Abstract
Age-related macular degeneration (AMD) is a leading cause of blindness worldwide. The pathogenesis of AMD involves dysfunction and loss of the retinal pigment epithelium (RPE), a monolayer of cells that provide nourishment and functional support for the overlying photoreceptors. RPE cells in mammals are not known to divide, renew or regenerate in vivo, and in advanced AMD, RPE loss leads to degeneration of the photoreceptors and impairment of vision. One possible therapeutic approach would be to support and replace the failing RPE cells of affected patients, and indeed moderate success of surgical procedures in which relatively healthy autologous RPE from the peripheral retina of the same eye was transplanted under the retina in the macular area suggested that RPE replacement could be a means to attenuate photoreceptor cell loss. This prompted exploration of the possibility to use pluripotent stem cells (PSCs) as a potential source for “healthy and young” RPE cells for such cell-based therapy of AMD. Various approaches ranging from the use of allogeneic embryonic stem cells to autologous induced pluripotent stem cells are now being tested within early clinical trials. Such PSC-derived RPE cells are either injected into the subretinal space as a suspension, or transplanted as a monolayer patch upon scaffold support. Although most of these approaches are at early clinical stages, safety of the RPE product has been demonstrated by several of these studies. Here, we review the concept of cell-based therapy of AMD and provide an update on current progress in the field of RPE transplantation.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
11.1 Introduction
As per World Health Organization estimates, AMD is one of the leading causes of visual impairment in developed countries. In its advanced stages, the disease severely worsens the patient’s quality of life. Globally, it is the third leading cause of blindness [1]. AMD is a complex multifactorial disease. Besides the main factor of age, its onset and progression are linked to both genetic and environmental factors including diet, smoking, chronic inflammation and hypertension [2]. The disease has two advanced stages, geographic atrophy (GA) as a severe form of “dry” (non-neovascular) AMD and choroidal neovascularization (CNV) or “wet” (neovascular) AMD (NVAMD). Vision loss in both stages is due to the death of photoreceptors, but the disease etiology is different in these two stages. NVAMD is caused by hyperproliferation of choroidal vessels, some of which penetrate the subretinal space where they leak fluid and blood separating photoreceptors from their supporting cell type, the retinal pigment epithelium (RPE). In the case of dry AMD progressing to GA, photoreceptor cell death is thought to be triggered and secondary to dysfunction and atrophy of RPE cells [3]. In both conditions, the pathology mainly affects the macula resulting in central vision loss and slowly progressing to perimacular regions [4]. While the pathogenesis of AMD is multifactorial and complex, involving interactions between RPE, choroid and the photoreceptors and involving multiple mechanisms including oxidative injury, immune system activation and inflammation (among others), the RPE has become one of the main therapeutic targets because dysfunction and loss of RPE cells seems to precede and play a key role in the changes that then occur in the photoreceptors, the retina, and the choroid.
The RPE is a monolayer of epithelial cells located adjacent to the retinal photoreceptor (PR) outer segments. This monolayer is supported on a proteinaceous Bruch’s membrane (BM) that separates it from the choroidal vasculature. The BM actually serves as a bilateral basement membrane, both to the overlying RPE cells and the underlying choriocapillaris. RPE cells within the monolayer are connected via tight junctions [5], and in a fully mature RPE monolayer, a mechanical barrier is thus formed between the retina and the choroidal blood supply, which is part of the blood-retinal barrier. Because of this feature, RPE cells control nutrient and oxygen flow from the choriocapillaris to the PRs, and metabolite flow back from PRs to the choriocapillaris. In addition, RPE cells are critically important for maintaining the health and integrity of PRs: RPE pigment granules absorb scattered light; replenish light-isomerized visual pigment; phagocytose shed outer segments of PRs; and secrete cytokines in a polarized fashion. The polarized nature of the RPE, with characteristic receptors, channels and cellular structures on its apical and basal sides, is a key feature that is required for most of its functions [5]. This apical-basal polarization is induced in cells as they form a confluent monolayer with tight junctions between neighboring cells. Once the tight junctions fully mature, they suppress free flow of receptors and channels between the apical and basal sides of RPE cells, allowing the two membrane sides to behave differently. For instance, predominant location of potassium channels on the apical side and chloride channels on the basal side allow vectorial fluid flow from the apical to the basal sides. Several different retinal degenerative diseases are known to be caused by specific gene mutations that affect some of these RPE functions, underscoring the importance of the RPE in PR and retinal health and survival [3, 6, 7].
With age, RPE cells (that like PRs and other retinal and CNS cells do not naturally divide, regenerate or renew), undergo metabolic changes that may reduce their ability to perform some of their functions. These changes can be exacerbated by environmental and genetic factors. Some of these changes include accumulation of lipofuscin deposits called drusen inside and below the RPE, and these accumulations of “debris” are the hallmark of AMD. Along with reduction of melanin content in the RPE, the changes likely lead to reduced anti-oxidative capacity. These pathological changes in the RPE cells and chronic aberrant inflammatory responses progress over time, and have been linked to development of the AMD [3, 7]. Dry AMD is marked by multiple and often confluent areas of drusen, which can be both internal and external to the RPE. Drusen accumulation has been associated with RPE cell death and eventually retinal degeneration in dry AMD. Furthermore, degeneration and/or death of RPE cells and subsequent weakening and formation of gaps and breaches in the RPE monolayer can result in choroidal neovascularization, leading to NVAMD.
Attempts to limit vision loss in neovascular AMD included surgical removal of the subretinal pathological choroidal neovascular membrane between the RPE and PRs, laser treatment of extra-foveal CNV and also photodynamic therapy [8,9,10]. Currently, the mainstay of treatment for curbing and attenuating this form of disease is through the use of anti-VEGF agents that temporarily block choroidal vessel proliferation and reduce permeability of such vessels. Anti-VEGF treatments, that revolutionized and altered the rapid and dramatic course of neovascular AMD, do not, however, fully prevent chronic fibrosis caused by slow leakage of ectopic choroidal vessels and do not arrest the underlying process of progressive RPE dysfunction and loss. Similarly, in the advanced form of dry AMD, formation and expansion of GA is currently largely untreatable. Besides limited benefit obtained by certain dietary modifications (as defined by the AREDS trials [11]) and protection from sunlight and short wavelength light, there is currently no approved drug available that can suppress RPE atrophy or significantly prevent/attenuate GA lesion expansion.
The observations that RPE dysfunction and loss play a critical role in the pathogenesis of AMD coupled with the fact that in humans these cells have an extremely limited potential for regeneration (if at all), suggest the possibility that RPE cell replacement/support may serve as a possible beneficial therapeutic approach. To be effective and maintain visual function, such transplantation needs to occur prior to the secondary loss of the overlying PRs. Once PRs are lost, replacement of both RPE and PR cells, as well as possibly a proper substrate equivalent to Bruch’s membrane, may be required. In the following sections, data from basic research studies and early clinical trials that attempt to address this challenge will be presented, with emphasis on generation and use of RPE cells, which in many ways are an easier therapeutic target, as their effects do not require the formation of functional neuronal/synaptic connections. Rather, if a monolayer of healthy RPE cells can be formed in the subretinal space by transplantation, evidence from animal experiments and clinical observations suggest this can support function and viability of the overlying PRs. In advanced stages of disease, once PRs are lost as well, combined RPE + PR grafts may provide regenerative capabilities, but at present this is a more challenging goal and while animal studies show some ability of PR progenitors to survive and partially integrate into the retina, this is as yet not being explored in clinical trials in AMD.
11.1.1 Proof of Concept Studies Suggesting Cell Replacement May Be Beneficial
The etiology of the two advanced stages of AMD and the post-mitotic, non-regenerative nature of RPE cells and PRs suggest that replacement cell-based therapy may be a possible therapeutic approach. This is supported by various proof-of-concept procedures and studies that aimed to replace/support the degenerating RPE in order to halt or slow down PR cell death. These included attempts to perform autologous transplantation of RPE from the retinal periphery to the macula, retinal rotation procedures to overlay the macular and particularly foveal cone PRs over healthier RPE, and early attempts to transplant RPE. As detailed here in different sections of this chapter, these studies indeed lend support to the notion that delivery of healthy RPE has the potential to attenuate PR loss.
Gouras et al. reported in 1984 the first ever RPE transplant, which was performed in a monkey eye. Adult post-mortem human RPE cells were cultured in vitro and transplanted as a cell suspension into the subretinal space where the native RPE had been surgically removed [6]. Similar RPE transplants were subsequently performed in rabbits [12] and in rats [13]. All these animal studies confirmed survival of human xenografts. Functional efficacy of RPE transplants was first tested in 1988 in Royal College of Surgeons (RCS) rats in which PR degeneration is secondary to RPE dysfunction, with a mutation in the MERTK gene causing defective phagocytosis of photoreceptor outer segments (POS) by the RPE cells [14]. As the majority of animal species apart from primates and certain birds do not develop macular structures, there is a lack of appropriate animal models for AMD. Thus, the RCS rat has been extensively used to examine therapeutic approaches for RPE-induced retinal degeneration. Transplanted human RPE cells were indeed demonstrated to phagocytose rat POS in this model [14, 15]. Later on, donor RPE from wild type rats, a human RPE cell line, human embryonic stem cell as well as iPSC-derived RPE cells were also tested and shown to affect disease progression in RCS rats [16,17,18,19,20]. These animal studies, that demonstrated survival and function of transplanted human RPE, provided the first proof-of-concept that RPE transplantation can be developed as a therapy for AMD.
In humans, lack of effective treatments for NVAMD prior to the development of PDT and later anti-VEGF therapies prompted retinal surgeons to attempt macular translocation surgeries, in which foveal PRs were re-positioned above healthier RPE outside the area affected by CNV and injury [21, 22]. These procedures showed that indeed improved survival of PRs and even significant visual acuity gains could be obtained in some cases, but the surgeries are highly complex and the rate of complications is high, including retinal detachment, diplopia, and recurrence of CNV [23]. As a routine treatment for NVAMD this was not practical and now is largely unnecessary, thanks to anti-VEGF treatments, but it did support the notion that approximating macular and foveal PRs to healthier RPE could be a valid therapeutic approach. This approach gained further support from studies of autologous RPE transplantation within the eyes of patients, as outlined here in Section 2a-III.
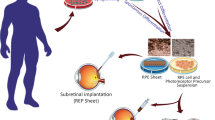
11.1.2 Delivery as a Cell Suspension or as Sheets of Cells on Scaffolds (Fig. 11.1)
Native RPE cells exhibit several critical characteristics that must also ultimately manifest in transplanted RPE cells to ensure their efficacy and proper function. These include maintaining cell polarity with the correct basal and apical orientation of different proteins and structures such as the Na+/K+ ATPase, proteins associated with tight junctions, retinol cycling, blood-retinal barrier and phagocytosis [31, 32]. Thus, structure of the cells and transplantation approach may markedly affect outcome. In general, two main forms of delivery are being tested, the first being injecting the cells in suspension, and the second as cell sheets, with or without a scaffold (Fig. 11.1) [30, 33, 34]. Both approaches have their advantages and disadvantages: subretinal or transchoroidal injection of cells in suspension is simpler and generally less surgically challenging and traumatic (Fig. 11.1a). In contrast, more complex biological formulations such as 3-dimensional RPE sheets on different types of scaffolds, while requiring a more complicated surgical approach, provide the possibility to deliver the RPE cells as an intact, functioning unit with the cells already in the correct polarity, enabling better formation of tight junctions (Fig. 11.1b–f) [35, 36]. In addition, this creates a natural monolayer anatomical structure rather than single cells, RPE clumps or multilayered structures with random orientation and phenotypic variability that can form following simple subretinal cell suspension implantation [25, 37, 38]. This being said, also after delivery in suspension, over time the transplanted cells are often able to layer out, and adjust their polarity by intracellular trafficking of the relevant proteins.
Schematic adapted from Sharma et al. [24] of various ongoing and planned retinal pigment epithelium (RPE) transplant approaches, showing fundus views of the transplants (left) and how the transplants (purple) would be integrated into the subretinal space, their possible impact on retina and choroid, and the various scaffold materials involved (right). (a) In the study by Schwartz et al. [25] and in additional trials such as the Cell Cure trial (Table 11.1), human embryonic stem cell (hESC)-derived RPE cells in suspension are injected into the subretinal space in the macular region. hESC-RPE cells in suspension do not initially form a polarized monolayer, but may over time layer out and adopt the correct polarity by intracellular trafficking of the appropriate proteins. (b) Da Cruz et al. [26] transplanted an hESC-RPE patch over the area of acute choroidal neovascularization (CNV) in two patients. This 3 × 6-mm transplant was intended to help in stopping CNV growth and activity while rescuing photoreceptors that are still viable in this area. (c) Kashani et al. [27] used an hESC-RPE patch on a parylene scaffold, transplanted subretinally in the area of geographic atrophy (GA). Shifting of the preferred retinal location for fixation to the area of the hESC-RPE patch was observed in three patients, suggesting that the patch was able to recover and support activity of some photoreceptors in the transplanted region. (d) Mandai et al. [28] tested the first autologous iPSC-RPE patch in an acute wet (neovascular) AMD patient. This patch was transplanted in a macular region that was fibrotic due to chronic vessel leakage. One-year follow-up with this patient revealed the absence of new leaks. (e) Sharma et al. [29] propose to transplant an autologous iPSC-RPE patch using a poly-(lactic-coglycolic) acid (PLGA) scaffold at the border of the GA lesion. This patch is intended to cover parts of the transition zone where the PRs are still alive to slow down or halt the expansion of the GA lesion. (f) Ben M’Barek et al. [30] propose to test a gelatin-embedded hESC-RPE patch on an amniotic membrane in patients with Leber congenital amaurosis (LCA). This patch will be transplanted on top of dysfunctional native RPE cells such that over the long term, the new RPE patch will integrate into the host RPE monolayer in place of diseased cells [Adapted and printed by permission from the review by Sharma R, Bose D, Maminishkis A, and Bharti K.: Retinal Pigment Epithelium Replacement Therapy for Age-Related Macular Degeneration: Are We There Yet? Annu Rev. Pharmacol Toxicol. 2020 Jan 6;60:553–572. doi: https://doi.org/10.1146/annurev-pharmtox-010919-023245. PMID: 31914900]
To date, various natural and synthetic scaffolds were used to seed RPE cells and to mimic BM. The scaffold material, design of the surface and dimensions highly affect cell adherence, growth, differentiation, and function [39]. Natural scaffolds include human lens capsule [40,41,42,43], extracellular matrix-coated basement membrane [44,45,46], human amniotic membrane [30, 47, 48] and Descemet’s membranes [49]. Human lens capsules are highly available endogenous basement membranes composed mainly of collagen type IV, laminin, and fibronectin and were shown to be biologically tolerated as a RPE scaffold [43]. Preliminary tests showed that RPE cells well adhere and survive on human lens capsule in culture, enabling the cells to create tight junctions more efficiently than on hydrogel scaffolds [40, 41, 43]. In addition, this tissue was found to have the potential to act as a substitute to BM, which is also damaged in AMD [42]. Human amniotic membranes have been in clinical use for many indications [50, 51], and in particular in ophthalmology [52, 53]. They were found to be well tolerated subretinally and reduce choroidal neovascularization [47]. All naturally occurring membranes that were investigated demonstrated high ability to support RPE growth and function, and to preserve the polarized monolayer structure of RPE cells grown upon them [40, 41, 47,48,49, 54] (Fig. 11.1f demonstrates schematic of the proposed use of a gelatin-embedded ESC-RPE patch on an amniotic membrane in patients with Leber congenital amaurosis [30]).
Natural polymer scaffolds were also investigated for their potential to serve as a scaffold for RPE growth and delivery. These include collagen [55], gelatin films [56], hydrogel [41], basement membrane explant layers [44, 57], cryoprecipitate [58], bacterial cellulose [59], microspheres of cross-linked fibrinogen [60], and silk fibroin [61,62,63,64]. Overall, they showed diverse potential in terms of cell survival and biocompatibility [58, 65, 66], but in general were less effective than natural scaffolds [41] and were associated with a serious risk of inducing significant inflammatory responses leading to transplant rejection and cell death.
A third group of scaffolds are the synthetic polymers such as poly lactic-co-glycolic acid(PLGA) [18, 67], polydimethylsiloxane (PDMS) sheets with laminin coating (PDMS-PmL) [68], poly-l-lactic and poly lactic-co-glycolic acid (PLLA/PLGA) [29, 69, 70], methacrylate/methacrylamide copolymer (PEGDMA) hydrogel [41], mesh-supported submicron parylene-C membrane (MSPM) [71], polyester [26], poly(ε-caprolactone) (PCL) [72] and parylene C synthetic material [27] (Fig. 11.1). Studies have shown the effectiveness of these artificial scaffolds in maintaining normal RPE function and morphology, and some of them have advantages that include biodegradability, good microstructure control enabling selective transport, cell adhesion, and tissue support. On the other hand, the biodegradable materials may carry a higher risk of provoking subretinal inflammatory reactions as compared with nondegradable materials [73]. In order to gain the synthetic scaffold properties on the one hand and the biocompatibility of the natural scaffolds on the other hand, combination scaffolds were created including chitosan-graft-poly(varepsilon-caprolactone)/polycaprolactone (CS-PCL/PCL) [74], Antheraea pernyi silk fibroin (RWSF), polycaprolactone (PCL), and gelatin (Gt) [75]. RPE cells grew and differentiated on these scaffolds without inflammatory response or rejection [75, 76].
The second strategy of RPE replacement therapy is delivery as a cell suspension, which was shown to be successful in terms of PR rescue [77] and phagocytosis activity [78]. The cells can be injected into the subretinal space following vitrectomy using a narrow gauge cannula with minimal surgical trauma, and transchoroidal injections (penetrating into the subretinal space from the choroidal side after advancing a cannula via the suprachoroidal approach) have also been used [79]. Healing following delivery of cells in suspension is rapid, there is no need to leave silicone oil or gas in the eye, which is required when scaffolds are inserted via relatively large retinotomies and collateral retinal injury is minimal. However, the cells are initially not in their correct polarity and may form cell aggregates and clumps in animal models and humans [25, 38]. Over time, the cells do seem to layer out and the fact that they can maintain viable PRs above the grafts in both animal models and in human patients supports the view that they are functional. In general, if only RPE transplantation is performed, both forms of RPE cell transplantation, whether as a cell suspension or as a monolayer on different types of scaffolds can be considered, and both show good safety profiles in terms of tumorigenicity and teratoma formation [38, 80]. In the future, if composite RPE and PR grafts will be developed, delivery on scaffolds will probably be required.
11.1.3 Surgical Approach
The surgical technique used to deliver the cells may affect their therapeutic potential and function. Subretinal transplantation of RPE sheets is a challenging surgical procedure, which requires formation of relatively large retinotomies, the use of special instruments and induction of large areas of retinal detachment to deliver the scaffold. In addition, the subretinal manipulation of the sheets and scaffolds may injure the remaining PRs. In these surgeries, the retinotomies usually are sealed using laser photocoagulation retinopexy and silicone oil (that later needs to be removed) is often used at the end of surgery. In contrast, retinotomies caused by injection of RPE cell suspension using 38–41 gauge needles are self-sealing [81]. This technical difference increases the risk of postoperative retinal detachment and epiretinal membrane for RPE sheet implantation and perhaps demands better surgical skills than subretinal injection of cells in suspension, especially in cases in which multiple areas of geographic atrophy need to be treated [81]. No standardized concentration or volume of transplanted cells for either cell formulation was established, and most trials in animal models and humans use a scale-up strategy in order to identify the optimal number and volume to be used. It was found that approximately 60,000 cells are needed to cover the macular area [82], but a larger number may need to be delivered as viability following delivery is partial.
The subretinal transplantation of RPE cells, both as cells in suspension and as sheet formulations, can be accomplished using two main routes [83]:
-
1.
Internal/trans-vitreal [84]—based on pars plana vitrectomy (PPV).
-
2.
External/trans-scleral [85, 86]—through the choroid and Bruch’s membrane without penetrating the retina itself.
Many factors influence the choice of surgical approach including eye size, rigidity of the implant, cell properties, and surgical abilities. To date, RPE cells in suspension were implanted mainly using the trans-vitreal approach. This procedure is considered minimally invasive and traumatic and is based on creation of a subretinal bleb of localized retinal detachment following PPV. The retinotomies can be made using 38–41 gauge needles, which form self-sealing holes [25, 81, 87, 88]. Some surgeons prefer forming a pre-bleb using a solution (such as buffered saline solution, BSS) or air, followed by injection of the cell suspension. Of note, a small air bubble may assist in preventing cell reflux [89]. The complications of this strategy are similar to the well-known ones of PPV surgery in addition to PR atrophy, subretinal hemorrhage and secondary choroidal neovascularization due to bruch’s membrane rupture. Also, increased risk of epiretinal membrane formation and proliferative vitreoretinopathy (PVR) were described, which may be related to RPE cell reflux into the vitreal cavity during and after the injection.
Several devices have been developed in order to transplant RPE sheets using a trans-vitreal approach [90,91,92]. In general, most trans-vitreal transplantation procedures are based on performing a standard 3 port vitrectomy followed by formation of a localized retinal detachment bleb by injection of BSS into the subretinal space. Afterward, a retinotomy is created (usually at the border of the bleb), and the sheet of cells is delivered into the bleb using various devices, needles or manipulators, including devices that allow to partially roll the sheets in order to minimize the size of the retinotomies [90,91,92]. Finally, laser is often applied along the retinotomy, an air-fluid exchange is performed and intravitreal tamponade using either gas or silicone oil is left in the eye [91, 93,94,95].
In many animal studies and in one human clinical trial [91,92,93, 96], subretinal delivery of cell preparations in suspension was performed via a trans-scleral, trans-choroidal approach. In rodents and particularly rats (such as the RCS rat model), in view of the large size of the lens and small vitreal cavity, this is often the preferred mode of delivery. In a study delivering umbilical cord-derived cells to the subretinal space in patients with AMD, a microcatheter inserted into the suprachoridal space through a peripheral scleral cutdown was used to reach the macular area and then a small needle was advanced to penetrate into the subretinal space from the choroidal side. The cells were then injected following formation of a small pre-bleb. The rate of surgical complications including inadvertent retinal perforations into the vitreal space and retinal detachments was high, leading the authors to conclude that this surgical approach requires improvement [79]. This study is further described in section 11.2(b), and indeed a modified device for subretinal delivery via the trans-choroidal approach is being developed by Orbit Biomedical and currently being tested in a hESC-derived RPE clinical trial conducted by Cell Cure Neurosciences Ltd. (NCT01226628, unpublished data).
The different routes of delivery share complications associated with the surgical technique including retinal detachment, PVR, subretinal hemorrhage, PR injury secondary to mechanical disruption, flow, and toxicity. Specific potential complications of the trans-scleral trans-choroidal approach include severe rupture of Bruch’s membrane, retinal breaching, choroidal trauma, suprachoroidal hemorrhage, and an increased risk of inflammatory and immune responses [97].
11.2 Overview of Clinical Trials
Currently, transplantation of RPE cells from various sources, via different modes of delivery and at different stages/forms of disease, form the main efforts for treatment of AMD that are already being tested in early clinical trials (Table 11.1). These efforts and studies will be the focus of this section. Other cell types including bone marrow and umbilical cord-derived cells and neural stem cells were tested in a limited fashion in a small number of trials and will also be described. Attempts to transplant PRs or PR-progenitors have not yet matured to clinical trials in AMD, but retinal progenitor cells (RPCs) derived from fetal tissue are being transplanted into the vitreous (jCyte, NCT03073733) and in the subretinal space (ReNeuron, NCT02464436) in patients with retinitis pigmentosa.
-
(a)
RPE cell replacement
For RPE replacement therapy in humans, attempts have been made using RPE cells from the following main sources:
-
I.
Allogeneic adult RPE: Harvested from adult cadaver eyes
-
II.
Allogeneic fetal RPE: Harvested from aborted fetus eyes
-
III.
Autologous RPE: Obtained from the same eye of the same patient
-
IV.
Allogeneic ESC-derived RPE: Generated in vitro from embryonic stem cells
-
V.
Autologous iPSC-derived RPE: Generated in vitro from induced pluripotent stem cells derived from the same patient
-
VI.
Allogeneic iPSC-derived RPE: Generated in vitro using HLA-matched, non-matched, or a universal donor induced pluripotent stem cells
In the following sections, these approaches and the relevant clinical trials will be detailed.
I. Allogeneic Adult RPE
The idea of “patching” or “bandaging” the area of choroidal neovascularization in NVAMD was raised and explored two decades ago. In the 1990s, much before researchers differentiated RPE cells from ESCs or iPSCs, retinal surgeons were testing allogeneic adult RPE transplants in the eyes of AMD patients [12–14]. Patients with CNV were considered an ideal choice for two reasons: (1) for lack of better treatment, subretinal CNV removal surgeries were a common practice, performed to try and arrest the neovascular process, (2) because of the rapid onset of symptoms in NVAMD, at least some photoreceptors were usually still viable when patients would come to the clinic with signs of vision loss. The rationale was that in addition to removal of the CNV, if the area of CNV could be “patched” with a “new” RPE sheet, this would stop further bleeding and protect overlying photoreceptors from degenerating. The easiest source of RPE tissue available at that time was from cadaver eyes. Since this was an allogeneic source, it required heavy immunosuppression limiting the success of this procedure. Tezel et al. reported a clinical trial of 12 NVAMD patients in which along with surgical removal of subfoveal CNV, a sheet of adult allogeneic RPE was transplanted [98]. No significant change in best-corrected visual acuity, contrast sensitivity, or reading speed was observed in the follow-up visit after 1 year. All patients were administered a triple immune-suppression drug regimen employed for renal transplantation both preoperatively and postoperatively. Five out of the twelve patients were able to continue the immune-suppression regimen for 6 months post-surgery and no sign of transplant rejection was observed in these patients during this period. The immunosuppression regimen had to be stopped midway in the remaining seven patients because of development of serious adverse effects. Soon after immunosuppression was stopped, at 6 months or before 6 months, intragraft fibrosis was observed in all patients, indicating that systemic immunosuppression is required for allogeneic RPE transplant. Primarily because of immunosuppression-related complications and a rather invasive procedure that resulted in moderate success, this form of treatment for NVAMD was not frequently used. Additional pertinent complications observed were PVR and transplanted cell migration to extrafoveal locations. Nevertheless, this study helped to establish that transplantation of RPE sheets was a feasible approach as a potential treatment for AMD patients.
II. Allogeneic Fetal RPE
Alongside allogeneic adult RPE transplants, attempts were also made to transplant RPE cells from fetal eye tissue (fRPE) [99, 100]. Using microarray-based analysis, the mRNA expression profile of cultured fRPE was found to closely resemble both native fetal and native adult RPE cells [101]. Initial results with encouraging outcomes led to the suggestion that PR rescue was likely induced by cytokines secreted by fRPE cells [101,102,103]. Algvere et al. (in a study that included 13 patients), reported that fRPE transplants were relatively better accepted by non-exudative AMD patients even without immunosuppression. However, multiple cases with leakage on fluorescein angiography and fibrosis were still observed. Similar observations were made by Weisz et al. (1 patient) [100]. In both of these studies, no change in vision of the patients was observed in the follow-up visits. Further use of fRPE was limited because of two main concerns and limitations: ethical and lawful procurement of fRPE is one major concern, and limited amount of material obtained from a given donor eye, combined with limited ability to amplify these cells, prevent scaling up of this potential therapy. Recently, attempts have been made to optimize fRPE culture conditions for increased production of cells [104]. This then prompted researchers in China to conduct a prospective study, which included six AMD patients treated by three different doses of fRPE cells ranging from 100,000 to 500,000 cells (NCT02868424). Results of this study are not yet publicly available. The use of fetal RPE cells, which seemed to fare better than allogeneic adult RPE cells, suggested that success of transplant may depend on the source of RPE cells and that “young” RPE cells may provide an advantage.
III. Autologous RPE
The limited success of allogeneic RPE transplants when not accompanied by aggressive immunosuppression confirmed the notion that the diseased eye, and particularly in the case of NVAMD, may not be immune-privileged [56, 104, 105]. Although improved transplant survival was observed with long-term systemic immunosuppression [7, 98, 106], this regimen led to severe systemic side-effects in the elderly AMD patients. Autologous transplantation, utilizing cells of the host, can prevent rejection and circumvent the need for such immunosuppressive treatment and its complications.
Autologous RPE transplantation was pioneered in 2002 by Binder and colleagues in NVAMD patients [107, 108]. Following vitrectomy and CNV excision, a subretinal transplant of at least 1500 autologous RPE cells was delivered. The autologous RPE cells for this purpose were harvested from the nasal side of the optic disc. Improved multifocal electroretinographic (mfERG) response density was observed in the treatment group at 3 and 6 months post-transplantation, as compared with patients treated by CNV excision alone, without autologous RPE transplant. Reading acuity also improved in these patients. However, no significant improvement was detected in distance acuity and the improvement of mfERG responses was not maintained at longer follow-up visits [107]. It is possible that the transient improvement of mfERG responses observed was due to excision of the CNV and control of the subretinal neovascular process, and/or transient cytokine secretion from the transplanted RPE cells. Although RPE cells transplanted in suspension were able to orient themselves to form a monolayer in vivo, it is possible that because of AMD-related changes in Bruch’s membrane, not all transplanted RPE cells formed a polarized monolayer [45]. If RPE cells do not form a monolayer, these non-adherent cells, in the long term, can either form aggregates or undergo apoptosis [38, 57].
Considering the importance of forming a polarized monolayer by attaching to Bruch’s membrane, in some studies adhesion promoting supplements were co-transplanted with the autologous RPE cells. In a study by van Meurs et al., poly-L-lysine, which when absorbed to a substrate (in this case Bruch’s membrane) increases the number of positively charged sites available for cell binding, was injected in the subretinal space prior to injection of autologous RPE cells in suspension [109]. However, while vision stabilized in five of the eight patients treated in this fashion, only one showed pigmentation at the site of transplant and the other three patients developed retinal detachment secondary to PVR. The authors concluded that translocation of autologous peripheral RPE cells after membrane extraction was technically possible, but was associated with a high rate of PVR and had no measurable positive effect on functional outcome.
Because of RPE cell suspension-associated complications and the lack of long-term visual benefits, an alternative approach using autologous RPE monolayer sheets was then tested. Post CNV excision, transplantation of autologous RPE sheet transplants was attempted in NVAMD patients in a study by Lu et al. [110]. The donor RPE monolayer (relatively healthy RPE) was harvested from a peripheral site of the retina and translocated to the macula. Vision improvement was observed in this study, suggesting that autologous transplantation of intact RPE sheets could improve visual outcomes of AMD patients. However, the surgical procedure was traumatic because of the large retinotomy required, retinal detachment of the donor site and the treatment site, and the very complex and risky maneuver required to translocate the transplant.
Although, none of these early clinical interventions was safe and efficient enough to mature into a commercially approved therapy, they helped develop surgical procedures for delivery of RPE cells and RPE sheets in the subretinal space. These studies also provided sufficient proof-of-concept that RPE transplantation has the potential of slowing down AMD disease progression.
IV. Allogeneic hESC-Derived RPE
As RPE transplantation strategies were evolving, regenerative medicine based on pluripotent cells was also progressing rapidly. Stable cell lines of human embryonic stem cells (hESCs) extracted at the blastocyst phase were first established in 1998 [111] and soon thereafter protocols were developed for differentiation of hESCs to RPE cells, starting in 2004 [112,113,114,115]. Initially, protocols relied on spontaneous differentiation: investigators, while growing ESCs in culture to explore their ability to form different cell types, identified and enriched by selection pigmented cells that spontaneously appeared in culture. Characterization of these cells showed that they closely resembled RPE cells [113, 114]. In 2009, Idelson and colleagues developed a directed differentiation protocol based upon the addition of Nicotinamide and Activin A to culture media at specific time-points. This allowed enhancement of the differentiation of hESCs into RPE [19]. Additional protocols were consequently identified by multiple groups to developmentally guide ESCs into retinal and RPE lineages by the use of other specific growth factors. This change not only improved the differentiation efficiency, it also improved reproducibility of differentiation, homogeneity and hence safety of the product, and also likely improved functionality of derived RPE cells [112, 115, 116].
The ability to derive RPE-like cells from hESCs led to the first attempts to transplant these cells in AMD (as well as Stargardt disease) eyes. The first preliminary report by Schwartz, Lanza, and colleagues on the use of hESC-derived RPE cells in suspension to treat AMD patients with GA appeared in 2012 [25] (Fig. 11.1a), followed by a more comprehensive report of this phase I safety study in nine AMD patients (as well as nine Stargardt patients) in 2015 [117]. Following vitrectomy, spontaneously-differentiated hECS-RPE cells in suspension were administered via subretinal injection at the border area of GA lesions. Patients were given systemic immunosuppression to avoid rejection of these allografts. Although no adverse events related to transplanted cells were observed, immunosuppression related side-effects were noted. Post-surgery, transplanted cells identified by their pigmentation and by OCT changes were observed in the subretinal space in the area of transplant in some of the eyes. Visual improvement was reported in some of the eyes that received transplanted cells, but no correlation could be drawn between the density and location of transplanted RPE cells and vision improvement [81].
An additional study delivering hESC-derived RPE cells in suspension was launched in 2015 by Banin, Reubinoff and colleagues (NCT02286089). In this case RPE cells were derived from hESCs according to the directed-differentiation protocol developed by Idelson et al. [19]. To date, 16 patients with advanced dry AMD and GA were transplanted, and systemic immune suppression was used for the first few months. The overall safety profile is good with epiretinal membranes, the majority of them mild and not requiring intervention, being the main adverse event. Various imaging observations suggest survival and possible efficacy of the cells (unpublished data).
In 2018, Da Cruz, Coffee and colleagues reported on the transplantation of an hESC-derived RPE patch in two AMD patients with severe exudative AMD [26] (Fig. 11.1b). hESCs were differentiated into RPE cells using a spontaneous differentiation protocol similar to the Schwartz et al. approach [25] (Fig. 11.1a). However, differentiated cells were seeded on a vitronectin-coated polyester sheet to form a confluent monolayer. A 6 mm × 3 mm patch of hESC-RPE on the polyester sheet was then transplanted into the subretinal space under the macula. Since this was again an allograft, patients were immunosuppressed, but the authors adopted a local immunosuppression protocol to avoid systemic side-effects. Both patients showed improved visual acuity and preferred fixation in retinal areas above the grafts. It is also worth noting that neither of the two patients showed new signs of subretinal bleeding, suggesting that the RPE patch can at least stop further incidence of CNV. One of the two patients developed an inferior retinal detachment due to PVR and there were some adverse events that were not deemed to be associated with the cells or patch, but in general safety signals were good. Overall, this two patient study provided hope that transplanting hESC-RPE on a scaffold is possible and has the potential to change disease course.
An additional study transplanting hESC-RPE grown on a synthetic scaffold in the eyes of dry AMD patients with GA was reported by the group of Kashani, Humayun and colleagues [27] (Fig. 11.1c). In this case hESC-derived RPE cells were grown on a nano-engineered parylene C scaffold and a 3.5 mm × 6.25 mm patch was transplanted into the subretinal space following vitrectomy. The parylene C scaffold used was 6 μm thick and contained multiple circular ultrathin areas of 0.3–0.4 μm to mimic the diffusion properties of Bruch’s membrane. Vision improvement was observed at 4 months post-surgery in one out of the four patients who received a successful transplant and the other three did not lose vision. Microperimetry testing suggested fixation over the area of the patch in some of the patients. Furthermore, no adverse events related to the patch or the transplantation procedure were observed. The patch was found to integrate under the retina, suggesting that RPE-patch transplantation may be a viable approach for dry AMD-GA patients as well.
V. Autologous iPSC-Derived RPE
Development and advances in deriving autologous induced pluripotent stem cells (iPSC) has provided another source for RPE cells. Using iPSC technology, the patient’s own cells can be reprogrammed to a pluripotent state and differentiated to an RPE fate, thereby providing an avenue for autologous therapy, which likely eliminates the requirement for immunosuppression. An autologous iPSC-derived cell therapy approach, however, does require an extremely robust manufacturing process so that RPE cells can be derived from multiple different patients in a safe and timely manner. Several reports have suggested that iPSCs may acquire potentially oncogenic mutation or chromosomal alterations during the reprograming process [118,119,120]. Therefore, much effort is focused on manufacturing of iPSCs that are free of such changes [118, 120]. Furthermore, the autologous iPSC manufacturing process is logistically challenging. Despite these difficulties, two autologous iPSC-RPE studies have reached clinical application.
In Japan, the group of Mandai, Takahashi and colleagues from the Riken Center for Developmental Biology initiated the first iPSC-derived RPE clinical trial [28] (Fig. 11.1d). In this case a NVAMD patient with active CNV refractory to anti-VEGF treatment was treated using an autologous iPSC-RPE patch without scaffold support [28]. A 1.3 mm × 3 mm sheet of RPE monolayer was transplanted in the subretinal space after surgical excision of the CNV. The authors noted that part of the patch folded over itself, but they were able to deliver it into the subretinal space. No immunosuppression was given and no associated complications were observed. Following surgery additional anti-VEGF injections were not required and no new signs of subretinal hemorrhage were seen. Visual acuity did not significantly change in this one patient over 4 years of follow up [34]. Unfortunately, this trial was suspended when chromosomal alterations were detected in iPSCs generated during the manufacturing of the RPE patch for the second patient [121]. This landmark study provided the first ever autologous iPSC-RPE-patch transplant in AMD patients, but it also highlighted manufacturing challenges associated with an autologous iPSC-based therapeutic approach.
Recently, the team of Bharti and colleagues from the National Eye Institute at the NIH developed an autologous iPSC-RPE patch on a biodegradable PLGA scaffold for administration in AMD patients with GA [29] (Fig. 11.1e). This group demonstrated successful clinical-grade manufacturing of iPSCs and iPSC-derived RPE from three AMD patients, and the iPSCs were shown to be free of potentially oncogenic mutations. While a thorough analysis of differences between the Mandai et al. and Sharma et al. manufacturing processes was not presented, the authors suggested that the use of CD34+ blood progenitor cells as the source for iPSCs may have assisted in preventing occurrence of oncogenic alterations [28, 29]. In comparison, Mandai et al. used patient fibroblasts for deriving the iPSCs. Because of their progenitor cell nature, CD34+ cells retain their proliferative potential and when forced to divide under reprograming conditions may not undergo genomic stress. However, more work is required to test this hypothesis. The group also published safe integration of a 4 mm × 2 mm iPSC-derived RPE patch in a laser-induced RPE injury pig model. In these injured pig eyes, the AMD iPSC-RPE patch outperformed the control group (PLGA scaffold transplant without any cells) in protecting the overlying PRs. Furthermore, functional integration of the RPE-patch was observed, including the ability to phagocytose pig photoreceptor outer segments. This project provides additional evidence that autologous iPSC-based therapy is feasible and a Phase I/IIa clinical trial is expected to begin in the very near future.
VI. Allogeneic iPSC-RPE
Using similar iPSC technologies and RPE differentiation processes, allogeneic iPSC-RPE transplants have also being proposed, and this approach may be especially useful in relatively genetically homogenous populations in which a limited number of iPSC lines may allow immune compatibility in a high percentage of patients. In more genetically diverse populations, as with the previously listed allogeneic sources of RPE cells, the immune response to the transplanted cells will need to be addressed. One of the key underlying causes of immune rejection is the expression of HLA class I antigens by the RPE cells [122] and also their capability to turn on the expression of MHC class II antigens [123]. Some innovative ideas are being tested including generation of HLA-matched iPSC lines and universal donor stem cell lines. Studies have shown that transplantation of MHC homozygous cells in matched recipients reduces infiltration of inflammatory cells and allows reduced use of immunosuppressive drugs [124, 125]. HLA complex genes located on chromosome 6 represent one of the most polymorphic genes in the human genome. The HLA is divided into three groups of antigens: class I, class II, and class III, and each class has multiple genes allowing many possible variations. Because of this diversity, HLA homozygous iPSC banks that include multiple HLA haplotypes will have to be generated for each geographic and ethnic location. To address this concern, studies are presently ongoing to generate hypoimmunogenic iPSCs [126]. Deuse et al. [126] have shown that inactivating MHC class I and II genes and overexpressing CD47 allows iPSCs to retain their pluripotent potential while allowing to prevent an immune-response even in MHC-mismatched transplants. These findings are promising and suggest a technology to generate universal banks of iPSCs for an off-the-shelf RPE product as opposed to the lengthy, costly and complicated process of deriving a separate product for each individual patient. However, such cells need to be further tested for complications that may be associated with their immune-cloaking such as increased risk for viral infections and formation of evasive tumors.
-
(b)
Bone marrow and umbilical cord derived cells
Cell therapy in AMD has and is being attempted using additional cell types besides RPE. Palucorcel [CNTO-2476] is a preparation of human umbilical tissue-derived cells that were shown to preserve outer nuclear layer structure and attenuate visual function loss following transplantation into the subretinal space in the RCS model [127]. This prompted a clinical safety and dose escalation Phase I/IIa trial of transplantation of these cells in patients with bilateral AMD and GA (NCT01226628). Delivery of the cells to the subretinal space in this trial was performed via a trans-choroidal and not a trans-vitreal approach: a microcatheter was advanced to the posterior pole of the eye in the suprachoridal space through a peripheral scleral cutdown, and then the choroid, Bruch’s membrane and RPE were penetrated and cells delivered after forming a small subretinal pre-bleb with viscoelastic. Transplantation was achieved in 33/35 patients in which surgery was attempted, and gains of ≥ 10 ETDRS letters and ≥15 letters were seen in 10 and 7 eyes, respectively. However, the rate of surgical complications was high with retinal perforation (into the vitreous) occurring in 13/35 cases and retinal detachment developing in 6/35 eyes. The authors concluded that palucorcel was well tolerated and may be associated with improvement in visual acuity, but that the surgical approach requires modification [79].
Bone marrow-derived stem cells (BSMCs) have also been transplanted in patients with AMD. In a trial in Brazil (NCT01518127) autologous CD34+ cells separated from the bone marrow were injected into the vitreous of 10 patients with dry AMD and GA. The study concluded that the procedure is safe, and is associated with significant improvements in BCVA and macular sensitivity threshold, with patients who have small areas of atrophy showing a better response. The assumption is that a paracrine effect of CD34+ cells underlies the functional improvement. It should be noted that while all patients completed the 6 month follow up, only 6 patients were evaluated at the 12 month time point [128]. Intravitreal delivery of autologous CD34+ BMSCs was also reported to be safe by Park et al., but this study included only six eyes, two of which were in patients with AMD (NCT01736059) [129].
-
(c)
Neural stem cells
The human central nervous system stem cell line (HuCNS-SC, StemCells, Inc., USA) was authorized by the US Food and Drug Administration (FDA) for testing in the lysosomal storage disorder neuronal ceroid lipofuscinosis (NCL) (NCT00337636). The first-in-human clinical trial involving transplantation of a purified population of human neural stem cells for a neurodegenerative disorder was completed in 2009 when six patients with NCL underwent direct neurosurgical transplantation of allogeneic HuCNS-SCs into the cerebral hemispheres and lateral ventricles. The study showed surgical feasibility without adverse effects directly attributed to the donor cells [130]. A similar 1-year, open-label phase I study was undertaken to evaluate safety in four patients with Pelizaeus-Merzbacher disease (PMD) (NCT01005004) [131]. In addition, the same cells were used in a phase I/II trial for the treatment of thoracic spinal cord injury (SCI), conducted in 12 patients in Zurich and two North America sites (NCT01321333) and in a phase II clinical trial (NCT02163876) examining safety and efficacy of HuCNS-SC for cervical SCI. No final results were published for these studies.
Attempts were then made to examine whether these cells may be beneficial in the context of retinal degenerative disease. In vivo preclinical studies in royal college of surgeons (RCS) rats using HuCNS-SC showed photoreceptor and visual function preservation with limited proliferation, phagocytic capacity and no tumor-like formation [132, 133]. StemCells Inc. then initiated a 1-year Phase I/II clinical trial using HuCNS-SC® human neural stem cells to treat dry AMD (NCT01632527). The study included 15 patients, divided into two sequential cohorts: cohort I included 8 patients with BCVA ≤20/400 in the study eye, who were transplanted with 200,000 (4 patients) and one million (4 patients) cells. Cohort II consisted of 7 patients with BCVA of 20/320 to 20/100 in the study eye, who underwent transplantation of one million cells. The cells were injected in one single subretinal injection. Interim results of cohort I showed a 70% reduction in the rate of geographic atrophy (GA) expansion as compared with the control eye and a 65% reduction in the rate of GA as compared with the expected natural history of the disease. In addition, a positive safety profile was observed. This study was due to end in June 2015, but after the interim results detailed here final results have not yet been posted. A long-term follow-up study over 4 years was recently terminated due to financial reasons and not due to safety concerns (NCT02137915) [134], but it is not clear whether the promising interim results were maintained in the long term.
The multiple studies and trials summarized so far are very positive for the field, as pursuing these varied approaches increases our understanding of the possibilities and challenges associated with cell therapy and particularly RPE-based therapy in AMD. There are some preliminary signs of success: long-term cell survival was seen in some cases, there is evidence of transplant-recipient tissue integration, PR rescue, and even vision improvement in few patients. Multiple obstacles were also observed, including limited tolerance for long-term systemic immunosuppression in elderly patients, cell migration and/or proliferation in the vitreous cavity, and surgical challenges that are particularly associated with delivery in NVAMD patients in which the CNV needs to be addressed/excised and also when subretinal delivery of cells on scaffolds is performed, which require a relatively large retinotomy.
11.3 What’s Under Development: Preclinical Studies to Derive PRs
While RPE transplantation using various sources and techniques of delivery is already in multiple clinical trials (as detailed above), this form of treatment will be effective for preservation of vision in AMD only if performed prior to loss of the PRs. Ideally, once proven safe and effective, such transplantation will be carried out in early phases of disease, as RPE changes and small areas of RPE loss and atrophy just begin to appear. Then, the new, healthy cells will be able to provide support and sustain the still viable PRs of the host. However, in advanced stages of disease, once significant numbers of PRs have been lost, transplantation of RPE cells alone will not suffice to regain vision. This situation occurs not only in advanced AMD, but also in other retinal and macular degenerations such as retinitis pigmentosa, Stargardt disease and others. As such, multiple groups are exploring the possibility of supporting/replacing not only RPE cells but also PRs, and perhaps as combined grafts. Preliminary studies showed that multiple intrinsic factors can induce formation of RPCs, followed by differentiation into PR cells (rods or cones) and finally subtypes of PRs by expressing their specific characteristics [135,136,137,138,139]. Pioneering research studies by Sasai and colleagues established protocols that allow the self-organization of eyecup-like structures consisting of self-organized, complex, stratified 3D retinal tissue, which in many ways follows the path of embryonic eye development, yielding PRs and additional retinal elements [140,141,142]. Reh and colleagues reported the differentiation of embryonic stem cells to retinal cells [143]. This protocol was extensively modified in order to increase the efficacy of the differentiation into PRs [144, 145]. Further enhancement was accomplished with the development of 2D/3D protocols [146,147,148,149]. Gamm and colleagues differentiated embryoid bodies (EB) in suspension, cells were plated on laminin-coated plates and then the neuroepithelial structures were grown in suspension leading to formation of optic vesicle (OV)-like structures [150]. These floating structures continued on a path of ocular and retinal differentiation, producing mature PRs [150,151,152]. Bi-layered optic cup-like structures developed, leading to differentiation into RPE and PRs organized in a rosette-like shape [141, 153,154,155,156]. Formation of PR outer segments in this model is very slow, but this ground-breaking progress in retinal organoid production brings us significantly closer to implementing retinal cell replacement therapy beyond RPE alone. Still, there are significant hurdles on the path of making this technique commercial and technically applicable for PR production and cell replacement therapy including large scale-production, assuring homogeneity of the product, automation of the procedure, and cryopreservation [157].
11.4 Challenges and Conclusion
While progress in the development of cell-based therapies for AMD is accelerating, major challenges still exist. The main impediments to large scale clinical translation of such treatment include the following: (i) identifying the optimal ways of delivering the transplants, especially patches, to the treatment site in the subretinal space, (ii) proving and improving survival and retention of the transplanted RPE cells at the transplant site, (iii) overcoming and managing the innate immune response in the case of allogeneic transplants, (iv) enhancing integration of the transplanted cells with recipient tissue such that in the case of RPE transplantation a polarized monolayer of RPE cells is formed and can physiologically interact with the overlying PRs, and in the case of PRs, the correct synaptic connectivity is achieved, (v) addressing and eliminating the risk of tumor formation and oncogenic transformation in stem cell- and iPSC-derived transplants. The fact that multiple groups are testing varied cell preparations and delivery methods is of much benefit at this early stage of development of cell-based therapies and increases the likelihood that safe and efficient treatments will be forthcoming.
An important cautionary note to be made is that in this current era of much “hype” that is associated with cell-based therapy, there are occasionally attempts to provide treatments that are not properly tested, regulated or approved. One of the worst outcomes in this regard occurred when three women with AMD received bilateral intravitreal injections of autologous adipose tissue-derived “stem cells” in a clinic in Florida. While a “trial” was listed on ClinicalTrials.gov (NCT02024269), it was not regulated in any manner and was not FDA approved. Furthermore, the patients paid for the treatment. Within a week, the patients experienced severe complications including ocular hypertension, hemorrhagic retinopathy, vitreous hemorrhage and combined traction and rhegmatogenous retinal detachment. Despite attempts to treat the complications at other centers, all patients ultimately suffered severe and permanent visual loss, to the level of no light perception in 2/6 eyes, light perception in one eye, two eyes at hand motion acuity and one eye at 20/200 [158]. Additional “stem-cell clinics” are apparently treating patients using unproven and unregulated therapies, and it is important to warn patients not to fall for such bogus “trials” [159].
In summary, there is good reason to believe that cell-based therapy and especially stem cell-based treatments are poised to become the next big revolution in medicine in general and in the eye and retina in particular. Indeed, retinal and macular degenerations, with emphasis on AMD, are currently the “testing ground” for these novel therapies that carry the potential to support and replace dysfunctional and degenerating retinal cells, with RPE cells being a main target. Preliminary results of the efforts and trials described here provide hope that better treatments for these blinding diseases are forthcoming.
References
Resnikoff S, Pascolini D, Etya’ale D et al (2004) Global data on visual impairment in the year 2002. Bull World Health Organ 82(11):844–851
Jin ZB, Gao ML, Deng WL et al (2019) Stemming retinal regeneration with pluripotent stem cells. Prog Retin Eye Res. https://doi.org/10.1016/j.preteyeres.2018.11.003
Ambati J, Fowler BJ (2012) Mechanisms of age-related macular degeneration. Neuron. https://doi.org/10.1016/j.neuron.2012.06.018
Holz FG, Strauss EC, Schmitz-Valckenberg S, Van Lookeren Campagne M (2014) Geographic atrophy: clinical features and potential therapeutic approaches. Ophthalmology. https://doi.org/10.1016/j.ophtha.2013.11.023
Bharti K, Nguyen MTT, Skuntz S et al (2006) The other pigment cell: specification and development of the pigmented epithelium of the vertebrate eye. Pigment Cell Res. https://doi.org/10.1111/j.1600-0749.2006.00318.x
Gouras P, Flood MT, Kjeldbye H (1984) Transplantation of cultured human retinal cells to monkey retina. An Acad Bras Cienc 56(4):431–443
Zarbin M, Sugino I, Castellarin A, Fine S, Berger JMM (1999) RPE transplantation for age-related macular degeneration. Age-related macular degeneration. Mosby Year Book Inc, Philadelphia
Bressler NM (1999) Photodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin: one-year results of 2 randomized clinical trials - TAP report 1. Arch Ophthalmol. https://doi.org/10.1001/archopht.117.10.1329
Merrill PT, LoRusso FJ, Lomeo MD et al (1999) Surgical removal of subfoveal choroidal neovascularization in age-related macular degeneration. Ophthalmology. https://doi.org/10.1016/S0161-6420(99)90167-7
Virgili G, Bini A (2007) Laser photocoagulation for neovascular age-related macular degeneration. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD004763.pub2
Chew EY (2017) Nutrition, genes, and age-related macular degeneration: what have we learned from the trials? Ophthalmologica. https://doi.org/10.1159/000473865
Lopez R, Gouras P, Brittis M, Kjeldbye H (1987) Transplantation of cultured rabbit retinal epithelium to rabbit retina using a closed-eye method. Investig Ophthalmol Vis Sci 28:1131–1137
Li L, Turner JE (1988) Transplantation of retinal pigment epithelial cells to immature and adult rat hosts: short- and long-term survival characteristics. Exp Eye Res. https://doi.org/10.1016/0014-4835(88)90044-9
Li L, Turner JE (1988) Inherited retinal dystrophy in the RCS rat: prevention of photoreceptor degeneration by pigment epithelial cell transplantation. Exp Eye Res. https://doi.org/10.1016/0014-4835(88)90073-5
Li LX, Sheedlo HJ, Turner JE (1990) Long-term rescue of photoreceptor cells in the retinas of RCS dystrophic rats by RPE transplants. Prog Brain Res 82:179–185. https://doi.org/10.1016/s0079-6123(08)62603-5
Lavail MM, Li L, Turner JE, Yasumura D (1992) Retinal pigment epithelial cell transplantation in RCS rats: Normal metabolism in rescued photoreceptors. Exp Eye Res. https://doi.org/10.1016/S0014-4835(05)80168-X
Coffey PJ, Girman S, Wang SM et al (2002) Long-term preservation of cortically dependent visual function in RCS rats by transplantation. Nat Neurosci. https://doi.org/10.1038/nn782
Lu L, Garcia CA, Mikos AG (1998) Retinal pigment epithelium cell culture on thin biodegradable poly(DL-lactic-co-glycolic acid) films. J Biomater Sci Polym Ed. https://doi.org/10.1163/156856298X00721
Idelson M, Alper R, Obolensky A et al (2009) Directed differentiation of human embryonic stem cells into functional retinal pigment epithelium cells. Cell Stem Cell. https://doi.org/10.1016/j.stem.2009.07.002
Carr AJ, Vugler AA, Hikita ST et al (2009) Protective effects of human iPS-derived retinal pigment epithelium cell transplantation in the retinal dystrophic rat. PLoS One. https://doi.org/10.1371/journal.pone.0008152
Ninomiya Y, Lewis JM, Hasegawa T, Tano Y (1996) Retinotomy and foveal translocation for surgical management of subfoveal choroidal neovascular membranes. Am J Ophthalmol. https://doi.org/10.1016/S0002-9394(14)70479-9
Au Eong KG, Pieramici DJ, Fujii GY et al (2001) Macular translocation: unifying concepts, terminology, and classification. Am J Ophthalmol. https://doi.org/10.1016/S0002-9394(00)00788-1
Eandi CM, Giansanti F, Virgili G (2008) Macular translocation for neovascular age-related macular degeneration. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.cd006928.pub2
Sharma R, Bose D, Maminishkis A, Bharti K (2020) Retinal pigment epithelium replacement therapy for age-related macular degeneration: are we there yet? Annu Rev Pharmacol Toxicol 60:553–572. https://doi.org/10.1146/annurev-pharmtox-010919-023245
Schwartz SD, Hubschman JP, Heilwell G et al (2012) Embryonic stem cell trials for macular degeneration: a preliminary report. Lancet. https://doi.org/10.1016/S0140-6736(12)60028-2
Da Cruz L, Fynes K, Georgiadis O et al (2018) Phase 1 clinical study of an embryonic stem cell-derived retinal pigment epithelium patch in age-related macular degeneration. Nat Biotechnol. https://doi.org/10.1038/nbt.4114
Kashani AH, Lebkowski JS, Rahhal FM et al (2018) A bioengineered retinal pigment epithelial monolayer for advanced, dry age-related macular degeneration. Sci Transl Med. https://doi.org/10.1126/scitranslmed.aao4097
Mandai M, Watanabe A, Kurimoto Y et al (2017) Autologous induced stem-cell-derived retinal cells for macular degeneration. N Engl J Med. https://doi.org/10.1056/NEJMoa1608368
Sharma R, Khristov V, Rising A et al (2019) Clinical-grade stem cell-derived retinal pigment epithelium patch rescues retinal degeneration in rodents and pigs. Sci Transl Med. https://doi.org/10.1126/scitranslmed.aat5580
M’Barek KB, Habeler W, Plancheron A et al (2017) Human ESC–derived retinal epithelial cell sheets potentiate rescue of photoreceptor cell loss in rats with retinal degeneration. Sci Transl Med. https://doi.org/10.1126/scitranslmed.aai7471
Vugler A, Carr AJ, Lawrence J et al (2008) Elucidating the phenomenon of HESC-derived RPE: anatomy of cell genesis, expansion and retinal transplantation. Exp Neurol. https://doi.org/10.1016/j.expneurol.2008.09.007
Kokkinaki M, Sahibzada N, Golestaneh N (2011) Human induced pluripotent stem-derived retinal pigment epithelium (RPE) cells exhibit ion transport, membrane potential, polarized vascular endothelial growth factor secretion, and gene expression pattern similar to native RPE. Stem Cells. https://doi.org/10.1002/stem.635
Matsumoto E, Koide N, Hanzawa H et al (2019) Fabricating retinal pigment epithelial cell sheets derived from human induced pluripotent stem cells in an automated closed culture system for regenerative medicine. PLoS One. https://doi.org/10.1371/journal.pone.0212369
Takagi S, Mandai M, Gocho K et al (2019) Evaluation of transplanted autologous induced pluripotent stem cell-derived retinal pigment epithelium in exudative age-related macular degeneration. Ophthalmol Retin. https://doi.org/10.1016/j.oret.2019.04.021
Ramsden CM, Powner MB, Carr AJF et al (2013) Stem cells in retinal regeneration: past, present and future. Development 140(12):2576–2585
Carr AJF, Smart MJK, Ramsden CM et al (2013) Development of human embryonic stem cell therapies for age-related macular degeneration. Trends Neurosci 36(7):385–395
Hsiung J, Zhu D, Hinton DR (2015) Polarized human embryonic stem cell-derived retinal pigment epithelial cell monolayers have higher resistance to oxidative stress-induced cell death than nonpolarized cultures. Stem Cells Transl Med. https://doi.org/10.5966/sctm.2014-0205
Diniz B, Thomas P, Thomas B et al (2013) Subretinal implantation of retinal pigment epithelial cells derived from human embryonic stem cells: improved survival when implanted as a monolayer. Investig Ophthalmol Vis Sci. https://doi.org/10.1167/iovs.12-11239
Steedman MR, Tao SL, Klassen H, Desai TA (2010) Enhanced differentiation of retinal progenitor cells using microfabricated topographical cues. Biomed Microdevices. https://doi.org/10.1007/s10544-009-9392-7
Hartmann U, Sistani F, Steinhorst UH (1999) Human and porcine anterior lens capsule as support for growing and grafting retinal pigment epithelium and iris pigment epithelium. Graefes Arch Clin Exp Ophthalmol. https://doi.org/10.1007/s004170050390
Singh S, Woerly S, Mclaughlin BJ (2001) Natural and artificial substrates for retinal pigment epithelial monolayer transplantation. Biomaterials. https://doi.org/10.1016/S0142-9612(01)00171-5
Lee CJ, Vroom JA, Fishman HA, Bent SF (2006) Determination of human lens capsule permeability and its feasibility as a replacement for Bruch’s membrane. Biomaterials. https://doi.org/10.1016/j.biomaterials.2005.09.008
Nicolini J, Kiilgaard JF, Wiencke AK et al (2000) The anterior lens capsule used as support material in RPE cell-transplantation. Acta Ophthalmol Scand. https://doi.org/10.1034/j.1600-0420.2000.078005527.x
Gullapalli VK, Sugino IK, Van Patten Y et al (2005) Impaired RPE survival on aged submacular human Bruch’s membrane. Exp Eye Res 80(2):235–248
Tezel TH, Del Priore LV, Kaplan HJ (2004) Reengineering of aged Bruch’s membrane to enhance retinal pigment epithelium repopulation. Investig Ophthalmol Vis Sci. https://doi.org/10.1167/iovs.04-0193
Sugino IK, Rapista A, Sun Q et al (2011) A method to enhance cell survival on Bruch’s membrane in eyes affected by age and age-related macular degeneration. Investig Ophthalmol Vis Sci. https://doi.org/10.1167/iovs.11-8400
Kiilgaard JF, Scherfig E, Prause JU, La Cour M (2012) Transplantation of amniotic membrane to the subretinal space in pigs. Stem Cells Int https://doi.org/10.1155/2012/716968
Capeéans C, Piñeiro Ces A, Pardo M et al (2003) Amniotic membrane as support for human retinal pigment epithelium (RPE) cell growth. Acta Ophthalmol Scand. https://doi.org/10.1034/j.1600-0420.2003.00076.x
Thumann G, Schraermeyer U, Bartz-Schmidt KU, Heimann K (1997) Descemet’s membrane as membranous support in RPE/IPE transplantation. Curr Eye Res. https://doi.org/10.1076/ceyr.16.12.1236.5031
Fénelon M, Catros S, Fricain JC (2018) What is the benefit of using amniotic membrane in oral surgery? A comprehensive review of clinical studies. Clin Oral Investig 22(5):1881–1891
Kogan S, Sood A, Granick MS (2018) Amniotic membrane adjuncts and clinical applications in wound healing: a review of the literature. Wounds a compend. Clin Res Pract 30(6):168–173
Paolin A, Cogliati E, Trojan D et al (2016) Amniotic membranes in ophthalmology: long term data on transplantation outcomes. Cell Tissue Bank. https://doi.org/10.1007/s10561-015-9520-y
Costa E, Neto Murta J (2015) Amniotic membrane in ophthalmology. In: Amniotic membrane: origin characterization and medical applications. Springer, New York
M’Barek KB, Habeler W, Plancheron A, Jarraya M, Goureau O, Monville C et al (2018) Engineering transplantation-suitable retinal pigment epithelium tissue derived from human embryonic stem cells. JoVE. https://doi.org/10.3791/58216
Bhatt NS, Newsome DA, Fenech T et al (1994) Experimental transplantation of human retinal pigment epithelial cells on collagen substrates. Am J Ophthalmol. https://doi.org/10.1016/S0002-9394(14)73079-X
Tezel TH, Del Priore LV, Berger AS, Kaplan HJ (2007) Adult retinal pigment epithelial transplantation in exudative age-related macular degeneration. Am J Ophthalmol. https://doi.org/10.1016/j.ajo.2006.12.007
Tezel TH, Kaplan HJ, Del Priore L V. (1999) Fate of human retinal pigment epithelial cells seeded onto layers of human Bruch’s membrane. Investig Ophthalmol Vis Sci 40(2):467-476
Farrokh-Siar L, Rezai KA, Patel SC, Ernest JT (1999) Cryoprecipitate: an autologous substrate for human fetal retinal pigment epithelium. Curr Eye Res. https://doi.org/10.1076/ceyr.19.2.89.5331
Gonçalves S, Rodrigues IP, Padrão J et al (2016) Acetylated bacterial cellulose coated with urinary bladder matrix as a substrate for retinal pigment epithelium. Colloids Surfaces B Biointerfaces. https://doi.org/10.1016/j.colsurfb.2015.11.051
Oganesicm A, Gabrielian K, Terry Ernest J, Patel SC (1999) A new model of retinal pigment epithelium transplantation with microspheres. Arch Ophthalmol
Chirila T V., Barnard Z, Zainuddin, et al (2008) Bombyx mori silk fibroin membranes as potential substrata for epithelial constructs used in the management of ocular surface disorders. Tissue Eng Part A. https://doi.org/10.1089/ten.tea.2007.0224
Kundu B, Rajkhowa R, Kundu SC, Wang X (2013) Silk fibroin biomaterials for tissue regenerations. Adv Drug Deliv Rev. https://doi.org/10.1016/j.addr.2012.09.043
Harkin DG, George KA, Madden PW et al (2011) Silk fibroin in ocular tissue reconstruction. Biomaterials. https://doi.org/10.1016/j.biomaterials.2010.12.041
Shadforth AMA, George KA, Kwan AS et al (2012) The cultivation of human retinal pigment epithelial cells on Bombyx mori silk fibroin. Biomaterials. https://doi.org/10.1016/j.biomaterials.2012.02.040
Del Priore LV, Tezel TH, Kaplan HJ (2004) Survival of allogeneic porcine retinal pigment epithelial sheets after subretinal transplantation. Investig Ophthalmol Vis Sci. https://doi.org/10.1167/iovs.03-0662
Tezel TH (1997) Reattachment to a substrate prevents apoptosis of human retinal pigment epithelium. Graefes Arch Clin Exp Ophthalmol. https://doi.org/10.1007/BF01007836
Warnke PH, Alamein M, Skabo S et al (2013) Primordium of an artificial Bruch’s membrane made of nanofibers for engineering of retinal pigment epithelium cell monolayers. Acta Biomater. https://doi.org/10.1016/j.actbio.2013.07.029
Peng CH, Chuang JH, Wang ML et al (2016) Laminin modification subretinal bio-scaffold remodels retinal pigment epithelium-driven microenvironment in vitro and in vivo. Oncotarget. https://doi.org/10.18632/oncotarget.11502
Lu L, Yaszemski MJ, Mikos AG (2001) Retinal pigment epithelium engineering using synthetic biodegradable polymers. Biomaterials
Hadlock T, Singh S, Vacanti JP, McLaughlin BJ (1999) Ocular cell monolayers cultured on biodegradable substrates. Tissue Eng. https://doi.org/10.1089/ten.1999.5.187
Lu B, Zhu D, Hinton D et al (2012) Mesh-supported submicron parylene-C membranes for culturing retinal pigment epithelial cells. Biomed Microdevices. https://doi.org/10.1007/s10544-012-9645-8
McHugh KJ, Tao SL, Saint-Geniez M (2014) Porous poly(ε-caprolactone) scaffolds for retinal pigment epithelium transplantation. Investig Ophthalmol Vis Sci. https://doi.org/10.1167/iovs.13-12833
Sorkio A, Hongisto H, Kaarniranta K et al (2014) Structure and barrier properties of human embryonic stem cell-derived retinal pigment epithelial cells are affected by extracellular matrix protein coating. Tissue Eng Part A. https://doi.org/10.1089/ten.tea.2013.0049
Chen H, Fan X, Xia J et al (2011) Electrospun chitosan-graft-poly (varepsilon-caprolactone)/poly (varepsilon-caprolactone) nanofibrous scaffolds for retinal tissue engineering. Int J Nanomedicine 6:453–461. https://doi.org/10.2147/IJN.S17057
Xiang P, Wu KC, Zhu Y et al (2014) A novel Bruch’s membrane-mimetic electrospun substrate scaffold for human retinal pigment epithelium cells. Biomaterials. https://doi.org/10.1016/j.biomaterials.2014.08.040
Chen H, Fan X, Xia J et al (2011) Electrospun chitosan-graft-poly (e-caprolactone)/poly (e-caprolactone) nanofibrous scaffolds for retinal tissue engineering. Int J Nanomedicine 6:453–461
Wang S, Lu B, Wood P, Lund RD (2005) Grafting of ARPE-19 and Schwann cells to the subretinal space in RCS rats. Investig Ophthalmol Vis Sci. https://doi.org/10.1167/iovs.05-0279
Carr AJ, Vugler A, Lawrence J et al (2009) Molecular characterization and functional analysis of phagocytosis by human embryonic stem cell-derived RPE cells using a novel human retinal assay. Mol Vis 15:283–295
Ho AC, Chang TS, Samuel M et al (2017) Experience with a subretinal cell-based therapy in patients with geographic atrophy secondary to age-related macular degeneration. Am J Ophthalmol. https://doi.org/10.1016/j.ajo.2017.04.006
Lu B, Malcuit C, Wang S et al (2009) Long-term safety and function of RPE from human embryonic stem cells in preclinical models of macular degeneration. Stem Cells. https://doi.org/10.1002/stem.149
Zarbin M, Sugino I, Townes-Anderson E (2019) Concise review: update on retinal pigment epithelium transplantation for age-related macular degeneration. Stem Cells Transl Med 8:466–477. https://doi.org/10.1002/sctm.18-0282
Bharti K, Miller SS, Arnheiter H (2011) The new paradigm: retinal pigment epithelium cells generated from embryonic or induced pluripotent stem cells. Pigment Cell Melanoma Res. https://doi.org/10.1111/j.1755-148X.2010.00772.x
Peng Y, Tang L, Zhou Y (2017) Subretinal injection: a review on the novel route of therapeutic delivery for vitreoretinal diseases. Ophthalmic Res 58:217–222
Mühlfriedel R, Michalakis S, Garrido MG et al (2013) Optimized technique for subretinal injections in mice. Methods Mol Biol. https://doi.org/10.1007/978-1-62703-080-9-24
Gerding H (2007) A new approach towards a minimal invasive retina implant. J Neural Eng. https://doi.org/10.1088/1741-2560/4/1/S05
Parikh S, Le A, Davenport J et al (2016) An alternative and validated injection method for accessing the subretinal space via a transcleral posterior approach. J Vis Exp. https://doi.org/10.3791/54808
Komáromy AM, Varner SE, De Juan E, et al (2006) Application of a new subretinal injection device in the dog. Cell Transplant https://doi.org/10.3727/000000006783981701
Westenskow PD, Kurihara T, Bravo S et al (2015) Performing subretinal injections in rodents to deliver retinal pigment epithelium cells in suspension. J Vis Exp. https://doi.org/10.3791/52247
Xue K, Groppe M, Salvetti AP, MacLaren RE (2017) Technique of retinal gene therapy: delivery of viral vector into the subretinal space. Eye. https://doi.org/10.1038/eye.2017.158
Ghosh F, Arnér K (2002) Transplantation of full-thickness retina in the normal porcine eye: surgical and morphologic aspects. Retina. https://doi.org/10.1097/00006982-200208000-00013
Stanzel BV, Liu Z, Brinken R et al (2012) Subretinal delivery of ultrathin rigid-elastic cell carriers using a metallic shooter instrument and biodegradable hydrogel encapsulation. Investig Ophthalmol Vis Sci. https://doi.org/10.1167/iovs.11-8260
Thumann G, Aisenbrey S, Schaefer F, Bartz-Schmidt KU (2006) Instrumentation and technique for delivery of tissue explants to the subretinal space. Ophthalmologica. https://doi.org/10.1159/000091760
Kamao H, Mandai M, Ohashi W et al (2017) Evaluation of the surgical device and procedure for extracellular matrix–scaffold–supported human iPSC–derived retinal pigment epithelium cell sheet transplantation. Investig Ophthalmol Vis Sci. https://doi.org/10.1167/iovs.16-19778
Thumann G, Viethen A, Gaebler A et al (2009) The in vitro and in vivo behaviour of retinal pigment epithelial cells cultured on ultrathin collagen membranes. Biomaterials. https://doi.org/10.1016/j.biomaterials.2008.09.039
Maaijwee K, Koolen T, Rosenbrand D et al (2008) Threshold amplitude and frequency for ocular tissue release from a vibrating instrument: an experimental study. Investig Ophthalmol Vis Sci. https://doi.org/10.1167/iovs.07-1220
Zhao C, Boles NC, Miller JD et al (2017) Development of a refined protocol for trans-scleral subretinal transplantation of human retinal pigment epithelial cells into rat eyes. J Vis Exp. https://doi.org/10.3791/55220
Alexander P, Thomson HAJ, Luff AJ, Lotery AJ (2015) Retinal pigment epithelium transplantation: concepts, challenges, and future prospects. Eye. https://doi.org/10.1038/eye.2015.89
Peyman GA, Blinder KJ, Paris CL et al (1991) A technique for retinal pigment epithelium transplantation for age-related macular degeneration secondary to extensive subfoveal scarring. Ophthalmic Surg. https://doi.org/10.3928/1542-8877-19910201-12
Algvere PV, Berglin L, Gouras P et al (1997) Transplantation of RPE in age-related macular degeneration: observations in disciform lesions and dry RPE atrophy. Graefes Arch Clin Exp Ophthalmol. https://doi.org/10.1007/BF00941722
Weisz JM, Humayun MS, De Juan E, et al (1999) Allogenic fetal retinal pigment epithelial cell transplant in a patient with geographic atrophy. Retina https://doi.org/10.1097/00006982-199919060-00011
Strunnikova NV, Maminishkis A, Barb JJ et al (2010) Transcriptome analysis and molecular signature of human retinal pigment epithelium. Hum Mol Genet. https://doi.org/10.1093/hmg/ddq129
Zhang Z, Zhang Y, Xiao H et al (2012) A gene expression profile of the developing human retinal pigment epithelium. Mol Vis
Liao JL, Yu J, Huang K et al (2010) Molecular signature of primary retinal pigment epithelium and stem-cell-derived RPE cells. Hum Mol Genet. https://doi.org/10.1093/hmg/ddq341
Algvere PV, Gouras P, Kopp ED (1999) Long-term outcome of RPE allografts in non-immunosuppressed patients with AMD. Eur J Ophthalmol. https://doi.org/10.1177/112067219900900310
Morohoshi K, Goodwin AM, Ohbayashi M, Ono SJ (2009) Autoimmunity in retinal degeneration: autoimmune retinopathy and age-related macular degeneration. J Autoimmun. https://doi.org/10.1016/j.jaut.2009.09.003
Del Priore L V., Kaplan HJ, Tezel TH, et al (2001) Retinal pigment epithelial cell transplantation after subfoveal membranectomy in age-related macular degeneration: clinicopathologic correlation. Am J Ophthalmol. https://doi.org/10.1016/S0002-9394(00)00850-3
Binder S, Krebs I, Hilgers RD et al (2004) Outcome of transplantation of autologous retinal pigment epithelium in age-related macular degeneration: a prospective trial. Investig Ophthalmol Vis Sci. https://doi.org/10.1167/iovs.04-0118
Binder S, Stolba U, Krebs I et al (2002) Transplantation of autologous retinal pigment epithelium in eyes with foveal neovascularization resulting from age-related macular degeneration: a pilot study. Am J Ophthalmol. https://doi.org/10.1016/S0002-9394(01)01373-3
Van Meurs JC, Ter Averst E, Hofland LJ et al (2004) Autologous peripheral retinal pigment epithelium translocation in patients with subfoveal neovascular membranes. In: Br J Ophthalmol. https://doi.org/10.1136/bjo.88.1.110
Lu Y, Han L, Wang C et al (2017) A comparison of autologous transplantation of retinal pigment epithelium (RPE) monolayer sheet graft with RPE–Bruch’s membrane complex graft in neovascular age-related macular degeneration. Acta Ophthalmol. https://doi.org/10.1111/aos.13054
Thomson JA (1998) Embryonic stem cell lines derived from human blastocysts. Science. https://doi.org/10.1126/science.282.5391.1145
Osakada F, Ikeda H, Sasai Y, Takahashi M (2009) Stepwise differentiation of pluripotent stem cells into retinal cells. Nat Protoc. https://doi.org/10.1038/nprot.2009.51
Haruta M, Sasai Y, Kawasaki H et al (2004) In vitro and in vivo characterization of pigment epithelial cells differentiated from primate embryonic stem cells. Investig Ophthalmol Vis Sci. https://doi.org/10.1167/iovs.03-1034
Klimanskaya I, Hipp J, Rezai KA et al (2004) Derivation and comparative assessment of retinal pigment epithelium from human embryonic stem cells using transcriptomics. Cloning Stem Cells. https://doi.org/10.1089/clo.2004.6.217
Buchholz DE, Pennington BO, Croze RH et al (2013) Rapid and efficient directed differentiation of human pluripotent stem cells into retinal pigmented epithelium. Stem Cells Transl Med. https://doi.org/10.5966/sctm.2012-0163
Reh TA, Lamba D, Gust J (2010) Directing human embryonic stem cells to a retinal fate. Methods Mol Biol. https://doi.org/10.1007/978-1-60761-691-7_9
Schwartz SD, Regillo CD, Lam BL et al (2015) Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt’s macular dystrophy: follow-up of two open-label phase 1/2 studies. Lancet. https://doi.org/10.1016/S0140-6736(14)61376-3
Attwood S, Edel M (2019) iPS-cell technology and the problem of genetic instability—can it ever be safe for clinical use? J Clin Med. https://doi.org/10.3390/jcm8030288
D’Antonio M, Benaglio P, Jakubosky D et al (2018) Insights into the mutational burden of human induced pluripotent stem cells from an integrative multi-omics approach. Cell Rep. https://doi.org/10.1016/j.celrep.2018.06.091
Turinetto V, Orlando L, Giachino C (2017) Induced pluripotent stem cells: advances in the quest for genetic stability during reprogramming process. Int J Mol Sci 18(9):1952
Garber K (2015) RIKEN suspends first clinical trial involving induced pluripotent stem cells. Nat Biotechnol. https://doi.org/10.1038/nbt0915-890
Rezai KA, Semnani RT, Patel SC et al (1997) The immunogenic potential of human fetal retinal pigment epithelium and its relation to transplantation. Investig Ophthalmol Vis Sci 38(12):2662–2671
Binder S, Stanzel BV, Krebs I, Glittenberg C (2007) Transplantation of the RPE in AMD. Prog Retin Eye Res 26(5):516–554
Opelz G, Döhler B (2010) Impact of HLA mismatching on incidence of posttransplant non-hodgkin lymphoma after kidney transplantation. Transplantation. https://doi.org/10.1097/TP.0b013e3181c69855
Sugita S, Iwasaki Y, Makabe K et al (2016) Successful transplantation of retinal pigment epithelial cells from MHC homozygote iPSCs in MHC-matched models. Stem Cell Rep. https://doi.org/10.1016/j.stemcr.2016.08.010
Deuse T, Hu X, Gravina A et al (2019) Hypoimmunogenic derivatives of induced pluripotent stem cells evade immune rejection in fully immunocompetent allogeneic recipients. Nat Biotechnol 37:252–258
Lund RD, Wang S, Lu B et al (2007) Cells isolated from umbilical cord tissue rescue photoreceptors and visual functions in a rodent model of retinal disease. Stem Cells. https://doi.org/10.1634/stemcells.2006-0308erratum
Cotrim CC, Toscano L, Messias A et al (2017) Intravitreal use of bone marrow mononuclear fraction containing CD34+ stem cells in patients with atrophic age-related macular degeneration. Clin Ophthalmol. https://doi.org/10.2147/OPTH.S133502
Park SS, Bauer G, Abedi M et al (2015) Intravitreal autologous bone marrow cd34+ cell therapy for ischemic and degenerative retinal disorders: preliminary phase 1 clinical trial findings. Investig Ophthalmol Vis Sci. https://doi.org/10.1167/iovs.14-15415
Selden NR, Al-Uzri A, Huhn SL et al (2013) Central nervous system stem cell transplantation for children with neuronal ceroid lipofuscinosis. J Neurosurg Pediatr. https://doi.org/10.3171/2013.3.PEDS12397
Gupta N, Henry RG, Strober J et al (2012) Neural stem cell engraftment and myelination in the human brain. Sci Transl Med. https://doi.org/10.1126/scitranslmed.3004373
Cuenca N, Fernández-Sánchez L, McGill TJ et al (2013) Phagocytosis of photoreceptor outer segments by transplanted human neural stem cells as a neuroprotective mechanism in retinal degeneration. Investig Ophthalmol Vis Sci. https://doi.org/10.1167/iovs.13-12860
McGill TJ, Cottam B, Lu B et al (2012) Transplantation of human central nervous system stem cells - neuroprotection in retinal degeneration. Eur J Neurosci. https://doi.org/10.1111/j.1460-9568.2011.07970.x
Jones MK, Lu B, Girman S, Wang S (2017) Cell-based therapeutic strategies for replacement and preservation in retinal degenerative diseases. Prog Retin Eye Res 58:1–7
Swaroop A, Kim D, Forrest D (2010) Transcriptional regulation of photoreceptor development and homeostasis in the mammalian retina. Nat Rev Neurosci 11(8):563–576
Brzezinski JA, Reh TA (2015) Photoreceptor cell fate specification in vertebrates. Development. https://doi.org/10.1242/dev.127043
Mears AJ, Kondo M, Swain PK et al (2001) Nrl is required for rod photoreceptor development. Nat Genet. https://doi.org/10.1038/ng774
Ng L, Hurley JB, Dierks B et al (2001) A thyroid hormone receptor that is required for the development of green cone photoreceptors. Nat Genet. https://doi.org/10.1038/83829
Roberts MR, Hendrickson A, McGuire CR, Reh TA (2005) Retinoid X receptor γ is necessary to establish the S-opsin gradient in cone photoreceptors of the developing mouse retina. Investig Ophthalmol Vis Sci. https://doi.org/10.1167/iovs.05-0093
Eiraku M, Takata N, Ishibashi H et al (2011) Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature. https://doi.org/10.1038/nature09941
Nakano T, Ando S, Takata N et al (2012) Self-formation of optic cups and storable stratified neural retina from human ESCs. Cell Stem Cell. https://doi.org/10.1016/j.stem.2012.05.009
Ikeda H, Osakada F, Watanabe K et al (2005) Generation of Rx+/Pax6+ neural retinal precursors from embryonic stem cells. Proc Natl Acad Sci U S A. https://doi.org/10.1073/pnas.0500010102
Lamba DA, Karl MO, Ware CB, Reh TA (2006) Efficient generation of retinal progenitor cells from human embryonic stem cells. Proc Natl Acad Sci U S A. https://doi.org/10.1073/pnas.0601990103
Meyer JS, Shearer RL, Capowski EE et al (2009) Modeling early retinal development with human embryonic and induced pluripotent stem cells. Proc Natl Acad Sci U S A. https://doi.org/10.1073/pnas.0905245106
Osakada F, Ikeda H, Mandai M et al (2008) Toward the generation of rod and cone photoreceptors from mouse, monkey and human embryonic stem cells. Nat Biotechnol. https://doi.org/10.1038/nbt1384
Osakada F, Jin ZB, Hirami Y et al (2009) In vitro differentiation of retinal cells from human pluripotent stem cells by small-molecule induction. J Cell Sci. https://doi.org/10.1242/jcs.050393
Boucherie C, Mukherjee S, Henckaerts E et al (2013) Brief report: self-organizing neuroepithelium from human pluripotent stem cells facilitates derivation of photoreceptors. Stem Cells. https://doi.org/10.1002/stem.1268
Nistor G, Seiler MJ, Yan F et al (2010) Three-dimensional early retinal progenitor 3D tissue constructs derived from human embryonic stem cells. J Neurosci Methods. https://doi.org/10.1016/j.jneumeth.2010.04.025
Chen HY, Kaya KD, Dong L, Swaroop A (2016) Three-dimensional retinal organoids from mouse pluripotent stem cells mimic in vivo development with enhanced stratification and rod photoreceptor differentiation. Mol Vis 22:1077–1094
Meyer JS, Howden SE, Wallace KA et al (2011) Optic vesicle-like structures derived from human pluripotent stem cells facilitate a customized approach to retinal disease treatment. Stem Cells. https://doi.org/10.1002/stem.674
Phillips MJ, Wallace KA, Dickerson SJ et al (2012) Blood-derived human iPS cells generate optic vesicle-like structures with the capacity to form retinal laminae and develop synapses. Invest Ophthalmol Vis Sci. https://doi.org/10.1167/iovs.11-9313
Zhong X, Gutierrez C, Xue T et al (2014) Generation of three-dimensional retinal tissue with functional photoreceptors from human iPSCs. Nat Commun. https://doi.org/10.1038/ncomms5047
Kuwahara A, Ozone C, Nakano T et al (2015) Generation of a ciliary margin-like stem cell niche from self-organizing human retinal tissue. Nat Commun. https://doi.org/10.1038/ncomms7286
Reichman S, Slembrouck A, Gagliardi G et al (2017) Generation of storable retinal organoids and retinal pigmented epithelium from adherent human iPS cells in Xeno-free and feeder-free conditions. Stem Cells. https://doi.org/10.1002/stem.2586
Reichman S, Goureau O (2016) Production of retinal cells from confluent human iPS cells. In: Methods in molecular biology. Springer, New York
Gonzalez-Cordero A, Kruczek K, Naeem A et al (2017) Recapitulation of human retinal development from human pluripotent stem cells generates transplantable populations of cone photoreceptors. Stem Cell Rep. https://doi.org/10.1016/j.stemcr.2017.07.022
Gagliardi G, Ben M’Barek K, Goureau O (2019) Photoreceptor cell replacement in macular degeneration and retinitis pigmentosa: a pluripotent stem cell-based approach. Prog Retin Eye Res. https://doi.org/10.1016/j.preteyeres.2019.03.001
Kuriyan AE, Albini TA, Townsend JH et al (2017) Vision loss after intravitreal injection of autologous “stem cells” for AMD. N Engl J Med. https://doi.org/10.1056/NEJMoa1609583
Taylor-Weiner H, Zivin JG (2015) Medicine’s wild west - unlicensed stem-cell clinics in the United States. N Engl J Med. https://doi.org/10.1056/NEJMp1504560
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply
About this chapter
Cite this chapter
Khateb, S., Jha, S., Bharti, K., Banin, E. (2021). Cell-Based Therapies for Age-Related Macular Degeneration. In: Chew, E.Y., Swaroop, A. (eds) Age-related Macular Degeneration. Advances in Experimental Medicine and Biology, vol 1256. Springer, Cham. https://doi.org/10.1007/978-3-030-66014-7_11
Download citation
DOI: https://doi.org/10.1007/978-3-030-66014-7_11
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-66013-0
Online ISBN: 978-3-030-66014-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)