Abstract
Infections related to cardiac implantable electrical devices (CIEDI) represent a relevant issue both for clinical and economic perspective. Since several reports showed an increased incidence of CIEDI in replacement procedures (vs. first implant), many efforts were devoted to improve battery longevity. However, it was early discovered that leads represented the main factor coupled with comorbidities. In this chapter we will start explaining this change in perception. Later we will provide a complete view of the historical perspective of leadless pacing from the very early pioneering experiences until last developments. Moreover, we will discuss pros and cons of the available devices in the light of CIEDI prevention and possible future improvements. The second part of the chapter covers leadless defibrillator describing its role for CIEDI prevention and management (i.e., reimplantation after lead extraction). We will conclude depicting future devices and approaches to provide new effective devices able to completely substitute current transvenous standards.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
10.1 Introduction: From CIED with Extended Batteries to Leadless Technology
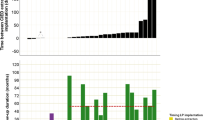
Infections related to cardiac implantable electrical devices (CIEDI) represent a relevant issue both for clinical and economic perspective [1]. The relevance of this complication progressively raised from the beginning of the 2000s as pointed out by the report of Voigt et al. [2]. The authors showed an alarming rising trend in the incidence of CIEDI emphasizing that this phenomenon did not parallel the increase in device implantation during the same period, being much higher in reality. In particular they underlined that in the period 1996–2003, there were no significant changes in the demographic characteristics of patients receiving CIED implantations except that the proportion of patients receiving an implantable cardiac defibrillator (ICD) increased significantly with respect to pacemaker (PM) (from 14% to 27%, p < 0.001). In the same period, hospitalizations for CIEDI increased 3.1-fold (2.8-fold for PMs and sixfold for ICDs). These findings coupled with the evidence of an increased incidence of CIEDI associated with replacement of cardiac implantable electrical devices (CIED) vs. first implant procedures (2.06% vs. 0.75%, p < 0.01) [3] provided a first possible explanation [4]. In particular, it was highlighted the unbalance between carrier longevity and device longevity for PM vs. ICD recipients leading to a replacement rate of around 80% for ICD carriers vs. 50% for PM carriers [2, 5,6,7]. These data provided an attractive explanation for the steep slope shown by the incidence of CIEDI hospitalization occurring after 4–5 years from publication of the results of the multicenter automatic defibrillator implantation (MADIT) [5]. This period was equivalent to the average longevity of ICD battery at this time leading to the consideration that the rising in ICD replacements caused this fast increase in CIEDI years before completion of the MADIT II trial [6] (see Fig. 10.1). These considerations prompted the development of new CIED with extended longevity, aimed not only at reducing costs related to battery exchange but also to reduce occurrence of CIEDI [4]. However, two additional factors hampered the benefit of extending CIED longevity: comorbidities and lead failures. The first factor derives from the progressive modification of the clinical profile of candidates to CIED implant driven by the broadening of indications to ICD and cardiac resynchronization therapy (CRT) coupled with an increased survival of patients with comorbidities [see Chap. 3 for additional insights]. However, lead failure probably represents the main factor who forwarded the development of the new leadless technologies, especially after the occurrence of two major recalls on ICD leads: the Sprint Fidelis (Medtronic Corp.) and Riata (St. Jude Medical Inc.) [8]. It is interesting to note that both these issues promoted the development and spreading of two great advancements in current CIED technology: remote CIED monitoring (to evidence early signs of lead malfunction before occurrence of clinical events) and leadless technology. It has to be stated that the leadless revolution was not only driven by recalls on CIED leads but also by the presence of several reports regarding a suboptimal performance of CIED leads, especially high-voltage leads [9]. These reports highlighted the presence of a huge gap between the longevity of CIED leads declared by manufacturers and the real service life in current clinical practice. Noticeably, it has to be stated that a reduced survival of CIED leads can also be attributed to several factors not connected with their production, such as: (a) implanting technique, (b) patients’ anatomy, (c) patients’ behavior (e.g., work and sports involving repetitive shoulder movement) [10], (d) modifications of the interface between lead and heart (e.g., development of fibrosis and/or ischemia), (e) micro/macro lead dislodgment, and (f) need for CIED system upgrade. However, independently from the case-specific source of suboptimal lead performance, in the last decade the presence of intravascular lead has been pointed out as the Achilles’ heel of conventional CIED [11].
Relationship between publication date of three leading randomized trials on implantable defibrillators for primary prevention of sudden cardiac death (Panel a [5,6,7]) and the disproportionate increase in the number of CIEDI with respect to implantation rate, as reported by Voigt et al. (Panel b; reproduced with permission [2]). CMRD = cardiac rhythm management devices
10.2 Leadless Pacing
Despite the relatively recent introduction of leadless PM the concept that transvenous leads are the weakest link of conventional PM systems prompted the investigation on possible solutions for leadless cardiac pacing just after development of permanent pacing, more than 40 years ago [11]. This preclinical report demonstrated the feasibility of a totally self-contained intracardiac PM inserted under fluoroscopy through the jugular vein in a dog with an iatrogenic heart block. The cylindrical device was attached to the ventricle by radially directed spiral barbs. Pacing was effectively delivered for >2 months. We had to wait until 1991 years for a second preclinical experience aimed at replicating this pioneering experience in eight dogs [12, 13] with good results and without any complication (Fig. 10.2) [14]. Noteworthy, these devices were made in a university hospital, representing a major achievement for independent research. However, we had to wait for several technological advancements to make from this pioneering experience an implantable CIED to be used in clinical practice: catheter-based delivery systems, miniaturized high-density energy sources, low-power electronics, novel packaging capabilities, and novel communication technologies. Three devices are currently available with two very different concepts (Fig. 10.3). Two devices are self-contained leadless intracardiac PM developed for right ventricular pacing, i.e., the Nanostim™ leadless pacemaker (St Jude Medical, St.Paul, MN, USA) and the Micra™ transcatheter pacing system (Medtronic, Minneapolis, MN, USA). Both these PM do not need additional devices for properly working as a PM but only for follow-up (on-site or remote) and programming through radiofrequency transmission (i.e., similar to standard PM) (Table 10.1) [15,16,17,18,19]. A different approach entails the development of multicomponent devices, like the recently introduced Wise™ CRT (Wireless Stimulation Endocardially for CRT; EBR Systems Inc., CA, USA) pacing system, adopting an intracardiac receiver activated through ultrasounds by a subcutaneous pulse generator.
The original representation of the leadless working pacemaker implanted by Vardas et al. in eight dogs (Reproduced from Vardas et al. with permission [13]). a = guiding catheter, b = pushing catheter, c = miniature pacemaker, d = steering arm
10.3 Self-Contained Leadless Pacing
The Nanostim™ and the Micra™ PM are the two currently commercially available self-contained leadless PM developed for single-chamber pacing of the right ventricle (Figs. 10.3 and 10.4) [20]. These devices are characterized by a single unit fully containing both the pulse generator and sensing/pacing electrodes, thereby eliminating not only the leads but also the need for surgical pocket and within-system connections. The device is delivered to the right ventricle with a dedicated delivery system through the femoral vein. There are some differences in device size between the Nanostim™ and the Micra™ PM (Table 10.1) but the two main differences are the outer diameter of the delivery sheath (24-F for Micra™ PM vs. 18-F for Nanostim™ PM) and the fixation mechanism. In comparison with standard PM, both devices are significantly smaller, being approximately 1:10 of the volume without considering the length of the intravascular lead. With regard to retention mechanisms, the Nanostim™ PM incorporate an active screw-in helix coupled with three angled nitinol tines perpendicular to the helix as a secondary fixation mechanism. On the contrary, the Micra™ PM includes four self-expanding nitinol tines to attach to the myocardium (Figs. 10.3 and 10.4). Notably, both devices use a tethering mechanism to maintain a connection between the delivery catheter and the device to test positional integrity before final deployment and both devices are reportedly retrievable [17, 21] although data on human being are limited in view of the relatively recent introduction of these devices. In brief, patients are considered eligible if they have indications for single-chamber, right ventricular pacing (VVI [R]) indications. The Nanostim™ PM received the CE mark in 2013, more than 40 years after the first preclinical experience [22, 23]. Between 2013 and 2016, a total of 1423 Nanostim™ PM were implanted worldwide and three clinical trials were initiated. However, reports of (rare) lost telemetry and pacing output due to abrupt battery failure starting >24 months after implant led to a Medical Device Advisory in October 2016 with a global stop to Nanostim™ PM implants [22]. While patients enrolled in the trial continued to be followed according to the protocol, the decision to explant, abandon, or replace was left to clinicians, according to pacemaker dependency and individual patient’s clinical history and overall medical condition. Two clinical trials evaluated the safety and efficacy of Nanostim (Table 10.2) [18, 19, 24,25,26]. After the first LEADLESS pilot study, analyzing 33 patients for 12 weeks for safety purposes, the large multicenter LEADLESS II IDE trial, evaluated PM performance and safety at 6 months. Inclusion criteria of both trials were as follows: (a) permanent atrial fibrillation with atrioventricular block and/or slow ventricular response, (b) normal sinus rhythm with second- or third-degree atrioventricular block, (c) sinus bradycardia with infrequent pauses, or (d) unexplained syncope with electrophysiological findings justifying a single-chamber PM. On the opposite the exclusion criteria were (a) complete pacemaker dependency, (b) significant pulmonary hypertension, (c) presence of a mechanical tricuspid valve prosthesis, (d) pacemaker/defibrillator leads, and (e) presence of an inferior vena cava filter. Later, the enrollment criteria of the LEADLESS Observational Study Europe were broader, being limited to indication for single-chamber pacing, a life expectancy of at least 1 year, and were believed to be suitable candidates based on overall health and well-being. Despite a general good performance with a successful implantation rate > 90% the subsequent evidence of a learning curve of about ten procedures coupled with the occurrence of two lethal cardiac perforations led to suggest operator training while limiting indications to those of the LEADLESS studies [22]. About 3000 Micra™ PM have been implanted until now, and the principal data derive from the Micra™ IDE study and the global Micra™ registry. The Micra™ PM received both CE mark and Food and Drug Administration (FDA) (2015 and 2016, respectively). The Micra™ IDE study assessed device efficacy and safety [19] among 725 patients, suitable candidates for VVI pacing and with a class I or II guideline-based indication for pacing [27, 28]. The study also excluded patients with recent acute coronary syndrome, presence of neurostimulator or any other chronically implanted device which uses electrical current, left ventricular assist device, morbidly obese, femoral venous anatomy unable to accommodate the 23F introducer, life expectancy <12 months, and pregnant or breastfeeding women [23]. The Micra™ PM was successfully implanted in 99% of the patients, with 3.4% experiencing device-related major complications, including: cardiac perforation (1.5%), vascular complications (0.7%), and venous thromboembolism (0.3%). Notably no systemic infection was observed (Table 10.2). This was confirmed at 12-month follow-up reporting four additional major complications: three heart failure events and one pacemaker syndrome [23]. The recently published results of the MICRA™ post-approval registry on 1817 patients substantially confirmed the results of the IDE study with an effective implantation in >99% of the patients, with a low complication rate (2.7% at 12 months, 95%CI 2.0%–3.7%). Indirect comparisons with historical cohorts of standard single-chamber PM seem to support a reduced incidence of major complications with self-contained leadless PM, evidencing a reduction in risk of pneumothorax, subclavian vein thrombosis/occlusion, lead-related complications, and pocket hematoma, but increased risk of femoral vein complications [21, 23, 26]. These findings claim for a randomized comparison of these technologies. In the meantime other considerations should be made when considering these devices which are costs, especially for older patients and device longevity for younger candidates. However, there are several candidates who can potentially obtain a great advantage by this technology and above all patients at increased risk of pacemaker-related infection or after lead extraction for CIEDI (Fig. 10.5).
Chest X-ray of two patients implanted with a leadless pacemaker: a Nanostim™ on the left and a Micra™ on the right (Reproduced from Madhavan et al. [20] with permission)
10.4 Multicomponent Leadless Pacing
The only multicomponent leadless PM available is the WiSE-CRT™ System (EBR Systems, Sunnyvale, California). This device was not developed to substitute standard PM, but to overcome some issues associated with cardiac resynchronization therapy (CRT). CRT is an effective treatment for patients with wide QRS and heart failure to reduce hospitalization and mortality. However, there is still a large proportion of candidates (30–40%) who will not respond to this treatment for several reasons or who present anatomical constraints preventing an effective epicardial stimulation via the coronary sinus: absence of appropriate venous site, occlusion of the upper extremity venous system, phrenic nerve stimulation, or high pacing threshold [29,30,31]. For this reason it has been previously tested the possibility of transeptal implantation of an endocardial left ventricular pacing lead with interesting results as confirmed by a prospective multicenter study [32] (Fig. 10.6). However, while transeptal LV endocardial stimulation may provide a more physiological ventricular activation (compared with epicardial left ventricular pacing), this approach is limited by the need for lifelong systemic anticoagulation and theoretical concern for mechanical effects on the mitral valve. These considerations provided the basis for the development of the WiSE-CRT™ system. This device consists of four components (Fig. 10.7): (a) a 12-F steerable delivery catheter system with an atraumatic inflatable polyester balloon at the catheter tip, (b) an 8F retractable delivery catheter with a pre-mounted receiver electrode capable of converting ultrasounds to electrical energy through piezoelectric crystals (implanted in the endocardium of the left ventricle via a transaortic retrograde approach), (c) a pulse generator (containing an ultrasound energy pulse transmitter and a battery) implanted in a subcutaneous pocket, and (d) the programmer. This system was investigated in the WiSE-CRT study and the more recent SELECT-LV study [33, 34]. The WiSE-CRT study was a multicenter, prospective feasibility study aimed at enrolling 100 patients with conventional PM/ICD who met standard criteria for CRT implantation, along with failed LV lead patients/nonresponders. Despite promising results in terms of efficacy, it was terminated early due to three cases of pericardial effusions associated with implantation of the left ventricular device, resulting in one death [17, 21, 23]. The delivery system was redesigned and reassessed in the SELECT-LV study that enrolled 35 patients with an indication for CRT and a failed conventional CRT implantation. The authors reported no perforation/pericardial effusion with the new delivery system but there were three serious procedure-related or device-related events: a ventricular fibrillation during implantation of the LV electrode (resulting in patient death), embolization of the left ventricular transducer to the left tibial artery, and development of a femoral artery fistula that required surgical intervention. Notably, the authors reported an improvement in NYHA class in 85% and 66% showed an absolute increase in left ventricular ejection fraction ≥5%. On these basis, a new multicenter randomized trial has started the SOLVE-CRT (Stimulation Of the Left Ventricular Endocardium for Cardiac Resynchronization Therapy in Non-Responders and Previously Untreatable Patients) study (Clinical-Trials.gov NCT02922036) [21, 23]. Patients who are nonresponders to conventional CRT or failed to have a successful coronary sinus left ventricular lead will receive a WiSE-CRT system and then be randomized to system on or off (sham comparator). The endpoints include assessment of left ventricular end systolic volume, heart failure events, functional class, quality of life measures, and death at 6 months. Moreover, safety outcomes related to the device and the implantation procedure will also be assessed. Finally, coupled with these studies it has been recently started the WiCS Post Market Surveillance Registry (Clinical-Trials.gov NCT02610673) that enrolled until August 2018 68 patients with a 97% effective pacing of the left ventricle [35]. Table 10.3 [33, 34] reports the principal studies on WiSE-CRT system with the reported incidence of CIEDI.
Intra-operatory X-ray of a nonresponder to resynchronization therapy implanted with a left ventricular endocardial lead (Cortesy of Dr. Mauro Biffi, Institute of Cardiology University Hospital of Bologna Italy). 1 = left endocardial lead making a loop (a) after crossing the interatrial septum, 2 = coronary sinus lead (originally used for resynchronization), 3 = defibrillator lead, 4 = atrial lead, 5 = CRT-D device
Chest X-ray of a patient with a previous CRT-D system (1 = CRT-D device, 2 = multipolar coronary sinus lead) later upgraded with implantation of an EBR system for absence of response and limited venous access. a = ultrasound transmitter implanted submuscular, b = receiver electrode, c = WiSE-CRT™ can and battery
10.5 Leadless Pacemaker and CIED-Related Infections
Looking in more detail self-contained leadless PM are expected to reduce CIED infections because this system does not create physical connections between the endocardium and the subcutaneous pocket. It is also hypothesized that the small size, the potential for encapsulation, and the absence of cutaneous incision may all lead to a reduced infection rate. In self-contained PM the lack of a generator pocket and the absence of long-term venous hardware also have obvious potential advantages in terms of infectious risk. This is different for the only currently available multicomponent system. The current WiSE-CRT™ system both requires a device pocket (for the pulse generator) and a second intracardiac device for synchronizing the pacing stimulus, which is usually performed by a transvenous PM/ICD. However, it could be speculated to have both a self-contained PM and a WiSE-CRT™ system in a patient with atrial fibrillation and (spontaneous or induced) AV block. A similar approach has been recently reported by a French group who implanted an 81-year-old man with a WiSE-CRT™ system coupled with a Micra™ PM after lead extraction for pocket CIEDI (Fig. 10.8) [36]. To date, limited data are still available on the true infection risk of leadless pacemakers but the majority of leadless pacing datasets did not report relevant infectious complications (Tables 10.2 and 10.3) [18, 19, 37]. Of note, in “real life” many of the patients implanted with a leadless pacemaker carry a high risk of infection. In the Micra Transcatheter Pacing System Post-Approval Registry [37], 20.9% of 795 patients were allocated to a leadless cardiac system owing to at least one condition contraindicating a transvenous approach including a history of or risk for infection in 9% of the patients and dialysis in 5%. Bilaterally infected patients were also shown to be candidates for leadless pacing [38]. Kypta et al. reported the implantation of a leadless pacemaker in six patients with severe device infection who were pacemaker dependent [39]. Three patients had pocket infection only, whereas the other three had both pocket and lead infection. Lead extraction was performed in all patients and four were bridged with a temporary pacemaker before leadless implantation (2 h to 2 days after extraction), whereas two patients had the leadless pacemaker implanted during the same procedure just before lead extraction. All patients stayed free of infection during 12 weeks of follow-up and positron emission tomography imaging indicated no signs of an infection around the leadless pacemaker. In contrast with this reassuring data, Koay et al. reported the world’s first case of infected leadless pacemaker which eventually led to its percutaneous extraction 1 month after implantation [40]. While the patient developed fever, chills, and rigors 1 month after implantation, methicillin-resistant Staphylococcus aureus was isolated in two separate blood cultures and transesophageal echocardiography demonstrated a vegetation on the device. After unsuccessful antibiotic therapy the device was removed percutaneously and an infected vegetation was identified on the device. The reimplantation strategy was not reported. In summary, device infection in leadless PM has been extremely rarely reported. This is all the more encouraging as, to date, a significant proportion of patients at high risk for infection have received a leadless pacemaker. Longer-term data are still needed to confirm that device encapsulation has a protecting effect against late infection.
(a, b) First reported case of reimplantation of a leadless CRT system after lead extraction for CIEDI showing the two intracardiac devices (a Micra™ PM in the bottom of both X-ray and the WiSE-CRT receiver indicated by the arrow). In the bottom it is represented patient’s rhythm under right ventricular (c) and biventricular (d) pacing (Reproduced with permission from Galand et al. [36])
10.6 The Subcutaneous ICD
Since the introduction, ICD technology proved to be cost-effective in reducing sudden death and overall mortality both in primary and secondary prevention [41]. However, as previously reported, the incidence of device-related complications challenged the benefits provided by widespread adoption of ICD therapy in a long-term perspective with some authors advocating for a “non-replacement approach” in patients without ICD intervention from implant to the physiologic exhaustion of ICD battery [42]. In particular, a meta-analysis found an overall ICD complication rate of 9.1% in randomized controlled studies being about three times greater to figures reported in ICD registries suggesting un underreporting from real-world studies [43]. Notably, these findings are quite complete for acute complications (e.g., pneumothorax, pericardial effusion, lead dislodgment, and hematoma) but it can be hardly extended to CIEDI since a relevant amount of these complications can manifest late and in a greater proportion after upgrade/replacement procedures [4, 29]. Moreover, among these issues the lead-related complications represent the vast majority especially in light of the recalls affecting several ICD leads and the underperformance of ICD leads in real-life service beyond the field actions [9, 44, 45]. All these considerations prompted the development of a completely subcutaneous ICD (SC-ICD) was developed as an alternative to the transvenous-ICD (TV-ICD) system. The SC-ICD system provides high-energy defibrillation shock (80 J) for the treatment of ventricular tachyarrhythmias through a pulse generator and a subcutaneous electrode. The generator is placed subcutaneously in a left lateral position and connected to a subcutaneous tripolar parasternal electrode (Fig. 10.9). The SC-ICD has not capability for bradycardia or anti-tachycardia pacing (ATP), but can deliver up to 30 s of post-shock transthoracic pacing. The device has two programmable zones of tachycardia detection: a conditional VT zone and a VF zone. In the conditional zone, complex morphology-based algorithms discriminate VT/VF from supraventricular tachycardia (SVT), while in the VF zone heart rate is the only criterion to determine whether the DC shock will be delivered or not (Fig. 10.10). In 2010 the initial feasibility study was published reporting both the initial evaluation of optimal configuration of generator and defibrillator coil and outcomes on a total of 61 patients [46]. After this publication three other multicenter studies have been reported showing interesting results both in terms of efficacy and safety [47,48,49] with conversion rates >97% in both spontaneous and induced VT/VF with complications well below the figures previously shown by the meta-analysis by Ezzat et al. [43]. Outcomes in particular patient populations have been studied, supporting the safety/efficacy of SC-ICD also in challenging situations: (1) patients with concurrent pacing either transvenous [43], leadless [50], or epicardial [51]; (2) end-stage renal disease [52] and dialysis [53], who are at very high risk for CIEDI [4, 54]; (3) hypertrophic cardiomyopathy [55, 56]; (4) arrhythmogenic right ventricular cardiomyopathy [57]; and (4) congenital heart disease [58, 59].
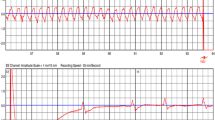
Example of effective conversion of a ventricular fibrillation to sinus rhythm by a subcutaneous defibrillator. Polymorphic non-sustained ventricular tachycardia in a patient with prolonged QTc (1). R-on-T phenomenon (2) degenerating in polymorphic ventricular tachycardia and ventricular fibrillation which is recognized by the device (3) and after few seconds it charges the capacitor (4) and finally delivers the shock (5)
Focusing on infective complications, Table 10.4 [47, 60,61,62,63,64] summarizes the data on CIEDI reported in various SC-ICD studies and registries. The rate of infections resulting in explanation or revision of this new device was not lower than that reported in TV ICD registries. However, it should be emphasized that none of the documented device infections were systemic.
In a recent meta-analysis comparing efficacy and safety outcomes between SC-ICD and TV-ICD, Basu-Ray et al. did not demonstrate a significant difference in infections between the SC-ICD and TV-ICD groups (OR, 0.75; 95% CI, 0.30 to 1.89) [65]. The total infection rate among SC-ICD recipients was 0.35% in this meta-analysis. This is much lower than the infection rate of 3.9% (95% CI, 2.2% to 5.7%) among SC-ICD recipients reported in the first large international cohort of real-world data from SC-ICD population [49]. Patients in this registry had been implanted since 2009 and followed-up over 60 months post-implant. The higher rates of infection in the registry may be related to procedural inexperience of and unfamiliarity with the surgical approach of left lateral thoracotomy and placement of the lead. The long observation time in the registry may have also partly contributed to the higher infection rate. Another plausible explanation may be that SC-ICD infections were primarily related to device implantation, which is not expected to be different from TV-ICD. However, the evolving technique of SC-ICD implant brings the potential for greater reduction in procedure-related complications, including CIEDI [66,67,68]. Regardless, the consequences of SC-ICD infection appear to be less severe, as no intravascular infection has been noted with SC-ICD infection. Once available, long-term data will be of high importance, particularly the infection rate after generator changes which is expected to be higher than the initial implant. It will be also helpful to collect not only cases which needed complete hardware removal but also the ones which have been successfully treated conservatively. At this regard there are some interesting reports on the use of SC-ICD for reimplantation after device extraction for CIEDI. These reports evidenced a good performance of SC-ICD also in this particular setting [69, 70].
10.7 Future Perspectives
Despite the high interest in the development of leadless PM, after several years from their introduction leadless PM remains a minority option. According to a survey promoted by the European Heart Rhythm Association in 2018 [71] standard PM represent >90% of implanted PM. Among the different reasons underling this phenomenon, more are related to economic considerations and above all there are device costs and reimbursement barriers. Despite these considerations it has been estimated that the global leadless PM market will reach from 47 million US dollars in 2017 to about 270 million US dollars by 2026 with a compound annual growth rate of 21.9% from 2018 to 2026 [72]. The second main obstacle to a broader adoption of leadless PM represents the single-chamber nature of these devices which are not well suited for the majority of patients requiring to preserve atrioventricular (and interventricular when possible) synchrony. To obtain a multichamber leadless system (Fig. 10.11) [73] these devices must communicate wirelessly with each other. However, a typical scenario entails a quick inter-device communication (to permit response from the receiving device, e.g., the ventricular PM after atrial pacing) at low energy consumption (due to the highly restricted battery volumes). Therefore, the communication must be very energy efficient and should not significantly reduce the lifetime of a PM. This cannot be met with wireless data communications based on radiofrequency telemetry and inductive coupling. For this reason galvanic coupled intra-body communication has been tested with in a proof-of-concept experiment involving three animal models [74] with promising, albeit pioneering, results also in the field of CRT devices [75]. There is a challenging equilibrium between device endothelialization/integration and the possibility of device retrieval/extraction which entails several potential risks: infection, embolization, thromboembolism, “overcrowding,” and device-device interaction. Long-term data in younger patients are needed to clarify these questions. A possible answer can derive from different approaches of powering CIED to address many of the current limitations of current devices. The use of piezoelectric systems that harness the kinetic energy of cardiac motion into electrical energy is one of the possibilities [76]. On the contrary the possibility to implant a small magnet inside the atrial chamber could enable atrioventricular synchronization even without an additional powered device (Fig. 10.12) [77]. Another attractive option is the development of biologic pacemakers by insertion of “pacing” genes into patient’s own myocytes to provide automaticity or through stem cell therapies [78, 79] as shown in a proof-of-concept study in a porcine model [80]. In the field of the treatment of cardiac tachyarrhythmias two additional devices are under development with a completely different approach: the implantable string subcutaneous defibrillator (Newpace Ltd., Israel) developed to eliminate the need for an active can by integrating the ICD components and functionality into a single flexible string shape device that is inserted subcutaneously [81] and the development of a novel lead designed specifically for pacing/sensing/defibrillation after being inserted into the substernal space. The ASD2 study was a prospective multicenter, worldwide, nonrandomized, acute, proof-of-concept clinical study showing highly promising results both in terms of PM and ICD function. However, further experimentations are needed to support these new approaches. Finally, it has been recently introduced the Empower system which includes a rate-responsive, single-chamber leadless PM pacemaker and an SC-ICD [82]. Firstly developed to provide anti-tachycardia pacing to SC-ICD carriers, this solution introduces the concept of “modular” CIED systems (like to what is proposed in Fig. 10.11). The availability of different devices which can interact combining their function can potentially create a CIED system able to overcome the evolution of patient needs without removing/abandoning previous hardware.
A theoretical entirely leadless CRT-D system including (a) atrial pacing device (b right ventricle pacing device), (c) left ventricle pacing device, (d) extravascular defibrillator (Reproduced with permission from Boriani et al. [73])
Leadless monitoring of heart activity. A magnet (1) is placed in the right atrium and, through an external subcutaneous Hall effect sensor (HES) (2), its movements are revealed during the cardiac cycle. Starting from the atrial diastole (a), the distance between the magnet and the HES decreases, with a consequent increase of the magnetic field that reaches a maximum when the distance is minimal (atrial systole b). Once the atrial activity is revealed, the external device (4) can drive a ventricular leadless PM (3). To reduce the number of external subcutaneous devices, an HES can be probably inserted directly in the leadless ventricular PM. (Courtesy of Ivan Corazza BS, Department of Experimental, Diagnostic and Specialty Medicine. University of Bologna. Italy) Based on the paper by Corazza et al. [77]
10.8 Conclusion
Newly developed technologies and devices represent attractive options to reduce the incidence of the extremely concerning issue of CIED infections, especially by eliminating CIED leads. The leadless PM seems associated with fewer infections but longer-term follow-up data are needed, while it is highly advocated the development of multichamber leadless devices. The infection rate observed with the subcutaneous ICD seems to be in the range of the transvenous ICD infection rate but, importantly, the infections are not systemic and at least in some of them can be treated without device removal. Several novelties are under development and in the forthcoming years we will probably see a completely different scenario in CIED-based medicine.
References
Clementy N, et al. Infections and associated costs following cardiovascular implantable electronic device implantations: a nationwide cohort study. Europace. 2018;20(12):1974–80.
Voigt A, Shalaby A, Saba S. Rising rates of cardiac rhythm management device infections in the United States: 1996 through 2003. J Am Coll Cardiol. 2006;48(3):590–1.
Johansen JB, et al. Higher incidence of pacemaker infection after replacement than after first implantation: experiences from 36,076 consecutive patients. Heart Rhythm. 2006;3(5):S102–3.
Diemberger I, et al. From lead management to implanted patient management: indications to lead extraction in pacemaker and cardioverter-defibrillator systems. Expert Rev Med Devices. 2011;8(2):235–55.
Moss AJ, et al. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. Multicenter Automatic Defibrillator Implantation Trial Investigators. N Engl J Med. 1996;335(26):1933–40.
Moss AJ, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346(12):877–83.
Bardy GH, et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352(3):225–37.
Providencia R., et al. Transvenous implantable cardioverter-defibrillator (ICD) lead performance: a meta-analysis of observational studies. J Am Heart Assoc, 2015:4(11).
Maisel WH, Kramer DB. Implantable cardioverter-defibrillator lead performance. Circulation. 2008;117(21):2721–3.
Diemberger I, et al. Implantation of cardioverter-defibrillator: effects on shoulder function. Int J Cardiol. 2013;168(1):294–9.
Diemberger I, et al. From lead management to implanted patient management: systematic review and meta-analysis of the last 15 years of experience in lead extraction. Expert Rev Med Devices. 2013;10(4):551–73.
Sutton R. The first European journal on cardiac electrophysiology and pacing, the European journal of cardiac pacing and electrophysiology. Europace. 2011;13(12):1663–4.
Vardas P, et al. A miniature pacemaker introduced intravenously and implanted Endocardially. Preliminary Findings from an Experimental Study. 1991;1:27–30.
Greenspon AJ, et al. 16-year trends in the infection burden for pacemakers and implantable cardioverter-defibrillators in the United States 1993 to 2008. J Am Coll Cardiol. 2011;58(10):1001–6.
Beurskens NE, Tjong FV, Knops RE. End-of-life Management of Leadless Cardiac Pacemaker Therapy. Arrhythm Electrophysiol Rev. 2017;6(3):129–33.
Dayal N, Burri H. Leadless cardiac stimulation: ready to take Centre stage? 2016;19:83–9.
Miller MA, et al. Leadless cardiac pacemakers: back to the future. J Am Coll Cardiol. 2015;66(10):1179–89.
Reddy VY, et al. Percutaneous implantation of an entirely intracardiac leadless pacemaker. N Engl J Med. 2015;373(12):1125–35.
Reynolds D, et al. A leadless intracardiac transcatheter pacing system. N Engl J Med. 2016;374(6):533–41.
Madhavan M, et al. Advances and future directions in cardiac pacemakers: part 2 of a 2-part series. J Am Coll Cardiol. 2017;69(2):211–35.
Chew DS, Kuriachan V. Leadless cardiac pacemakers: present and the future. Curr Opin Cardiol. 2018;33(1):7–13.
Sperzel J, Hamm C, Hain A. Nanostim-leadless pacemaker. Herzschrittmacherther Elektrophysiol. 2018;29(4):327–33.
Lee JZ, Mulpuru SK, Shen WK. Leadless pacemaker: performance and complications. Trends Cardiovasc Med. 2018;28(2):130–41.
El-Chami MF, et al. Updated performance of the Micra transcatheter pacemaker in the real-world setting: a comparison to the investigational study and a transvenous historical control. Heart Rhythm. 2018;15(12):1800–7.
Martinez-Sande JL, et al. Acute and long-term outcomes of simultaneous atrioventricular node ablation and leadless pacemaker implantation. Pacing Clin Electrophysiol. 2018;41(11):1484–90.
Sperzel J, et al. Primary safety results from the LEADLESS observational study. Europace. 2018;20(9):1491–7.
Epstein AE, et al. ACC/AHA/HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices) developed in collaboration with the American Association for Thoracic Surgery and Society of Thoracic Surgeons. J Am Coll Cardiol. 2008;51(21):e1–62.
Zipes DP, et al. ACC/AHA/ESC 2006 guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death--executive summary: A report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Develop Guidelines for Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death) Developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Eur Heart J. 2006;27(17):2099–140.
Boriani G, Diemberger I. Cardiac resynchronization therapy in the real world: need to upgrade outcome research. Eur J Heart Fail. 2018;20(10):1469–71.
Ziacchi M, et al. Cardiac resynchronization therapy: a comparison among left ventricular bipolar, quadripolar and active fixation leads. Sci Rep. 2018;8(1):13262.
Biffi M, et al. Phrenic stimulation: a challenge for cardiac resynchronization therapy. Circ Arrhythm Electrophysiol. 2009;2(4):402–10.
Biffi M, et al. Benefits of left ventricular endocardial pacing comparing failed implants and prior non-responders to conventional cardiac resynchronization therapy: a subanalysis from the ALSYNC study. Int J Cardiol. 2018;259:88–93.
Auricchio A, et al. Feasibility, safety, and short-term outcome of leadless ultrasound-based endocardial left ventricular resynchronization in heart failure patients: results of the wireless stimulation endocardially for CRT (WiSE-CRT) study. Europace. 2014;16(5):681–8.
Reddy VY, et al. Cardiac resynchronization therapy with wireless left ventricular Endocardial pacing: the SELECT-LV study. J Am Coll Cardiol. 2017;69(17):2119–29.
Sieniewicz J, et al. Real world experience of leadless LV endocardial CRT with the WiSE CRT pacing system. Int Study. 2018;24:S66.
Galand V, et al. An entirely leadless cardiac resynchronization therapy. Eur Heart J. 2019;40(10):858–9.
Roberts PR, et al. A leadless pacemaker in the real-world setting: the Micra transcatheter pacing system post-approval registry. Heart Rhythm. 2017;14(9):1375–9.
Da Costa A, et al. Transcatheter leadless cardiac pacing: the new alternative solution. Int J Cardiol. 2017;227:122–6.
Kypta A, et al. Leadless cardiac pacemaker implantation after Lead extraction in patients with severe device infection. J Cardiovasc Electrophysiol. 2016;27(9):1067–71.
Koay A, et al. Treating an infected transcatheter pacemaker system via percutaneous extraction. Heart Rhythm Case Rep. 2016;2(4):360–2.
Boriani G, et al. Expenditure and value for money: the challenge of implantable cardioverter defibrillators. QJM. 2009;102(5):349–56.
Merchant FM, et al. Implantable Cardioverter-defibrillators at end of battery life: opportunities for risk (re)-stratification in ICD recipients. J Am Coll Cardiol. 2016;67(4):435–44.
Ezzat VA, et al. A systematic review of ICD complications in randomised controlled trials versus registries: is our ‘real-world’ data an underestimation? Open Heart. 2015;2(1):e000198.
Kleemann T, et al. Annual rate of transvenous defibrillation lead defects in implantable cardioverter-defibrillators over a period of >10 years. Circulation. 2007;115(19):2474–80.
Dorwarth U, et al. Transvenous defibrillation leads: high incidence of failure during long-term follow-up. J Cardiovasc Electrophysiol. 2003;14(1):38–43.
Bardy GH, et al. An entirely subcutaneous implantable cardioverter-defibrillator. N Engl J Med. 2010;363(1):36–44.
Burke MC, et al. Safety and efficacy of the totally subcutaneous implantable defibrillator: 2-year results from a pooled analysis of the IDE study and EFFORTLESS registry. J Am Coll Cardiol. 2015;65(16):1605–15.
Gold MR, et al. Subcutaneous implantable cardioverter-defibrillator post-approval study: clinical characteristics and perioperative results. Heart Rhythm. 2017;14(10):1456–63.
Lambiase PD, et al. Worldwide experience with a totally subcutaneous implantable defibrillator: early results from the EFFORTLESS S-ICD registry. Eur Heart J. 2014;35(25):1657–65.
Ahmed FZ, et al. Totally leadless dual-device implantation for combined spontaneous ventricular tachycardia defibrillation and pacemaker function: a first report. Can J Cardiol. 2017;33(8):1066 e5–7.
Erath JW, et al. Epicardial CRT-P- and S-ICD implantation in a young patient with persistent left superior vena cava. Herzschrittmacherther Elektrophysiol. 2016;27(4):396–8.
El-Chami MF, et al. Outcome of subcutaneous implantable cardioverter defibrillator implantation in patients with end-stage renal disease on Dialysis. J Cardiovasc Electrophysiol. 2015;26(8):900–4.
Koman E, et al. Outcomes of subcutaneous implantable cardioverter-defibrillator implantation in patients on hemodialysis. J Interv Card Electrophysiol. 2016;45(2):219–23.
De Maria E, et al. Prevention of infections in cardiovascular implantable electronic devices beyond the antibiotic agent. J Cardiovasc Med (Hagerstown). 2014;15(7):554–64.
Maurizi N, et al. Effectiveness of subcutaneous implantable cardioverter-defibrillator testing in patients with hypertrophic cardiomyopathy. Int J Cardiol. 2017;231:115–9.
Friedman DJ, et al. Ventricular fibrillation conversion testing after implantation of a subcutaneous implantable cardioverter defibrillator: report from the national cardiovascular data registry. Circulation. 2018;137(23):2463–77.
Migliore F, et al. Subcutaneous implantable cardioverter defibrillator in patients with arrhythmogenic right ventricular cardiomyopathy: results from an Italian multicenter registry. Int J Cardiol. 2019;280:74–9.
Ferrero P, et al. Entirely subcutaneous defibrillator and complex congenital heart disease: data on long-term clinical follow-up. World J Cardiol. 2017;9(6):547–52.
D’Souza BA, et al. Outcomes in patients with congenital heart disease receiving the subcutaneous implantable-cardioverter defibrillator: results from a pooled analysis from the IDE study and the EFFORTLESS S-ICD registry. JACC Clin Electrophysiol. 2016;2(5):615–22.
Brouwer TF, et al. Long-term clinical outcomes of subcutaneous versus Transvenous implantable defibrillator therapy. J Am Coll Cardiol. 2016;68(19):2047–55.
Friedman DJ, et al. Trends and in-hospital outcomes associated with adoption of the subcutaneous implantable cardioverter defibrillator in the United States. JAMA Cardiol. 2016;1(8):900–11.
Honarbakhsh S, et al. A propensity matched case-control study comparing efficacy, safety and costs of the subcutaneous vs. transvenous implantable cardioverter defibrillator. Int J Cardiol. 2017;228:280–5.
Kobe J, et al. Implantation and follow-up of totally subcutaneous versus conventional implantable cardioverter-defibrillators: a multicenter case-control study. Heart Rhythm. 2013;10(1):29–36.
Mithani AA, et al. Characteristics and early clinical outcomes of patients undergoing totally subcutaneous vs. transvenous single chamber implantable cardioverter defibrillator placement. Europace. 2018;20(2):308–14.
Basu-Ray I, et al. Subcutaneous versus Transvenous implantable defibrillator therapy: a meta-analysis of case-control studies. JACC Clin Electrophysiol. 2017;3(13):1475–83.
Winter J, et al. Intermuscular technique for implantation of the subcutaneous implantable cardioverter defibrillator: long-term performance and complications. Europace. 2017;19(12):2036–41.
Migliore F, et al. Intermuscular two-incision technique for subcutaneous implantable cardioverter defibrillator implantation: results from a multicenter registry. Pacing Clin Electrophysiol. 2017;40(3):278–85.
Droghetti A, et al. Ultrasound-guided serratus anterior plane block combined with the two-incision technique for subcutaneous ICD implantation. Pacing Clin Electrophysiol. 2018;41(5):517–23.
Boersma L, et al. Infection and mortality after implantation of a subcutaneous ICD after transvenous ICD extraction. Heart Rhythm. 2016;13(1):157–64.
Viani S, et al. Use and outcomes of subcutaneous implantable cardioverter-defibrillator (ICD) after transvenous ICD extraction: an analysis of current clinical practice and a comparison with transvenous ICD reimplantation. Heart Rhythm. 2019;16(4):564–71.
Boveda S, et al. Use of leadless pacemakers in Europe: results of the European heart rhythm association survey. Europace. 2018;20(3):555–9.
Markets, R.a., Global Leadless Cardiac Pacemakers Market Size, Market Share, Application Analysis, Regional Outlook, Growth Trends, Key Players, Competitive Strategies and Forecasts, 2018 To 2026.
Boriani G, Elsner C, Diemberger I. The struggle against infections of cardiac implantable electrical devices: the burden of costs requires new personalized solutions. Europace. 2018;20(12):1877–9.
Bereuter L, et al. Leadless dual-chamber pacing: a novel communication method for wireless pacemaker synchronization. JACC Basic Transl Sci. 2018;3(6):813–23.
Bereuter L., et al. Leadless cardiac resynchronization therapy: an in vivo proof-of-concept study of wireless pacemaker synchronization. Heart Rhythm, 2019.
Hwang GT, et al. Self-powered cardiac pacemaker enabled by flexible single crystalline PMN-PT piezoelectric energy harvester. Adv Mater. 2014;26(28):4880–7.
Corazza I, et al. Wireless Endocardial atrial (and ventricular) sensing with no implanted power source: a proposal. J Med Syst. 2019;43(6):159.
Marban E, Cho HC. Biological pacemakers as a therapy for cardiac arrhythmias. Curr Opin Cardiol. 2008;23(1):46–54.
Robinson RB. Engineering a biological pacemaker: in vivo, in vitro and in silico models. Drug Discov Today Dis Models. 2009;6(3):93–8.
Hu YF, et al. Biological pacemaker created by minimally invasive somatic reprogramming in pigs with complete heart block. Sci Transl Med. 2014;6(245):245ra94.
Neuzil P, First-in-man Feasibility Study Of Subcutaneous Defibrillation Utilizing An Integrated Flexible String Shaped Defibrillator. Heart Rhythm Society Congress (Chicago) Session C-LBCT03–01 - 12.05.2017, 2017.
Tjong FVY, Koop BE. The modular cardiac rhythm management system: the EMPOWER leadless pacemaker and the EMBLEM subcutaneous ICD. Herzschrittmacherther Elektrophysiol. 2018;29(4):355–61.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Deharo, JC., Martignani, C. (2020). Prevention of Device Infection: New Implantable Devices. In: Diemberger, I., Boriani, G. (eds) Infections of Cardiac Implantable Devices. Springer, Cham. https://doi.org/10.1007/978-3-030-46255-0_10
Download citation
DOI: https://doi.org/10.1007/978-3-030-46255-0_10
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-46254-3
Online ISBN: 978-3-030-46255-0
eBook Packages: MedicineMedicine (R0)