Abstract
Peroxisomopathies are qualitative or quantitative deficiencies in peroxisomes which lead to increases in the level of very-long-chain fatty acids (VLCFA) and can be associated with more or less pronounced dysfunction of central nervous system cells: glial and microglial cells. Currently, in frequent neurodegenerative diseases, Alzheimer’s disease (AD) and multiple sclerosis (MS), peroxisomal dysfunction is also suspected due to an increase in VLCFA, which can be associated with a decrease of plasmalogens, in these patients. Moreover, in patients suffering from peroxisomopathies, such as X-linked adrenoleukodystrophy (X-ALD), AD, or MS, the increase in oxidative stress observed leads to the formation of cytotoxic oxysterols: 7-ketocholesterol (7KC) and 7β-hydroxycholesterol (7β-OHC). These observations led to the demonstration that 7KC and 7β-OHC alter the biogenesis and activity of peroxisomes in glial and microglial cells. In X-ALD, AD, and MS, it is suggested that 7KC and 7β-OHC affecting the peroxisome, and which also induce mitochondrial dysfunctions, oxidative stress, and inflammation, could promote neurodegeneration. Consequently, the study of oxisome in peroxisomopathies, AD and MS, could help to better understand the pathophysiology of these diseases to identify therapeutic targets for effective treatments.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Oxysterol
- 7-ketocholesterol
- 7β-hydroxycholesterol
- Peroxisome
- Very-long-chain fatty acids
- Alzheimer’s disease
- Multiple sclerosis
- X-linked adrenoleukodystrophy
1 Neurodegeneration and Peroxisomal Disorders
1.1 Peroxisome and Peroxisomopathies
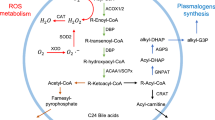
Peroxisomes, which are devoid of DNA, are single cell membrane organelles present in nearly all eukaryotic cells [1]. Depending on the cell type, elongated tubular (>2 μm in length) or spherical (0.1–1 μm) peroxisomes can be observed by transmission electron microscopy (TEM) [2] (Fig. 3.1). The identification of peroxisomes by immunofluorescence with the use of appropriated antibodies directed against specific peroxisomal antigens, such as the Abcd3, gives information on the peroxisomal topography (conventional immunofluorescence, confocal microscopy) and on the peroxisomal mass per cell (flow cytometry) (Fig. 3.2). Peroxisomes are essential to maintain Redox homeostasis and have important roles in lipid metabolism: β-oxidation of very-long-chain fatty acids (VLCFAs) and branched fatty acids, synthesis of docosahexaenoic acid (DHA, C22:6 n-3) and of plasmalogens which are major components of the myelin sheath [1]. There are also several evidences that peroxisome and mitochondria are tightly connected organelles playing key roles in cellular ageing [3]. It is now well-established that peroxisomal changes can directly or indirectly affect mitochondrial activity, and reciprocally [3].
Identification of peroxisomes in neural cells by transmission electron microscopy. Peroxisomes can be identified by various techniques, including transmission electron microscopy (TEM). Dark arrows point towards peroxisomes and white arrows towards mitochondria. Round peroxisomes were observed. The diameters were in the range of 0.5 μm in mouse liver, murine oligodendrocytes 158 N, and human neuronal SK-NB-E cells and of 0.1 μm in murine neuronal N2a cells
Analysis of peroxisomes in neural cells by flow cytometry and fluorescence microscopy. The peroxisomes were revealed by indirect immunofluorescence with a rabbit polyclonal primary antibody directed against Abcd3 (ref # 11523651, Pierce / Thermo Fisher Scientific, Montigny le Bretronneux, France) and with a secondary goat anti-rabbit antibody coupled with 488-Alexa (Thermo Fisher Scientific). The peroxisomal mass was quantified by flow cytometry. A fluorescent microscope coupled to an Apotome-structured illumination system (Imager M2, Zeiss) was used to visualized the peroxisomes; the fluorescent signals of the samples were collected with the ZEN software (Zeiss). For microscopical observations, the nuclei were stained with Hoechst 33342 (2 μg/mL)
Peroxisomopathies, which are rare diseases of genetic origin affecting the central and/or peripheral nervous system, include peroxisome biogenesis disorders (Zellweger syndrome), multiple peroxisomal enzyme deficiency (rhizomelic chondrodysplasia punctata), and single peroxisomal enzyme deficiency, such as X-linked adrenoleukodystrophy (X-ALD, MIM 300100), Acyl-CoA oxidase deficiency, and D-Bifunctional protein deficiency [4]. X-ALD, which is the most frequent peroxisomal leukodystrophy of childhood, is characterized by progressive central nervous system (CNS) demyelination [5]. X-ALD is caused by mutations of the ABCD1 gene which encodes for a peroxisomal ABC half-transporter (ATP-binding cassette member 1 (ABCD1) also named adrenoleukodystrophy protein (ALDP)) involved in the import of very-long-chain fatty acids (VLCFAs) into the peroxisome [6]. These different forms of peroxisomopathies are all characterized by increased plasma levels of VLCFAs due to an impaired β-oxidation in the peroxisome and/or increased elongation [7]. The initial diagnosis of peroxisomopathies relies on the clinical presentation, brain-imaging, and biochemical analyses of VLCFAs, especially C24:0 and C26:0, which are elevated in the plasma and tissues of patients [7]. Newborn screening is based on the measurement of C26:0 lysophosphatidylcholine (26:0-lyso-PC) in dried blood spots [7]. At the moment, several studies have shown cytotoxic effects of VLCFA in vitro and in vivo [8, 9]. In vitro, on different types of nerve cells (neurons, glial, and microglial cells), C24:0 and C26:0 often induce a non-apoptotic mode of cell death associated with Ca2+ raise [10], K+ homeostasis disruption [11], reactive oxygen species (ROS) and reactive nitrogen species (RNS) overproduction [8, 12], and autophagic criteria [13]. In vivo, lysophosphatidylcholine (C24:0) injection into the parietal cortex of mice led to widespread microglial activation and apoptosis [14]. However, in human, there is no apparent correlation between the VLCFA level and the phenotype in X-ALD patients [7]. Therefore, other factors than VLCFA, including lipid and non-lipid compounds, are suspected to trigger neurodegeneration in peroxisomopathies. At the moment, some signs of oxidative stress, considered as a hallmark of neurodegeneration, have been detected in the plasma and brain of X-ALD patients [15] and in patients with frequent neurodegenerative diseases: Alzheimer’s disease (AD), Parkinson’s disease (PD), and multiple sclerosis (MS) [16]. As VLCFA (C24:0; C26:0) are potent inducers of oxidative stress [8, 12], they can favor the production of protein and lipid oxidation products capable to trigger cytotoxic effects. The lipid peroxidation products generated include several aldehydes [8], which are known to induce protein carbonylation, as well as some cholesterol oxidation products (oxysterols), including 7-ketocholesterol (7KC; also named 7-oxocholesterol) and 7β-hydroxycholesterol (7β-OHC) [8]. 7KC and 7β-OHC, which are mainly formed by cholesterol auto-oxidation, are strongly cytotoxic [17]. These oxysterols induce a mode of cell death by oxiapoptophagy involving oxidative stress, apoptosis, and autophagy [18], which are hallmarks of neurodegeneration. Currently, increased levels of 7KC and 7β-OHC have been described in the plasma of patients with different forms of X-ALD [19]: (i) cerebral demyelination, inflammatory childhood phenotypes (CCALD: childhood cerebral ALD), which is associated with a poor prognosis and rapidly progresses to dementia and death; (ii) cerebral juvenile and adult forms with demyelination in the CNS (ACALD: adolescent cerebral ALD); (iii) adulthood forms without CNS demyelination (AMN: adrenomyeloneuropathy) defined as a spinal cord and peripheral nerve disease; and (iv) Addison’s disease, which is characterized by adrenal insufficiency only. It is therefore hypothesized that 7KC and 7β-OHC could contribute to neurodegeneration in peroxisomopathies, especially in X-ALD, and also in other major neurodegenerative diseases [20].
1.2 Potential Involvement of Peroxisomal Changes in Major Neurodegenerative Diseases: Alzheimer’s Disease and Multiple Sclerosis
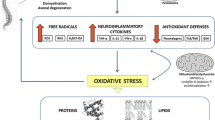
Peroxisomal abnormalities associated with peroxisomopathies led to the clarification of the role of this organelle in neurodegeneration occurring in frequent neurodegenerative diseases, such as AD and MS. Currently, potential roles of peroxisomes are suspected in AD and in dementia of the Alzheimer’s type. Indeed, an accumulation of C22:0 and VLCFAs (C24:0; C26:0), all substrates for peroxisomal β-oxidation, was observed in the cortical regions of AD patients with stages V–VI of the disease compared with those modestly affected (stages I–II); conversely, the level of plasmalogens, which need intact peroxisomes for their biosynthesis, was decreased [21]. In addition, in demented patients, including AD patients, the variations of fatty acid levels and the accumulation of C26:0 in the plasma and red blood cells highlight an alteration of fatty acid metabolism and point towards possible peroxisomal dysfunction [22]. In MS patients, a reduction in neuronal peroxisomes in MS grey matter has been reported [23], and alteration in the metabolism of VLCFAs, which could be a consequence of defective peroxisomes, has been described [24]. In the brain of patients with AD, increased levels of 7KC and 7β-OHC have also been shown [25]. It is important to underline that these oxysterols can be formed under the action of VLCFA-induced oxidative stress [8] and under stress conditions induced by amyloid-β (Aβ) proteins, mainly Aβ42, which accumulates in brain lesions of patients with AD. High amount of 7KC have been reported in the cerebrospinal fluid of MS patients [26] as well as low levels [27]. 7KC values might depend on the type of MS considered (Progressive-MS (P-MS) versus remittent recurrent – MS (RR-MS)) [28]. It is however widely accepted that oxysterols are considered as bonafide lipid mediators. To be qualified as a bonafide lipid mediator, a lipid compound should meet three conditions: (i) to be endogenous, (ii) to have its levels altered depending on the physiological or pathological situation, and (iii) to induce a signaling response when its levels are altered. As a result, abnormal levels of 7KC (low or high) can alter cellular behavior. It is therefore hypothesized that 7KC, 7β-OHC could favor peroxisomal and mitochondrial dysfunction leading to neurodegeneration in patients with AD and MS [20] (Fig. 3.3). This led us to specify the impact of different concentrations of 7KC and/or 7β-OHC on murine glial cells (murine oligodendrocyte 158 N [29], murine microglial BV-2 cells [19], murine neuronal N2a cells, human neuroblastoma SK-N-BE cells, and on rat C6 astrocytoma cells [30, 31], taking into account the effects on organelles (peroxisomes, mitochondria and lysosomes), oxidative stress, and the ability to induce cell death (apoptosis/necrosis) and to activate autophagy.
Incidence of peroxisomal dysfunction in neurodegeneration: hypothetic model. Environmental factors (oxysterols: 7-ketocholesterol (7KC) and 7β-hydroxycholesterol (7β-OHC)) resulting from rupture or Redox homeostasis, which is a hallmark of neurodegenerative diseases, are often enhanced in the biological fluids and in the brain of patients with neurodegenerative disease. These oxysterols could trigger peroxisomal and mitochondrial damages which could further favor bioenergetic failure, overproduction of ROS, and activation of inflammatory processes. In turn, these events could contribute to amplify brain damages via secondary mitochondrial and peroxisomal dysfunctions contributing to the overproduction of oxysterols, thereby creating an amplification loop [20]
2 In Vitro Evidence of 7-Ketocholesterol- and 7β-Hydroxycholesterol-Induced Peroxisomal Damages in Nerve Cells
The cytotoxic effects of 7KC and 7β-OHC have been studied on several nerve cell lines: murine oligodendrocyte 158 N cells, murine microglial BV-2 cells, murine neuronal N2a cells, and C6 rat glioma cells. On 158 N and/or BV-2 cells, 7KC and 7β-OHC induce a mode of cell death by oxiapoptophagy (OXIdative stress + APOPTOsis + autoPHAGY) characterized by ROS overproduction revealed by dihydroethidium staining, a decrease of oxidative phosphorylation associated with a loss of transmembrane mitochondrial potential (ΔΨm) measured with DiOC6(3), reduced expression of Bcl-2, caspase-3 activation, poly-ADP-ribose polymerase (PARP) degradation, and condensation and/or fragmentation of the nuclei which are typical criteria of oxidative stress and apoptosis [18, 19, 32, 33]. Moreover, 7KC and 7β-OHC also enhance cytoplasmic membrane permeability to propidium iodide and induce acidic vesicular organelle formation evaluated with acridine orange which evocate autophagic vesicles in agreement with observations realized by TEM [32, 33]. In addition, 7KC and 7β-OHC promote conversion of microtubule-associated protein light chain 3 (LC3-I) to LC3-II which is a characteristic of autophagy [18, 32], and 7KC increases the expression of p62 an autophagosome cargo protein involved in the sequestosome/aggresome formation preceding the autophagosome formation [19, 29]. On C6 rat glioma cells, it has been demonstrated that 7β-OHC induces survival autophagy, since rapamycin, an autophagic inducer, and 3-methyladenine, an autophagic inhibitor, reduce and increase 7β-OHC-induced cell death, respectively [31]. On 158 N cells, under treatment with 7KC used at sub-toxic (25 μM, 24 h) and toxic (50 μM, 24 h) concentrations, important peroxisomal changes were observed even in the absence of oxiapoptophagy which was only induced at 50 μM [31] (Fig. 3.4). In the presence of 7KC (25 μM), only slight mitochondrial dysfunction and oxidative stress were found as well as modifications of the cytoplasmic distribution of mitochondria: clusters of mitochondria were detected. Thus, the peroxisomal alterations observed were similar with 7KC used at 25 and 50 μM [29]: presence of peroxisomes with abnormal sizes and shapes observed by TEM; lower cellular level of ATP-binding cassette transporter member 3 (ABCD3) used as a marker of peroxisomal mass (measured by flow cytometry); lower mRNA and protein levels (measured by RT-qPCR and western blotting) of ABCD1 and ABCD3 (two ATP-dependent peroxisomal transporters), of two peroxisomal enzymes Acyl-CoA Oxidase 1 (ACOX1) and multifunctional protein 2 (MFP2) enzymes involved in peroxisomal β-oxidation, and lower mRNA level of dihydroxyacetone-phosphate acyltransferase (DHAPAT), involved in peroxisomal β-oxidation and plasmalogen synthesis; and increased levels of VLCFAs (C24:0, C24:1, C26:0, and C26:1) quantified by gas chromatography coupled with mass spectrometry metabolized by peroxisomal β-oxidation. On 158 N cells, under treatment with 7β-OHC (50 μM, 24 h), morphological alterations of mitochondria and peroxisomes were simultaneously observed [33]. These data obtained on 158 N cells support the following hypotheses: (i) the peroxisome would be more sensitive to 7KC than the mitochondria (7KC used at 25 μM induced important peroxisomal changes, whereas the mitochondria was slightly affected); (ii) peroxisomal changes can occur without signs of cytotoxicity (induction of oxiapoptophagy); and (iii) peroxisomal changes could precede mitochondrial dysfunctions required to induce oxiapoptophagy. These peroxisomal dysfunctions may be associated with oligodendrocyte degeneration and may contribute to demyelination in X-ALD and MS. On BV-2 cells, 7KC also induced several peroxisomal modifications: decreased Abcd1, Abcd2, Abcd3, Acox1, and/or Mfp2 mRNA and protein levels, increased catalase activity, and decreased Acox1-activity [19]. In microglial cells, 7-ketocholesterol- and 7β-hydroxycholesterol-induced peroxisomal alterations may favor the pro-inflammatory phenotype of these cells, thereby contributing to brain inflammation often seen in neurodegenerative diseases.
Association of peroxisomal damages with mitochondrial dysfunctions in 7-ketocholesterol-treated 158 N murine oligodendrocytes. 7-ketocholesterol (7KC)-induced topographical, morphological, and functional peroxisomal changes are observed either with slight or strong mitochondrial dysfunctions, slight or strong ROS overproduction, and without or with cell death induction (oxiapoptophagy) on 158 N cells. The cellular model (158 N cells treated with 7KC) has been used to clarify the relationships between peroxisome and mitochondria (and vis versa) during 7KC-induced side effects leading either to slight cytotoxic effects (7KC: 25 μM) or oxiapoptophagy (7KC: 50 μM) The figure was reproduced with the written consent of Elsevier Editor (Nury T et al., Induction of peroxisomal changes in oligodendrocytes treated with 7-ketocholesterol: Attenuation by α-tocopherol [29])
3 Pharmacological Consequences of the Involvement of 7-Ketocholesterol- and 7β-Hydroxycholesterol-Induced Peroxisomal Damages for the Treatment of Neurodegenerative Diseases
Under the effect of 7KC and 7β-OHC, the signaling pathways involved in oxidative stress, inflammation, and cell death, associated with apoptotic and autophagic criteria, have the advantage of being fairly well-known [17, 33]. This made it possible to identify natural and synthetic molecules as well as mixtures of molecules (argan, olive, and milk thistle oils) [34] having cytoprotective activities with respect to 7KC and 7β-OHC and capable to prevent peroxisomal and mitochondrial dysfunctions. Several molecules are able to attenuate the cytotoxic effects of 7KC on different cell types, but only few are very efficient. Currently, the only molecules identified strongly counteracting the toxicity of 7KC and 7β-OHC are α-tocopherol, docosahexaenoic acid (DHA, C22:6 n-3), and oleic acid (C18:1 n-9) [18]; biotin is only cytoprotective for 7β-OHC. Among the synthetic molecules, only dimethyl fumarate (DMF), marketed under the name Tecfidera for the treatment of MS, as well as its major metabolite, monomethyl fumarate (MMF), protect against oxiapoptophagy [33]. As more and more arguments are in favor of the relationships between neurodegeneration, peroxisomal, and mitochondrial dysfunction and the involvement of oxysterols, to oppose the cytotoxic activities of these molecules could pave the way for new therapeutic approaches in both peroxisomopathies and frequent neurodegenerative diseases, such as AD and MS.
4 Conclusion
Although the auto-oxidation of cholesterol essentially leads to the formation of oxidized cholesterol derivatives in the 7-position, the formation of oxidized cholesterol derivatives at the 4-, 5-, and 6-positions should be studied. An analysis of the oxisome by appropriate analytical biochemistry methods could also be informative. This approach would also take into account the metabolites of 7KC and 7β-OHC and also other enzymatically formed oxysterols (24S-hydroxycholesterol (also named cerebrosterol), 27-hydroxycholesterol, 25-hydroxycholesterol, and derivatives) known to be involved in neurodegeneration. The study of the impact of oxysterols on the organelles of nerve cells (glial and microglial cells, neurons) in relation to oxidative stress, inflammation, and cell death as well as the incidence of these molecules on certain nuclear receptors (peroxisome proliferator-activated receptors (PPARs), Liver X receptors (LXRs) in particular) offers many perspectives to better know and better treat neurodegenerative diseases.
Abbreviations
- ABC:
-
ATP-binding cassette
- ACALD:
-
adolescent cerebral adrenoleukodystrophy
- ACOX1:
-
Acyl-CoA oxidase 1
- AD:
-
Alzheimer’s disease
- ALDP:
-
adrenoleukodystrophy protein
- AMN:
-
adrenomyeloneuropathy
- CCALD:
-
childhood cerebral adrenoleukodystrophy
- CNS:
-
central nervous system
- DHA:
-
docosahexaenoic acid
- DHAPAT:
-
dihydroxyacetone-phosphate acyltransferase
- DMF:
-
dimethyl fumarate
- DNA:
-
deoxyribonucleic acid
- LC3:
-
protein light chain 3
- LXR:
-
Liver X receptor
- MFP2:
-
multifunctional protein 2
- MMF:
-
monomethyl fumarate
- MS:
-
multiple sclerosis
- PARP:
-
poly-ADP-ribose polymerase
- PD:
-
Parkinson’s disease
- P-MS:
-
progressive MS
- PPAR:
-
peroxisome proliferator-activated receptor
- RNS:
-
reactive nitrogen species
- RR-MS:
-
remittent recurrent-MS
- ROS:
-
reactive oxygen species
- TEM:
-
transmission electron microscopy
- VLCFA:
-
very-long-chain fatty acid
- X-ALD:
-
X-linked adrenoleukodystrophy
- 7β-OHC:
-
7β-hydroxycholesterol
- 7KC:
-
7-ketocholesterol
References
Schrader M, Fahimi HD (2008) The peroxisome: still a mysterious organelle. Histochem Cell Biol 129(4):421–440
Schrader M, Fahimi HD (2006) Growth and division of peroxisomes. Int Rev Cytol 255:237–290
Lismont C, Nordgren M, Van Veldhoven PP, Fransen M (2015) Redox interplay between mitochondria and peroxisomes. Front Cell Dev Biol 3:35
Depreter M, Espeel M, Roels F (2003) Human peroxisomal disorders. Microsc Res Tech 61(2):203–223
Kemp S, Huffnagel IC, Linthorst GE, Wanders RJ, Engelen M (2016) Adrenoleukodystrophy - neuroendocrine pathogenesis and redefinition of natural history. Nat Rev Endocrinol 12(10):606–615
Berger J, Forss-Petter S, Eichler FS (2014) Pathophysiology of X-linked adrenoleukodystrophy. Biochimie 98:135–142
Engelen M, Kemp S, de Visser M, van Geel BM, Wanders RJ, Aubourg P, Poll-The BT (2012) X-linked adrenoleukodystrophy (X-ALD): clinical presentation and guidelines for diagnosis, follow-up and management. Orphanet J Rare Dis 7:51
Baarine M, Andréoletti P, Athias A, Nury T, Zarrouk A, Ragot K, Vejux A, Riedinger JM, Kattan Z, Bessede G, Trompier D, Savary S, Cherkaoui-Malki M, Lizard G (2012) Evidence of oxidative stress in very long chain fatty acid—treated oligodendrocytes and potentialization of ROS production using RNA interference-directed knockdown of ABCD1 and ACOX1 peroxisomal proteins. Neuroscience 213:1–18
Wanders RJ, Ferdinandusse S, Brites P, Kemp S (2010) Peroxisomes, lipid metabolism and lipotoxicity. Biochim Biophys Acta 1801(3):272–280
Hein S, Schönfeld P, Kahlert S, Reiser G (2008) Toxic effects of X-linked adrenoleukodystrophy-associated, very long chain fatty acids on glial cells and neurons from rat hippocampus in culture. Hum Mol Genet 17(12):1750–1761
Bezine M, Debbabi M, Nury T, Ben-Khalifa R, Samadi M, Cherkaoui-Malki M, Vejux A, Raas Q, de Sèze J, Moreau T, El-Ayeb M, Lizard G (2017) Evidence of K(+) homeostasis disruption in cellular dysfunction triggered by 7-ketocholesterol, 24S-hydroxycholesterol, and tetracosanoic acid (C24:0) in 158N murine oligodendrocytes. Chem Phys Lipids 207(Pt B):135–150
López-Erauskin J, Fourcade S, Galino J, Ruiz M, Schlüter A, Naudi A, Jove M, Portero-Otin M, Pamplona R, Ferrer I, Pujol A (2011) Antioxidants halt axonal degeneration in a mouse model of X-adrenoleukodystrophy. Ann Neurol 70(1):84–92
Doria M, Nury T, Delmas D, Moreau T, Lizard G, Vejux A (2019) Protective function of autophagy during VLCFA-induced cytotoxicity in a neurodegenerative cell model. Free Radic Biol Med 137:46–58
Eichler FS, Ren JQ, Cossoy M, Rietsch AM, Nagpal S, Moser AB, Frosch MP, Ransohoff RM (2008) Is microglial apoptosis an early pathogenic change in cerebral X-linked adrenoleukodystrophy? Ann Neurol 63(6):729–742
Deon M, Marchetti DP, Donida B, Wajner M, Vargas C (2016) Oxidative stress in patients with X-linked Adrenoleukodystrophy. Cell Mol Neurobiol 36(4):497–512
Niedzielska E, Smaga I, Gawlik M, Moniczewski A, Stankowicz P, Pera J, Filip M (2016) Oxidative stress in neurodegenerative diseases. Mol Neurobiol 53(6):4094–4125
Vejux A, Lizard G (2009) Cytotoxic effects of oxysterols associated with human diseases: induction of cell death (apoptosis and/or oncosis), oxidative and inflammatory activities, and phospholipidosis. Mol Asp Med 30(3):153–170
Nury T, Zarrouk A, Mackrill JJ, Samadi M, Durand P, Riedinger JM, Doria M, Vejux A, Limagne E, Delmas D, Prost M, Moreau T, Hammami M, Delage-Mourroux R, O’Brien NM, Lizard G (2015) Induction of oxiapoptophagy on 158N murine oligodendrocytes treated by 7-ketocholesterol-, 7β-hydroxycholesterol-, or 24(S)-hydroxycholesterol: protective effects of α-tocopherol and docosahexaenoic acid (DHA; C22:6 n-3). Steroids 99(Pt B):194–203
Nury T, Zarrouk A, Ragot K, Debbabi M, Riedinger JM, Vejux A, Aubourg P, Lizard G (2017) 7-Ketocholesterol is increased in the plasma of X-ALD patients and induces peroxisomal modifications in microglial cells: potential roles of 7-ketocholesterol in the pathophysiology of X-ALD. J Steroid Biochem Mol Biol 169:123–136
Trompier D, Vejux A, Zarrouk A, Gondcaille C, Geillon F, Nury T, Savary S, Lizard G (2014) Brain peroxisomes. Biochimie 98:102–110
Kou J, Kovacs GG, Höftberger R, Kulik W, Brodde A, Forss-Petter S, Hönigschnabl S, Gleiss A, Brügger B, Wanders R, Just W, Budka H, Jungwirth S, Fischer P, Berger J (2011) Peroxisomal alterations in Alzheimer’s disease. Acta Neuropathol 122(3):271–283
Zarrouk A, Riedinger JM, Ahmed SH, Hammami S, Chaabane W, Debbabi M, Ben Ammou S, Rouaud O, Frih M, Lizard G, Hammami M (2015) Fatty acid profiles in demented patients: identification of hexacosanoic acid (C26:0) as a blood lipid biomarker of dementia. J Alzheimers Dis 44(4):1349–1359
Gray E, Rice C, Hares K, Redondo J, Kemp K, Williams M, Brown A, Scolding N, Wilkins A (2014) Reductions in neuronal peroxisomes in multiple sclerosis grey matter. Mult Scler 20(6):651–659
Senanayake VK, Jin W, Mochizuki A, Chitou B, Goodenowe DB (2015) Metabolic dysfunctions in multiple sclerosis: implications as to causation, early detection, and treatment, a case control study. BMC Neurol 15:154
Testa G, Staurenghi E, Zerbinati C, Gargiulo S, Iuliano L, Giaccone G, Fantò F, Poli G, Leonarduzzi G, Gamba P (2016) Changes in brain oxysterols at different stages of Alzheimer’s disease: their involvement in neuroinflammation. Redox Biol 10:24–33
Leoni V, Lütjohann D, Masterman T (2005) Levels of 7-oxocholesterol in cerebrospinal fluid are more than one thousand times lower than reported in multiple sclerosis. J Lipid Res 46(2):191–195
Fellows Maxwell K, Bhattacharya S, Bodziak ML, Jakimovski D, Hagemeier J, Browne RW, Weinstock-Guttman B, Zivadinov R, Ramanathan M (2019) Oxysterols and apolipoproteins in multiple sclerosis: a 5 year follow-up study. J Lipid Res 60(7):1190–1198
Mukhopadhyay S, Fellows K, Browne RW, Khare P, Krishnan Radhakrishnan S, Hagemeier J, Weinstock-Guttman B, Zivadinov R, Ramanathan M (2017) Interdependence of oxysterols with cholesterol profiles in multiple sclerosis. Mult Scler 23(6):792–801
Nury T, Sghaier R, Zarrouk A, Ménétrier F, Uzun T, Leoni V, Caccia C, Meddeb W, Namsi A, Sassi K, Mihoubi W, Riedinger JM, Cherkaoui-Malki M, Moreau T, Vejux A, Lizard G (2018) Induction of peroxisomal changes in oligodendrocytes treated with 7-ketocholesterol: attenuation by α-tocopherol. Biochimie 153:181–202
Nury T, Samadi M, Zarrouk A, Riedinger JM, Lizard G (2013) Improved synthesis and in vitro evaluation of the cytotoxic profile of oxysterols oxidized at C4 (4α- and 4β-hydroxycholesterol) and C7 (7-ketocholesterol, 7α- and 7β-hydroxycholesterol) on cells of the central nervous system. Eur J Med Chem 70:558–567
Sassi K, Nury T, Zarrouk A, Sghaier R, Khalafi-Nezhad A, Vejux A, Samadi M, Aissa-Fennira FB, Lizard G (2019) Induction of a non-apoptotic mode of cell death associated with autophagic characteristics with steroidal maleic anhydrides and 7β-hydroxycholesterol on glioma cells. J Steroid Biochem Mol Biol 191:105371
Nury T, Zarrouk A, Vejux A, Doria M, Riedinger JM, Delage-Mourroux R, Lizard G (2014) Induction of oxiapoptophagy, a mixed mode of cell death associated with oxidative stress, apoptosis and autophagy, on 7-ketocholesterol-treated 158N murine oligodendrocytes: impairment by α-tocopherol. Biochem Biophys Res Commun 446(3):714–719
Sghaier R, Nury T, Leoni V, Caccia C, Pais De Barros JP, Cherif A, Vejux A, Moreau T, Limem K, Samadi M, Mackrill JJ, Masmoudi AS, Lizard G, Zarrouk A (2019) Dimethyl fumarate and monomethyl fumarate attenuate oxidative stress and mitochondrial alterations leading to oxiapoptophagy in 158N murine oligodendrocytes treated with 7β-hydroxycholesterol. J Steroid Biochem Mol Biol 194:105432
Zarrouk A, Martine L, Grégoire S, Nury T, Meddeb W, Camus E, Badreddine A, Durand P, Namsi A, Yammine A, Nasser B, Mejri M, Bretillon L, Mackrill JJ, Cherkaoui-Malki M, Hammami M, Lizard G (2019) Profile of fatty acids, Tocopherols, Phytosterols and polyphenols in Mediterranean oils (Argan oils, olive oils, Milk thistle seed oils and nigella seed oil) and evaluation of their antioxidant and Cytoprotective activities. Curr Pharm Des 25(15):1791–1805
Acknowledgements
This work was supported by grants from: Univ. Bourgogne (Dijon, France); Univ. Monastir (Monastir, Tunisia); ELA fondation (grant application ELA 2010-030I4); ASSAD (Louhans, France); Applications des Sciences de l’Information en Médecine (ABASIM, Dijon, France), and Department of Neurology (University Hospital, Dijon, France).
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Nury, T., Yammine, A., Menetrier, F., Zarrouk, A., Vejux, A., Lizard, G. (2020). 7-Ketocholesterol- and 7β-Hydroxycholesterol-Induced Peroxisomal Disorders in Glial, Microglial and Neuronal Cells: Potential Role in Neurodegeneration. In: Lizard, G. (eds) Peroxisome Biology: Experimental Models, Peroxisomal Disorders and Neurological Diseases. Advances in Experimental Medicine and Biology, vol 1299. Springer, Cham. https://doi.org/10.1007/978-3-030-60204-8_3
Download citation
DOI: https://doi.org/10.1007/978-3-030-60204-8_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-60203-1
Online ISBN: 978-3-030-60204-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)