Abstract
The realization of future energy based on safe, clean, sustainable, and economically viable technologies is one of the grand challenges faced by modern society. Electrochemical energy technologies underpin the potential success of this effort to divert energy sources away from fossil fuels, whether one considers alternative energy conversion strategies through photoelectrochemical (PEC) production of chemical fuels or fuel cells run with sustainable hydrogen, or energy storage strategies, such as in batteries and supercapacitors. This dissertation builds on recent advances in nanomaterials design, synthesis, and characterization to develop novel electrodes that can electrochemically convert and store energy. With the improvement of global economy, the fatigue of energy becomes inevitable in the twenty-first century. It is expected that the increase in world energy requirements will be triple at the end of this century. Thus, there is an imperative need for the development of renewable energy sources and storage systems.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
The accelerated consumption of non-renewable sources of fuels (i.e. coal, petroleum, gas) along with the consequent global warming issues have intrigued immense research interest for the advancement and expansion of an alternate efficient energy conversion and storage technique in the form of clean renewable resource. The global demand for energy production is predicted to be at least double by 2050, while the rate at which the non-renewable fossil fuels are being consumed today; it will take not more than 40 years to run all the known oil repositories dry leaving the entire world into an era of complete darkness. In addition to this, the combustion of fossil fuels at a faster rate also leads to the excessive emission of harmful gases such as CO2, CH4, N2O etc. in the atmosphere because of which the temperature of the earth is getting raised. Therefore in order to overcome these issues, we need to focus at sustainable green energy origins such as wind, solar, hydroelectric, geothermal, biological, nuclear, etc. However, the infrequent availability of abovementioned sustainable green energy origins encourages the focus of research on efficient ways of storage systems for stockpile and provides energy in a steady mode. The research work in the direction of storing electrochemical energy has expanded significantly during the last few decades and a huge range of active materials have been reported, both for supercapacitor and battery type energy storage [1, 2]. But till today among all the systems for storing energy electrochemical energy storage/conversion system found to be prominent candidate to get rid of the prevailing energy crisis. Based on the energy conversion mechanisms electrochemical energy storage systems can be divided into three broader sections namely batteries, fuel cells and supercapacitors. In batteries and fuel cells, chemical energy is the actual source of energy which is converted into electrical energy through faradic redox reactions while in case of the supercapacitor, electric energy is stored at the interface of electrode and electrolyte material forming electrochemical double layer resulting in non-faradic reactions.
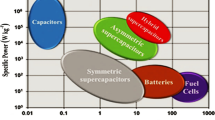
The selection of an energy storage device for various energy storage applications depends upon several key factors such as cost, environmental conditions and mainly on the power along with energy density present in the device. Basically an ideal energy storage device must show a high level of energy with significant power density but in general compromise needs to be made in between the two and the device which provides the maximum energy at the most power discharge rates are acknowledged as better in terms of its electrical performance. The variety of energy storage systems can be compared by the “Ragone plot”. Ragone plot comprises of performance of energy storage devices, such as capacitors, supercapacitors, batteries, and fuel cells are shown in Fig. 1.
The relationship of specific energy (E) with specific power (P) is provided by the expression [3, 4]
where c represents the specific capacitance (F g−1), ∆V represents the operating potential window (V), and tdis represents the discharge time (s).
Ragone plot is a plot in which the values of the specific power density are being plotted against specific energy density, in order to analyze the amount of energy which can be accumulate in the device along with the efficiency of the energy’s release. According to the Ragone plot batteries and fuel cells both acquire large value of specific energy density with small value of specific power density in contrast capacitors have high value of specific power density with a small value of specific energy density. Whereas supercapacitor possess intermediate specific energy density together with power density and also possess a longer lifetime due to the absence of chemical reactions [5]. The low energy density of the supercapacitor is the only shortcoming in comparison to the batteries and fuel cell which is act as an obstacle for their commercialization. The type of material is being used with its structure for the preparation of electrode material of supercapacitor decides the performance of the supercapacitor.
Conducting polymers has immense electrical conductivity and undergoes reversible redox reactions due to its intrinsic tendency making it promising cathode materials for energy storage devices but suffers limitation owing to lesser specific capacity and tendency of large extent of self-discharge. The fusion of conducting polymer with other material having high specific capacity could create hybrid conducting polymer having beneficial properties of both component widening the applicability in storage devices with broadening application. In hybrid conducting polymers, conducting polymer can contribute towards the cyclic efficiency, high discharge capacity while other component contributes towards high capacity and ability to intercalate. The presence of inorganic materials in hybrid conducting polymer contributes towards the enhancement in the electron transfer tendency at the interface of the surface of the electrode and electrolyte material [6]. Hybrid conducting polymer can be classified into two major groups depending upon the conduction polymer and nanoparticle arrangement. The conducting polymer can serve as a matrix for inorganic nanomaterials or can be inserted inside the layer of the inorganic matrix.
The synthesis of hybrid conducting polymer can be classified into three categories [7]. In the first approach, the synthesis of nanoparticles occurs in the presence of a polymer matrix. It may cause aggregation which makes it difficult strategy to synthesize hybrid conducting polymer. In the second approach nanoparticles are generated in situ while the polymerization of monomer taking place resulting in homogeneous distribution of nanoparticles in the polymer matrix. In this, the polymerization reaction and the synthesis of nanoparticles proceed simultaneously leading to the synthesis of homogeneous hybrid conducting polymer. The third synthetic method includes polymerization of the monomer of the requisite polymer around the nanoparticles by means of chemically compatible ligands [8] or polymeric structures [9].
Controlling the size and shape of content phases, degree of mixing and synthetic protocol participates in the improvement of as-prepared nanocomposite properties. Different properties can be expected by varying attributes of the content phases and synthetic method used for hybrid conducting polymers.
2 Proposed Mechanism for Designing Hybrid Conducting Polymer
Conducting polymers behave as insulators or semiconductors in pure state. The doping of donor or acceptor substituents by using oxidizing or reducing agents respectively increases conductivity by several orders. The oxidation leads to p-doping in conducting polymers by using electron acceptor species such as I2, H2SO4 etc. The reduction of conducting polymers leads to n-doping introduced by using electron donor species i.e. alkali metal ions (Na, K) and Li. Doping creates charged defects such as polaron, bipolaron and soliton acting as charge carrier for conduction. Hence increase in concentration of doping species enhances conductivity.
Although conductivity in conducting polymer has been explained and discussed by many research group but exact reason of conductivity in hybrid conducting polymer has been expected to be similar to conducting polymers and not explored extensively. The synergic effect arises due to the proper alignment of polymer chains may help in enhancement in conductivity. The interfacial interaction, photoinduced electron transfer, electrostatic interaction found to increase the conductivity of hybrid conducting polymers [9,10,11].
3 Batteries
Batteries have become the typical power source utilized for numerous purposes in industrial and consumer electronics because of its compactness, efficiency, reliability, and economical point of view. Battery maintains virtual instantaneous input and output response from the battery to network and vice-versa. Basically batteries are electrochemical devices exploiting redox reactions for converting the accumulated chemical energy of batteries into required electrical energy. Typically a battery consists of electrochemical cells containing electrically connected electrodes using a conductive electrolyte containing negatively charged ions and positively charged ions. The transportation of ions in the cell determines the polarity of a cell. The anion transporting electrode is defined as anode and cation transporting electrode defined as cathode taking place during charging process. Mostly, in batteries redox reaction takes place with oxidation and reduction reactions at the anode and cathode respectively.
On the basis of charging capabilities of batteries are classified in four classes: Primary batteries (non-rechargeable), secondary (rechargeable batteries), Grid-scale battery systems and Fuel cells.
Primary Batteries
These batteries are not rechargeable, cannot be recycled and simple electric devices. These are simple and convenient to use involving irreversible processes. Primary batteries utilize aqueous and non-aqueous electrolytes. In these devices, electrolyte reacts with electrodes for creating flow of electric current along with formation of by-products that cannot be reused. The commonly employed primary batteries include zinc-carbon battery, alkaline battery and lithium primary batteries. It suffers from less energy density, reduced leakage resistance, and drop in voltage through discharge. These batteries commonly used in flashlight and many portable devices.
Secondary Batteries
These batteries are rechargeable broadening the range of application for portable electronic devices. The longer charge–discharge cycles commercializes secondary batteries for residential power storage and for electric vehicles. Secondary batteries use reversible process having two distinct charge cycle and discharge cycles, marked by distinctive chemical reactions and peculiar electrical properties. In course of charging cycle, electrical energy transforms electrolyte storing electrical energy in form of chemical bonds. In discharge cycle, energy is released from chemical bonds and generates electrical energy by the transformation of electrolyte. Secondary rechargeable batteries comprise of lead-acid batteries, lithium-ion batteries, lithium-sulfur batteries, nickel-metal hydride batteries, and nickel-metal batteries depending upon their electrode component. The secondary batteries offer superior battery performance, high-quality performance in altering temperature range, elevated voltage, and fine charge retention. Amidst other secondary batteries, lithium–ion batteries found to show the highest storage efficiency valued nearly 83%, and have been installed in renewable energy systems widely along with micro-grid systems. The assets of using lithium-ion batteries includes the least maintenance, extended life-cycle, stability over a wide range of temperature, efficient charging-discharging ability, and elevated energy density. Secondary batteries are included in laptops and mobile phones.
Grid-Scale Battery Systems
Grid scale storage provides peak power and stability for a sustained period. It includes red-ox flow batteries, Na–S batteries using advance level lead-A and Lithium-ion batteries.
Fuel Cells
Fuel cells use either hydrogen, or indirect systems using fossil fuels such as methanol by the means of catalysed and thermal reaction. Fuel cells are resourceful in the output power supply, high reliability factor, and negligible amount of degradation process.
Thus batteries are storage option for the electrical energy providing smooth and steady electrical power for micro systems and are assembly of pseudocapacitive electrodes storing charge using faradic reactions. For various purposes batteries are preferred over supercapacitors due to their characteristics of slower discharge time providing lower energy densities available for much longer lifetime. The battery type electrodes exhibit non-reversible oxidation and reduction phenomenon. The galvanostatic charge-discharge curves presents typical non-linear behavior of the curve having flat discharge plateau reasoning for their ability to store large amount of energy. The flat discharge plateau represents phase transformation phenomenon occurring at the surface of electrode materials. The electrode materials due to phase transformation cause depletion in rate capability of the battery electrode limiting their use for longer run. The batteries used in industries for securing power in telecommunications, data networks etc. maintaining the continuous electricity supply. A range of battery chemistries is used for various types of energy storage applications. Extensive research has been performed to increase the capacitance and cyclic performance. Among various types of batteries, the commercialized batteries are lithium-ion batteries, sodium-sulfur batteries, lead-acid batteries, flow batteries and supercapacitors.
As we will be dealing with hybrid conducting polymer applicable for the energy storage devices in this chapter, here describing some important categories of hybrid conducting polymers consisting of conducting polymers with other constituents.
3.1 Metal Oxide/conducting Polymer
Metal oxides majorly oxides of iron, copper, cobalt and nickel have been investigated for the battery materials due to their high theoretical storage capacity shown for sodium. The reactions taking place reduces their life span due to large volume changes associated with it and therefore limited their exploitation in battery systems. However incorporation of conducting polymers not only increases electrical conductivity also improves its mechanical stress. The TiO2 charge capacity is increased by sandwiching in between polyaniline (PANI) and graphene nanosheets serving in lithium ion batteries as anode material. It is accompanied by 99.19% efficiency after 100 cycles [12]. Metal oxides do not have higher capacity, however blending with conducting polymers can lead to the enhancement in the reversible capacity approximately ten times compared to the bare MnO2 devices. The swelled layered structure of PANI intercalated MnO2 possess high capacity along with excellent cycle stability and is therefore considered to be a promising cathode material for high-capacity lithium ion batteries [13]. The PANI/β-AgVO3 triaxial nanowire indicates faster kinetics and better capacity compared to β-AgVO3 nanowires which is expected owing to the decrease in charge transfer resistance.[14]. Polypyrrole (PPy) nanowires blended with silicon particles has been synthesized by mechanical milling showing improved reversibility and enhanced life cycle than that of silicon, because PPy nanowire act as a polymer matrix layer supporting active silicon granule since they have capability to continually get alloy with lithium ions during the charging-discharging process of lithium ion batteries [15]. PPy have been found to decrease the resistance of charge transfer reactions connected to the Li ions intercalation-deintercalation processes in PPy-metal oxide hybrid polymers such as MoO3, V2O5, LiCoO2 and LiV3O8 [16,17,18,19,20]. Hybrid composite of PPy/Polyethylene glycol (PEG) with LiFePO4 showed higher discharge capacity than pristine LiFePO4, PEG presence reduces structural defects due to stabilization effect provided by PEG [21].
3.2 Metal Chalcogenides/conducting Polymer
Metal chalcogenides owing to their tunable properties, unique compositional and structural features motivated for fundamental understanding and industrial advancement for varied applications. The two-dimensional metal chalcogenides have been explored extensively for lithium-ion batteries because of their aptitude to provide a platform by forming structures which support reversible intercalation of lithium-ion at very large applied potential and high surface-to-volume ratio. Due to structural instability and less electrical conductivity suffers these materials from low life-time and small rate capability. Integration of metal chalcogenides with conducting polymer creating hybrid conducting polymer combine valuable properties of both. The remarkable electrochemical performance of the devices was observed due to the synergic effect involved in between metal sulfides and the conducting polymer. Molybdenum sulfide (MoS2) in combination with conductive PANI proved the reduced resistance of charge-transfer at the interface of electrode and electrolyte resulting in high charge capacitance and cycling behaviour as seen from the Nyquist plots of bare MoS2 and MoS2/PANI. The MoS2/PANI showed improved properties through large charging capacity valued 1063.9 mAhg−1 with 90.2% retention in specific capacitance after long cycle-life of 95 cycles [22]. Hybridization of PANI with MoS2 found to decrease the resistance of charge-transfer at the interface of electrode-electrolyte [22]. The PPy hybrid composite by SnS2 exhibits specific capacitance of 1000 mAhg−1 by retaining specific capacitance upto 703 mAhg−1 after 500 cycles [23]. The lamellar structure of hybrid conducting polymer provides conductive network for the transportation of ions and enhancing the tendency of the expansion and contraction processes at the electrode material occurring in charge–discharge process [21]. The specific capacitance in batteries has also been found to increase by constructing MoS2 nanocomposites with PPy and poly(3,4-ethylenedioxythiophene) polystyrene sulfonate (PEDOT: PSS) [24, 25]. Binary composite of PPy/MoS2 illustrated the high capacitance with outstanding cycling stability that proves its excellence as an electrode material for supercapacitors and a schematic diagram for the blending has been presented in Fig. 2.
Similarly, The ternary hybrid conducting polymer are found to observe further improvement in the capacitance properties of conducting polymer reasoned out by blending the properties of three components present in hybrid conducting polymer [25,26,27].
3.3 Carbon Supported Hybrid Materials
During recent years, enormous efforts have been made to synthesize graphene hybrid materials as electrodes for novel energy storage devices. Graphene is two-dimensional layered material having total specific area of 2630 m2/g along with 2000–5000 cm2/V s of charge carrier mobility which is suitable for energy storage devices [28]. The principle of using graphene is to enhance the surface area which helps in allowing superior charge adsorption processes. Designing graphene using inorganic materials exploits the flexibility in graphene and reduces some stress off the inorganic material during operation cycles thereby improving specific capacitance as well as cyclic performance. Graphene oxide in reality is functionalized form of graphene obtained by chemically modification of graphene resulting in functional groups containing oxygen such as alcohols, epoxides, and acid groups dispersible in organic solvents, water, and different matrixes. The application of graphene in batteries is exploiting properties such as large surface area, remarkable electrical conductivity, excellent stability towards chemicals, thermal conductivity, electronic tunability, and mechanical strength. Graphene due to high mechanical strength and flexibility found to improve the storage of lithium ion in its hybrid material by reducing the stress cracking after repeated charge/discharge process which discontinues the connection between charge storing material and current collectors. According to the report, graphene nanosheets hybrid tin oxide exhibits a capacitance of 810 mAh/g retaining 70% efficiency after completing 30 charge-discharge cycles, whilst bare SnO2 exhibited capacitance of only 550 mAh/g and also decreased to 60 mAh/g after completing 15th cycles [29]. The presence of graphene nanosheets in tin oxide creates enough void spaces buffering volume change occurs during the process of lithium insertion along with electronic conductive channels improves electrochemical performance. Sulfur based cathodic material has a theoretical specific capacity of 1672 mAh/g but has limited application due to their tendency to dissolve into electrolyte and experience swelling. However, sulfur hybrid material with graphene and PEG exhibit specific capacitance of 600 mAh/g reported for completing 100 cycles [30]. The metal oxide nanoparticles such as MnO and Fe3O4 integrated with graphene to achieve high specific capacity and stability [31, 32]. More than 90% of capacity retention exhibited by MoS2/graphene oxide hybrid and rGO/SnS2 shows 84% retention in charge capacity upto 500 cycles [33, 34]. ZnMn2O4–graphene hybrid nanosheets exploited in Li–ion battery as electrode exhibits higher rate capability and cycling stability compared to conventional graphite anode [35].
PANI based graphene hybrid material are examined for lithium ion batteries as electrode materials [36]. Graphene/PANI is the extensively studied hybrid materials as supercapacitor electrode attributed to their wide potential window, ease of its synthesis, and improved stability among other conducting polymers hybrid. A schematic diagram for the interaction showing between PANI and Graphene is presented in Fig. 3. MoS2-graphene a combination of two-dimensional materials which has a high surface area, versatile electronic structure and high electrical conductance outperforms as an excellent candidate for energy storage application [37]. Although lithium-ion battery has been used mainly for practical purposes but sodium ion batteries have also been explored with MoS2 composites with carbon and graphene materials [38].
Other than conducting polymer hybrids, nanoengineered two-dimensional MXenes and their derivatives attracted attention for energy storage devices improving limitations for practical applications [39]. MXene/polymer composites are widely used as electrode materials for hybrid supercapacitors. The frequently used polymers are electroactive in nature i.e. PVA, PPy, PANI, PEDOT and its derivatives, polyfluorene derivatives (PFDs), and polydiallyldimethylammonium chloride (PDAC). MXenes materials are including two-dimensional (2D) structure of metal carbides and nitrides behaving as hopeful electrode material having high electrical conductivity however suffer from low mechanical strength. MXenes suffers from the disadvantage of forming aggregation which is irreversible in nature and easily form stacking. The integration of graphene/conducting polymers linking the MXenes interlayer results in the formation of new types of material showing advanced functional and mechanical properties. The presence of conducting spacers in MXenes improves the kinetics and rate performance. For example, Ling et al. [40] have reported the synthesis of MXene/PVA nanocomposite composite having outstanding mechanical properties in comparison to their pure MXene/PVA counterparts [40]. Boota et al. [41] reported the addition of PPy (8 wt %) to MXene yields volumetric and gravimetric capacitances of 1000 F/cm3 and 416 F/g, relatively higher than that of the pure MXene electrode [41]. Qin et al. [42] reported nanocomposite film based on MXene and PPy self-assembly as electrode-based supercapacitors, having excellent capacitance (69.5 mF cm−2), ultrahigh energy density (250.1 mWh cm−3) and outstanding cycling stability [42]. The enhanced electrochemical performance MXene/PPy nanocomposite may be attributed to the synergistic effect existing between MXene’s (electric double layer capacitance mechanism) and PPy’s (pseudocapacitance mechanism). Zhu et al. [43] also reported the synthesis of conducting hybrid films based on PPy/Ti3C2Tx having excellent electrochemical performance as a supercapacitor electrode. However, still efforts need to be done on identifying novel organic materials that can be easily inserted in between the interlayer region of MXene to develop hybrid structures for high-performance energy storage devices [43].
Batteries have disadvantages in concern with the environment through hazardous waste and toxic fumes during manufacturing in addition with disposal and recycling. The use of heavy metal as electrode material when exposed causes serious effects on the health of animals and humans. There are some limitations in using batteries if not handled properly. A battery explosion is very common problem being faced and caused by misuse, short-circuit and excessive charging of batteries. The excessive charging or rate of charging leads to the formation of mixture of hydrogen and oxygen building up excessive pressure inside the battery. In extreme situation, battery chemicals may spray causing irreversible damage. The short-circuit generates large amount of current responsible for explosion. Another problem associated with batteries is leakage, releasing of dangerous chemical damaging the equipment or the environment.
4 Supercapacitors
In comparison to the batteries, supercapacitors are evolving as one of the most exciting innovative developments in the field of devices storing energy for future perspective. Supercapacitors fill the space having amid batteries quality and capacitors quality since its specific power density is higher compared to batteries and specific energy density is higher than that of the capacitor. Other significant features of supercapacitors include faster charge-discharge rate, longer cycling life time, simple fabrication with low maintenance, and without short circuit issues which are major concern in using available batteries. In addition these are exceptionally safe for storage since they can be easily discharged and do not release any toxic waste in the environment. Therefore supercapacitors are attractive and appropriate efficient energy storage devices mainly utilized in mobile electronic devices, hybrid electric vehicles, manufacturing equipment’s, backup systems, defence devices etc. where the requirement of power density is high and cycling-life time required is longer are highly desirable [44,45,46,47,48]. Although the working of the supercapacitors is comparable to the conventionally used capacitors but supercapacitors are capable of storing greater charge through the presence of pores present within the electrodes having large surface area and also high charge separation between the electrolyte and an electrode occurs at a very small distance.
The components of a supercapacitor are two electrode system immersed in electrolyte having a separator. The electrodes possess high specific surface area and are separated by a separator i.e. membrane that permits the mobility of charged ions. The electrolyte is the mixture of positively and negatively charged ions dissolved in water. They are capable of storing a large amount of energy that can be released very fast. An ionic layer forms in between the electrodes sharing common electrolyte accumulate electric charge in the supercapacitor. Based on the mechanism involved in the charge storage and the active material of electrode, supercapacitors classified in three broader types, i.e. electrochemical double layer capacitors (EDLCs), pseudocapacitor and hybrid capacitors (Fig. 4). Each type has its own charge storage mechanism i.e. Faradic mechanism, Non-Faradic mechanism and the combination of Faradic and Non-Faradic mechanism respectively [44, 49, 50].
EDLC are storing their energy by non-Faradaic mechanism in which EDL charges at the interface of electrically conducting porous electrode such as carbon-based materials and ionically conducting electrolytes (Fig. 5). In EDLC, there do not occur any charge transformation at the interface of the electrode and electrolyte material. In EDLCs charges are distributed on the surface by physical mechanism without formation or cleavage of any chemical bond. Thus the electrode material remains inert at all working voltage. The model of EDLCs was first proposed by Helmholtz in 1999 that was supplemented by Gouy and Chapman [51,52,53]. According to the theory, they proposed that the two opposite charge layers built up at the interface of electrode/electrolyte named as Inner Helmholtz Plane and Outer Helmholtz Plane. Stern modified the model presented by Gouy and Chapman, he united the model given by Helmholtz with Gouy-Chapman model to clearly distinguish between the two types of ion distribution layers i.e. the compacted layer and the diffused layer [54]. For ideal EDLCs, the specific capacitance, C (F/g), of electrode is generally given by the expression
where, Ɛ0 represents dielectric constant of the free space, Ɛr represents dielectric constant of the insulating material present amid the electrodes, A is the total surface area of the electrode available for the electrolyte ions, and d represents the effective thickness of the EDLCs. Therefore, by making use of active materials with large surface area, significant increase in specific capacitances easily attained.
The pseudocapacitor or redox capacitor stores energy by Faradaic mechanism by means of the pseudocapacitive behaviour of the used redox-active material. The charge transfer reaction occur between the interface of electrode and electrolyte exploits redox-reactions, electro-sorption and intercalation processes. The rapid as well as reversible faradic reactions occur at the surface as well as in the vicinity of the surface of active electrode material. In general, pseudocapacitors are based on the materials possessing variable oxidation states. Pseudocapacitors possess superior capacitances along with energy densities compared to EDLCs (Fig. 6).
Additionally, the hybrid capacitors operate by means of both mechanisms i.e. using faradic and non-faradic mechanism. Commonly, the electrode materials of hybrid capacitor are composite of carbon based porous material blended with either conducting polymers or metal oxide or both. Electrode materials employed in pseudo-capacitors are usually made up of metal oxides and conducting polymers while EDLCs make use of large surface area based carbon electrode [55, 56].
The commonly employed electrode material in supercapacitor is carbon based materials attributed to their exceptional properties mainly superior conductivity, controlled pore size, variable allotropic forms, superior corrosion resistance, large surface area, high temperature stability, relatively low cost, environmental friendliness, easy and processability. Carbon based materials exists in variety of forms such as fine particles, fibre, composites, textile, etc. and showing various dimensional variety from zero-dimensional to three dimensional. The different forms of carbon that are mainly used as electrode for enhancing the performance of supercapacitor are discussed below:
Activated Carbon
Activated carbon is usually employed in EDLCs as electrode material by virtue of their low cost, easy processing and large surface area (500–3000 m2g−1). Specific capacitance (SC) offered lies from 25 to 150 F/gm in aqueous electrolyte as well as organic electrolyte in the activated carbon electrodes. Along with large surface area the capacitive performances in activated carbons also depends upon several other factors such as pore structures and pore size distributions. Large pore size results in high power densities where as the small pore size results in high energy density. Zhang and Zhao [47] have reported that activated carbon makes use of complex porous structure consisting of variable size macropores (more than 50 nm), mesopores (from 2 to 50 nm) and micropores (less than 2 nm) wide to achieve a high value of surface area (BET) [47]. Also the surface functionality has profound effect on the capacitance and is exercised as additive for enhancing the conductivity performance in supercapacitor. Tang et al. [57] have reported that the oxygen and nitrogen functionalities present at the surface of carbon can enhance the specific capacitance by enhancing the wettability and by pseudo-Faradaic reaction [57]. Apart from activated carbon, carbon black is also exploited as active electrode material for supercapacitors as a result of its large surface area to volume ratio and high conductivity.
Carbon Nanotubes (CNTs)
CNTs were first discovered by Iijima in the year 1991 in carbon soot and are amongst the most important forms of carbon gained enormous interest of researchers worldwide owing to its unique pore size, high thermal stability, superior electrical and mechanical properties. In comparison to activated carbon, CNTs possess mesoporous structure that allows facile movement of ions and lesser series resistance resulting in the improvement of specific energy density along with specific power density. CNTs can principally be classified in two type’s i.e. multiwalled CNT (MWCNTs) and single walled CNT (SWCNTs) which bend on themselves forming tubes with hollow internal core area (Zhang and Zhao [47]). The diameter of a tube is the most important characteristic of CNTs that lie from 1 to 3 nm order and in length it has tens of microns orders. Niu etal.1997 first suggested the use of MWCNT based electrode material in supercapacitors exhibiting a power density and specific capacitance of 8 kW kg−1 and 102 F g−1 respectively in presence of H2SO4 solution as an electrolyte [58]. But the utilization of CNTs for various roles is still limited owing to its huge manufacturing cost. Recently helical carbon nanotubes have also attracted enormous consideration because of comparatively high specific surface area and excellent elasticity. Zeng et al. [59] have reported the synthesis of high-purity helical carbon nanotubes (HCNTs) by using one-step chemical synthetic method resulting in superior specific capacitance of 95 F g−1 at a current density of 0.1 A g−1 [59].
Carbon Fiber
Carbon fibers possess one dimensional structure resulting in superior charge transportation property and also the presence of numerous pores present at the surface of the carbon fiber offers immense adsorption sites for ions to get adsorb and thus proves to be the most promising candidate electrode material.
Graphene
Recently, a considerable shift in the attention of researchers from other frequently used carbon based materials such as CNTs, activated carbon etc. towards graphene has been observed due to their large surface area, appropriate pore size distribution, superior chemical stability, high conductivity, thermal stability, high elasticity, superior mechanical and thermal properties and fast heterogeneous electron transfer. Gonzalez et al. [60] reported intrinsic properties of graphene are responsible for make them an ideal electrode material for use in supercapacitor [60].
Metal Oxides
Ruthenium oxide is the most commonly used metal oxide in pseudo-capacitors because of its wide potential window, excellent stability towards heat, longer life time, high conductivity, high energy density as well as high power density. However, main drawback that limits the application of it is its limited availability leading to the higher cost and toxic nature. Therefore various transition metal oxides have been explored for the utilization in pseudocapacitor to act as an electrode material, as they possess appreciable conductivity and variable oxidation state. Extensively researched transition metal oxides such as MnO2, CoO, NiO, Fe2O3, V2O3, etc. are considered as a potential candidate as an electrode material for supercapacitor owing to owing to its low price, environmental benign nature, excellent theoretical specific capacitance and superior capacitive behavior. Manganese dioxide is considered to be a better substitute for RuO2 owing to its small cost, environmental benign nature, percentage abundance and excellent theoretical specific capacitance. Nickel oxide, vanadium oxide, iron oxide also behave as a superior candidate in supercapacitor due to its large theoretical specific capacitance, environmental stability, thermal stability, accessible layered structure (V2O5), mixed oxidation states, low cost and simple synthesis. Additionally, iron oxide (Fe3O4) based materials in aqueous solutions shows broad potential window up to 1.2 V which is higher when compared to the potential window of RuO2, MnO2 and NiO (less than 1 V) there by will enhance energy density. However, in order to enhance to supercapacitive performance of the metal oxides, research have been done to study the metal oxide composites, with CNTs, activated carbon and conducting polymers, since best features of variety of materials can be integrated to form a novel material having advanced applications. Ideally, the composite materials have low weight with attractive mechanical, thermal, chemical, electrical, magnetic and optical properties. Zhang et al. [61] have reported ZnO-CNT as electrodes in presence of poly vinyl alcohol and phosphomolybdic acid as a gel polymer electrolyte to improve the capacitance of electrode material [61]. Hu et al. [62] shown the enhanced specific capacitance and energy density of 305.3 F/g and 42.4 Wh/kg, respectively for the PANI and tin oxide based nano-composite material [62].
Conducting Polymers
Polymeric materials are much more cost effective than RuO2 and can produce comparatively higher specific capacitance. The generally used conducting polymers utilized in supercapacitor applications include PANI, PPy, PEDOT and their corresponding derivatives [63] (Fig. 7).
Conducting polymers exhibits red-ox reactions occurring both at the surface of polymer along with entire bulk polymer demonstrating excellent capacitive behaviour. The redox phenomenon in conducting polymers is highly reversible in nature since there is not any phase transformation or structural change. Among the various conducting polymers, PANI is the highly studied polymer due to its inherent exceptional properties for instance economical, chemical stability, environment stability, facile synthesis (chemical or electrochemical synthesis), doping process, dedoping process, mechanical flexibility and wide variety of applications in various fields. Conducting conjugated polymers and their derivatives, act as potential material for energy storage applications due to its exceptionally high electrical conductivity (up to 4.6 × 105 S m−1) and excellent capacitance values (2000 F g−1 for PANI, 620 F g−1 for PPy, depending on the doping level).
PANI mainly occurs in three distinct oxidation states that make it a promising candidate as an electrode material for supercapacitor application. The reduced structure of PANI consists of only benzenoid units and is known as leucoemeralidine i.e. either faded yellow or colorless, where as the fully oxidized form of PANI is composed of quinoid units and is known as pernigraniline having blue/violet color. On the other hand semi oxidized of PANI is composed of both benzenoid and quinoid units and is known as emeralidine i.e. green or slightly blue in colour (Fig. 8).
Although conducting polymers exhibit unique properties such as low cost, light weight, corrosion resistance, large scale production, easy processing, fast redox reactions, and high conductivity but their reduced cycling stability along with the poor mechanical stability has declined the progress of conducting polymer based pseudo-capacitors. Conducting polymers experience a range of physical properties variation with time such as swelling, shrinkage, doping etc. that deteriorates polymer’s performance. However remarkable performance improvement of the conducting polymer based supercapacitor is obtainable using hybrid capacitors that store charge by exploiting Faradic and non-Faradic processes combining the best features of EDLCs and pseudocapacitors together into a unified supercapacitor resulting in advancement of the novel materials with outstanding properties. The key benefit in using these hybrid capacitors over the conducting polymer is that they present superior cyclic stability andexcellent power performance along with higher specific capacitance.
Du et al. [64] reported capacitance of 154.5 F g−1 for CNT/PPY composite (in organic electrolyte) which was considerably higher as compare to that of pure PPy and CNT [64]. In addition reinforcement of PPy with CNT reduces the swelling and shrinkage of polymer during the charge discharge process. Zhang et al. [65] reported synthesis of PPY/MnO2 nanocomposite having higher conductivity (about 4–5 orders of magnitude) and specific capacitance (290 F g−1) than that of MnO2 (221 F g−1) [65]. Huang et al. [66] reported ruthenium oxide/PEDOT:PSS composite having capacitance value of 1409 F g−1 [66]. However use of this composite material is still limited due to its exceptionally high cost. Therefore it’s desirable to make use of cost effective metal oxide that can produce supercapacitor electrode of high specific energy, while being reasonably affordable. Xu et al. [67] reported synthesis of PPy/iron oxide nanohybrid having high capacitance value of 560 F g–1 and outstanding cycling stability [67]. Lee et al. [68] reported composites of PEDOT:PSS wrapped CNT/MnO2 for flexible supercapacitors having capacitance of 428.2 F g−1 and high energy density of 63.8 Wh kg−1 [68].
Thus by using a proper combination of electrode, improvement in the cell voltage, directly resulting in the enhancement of energy and power densities is possible. In general, carbon based materials acts as best electrodes for EDLCs, while for pseudocapacitor the best candidate found to be transition metal oxides and conducting polymers. However, the rapid vanishing of the power density, reduced capacitance retention along with reduced cyclability at high power rates are the some of the major issues hindering the expansion of Hybrid supercapacitors. At present, researchers are focusing mainly on hybrid supercapacitors that are distinguishable by means of the configuration of their electrode and the hybrid supercapacitors are composite-type, asymmetric-type and battery-type.
Composite Electrodes
It involves the combination of carbon-based materials together with either conducting polymer or with metal oxide forming a single electrode, showing charge storage mechanisms involving physical and chemical processes. The process of storage of charge involved in composite electrode is capacitive double layer mechanism obtained from carbon-based materials and the large surface area provided by carbon-based materials improves the contact between electrolyte and pseudocapacitive materials. Composite of MnO2/CNT synthesized using simple hydrothermal approach revealed a large improvement in rate capability and capacitance in comparison to the bare MnO2 or CNT electrode endorsed by the large surface area and porous structure of MnO2 [69].
Asymmetric Hybrids
It involves the coupling of EDLC with a Pseudocapacitor electrode by the integration of faradic and non-faradic processes. Asymmetric hybrids are fabricated with one electrode composed of a double-layer carbon material while another electrode made of a pseudo-capacitance material which can be metal oxide or conducting polymer. By opting an appropriate material for electrode, achieving a high working voltage along with high energy density is possible which contributes in raising the total energy density in supercapacitor. The main advantage of conductive polymers in asymmetric hybrids is their processability but the lack of efficiency has limited their application. This problem can be overcome using negatively charged, activated carbon electrode in place of negatively charged, conducting polymer. Although the conducting polymer electrodes exhibits high capacitance and low resistance compared with activated carbon electrodes, but shows poorer maximum voltage along with lesser cycling stability. Cheng et al. reported synthesis of MnO2/CNT composite used as a asymmetric supercapacitor having MnO2-coated/graphene as cathode electrode while anode electrode is formed by using pure graphene presented higher capacitances and energy densities exhibiting higher capacitances along with the energy and power densities [70].
Battery Type
battery type hybrid is consists of dissimilar electrodes such as a supercapacitor electrode with battery electrode. The obtained combination utilises the properties of supercapacitors as well as batteries within single assembled cell. This specific configuration highlights the requirement of higher energy supercapacitors and higher power batteries, by merging the power, cycle life, energy qualities of batteries by the recharging time of supercapacitors. The first hybrid system was first proposed by Amatucci et al. [71] having Li4Ti5O12 battery-type and AC supercapacitor-type, exhibiting energy density obtained 20 Wh kg−1 inside acetonitrile solution [71]. Positive electrodes of nickel hydroxide, lead dioxide, LiCoO2, Li4Mn5O12, LiMn2O4, LiMn2O4/activated carbon (AC) composite etc. and are used to create the battery type hybrid materials. They helped to accomplish the enhancement in energy density along with power density, high charge-discharge, enhance cycle life time, specific capacitance, etc. Zhang et al. reported hybrid capacitors having LiMn2O4 (LMO) as positive electrode with activated carbon (AC) as negative electrode having excellent performance [72]. Hu et al. [73] presented the battery-supercapacitor hybrid of Li4Ti5O12 acting as an anode, while cathode is made by LiMn2O4/activated carbon composite with superior properties [73]. Although the available experimental figures utilizing hybrid battery type is comparatively lesser than that of other supercapacitors, but still there is ample opportunity to work so that gap present in between the supercapacitors and batteries can be filled in coming future.
5 Conclusion
Global warming and the inevitable depletion of fossil fuels, coupled with the growth of the human population and technology development has resulted in a rapidly increasing global energy demand. Therefore, it is of utmost importance to concentrate on an active search for efficient, rechargeable, renewable, electrical energy storage devices. The current chapter embodies an overview of the advanced hybrid conducting polymer for energy storage applications. The performance of these hybrid conducting polymers depends upon several factors i.e. environmental stability, surface area, conductivity, etc. In this chapter, we have discussed various conducting polymers based composites for energy storage applications. The improvement in the performance values of energy storage devices using these conducting polymer composites gives an indication that these hybrid conducting polymers are capable of bridging the gap existing between supercapacitor and batteries. In addition, they can also play a lead role in the development of smart, efficient, flexible and cost-effective energy storage systems in the coming future.
References
Zhang, C., Wei, Y.L., Cao, P.F., Lin, M.C.: Energy storage system: current studies on batteries and power condition system Renew. Sust. Energ. Rev. 82, 3091 (2018)
Nitta, N., Wu, F., Lee, J.T., Yushin, G.: Li-ion battery materials: present and future Mater. Today 18, 252 (2015)
Gunawardane, K.: Capacitors as energy storage devices—Simple basics to current commercial families. In: Energy Storage Devices for Electronic Systems, p. 137. Academic Press, Elsevier
Kularatna, N.: Capacitors as energy storage devices—simple basics to current commercial families. In: Energy Storage Devices—A General Overview, p. 1. Academic Press, Elsevier (2015)
Zuo, W., Li, R., Zhou, C., Li, Y., Xia, J., Liu, J.: Battery-Supercapacitor Hybrid Devices: Recent Progress and Future Prospects Adv. Sci. 4, 1600539 (2017)
Ballarin B., Masiero S., Seeber R., Tonelli D.: Modification of electrodes with porphyrin-functionalised conductive polymers J. Electroanal. Chem. 449, 173 (1998)
Iqbal, S., Ahmad, S.: Recent development in hybrid conducting polymers: Synthesis, applications and future prospects J. Ind. Eng. Chem. 60, 53 (2018)
Mandal, T.K., Fleming, M.S., Walt, D.R.: Preparation of polymer coated gold nanoparticles by surface-confined living radical polymerization at ambient temperature Nano Lett. 2, 3 (2002)
Corbierre, M.K., Cameron, N.S., Sutton, M., Mochrie, S.G.J., Lurio, L.B., Rühm, A., Lennox, R.B.: Polymer-stabilized gold nanoparticles and their incorporation into polymer matrices J. Am. Chem. Soc. 123, 10411 (2001)
Girtan, M.: On the stability of the electrical and photoelectrical properties of P3HT and P3HT:PCBM blends thin films Org. Electron. Phys. Mater. Appl. 14, 200 (2013)
Chen, B., Liu, C., Ge, L., Hayashi, K.: Electrical conduction and gas sensing characteristics of P3HT/Au nano-islands composite Sens. Actuators B Chem. 241, 1099 (2017)
Zhang, F., Cao, H., Yue, D., Zhang, J., Qu, M.: Enhanced anode performances of polyaniline-TiO2-reduced graphene oxide nanocomposites for lithium ion batteries Inorg. Chem. 51, 9544 (2012)
Wang, Y.G., Wu, W., Cheng, L., He, P., Wang, C.X., Xia, Y.Y.: A Polyaniline‐Intercalated Layered Manganese Oxide Nanocomposite Prepared by an Inorganic/Organic Interface Reaction and Its High Electrochemical Performance for Li Storage Adv. Mater. 20, 2166 (2008)
Mai, L., Xu, X., Han, C., Luo, Y., Xu, L., Wu, Y.A., Zhao, Y.: Rational synthesis of silver vanadium oxides/polyaniline triaxial nanowires with enhanced electrochemical property NanoLett. 11, 4992 (2011)
Zhou, X.Y., Tang, J., Yang, J., Zou, Y.L., Wang, S.C., Xie, J., Ma, L.L.: Effect of polypyrrole on improving electrochemical performance of silicon based anode materials Electrochim. Acta 70, 296 (2012)
Wang, G.J., Yang, L.C., Qu, Q.T., Wang, B., Wu, Y.P., Holze, R.: An aqueous rechargeable lithium battery based on doping and intercalation mechanisms J. Solid State Electrochem. 14, 865 (2010)
Tang, W., Liu, L., Zhu, Y., Sun, H., Wu, Y., Zhu, K.: An aqueous rechargeable lithium battery of excellent rate capability based on a nanocomposite of MoO3 coated with PPy and LiMn2O4 Energy Environ. Sci. 5, 6909 (2012)
Tang, W., Gao, X.W., Zhu, Y.S., Yue, Y.B., Shi, Y., Wu, Y.P., Zhu, K.: An aqueous rechargeable lithium battery of excellent rate capability based on a nanocomposite of MoO3 coated with PPy and LiMn2 O4 J. Mater. Chem. 22, 20143 (2012)
Liu, L.L., Wang, X.J., Zhu, Y.S., Hu, C.L., Wu, Y.P., Holze, R.: A hybrid of V2 O2 nanowires and MWCNTs coated with polypyrrole as an anode material for aqueous rechargeable lithium batteries with excellent cycling performance J. Power Sources 224, 290 (2013)
Tian, F., Liu, L., Yang, Z., Wang, X., Chen, Q., Wang, X.: Polypyrrole-coated LiV3 O8-nanocomposites with good electrochemical performance as anode material for aqueous rechargeable lithium batteries Mater. Chem. Phys. 127, 151 (2011)
Liu, H., Zhang, F., Li, W., Zhang, X., Lee, C., Wang, W., Tang, Y.: Porous tremella-like MoS /polyaniline hybrid composite with enhanced performance for lithium-ion battery anodes Electrochim. Acta 167, 132 (2015)
Yang, L., Wang, S., Mao, J., Deng, J., Gao, Q., Tang, Y., Schmidt, O.G.: Hierarchical MoS2 /Polyaniline Nanowires with Excellent Electrochemical Performance for Lithium‐Ion Batteries Adv. Mater. 25, 1180 (2013)
Liu, J., Gu, M., Ouyang, L., Wang, H., Yang, L., Zhu, M.: Sandwich-like SnS/Polypyrrole Ultrathin Nanosheets as High-Performance Anode Materials for Li-Ion Batteries ACS Appl. Mater. Interfaces 8, 8502 (2016)
Zhao, X., Mai, Y., Luo, H., Tang, D., Lee, B., Huang, C., Zhang, L.: Nano-MoS2 /poly (3,4-ethylenedioxythiophene): Poly(styrenesulfonate) composite prepared by a facial dip-coating process for Li-ion battery anode Appl. Surf. Sci. 288, 736 (2014)
Xie, D., Wang, D.H., Tang, W.J., Xia, X.H., Zhang, Y.J., Wang, X.L., Gu, C.D., Tu, J.P.: Binder-free network-enabled MoS2-PPY-rGO ternary electrode for high capacity and excellent stability of lithium storage J. Power Sources 307, 510 (2016)
Azman, N.H.N., Mamat, M.S., Ngee, L.H., Sulaiman, Y.: Graphene‐based ternary composites for supercapacitors Int. J. Energy Res. 42, 2104 (2018)
Han, S., Ai, Y., Tang, Y., Jiang, J., Wu, D.: Carbonized polyaniline coupled molybdenum disulfide/graphene nanosheets for high performance lithium ion battery anodes RSC Adv. 5, 96660 (2015)
Novoselov, K.S., Jiang, D., Schedin, F., Booth, T.J., Khotkevich, V.V., Morozov, S.V., Geim, A.K.: Two-dimensional atomic crystals Proc. Natl. Acad. Sci. U.S.A. 102, 10451 (2005)
Paek, S.M., Yoo, E., Honma, I.: Enhanced Cyclic Performance and Lithium Storage Capacity of SnO2 /Graphene Nanoporous Electrodes with Three-Dimensionally Delaminated Flexible Structure Nano Lett. 9, 72 (2009)
Wang, H., Yang, Y., Liang, Y., Robinson, J.T., Li, Y., Jackson, A., Cui, Y., Dai, H.: Graphene-Wrapped Sulfur Particles as a Rechargeable Lithium–Sulfur Battery Cathode Material with High Capacity and Cycling Stability Nano Lett. 11, 2644 (2011)
Luo, J., Liu, J., Zeng, Z., Ng, C.F., Ma, L., Zhang, H., Lin, J., Shen, Z., Fan, H.J.: Three-Dimensional Graphene Foam Supported Fe3 O3 Lithium Battery Anodes with Long Cycle Life and High Rate Capability Nano Lett. 13, 6136 (2013)
Gao, F., Qu, J.Y., Zhao, Z.B., Dong, Y.F., Yang, J., Dong, Q., Qiu, J.S.: Easy synthesis of MnO-graphene hybrids for high-performance lithium storage New Carbon Mater. 29, 316 (2014)
David, L., Bhandavat, R., Singh, G.: MoS /Graphene Composite Paper for Sodium-Ion Battery Electrodes ACS Nano 8, 1759 (2014)
Qu, B., Ma, C., Ji, G., Xu, C., Xu, J., Meng, Y.S., Wang, T., Lee, J.Y.: Layered SnS2-reduced graphene oxide composite--a high-capacity, high-rate, and long-cycle life sodium-ion battery anode material Adv. Mater. 26, 3854 (2014)
Yuan, F.W., Tuan, H.Y.: Scalable Solution-Grown High-Germanium-Nanoparticle-Loading Graphene Nanocomposites as High-Performance Lithium-Ion Battery Electrodes: An Example of a Graphene-Based Platform toward Practical Full-Cell Applications Chem. Mater. 26, 2172 (2014)
Murugan, A.V., Muraliganth, T., Manthiram, A.: Rapid, Facile Microwave-Solvothermal Synthesis of Graphene Nanosheets and Their Polyaniline Nanocomposites for Energy Strorage Chem. Mater. 21, 5004 (2009)
Wang, H., Tran, D., Qian, J., Ding, F., Losic, D.: MoS2 /Graphene Composites as Promising Materials for Energy Storage and Conversion Applications Adv. Mater. Interfaces 1900915 (2019)
Li, Q., Guo, X., Zheng, M., Pang, H.: Some MoS2-based materials for sodium-ion battery Funct. Mater. Lett. 11, 1840004 (2018)
Nan J., Guo X., Xiao J., Li X., Chen W., Wu W., Liu H., Wang Y., Wu M., Wang G.: Nanoengineering of 2D MXene‐Based Materials for Energy Storage Applications Small 1902085 (2019)
Ling, Z., Ren, C.E., Zhao, M.Q., Yang, J., Giammarco, J.M., Qiu, J., Barsoum, M.W., Gogotsi, Y.: Flexible and conductive MXene films and nanocomposites with high capacitance Proc. Natl. Acad. Sci. USA 111, 16676 (2014)
Boota, M., Anasori, B., Voigt, C., Zhao, M.Q., Barsoum, M.W., Gogotsi, Y.: Pseudocapacitive Electrodes Produced by Oxidant-Free Polymerization of Pyrrole between the Layers of 2D Titanium Carbide (MXene) Adv. Mater. 28, 1517 (2016)
Qin, L., Tao, Q., Liu, X., Fahlman, M., Halim, J., Persson, P.O., Rosen, J., Zhang, F.: Polymer-MXene composite films formed by MXene-facilitated electrochemical polymerization for flexible solid-state microsupercapacitors Nano Energy 60, 734 (2019)
Zhu, M., Huang, Y., Deng, Q., Zhou, J., Pei, Z., Xue, Q., Huang, Y., Wang, Z., Li, H., Huang, Q., Zhi, C.: Highly Flexible, Freestanding Supercapacitor Electrode with Enhanced Performance Obtained by Hybridizing Polypyrrole Chains with MXene Adv. Energy Mater. 6, 1600969 (2016)
Conway, B.E.: Electrochemical Supercapacitors: Scientific Fundamentals and Technological Applications. Kluwer-Plenum, New York (1999)
Winter, M., Brodd, R.J.: What Are Batteries, Fuel Cells, and Supercapacitors? Chem. Rev. 104, 4245 (2004)
Wang, Y., Shi, Z., Huang, Y., Ma, Y., Wang, C., Chen, M., Chen, Y.: Supercapacitor Devices Based on Graphene Materials J. Phys. Chem. C 113, 13103 (2009)
Zhang, L.L., Zhao, X.S.: Carbon-based materials as supercapacitor electrodes Chem. Soc. Rev. 38, 2520 (2009)
Wu, Z., Li, L., Yan, J., Zhang, X.: Materials Design and System Construction for Conventional and New‐Concept Supercapacitors Adv. Sci. 4(6), 1600382 (2017)
Zhang J., Zhang L., Sun X., Liu H., Sun A., Liu R.S., Zhang, J.: Electrochemical Technologies for Energy Storage and Conversion, 1st edn. Wiley (2012)
Simon, P., Gogotsi, Y.: Materials for electrochemical capacitors Nat. Mater. 7, 845 (2008)
Helmholtz, H.V.: Some laws concerning the distribution of electrical currents in conductors Ann. Phys. (Leipzig) 89, 21 (1853)
Gouy, G.: Constitution of the Electric Charge at the Surface of an Electrolyte J. Phys. 4, 457 (1910)
Chapman, D.L.: A contribution to the theory of electrocapillarity Philos. Mag. 6, 475 (1913)
Stern, O.: The theory of the electrolytic double shiftThe theory of the electrolytic double shift Z. Electrochem. 30, 508 (1924)
Lota, K., Khomenko, V., Frackowiak, E.: Capacitance properties of poly(3,4-ethylenedioxythiophene)/carbon nanotubes composites J. Phys. Chem. Solids 65, 295 (2004)
Chen, L.M., Lai, Q.Y., Hao, Y.J., Huang, J.H., Ji, X.Y.: Pseudo-capacitive properties of LiCoO2/AC electrochemical capacitor in various aqueous electrolytes Ionics 14, 441 (2010)
Tang, C., Liu, Y., Yang, D., Yang, M., Li, H.: Oxygen and nitrogen co-doped porous carbons with finely-layered schistose structure for high-rate-performance supercapacitors Carbon 122, 538 (2017)
Niu, C., Sichel, E.K., Hoch, R., Moy, D., Tennent, H.: High power electrochemical capacitors based on carbon nanotube electrodes Appl. Phys. Lett. 70, 1480 (1997)
Zeng, Q., Tian, H., Jiang, J., Ji, X., Gao, D., Wang, C.: High-purity helical carbon nanotubes with enhanced electrochemical properties for supercapacitors RSC Adv. 7, 7375 (2017)
González, A., Goikole, E., Barrena, J.A., Mysyk, R.: Review on supercapacitors: Technologies and materials Renew. Sust. Energ. Rev. 58, 1189 (2016)
Zhang Y., Sun X., Pan L., Li H., Sun Z., Sun C., Tay B.K.: Carbon nanotube–zinc oxide electrode and gel polymer electrolyte for electrochemical supercapacitors J. Alloys Compd. 480, L17 (2009)
Hu, Z.A., Xie, Y.L., Wang, Y.X., Mo, L.P., Yang, Y.Y., Zhang, Z.Y.: Polyaniline/SnO nanocomposite for supercapacitor applications Mater. Chem. Phys. 114, 990 (2009)
Yan, Y., Wang, T., Li, X., Pang, H., Xue, H.: Noble metal-based materials in high-performance supercapacitors Inorg. Chem. Front. 4, 33 (2017)
Du, B., Jiang, Q., Zhao, X.F., Huang, B., Zhao, Y.: Preparation of PPy/CNT Composite Applications for Supercapacitor Electrode Material Mater. Sci. Forum 610–613, 502 (2009)
Zhang, X., Yang, W., Ma, Y.: Synthesis of Polypyrrole-Intercalated Layered Manganese Oxide Nanocomposite by a Delamination⁄Reassembling Method and Its Electrochemical Capacitance Performance Electrochem. Solid St. 12, A95 (2009)
Huang, L.M., Wen, T.C., Gopalan, A.: Electrochemical and spectroelectrochemical monitoring of supercapacitance and electrochromic properties of hydrous ruthenium oxide embedded poly(3,4-ethylenedioxythiophene)–poly(styrene sulfonic acid) composite Electrochim. Acta 51, 3469 (2006)
Xu, C., Puente-Santiago, A.R., Padron, D.R., Caballero, A., Balu, A.M., Romero, A.A., Muñoz-Batista, M.J., Luque, R.: Controllable Design of Polypyrrole-Iron Oxide Nanocoral Architectures for Supercapacitors with Ultrahigh Cycling Stability ACS Appl. Energy Mater. 2(3), 2161 (2019)
Lee, H.U., Yin, J.L., Park, S.W., Park, J.Y.: Preparation and characterization of PEDOT:PSS wrapped carbon nanotubes/MnO2 composite electrodes for flexible supercapacitors Synth. Met. 228, 84 (2017)
Xia, H., Wang, Y., Lin, J., Lu, L.: Hydrothermal synthesis of MnO2/CNT nanocomposite with a CNT core/porous MnO2 sheath hierarchy architecture for supercapacitors Nanoscale Res. Lett. 7, 33 (2012)
Cheng, Q., Tang, J., Ma, J., Zhang, H., Shinya, N., Qin, L.C.: Graphene and nanostructured MnO2 composite electrodes for supercapacitors Carbon 49, 2917 (2011)
Amatucci, G.G., Badway, F., Pasquier, A.D., Zheng, T.: An Asymmetric Hybrid Nonaqueous Energy Storage Cell J. Electrochem. Soc. 148, A930 (2001)
Zhang, S., Li, C., Zhang, X., Sun, X., Wang, K., Ma, Y.: High Performance Lithium-Ion Hybrid Capacitors Employing Fe3O4–Graphene Composite Anode and Activated Carbon Cathode ACS Appl. Mater. Interfaces 20, 17136 (2017)
Hu, X., Deng, Z., Suo, J., Pan, Z.: A high rate, high capacity and long life (LiMn2O4 + AC)/Li4 Ti5O12 hybrid battery–supercapacitor J. Power Sources 187, 635 (2009)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Gusain, M., Singh, P., Zhan, Y. (2021). Energy Storage Devices (Supercapacitors and Batteries). In: Shahabuddin, S., Pandey, A.K., Khalid, M., Jagadish, P. (eds) Advances in Hybrid Conducting Polymer Technology. Engineering Materials. Springer, Cham. https://doi.org/10.1007/978-3-030-62090-5_3
Download citation
DOI: https://doi.org/10.1007/978-3-030-62090-5_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-62089-9
Online ISBN: 978-3-030-62090-5
eBook Packages: EngineeringEngineering (R0)