) Treatment of high-strength wastewater in tropical constructed wetlands planted with Sesbania sesban: horizontal subsurface flow versus vertical downflow. Ecol Eng 37:711–720
Cui L, Ouyang Y, Lou Q, Yang F, Chen Y, Zhu W, Luo S (2010) Removal of nutrients from wastewater with Canna indica L. under different vertical-flow constructed wetland conditions. Ecol Eng 36:1083–1088
Kantawanichkul S, Kladprasert S, Brix H (2009) Treatment of high-strength wastewater in tropical vertical flow constructed wetlands planted with typha angustifolia and cyperus involucratus. Ecol Eng 35:238–247
Landry GM, Maranger R, Brisson J, Chazarenc F (2009) Nitrogen transformations and retention in planted and artificially aerated wetlands. Water Res 43:535–545
Huett DO, Morris SG, Smith G, Hunt N (2005) Nitrogen and phosphorus removal from plant nursery runoff in vegetated and unvegetated subsurface flow wetlands. Water Res 39:3259–3272
Shamir E, Thompson TL, Karpiscak MM, Freitas RJ, Zauderer J (2001) Nitrogen accumulation in a constructed wetland for dairy wastewater treatment. J Am Water Resour Assoc 37:315–325
Drizo A, Frost CA, Smith KA, Grace J (1997) Phosphate and ammonium removal by constructed wetlands with horizontal subsurface flow, using shale as a substrate. Water Sci Technol 35:95–102
Bayley ML, Davison L, Headley TR (2003) Nitrogen removal from domestic effluent using subsurface flow constructed wetlands: influence of depth, hydraulic residence time and pre-nitrification. Water Sci Technol 48:175–182
Kaseva ME (2004) Performance of a sub-surface flow constructed wetland in polishing pre-treated wastewater e a tropical case study. Water Res 38:681–687
Langergraber G (2005) The role of plant uptake on the removal of organic matter and nutrients in subsurface flow constructed wetlands: a simulation study. Water Sci Technol 51:213–223
Brix H (1997) Do macrophytes play a role in constructed treatment wetlands? Water Sci Technol 35:11–17
Masi F (2008) Enhanced denitrification by a hybrid HF-FWS constructed wetland in a large-scale wastewater treatment plant. In: Vymazal J (ed) Wastewater treatment, plant dynamics and management in constructed and natural wetlands. pp 267–275
Osorio AC, Villafañe P, Caballero V, Manzan Y (2011) Efficiency of mesocosm scale constructed wetland systems for treatment of sanitary wastewater under tropical conditions. Water Air Soil Pollut 220:161–171
Wang R, Baldy V, Périssol C, Korboulewsky N (2012) Influence of plants on microbial activity in a vertical-downflow wetland system treating waste activated sludge with high organic matter concentrations. J Environ Manage 95:S158–S164
Wetzel RG (11–16 Nov, 2000) Fundamental processes within natural and constructed wetland ecosystems: short-term versus long-term objectives. 7th International conference on wetland systems for water pollution control. Lake Buena Vista pp 3–12
Garnett TP, Shabala SN, Smethurst PJ, Newman IA (2001) Simultaneous measurement of ammonium, nitrate and proton fluxes along the length of eucalyptus roots. Plant Soil 236:55–62
Brodrick SJ, Cullen P, Maher W (1988) Denitrification in a natural wetland receiving secondary treated effluent. Water Res 22:431–439
Biddlestone AJ, Gray KR, Job GD (1991) Treatment of dairy farm wastewaters in engineered reed bed systems. Process Biochem 26:265–268
Armstrong W, Armstrong J, Beckett RM (1990) Measurement and modeling of oxygen release from roots of phragmites Australis. Constructed wetlands for water pollution control. Pergamon Press, Oxford, UK
Brix H, Schierup H (1990) Soil oxygeneration in constructed reed beds: the role of macrophyte and soil atmosphere interface oxygen transport. Constructed wetlands for water pollution control. Pergamon Press, Oxford, UK
Bavor HJ, Roser DJ, McKersie SA, Breen P (1988) Treatment of secondary effluent. Report to Sydney Water Board, Australia
Cameron SG, Schipper LA (2010) Nitrate removal and hydraulic performance of organic carbon for use in denitrification beds. Ecol Eng 36:1588–1595
Sirivedhin T, Gray KA (2006) Factors affecting denitrification rates in experimental wetlands: field and laboratory studies. Ecol Eng 26:167–181
Aslan Ş, Türkman A (2004) Simultaneous biological removal of endosulfan (α +β) and nitrates from drinking waters using wheat straw as substrate. Environ Int. 30:449–455
Ingersoll TL, Baker LA (1998) Nitrate removal in wetland microcosms. Water Res 32:677–684
Kuschk P, Wiessner A, Kappelmeyer U, Weissbrodt E, Kaestner M, Stottmeister U (2003) Annual cycle of nitrogen removal by a pilot-scale subsurface horizontal flow in a constructed wetland under moderate climate. Water Res 37:4236–4242
Zhong F, Huang S, Wu J, Cheng S, Deng Z (2019) The use of microalgal biomass as a carbon source for nitrate removal in horizontal subsurface flow constructed wetlands. Ecol Eng 127:263–267. https://doi.org/10.1016/j.ecoleng.2018.11.029
Glass C, Silverstein J (1998) Denitrification kinetics of high nitrate concentration water: pH effect on inhibition and nitrite accumulation. Water Res 32:831–839
Shen Z, Zhou Y, Liu J, Xiao Y, Cao R, Wu F (2015) Enhanced removal of nitrate using starch/PCL blends as solid carbon source in a constructed wetland. Biores Technol 175:239–244
Áséy A, Édegaard H, Bach K, Pujol R, Hamon M (1998) Denitrification in a packed bed biofilm reactor (Biofor)—experiments with different carbon sources. Water Res 32(5):1463–1470
Liu X, Fu X, Pu A, Zhang K, Luo H, Anderson BC, Li M, Huang B, Hu L, Fan L, Chen W, Chen J, Fu S (2019) Impact of external carbon source addition on methane emissions from a vertical subsurface-flow constructed wetland. Greenhouse Gases: Sci Technol 9:331–348. https://doi.org/10.1002/ghg.1847
Zhao Q, Chen Y (2015) Nitrogen removal effect in different locations of tidal flow constructed wetland and the effect of external carbon source on it. Water Supply Technol 9(4):32–37
Xiao L, He F, Liang X (2012) Effect of adding solid carbon source on the treatment effect of vertical flow constructed wetland sewage. Lake Sci 24:843–848
Nikolausza M, Kappelmeyera U, Szekelyb A, Rusznyakb A, Marialigetib K, Kastnera M (2008) Diurnal redox fluctuation and microbial activity in rhizosphere of wetland plants. Eur J Soil Biol 44:324–333
Fu G, Huangshen L, Guo Z, Zhou Q, Wu Z (2017) Effect of plant-based carbon sources on denitrifying microorganisms in a vertical flow constructed wetland. Biores Technol 224:214–221
Hang Q, Wang H, Chu Z, Ye B, Li C, Hou Z (2016) Application of plant carbon source for denitrification by constructed wetland and bioreactor: review of recent development. Environ Sci Pollut Res 23:8260–8274. https://doi.org/10.1007/s11356-016-6324-y
Chang JJ, Lu YF, Chen JQ, Wang XY, Luo T, Liu H (2016) Simultaneous removals of nitrate and sulfate and the adverse effects of gravel-based biofilters with flower straws added as exogenous carbon source. Ecol Eng 95(2016):189–197
Soares MIM, Abeliovich A (1998) Wheat straw as substrate for water denitrification. Water Res 32:3790–3794
Schipper LA, Vojvodić-Vuković M (2001) Five years of nitrate removal, denitrification and carbon dynamics in a denitrification wall. Water Res 35:3473–3477
Ovez B (2006) Batch biological denitrification using arundo donax, glycyrrhiza glabra, and gracilaria verrucosa as carbon source. Process Biochem 41:1289–1295
Ovez B, Ozgen S, Yuksel M (2006) Biological denitrification in drinking water using glycyrrhiza glabra and arunda donax as the carbon source. Process Biochem 41:1539–1544
Singer A, Parnes S, Gross A, Sagi A, Brenner A (2008) A novel approach to denitrification processes in a zero-discharge recirculating system for small-scale urban aquaculture. Aquacult Eng 39:72–77
Trois C, Pisano G, Oxarango L (2010) Alternative solutions for the biodenitrification of landfill leachates using pine bark and compost. J Hazard Mater 178:1100–1105
Warneke S, Schipper LA, Matiasek MG, Scow KM, Cameron S, Bruesewitz DA, McDonald IR (2011) Nitrate removal, communities of denitrifiers and adverse effects in different carbon substrates for use in denitrification beds. Water Res 45:5463–5475
Lu S, Hu H, Sun Y, Yang J (2009) Effect of carbon source on the denitrification in constructed wetlands. J Environ Sci 21:1036–1043. https://doi.org/10.1016/S1001-0742(08)62379-7
Zhang M, Zhao L, Mei C, Yi L, Hua G (2014) Effects of plant material as carbon sources on TN removal efficiency and N2O flux in vertical-flow-constructed wetlands. Water Air Soil Pollut 225:11. https://doi.org/10.1007/s11270-014-2181-9
Wu S, Kuschk P, Brix H, Vymazal J, Dong R (2014) Development of constructed wetlands in performance intensifications for wastewater treatment: a nitrogen and organic matter targeted review. Water Res 57(5):40–55
Gomez MA, Gonzalez-Lopez J, Hontoria-Garcia E (2006) Influence of carbon source on nitrate removal of contaminated groundwater in a denitrifying submerged filter. J Hazard Mater 80:69–80
Hareendran RA (2010) Study of denitrification kinetics at low temperatures using methanol as the external carbon sources. The thesis, George Washington University. p 47
Chen X, He S, Zhang Y, Huang X, Huang Y, Chen D, Huang XC, Tang J (2015) Enhancement of nitrate removal at the sediment-water interface by carbon addition plus vertical mixing. Chemosphere 136:305–310. https://doi.org/10.1016/j.chemosphere.2014.12.010
Rustige H, Nolde E (2007) Nitrogen elimination from landfill leachates using an extra carbon source in subsurface flow constructed wetlands. Water Sci Technol 56(3):125–133
Chu L, Wang J (2016) Denitrification of groundwater using PHBV blends in packed bed reactors and the microbial diversity. Chemosphere 155:463–470
Wu W, Yang L, Wang J (2013) Denitrification using PBS as carbon source and biofilm support in a packed-bed bioreactor. Environ Sci Pollut Res 20:333–339
Chu L, Wang J (2011) Nitrogen removal using biodegradable polymers as carbon source and biofilm carriers in a moving bed biofilm reactor. Chem Eng J 170:220–225
Zhang Q, Ji F, Xu X (2016) Effects of physicochemical properties of poly-ε-caprolactone on nitrate removal efficiency during solid-phase denitrification. Chem Eng J 283:604–613
Gutierrez-Wing MT, Malone RF, Rusch KA (2012) Evaluation of polyhydroxybutyrate as a carbon source for recirculating aquaculture water denitrification. Aquacult Eng 51:36–43
Marusincova H, Husarova L, Ruzicka J, Ingr M, Navrátil V, Buňková L, Koutny M (2013) Polyvinyl alcohol biodegradation under denitrifying conditions. Int Biodeterior Biodegradation 84:21–28. https://doi.org/10.1016/j.ibiod.2013.05.023
Shen Z, Zhou Y, Liu J, Xiao Y, Cao R, Wu F (2014) Enhanced removal of nitrate using starch/PCL blends as solid carbon source in a constructed wetland. Biores Technol 175C:239–244
Li P, Zuo J, Xing W, Tang L, Ye X, Li Z, Yuan L, Wang K, Zhang H (2013) Starch/polyvinyl alcohol blended materials used as solid carbon source for tertiary denitrification of secondary effluent. J Environ Sci 25:1972–1979. https://doi.org/10.1016/S1001-0742(12)60259-9
Wu W, Yang F, Yang L (2012) Biological denitrification with a novel biodegradable polymer as carbon source and biofilm carrier. Biores Technol 118:136–140
Srinandan CS, D’souza, G., Srivastava, N., Nayak, B.B., Nerurkar, A.S, (2012) Carbon sources influence the nitrate removal activity, community structure and biofilm architecture. Bioresour Technol 117:292–299
Shen Z, Zhou Y, Wang J (2013) Comparison of denitrification performance and microbial diversity using starch/polylactic acid blends and ethanol as electron donor for nitrate removal. Bioresour Technol 131:33–39
Boley A, Müller WR, Haider G (2000) Biodegradable polymers as solid substrate and biofilm carrier for denitrification in recirculated aquaculture systems. Aquacult Eng 22:75–85
Huai J, Wu J, Zhong F, Cheng S (2018) Level. Advances in research on carbon source replenishment strategies for constructed wetlands. Environ Prot Frontiers 8(6):475–481. https://doi.org/10.12677/aep.2018.86059
Qian Z (2016) Research on nitrogen and phosphorus removal of low carbon source wastewater based on solid phase nitrification and adsorption phosphorus [D]: [Ph.D. Thesis]. Chongqing University, Chongqing
Rørslett B, Berge D, Johansen SW (1986) Lake enrichment by submersed macrophytes: a Norwegian whole-lake experience with Elodea canadensis. Aquat Bot 26:325–340
US EPA (1975) Process design manual for nitrogen control. Office of Technology Transfer, Washington, DC
Wang J, Chu L (2016) Biological nitrate removal from water and wastewater by solid-phase denitrification process. Biotechnol Adv 34:1103–1112
Phipps RG, Crumpton WG (1994) Factors affecting nitrogen loss in experimental wetlands with different hydrologic loads. Ecol Eng 3:399–408
Katayon S, Fiona Z, Noor MM, Halim GA, Ahmad J (2008) Treatment of mild domestic wastewater using subsurface constructed wetlands in Malaysia. Int J Environ Stud 65:87–102
Langergraber G, Prandtstetten Ch, Pressl A, Rohrhofer R, Haberl R (2007) Optimization of subsurface vertical flow constructed wetlands for wastewater treatment. Water Sci Technol 55:71–78
Nivala J, Hoos MB, Cross C, Wallace S, Parkin G (2007) Treatment of landfill leachate using an aerated, horizontal subsurface flow constructed wetland. Sci Total Environ 380:19–27
Tuncsiper B (2007) Removal of nutrient and bacteria in pilot-scale constructed wetlands. J Environ Sci Health, Part A 42:1117–1124
Herkowitz J (1986) Listowel artificial marsh project report. Ontario Ministry of the Environment, Water Resources Branch, Toronto
Oostrom AJ, Russell JM (1994) Denitrification in constructed wastewater wetlands receiving high concentrations of nitrate. Water Sci Technol 29:7–14
Akratos CS, Tsihrintzis VA (2007) Effect of temperature, HRT vegetation and porous media on removal efficiency of pilot scale horizontal subsurface flow constructed wetlands. Ecol Eng 29:173–191
Huang J, Reneau RB, Hagedorn C (2000) Nitrogen removal in constructed wetlands employed to treat domestic wastewater. Water Res 34:2582–2588
Zhou W, Sun Y, Wu B, Zhang Y, Huang M, Miyanaga T, Zhang Z (2011) Autotrophic denitrification for nitrate and nitrite removal using sulfur limestone. J Environ Sci 23:1761–1769. https://doi.org/10.1016/S1001-0742(10)60635-3
Boley A, Muller WR (2005) Denitrification with polycaprolactone as solid substrate in a laboratory-scale recirculated aquaculture system. Water Sci Technol 52:495–502
Viotti P, Collivignarelli MC, Martorelli E, Raboni M (2016) Oxygen control and improved denitrification efficiency by dosing ferrous ions in the anoxic reactor. Desalin Water Treat 57:18240–18247
Hiraishi A, Khan ST (2003) Application of polyhydroxyalkanoates for denitrification in water and wastewater treatment. Appl Microbiol Biotechnol 61:103–109
Xu ZX, Shao L, Yin HL, Chu HQ, Yao YJ (2009) Biological denitrification using corncobs as a carbon source and biofilm carrier. Water Environ Res 81:242–247
Button M, Nivala J, Weber KP, Aubron T, Müller RA (2015) Microbial community metabolic function in subsurface flow constructed wetlands of different designs. Ecol Eng 80:162–171
Chen Y, Wen Y, Zhou Q, Vymazal J (2014) Effects of plant biomass on nitrogen transformation in subsurface-batch constructed wetlands: a stable isotope and mass balance assessment. Water Res 63:158–167
Abou-Elela SI, Golinielli G, Abou-Taleb EM, Hellal MS (2013) Municipal wastewater treatment in horizontal and vertical flows constructed wetlands. Ecol Eng 61:460–468
Karathanasis AD, Potter CL, Coyne MS (2003) Vegetation effect on fecal bacteria BOD, and suspended solid removal in constructed wetlands treating domestic wastewater. Ecol Eng 20:157–169
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Ajibade, F.O. et al. (2021). Removal of Nitrogen Oxyanion (Nitrate) in Constructed Wetlands. In: Oladoja, N.A., Unuabonah, E.I. (eds) Progress and Prospects in the Management of Oxyanion Polluted Aqua Systems. Environmental Contamination Remediation and Management. Springer, Cham. https://doi.org/10.1007/978-3-030-70757-6_12
Download citation
DOI: https://doi.org/10.1007/978-3-030-70757-6_12
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-70756-9
Online ISBN: 978-3-030-70757-6
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)