Abstract
Groundwater plays a dominant role in arid and semi-arid regions; it is among the most available water resources in Essaouira basin, where groundwater is the most important source of water supply. The aim of the study is to assess water quality with respect to agriculture and drinking for a better management of groundwater resources. To achieve such objectives, water analysis was carried out on 50 groundwater samples. The Piper plot reveals that the facies characterizing Ouazi basin was a combination of Ca-Mg-Cl type, Mg-Ca-HCO3 type, SO4-Ca-Mg and Na-Cl water type. The sustainability of groundwater for drinking and irrigation was assessed based on the World Health Organization (WHO), Wilcox and Richards’s diagrams. The obtained results show that the consumption of groundwater in the study area requires a treatment before use as drinking water and most of the groundwater samples fall in C3S1–C4S1 indicating very high salinity and high to low sodium alkalinity hazard. Thus, groundwater quality is ranging between good to permissible and doubtful to unsuitable for irrigation uses under normal condition, and further action for salinity control is required in remediating such problem. The groundwater remains suitable for plants supporting high salinity.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Groundwater quality is equally important to its quantity owing to the suitability of water for various purposes. Water quality analysis is an important issue in groundwater studies. Variation of groundwater quality in an area is a function of physical and chemical parameters that are greatly influenced by geological formations and anthropogenic activities (El Mountassir et al. 2020). The hydrochemical study reveals the quality of water that is suitable for drinking, agriculture and industrial purposes and helps to understand the change in quality due to rock–water interaction or any type of anthropogenic influence (Carreira et al. 2018; Bahir et al. 2019a). Several studies have been realized in the Essaouira basin having partially identified and characterized some of the main mechanisms causing degradation of water quality in some coastal areas of this region, based on the application of geochemical and isotopic approaches (Bahir et al. 2019b; Ouhamdouch et al. 2019). These studies have demonstrated that (a) the groundwater recharge in Essaouira basin is supported by water runoff and precipitation; (b) important contribution of water–rock interaction in groundwater mineralization; (c) anthropogenic contamination was also identified.
The present study had the objective to understand the spatial distribution of hydrochemical constituents of groundwater related to its suitability for agriculture and domestic use, with application of cluster analysis.
2 Methodology
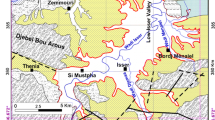
The Ouazzi basin is located in the north-eastern part of Essaouira city (Fig. 1). The basin is under semi-arid climate with irregular rainfall of about 300 mm year, and the temperature oscillates about 20 °C (El Mountassir et al. 2020). A set of 50 groundwater samples was analysed for 11 physical and chemical parameters comprising major ion concentrations. The samples were collected in 2019 from the Plio-Quaternary (downstream) and Cenomanian–Turonian (upstream) aquifers (depth 9.20–170 m) of Ouazzi basin. The physical parameters (temperature, pH, electrical conductivity) were measured in situ, and the major chemical parameters (Ca2+, HCO3−, Mg2+, Na+, K+, Cl−, SO4− and NO3−) were determined in the Laboratory of Geosciences and Environment—ENS (LGE–ENS) at ‘École Normale Supérieure’ of Marrakech (Cadi Ayyad University, Marrakech, Morocco). To identify possible groups and relationships among the samples analysed based on major chemical compositions, ion species (Ca2+, HCO3−, Mg2+, Na+, K+, Cl−, SO4− and NO3−) were considered as variables for application in Q-mode cluster analysis. The clustering procedure was performed by the Ward’s linkage method with the Euclidean distance as a measure of similarity of samples using the XLSTAT statistical tool.
3 Results and Discussions
3.1 Hydrochemical Characterization and Water Type
The electrical conductivity of groundwater samples ranges from 615 to 5738 μS/cm with a mean value of 4479 μS/cm. The salinity increases in the direction of groundwater flow from north to south. The pH ranges between 7 and 8.4 with a mean value of 7.5, which shows that the groundwater of the study area is of alkaline nature. The mean temperature of groundwater samples was 20.4 °C. The maximum Ca2+ and Mg2+ concentrations of 769.5 and 297.4 mg/L, respectively, are however higher than their respective WHO standards of 200 and 30 mg/L. The mean sodium and potassium concentrations in the groundwater are 168 and 18.4 mg/L, respectively. The concentration of HCO3− in the study area ranges between 244 and 898 mg/L. Most Cl− in the groundwater is from three sources including ancient sea water entrapped in sediment, solution of halite and related minerals in evaporate deposits in the region. The chloride value in the study area ranges between 113 and 1817 mg/L. The projection of 50 samples analysed on the Piper diagram (Fig. 2) shows four water types: Ca-Mg-Cl type, Mg-Ca-HCO3 type, SO4-Ca-Mg and Na-Cl water type. The dispersion of the points in the Piper diagram suggests that the groundwater mineralization is of several origins.
3.2 Cluster Analysis
The output of the Q-mode cluster analysis is given as a dendrogram (Fig. 3). Three preliminary groups are selected based on visual examination of the dendrogram, each representing a hydrochemical facies (Table 1). The first group of waters, group 1, has high salinity (mean EC = 4275.67 μS/cm) and abundance orders (meq/L): Na+ > Ca2+ > Mg2+ > K+ and Cl− > HCO3− > SO42− > NO3− (Table 1). These waters are classified as Ca-Mg-Cl and Na-Cl water type. Group 2 is made up of water samples wherein the anion composition was dominated by SO42− and Cl−, with cation composition varying from dominantly Ca2+ and Mg2+ to dominantly Na+ plus K+ (Table 1). EC values (mean 4007.86 μS/cm) are significantly less than those of group 1, reflecting a more effective weathering process. These waters are classified as Ca-Cl and Ca-Mg-Cl. Group 3, made up of 37 water samples, has a salinity range (615 μS/cm < EC < 3842 μS/cm); mean 1857.9 μS/cm overlapping those of the former two groups (G1 and G2). On the basis of overall chemical composition, characterized by ion abundances Ca2+ > Mg2+ > Na+ > K+ and Cl− > HCO3− > SO42− > NO3−, these waters are classified as Ca-Mg-Cl, Ca-Cl and SO4-Ca-Mg.
3.3 Irrigation Water Quality
The suitability of groundwater for agricultural purposes depends on the effect of mineral constituents of water on both plants and soil. Effects of salts on soils causing changes in soil structure, permeability and aeration indirectly affect plant growth. Wilcox and US Salinity Laboratory Staff proposed irrigational specifications for evaluating the suitability of water for irrigation use. There is a significant relationship between sodium adsorption ratio (SAR) values for irrigation water and the extent to which sodium is adsorbed by the soils. SAR was computed using the equation given below :
where the concentrations are reported in meq/L.
The SAR values ranged from 0.22 to 5.83 with a mean varying from value of 1.95 to 4.8 in the three groups. Approximately, all samples fall in low SAR class (S1) and (S2) (Fig. 4). This implies that no alkali hazard is anticipated to the crops. If the SAR value is greater than 6–9, the irrigation water will cause permeability problems on shrinking and swelling of clayey soils types (Saleh et al. 1999).
The SAR and electrical conductivity values plotted on the US salinity richards diagram illustrate that most of the groundwater samples of the group 1 belong to the categories C4S2 (very high salinity and medium sodium) (Fig. 4). Most samples of the group 2 fall in the field of C4S1, indicating high salinity and low sodium water, and thus, they can be used for irrigation on almost all types of soil with little danger of exchangeable sodium (Fig. 4). The samples of the group 3 fall in the field of C4S2 and C4S1, indicating very high salinity and medium sodium hazard. These samples will be suitable for plants having good salt tolerance and hence restricted suitability for irrigation, especially in soils with limited drainage (Saleh et al. 1999).
The sodium percentage (Na %) is calculated using the formula given below, where all the concentrations are expressed in meq/L:
The Na% of groundwater samples ranges from 4.3 to 53.18 with a mean of 40.92 for group 1, 18.76 in group 2 and 25.15 in group 3. The Wilcox diagram relating sodium percentage and electrical conductivity values shows that most of the groundwater samples fall in the field of good to permissible (group 3) except a few samples falling in the fields of doubtful and unsuitable category for irrigation (Fig. 5). The Na% indicates that the groundwater is unsuitable for irrigation in the group (1 and 2) (Fig. 5).
4 Concluding Remarks
This study was conducted to evaluate factors regulating groundwater quality in an area with agriculture as main use. Fifty groundwater samples have been collected from Ouazi basin (Essaouira) for hydrochemical investigations, to understand the sources of dissolved ions and assess the chemical quality of the groundwater. Q-mode cluster analysis was applied to groundwater quality data sets and generated three clusters (groups 1, 2 and 3). The values of sodium absorption ratio and electrical conductivity of the ground water were plotted in the US salinity laboratory diagram for irrigation water. Most of the samples fall in C3S1–C4S1 quality with high and very high salinity hazard to low sodium hazard. However, the study has helped to improve understanding of water quality of the area for effective management and proper utilization of groundwater resources for better living conditions of the people. A continuous monitoring program of water quality is required to avoid further deterioration of the water quality of the study area. The hydroclimatic, hydrodynamic, hydrochemical and isotopic approaches have led to the diagnosis of the state of vulnerability of the aquifers of the Essaouira basin. Thus, the use of unconventional resources, such as desalinated sea water for drinking water supply or treated wastewater for agriculture, must be considered an immediate priority to avoid severe water shortage.
References
Bahir M, Ouazar D, Goumih A, Ouhamdouch S (2019a) Evolution of the chemical and isotopic composition of groundwater under a semi-arid climate; the case of the Cenomano-Turonian aquifer within the Essaouira basin (Morocco). Environ Earth Sci 78:353
Bahir M, Ouazar D, Ouhamdouch S (2019b) Hydrogeochemical mechanisms and recharge mode of the aquifers under semiarid climate from Morocco. Appl Water Sci 9:103
Carreira PM, Bahir M, Ouhamdouch S, Fernandes PG, Nunes D (2018) Tracing salinization processes in coastal aquifers using an isotopic and geochemical approach: comparative studies in western Morocco and southwest Portugal. Hydrogeol J 26:2595–2615
El Mountassir O, Bahir M, Ouazar D, Ouhamdouch S, Chehbouni A, Ouarani M (2020) The use of GIS and water quality index to assess groundwater quality of Krimat aquifer (Essaouira, Morocco). SN Appl Sci 2:871
Ouhamdouch S, Bahir M, Ouazar D, Carreira PM, Zouari (2019) Evaluation of climate change impact on groundwater from semi-arid environment (Essaouira Basin, Morocco) using integrated approaches. Environ Earth Sci 78:449
Saleh A, Al-Ruwih F, Shehata M (1999) Hydrogeochemical processes operating within the main aquifers of Kuwait. J Arid Environ 42:195–209
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this paper
Cite this paper
Bahir, M., El Mountassir, O., Ouazar, D., Carreira, P.M. (2021). Hydrochemical Analysis and Evaluation of Groundwater Quality in Ouazi Basin (Essaouira, Morocco). In: Abrunhosa, M., Chambel, A., Peppoloni, S., Chaminé, H.I. (eds) Advances in Geoethics and Groundwater Management : Theory and Practice for a Sustainable Development. Advances in Science, Technology & Innovation. Springer, Cham. https://doi.org/10.1007/978-3-030-59320-9_50
Download citation
DOI: https://doi.org/10.1007/978-3-030-59320-9_50
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-59319-3
Online ISBN: 978-3-030-59320-9
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)