Abstract
Campylobacter jejuni and Campylobacter coli can be frequently isolated from poultry and poultry-derived products, and in combination these two species cause a large portion of human bacterial gastroenteritis cases. While birds are typically colonized by these Campylobacter species without clinical symptoms, in humans they cause (foodborne) infections at high frequencies, estimated to cost billions of dollars worldwide every year. The clinical outcome of Campylobacter infections comprises malaise, diarrhea, abdominal pain and fever. Symptoms may continue for up to two weeks and are generally self-limiting, though occasionally the disease can be more severe or result in post-infection sequelae. The virulence properties of these pathogens have been best-characterized for C. jejuni, and their actions are reviewed here. Various virulence-associated bacterial determinants include the flagellum, numerous flagellar secreted factors, protein adhesins, cytolethal distending toxin (CDT), lipooligosaccharide (LOS), serine protease HtrA and others. These factors are involved in several pathogenicity-linked properties that can be divided into bacterial chemotaxis, motility, attachment, invasion, survival, cellular transmigration and spread to deeper tissue. All of these steps require intimate interactions between bacteria and host cells (including immune cells), enabled by the collection of bacterial and host factors that have already been identified. The assortment of pathogenicity-associated factors now recognized for C. jejuni, their function and the proposed host cell factors that are involved in crucial steps leading to disease are discussed in detail.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Zoonotic infections by bacterial, viral and parasitic microbes represent a significant health burden to humans (Cunningham et al. 2017; Logue et al. 2017; Plowright et al. 2017). Among these, a number of important foodborne pathogens are responsible for high degrees of morbidity and mortality worldwide. Examples from the bacterial kingdom include Campylobacter species, which are often detected in the natural environment such as water surface habitats as well as in the gastrointestinal tract of certain birds and mammals, where they colonize asymptomatically as commensals (Young et al. 2007; Burnham and Hendrixson 2018). In addition, the presence of Campylobacter species, in particular C. jejuni and C. coli, in poultry flocks and other farm animals such as dairy cows provides a high zoonotic potential. By means of multilocus sequence typing (MLST), it was demonstrated that certain genetic C. jejuni variants can better survive in the environment than others and are frequently found in natural soil and water reservoirs, especially during the warm summer seasons (Epps et al. 2013; Bronowski et al. 2014). From such sources, the bacteria can transfer to new hosts, e.g., to poultry flocks through rodents, flies or direct contact (Jorgensen et al. 2011; Bronowski et al. 2014). Even though Campylobacter spp. represent fastidious microaerophilic bacteria, they are well adapted to persistence in natural ecosystems, for instance through biofilm formation, aerotolerance mechanisms and starvation strategies (Gölz et al. 2012; Tram et al. 2020). Multiple surveys have demonstrated that the majority of commercial poultry flocks become colonized with C. jejuni or C. coli within about 2–4 weeks after hatching (Potturi-Venkata et al. 2007; van Gerwe et al. 2009). This colonization of chicks most frequently proceeds through horizontal transfer from the environment, rather than by vertical transmission from mother hens. Cross-transmission from other Campylobacter-positive flocks present on the same farm or from previous flocks can be prevented by strict hygiene procedures and fumigation (Herman et al. 2003; Wedderkopp et al. 2003; Bronowski et al. 2014). Taken together, although we now have a better understanding of the transmission routes by which Campylobacter enters the farm environment, it appears that more studies are required to find ways to effectively combat this pathogen on farms (see also Chaps. 4 and 5 of this book).
The major transmission route of C. jejuni (unless specifically stated otherwise, all reference to C. jejuni in this chapter also applies to C. coli) to humans mostly proceeds via the handling and consumption of contaminated poultry meat, raw milk, cross-contamination to other food products and, less frequently, by contact with freshwater or consumption of well water (Kaakoush et al. 2015). Occasionally, close contact to infected animals such as pets, particularly young dogs with diarrhea, can be sources of infection by C. jejuni (Campagnolo et al. 2018; Bronowski et al. 2014). Thus, the main infection route toward humans occurs by a fecal-to-oral pathway. Nevertheless, human-to-human spread is relatively uncommon. However, upon ingestion Campylobacter enters the gastrointestinal tract and colonizes the jejunal mucosa of the intestine by successfully competing with the intestinal microbiota (Masanta et al. 2013). The overall prevalence of C. jejuni infections is very high and represents a major fraction of all bacteria-caused gastroenteritis cases: It was estimated to account for about 400–500 million human incidences across the entire planet annually (Friedman et al. 2000). Infection by C. jejuni can lead to watery or bloody diarrheal disease, which can vary from non-inflammatory and self-limiting to a severe and inflammatory nature, resulting in significant medical and socioeconomic consequences (Nachamkin et al. 2008; Oyarzabal and Backert 2012). In a small subset of persons, the infection can be accompanied by more serious complications such as bacteraemia, or may result in the development of reactive arthritis and Reiter's syndrome or the neurological sequelae Guillain–Barré and Miller Fisher syndrome (Smith 2002; Yuki and Koga 2006). The rate of human infections by zoonotic foodborne C. jejuni has been progressively growing over the years, which creates a significant public health burden worldwide (Kaakoush et al. 2015).
Genetic typing tools and whole-genome sequencing have clearly demonstrated that C. jejuni isolates obtained from chicken meat can result in human campylobacteriosis (see Chap. 3 of this book). Nevertheless, the collective genetic diversity among Campylobacter isolates suggests that other transmission routes also exist, and that highly mutable sequences in multiple genetic loci across the chromosome appear to have an important function for bacterial adaptation in a new host (Sheppard and Maiden 2015). For example, comparative genomics and phenotypic analyses identified the emergence of specific C. jejuni lineages to become cattle specialists, which coincided with the enormous rise in the global cattle population in recent decades (Mourkas et al. 2020). Genome sequencing and other approaches have demonstrated the presence of various virulence factors, some of which have been well characterized in recent years. Once the bacteria have reached the human intestine, C. jejuni has been shown to interact with the gut epithelium as well as with immune cells (as summarized in a model in Fig. 1). A major difference between the infection of chicken and human hosts is the markedly higher capability of C. jejuni to invade human vs. avian epithelial cells (Young et al. 2007; Ó Cróinín and Backert 2012; Burnham and Hendrixson 2018). These findings imply that C. jejuni adhesion to and invasion into the epithelium is most likely associated with disease outcome. Consequently, it can be assumed that pinpointing factors involved in bacteria-host interactions are crucial to understand C. jejuni pathogenesis and for the development of new antimicrobial therapies. This chapter reviews in detail the infection strategy by C. jejuni toward the human host and the interplay of bacterial factors with epithelial as well as immune cells, which is important for the development of gut disease.
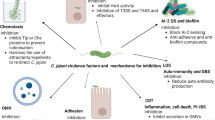
Model for crucial steps and C. jejuni mechanisms during infection in the human intestine. The intestinal epithelium functions as a tight physical barrier and serves as sensor of microbial infections such as C. jejuni. Various indicated surface-exposed and secreted bacterial factors are proposed to enable colonization of the mucus layer, adhere to epithelial cells, open the tight and adherens junctions, allow cell invasion as well as trafficking and survival in intracellular vacuoles. In addition, factors produced by C. jejuni can trigger nuclear responses such as cell cycle arrest, DNA damage, apoptosis and pro-inflammatory cytokine production. The latter leads to the infiltration of various immune cell types to the sites of infection. Production of reactive oxygen species and other anti-bacterial responses enhance cell damage, leading to campylobacteriosis and eventually to bacterial clearance. Furthermore, modified lipooligosaccharide (LOS) structures of C. jejuni mimic human gangliosides, which can lead to auto-antibody production and neural disorders in some patients
2 Bacterial Virulence Factors and Epithelial Cell Responses
2.1 Specialized Metabolism and Enteric Life Style
It is well established that C. jejuni does not utilize sugar metabolites as a carbon source, which was shown to be due to absence of the glycolytic enzyme phosphofructokinase (Parkhill et al. 2000; Velayudhan and Kelly 2002). Instead, C. jejuni growth depends on the presence of single amino acids or keto acids, either supplied by the host or by the residual gut microbiota (Lee and Newell 2006; Hofreuter 2014). The amino acids present in the chicken gut were quantified, and the most abundant ones were those that C. jejuni depends on for its metabolism, demonstrating its adaptation to this host (Parsons et al. 1983). Early C. jejuni growth experiments in vitro have shown that aspartate, serine, proline and glutamate are favorably utilized as nutritive substances (Leach et al. 1997; Elharrif and Mégraud 1986; Leon-Kempis et al. 2006; Velayudhan et al. 2004; Guccione et al. 2008). The bacteria contain dedicated membrane transporters for certain amino acids that are essential for serine metabolism. By means of mutagenesis, it was demonstrated that these transporter systems are required for colonization in the intestine of chickens (Hendrixson and DiRita 2004; Velayudhan et al. 2004; Ribardo and Hendrixson 2011). Furthermore, it appears that natural C. jejuni strains display a high genetic diversity and sometimes utilize specific pathways to metabolize a given amino acid (Hofreuter 2014; Gao et al. 2017). In addition, specific genetic polymorphisms have been associated with varying capabilities to acquire nutrients and establish colonization in mice. For example, C. jejuni strains expressing a certain γ-glutamyltranspeptidase (GGT) enzyme were also able to metabolize glutathione and glutamine, leading to elevated colonization rates in the murine gut (Hofreuter et al. 2008; Floch et al. 2014). Similarly, when an asparaginase enzyme was present with a sec-mediated secretion motif, C. jejuni could acquire asparagine from the environment, which not only improved colonization of the intestinal tract, but also of the liver of mice (Hofreuter et al. 2008). Finally, a recent comprehensive in vitro analysis of a C. jejuni transposon mutant library was performed for bacterial multiplication in distinct broth media and subsequently for the capability to colonize mice, and this was merged with isotopolog profiling experiments and metabolic flow studies (Gao et al. 2017). This work identified that C. jejuni can consume various metabolic end products from the gut microbiome, including acetate and carbon dioxide in the form of hydrogen carbonate, and particularly single amino acids as mentioned above, as well as oligo-peptides made available from food and degraded host proteins. Furthermore, it was observed that single amino acids and di-peptides are present in the intestinal mucus layer in considerable quantities. However, certain required amino acids may not be available in abundances high enough to support C. jejuni growth, as demonstrated by auxotrophic mutations that prevented the production of serine or aromatic and branched amino acids and led to the inability to colonize mice (Gao et al. 2017). It seems that C. jejuni surmounts these metabolic substrate constraints by utilizing the tricarboxylic acid cycle, the non-oxidative pentose phosphate pathway and gluconeogenesis, which can collectively promote growth in vitro and in vivo (Gao et al. 2017). Together, this detailed study has pinpointed multiple routes of a highly specialized metabolism and life style of C. jejuni to achieve bacterial fitness in the gut.
2.2 Campylobacter Motility and Chemotaxis
Motility is a characteristic property of C. jejuni and is essential for effective colonization in the avian, murine or human host (Wassenaar et al. 1993; Guerry 2007; Chang and Miller 2006; Artymovich et al. 2013; Black et al. 1988; Schmidt et al. 2019). The bacteria are motile by means of two flagella, one on each end of the spirally-shaped bacterial cell body. The flagellum functions as a propeller driven by a rotating motor, which enables the bacterium to swim by a corkscrew-like mechanism (Purcell 1997; Karim et al. 1998; Shigematsu et al. 1998; Cohen et al. 2020). The C. jejuni flagellar structure resembles that of Salmonella or E. coli, but C. jejuni has three additional disk structures making up the motor, called the basal disk (mainly consisting of FlgP), the medial disk (composed of PflA) and the proximal disk (formed by PflB and MotAB stator units) (Chen et al. 2011; Beeby et al. 2016). These disk structures are located in the periplasm, where they surround the flagellar rod (Beeby et al. 2016). In addition, the C. jejuni flagellum has an MS ring and a C ring of higher complexity that contributes to enhanced activity of the flagellar type III secretion system (Henderson et al. 2020), which will be further described in Sect. 2.6 below. Connected to this structure is the so-called surface hook (FlgE) to which the flexible extracellular flagellar filament (composed of FlaA and FlaB) is attached (Guerry et al. 1991; Hendrixson and DiRita 2003; Chen et al. 2011; Beeby et al. 2016). In addition to a function in bacterial motility, the flagellum may also be used for the secretion of proteins into the extracellular space (Konkel et al. 1999; Poly et al. 2007; Christensen et al. 2009; Barrero-Tobon and Hendrixson 2012; Faber et al. 2015). It was also reported that the flagellum can play a role in the adhesion of C. jejuni to certain host cells (Yao et al. 1994). In line with that observation, it was described that the secreted flagellin-like protein FlaC binds to the host cell, may contribute to C. jejuni invasion and as such may play a role by modulation of the host immune response (Song et al. 2004). The flagellar filament undergoes O-linked glycosylation, which enables it to colonize chickens (Howard et al. 2009). Protein glycosylation is also important for the correct assembly of the filament’s building blocks, flagellin (FlaA and FlaB). To this end, it appears that glycosylation allows the flagellin subunits to interact with each other (Goon et al. 2003; Guerry et al. 2006; Kreutzberger et al. 2020). A two-component signal transduction system (FlgS and FlgR) regulates transcription of many flagellar genes essential for flagellar biosynthesis, some of which are produced with Sigma 54 (Hendrixson and DiRita 2003; Wösten et al. 2004). They are controlled by supercoiling of chromosomal DNA (Shortt et al. 2016) as well as by phase variation and phosphorylation, which is unique in this bacterium (Hendrixson 2006, 2008).
By means of chemotactic sensors, C. jejuni is able to senses metabolic concentration gradients, such as those represented by certain components surrounding the mucosa of the gut (Korolik 2019). For the human intestine, these are mainly aspartate, asparagine and lactate, while in the chicken intestine l-fucose is present (Vegge et al. 2009; Hartley-Tassell et al. 2010; Rahman et al. 2014; Dwivedi et al. 2016). Thus, chemotaxis plays an important role both in commensal and pathogenic microbe–host interactions. Through a broad genome sequence analysis among multiple C. jejuni strains, a number of orthologous chemotaxis genes including cheA, cheW, cheV, cheY, cheR and cheB were identified (Marchant et al. 2002). It was shown that CheY acts as a response regulator and interacts with the flagellar motor to affect the rotation direction to turn either clockwise or counterclockwise. Therefore, it is a particularly important factor for flagellar function (Yao et al. 1997). A recent publication demonstrated that CheY has no effect on the speed of rotation, because speed was not affected by deletion of cheY (Cohen et al. 2020). Deletion of one of the above mentioned chemotaxis components leads to a colonization defect as shown in ferret and mouse models (Yao et al. 1997; Chang and Miller 2006). As a result, C. jejuni is no longer able to induce the associated disease during infection. Together, it can be concluded that motility in combination with the chemotaxis cascade is important for colonization and proper interaction of C. jejuni with its host, either asymptomatic as in chickens or symptomatic as in humans (Korolik 2019).
2.3 CDT Toxin Production
The cytolethal distending toxin (CDT) is the only known toxin found in most, though not all C. jejuni strains (Mortensen et al. 2011). In contrast to other diarrheal pathogens, C. jejuni does not encode other toxins, which makes the CDT unique for this bacterium (Lai et al. 2016). When cultured host cells are exposed to CDT, it leads to a characteristically enlarged cell surface and to cell death, which gave the toxin its name. This was originally shown for several sensitive cell lines upon infection with C. jejuni (Johnson and Lior 1988). CDT is a holotoxin comprising of three subunits, CdtA, CdtB and CdtC. Of these, CdtB exhibits enzymatic Dnase activity that ultimately results in cell-cycle arrest and cell death, while CdtA and CdtC are responsible for the translocation of CdtB across the target cell membrane (Lara-Tejero and Galán 2001). Subunits CdtA, CdtB and CdtC are located on the bacterial cell surface, where they assist in binding to the host cell (Guerra et al. 2011); however, the exact mechanism of CdtA/CdtC-assisted CdtB translocation remains controversial. Similarities to the B chain of ricin toxin, which is important for the receptor-induced endocytosis of ricin, have been demonstrated (Lara-Tejero and Galán 2001). It has also been shown that the CDT holotoxin is either directly secreted into the extracellular space or packaged into outer membrane vesicles (OMVs) that are continuously being shed from the bacteria (Lindmark et al. 2009). OMVs are commonly formed by Gram-negative bacteria and fulfil a number of tasks such as the delivery of toxins (Wai et al. 2003). So far it remains unclear, however, how the OMVs carrying CDT bind to target cells, or whether the OMVs enter the cell. It appears that the holotoxin, when packed in OMVs, can enter the host cell by membrane fusion (DiRienzo 2014). Thus, it remains to be determined how exactly CdtB is translocated into the host cell.
Once CdtB has reached the cytoplasm of the target cell, it is transported to the endoplasmic reticulum with the help of the Golgi apparatus to get into the cell nucleus (Heywood et al. 2005). Both actin and the microtubulin systems assist in this transport of CdtB into the nucleus (Méndez-Olvera et al. 2016). Inside the cell nucleus, CdtB induces DNA double-strand breaks, which lead to arrest of the cell cycle in the G2/M phase (Whitehouse et al. 1998). This mechanism results in the activation of DNA repair mechanisms and blocking of the nuclear CDC2 kinase via phosphorylation, which is responsible for entering mitosis. This ultimately leads to apoptosis of the cell (Pickett and Whitehouse 1999; Guerra et al. 2005). In some human cell lines (Hela, Caco-2), cell enlargement is indeed observed during infection with C. jejuni, leading to cell death (Johnson and Lior 1988; Elmi et al. 2016). Further studies showed that CDT is able to trigger the secretion of IL-8 as an immune response, which leads to the recruitment of macrophages and neutrophils to the infected site (Hickey et al. 2000; Purdy et al. 2000; Zheng et al. 2008). This leads to a massive infiltration of immune cells into the infected tissue, which is a typical histopathological hallmark of campylobacteriosis. In support of this, a ∆cdt knockout mutant was still able to colonize immuno-suppressed mice but was no longer able to induce symptoms or systemic infection (Purdy et al. 2000; Fox et al. 2004). Thus, these results clearly show that CDT plays a role in the pathogenicity of C. jejuni and can therefore be counted as virulence factor. Nevertheless, human symptomatic infections are sometimes caused by strains defective in CDT production (Mortensen et al. 2011).
2.4 Serine Protease HtrA and Epithelial Barrier Disruption
The trypsin-like serine protease HtrA (high-temperature requirement A) is a highly conserved enzyme found in both prokaryotes and eukaryotes and was first described in Escherichia coli (Lipinska et al. 1989). That species typically contains three htrA orthologous genes, called degQ, degP and degS, while other bacteria such as C. jejuni encode only one htrA gene copy, whose product is most similar to DegQ. HtrA has a domain-like structure and consists of a signal peptide required for secretion, a protease domain and two PDZ domains. The bi-functional protein acts as a chaperone and also has protease activity. The protease domain contains a catalytic triad which is composed of the amino acids histidine (His), aspartate (Asp) and serine (Ser). Following removal of the signal peptide, the protein reaches the periplasm, where it forms proteolytically active multimers that carry out protein quality control functions (Clausen et al. 2002; Kim and Kim 2005; Krojer et al. 2010). Cryo-electron microscopy of C. jejuni HtrA revealed that it forms a dodecamer, built of four trimers (Zarzecka et al. 2020). Biochemical studies disclosed proteolytically active hexamers, dodecamers and even larger oligomers with a remarkable stability compared to previously investigated HtrA orthologs in other bacteria (Zarzecka et al. 2020). It was further shown that HtrA protects the C. jejuni bacteria against the enrichment of denatured or non-properly folded proteins in the periplasm under stress conditions (Brøndsted et al. 2005; Bæk et al. 2011a). Indeed, a C. jejuni ∆htrA deletion mutant exhibited reduced growth compared to the wild-type bacteria. This was attributed to the chaperone activity of the protein, since a protease-inactive S197A mutant showed no impairment in terms of growth (that single amino acid mutation does not affect the chaperonin activity of HtrA). Similar behavior was also observed in response to oxidative stress (Brøndsted et al. 2005; Bæk et al. 2011a). Further studies demonstrated that the ∆htrA deletion mutant had defects to adhere and invade into host cells. The defective phenotype was restored after genetic complementation with the wild-type htrA gene (Bæk et al. 2011a; Boehm et al. 2012, 2013, 2015). However, the ability to adhere to the host cell did not require protease activity of HtrA (Bæk et al. 2011b). Altogether, these findings suggest that the chaperone part of HtrA has an important role in the adherence and invasion process during C. jejuni infection (Konkel et al. 2001).
It has been demonstrated that HtrA of C. jejuni is also secreted into the extracellular space, which suggests stress response and survival is not its only function (Boehm et al. 2012, 2018; Backert et al. 2018). A plausible hypothesis is that the HtrA protein is released as a soluble enzyme and/or packaged as cargo into OMVs (Boehm et al. 2012; Elmi et al. 2012, 2016; Yoon 2016). The number of HtrA molecules secreted per C. jejuni cell has been quantified and is considerably high: On average, about 4000–5000 HtrA molecules can be secreted by a single bacterium during 2 h of culture in liquid broth (Neddermann and Backert 2019). When the protein is secreted into the extracellular environment during infection, HtrA comes in direct contact with host cell surface proteins, where it may exhibit protease activity, as has been experimentally demonstrated. The first described HtrA target was the adherens junction and tumor suppressor protein E-cadherin, which HtrA cleaves into various fragments (Boehm et al. 2012). Another recently discovered target protein which is cleaved by C. jejuni HtrA is the tight junction protein occludin (Harrer et al. 2019). These cleavage events lead to the temporary opening of cell-to-cell junctions in the epithelium, allowing C. jejuni to transmigrate between two neighboring cells (Fig. 1). Interestingly, it was observed that such a temporary opening of the junctions has no major impact on the transepithelial electrical resistance (TER), which demonstrates the overall tightness of an epithelial layer (Boehm et al. 2012; Harrer et al. 2019). It was also shown that both the ∆htrA and an S197A mutants were unable to transmigrate across a cell monolayer, despite being fully motile, which clearly demonstrated the importance of the HtrA protease of C. jejuni for crossing the epithelial cell monolayer. Thus, our hypothesis is that HtrA has a dual function for the pathogen: (i) intracellular protein quality control and (ii) extracellular cleavage of host cell junctional proteins to establish a proper infection (Boehm et al. 2012, 2013; Backert et al. 2018).
In two different mouse models (one using IL-10−/− knockout mice and the other using infant wild-type mice), the impact of C. jejuni HtrA on the infection process has been monitored (Boehm et al. 2018). When IL-10−/− knockout mice were infected with wild-type C. jejuni, strong changes in the crypt architecture were observed, accompanied by a strong infiltration of immune cells into the tissue. These features were diminished in mice infected with the ∆htrA mutant (Heimesaat et al. 2014a). Infection of infant mice with wild-type and ∆htrA mutant C. jejuni resulted in similar observations, and in this model presence or absence of HtrA did not affect the colonization rates of C. jejuni in the intestine (Heimesaat et al. 2014b). Taken together, these observations clearly identified HtrA as a virulence factor of C. jejuni. We therefore consider HtrA an ideal candidate for future development of new antimicrobial drugs. The development of new drugs against this foodborne pathogen is very important, as Campylobacter-mediated enteritis is highly frequent in a number of countries (Gölz et al. 2014). Efforts to develop an inhibitor against HtrA have been conducted in vitro, mostly targeting the proteins of E. coli or Helicobacter pylori HtrA (Hauske et al. 2009; Perna et al. 2014, 2015; Schmidt et al. 2016; Tegtmeyer et al. 2016). These attempts had shown some success; however, the identified inhibitors are still somewhat unspecific and can also inhibit HtrAs of other bacteria, including commensals. Thus, further research must be carried out in this area to narrow down the specificity of HtrA inhibitors toward the proteins of pathogenic bacteria only.
2.5 Outer Membrane Adhesins and Host Cell Binding
C. jejuni invasion into host epithelial cells is first initiated through cell adherence provided by various adhesion proteins (called adhesins), which recognize and bind to specific host cell receptors resulting in stable attachment. The binding activity of adhesins is widely accepted to be fundamental for the effective interaction of a given bacterium with host cells and is a necessary prerequisite for subsequent invasion (Hermans et al. 2011; Backert et al. 2013). Several major C. jejuni adhesins have been shown to result in attachment to the host cell (Fig. 2), with CadF (Campylobacter adhesin to fibronectin) believed to play a key role in this process. Other major adhesins include fibronectin like protein A (FlpA), jejuni lipoprotein A (JlpA), major outer membrane protein (MOMP), Campylobacter autotransporter protein A (CapA), a 95 kDa outer membrane protein (p95) and periplasmic binding protein 1 (PEB1) (Ó Cróinín and Backert 2012). CadF is a 37-kDa outer membrane protein that mediates bacterial attachment to the host cells through the extracellular matrix protein fibronectin (Konkel et al. 1997; Schmidt et al. 2019; Krause-Gruszczynska et al. 2007a). The CadF/fibronectin interaction during C. jejuni colonization was mostly studied using INT-407 cells as a model for infection. Originally thought to be derived from normal embryonic intestinal tissue, INT-407 was subsequently found to have been established via HeLa cell contamination, which should be taken into account when working with these cells (Nelson-Rees and Flandermeyer 1976; Neimark 2015). Disruption of the cadF gene resulted in reduction of bacterial adherence to the INT-407 cells (Monteville and Konkel 2002; Monteville et al. 2003; Krause-Gruszczynska et al. 2007b). CadF deficiency further rendered the bacteria less capable to colonize chickens in comparison with wild-type C. jejuni (Ziprin et al. 1999). It was proposed that CadF is responsible for the uptake of C. jejuni at the basolateral area of host cells, where fibronectin is linked to the integrin-based receptor complex (Monteville and Konkel 2002). However, in the IL-10−/− mouse model observations revealed that C. jejuni flagellin A and B, but not cell adhesion mediated by CadF, are essential for inducing murine campylobacteriosis (Schmidt et al. 2019).
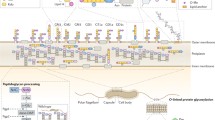
Major surface-exposed adhesins and involved host cell receptors described for C. jejuni. At least eight membrane-anchored bacterial proteins have been described to act as binding factors to host target cells. For three of these factors, the corresponding host cell interaction partner has been identified. For the others, the host receptor is still unknown and these are labeled with question marks. FlaC* is secreted via the flagellar T3SS and PEB1** has been described as an aspartate/glutamate-binding protein of an ABC transporter. For more details on these adhesins, see text
Another binding factor of C. jejuni acting through interaction with fibronectin is the 46-kDa protein FlpA. Deletion of flpA resulted in a significant reduction of bacterial attachment to INT407 cells, while in wild-type C. jejuni the FlpA protein interacted with cellular fibronectin in a dose-dependent manner (Konkel et al. 2010; Eucker and Konkel 2012). This interaction was studied in more detail, revealing that the FlpA/fibronectin interaction is mediated by the fibronectin-binding linear motif in domain-2 of FlpA with the gelatin-binding domain of fibronectin (Larson et al. 2013). Altogether, it seems that both CadF and FlpA proteins enable attachment of C. jejuni to host cells via fibronectin in a cooperative manner.
A third adhesin is the constitutively expressed JlpA, a 43-kDa surface lipoprotein that was shown to interact with intestinal heat shock protein 90α. Mutation in the jlpA gene led to reduced adherence of C. jejuni to cultured HEp-2 cells by 18–19.4% (Jin et al. 2001, 2003). In addition, pretreatment of HEp-2 cells with purified JlpA decreased C. jejuni adhesion in a dose-dependent manner (Jin et al. 2001), suggesting saturation of a receptor. In an interesting study, recombinant JlpA was expressed in Lactococcus lactis and when these bacteria were fed to chickens, IgA antibodies were raised against the protein that was present in chicken feces. When these antibodies were used for pretreatment of either human INT407 or primary chicken embryo intestinal cells, it resulted in a significant reduction in bacterial adherence and invasion of C. jejuni (Gorain et al. 2020), again suggesting the interaction between JlpA and its targets can be blocked, this time with JlpA-directed antibodies.
Similarly, inactivation of the peb1A gene, which encodes a 28-kDa protein PEB1, decreased adherence of C. jejuni to cultured HeLa cells, and prevented colonization of mice (Pei et al. 1998); however, the exact adhesion mechanism of this protein remains unclear. Later studies showed that PEB1 had less influence on adherence of C. jejuni to T84 cells or chicken epithelial cells (Novik et al. 2010; Flanagan et al. 2009). PEB1 actually functions as a periplasmic binding protein as part of an aspartate/glutamate ABC transporter system, which is required for optimal microaerobic growth on dicarboxylic amino acids (Leon-Kempis et al. 2006). This could explain why PEB1-deficient bacteria are less able to colonize a given host.
The MOMP protein, which is also known as PorA, has also been suggested to contribute to the adherence of C. jejuni to host cells, although its major role seems to be the transport of nutrients and other small molecules. Nevertheless, a recent study showed that the transcription terminator of the porA gene enhances the expression level of MOMP by stabilizing its mRNA and therefore influences the virulence of C. jejuni (Dai et al. 2019). Some of the proposed C. jejuni adhesins are controversial in the literature, and their contribution to the bacterial adhesive properties might be indirect, as may apply to, among others, PEB1, MOMP, CapA and p95 (Ó Cróinín and Backert 2012). Interestingly, novel genes regulating adhesion factors remain to be discovered, as illustrated by the recent report of a new two-component signal transduction system which is involved in regulating adhesion (Xi et al. 2020). These genes (cj1492c and cj1507c) encode a histidine kinase and a transcriptional regulator, respectively, and when inactivated, this impaired motility, adherence and invasion, and fewer bacteria survived intracellularly. The gene pair has been renamed BumR and BumS and has also been found to be involved in directing a response to butyrate (Luethy et al. 2015; Goodman et al., 2020). Together, these findings illustrate that adhesion is a key prerequisite for the successful colonization of host by C. jejuni.
2.6 The Flagellum as a Specialized Type III Secretion System
C. jejuni does not possess classical type III or type IV secretion systems (T3SS or T4SS) to inject effector molecules into host cells; however, T3SS functions were found to be provided by the flagellum, which has been demonstrated to export effector proteins that can control bacteria–host interactions as discussed below (Barrero-Tobon and Hendrixson 2012; Christensen et al. 2009; Young et al. 1999; Ziprin et al. 1999). The flagellar filament of C. jejuni consists of two glycosylated structural flagellins, FlaA and FlaB, as described above. One of the proteins secreted through the flagellar filament is the non-structural protein FlaC, which is implemented in host cell invasion. The flagellar proteins FliS and FliW in C. jejuni assist in the secretion of FlaC protein (Radomska et al. 2017). The FliS protein (a flagellar chaperone) preferentially binds to the glycosylated flagellins and is essential for flagellar assembly; it also directs FlaC toward the flagella for its secretion, while FliW mainly acts as sensor of intracellular FlaA/FlaB flagellin levels. The FlaC protein is thus secreted from the flagellar apparatus of C. jejuni cells and plays an important role in entry to epithelial cells (Song et al. 2004). Moreover, FlaC has been shown to directly interact with toll-like receptor 5 (TLR5), resulting in p38 activation (Faber et al. 2015). Preincubation with FlaC modulated the immune responses of chicken and human macrophage-like cells toward the bacterial TLR4 agonist lipopolysaccharide (LPS) by promoting cross-tolerance with subsequent reduction of interleukin-1β (IL-1β) expression (Faber et al. 2015). Consequently, the flagellum is a complex machinery that not only renders C. jejuni motile, but also enables protein secretion and administration into the host cell, providing a crucial step in the process leading to host invasion (Burnham and Hendrixson 2018). In this context, it is noteworthy that the flagellum is evolutionarily related to T3SSs used by, for instance, pathogenic Salmonella or Yersinia species, and was therefore named flagellar T3SS (fT3SS).
FlaC is not the only protein secreted by the C. jejuni fT3SS. There are two other distinct groups of proteins secreted this way, described as (i) flagellar co-expressed determinants (FedA-D) and (ii) Campylobacter invasion antigens (CiaA-I) (Konkel et al. 1999; Eucker and Konkel 2012; Burnham and Hendrixson 2018). The Fed proteins were found to be important in commensal colonization of chickens, while FedA is also involved in invasion of human intestinal cells (Barrero-Tobon and Hendrixson 2012). However, the individual functions of Fed proteins remain largely unknown (Burnham and Hendrixson 2018). The Cia proteins have been reported to influence C. jejuni interaction with human intestinal cells; however, their mechanisms of action during adhesion and invasion are also relatively unclear. One of the best-characterized Cia members, the 73-kDa protein CiaB, appears to be necessary for the secretion process itself, and is required for maximal invasion of C. jejuni into host target cells (Konkel et al. 1999). C. jejuni strain F38011 with a deleted ∆ciaB gene exhibited significantly lower invasion capacity into human cells, along with reduced colonization in chickens (Ziprin et al. 1999). However, inactivation of ciaB in strain 81–176 did not influence invasion capacity toward cultured intestinal epithelial cells (Novik et al. 2010). These conflicting outcomes may be related to strain differences or differences in experimental procedures. A gene screening of C. jejuni strain NCTC 11168 revealed at least 42 proteins with putative fT3SS amino-terminal sequences directing their export through the flagellum (Christensen et al. 2009). CiaC is an example and was reported to be essential for maximal invasion of epithelial cells, which took place through the recruitment and activation of small Rho GTPase member Rac1, while ciaC-deficient C. jejuni resulted in significant decrease of Rac1 activation (Eucker and Konkel 2012). Another Cia member, CiaI, may have a function in intracellular survival in human cells (Buelow et al. 2011) and/or colonization in chickens (Barrero-Tobon and Hendrixson 2012) and is discussed below. Taken together, it appears that the flagellar export machinery fT3SS represents a crucial secretory apparatus, which enables C. jejuni to invade and manipulate host cells.
2.7 Bacterial Factors and Signaling Involved in Host Cell Invasion
A major disease-associated feature of C. jejuni is its capability to invade host tissues, which is believed to represent a primary mechanism of pathogenesis associated with host tissue damage. A number of molecular players of the bacterium and host cell involved in invasion have been discovered (Fig. 3). High-resolution electron microscopy of infected epithelial cells revealed that C. jejuni can induce membrane rearrangements upon contact with a host membrane, which eventually leads to cell invasion (Boehm et al. 2011; Krause-Gruszczynska et al. 2011). According to multiple studies, there are two major strategies utilized by C. jejuni to enter cultured cells, either microtubule-dependent or actin-filament-dependent mechanisms, which seem to vary between C. jejuni strains (Ó Cróinín and Backert 2012). While the detailed signaling mechanism of the microtubule-dependent invasion pathway is unclear, members of the small Rho GTPase proteins are known as key regulators, which trigger actin polymerization, membrane ruffling and bacterial internalization (Stradal and Schelhaas 2018). Various experiments reported that entry of C. jejuni into intestinal epithelial cells depends on the activation of the small Rho GTPase members Cdc42 and Rac1 (Krause-Gruszczynska et al. 2007b; Krause-Gruszczynska et al. 2011; Boehm et al. 2011; Eucker and Konkel 2012). C. jejuni binds to fibronectin by means of its CadF and FlpA adhesins, which may act together, and in turn drive the phosphorylation of EGF receptor by activation of the integrin receptor (Eucker and Konkel 2012). Upon attachment to the host cell surface, CadF invokes two signaling cascades that lead to either Cdc42 or Rac1 activation, both resulting in F-actin-dependent engulfment and uptake of C. jejuni. This process involves a wide range of intermediate factors initiated by binding of CadF to fibronectin, followed by signal cascades involving PDGF and EGF receptors, integrins, cytosolic kinases and guanine exchange factors. The activation of Cdc42 and Rac1, respectively, takes place by the following proposed pathways. The difference of these two routes is the step following integrin/focal adhesion kinase (FAK) interaction: CadF → fibronectin → β1-integrin → FAK/Src → PDGFR/EGFR → PI3-kinase → Vav2 → Cdc42 (Krause-Gruszczynska et al 2011) (left side of Fig. 3); or CadF → fibronectin → β1-integrin → FAK → Tiam-1/DOCK180 → Rac1 (Krause-Gruszczynska et al. 2011; Boehm et al. 2011) (middle part of Fig. 3). The importance of all these host factors for efficient bacterial uptake was demonstrated by C. jejuni infection of fibroblasts derived from fibronectin−/−, integrin-β1−/−, FAK−/− and Src−/−/Yes−/−/Fyn−/− (SYF) triple knockout mice, each of which resulted in invasion failure (Krause-Gruszczynska et al. 2011; Boehm et al. 2011). Whether the activation of Cdc42 and Rac1 are involved in microtubule-dependent uptake of C. jejuni has not yet been investigated. In addition, various studies using inhibitors and gentamycin protection assays suggested that heterotrimeric G proteins, the mitogen-activated kinases ERK and p38, protein kinase C (PKC), phosphatidylinositol-3-kinase (PI3K) and Ca2+ release from host cytoplasmic compartments all play a role in C. jejuni host cell entry (Wooldridge et al. 1996; Biswas et al. 2000; Hu et al. 2005; 2006), but the involved bacterial factors are not yet clear and need further investigation.
Molecular signal transduction model for C. jejuni-induced events leading to bacterial invasion of the human intestinal epithelium. C. jejuni express two fibronectin-binding proteins, CadF and FlpA, which mediate attachment of the bacteria to integrin-based focal adhesion structures. In this way, integrin receptors are activated leading to various indicated signaling events. For example, the growth factor receptors EGFR and PDGFR are activated by phosphorylation, which leads to stimulation of PI3-kinase (PI3-K) and the guanine exchange factor Vav-2, activating the small Rho GTPase Cdc42. In addition, integrin engagement leads to autophosphorylation of the cytoplasmic kinases FAK and Src as well as paxillin, which in turn activate the guanine exchange factors Tiam-1 and Dock180, stimulating small Rho GTPase Rac1. Both Cdc42 and Rac1 can then trigger microtubule and F-actin polymerization/reorganization events leading to the membrane engulfment and subsequent uptake of C. jejuni into the host cells. Activation of additional host receptors such as G proteins by yet unknown bacterial factor(s) also appears to contribute to bacterial host cell entry. Finally, various secreted effector proteins of the C. jejuni flagellum (fT3SS) are proposed to function in bacterial attachment and invasion, but their exact mechanisms are yet unclear. For more details, see text
It should be mentioned that many of the above in vitro studies were performed with non-polarized host cells that lack proper cell-to-cell junctions, so that the basolateral fibronectin/integrin complex is easily accessible, a situation that vastly differs from an intact intestinal epithelium encountered in vivo. By means of polarized intestinal Caco-2 cells, it was demonstrated that the serine protease HtrA enables disruption of the cellular tight and adherens junctions as discussed above, which facilitates bacterial invasion of epithelial cells at the basolateral site (Harrer et al. 2019). Taken together, invasion of the epithelium by C. jejuni is a complex process involving dozens of bacterial effector molecules as well as a range of host receptors and proteins. While fibronectin-mediated effects of the proteins CadF and FlpA are well described, the molecular mechanisms of action of other proteins, such as adhesins PEB1, MOMP, p95 or CapA, remain to be elucidated.
2.8 Intracellular Survival and Trafficking of Campylobacter
Invasion of C. jejuni into the gut epithelium has been examined in detail; however, there are only a handful of studies investigating the fate and persistence of the bacteria once they are inside the host cells (Pesci et al. 1994; Gaynor et al. 2005; Naikare et al. 2006; Buelow et al. 2011; Bouwman et al. 2013). Gentamicin protection assays and electron microscopy studies have shown that C. jejuni can survive for up to 1–3 days in intestinal epithelial cell lines in vitro. In particular, intracellular C. jejuni were observed in a membrane-enclosed compartment in the cellular cytoplasm that was named CCV (short for Campylobacter-containing vacuole) (Watson and Galán 2008). These findings initiated investigations toward the C. jejuni factors that are involved in intracellular survival and trafficking. The first C. jejuni gene reported to contribute to survival within epithelial cells was sodB, encoding a superoxide dismutase catalyzing the breakdown of superoxide radicals, which represents an important defense mechanism against oxidative damage (Pesci et al. 1994). Another intracellular bacterial survival factor is spoT, a gene encoding a bifunctional ppGpp synthetase/ pyrophosphohydrolase (Gaynor et al. 2005). Microarray expression studies showed that SpoT regulates the so-called stringent stress response by C. jejuni. This response appears to be important for bacterial survival in the stationary phase as well as persistence during changing O2 or CO2 concentrations. In addition, the stringent response was necessary for various pathogenicity-related phenotypes including C. jejuni viability inside the cultured intestinal epithelial cells (Gaynor et al. 2005). Other genes essential to intracellular survival include aspA (aspartate ammonia-lyase) and aspB (aspartate aminotransferase) as demonstrated by their inactivation, which decreased intracellular survival, probably due to decreased viability and/or unidentified consequences of the bacterial physiology (Novik et al. 2010). However, in such studies it is difficult to differentiate factors specifically needed from intracellular survival, as mutation of many housekeeping enzymes would result in impaired intracellular survival as well. In another study, the gene of the fT3SS-delivered protein CiaI was inactivated, which compromised invasion and reduced intracellular survival levels (Buelow et al. 2011). However, the latter function is not yet fully clear because other studies supported the view that CiaI may exhibit a different function in establishing commensalism during colonization of chicken (Barrero-Tobon and Hendrixson 2011). Thus, more work is required to characterize the C. jejuni factors facilitating intracellular survival functions.
It appears that LOS enhances C. jejuni attachment and endocytosis into intestinal epithelial cells (Louwen et al. 2008, 2012). Survival of C. jejuni inside CCVs, a compartment in which other pathogens can be killed, may be due to a mechanism evading their maturation to a typical lysosome (Watson and Galán 2008). It has been reported that the CCV diverges from the conventional “canonical” endocytic route (Fig. 4). Interestingly, experimental recovery of intracellular C. jejuni from CCVs was only possible by culturing the bacteria under oxygen-limiting conditions (Watson and Galán 2008). This implies that the bacteria undergo crucial physiological adaptations inside the CCVs that may be irreversible. Furthermore, the CCVs were shown to interact with endosomal compartments, and since they can be stained for the early endosomal marker EEA-1 (Early Endosome Antigen 1) as well as for two trafficking GTPases (called Rab4 and Rab5) (Watson and Galán 2008; Louwen et al. 2012); the presence of these markers on the outside of CCVs can be assumed. Nevertheless, this early event obviously appears only temporary and does not continue along the canonical cascade of endocytosis and is evidently different from conventional lysosome formation. However, the CCVs stained positive for the well-known late endosomal marker protein Lamp-1 (Fig. 4). In contrast, the CCVs did not contain signals for cathepsin B, a well-known lysosomal marker protease, and it is also not stainable for specific other endocytic tracer proteins (Watson and Galán 2008). Altogether, the recruitment of Lamp-1, which happens at a very early stage of CCV development appears to progress by a unique C. jejuni-triggered signaling cascade and does not lead to fusion with lysosomes. And this pathway does not demand the functional expression of the GTPases Rab5 and Rab7, despite their presence in the CCVs. Thus, further analyses are necessary to clarify in better detail how C. jejuni hijacks endocytic compartments for trafficking and to cause disease in humans.
Model for the establishing and maturation of C. jejuni-containing vacuoles (CCVs) in the cytoplasm of infected epithelial cells. C. jejuni enters the intestinal epithelium via mechanisms described in Fig. 3. After internalization, a so-called CCV is formed by membrane engulfment of invading C. jejuni. This CCV transiently recruits various marker molecules of the endocytic cascade including flotilin-1, Rab4, Rab5, Rab7, Caveolin and phosphatidylinositol 3,4,5 trisphosphate (PIP3). This leads to trafficking and intracellular survival of the bacteria in this compartment, inhibiting the canonical endocytic route toward lysosome development. In particular, intracellular C. jejuni inhibit the fusion of the late endosome to form lysosomes, and thus avoid bacterial killing. The CCVs also contain the marker protein Lamp-1 and localize in close proximity to the host Golgi apparatus near the nucleus. For more details, see text
3 Bacterial Virulence Factors and Immune Cell Responses
Innate immunity identifies multiple microbes through the action of various immune receptors. The recognition of C. jejuni by such receptors has raised much consideration because their activities could explain the immune pathology of this pathogen (Phongsisay 2016). Below we discuss important receptors binding to C. jejuni factors and examine their downstream signaling cascades and resulting immune responses. Numerous pro- and anti-inflammatory pathways that are triggered by C. jejuni and control the infection, respectively, are highlighted in Figs. 5 and 6. In vitro and in vivo studies have demonstrated that cytokines including TNF-α, IL-1β, IL-6, IL-8, IFN-β, IFN-γ and others are induced by C. jejuni, which not only leads to a pronounced inflammatory response (Phongsisay 2016), but also disturbs the intestinal epithelial barrier function in various ways (Bücker et al. 2018; for details see Chapter 8 in this book).
C. jejuni targets various pro-inflammatory and anti-inflammatory immune signaling pathways by interaction of bacterial factors with indicated receptor molecules. C. jejuni passes the mucus layer in the human gut and interacts with the intestinal epithelial cells, triggering nuclear responses such as cytokine expression including IL-6, IL-8, IL-12 or TNF. C. jejuni-induced cytokine secretion can be stimulated by CDT toxin, adhesin JlpA, LOS and functional flagella. The pro-inflammatory signal transduction proceeds via the activation of host cell receptors HSP-90α, toll-like receptors (TLR2, TLR4 and TLR5), intracellular signaling proteins and transcription factor NF-κB; while engagement of Siglec receptors by C. jejuni stimulate the production of anti-inflammatory cytokine IL-10
Signal transduction events leading to inflammasome activation by C. jejuni. a C. jejuni stimulates NLRP3 inflammasome formation, typically assembling in infected human dendritic cells, macrophages or monocytes, which finally leads to bacterial clearance. The canonical pathway of inflammasome activation comprises two signals coming from the bacterium, signal-1 and signal-2. Signal-1 leads to activation of transcription factor NF-ҡB and mRNA production of NLRP3 and the pro-forms of IL-1β and IL-18. Engagement of the NLRP3 protein through signal-2 and adaptor protein ASC induces the recruitment and cleavage of autoproteolytic pro-caspase-1. Activated caspase-1 then cleaves interleukin pro-forms leading to the production of mature IL-1β and IL-18 cytokines. It was described that energy taxis protein CetA and CheY-controlled host cell invasion by C. jejuni play an important role, but the actual bacterial factors representing signal-1 and signal-2, respectively, are yet unknown and labeled with question marks. b Autoproteolytic processing events in pro-caspase-1 leading to the activation of caspase-1 and maturation steps of IL-1β and IL-18 pro-forms through caspase-1 are shown. For more details, please see text
3.1 Interaction with Toll-Like Receptors
Toll-like receptors (TLRs) are key players in the activation of innate immunity. These transmembrane proteins are typically expressed on the surface of macrophages and dendritic cells but can also be found on intestinal epithelial cells. TLRs comprise specific patter-recognition receptors that recognize structurally conserved components of pathogenic microbes, including fungi, bacteria and viruses (Trinchieri and Sher 2007). For example, TLR2 recognizes peptidoglycan and lipoteichoic acid from Gram-positive bacteria (Schwandner et al. 1999), TLR4 recognizes LPS of Gram-negative bacteria (Poltorak et al. 1998), while TLR5 commonly recognizes bacterial flagellin (Andersen-Nissen et al. 2005). In vitro studies using mouse dendritic cells revealed that contact with C. jejuni activates their signal transduction protein MyD88 via the receptors TLR2 and TLR4 (Rathinam et al. 2009). Specifically, it is the contact of TLR4 with specific bacterial glycoconjugates that stimulates this signal cascade. At least five different C. jejuni glycoconjugates were found to trigger this response, three of which may be components of low-molecular-weight LOS. A fourth glycoconjugate appears as a ladder-like band with a molecular size between 30 and 50 kDa on SDS–polyacrylamide gels, while the fifth is a large protein of approximately 150 kDa (Phongsisay et al. 2015). However, the exact C. jejuni factor(s) that trigger TLR2 receptor activation remain to be identified. In addition, C. jejuni flagellin FlaA is a very poor stimulator of TLR5 (Watson and Galán 2005). Instead, cellular contact to the C. jejuni flagellar factor FlaC activates TLR5 (Faber et al. 2015). Upon stimulation, TRL2, TLR4 and TLR5 activate MyD88, which in turn mediates an inflammatory response via the MyD88-NF-κB signaling pathway that involves IRAK, TRAF6 and possibly other mediators, and results in the release of pro-inflammatory cytokines such as IL-6, IL-8 and IL-12. In addition to MyD88-NF-κB, TRL4 also induces TIR-domain-containing adapter-inducing interferon-β (TRIF)-mediated phosphorylation of Interleukin release factor 3 (IRF3), which stimulates IFN-β secretion from the challenged cells (Rathinam et al. 2009). All these responses are part of immune stimulation that eventually recruits immune cells. Taken together, recognition of C. jejuni biomolecules by a variety of TLRs activates pro-inflammatory responses (Fig. 5) and finally results in clearance of the pathogen.
3.2 Role of Siglec Receptors
Sialic acid-binding immunoglobulin-like lectins (Siglecs) are transmembrane proteins comprising 15 members in humans and that are expressed by various immune cell types. The N-terminus is exposed to the extracellular space and binds to ligands containing sialic acid components (von Gunten and Bochner 2008). Due to their specificity, several of the known Siglecs can bind to a variety of pathogens, including Group B Streptococcus and Trypanosoma cruzi, resulting in the production of interleukins (Crocker et al. 2007). Systematic analyses of the binding capacity of 10 different Siglecs revealed that C. jejuni LOS interacted with Siglec7 (Avril et al. 2006). As expected, this binding was only observed for sialylated, but not with unsialylated LOS components. In addition, sialylated LOS, particularly α2,3-sialylated LOS, was shown to interact with the soluble Siglec-1/Sn (Heikema et al. 2010), while Siglec7 preferably binds to α2,8-sialylated LOS (Bax et al. 2011). Furthermore, modified LOS structures of C. jejuni mimic human gangliosides, which can lead to auto-antibody production and neural disorders in some patients (Yuki et al. 2004). However, in addition to Siglec1/Sn and Siglec7, C. jejuni is also recognized by Siglec10, which was reported to bind both live C. jejuni bacteria and purified flagella, suggesting that activation of Siglec10 might be mediated by the flagella. In contrast to TLRs that activate a pro-inflammatory response, flagellin-Siglec10 contact increased the expression of IL-10 (Stephenson et al. 2014) and thus anti-inflammatory signaling (Fig. 5). Further studies are required to confirm these findings in in vivo models of infection.
3.3 Activation of the NLRP3 Inflammasome
Inflammasomes represent cytosolic multiprotein complexes of the host innate immune system. A wide variety of pathogens, including yeasts, bacteria and viruses, induce inflammasome-dependent production of IL-1β (Schroder and Tschopp 2010). Upregulation of pro-IL-1β transcription and subsequent secretion of IL-1β by mouse macrophages was also observed following infection with C. jejuni, which suggested processing of the pro-cytokine by the inflammasome (Bouwman et al. 2014). There are several inflammasome types, e.g., NLRP1, NLRP3 and NLRC4 (Schroder and Tschopp 2010), of which C. jejuni activates NLRP3 (Bouwman et al. 2014). The NLRP3 inflammasome is triggered by two bacterial signals (signal-1 and signal-2, respectively) and assembles with pro-caspase-1 and adaptor protein ASC, which stimulates maturation and formation of active caspase-1 (Fig. 6a). Active caspase-1 then conventionally processes pro-IL-1β and pro-IL-18 into the respective mature cytokines (Fig. 6b). An analysis of a series of C. jejuni mutants defective for a variety of known virulence-associated factors, including LOS, flagella and adhesins, attempted to identify C. jejuni components involved in triggering this inflammasome response (Bouwman et al. 2014). Only two of the tested mutants, resulted in decreased IL-1β production, namely the deletion of the chemotaxis protein CheY and of the energy taxis protein CetA. This suggests that there is a direct correlation between the number of intracellular C. jejuni bacteria (which is regulated by at least CheY and CetA) and NLRP3 inflammasome activation. Yet, all engineered deletion mutants still induced mature IL-1β secretion, albeit at slightly lower levels, suggesting that multiple components may activate this response. It is suggested that contact of macrophages with C. jejuni induces a transmembrane ion flux (e.g., K+-efflux), as seen for other pathogens (Muñoz-Planillo et al. 2013), but the exact signals that trigger the increased transcription of pro-IL-1β and possibly pro-IL-18, as well as the formation of the NLRP3 inflammasome, remain to be elucidated.
4 Concluding Remarks
C. jejuni is a fascinating microorganism which colonizes the chicken intestinal tract as a commensal but is associated with various diseases when infecting humans. Interestingly, by comparison to other enteric pathogens, C. jejuni comprises only a relatively small array of known virulence factors. While other pathogens like Salmonella, Yersinia or Listeria species exhibit a broad collection of “weapons” including various toxins, multiple secretion systems and effector molecules, C. jejuni has a relatively limited number of identified disease-associated factors, most notably CDT, LOS, HtrA, CadF and fT3SS. As reviewed here, some of these are involved in adhesion to and invasion into host epithelial cells, where the bacteria can survive and even spread into neighboring tissue. Although all details are still not fully clear, most data support an invasion mechanism that depends on the fibronectin-integrin-β1 receptor and the small Rho GTPases Rac1 and Cdc42, which appear to dominate C. jejuni host cell entry (Fig. 3). However, the process of cell invasion would involve molecular dissection of the mechanic forces activated by host cells, which then trigger bacterial engulfment, allow entry and result in membrane closing behind the invading C. jejuni. Future studies should examine how this works in detail. It is also important to investigate in more detail the mechanisms by which C. jejuni survives and spreads intracellularly, or how the bacteria cause infection of other organs in the human body that can include the spleen, mesenteric lymph nodes and the liver (Burnham and Hendrixson 2018; Backert et al. 2013). It also appears that C. jejuni survives inside the macrophages using its catalase enzyme KatA (Day et al. 2000), a phenomenon which deserves further investigation. Furthermore, for many of the proposed C. jejuni virulence proteins, a general knowledge of how they function mechanistically in vivo is still missing. Also, the actual advantage of some bacterial factors such as the CDT toxin for the bacterium is still unclear. It might be that the DNA damage triggered by its DNase activity not only initiates apoptosis but also modulates the host immune response, operating as an immunoregulatory factor, which helps the bacteria producing CDT to establish a suitable niche in its host. In this context, the process of apoptotic cell death induced by C. jejuni is more complicated than originally envisaged. Besides CDT described above (Pickett and Whitehouse 1999; Guerra et al. 2005), the serine protease HtrA (Heimesaat et al. 2014b) and the fT3SS substrate FspA2 (Poly et al. 2007) were also reported to stimulate apoptosis in epithelial cells, which can be counteracted by the C. jejuni-induced ubiquitin-editing enzyme A20 that negatively regulates apoptotic cell death (Lim et al. 2017). Thus, the interplay of apoptotic and anti-apoptotic signal pathways modulated by C. jejuni are still not fully understood, deserving further investigation. Lastly, it must be mentioned that there are controversies and contradictions in various reports describing the functional mechanistics and importance of particular C. jejuni virulence factors, such as the Cia proteins or some proposed adhesins. Strain-dependent differences, variation in experimental procedures, and a disconnect between in vitro work, in vivo (mouse) models, and the human situation (where individual immunological variation may be of crucial importance to clinical outcome) further murken the waters. These important issues also need to be addressed and clarified in future studies. Thus, it occurs that C. jejuni will continue to be an attractive and gratifying research subject with high importance to public health.
References
Andersen-Nissen E, Smith KD, Strobe KL, Barrett SL, Cookson BT, Logan SM, Aderem A (2005) Evasion of Toll-like receptor 5 by flagellated bacteria. Proc Natl Acad Sci USA 102:9247–9252. https://doi.org/10.1073/pnas.0502040102
Artymovich K, Kim JS, Linz JE, Hall DF, Kelley LE, Kalbach HL, Kathariou S, Gaymer J, Paschke B (2013) A “successful allele” at Campylobacter jejuni contingency locus Cj0170 regulates motility; “successful alleles” at locus Cj0045 are strongly associated with mouse colonization. Food Microbiol 34(2):425–430. https://doi.org/10.1016/j.fm.2013.01.007
Avril T, Wagner ER, Willison HJ, Crocker PR (2006) Sialic acid-binding immunoglobulin-like lectin 7 mediates selective recognition of sialylated glycans expressed on Campylobacter jejuni lipooligosaccharides. Infect Immun 74:4133–4141. https://doi.org/10.1128/IAI.02094-05
Backert S, Bernegger S, Skorko-Glonek J, Wessler S (2018) Extracellular HtrA serine proteases: an emerging new strategy in bacterial pathogenesis. Cell Microbiol 20:e12845. https://doi.org/10.1111/cmi.12845
Backert S, Boehm M, Wessler S, Tegtmeyer N (2013) Transmigration route of Campylobacter jejuni across polarized intestinal epithelial cells: paracellular, transcellular or both? Cell Commun Signal 11:72. https://doi.org/10.1186/1478-811X-11-72
Bæk KT, Vegge CS, Brøndsted L (2011) HtrA chaperone activity contributes to host cell binding in Campylobacter jejuni. Gut Pathog 3:13. https://doi.org/10.1186/1757-4749-3-13.10.1186/1757-4749-3-13
Bæk KT, Vegge CS, Skorko-Glonek J, Brøndsted L (2011) Different contributions of HtrA protease and chaperone activities to Campylobacter jejuni stress tolerance and physiology. Appl Environ Microbiol 77:57–66. https://doi.org/10.1128/aem.01603-10
Barrero-Tobon AM, Hendrixson DR (2012) Identification and analysis of flagellar coexpressed determinants (Feds) of Campylobacter jejuni involved in colonization. Mol Microbiol 84:352–369. https://doi.org/10.1111/j.1365-2958.2012.08027.x
Bax M, Kuijf ML, Heikema AP, van Rijs W, Bruijns SC, Garcia-Vallejo JJ, Crocker PR, Jacobs BC, van Vliet SJ, van Kooyk Y (2011) Campylobacter jejuni lipooligosaccharides modulate dendritic cell-mediated T cell polarization in a sialic acid linkage-dependent manner. Infect Immun 79:2681–2689. https://doi.org/10.1128/IAI.00009-11
Beeby M, Ribardo DA, Brennan CA, Ruby EG, Jensen GJ, Hendrixson DR (2016) Diverse high-torque bacterial flagellar motors assemble wider stator rings using a conserved protein scaffold. Proc Natl Acad Sci USA 113:E1917-1926. https://doi.org/10.1073/pnas.1518952113
Biswas D, Itoh K, Sasakawa C (2000) Uptake pathways of clinical and healthy animal isolates of Campylobacter jejuni into INT-407 cells. FEMS Immunol Med Microbiol 29(3):203–211. https://doi.org/10.1111/j.1574-695X.2000.tb01524.x
Black RE, Levine MM, Clements ML, Hughes TP, Blaser MJ (1988) Experimental Campylobacter jejuni infection in humans. J Infect Dis 157(3):472–479. https://doi.org/10.1093/infdis/157.3.472
Boehm M, Haenel I, Hoy B, Brøndsted L, Smith TG, Hoover T, Wessler S, Tegtmeyer N (2013) Extracellular secretion of protease HtrA from Campylobacter jejuni is highly efficient and independent of its protease activity and flagellum. Eur J Microbiol Immunol (Bp) 3:163–173. https://doi.org/10.1556/EuJMI.3.2013.3.3
Boehm M, Hoy B, Rohde M, Tegtmeyer N, Bæk KT, Oyarzabal OA, Brøndsted L, Wessler S, Backert S (2012) Rapid paracellular transmigration of Campylobacter jejuni across polarized epithelial cells without affecting TER: role of proteolytic-active HtrA cleaving E-cadherin but not fibronectin. Gut Pathog 4:3. https://doi.org/10.1186/1757-4749-4-3
Boehm M, Krause-Gruszczynska M, Rohde M, Tegtmeyer N, Takahashi S, Oyarzabal OA, Backert S (2011) Major host factors involved in epithelial cell invasion of Campylobacter jejuni: role of fibronectin, integrin beta1, FAK, Tiam-1, DOCK180 in activating Rho GTPase Rac1. Front. In Cell Infect. Microbiol 1:17. https://doi.org/10.3389/fcimb.2011.00017
Boehm M, Lind J, Backert S, Tegtmeyer N (2015) Campylobacter jejuni serine protease HtrA plays an important role in heat tolerance, oxygen resistance, host cell adhesion, invasion, and transmigration. Eur J Microbiol Immunol (Bp) 5:68–80. https://doi.org/10.1556/eujmi-d-15-00003
Boehm M, Simson D, Escher U, Schmidt AM, Bereswill S, Tegtmeyer N, Backert S, Heimesaat MM (2018) Function of serine protease HtrA in the lifecycle of the foodborne pathogen Campylobacter jejuni. Eur J Microbiol Immunol (Bp) 8:70–77. https://doi.org/10.1556/1886.2018.00011
Bouwman LI, de Zoete MR, Bleumink-Pluym NM, Flavell RA, van Putten JP (2014) Inflammasome activation by Campylobacter jejuni. J Immunol 193:4548–4557. https://doi.org/10.4049/jimmunol.1400648
Bouwman LI, Niewold P, van Putten JP (2013) Basolateral invasion and trafficking of Campylobacter jejuni in polarized epithelial cells. PLoS ONE 8(1):e54759. https://doi.org/10.1371/journal.pone.0054759
Brøndsted L, Andersen MT, Parker M, Jorgensen K, Ingmer H (2005) The HtrA protease of Campylobacter jejuni is required for heat and oxygen tolerance and for optimal interaction with human epithelial cells. Appl Environ Microbiol 71:3205–3212. https://doi.org/10.1128/aem.71.6.3205-3212.2005
Bronowski C, James CE, Winstanley C, Bronowski C (2014) Role of environmental survival in transmission of Campylobacter jejuni. FEMS Microbiol Lett 356(1):8–19. https://doi.org/10.1111/1574-6968.12488
Bücker R, Krug SM, Moos V, Bojarski C, Schweiger MR, Kerick M, Fromm A, Janßen S, Fromm M, Hering NA, Siegmund B, Schneider T, Barmeyer C, Schulzke JD (2018) Campylobacter jejuni impairs sodium transport and epithelial barrier function via cytokine release in human colon. Mucosal Immunol 11(2):474–485. https://doi.org/10.1038/mi.2017.66
Buelow DR, Christentsen JE, Neal-McKinney JM, Konkel ME (2011) Campylobacter jejuni survival within human epithelial cells is enhanced by the secreted protein CiaI. Mol Microbiol 80:1296–1312. https://doi.org/10.1111/j.1365-2958.2011.07645.x
Burnham PM, Hendrixson DR (2018) Campylobacter jejuni: collective components promoting a successful enteric lifestyle. Nat Rev Microbiol 16(9):551–565. https://doi.org/10.1038/s41579-018-0037-9
Campagnolo ER, Philipp LM, Long JM, Hanshaw NL (2018) Pet-associated Campylobacteriosis: a persisting public health concern. Zoonoses Public Health 65(3):304–311. https://doi.org/10.1111/zph.12389
Chang C, Miller JF (2006) Campylobacter jejuni colonization of mice with limited enteric flora. Infect Immun 74:5261–5271. https://doi.org/10.1128/iai.01094-05
Chen S, Beeby M, Murphy GE, Leadbetter JR, Hendrixson DR, Briegel A, Li Z, Shi J, Tocheva EI, Muller A, Dobro MJ, Jensen GJ (2011) Structural diversity of bacterial flagellar motors. Embo J 30:2972–2981. https://doi.org/10.1038/emboj.2011.186
Christensen JE, Pacheco SA, Konkel ME (2009) Identification of a Campylobacter jejuni—secreted protein required for maximal invasion of host cells. Mol Microbiol 73:650–662. https://doi.org/10.1111/j.1365-2958.2009.06797.x
Clausen T, Southan C, Ehrmann M (2002) The HtrA family of proteases: implications for protein composition and cell fate. Mol Cell 10:443–455. https://doi.org/10.1016/s1097-2765(02)00658-5
Cohen EJ, Nakane D, Kabata Y, Hendrixson DR, Nishizaka T, Beeby M (2020) Campylobacter jejuni motility integrates specialized cell shape, flagellar filament, and motor, to coordinate action of its opposed flagella. PLoS Pathog 2;16(7):e1008620. https://doi.org/10.1371/journal.ppat.1008620
Crocker PR, Paulson JC, Varki A (2007) Siglecs and their roles in the immune system. Nat Rev Immunol 7:255–266. https://doi.org/10.1038/nri2056
Cunningham AA, Daszak P, Wood JLN (2017) One Health, emerging infectious diseases and wildlife: two decades of progress? Philos Trans R Soc Lond B Biol Sci 372(1725):20160167. https://doi.org/10.1098/rstb.2016.0167
Dai L, Wu ZW, Xu CY, Sahin O, Yaeger M, Plummer PJ, Zhang QJ (2019) The rho-independent transcription terminator for the porA gene enhances expression of the major outer membrane protein and Campylobacter jejuni virulence in abortion induction. Infect Immun 87(12):e00687-e719. https://doi.org/10.1128/IAI.00687-19
Day WA Jr, Sajecki JL, Pitts TM, Joens LA (2000) Role of catalase in Campylobacter jejuni intracellular survival. Infect Immun 68:6337–6345. https://doi.org/10.1128/iai.68.11.6337-6345.2000
DiRienzo JM (2014) Uptake and processing of the cytolethal distending toxin by mammalian cells. Toxins (Basel) 6:3098–3116. https://doi.org/10.3390/toxins6113098
Dwivedi R, Nothaft H, Garber J, Xin Kin L, Stahl M, Flint A, van Vliet AH, Stintzi A, Szymanski CM (2016) L-fucose influences chemotaxis and biofilm formation in Campylobacter jejuni. Mol Microbiol 101:575–589. https://doi.org/10.1111/mmi.13409
Elharrif Z, Mégraud F (1986) Characterization of thermophilic Campylobacter: carbon-substrate utilization tests. Curr Microbiol 13:117–122
Elmi A, Nasher F, Jagatia H, Gundogdu O, Bajaj-Elliott M, Wren B, Dorrell N (2016) Campylobacter jejuni outer membrane vesicle-associated proteolytic activity promotes bacterial invasion by mediating cleavage of intestinal epithelial cell E-cadherin and occludin. Cell Microbiol 18:561–572. https://doi.org/10.1111/cmi.12534
Elmi A, Watson E, Sandu P, Gundogdu O, Mills DC, Inglis NF, Manson E, Imrie L, Bajaj-Elliott M, Wren BW, Smith DG, Dorrell N (2012) Campylobacter jejuni outer membrane vesicles play an important role in bacterial interactions with human intestinal epithelial cells. Infect Immun 80:4089–4098. https://doi.org/10.1128/IAI.00161-12
Epps SV, Harvey RB, Hume ME, Phillips TD, Anderson RC, Nisbet DJ (2013) Foodborne Campylobacter: infections, metabolism, pathogenesis and reservoirs. Int J Environ Res Public Health 10(12):6292–6304. https://doi.org/10.3390/ijerph10126292
Eucker TP, Konkel ME (2012) The cooperative action of bacterial fibronectin-binding proteins and secreted proteins promote maximal Campylobacter jejuni invasion of host cells by stimulating membrane ruffling. Cell Microbiol 14:226–238. https://doi.org/10.1111/j.1462-5822.2011.01714.x
Faber E, Gripp E, Maurischat S, Kaspers B, Tedin K, Menz S, Zuraw A, Kershaw O, Yang I, Rautenschlein S, Josenhans C (2015) Novel Immunomodulatory Flagellin-Like Protein FlaC in Campylobacter jejuni and Other Campylobacterales. mSphere 1(1):e00028-15. https://doi.org/10.1128/mSphere.00028-15
Flanagan RC, Neal-McKinney JM, Dhillon AS, Miller WG, Konkel ME (2009) Examination of Campylobacter jejuni putative adhesins leads to the identification of a new protein, designated FlpA, required for chicken colonization. Infect Immunol 77:2399–23407. https://doi.org/10.1128/IAI.01266-08
Floch P, Pey V, Castroviejo M, Dupuy JW, Bonneu M, de la Guardia AH, Pitard V, Mégraud F, Lehours P (2014) Role of Campylobacter Jejuni gamma-glutamyl transpeptidase on epithelial cell apoptosis and lymphocyte proliferation. Gut Pathog 6:20. https://doi.org/10.1186/1757-4749-6-20
Fox JG, Rogers AB, Whary MT, Ge Z, Taylor NS, Xu S, Horwitz BH, Erdman SE (2004) Gastroenteritis in NF-kappaB-deficient mice is produced with wild-type Camplyobacter jejuni but not with C. jejuni lacking cytolethal distending toxin despite persistent colonization with both strains. Infect Immun 72:1116–1125. https://doi.org/10.1128/iai.72.2.1116-1125.2004
Friedman CR, Neimann J, Wegener HC, Tauxe RV (2000) Epidemiology of Campylobacter jejuni infections in the United States and other industrialized nations. In: Nachamkin I, Blaser MJ (eds) Campylobacter. ASM Press, Washington, DC, pp 121–138
Gao B, Vorwerk H, Huber C, Lara-Tejero M, Mohr J, Goodman AL, Eisenreich W, Galán JE, Hofreuter D (2017) Metabolic and fitness determinants for in vitro growth and intestinal colonization of the bacterial pathogen Campylobacter jejuni. PLoS Biol 15(5):e2001390. https://doi.org/10.1371/journal.pbio.2001390
Gaynor EC, Wells DH, MacKichan JK, Falkow S (2005) The Campylobacter jejuni stringent response controls specific stress survival and virulence-associated phenotypes. Mol Microbiol 56:8–27. https://doi.org/10.1111/j.1365-2958.2005.04525.x
Gölz G, Rosner B, Hofreuter D, Josenhans C, Kreienbrock L, Lowenstein A, Schielke A, Stark K, Suerbaum S, Wieler LH, Alter T (2014) Relevance of Campylobacter to public health—the need for a one health approach. Int J Med Microbiol 304:817–823. https://doi.org/10.1016/j.ijmm.2014.08.015
Gölz G, Sharbati S, Backert S, Alter T (2012) Quorum sensing dependent phenotypes and their molecular mechanisms in Campylobacterales. Eur J Microbiol Immunol (Bp) 2(1):50–60. https://doi.org/10.1556/EuJMI.2.2012.1.8
Goodman KN, Powers MJ, Crofts AA, Trent MS, Hendrixson DR (2020) Campylobacter jejuni BumSR directs a response to butyrate via sensor phosphatase activity to impact transcription and colonization. Proc Natl Acad Sci USA 117(21):11715–11726. https://doi.org/10.1073/pnas.1922719117
Goon S, Kelly JF, Logan SM, Ewing CP, Guerry P (2003) Pseudaminic acid, the major modification on Campylobacter flagellin, is synthesized via the Cj1293 gene. Mol Microbiol 50:659–671. https://doi.org/10.1046/j.1365-2958.2003.03725.x
Gorain C, Singh A, Bhattacharyya S, Kundu A, Lahiri A, Gupta S, Mallick AI (2020) Mucosal delivery of live Lactococcus lactis expressing functionally active JlpA antigen induces potent local immune response and prevent enteric colonization of Campylobacter jejuni in chickens. Vaccine 38:1630–1642. https://doi.org/10.1016/j.vaccine.2019.12.064
Guccione E, del Rocio L-K, Pearson BM, Hitchin E, Mulholland F, van Diemen PM, Stevens MP, Kelly DJ (2008) Amino acid-dependent growth of Campylobacter jejuni: key roles for aspartase (AspA) under microaerobic and oxygen-limited conditions and identification of AspB (Cj0762), essential for growth on glutamate. Mol Microbiol. 69(1):77–93. https://doi.org/10.1111/j.1365-2958.2008.06263.x.10.1016/j.vaccine.2019.12.064
Guerra L, Teter K, Lilley BN, Stenerlow B, Holmes RK, Ploegh HL, Sandvig K, Thelestam M, Frisan T (2005) Cellular internalization of cytolethal distending toxin: a new end to a known pathway. Cell Microbiol 7:921–934. https://doi.org/10.1111/j.1462-5822.2005.00520.x
Guerra L, Cortes-Bratti X, Guidi R, Frisan T (2011) The biology of the cytolethal distending toxins. Toxins (Basel) 3(3):172–190. https://doi.org/10.3390/toxins3030172
Guerry P (2007) Campylobacter flagella: not just for motility. Trends Microbiol 15(10):456–461. https://doi.org/10.1016/j.tim.2007.09.006
Guerry P, Alm RA, Power ME, Logan SM, Trust TJ (1991) Role of two flagellin genes in Campylobacter motility. J Bacteriol 173(15):4757–4764. https://doi.org/10.1128/jb.173.15.4757-4764.1991
Guerry P, Ewing CP, Schirm M, Lorenzo M, Kelly J, Pattarini D, Majam G, Thibault P, Logan S (2006) Changes in flagellin glycosylation affect Campylobacter autoagglutination and virulence. Mol Microbiol 60:299–311. https://doi.org/10.1111/j.1365-2958.2006.05100.x
Harrer A, Bucker R, Boehm M, Zarzecka U, Tegtmeyer N, Sticht H, Schulzke JD, Backert S (2019) Campylobacter jejuni enters gut epithelial cells and impairs intestinal barrier function through cleavage of occludin by serine protease HtrA. Gut Pathog 11:4. https://doi.org/10.1186/s13099-019-0283-z
Hartley-Tassell LE, Shewell LK, Day CJ, Wilson JC, Sandhu R, Ketley JM, Korolik V (2010) Identification and characterization of the aspartate chemosensory receptor of Campylobacter jejuni. Mol Microbiol 75:710–730. https://doi.org/10.1111/j.1365-2958.2009.07010.x
Hauske P, Meltzer M, Ottmann C, Krojer T, Clausen T, Ehrmann M, Kaiser M (2009) Selectivity profiling of DegP substrates and inhibitors. Bioorg Med Chem 17:2920–2924. https://doi.org/10.1016/j.bmc.2009.01.073
Heikema AP, Bergman MP, Richards H, Crocker PR, Gilbert M, Samsom JN, van Wamel WJB, Endtz HP, van Belkum A (2010) Characterization of the specific interaction between sialoadhesin and sialylated Campylobacter jejuni lipooligosaccharides. Infect Immun 78:3237–3246. https://doi.org/10.1128/IAI.01273-09
Heimesaat MM, Alutis M, Grundmann U, Fischer A, Tegtmeyer N, Böhm M, Kuhl AA, Göbel UB, Backert S, Bereswill S (2014) The role of serine protease HtrA in acute ulcerative enterocolitis and extra-intestinal immune responses during Campylobacter jejuni infection of gnotobiotic IL-10 deficient mice. Front Cell Infect Microbiol 4:77. https://doi.org/10.3389/fcimb.2014.00077
Heimesaat MM, Fischer A, Alutis M, Grundmann U, Boehm M, Tegtmeyer N, Gobel UB, Kuhl AA, Bereswill S, Backert S (2014) The impact of serine protease HtrA in apoptosis, intestinal immune responses and extra-intestinal histopathology during Campylobacter jejuni infection of infant mice. Gut Pathog 6:16. https://doi.org/10.1186/1757-4749-6-16
Henderson LD, Matthews-Palmer TRS, Gulbronson CJ, Ribardo DA, Beeby M, Hendrixson DR. (2020) Diversification of Campylobacter jejuni Flagellar C-Ring composition impacts its structure and function in motility, flagellar assembly, and cellular processes. mBio 11(1):e02286-19. https://doi.org/10.1128/mBio.02286-19
Hendrixson DR, DiRita VJ (2003) Transcription of sigma54-dependent but not sigma28-dependent flagellar genes in Campylobacter jejuni is associated with formation of the flagellar secretory apparatus. Mol Microbiol 50(2):687–702. https://doi.org/10.1046/j.1365-2958.2003.03731.x
Hendrixson DR, DiRita VJ (2004) Identification of Campylobacter jejuni genes involved in commensal colonization of the chick gastrointestinal tract. Mol Microbiol 52:471–484. https://doi.org/10.1111/j.1365-2958.2004.03988.x
Hendrixson DR (2006) A phase-variable mechanism controlling the Campylobacter jejuni FlgR response regulator influences commensalism. Mol Microbiol 61:1646–1659. https://doi.org/10.1111/j.1365-2958.2006.05336.x
Hendrixson DR (2008) Restoration of flagellar biosynthesis by varied mutational events in Campylobacter jejuni. Mol Microbiol 70:519–536. https://doi.org/10.1111/j.1365-2958.2008.06428.x
Herman L, Heyndrickx M, Grijspeerdt K, Vandekerchove D, Rollier I, De Zutter L (2003) Routes for Campylobacter contamination of poultry meat: epidemiological study from hatchery to slaughter. Epidemiol Infect 131:1169–1180. https://doi.org/10.1017/s0950268803001183
Hermans D, Van Deun K, Martel A, Van Immerseel F, Messens W, Heyndrickx M, Haesebrouck F, Pasmans F (2011) Colonization factors of Campylobacter jejuni in the chicken gut. Vet Res 42:14. https://doi.org/10.1186/1297-9716-42-82
Heywood W, Henderson B, Nair SP (2005) Cytolethal distending toxin: creating a gap in the cell cycle. J Med Microbiol 54:207–216. https://doi.org/10.1099/jmm.0.45694-0
Hickey TE, McVeigh AL, Scott DA, Michielutti RE, Bixby A, Carroll SA, Bourgeois AL, Guerry P (2000) Campylobacter jejuni cytolethal distending toxin mediates release of interleukin-8 from intestinal epithelial cells. Infect Immun 68:6535–6541. https://doi.org/10.1128/iai.68.12.6535-6541.2000
Hofreuter D, Novik V, Galán JE (2008) Metabolic diversity in Campylobacter jejuni enhances specific tissue colonization. Cell Host Microbe 4:425–433. https://doi.org/10.1016/j.chom.2008.10.002
Hofreuter D (2014) Defining the metabolic requirements for the growth and colonization capacity of Campylobacter jejuni. Front Cell Infect Microbiol 4:137. https://doi.org/10.3389/fcimb.2014.00137
Howard SL, Jagannathan A, Soo EC, Hui JP, Aubry AJ, Ahmed I, Karlyshev A, Kelly JF, Jones MA, Stevens MP, Logan SM, Wren BW (2009) Campylobacter jejuni glycosylation island important in cell charge, legionaminic acid biosynthesis, and colonization of chickens. Infect Immun 77(6):2544–2556. https://doi.org/10.1128/IAI.01425-08
Hu L, Raybourne RB, Kopecko DJ (2005) Ca2+ release from host intracellular stores and related signal transduction during Campylobacter jejuni 81–176 internalization into human intestinal cells. Microbiology (Reading) 151(Pt9):3097–3105. https://doi.org/10.1099/mic.0.27866-0
Hu L, McDaniel JP, Kopecko DJ (2006) Signal transduction events involved in human epithelial cell invasion by Campylobacter jejuni 81–176. Microb Pathog. 40(3):91–100. https://doi.org/10.1016/j.micpath.2005.11.004
Jin S, Joe A, Lynett J, Hani EK, Sherman P, Chan VL (2001) JlpA, a novel surface-exposed lipoprotein specific to Campylobacter jejuni, mediates adherence to host epithelial cells. Mol Microbiol 39:1225–1236. https://doi.org/10.1111/j.1365-2958.2001.02294.x
Jin S, Song YC, Emili A, Sherman PM, Chan VL (2003) JlpA of Campylobacter jejuni interacts with surface-exposed heat shock protein 90 and triggers signalling pathways leading to the activation of NF-kabbaB and p38 MAP kinase in epithelial cells. Cell Microbiol 5:165–174. https://doi.org/10.1046/j.1462-5822.2003.00265.x
Johnson WM, Lior H (1988) A new heat-labile cytolethal distending toxin (CLDT) produced by Campylobacter spp. Microb Pathog 4:115–126. https://doi.org/10.1016/0882-4010(88)90053-8
Jorgensen F, Ellis-Iversen J, Rushton S, Bull SA, Harris SA, Bryan SJ, Gonzalez A, Humphrey TJ (2011) Influence of season and geography on Campylobacter jejuni and C. coli subtypes in housed broiler flocks reared in Great Britain. Appl Environ Microbiol 77:3741–3748. https://doi.org/10.1128/AEM.02444-10
Kaakoush NO, Castaño-Rodríguez N, Mitchell HM, Man SM (2015) Global epidemiology of Campylobacter infection. Clin Microbiol Rev 28(3):687–720. https://doi.org/10.1128/CMR.00006-15
Karim QN, Logan RP, Puels J, Karnholz A, Worku ML (1998) Measurement of motility of Helicobacter pylori, Campylobacter jejuni, and Escherichia coli by real time computer tracking using the Hobson BacTracker. J Clin Pathol 51:623–628. https://doi.org/10.1136/jcp.51.8.623
Kim DY, Kim KK (2005) Structure and function of HtrA family proteins, the key players in protein quality control. J Biochem Mol Biol 38:266–274. https://doi.org/10.5483/bmbrep.2005.38.3.266
Konkel ME, Garvis SG, Tipton SL, Anderson DE Jr, Cieplak W Jr (1997) Identification and molecular cloning of a gene encoding a fibronectin-binding protein (CadF) from Campylobacter jejuni. Mol. Microbiol 24:953–963. https://doi.org/10.1046/j.1365-2958.1997.4031771.x
Konkel ME, Kim BJ, Rivera-Amill V, Garvis SG (1999) Bacterial secreted proteins are required for the internalization of Campylobacter jejuni into cultured mammalian cells. Mol Microbiol 32:691–701. https://doi.org/10.1046/j.1365-2958.1999.01376.x
Konkel ME, Monteville MR, Rivera-Amill V, Joens LA (2001) The pathogenesis of Campylobacter jejuni-mediated enteritis. Curr Issues Intest Microbiol 2:55–71
Konkel ME, Larson CL, Flanagan RC (2010) Campylobacter jejuni FlpA binds fibronectin and is required for maximal host cell adherence. J Bacteriol 192:68–76. https://doi.org/10.1128/JB.00969-09
Korolik V (2019) The role of chemotaxis during Campylobacter jejuni colonisation and pathogenesis. Curr Opin Microbiol 47:32–37. https://doi.org/10.1016/j.mib.2018.11.001
Krause-Gruszczynska M, Rohde M, Hartig R, Genth H, Schmidt G, Keo T, Koenig W, Miller WG, Konkel ME, Backert S (2007) Role of the small Rho GTPases Rac1 and Cdc42 in host cell invasion of Campylobacter jejuni. Cell Microbiol 9:2431–2444. https://doi.org/10.1111/j.1462-5822.2007.00971.x
Krause-Gruszczynska M, van Alphen LB, Oyarzabal OA, Alter T, Hänel I, Schliephake A, König W, van Putten JP, Konkel ME, Backert S (2007) Expression patterns and role of the CadF protein in Campylobacter jejuni and Campylobacter coli. FEMS Microbiol Lett 274(1):9–16. https://doi.org/10.1111/j.1574-6968.2007.00802.x
Krause-Gruszczynska M, Boehm M, Rohde M, Tegtmeyer N, Takahashi S, Buday L, Oyarzabal OA, Backert S (2011) The signaling pathway of Campylobacter jejuni-induced Cdc42 activation: role of fibronectin, integrin beta1, tyrosine kinases and guanine exchange factor Vav2. Cell Commun Signal 9:32. https://doi.org/10.1186/1478-811X-9-32
Kreutzberger MAB, Ewing C, Poly F, Wang F, Egelman EH (2020) Atomic structure of the Campylobacter jejuni flagellar filament reveals how ε Proteobacteria escaped Toll-like receptor 5 surveillance. Proc Natl Acad Sci U S a 117(29):16985–16991. https://doi.org/10.1073/pnas.2010996117
Krojer T, Sawa J, Huber R, Clausen T (2010) HtrA proteases have a conserved activation mechanism that can be triggered by distinct molecular cues. Nat Struct Mol Biol 17:844–852. https://doi.org/10.1038/nsmb.1840
Lai CK, Chen YA, Lin CJ, Lin HJ, Kao MC, Huang MZ, Lin YH, Chiang-Ni C, Chen CJ, Lo UG, Lin LC, Lin H, Hsieh JT, Lai CH (2016) Molecular Mechanisms and potential clinical applications of Campylobacter jejuni cytolethal distending toxin. Front Cell Infect Microbiol 6:9. https://doi.org/10.3389/fcimb.2016.00009
Lara-Tejero M, Galán JE (2001) CdtA, CdtB, and CdtC form a tripartite complex that is required for cytolethal distending toxin activity. Infect Immun 69:4358–4365. https://doi.org/10.1128/iai.69.7.4358-4365.2001
Larson CL, Samuelson DR, Eucker TP, O’Loughlin JL, Konkel ME (2013) The fibronectin-binding motif within FlpA facilitates Campylobacter jejuni adherence to host cell and activation of host cell signaling. Emerg Microbes Infect 2(10):e65. https://doi.org/10.1038/emi.2013.65
Leach S, Harvey P, Wali R (1997) Changes with growth rate in the membrane lipid composition of and amino acid utilization by continuous cultures of Campylobacter jejuni. J Appl Microbiol 82:631–640. https://doi.org/10.1111/j.1365-2672.1997.tb02873.x
Lee MD, Newell DG (2006) Campylobacter in poultry: filling an ecological niche. Avian Dis 50:1–9. https://doi.org/10.1637/7474-111605R.1
Leon-Kempis MR, Guccione E, Mulholland F, Williamson MP, Kelly DJ (2006) The Campylobacter jejuni PEB1a adhesin is an aspartate/glutamate-binding protein of an ABC transporter essential for microaerobic growth on dicarboxylic amino acids. Mol Microbiol 60:1262–1275. https://doi.org/10.1111/j.1365-2958.2006.05168.x
Lim MCC, Maubach G, Sokolova O, Feige MH, Diezko R, Buchbinder J, Backert S, Schlüter D, Lavrik IN, Naumann M (2017) Pathogen-induced ubiquitin-editing enzyme A20 bifunctionally shuts off NF-κB and caspase-8-dependent apoptotic cell death. Cell Death Differ 24(9):1621–1631. https://doi.org/10.1038/cdd.2017.89
Lindmark B, Rompikuntal PK, Vaitkevicius K, Song T, Mizunoe Y, Uhlin BE, Guerry P, Wai SN (2009) Outer membrane vesicle-mediated release of cytolethal distending toxin (CDT) from Campylobacter jejuni. BMC Microbiol 9:220. https://doi.org/10.1186/1471-2180-9-220
Lipinska B, Fayet O, Baird L, Georgopoulos C (1989) Identification, characterization, and mapping of the Escherichia coli htrA gene, whose product is essential for bacterial growth only at elevated temperatures. J Bacteriol 171:1574–1584. https://doi.org/10.1128/jb.171.3.1574-1584.1989
Logue CM, Barbieri NL, Nielsen DW (2017) Pathogens of food animals: sources, characteristics, human risk, and methods of detection. Adv Food Nutr Res 82:277–365. https://doi.org/10.1016/bs.afnr.2016.12.009
Louwen R, Heikema A, van Belkum A, Ott A, Gilbert M, Ang W, Endtz HP, Bergman MP, Nieuwenhuis EE (2008) The sialylated lipooligosaccharide outer core in Campylobacter jejuni is an important determinant for epithelial cell invasion. Infect Immunol 76:4431–4438. https://doi.org/10.1128/IAI.00321-08
Louwen R, Nieuwenhuis EE, van Marrewijk L, Horst-Kreft D, de Ruiter L, Heikema AP, van Wamel WJ, Wagenaar JA, Endtz HP, Samsom J, van Baarlen P, Akhmanova A, van Belkum A (2012) Campylobacter jejuni translocation across intestinal epithelial cells is facilitated by ganglioside-like lipooligosaccharide structures. Infect Immun 80(9):3307–3318. https://doi.org/10.1128/IAI.06270-11
Luethy PM, Huynh S, Parker CT, Hendrixson DR (2015) Analysis of the activity and regulon of the two-component regulatory system composed by Cjj81176_1484 and Cjj81176_1483 of Campylobacter jejuni. J Bacteriol 197(9):1592–1605. https://doi.org/10.1128/JB.02564-14
Marchant J, Wren B, Ketley J (2002) Exploiting genome sequence: predictions for mechanisms of Campylobacter chemotaxis. Trends Microbiol 10(4):155–159. https://doi.org/10.1016/s0966-842x(02)02323-5
Masanta WO, Heimesaat MM, Bereswill S, Tareen AM, Lugert R, Groß U, Zautner AE (2013) Modification of intestinal microbiota and its consequences for innate immune response in the pathogenesis of campylobacteriosis. Clin Dev Immunol 2013:526860. https://doi.org/10.1155/2013/526860
Méndez-Olvera ET, Bustos-Martinez JA, Lopez-Vidal Y, Verdugo-Rodriguez A, Martinez-Gomez D (2016) Cytolethal distending toxin from Campylobacter jejuni requires the cytoskeleton for toxic activity. Jundishapur J Microbiol 9:e35591. https://doi.org/10.5812/jjm.35591
Monteville MR, Konkel ME (2002) Fibronectin-facilitated invasion of T84-eukaryotic cells by Campylobacter jejuni occurs preferentially at the basolateral cell surface. Infect Immun 70:6665–6671. https://doi.org/10.1128/iai.70.12.6665-6671.2002
Monteville MR, Yoon JE, Konkel ME (2003) Maximal adherence and invasion of INT 407 cells by Campylobacter jejuni requires the CadF outer-membrane protein and microfilament reorganization. Microbiology 149:153–165. https://doi.org/10.1099/mic.0.25820-0
Mortensen NP, Schiellerup P, Boisen N, Klein BM, Locht H, Abuoun M, Newell D, Krogfelt KA (2011) The role of Campylobacter jejuni cytolethal distending toxin in gastroenteritis: toxin detection, antibody production, and clinical outcome. Apmis 119:626–634. https://doi.org/10.1111/j.1600-0463.2011.02781.x
Mourkas E, Taylor AJ, Méric G, Bayliss SC, Pascoe B, Mageiros L, Calland JK, Hitchings MD, Ridley A, Vidal A, Forbes KJ, Strachan NJC, Parker CT, Parkhill J, Jolley KA, Cody AJ, Maiden MCJ, Kelly DJ, Sheppard SK (2020) Agricultural intensification and the evolution of host specialism in the enteric pathogen Campylobacter jejuni. Proc Natl Acad Sci USA 117(20):11018–11028. https://doi.org/10.1073/pnas.1917168117
Muñoz-Planillo R, Kuffa P, Martínez-Colón G, Smith BL, Rajendiran TM, Núñez G (2013) K(+) efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity 38:1142–1153. https://doi.org/10.1016/j.immuni.2013.05.016
Nachamkin I, Szymanski CM, Blaser MJ (2008) Campylobacter, 3rd edn. ASM Press, Washington
Naikare H, Palyada K, Panciera R, Marlow D, Stintzi A (2006) Major Role for FeoB in Campylobacter Jejuni ferrous iron acquisition, gut colonization, and intracellular survival. Infect Immun 74(10):5433–5444. https://doi.org/10.1128/IAI.00052-06
Neddermann M, Backert S (2019) Quantification of serine protease HtrA molecules secreted by the foodborne pathogen Campylobacter jejuni. Gut Pathog 11:14. https://doi.org/10.1186/s13099-019-0295-8
Neimark J (2015) Line of attack. Science 347(6225):938–940. https://doi.org/10.1126/science.347.6225.938
Nelson-Rees WA, Flandermeyer RR (1976) HeLa cultures defined. Science 191(4222):96–98. https://doi.org/10.1126/science.1246601
Novik V, Hofreuter D, Galan JE (2010) Identification of Campylobacter jejuni genes involved in its interaction with epithelial cells. Infect Immun 78(8):3540–3553. https://doi.org/10.1128/IAI.00109-10
Ó Cróinín T, Backert S (2012) Host epithelial cell invasion by Campylobacter jejuni: trigger or zipper mechanism? Front Cell Infect Microbiol 2:25. https://doi.org/10.3389/fcimb.2012.00025
Oyarzabal OA, Backert S (2012) Microbial food safety: an introduction (food science text series). Springer, Heidelberg
Parkhill J, Wren B, Mungall K, Ketley J, Churcher C, Basham D, Chillingworth T, Davies R, Feltwell T, Holroyd S, Jagels K, Karlyshev AV, Moule S, Pallen MJ, Penn CW, Quail MA, Rajandream MA, Rutherford KM, van Vliet AH, Whitehead S, Barrell BG (2000) The genome sequence of the food-borne pathogen Campylobacter jejuni reveals hypervariable sequences. Nature 403:665–668. https://doi.org/10.1038/35001088
Parsons CM, Potter LM, Brown RD Jr (1983) Effects of dietary carbohydrate and of intestinal microflora on excretion of endogenous amino acids by poultry. Poult Sci. 62(3):483–489. https://doi.org/10.3382/ps.0620483
Pei ZH, Burucoa C, Grignon B, Baqar S, Huang XZ, Kopecko DJ, Bourgeois AL, Fauchère JL, Blaser MJ (1998) Mutation in the peb1A locus of Campylobacter jejuni reduces interactions with epithelial cells and intestinal colonization of mice. Infect Immun 66:938–943
Perna AM, Reisen F, Schmidt TP, Geppert T, Pillong M, Weisel M, Hoy B, Simister PC, Feller SM, Wessler S, Schneider G (2014) Inhibiting Helicobacter pylori HtrA protease by addressing a computationally predicted allosteric ligand binding site. Chem Sci 5:3583–3590. https://doi.org/10.1039/c4sc01443j
Perna AM, Rodrigues T, Schmidt TP, Bohm M, Stutz K, Reker D, Pfeiffer B, Altmann KH, Backert S, Wessler S, Schneider G (2015) Fragment-based De Novo design reveals a small-molecule inhibitor of Helicobacter pylori HtrA. Angew Chem Int Ed Engl 54:10244–10248. https://doi.org/10.1002/anie.201504035
Pesci EC, Cottle DL, Pickett CL (1994) Genetic, enzymatic, and pathogenic studies of the iron superoxide dismutase of Campylobacter jejuni. Infect Immun 62(7):2687–2694. https://doi.org/10.1128/IAI.62.7.2687-2694.1994
Phongsisay V, Hara H, Fujimoto S (2015) Toll-like receptors recognize distinct proteinase-resistant glycoconjugates in Campylobacter jejuni and Escherichia coli. Mol Immunol 64:195–203. https://doi.org/10.1016/j.molimm.2014.11.020
Phongsisay V (2016) The immunobiology of Campylobacter jejuni: innate immunity and autoimmune diseases. Immunobiol. 221(4):535–543. https://doi.org/10.1016/j.imbio.2015.12.005
Pickett CL, Whitehouse CA (1999) The cytolethal distending toxin family. Trends Microbiol 7:292–297. https://doi.org/10.1016/s0966-842x(99)01537-1
Plowright RK, Parrish CR, McCallum H, Hudson PJ, Ko AI, Graham AL, Lloyd-Smith JO (2017) Pathways to zoonotic spillover. Nat Rev Microbiol 15(8):502–510. https://doi.org/10.1038/nrmicro.2017.45
Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B (1998) Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282(5396):2085–2208. https://doi.org/10.1126/science.282.5396.2085
Poly F, Ewing C, Goon S, Hickey TE, Rockabrand D, Majam G, Lee L, Phan J, Savarino NJ, Guerry P (2007) Heterogeneity of a Campylobacter jejuni protein that is secreted through the flagellar filament. Infect Immun 75:3859–3867. https://doi.org/10.1128/IAI.00159-07
Potturi-Venkata LP, Backert S, Vieira SL, Oyarzabal OA (2007) Evaluation of logistic processing to reduce cross-contamination of commercial broiler carcasses with Campylobacter spp. J Food Prot 70:2549–2554. https://doi.org/10.4315/0362-028x-70.11.2549
Purcell EM (1997) The efficiency of propulsion by a rotating flagellum. Proc Natl Acad Sci USA 94:11307–11311. https://doi.org/10.1073/pnas.94.21.11307
Purdy D, Buswell CM, Hodgson AE, Mc AK, Henderson I, Leach SA (2000) Characterisation of cytolethal distending toxin (CDT) mutants of Campylobacter jejuni. J Med Microbiol 49:473–479. https://doi.org/10.1099/0022-1317-49-5-473
Radomska KA, Wosten M, Ordonez SR, Wagenaar JA, van Putten JPM (2017) Importance of Campylobacter jejuni FliS and FliW in flagella biogenesis and flagellin secretion. Front Microbiol 8:1060. https://doi.org/10.3389/fmicb.2017.01060
Rahman H, King RM, Shewell LK, Semchenko EA, Hartley-Tassell LE, Wilson JC, Day CJ, Korolik V (2014) Characterisation of a multi-ligand binding chemoreceptor CcmL (Tlp3) of Campylobacter jejuni. PLoS Pathog 10:e1003822. https://doi.org/10.1371/journal.ppat.1003822
Rathinam VA, Appledorn DM, Hoag KA, Amalfitano A, Mansfield LS (2009) Campylobacter jejuni-induced activation of dendritic cells involves cooperative signaling through Toll-like receptor 4 (TLR4)-MyD88 and TLR4-TRIF axes. Infect Immun 77:2499–2507. https://doi.org/10.1128/IAI.01562-08
Ribardo DA, Hendrixson DR (2011) Analysis of the LIV system of Campylobacter jejuni reveals alternative roles for LivJ and LivK in commensalism beyond branched-chain amino acid transport. J Bacteriol 193(22):6233–6243. https://doi.org/10.1128/JB.05473-11
Schmidt AM, Escher U, Mousavi S, Tegtmeyer N, Boehm M, Backert S, Bereswill S, Heimesaat MM (2019) Immunopathological properties of the Campylobacter jejuni flagellins and the adhesin CadF as assessed in a clinical murine infection model. Gut Pathogens 11:24. https://doi.org/10.1186/s13099-019-0306-9
Schmidt TP, Perna AM, Fugmann T, Bohm M, Jan H, Haller S, Gotz C, Tegtmeyer N, Hoy B, Rau TT, Neri D, Backert S, Schneider G, Wessler S (2016) Identification of E-cadherin signature motifs functioning as cleavage sites for Helicobacter pylori HtrA. Sci Rep 6:23264. https://doi.org/10.1038/srep23264
Schroder K, Tschopp J (2010) The inflammasomes. Cell 140:821–832. https://doi.org/10.1016/j.cell.2010.01.040
Schwandner R, Dziarski R, Wesche H, Rothe M, Kirschning CJ (1999) Peptidoglycan- and lipoteichoic acid-induced cell activation is mediated by toll-like receptor 2. J Biol Chem 274(25):17406–17409. https://doi.org/10.1074/jbc.274.25.17406
Sheppard SK, Maiden MC (2015) The evolution of Campylobacter jejuni and Campylobacter coli. Cold Spring Harb Perspect Biol 7(8):a018119:22. https://doi.org/10.1101/cshperspect.a018119
Shigematsu M, Umeda A, Fujimoto S, Amako K (1998) Spirochaete-like swimming mode of Campylobacter jejuni in a viscous environment. J Med Microbiol 47:521–526. https://doi.org/10.1099/00222615-47-6-521
Shortt C, Scanlan E, Hilliard A, Cotroneo CE, Bourke B, Ó Cróinín T (2016) DNA supercoiling regulates the motility of Campylobacter jejuni and is altered by growth in the presence of chicken mucus. mBio 7(5):e01227-16. https://doi.org/10.1128/mBio.01227-16
Smith JL (2002) Campylobacter jejuni infection during pregnancy: long-term consequences of associated bacteremia, Guillain-Barré syndrome, and reactive arthritist. J Food Prot 65(4):696–708. https://doi.org/10.4315/0362-028x-65.4.696
Song YC, Jin S, Louie H, Ng D, Lau R, Zhang Y, Weerasekera R, Al Rashid S, Ward LA, Der SD, Chan VL (2004) FlaC, a protein of Campylobacter jejuni TGH9011 (ATCC43431) secreted through the flagellar apparatus, binds epithelial cells and influences cell invasion. Mol Microbiol 53:541–553. https://doi.org/10.1111/j.1365-2958.2004.04175.x
Stephenson HN, Mills DC, Jones H, Milioris E, Copland A, Dorrell N, Wren BW, Crocker PR, Escors D, Bajaj-Elliott M (2014) Pseudaminic acid on Campylobacter jejuni flagella modulates dendritic cell IL-10 expression via Siglec-10 receptor: a novel flagellin-host interaction. J Infect Dis 210(9):1487–1498. https://doi.org/10.1093/infdis/jiu287
Stradal TEB, Schelhaas M (2018) Actin dynamics in host-pathogen interaction. FEBS Lett 592(22):3658–3669. https://doi.org/10.1002/1873-3468.13173
Tegtmeyer N, Moodley Y, Yamaoka Y, Pernitzsch SR, Schmidt V, Traverso FR, Schmidt TP, Rad R, Yeoh KG, Bow H, Torres J, Gerhard M, Schneider G, Wessler S, Backert S (2016) Characterisation of worldwide Helicobacter pylori strains reveals genetic conservation and essentiality of serine protease HtrA. Mol Microbiol 99(5):925–944. https://doi.org/10.1111/mmi.13276
Tram G, Day CJ, Korolik V (2020) Bridging the gap: a role for Campylobacter jejuni biofilms. Microorganisms 8(3):452. https://doi.org/10.3390/microorganisms8030452
Trinchieri G, Sher A (2007) Cooperation of Toll-like receptor signals in innate immune defense. Nat Rev Immunol 7:179–190. https://doi.org/10.1038/nri2038
van Gerwe T, Miflin JK, Templeton JM, Bouma A, Wagenaar JA, Jacobs-Reitsma WF, Stegeman A, Klinkenberg D (2009) Quantifying transmission of Campylobacter jejuni in commercial broiler flocks. Appl Environ Microbiol 75:625–628. https://doi.org/10.1128/AEM.01912-08
Vegge CS, Brøndsted L, Li YP, Bang DD, Ingmer H (2009) Energy taxis drives Campylobacter jejuni toward the most favorable conditions for growth. Appl Environ Microbiol 75:5308–5314. https://doi.org/10.1128/aem.00287-09
Velayudhan J, Kelly DJ (2002) Analysis of gluconeogenic and anaplerotic enzymes in Campylobacter jejuni: an essential role for phosphoenolpyruvate carboxykinase. Microbiology 148(3):685–694. https://doi.org/10.1099/00221287-148-3-685
Velayudhan J, Jones MA, Barrow PA, Kelly DJ (2004) L-serine catabolism via an oxygen-labile L-serine dehydratase is essential for colonization of the avian gut by Campylobacter jejuni. Infect Immun 72:260–268. https://doi.org/10.1128/iai.72.1.260-268.2004
von Gunten S, Bochner BS (2008) Basic and clinical immunology of Siglecs. Ann N Y Acad Sci 1143:61–82. https://doi.org/10.1196/annals.1443.011
Wai SN, Lindmark B, Soderblom T, Takade A, Westermark M, Oscarsson J, Jass J, Richter-Dahlfors A, Mizunoe Y, Uhlin BE (2003) Vesicle-mediated export and assembly of pore-forming oligomers of the enterobacterial ClyA cytotoxin. Cell 115:25–35. https://doi.org/10.1016/s0092-8674(03)00754-2
Wassenaar TM, van der Zeijst BA, Ayling R, Newell DG (1993) Colonization of chicks by motility mutants of Campylobacter jejuni demonstrates the importance of flagellin A expression. J Gen Microbiol 139(6):1171–1175. https://doi.org/10.1099/00221287-139-6-1171
Watson RO, Galán JE (2005) Signal transduction in Campylobacter jejuni-induced cytokine production. Cell Microbiol 7:655–665. https://doi.org/10.1111/j.1462-5822.2004.00498.x
Watson RO, Galán JE (2008) Campylobacter jejuni survives within epithelial cells by avoiding delivery to lysosomes. PLoS Pathog 4:e14. https://doi.org/10.1371/journal.ppat.0040014
Wedderkopp A, Nielsen EM, Pedersen K (2003) Distribution of Campylobacter jejuni Penner serotypes in broiler flocks 1998–2000 in a small Danish community with special reference to serotype 4-complex. Epidemiol Infect 131:915–921. https://doi.org/10.1017/s0950268803008975
Whitehouse CA, Balbo PB, Pesci EC, Cottle DL, Mirabito PM, Pickett CL (1998) Campylobacter jejuni cytolethal distending toxin causes a G2-phase cell cycle block. Infect Immun 66:1934–1940
Wooldridge KG, Williams PH, Ketley JM (1996) Host signal transduction and endocytosis of Campylobacter jejuni. Microb Pathog 21(4):299–305. https://doi.org/10.1006/mpat.1996.0063
Wösten MM, Wagenaar JA, van Putten JP (2004) The FlgS/FlgR two-component signal transduction system regulates the fla regulon in Campylobacter jejuni. J Biol Chem. 279(16):16214–16222. https://doi.org/10.1074/jbc.M400357200
Xi D, Alter T, Einspanier R, Sharbati S, Golz G (2020) Campylobacter jejuni genes Cj1492c and Cj1507c are involved in host cell adhesion and invasion. Gut Pathogens 12. https://doi.org/10.1186/s13099-020-00347-8
Yao R, Burr DH, Doig P, Trust TJ, Niu H, Guerry P (1994) Isolation of motile and non-motile insertional mutants of Campylobacter jejuni: the role of motility in adherence and invasion of eukaryotic cells. Mol Microbiol 14:883–893. https://doi.org/10.1111/j.1365-2958.1994.tb01324.x
Yao R, Burr DH, Guerry P (1997) CheY-mediated modulation of Campylobacter jejuni virulence. Mol Microbiol 23:1021–1031. https://doi.org/10.1046/j.1365-2958.1997.2861650.x
Yoon H (2016) Bacterial outer membrane vesicles as a delivery system for virulence regulation. J Microbiol Biotechnol 26:1343–1347. https://doi.org/10.4014/jmb.1604.04080
Young GM, Schmiel DH, Miller VL (1999) A new pathway for the secretion of virulence factors by bacteria: The flagellar export apparatus functions as a protein-secretion system. Proc Natl Acad Sci USA 96:6456–6461. https://doi.org/10.1073/pnas.96.11.6456
Young KT, Davis LM, Dirita VJ (2007) Campylobacter jejuni: molecular biology and pathogenesis. Nat Rev Microbiol 5:665–679. https://doi.org/10.1038/nrmicro1718
Yuki N, Susuki K, Koga M, Nishimoto Y, Odaka M, Hirata K, Taguchi K, Miyatake T, Furukawa K, Kobata T, Yamada M (2004) Carbohydrate mimicry between human ganglioside GM1 and Campylobacter jejuni lipooligosaccharide causes Guillain-Barré syndrome. Proc Natl Acad Sci USA 101(31):11404–11409. https://doi.org/10.1073/pnas.0402391101
Yuki N, Koga M (2006) Bacterial infections in Guillain-Barré and Fisher syndromes. Curr Opin Neurol 19(5):451–457. https://doi.org/10.1097/01.wco.0000245367.36576.e9
Zarzecka U, Grinzato A, Kandiah E, Cysewski D, Berto P, Skorko-Glonek J, Zanotti G, Backert S (2020) Functional analysis and cryo-electron microscopy of Campylobacter jejuni serine protease HtrA. Gut Microbes (in Press). https://doi.org/10.1080/19490976.2020.1810532
Zheng J, Meng J, Zhao S, Singh R, Song W (2008) Campylobacter-induced interleukin-8 secretion in polarized human intestinal epithelial cells requires Campylobacter-secreted cytolethal distending toxin- and Toll-like receptor-mediated activation of NF-kappaB. Infect Immun 76:4498–4508. https://doi.org/10.1128/iai.01317-07
Ziprin RL, Young CR, Stanker LH, Hume ME, Konkel ME (1999) The absence of cecal colonization of chicks by a mutant of Campylobacter jejuni not expressing bacterial fibronectin-binding protein. Avian Dis 43:586–589
Acknowledgements
We thank Drs. David Hendrixson (UT Southwestern, Dallas, Texas/USA) and Tadhg Ó Cróinín (University College Dublin, Dublin/Ireland) for helpful comments on the manuscript. This work is supported by the German Federal Ministry of Education and Research (BMBF) in frame of the zoonoses research consortium PAC-Campylobacter to S.B. (project IP9/01KI1725E).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Tegtmeyer, N., Sharafutdinov, I., Harrer, A., Soltan Esmaeili, D., Linz, B., Backert, S. (2021). Campylobacter Virulence Factors and Molecular Host–Pathogen Interactions. In: Backert, S. (eds) Fighting Campylobacter Infections. Current Topics in Microbiology and Immunology, vol 431. Springer, Cham. https://doi.org/10.1007/978-3-030-65481-8_7
Download citation
DOI: https://doi.org/10.1007/978-3-030-65481-8_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-65480-1
Online ISBN: 978-3-030-65481-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)