Abstract
Purpose
Degeneration or dysfunction of the retinal pigment epithelium (RPE) can induce secondary photoreceptor atrophy and catastrophic vision loss in patients with age-related macular degeneration (AMD). AMD is the leading cause of vision loss in the elderly in industrialized countries and no cure exists for the “dry” or atrophic form to date. However, recent pre-clinical data from several groups suggests that embryonic stem cell-derived RPE cell transplantation may prevent photoreceptor degeneration in animal models of RPE degeneration. Another approach may be to derive RPE cells from autologous induced pluripotent stem cells (iPSCs) reprogrammed from dermal tissue. However, the safety of this approach has been questioned on several levels. In this chapter we will summarize work reported by several groups, including our own, that clearly demonstrate that transplanted RPE cells can provide anatomical and functional photoreceptor rescue in animal models of retinal degeneration. We will also discuss some of the prevailing concerns and challenges associated with this technique.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
The retina consists of diverse cell types stratified into organized functional tiers. Rod and cone photoreceptors and retinal pigment epithelium (RPE) cells work in conjunction to perform the energy-demanding and complicated biochemical process of converting light energy into electrical signals that ultimately are processed into “vision.” The neurosensory retina rests on a monolayer of RPE cells that associates with the photoreceptors on one side while partitioning the retina from the choriocapillaris on the other. The RPE performs multiple critical diverse functions essential for maintaining photoreceptor homeostasis (for review, see [1]). In fact, death or dysfunction of RPE cells can induce devastating secondary effects on photoreceptors characteristic of age-related macular degeneration (AMD) . AMD is the leading cause of vision loss in the elderly in industrialized countries [2, 3], and demographic analyses predict that it will become more widespread [4].
AMD is a multifactorial polygenic disease characterized by a broad spectrum of signs and symptoms (for review see [5]). However, RPE dysfunction and photoreceptor degeneration are shared characteristics that may be amenable to cell replacement strategies for a majority of AMD patients if delivery could be optimized and graft rejection prevented [6–9]. Compared with photoreceptor transplantation, RPE transplantation strategies are more simple since RPE cells do not need to integrate into neuronal networks that begin to degenerate and/or remodel during retinal degeneration (for review see [10]). Pluripotent stem cells may provide an excellent source of RPE, and banks of histocompatibility antigen-typed RPE derived from human embryonic stem cells (hESC-RPE) cells can be generated and used to intervene therapeutically after a patient is diagnosed with the disease.
Several NIH approved pluripotent embryonic stem cells (ESCs) are currently available, however their widespread use is still controversial due to ethical concerns. These concerns may be obviated due to a remarkable observation that the transgenic manipulation of only four transcription factors, OSKM: Oct4, Sox2, Klf4, or c-Myc [11], or OSNL: Oct4, Sox2, Nanog, and Lin28 [12], in somatic cells can “reprogram” them into induced pluripotent stem cells (iPSCs) from which autologous RPE grafts can be generated. These iPSC-RPE could be derived from individual patients and used for therapeutic transplantation since the progression of AMD is relatively slow. However, there are some prevailing safety and immunogenic concerns regarding the use of iPSCs that must first be resolved [13–20].
2 Stem Cell Derived RPE Cells Have Been Well Characterized
Pigmented RPE can be readily derived from hESCs [21–25] and hiPSCs [24, 26–30]. These cells spontaneously differentiate with appropriate morphologies and functionality, but may be more efficiently generated if exogenous factors are added to the differentiation media [21, 24, 29, 30]. hES– and hiPS–RPE cells have been well characterized. They express RPE-specific terminal differentiation markers [21–30] in polarized planes [25, 26, 29]. Using high resolution mass spectrometry-based metabolomic analyses as a measure of functional genomics, we have shown that iPS-RPE strongly resemble primary human RPE cells [29].
hES–RPE and hiPS–RPE also function as well as their human primary RPE counterparts in vitro. As observed in primary RPE [31], tightly coupled stem cell-derived RPE form fluid-filled domes, demonstrating that the ion pumps are vectorially functional [32]. Stem cell-derived RPE can also phagocytose photoreceptor outer segments in vitro [21, 25–27, 29, 33]. We have developed a flow cytometry-based method to measure the dynamics of RPE phagocytosis. Using this strategy we have shown that iPS-RPE synthesize phagocytosis receptors that phagocytose outer segments as effectively as primary human RPE do [34].
An important measure of RPE function is to determine if the cells operate in a diseased context in vivo. Studies in animal models have provided very encouraging evidence that stem cell-derived RPE transplantation can effectively promote photoreceptor cell anatomical and functional rescue in dystrophic retinas [21, 23, 25, 27, 29]. Additionally, the extremely preliminary results of a Phase 1/2 clinical trial using hES–RPE managed by Advanced Cell Technology Inc. (ACT) have shown that after four months the transplanted cells do not induce any obvious adverse side effects and may have integrated into the subretinal space of the treated patients [35].
3 Current and Future Prospects for RPE Cell Transplantation
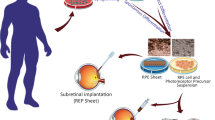
In the majority of studies published to date (and in the ACT managed clinical trial) a bolus of RPE cells were injected into the subretinal space. With a few exceptions (including in our study in which we see a monolayer of RPE cells integrated in the subretinal space up to 17 months post injection [29]), transplanted RPE cells generally survive only for a few months after implantation and not integrate into monolayers [21, 27, 36]. For these reasons the use of alternative approaches are being advocated, including the use of intact RPE sheets or RPE cells grown on porous engineered Bruch’s membrane mimics [37, 38]. The surgical techniques required to deliver sheets of cells are inherently more complicated. Furthermore, it must be demonstrated that the scaffolds can stably support RPE cells over extended periods of time and allow adequate RPE and choriocapillaris crosstalk.
The use of iPS–RPE, and personalized medicine in general, could be very expensive. One alternative approach may be to bank reduced complexity human leukocyte antigen (HLA)-homozygous iPSC (or perhaps even hESC cell-lines). It has been estimated that approximately 75 and 140 unique donors would be needed to cover ~ 80 % and ~ 90 % of the Japanese population, and roughly 64,000–160,000 individuals would need to be typed to find the donors [39]. However, it is also important to consider that long-term immunosuppressive therapies are expensive, associated with complications, and not well tolerated by elderly patients [40, 41]. Therefore, the expenses for both techniques, and potential consequences of life-long immunosuppression, should be directly compared before any approach is deemed to be cost prohibitive.
While still extremely premature, an alternative therapeutic approach may involve the replacement of whole degenerated regions of human retinas with large patches of intact ocular tissues grown from stem cells in 3D cultures. The idea of transplanting stratified layers of functional neural networks may be more realistic based on two ground-breaking recent reports demonstrating that aggregates of mouse and, more recently, human ES cells grown in 3-D cultures self-assembled into structures strongly resembling optic cups (rudimentary sensory retinas) with neural and RPE domains [42, 43]. If this approach could be optimized, theoretically entire autologous maculas may be generated as therapeutic interventions for AMD .
4 Conclusions
RPE that function in vitro and in vivo to maintain photoreceptor homeostasis can be readily generated from stem cells. While few conclusions can be drawn until long-term studies in human patients have been completed, and RPE derivation and delivery techniques are optimized, the evidence collected in animal models that RPE grafts can prevent continued retinal degeneration and maintain visual function is very encouraging. Therefore, stem cell-based RPE transplantation therapies for untreatable retinal degenerative diseases such as AMD may ultimately prove to be not only realistic, but also therapeutically effective.
Abbreviations
- RPE:
-
Retinal pigment epithelium
- AMD:
-
Age-related macular degeneration
- iPSC:
-
Induced pluripotent stem cell
- iPSC-RPE:
-
Induced pluripotent stem cell-derived RPE
- hESC:
-
Human embryonic stem cell
- hESC-RPE:
-
Human embryonic stem cell-derived RPE
- HLA:
-
Human leukocyte antigen
- OSKM:
-
Oct4, Sox2, Klf4, and c-Myc
- OSNL:
-
Oct4, Sox2, Nanog, and Lin28
- ACT:
-
Advanced Cell Technology, Inc
References
Strauss O (2005) The retinal pigment epithelium in visual function. Physiol Rev 85(3):845–881
Congdon N, O’Colmain B, Klaver CC, Klein R, Munoz B, Friedman DS et al (2004) Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol 122(4):477–485
Resnikoff S, Pascolini D, Etya’ale D, Kocur I, Pararajasegaram R, Pokharel GP et al (2004) Global data on visual impairment in the year 2002. Bull World Health Organ 82(11):844–851
Friedman DS, O’Colmain BJ, Munoz B, Tomany SC, McCarty C, de Jong PT et al (2004) Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol 122(4):564–572
Bird AC (2010) Therapeutic targets in age-related macular disease. J Clin Invest 120(9):3033–3041
Algvere PV, Gouras P, Dafgard Kopp E (1999) Long-term outcome of RPE allografts in non-immunosuppressed patients with AMD. Eur J Ophthalmol 9(3):217–230
Cahill MT, Freedman SF, Toth CA (2003) Macular translocation with 360 degrees peripheral retinectomy for geographic atrophy. Arch Ophthalmol 121(1):132–133
Joussen AM, Joeres S, Fawzy N, Heussen FM, Llacer H, van Meurs JC et al (2007) Autologous translocation of the choroid and retinal pigment epithelium in patients with geographic atrophy. Ophthalmology 114(3):551–560
Zhang X, Bok D (1998) Transplantation of retinal pigment epithelial cells and immune response in the subretinal space. Invest Ophthalmol Vis Sci 39(6):1021–1027
Marc R (2005) Retinal remodeling. J Vis 5(12):5
Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K et al (2007) Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131(5):861–872
Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S et al (2007) Induced pluripotent stem cell lines derived from human somatic cells. Science 318(5858):1917–1920
Aoi T, Yae K, Nakagawa M, Ichisaka T, Okita K, Takahashi K et al (2008) Generation of pluripotent stem cells from adult mouse liver and stomach cells. Science 321(5889):699–702
Bock C, Kiskinis E, Verstappen G, Gu H, Boulting G, Smith ZD et al (2011) Reference maps of human ES and iPS cell variation enable high-throughput characterization of pluripotent cell lines. Cell 144(3):439–452
Hussein SM, Batada NN, Vuoristo S, Ching RW, Autio R, Narva E et al (2011) Copy number variation and selection during reprogramming to pluripotency. Nature 471(7336):58–62
Laurent LC, Ulitsky I, Slavin I, Tran H, Schork A, Morey R et al (2011) Dynamic changes in the copy number of pluripotency and cell proliferation genes in human ESCs and iPSCs during reprogramming and time in culture. Cell Stem Cell 8(1):106–118
Markoulaki S, Hanna J, Beard C, Carey BW, Cheng AW, Lengner CJ et al (2009) Transgenic mice with defined combinations of drug-inducible reprogramming factors. Nat Biotechnol 27(2):169–171
Mayshar Y, Ben-David U, Lavon N, Biancotti JC, Yakir B, Clark AT et al (2010) Identification and classification of chromosomal aberrations in human induced pluripotent stem cells. Cell Stem Cell 7(4):521–531
Quinlan AR, Boland MJ, Leibowitz ML, Shumilina S, Pehrson SM, Baldwin KK et al (2011) Genome sequencing of mouse induced pluripotent stem cells reveals retroelement stability and infrequent DNA rearrangement during reprogramming. Cell Stem Cell 9(4):366–373
Zhao T, Zhang Z-N, Rong Z, Xu Y (2011) Immunogenicity of induced pluripotent stem cells. Nature 474(7350):212–215
Idelson M, Alper R, Obolensky A, Ben-Shushan E, Hemo I, Yachimovich-Cohen N et al (2009) Directed differentiation of human embryonic stem cells into functional retinal pigment epithelium cells. Cell Stem Cell 5(4):396–408
Klimanskaya I, Hipp J, Rezai KA, West M, Atala A, Lanza R (2004) Derivation and comparative assessment of retinal pigment epithelium from human embryonic stem cells using transcriptomics. Cloning Stem Cells 6(3):217–245
Lund RD, Wang S, Klimanskaya I, Holmes T, Ramos-Kelsey R, Lu B et al (2006) Human embryonic stem cell-derived cells rescue visual function in dystrophic RCS rats. Cloning Stem Cells 8(3):189–199
Meyer JS, Shearer RL, Capowski EE, Wright LS, Wallace KA, McMillan EL et al (2009) Modeling early retinal development with human embryonic and induced pluripotent stem cells. Proc Natl Acad Sci 106(39):16698–16703
Vugler A, Carr AJ, Lawrence J, Chen LL, Burrell K, Wright A et al (2008) Elucidating the phenomenon of HESC-derived RPE: anatomy of cell genesis, expansion and retinal transplantation. Exp Neurol 214(2):347–361
Buchholz DE, Hikita ST, Rowland TJ, Friedrich AM, Hinman CR, Johnson LV et al (2009) Derivation of functional retinal pigmented epithelium from induced pluripotent stem cells. Stem Cells 27(10):2427–2434
Carr AJ, Vugler AA, Hikita ST, Lawrence JM, Gias C, Chen LL et al (2009) Protective effects of human iPS-derived retinal pigment epithelium cell transplantation in the retinal dystrophic rat. PLoS One 4(12):e8152
Hirami Y, Osakada F, Takahashi K, Okita K, Yamanaka S, Ikeda H et al (2009) Generation of retinal cells from mouse and human induced pluripotent stem cells. Neurosci Lett 458(3):126–131
Krohne T, Westenskow P, Kurihara T, Friedlander D, Lehmann M, Dorsey A et al (2012) Generation of retinal pigment epithelial cells from small molecules and OCT4-reprogrammed human induced pluripotent stem cells. Stem Cells Trans Med 1(2):96–109
Osakada F, Jin ZB, Hirami Y, Ikeda H, Danjyo T, Watanabe K et al (2009) In vitro differentiation of retinal cells from human pluripotent stem cells by small-molecule induction. J Cell Sci 122(Pt 17):3169–3179
Mircheff AK, Miller SS, Farber DB, Bradley ME, O’Day WT, Bok D (1990) Isolation and provisional identification of plasma membrane populations from cultured human retinal pigment epithelium. Invest Ophthalmol Vis Sci 31(5):863–878
Kokkinaki M, Sahibzada N Golestaneh N (2011) Human induced pluripotent stem-derived retinal pigment epithelium (RPE) cells exhibit ion transport, membrane potential, polarized vascular endothelial growth factor secretion, and gene expression pattern similar to native RPE. Stem Cells 29(5):825–835
Carr AJ, Vugler A, Lawrence J, Chen LL, Ahmado A, Chen FK et al (2009) Molecular characterization and functional analysis of phagocytosis by human embryonic stem cell-derived RPE cells using a novel human retinal assay. Mol Vis 15:283–295
Westenskow PD, Moreno SK, Krohne TU, Kurihara T, Zhu S, Zhang ZN et al (2012) Using flow cytometry to compare the dynamics of photoreceptor outer segment phagocytosis in iPS-derived RPE cells. Invest Ophthalmol Vis Sci 53(10):6282–6290
Schwartz SD, Hubschman J-P, Heilwell G, Franco-Cardenas V, Pan CK, Ostrick RM et al (2012) Embryonic stem cell trials for macular degeneration: a preliminary report. Lancet 379(9817):713–720
Lu B, Malcuit C, Wang S, Girman S, Francis P, Lemieux L et al (2009) Long-term safety and function of RPE from human embryonic stem cells in preclinical models of macular degeneration. Stem Cells 27(9):2126–2135
Sheridan C, Williams R, Grierson I (2004) Basement membranes and artificial substrates in cell transplantation. Graefes Arch Clin Exp Ophthalmol 242(1):68–75
Williams RL, Krishna Y, Dixon S, Haridas A, Grierson I, Sheridan C (2005) Polyurethanes as potential substrates for sub-retinal retinal pigment epithelial cell transplantation. J Mater Sci Mater Med 16(12):1087–1092
Okita K, Ichisaka T, Yamanaka S. 2007 Generation of germline-competent induced pluripotent stem cells. Nature 448(7151):313–317
Karamehic J, Ridic O, Jukic T, Ridic G, Slipicevic O, Coric J et al (2011) Financial aspects of the immunosuppressive therapy. Med Arh 65(6):357–362
Tezel TH, Del Priore LV, Berger AS, Kaplan HJ (2007) Adult retinal pigment epithelial transplantation in exudative age-related macular degeneration. Am J Ophthalmol 143(4):584–595
Eiraku M, Takata N, Ishibashi H, Kawada M, Sakakura E, Okuda S et al (2011) Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature 472(7341):51–56
Nakano T, Ando S, Takata N, Kawada M, Muguruma K, Sekiguchi K et al (2012) Self-formation of optic cups and storable stratified neural retina from human ESCs. Cell Stem Cell 10(6):771–785
Acknowledgments
We wish to warmly thank all members of the Friedlander lab and our collaborators. Work discussed in this chapter was supported by generous funding to PDW, a Ruth Kirschstein Fellow (NEI EY021416), to TK (Manpei Suzuki Diabetes Foundation and The Japan Society for the Promotion of Science Postdoctoral Fellowships for Research Abroad), and to MF from the NEI (EY11254; EY017540), the California Institute for Regenerative Medicine (CIRM TR1–01219), the Lowy Medical Research Foundation (the MacTel Project), and the Rasmussen Foundation.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media, LLC
About this paper
Cite this paper
Westenskow, P., Kurihara, T., Friedlander, M. (2014). Utilizing Stem Cell-Derived RPE Cells as A Therapeutic Intervention for Age-Related Macular Degeneration. In: Ash, J., Grimm, C., Hollyfield, J., Anderson, R., LaVail, M., Bowes Rickman, C. (eds) Retinal Degenerative Diseases. Advances in Experimental Medicine and Biology, vol 801. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-3209-8_41
Download citation
DOI: https://doi.org/10.1007/978-1-4614-3209-8_41
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-3208-1
Online ISBN: 978-1-4614-3209-8
eBook Packages: MedicineMedicine (R0)