Abstract
Epidemiological evidence suggests that exercise improves survival in cancer patients. However, much is still unknown regarding the mechanisms of this positive survival effect and there are indications that exercise may not be universally beneficial for cancer patients. The key to understanding in which situations exercise is beneficial may lie in understanding its influence on the tumour microenvironment (TME)—and conversely, the influence of the tumour on physical functioning. The TME consists of a vast multitude of different cell types, mechanical and chemical stressors and humoral factors. The interplay of these different components greatly influences tumour cell characteristics and, subsequently, tumour growth rate and aggression. Exercise exerts whole-body physiological effects and can directly and indirectly affect the TME. In this chapter, we first discuss the possible role of exercise capacity (‘fitness’) and exercise adaptability on tumour responsiveness to exercise. We summarise how exercise affects aspects of the TME such as tumour perfusion, vascularity, hypoxia (reduced oxygenation) and immunity. Additionally, we discuss the role of myokines and other circulating factors in eliciting these changes in the TME. Finally, we highlight unanswered questions and key areas for future research in exercise oncology and the TME.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Exercise
- Physical activity
- Cancer
- Tumour microenvironment
- Hypoxia
- Anti-tumour immunity
- Tumour perfusion
- Myokines
- Exercise capacity
- Vascularity
- Exercise adaptation
- Immunology
- Preclinical
- Metastasis
- Physiology
3.1 Introduction
Gone are the days in which cancer patients are treated to the adage ‘rest is best’. A wealth of epidemiological studies over the past two decades have provided evidence that physical activity or exercise reduces the risk of developing a range of different cancers (such as breast, colorectal and lung) and is even associated with improved survival outcomes in breast and colorectal cancer patients [1, 2]. Furthermore, exercise exhibits a remarkable safety profile compared with cancer therapeutics—it is not associated with any toxicities of its own and may even reduce the rate or severity of treatment-associated adverse events [3]. In addition to survival benefits, exercise has been shown to improve cognitive and physical functioning, reduce anxious and depressive symptoms and improve health-related quality of life in cancer patients (reviewed in [3]).
However, there are indications that exercise is not universally beneficial for all cancer patients. Occasional preclinical studies have suggested that, in some situations, exercise may not affect tumour growth rate at all—or even increase tumour growth rate [4, 5]. It is unclear what the determinants of an exercise-responsive versus non-responsive tumour are, or how to identify which patients will benefit from exercise. The key to this may lie in how exercise affects the tumour microenvironment (TME).
The TME is the local cellular, physical, chemical and humoral environment that tumour cells inhabit. More specifically, it includes a range of cell types other than cancer cells, such as endothelial cells, immune cells, fibroblasts and adipocytes. Physical, chemical and humoral factors include the stiffness of the extracellular matrix, pH, oxygen tension, cytokines and growth factors. It is now well-known that tumour growth characteristics and metastatic capacity are in large part defined by the make-up of the TME. Therefore, understanding the effects of exercise on the TME is integral to understanding how exercise exerts its beneficial effects (or lack thereof).
This chapter will summarise and synthesise available preclinical (and clinical, where available) data on the effects of exercise on the TME. We will describe the factors affecting individual exercise capacity and exercise adaptability and briefly summarise preclinical and epidemiological evidence on how exercise affects tumour growth/patient survival. The main focus of this chapter will be to discuss the finer details of how exercise affects the TME, including the role of myokines, tumour vasculature/perfusion and hypoxia and immunity. Finally, we will suggest future avenues of investigation for the field of exercise oncology.
3.2 Exercise Capacity and Exercise Adaptability
3.2.1 Exercise Capacity
Exercise capacity is defined as the maximum amount of physical exertion that an individual can sustain and is colloquially referred to as ‘fitness’ [6]. The major variable affecting exercise capacity is, of course, the intensity and duration of exercise performed by an individual on a regular basis. However, other factors can play an important role, including nutrition, age, gender and genetics.
There is a clear heritable component to exercise capacity. Rodents can be selectively bred for high inherent exercise capacity; these animals can run for longer and at a higher speed (in an untrained state) than their low inherent exercise capacity counterparts [7]. In addition, different inbred mouse strains have significantly different exercise capacities in the untrained state [8]. In humans, studies have suggested that there is a large heritable component to exercise capacity (reviewed in [9]).
To our knowledge, there is just one study that has investigated the effect of inherent exercise capacity on cancer risk. Rats were selectively bred for high or low inherent exercise capacity and exposed to the carcinogen 1-methyl-1-nitrosurea (MNU) [10]. Rats with high inherent exercise capacity had lower tumour incidence (fewer rats with any breast malignancy), and those that did develop tumours had fewer tumours than rats with low inherent exercise capacity [10]. This suggests that there may be a large heritable component to the protective effect of exercise on cancer risk.
3.2.2 Exercise Adaptability
There also appears to be a heritable component to exercise adaptability, that is, the ability of an individual to effect the physiological changes required to improve exercise capacity in response to exercise. These include muscular adaptations (increased capillary density and mitochondrial expansion) and improved pulmonary and cardiovascular capacity [11]. In mice, different inbred strains exhibit significantly different changes in exercise capacity following the same training protocol [8]. Similarly, in humans, training-induced increases in maximal oxygen uptake (VO2 max) vary significantly more between families than within families [12]. It has been suggested that the heritability of exercise adaptability may be as much as 50% (reviewed in [9]).
To our knowledge, there are no published studies specifically investigating the role of exercise adaptability on risk of cancer. However, higher VO2 max was associated with improved survival in metastatic breast cancer patients and non-small cell lung cancer patients [13, 14]. We found a single clinical study in which exercise adaptations in the skeletal muscle of cancer patients were compared with healthy controls [15]. In that study, the authors found that a greater number of healthy individuals had an increase in muscle fibre cross-sectional area with exercise training compared with cancer patients [15]. In addition, healthy individuals had an increase in muscle capillarisation and quadriceps strength while cancer patients did not [15]. Although this study is limited by small numbers (n = 12–16 per group), it provides preliminary evidence that cancer patients may not adapt to exercise to the same degree as healthy individuals. Furthermore, comparison of a systematic review on improvements of VO2 max in cancer patients with data in healthy subjects suggests that the magnitude of improvement in VO2 max is lower in cancer patients despite following similar exercise programs to the healthy subjects [16].
It is largely unknown what role exercise adaptability and exercise capacity play in cancer patients’ response to exercise and, specifically, in exercise-induced changes in the TME. There are two possibilities: (1) the exercise adaptation response of the tumour is just as or more variable than the muscular/cardiopulmonary response or (2) the tumour response is less variable as tumour tissue is not directly involved in determining exercise capacity. In addition, the anatomical location of the tumour may be important. We speculate that tumours located in exercise-involved tissues (such as lung) may respond more strongly to exercise.
The potential role of (inherited) exercise capacity and exercise adaptability has thus far been largely unacknowledged in exercise oncology research. Given that higher exercise capacity is associated with lower tumour incidence in rodents [10] and improved survival in cancer patients [13], inherited exercise adaptability and exercise capacity may play a significant role in whether or not a particular cancer patient will benefit from exercise. In addition, impairments in exercise adaptability due to tumour or treatment burden may limit the effectiveness of exercise.
3.3 Effect of Exercise on Tumour Growth/Patient Survival
In this section we will give only a brief overview regarding the effect of exercise on tumour growth/progression and patient survival in order to retain the main focus on the effects of exercise on the TME. We refer the interested reader to the many comprehensive reviews on the effect of exercise on cancer patient survival [2, 3, 17, 18] and the effect of exercise as a sole intervention in preclinical studies [3, 19,20,21,22].
3.3.1 Clinical Studies
The majority of clinical studies investigating the effect of exercise or physical activity on cancer patient survival have been observational studies. There is evidence that meeting the World Health Organization (WHO) guidelines of 150 min of moderate-intensity or 75 min of vigorous-intensity exercise per week confers a significant survival benefit for cancer patients and for some cancer types, such as colorectal cancer, a dose-response relationship between exercise volume and survival has been reported [2]. Current evidence supports a 40–50% reduction in all-cause mortality for breast, colorectal and prostate cancer survivors engaging in high levels of physical activity; other cancers have not yet been sufficiently studied in this context [18]. However, there is a large possibility of reverse causation for the relationship between cancer survival and physical activity level (patients who are less well may exercise less, rather than high activity levels causing an improved outcome) [18], which emphasises the importance of conducting randomised controlled intervention trials to fully investigate the role of exercise in improving patient survival. Two large intervention studies (the CHALLENGE trial and the INTERVAL trial) are ongoing to address this and will shed more light on whether a targeted exercise intervention can improve survival [23, 24].
3.3.2 Preclinical Studies
The effect of exercise on tumour growth in preclinical studies is less clear than in epidemiological studies. This is likely to be largely due to heterogeneity in study design. Key factors that influence outcomes of preclinical studies are the rodent strain used (as discussed above, different strains have different inherent exercise capacity and adaptability), immunocompetency of the rodent strain, timing of exercise initiation (pre- vs post-‘diagnosis’), tumour type and anatomical location, exercise modality (forced, swimming or treadmill; voluntary, wheel running), tumour burden and study endpoint (predetermined time after tumour initiation or ethically determined by tumour size). Very few studies have more than a few of these factors in common, making comparisons difficult. Comprehensive discussion of these parameters is beyond the scope of this chapter (refer to [20] for comparison of the effects of some of these parameters on tumour growth), but we will briefly discuss the role of pre- versus post-implantation (mimicking pre- vs post-diagnosis) exercise on tumour growth.
In many preclinical studies, post-implantation exercise either only marginally affects tumour growth rate [25,26,27,28] or does not affect tumour growth rate at all [29,30,31,32,33]. In agreement with this, only a ‘small to moderate’ effect size of exercise on final tumour size was reported in a recent systematic review [20]. Of those studies included in the analysis that did find a statistically significant difference in final tumour size, 4/8 had a ‘probably high’ risk of bias and one even showed an increased tumour size with exercise. Together with the observation that many studies find a statistically but not clinically significant result, this suggests that exercise as a sole intervention (monotherapy) is minimally effective at slowing primary tumour growth rate.
The documented effect of pre-implantation exercise is more consistent than that of post-implantation exercise. In the above-mentioned systematic review [20], studies in which exercise was performed both pre- and post-implant had a larger effect size for exercise to reduce tumour growth than studies in which exercise was performed only after tumour implant [20]. In addition, Pedersen et al. found that growth rate of B16-F10 melanoma was slowed with pre-implantation or pre-and post-implantation exercise, but not post-implantation exercise only [30]. Similarly, a number of studies using carcinogen-induced models (which typically start exercise after carcinogen administration but before tumours become detectable) have found a reduction in malignant tumour incidence and/or overall tumour burden (by number of tumours per animal or combined weight of tumours) [30, 34]. This suggests that while exercise monotherapy may not be very effective (as discussed above), exercise preconditioning may be important both for prevention and slower growth of cancer once it has arisen. In a clinical setting, this may also translate to reduced rates of recurrence after tumour control following treatment, although this remains speculative.
It seems clear that exercise can reduce tumour growth rate, but likely only to a significant extent in a pre-implantation setting or possibly in combination with cancer therapies (discussed in Sect. 3.7). However, some studies have found differences in the exercise responsiveness of different tumours, using the same exercise protocol, with some tumours (of the same subtype) exhibiting either no change in growth rate or even an increased growth rate with exercise [4, 5]. This is important, as it indicates that exercise treatment is far from a one-size-fits-all approach and some patients may not benefit from exercise.
3.3.3 The Effect of Exercise on Metastasis
Metastasis is the process whereby tumour cells migrate from the site of the primary tumour to establish secondary tumours in different tissues. For many cancer types, it is the emergence of secondary tumours in vital tissues which ultimately causes death.
Before cancer cells can seed in secondary sites, they must survive transport through the circulation. Regmi et al. have shown that high shear stresses (such as those present in the vasculature during intense exercise) can kill circulating tumour cells using an in vitro microfluidic system [35]. However, most of the time points used were not clinically relevant. Cells were circulated under high shear stress for up to 18 h—this mimics the scenario of vigorous exercise for 18 h, which is highly unrealistic for the vast majority of the healthy population, let alone cancer patients. In one experiment, increased lactate dehydrogenase (LDH) release (a proxy for necrotic cell death) was seen after 1 h of circulating under high shear stress, which represents a more achievable length of exercise time. Much longer than this is not realistically achievable in a clinical setting. It would be prudent to repeat these experiments with shorter time points that more accurately mimic the exercise behaviour of the average population (and specifically, cancer patients).
As with preclinical data examining the effect of exercise on primary tumour growth, it is unclear how exercise affects metastasis due to varying results. However, there are several studies describing a reduced number or mass of metastases with both spontaneous [32, 36, 37] and experimental metastasis [25, 30, 38,39,40], with fewer studies reporting no change [39, 41] or an increase in the number/mass of metastases [25, 42], suggesting that in many situations, exercise can inhibit metastatic tumour formation. Stress may play a role in how exercise affects metastasis. Zhang et al. found that swimming for 8 min/day (which mice performed without added encouragement) reduced the relative size of experimental lung metastases, whereas when mice were forced to swim for 16 or 32 min/day, the relative size of metastases was increased [25]. More mechanistic studies are required to help delineate how exercise affects different aspects of the metastatic cascade.
3.4 Myokines and Other Circulating Factors
During exercise, muscle tissue releases a vast array of factors into the circulation, collectively termed ‘myokines’ (from ‘muscle-derived cytokines’). These myokines have known effects on peripheral tissues, such as skeletal muscle remodelling in response to exercise and improvements in cognitive function (reviewed in [43]). It is thought that the action of myokines (and other factors) is directly (affecting cancer cells) and/or indirectly (affecting other cells of the TME) responsible for many of the changes seen in the TME with exercise.
A number of in vitro studies have found that post-exercise serum (serum harvested from humans or animals following an acute exercise bout) can directly inhibit cancer cell proliferation, viability or survival when supplemented into the cell culture media [44,45,46,47,48]. This has been attributed to a few different myokines, including secreted protein acidic and rich in cysteine (SPARC) [49], irisin [50] and oncostatin M [47].
Direct in vivo data indicating the effect of select myokines on tumour growth is still largely lacking. However, Aoi et al. found that the protective effect of exercise against azoxymethane-induced colon tumourigenesis was nullified in SPARC knockout mice, and SPARC was able to induce colon cancer cell apoptosis in vitro [49]. This suggests that the myokine SPARC plays an important role in the protective effect of exercise on cancer development and highlights the value of further investigating the effects of exercise-induced myokines on tumour growth.
Research by Hojman and colleagues indicates that exercise-induced catecholamines (epinephrine and norepinephrine) have both an indirect and a direct effect resulting in the reduction of tumour growth rate [30, 45]. Human breast tumour cells preconditioned with exercise serum were less able to form xenograft tumours in mice, and this effect was completely abolished when the beta-blocker propranolol was also added to the pretreatment [45]. In addition, daily injections of epinephrine or norepinephrine were able to significantly slow MCF-7 and MDA-MB-231 xenograft growth rate [45]. Indirectly, catecholamines (and IL-6) are essential for the exercise-induced mobilisation of natural killer (NK) cells to the tumour site, which are themselves essential for the exercise-induced delay in tumour growth seen in this study [30].
A third factor that has been linked to an exercise-associated delay in tumour progression is dopamine. Zhang et al. found that moderate swimming exercise reduced the tumour weight of subcutaneous and pulmonary hepatomas in mice, and this was mirrored by an increase in dopamine levels in the prefrontal cortex, serum and tumour tissue [25]. Moreover, dopamine treatment was able to reduce tumour weight to the same extent as swimming, and a dopamine receptor 2 antagonist (domperidone) abolished the tumour growth inhibitory effect of both dopamine and swimming exercise [25].
Although there is still a scarcity of data investigating the effects of exercise factors on tumour growth and the tumour microenvironment, those studies that have been done indicate that exercise-induced systemic factors may mediate the effects of exercise on the tumour microenvironment.
3.5 Effect of Exercise on Tumour Vascularity, Hypoxia and Perfusion
3.5.1 Effect of Exercise on Normal Vasculature
Acute exercise modulates blood flow to different organ systems, with some receiving increased (such as skeletal muscle) and some receiving decreased (such as skin) blood flow during exercise [51]. This enables the body to cope with the stress of acute exercise by providing those tissues directly involved in exercise with more oxygen and nutrients. Meanwhile, chronic exercise can induce vascular remodelling [52]. Skeletal muscle is the tissue most affected by these changes, but many tissue types are affected to some degree, including the brain, heart and bone [53,54,55].
It has been shown in vitro and in vivo that exercise can directly affect endothelial cell behaviour. Schadler et al. transplanted Matrigel plugs (an artificial matrix containing gelatine and basement proteins) containing primary mouse endothelial cells into mice and found that those implanted into exercising mice were better perfused and showed elongated vessels compared with those implanted into non-exercising mice [31]. In addition, endothelial cells exposed to exercise-conditioned serum in a microfluidic system showed reduced sprouting (i.e. less angiogenesis), as did those exposed to high shear stress (mimicking that present during exercise) [31]. This seems counterintuitive, but the authors argue that this reflects increased vascular maturity which is ultimately conducive to more stable vascular networks. Interestingly, exercise may also reduce age-associated venous endothelial cell senescence in humans [56].
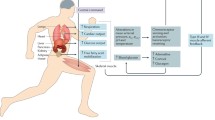
Given that exercise can affect endothelial cell behaviour and induce vascular remodelling in a variety of normal tissues, it is possible that tumour vasculature may also be affected by exercise. A number of preclinical studies have investigated how exercise affects tumour hypoxia, perfusion and vascularity in various tumour types and locations (Fig. 3.1).
Effects of acute and chronic exercise on tumour vascular characteristics and hypoxia. Acute exercise increases blood flow to tumours located in tissues that receive constant or increased blood flow during exercise while decreasing blood flow to tumours located in tissues that are poorly perfused during exercise. This may alter levels of hypoxia and affect drug delivery in the indicated directions. Vascular contractility and dilatory responsiveness are impaired in tumour vessels and this impairment is unchanged by acute exercise. On the other hand, long-term training (chronic exercise) may improve vascular maturation by improving responsiveness and pericyte coverage, which may improve perfusion, oxygen extraction and drug delivery and reduce hypoxia. Areas in need of further research are indicated with?
3.5.2 Effect of Exercise on Tumour Hypoxia
Just like any other tissue, tumours require blood flow delivering oxygen and nutrients in order to survive. However, tumours are not evenly perfused and are characterised by disorganised, dysfunctional vasculature and regions of hypoxia [57]. This leads to the activation of hypoxia factors such as the hypoxia-inducible factors (HIFs) , which stimulate the transcription of a large array of genes designed to help the cell adapt to low oxygen conditions. These include genes central to angiogenesis, cell metabolism and metastasis [58]. In normal tissue, this results in improved vascular coverage and subsequent improved perfusion and alleviation of hypoxia. However, in tumours, the hypoxic response does not improve perfusion as the new blood vessels formed are often immature and may be leaky or lack proper haemodynamic control [59, 60]. This perpetuates tumour hypoxia rather than alleviating it.
In hyperlipidaemic ApoE−/− mice bearing orthotopic breast tumours, we found that mice with high levels of cytochrome c oxidase subunit 4 (COX-IV, a marker of mitochondrial content and proxy for training status) in the quadriceps femoris muscle exhibited greatly reduced tumour hypoxia, although there was no change in tumour perfusion or CD31+ vessel density [37]. A second study found that hypoxia in orthotopic prostate tumours was reduced during exercise [61], and this was also evident in tumours from trained rats [33]. Furthermore, Betof et al. observed that tumour hypoxia was decreased in orthotopic 4T1 breast tumours from exercising mice [28].
Levels of the hypoxic response protein HIF-1α were decreased with exercise in an orthotopic breast cancer model [4]. In contrast, HIF-1α levels were increased with exercise in human-derived breast xenografts in athymic mice [62] and in orthotopic breast and prostate cancer in immunocompetent mice [4, 32]. In subcutaneous Ewing sarcoma xenografts, HIF-1α and carbonic anhydrase IX (CA-IX, a HIF-1 target gene) mRNA were decreased with exercise in one of two tumour cell lines used [63]. It is unclear why this disparity exists between measured hypoxia and levels of HIF-1α, given that HIF-1α protein stability is strongly dependent on oxygen levels. However, it can partially be regulated independently of hypoxia, which may explain the above-described results [58].
To our knowledge, hypoxia in tumours from mice starting exercise prior to tumour cell inoculation has not yet been investigated nor has tumour hypoxia in exercising cancer patients. It is also unknown whether the reduction in tumour hypoxia occurs in tumour types other than breast and prostate cancer.
3.5.3 Tumour Perfusion and Vessel Density
Initial anticancer strategies targeting tumour blood vessels focussed on inhibiting angiogenesis, as tumour cells will die if completely deprived of oxygen and nutrients. However, as with all cancer therapies, many tumours develop resistance to anti-angiogenic agents. A more recent strategy is vascular normalisation [59], which aims to promote the normal development of tumour vessels to form a functional, evenly perfused network which more closely resembles that of normal tissue. This would reduce hypoxia, thereby reducing metastatic potential and enhancing radiosensitivity. In addition, drug delivery throughout the tumour would be improved.
The effect of exercise on tumour vascularity and perfusion remains unclear. Studies by various groups have demonstrated an increase in perfusion homogeneity, the level of perfusion and/or vessel density or Cd31 mRNA levels (breast, prostate and pancreatic cancer) [28, 31, 32, 64, 65], but others have found no change in the mean level of perfusion [15, 19] or vessel density in breast and/or prostate cancer [66]. Further studies even found reduced numbers of blood vessels in breast tumours or lymphomas from exercising mice [67, 68].
McCullough et al. observed increased blood flow to orthotopic prostate tumours during acute exercise (but not with exercise training) which was associated with a reduction in tumour hypoxia, which suggests that levels of tumour perfusion may change with acute exercise but this is not necessarily maintained after exercise cessation [61].
A potential reason for the differing results observed in different studies may be the method used to detect tumour perfusion. We and McCullough et al. used IV injection of Hoechst 33342 prior to euthanasia to label perfused blood vessels [33, 37], while other studies used MRI to generate a perfusion map of the entire tumour while the animal was still alive [28, 32]. MRI is likely a more representative method of whole-tumour perfusion, as Hoechst injection and subsequent imaging of thin tissue sections presents only a snapshot of tumour perfusion in time and space, which fails to take into account the dynamic nature of tumour blood flow. Nevertheless, this method has been able to detect differences in perfusion in the past [62] and has been validated for this purpose [69].
A further important consideration is the impact of anatomical location of the tumour on blood flow responses to exercise. Garcia et al. elegantly demonstrated that blood flow during exercise is increased to orthotopic prostate tumours in rats, but decreased to subcutaneous tumours of the same type [51]. They further measured blood flow to different organs during exercise, including the bladder, prostate (location of the orthotopic tumour), soleus muscle, kidneys, skin, subcutaneous adipose (location of the ectopic tumour) and visceral adipose tissue. Blood flow to the bladder and prostate was unchanged, but increased to the soleus muscle and decreased to the kidneys, skin, subcutaneous and visceral adipose tissue [51]. This suggests that host tissue haemodynamics in response to exercise also play a role in regulating blood flow to the tumour. As such, tumours located in tissues that become less well perfused during exercise may not benefit from the increased perfusion seen in prostate tumours and may even become more poorly perfused during exercise, which could exacerbate tumour hypoxia. This has yet to be further investigated in different tumour models.
Tissue blood flow is largely regulated by vascular contractility and myogenic tone. Due to their poor maturation (lack of smooth muscle cells and innervation), tumour vessels have poor contractile and dilatory responsiveness, which limits their ability to regulate blood flow [51, 61, 70]. Contractile responsiveness to norepinephrine does not change with exercise training, indicating that tumour vessel response to both acute and chronic exercise is impaired compared with normal tissue [51, 61]. Due to this, tumour blood flow cannot be regulated to provide optimal conditions for oxygen extraction. Optimal oxygen extraction relies on complex haemodynamics—parameters such as capillary transit time, microvascular pressure and apparent blood viscosity all affect how much oxygen can be extracted from the bloodstream [57, 71]. Thus, optimal tissue oxygenation relies on more than just the presence of perfused vessels. As mentioned above, acute exercise does not alter tumour vessel contractility, but it is unknown whether long-term exercise could improve vascular maturation to a point where vessel contractile and dilatory responses are restored, thus improving tissue perfusion and oxygen extraction.
Part of the therapeutic appeal of vascular normalisation is the enhanced delivery of anticancer agents to the tumour. Two preclinical studies have investigated how exercise affects chemotherapy delivery to the tumour [31, 63]. Schadler et al. found that although exercise alone did not reduce tumour growth rate of subcutaneous, pancreatic PDAC or B16-F10 melanoma, chemotherapy in combination with exercise significantly slowed tumour growth rate over and above the effect of chemotherapy alone [31]. Immunofluorescence analysis revealed that there was increased expression of the DNA damage marker γH2AX in PDAC tumours from mice receiving both chemotherapy and exercise compared with those only receiving chemotherapy and higher levels of doxorubicin fluorescence in B16-F10 tumours from exercised mice receiving chemotherapy compared with those receiving chemotherapy only. This was only the case in tumours from trained mice; one acute exercise session was insufficient to enhance doxorubicin delivery to the tumour [31]. Furthermore, the authors demonstrated that pharmacologically increasing tumour blood velocity by the use of an antihypertensive agent (prazosin) also enhanced the growth inhibitory effect of gemcitabine on PDAC tumours [31]. Similarly, Morrel et al. found that exercise improved doxorubicin delivery to subcutaneous Ewing sarcomas, and this was associated with a further reduced tumour growth rate compared with exercise or doxorubicin alone (although exercise alone also had a strong growth inhibitory effect) [63]. These data suggest that exercise can induce vascular changes leading to improved tumour blood flow even in tumours that are located in tissue that does not receive enhanced blood flow during acute exercise (i.e. subcutaneous adipose tissue), although this is yet to be corroborated by other groups.
3.5.4 Markers of Angiogenesis and Vascular Maturation
Further to the above-described effects of exercise on tumour hypoxia, vascularity and perfusion, a few studies have investigated markers of angiogenesis and vascular maturation in tumours following exercise.
Betof et al. found that voluntary wheel running not only increased CD31+ vessel density, but also enhanced pericyte coverage (a marker of vascular maturation) in orthotopic 4T1 breast tumours [28]. In addition, pericyte coverage was increased by exercise in two Ewing sarcoma models [63]. Conversely, Schadler et al. found that the α-smooth muscle actin (α-SMA, a pericyte marker) to CD31 ratio did not change with exercise in subcutaneous B16-F10 tumours [31]. This discrepancy may simply be due to differing tumour models, but future studies should aim to clarify this.
A few studies have investigated tumour levels of the angiogenic factor vascular endothelial growth factor (VEGF, a HIF-1 target) in mammary tumours. One study observed increased mRNA levels of Vegfa [28]. In agreement with this, another group found increased VEGFA protein expression in mammary tumours from exercised rats [64]. In contrast, two other studies found that VEGF protein expression was reduced in tumours from mice exercising after tumour implant [27, 67].
3.5.5 Conclusions on Tumour Vascularity, Hypoxia and Perfusion
It seems clear that acute exercise can regulate tumour blood flow, either increasing or decreasing blood flow depending on tumour location [51]. However, it remains unclear whether or how long this persists after exercise cessation and whether chronic exercise can remodel the TME in such a way as to normalise the vasculature to improve perfusion and oxygen extraction even at rest. These questions are central to future work in this area, as a thorough understanding of tumour blood flow and perfusion dynamics (with respect to acute and chronic exercise) is required to inform relevant intervention trials and subsequent clinical practice to achieve the greatest benefit from exercise together with standard cancer therapies.
3.6 Effect of Exercise on the Immune Microenvironment
3.6.1 Effect of Exercise on Immunity in Healthy Individuals
Acute exercise causes a rapid rise in the number of circulating immune cells; this includes an increase in numbers of all major subclasses (lymphocytes, monocytes and granulocytes) [72]. Lymphocytes, in particular NK cells, are among those that respond most strongly to acute exercise [73]. Following exercise cessation, lymphocyte counts in the blood rapidly decrease, falling below pre-exercise levels by 1 h post-exercise [72]. This was previously thought to be due to lymphocyte apoptosis and attributed to an immunosuppressive effect of exercise, but based on evidence that lymphocyte apoptosis post-exercise only accounts for a small fraction of the observed lymphocytopenia, it seems more likely that the bulk of this is due to egress into peripheral tissues and may present a mechanism for heightened immune surveillance of tissues post-exercise [74]. Direct evidence for this is still lacking, but is supported by evidence that leukocyte subtypes that are preferentially mobilised by exercise tend to be cytotoxic subtypes and express markers associated with extravasation and tissue migration (such as integrins and chemokine receptors) [75,76,77].
Regular moderate-intensity exercise has been linked with enhanced overall immunity, such as improved NK cell cytotoxic activity, increased lymphocyte proliferation, reduced T cell senescence and enhanced vaccine responses [78,79,80]. There is some controversy regarding the effect of intensive exercise on immunity, with the open window hypothesis stating that intense exercise is followed by a transient state of immune depression, which becomes chronic if regular intense exercise is performed [81]. This has recently been challenged by Campbell and Turner, who argue that the evidence supposedly supporting the open window hypothesis (increased frequency of upper respiratory tract infections, a fall in salivary IgA and lymphocytopenia following intense, acute exercise such as a marathon) has been largely misinterpreted [82]. They argue that the supposed increase in incidence of upper respiratory tract infections is either due to symptoms of an infection but no actual infection (rather caused by airway irritation due to increased ventilation or non-specific inflammation) or an actual infection caused by factors not directly related to intense exercise such as increased exposure to pathogens due to a large accumulation of people. As discussed above, acute lymphocytopenia following exercise is now thought to be due to lymphocyte egress into peripheral tissues.
3.6.2 Effect of Exercise on Immunity in the TME
3.6.2.1 Peripheral Immunity in Cancer Survivors
Changes in circulating levels of immune cells with exercise may provide an indication of whole-body immunity, including effects on the tumour, in cancer patients. In some patients, the acute exercise-induced increase in circulating immune cells is attenuated or even abolished [83, 84]. Lymphocytes seem to be most strongly affected by this, with two studies showing a nullified or attenuated lymphocytosis but intact neutrophil [84], granulocyte and monocyte response with acute exercise [83]. Another study has found an increase in both lymphocytes and granulocytes immediately following acute exercise in chronic myeloid leukaemia patients [85]. However, other studies were in patients with solid tumours [83, 84], which may impact systemic immune responses differently. These results suggest that either tumour burden or treatment may negatively affect immune cell mobilisation in response to exercise, which may reduce immune surveillance of peripheral tissues.
Chronic exercise does not alter numbers of circulating immune cells in most studies [84, 86,87,88,89,90]. However, occasionally some studies have found an increase in various immune cell types with chronic exercise, including granulocytes, leukocytes, lymphocytes and neutrophils (systematically reviewed in [89]). Others have reported a decrease in lymphocytes or monocytes [89]. In addition, exercise training was unable to prevent the chemotherapy-associated decline in immune cell numbers [90]. Taken together, this suggests that exercise training does not alter numbers of circulating immune cells in cancer patients and other factors may be responsible for the observed increases or decreases in certain components in some studies.
3.6.2.2 Ex Vivo Immunity
Preclinical exercise studies reporting on ex vivo or intratumoural immunity are summarised in Fig. 3.2.
Effects of exercise on peripheral immunity and the immune TME. Exercise increases cytotoxicity of peripheral, IL-2 activated NK cells and macrophages against tumour targets and enhances phagocytic activity of macrophages ex vivo. In addition, exercise may increase recruitment of cytotoxic lymphocytes (NK cells and CTLs) to the tumour site while decreasing number and/or changing phenotype of myeloid cells such as neutrophils and macrophages to an anti- (M1/N1) rather than pro-tumour (M2/N2) state. CTL cytotoxic T lymphocyte, NK cell natural killer cell, IL-2 interleukin 2
The effect of exercise on immune cell function is difficult to measure in vivo. However, a number of studies have isolated immune cells from either the spleen, tumour or peritoneum of exercising and non-exercising animals and compared their cytotoxic capacity, phagocytic capacity or cytokine production in vitro.
The first studies investigating ex vivo immune function against tumour targets were conducted by MacNeil and Hoffman-Goetz in the early 1990s. Splenic NK cells isolated from healthy mice immediately following an acute exercise session had higher activity when stimulated with IL-2 than those from non-exercised mice [91]. In addition, splenic NK cells isolated from tumour-bearing mice performing chronic exercise beginning prior to tumour implant exhibited increased activity against tumour targets [92,93,94,95]. In contrast, Pedersen et al. found no change in the cytotoxic activity of splenic NK cells isolated from trained compared with non-exercised mice bearing B16-F10 melanoma [30]. This discrepancy may be due to the activation status of the NK cells. In a few of the above-mentioned early studies, the authors showed that only IL-2 activated but not unactivated NK cells from exercised mice had increased cytotoxicity against tumour targets [91, 93]. In addition, unactivated NK cells are poorly effective against lysis-resistant tumour cell lines, but are able to achieve up to ~60% lysis when pre-stimulated with IL-2 and IL-12 [96]. This suggests that the in vivo antitumour activity of NK cells may be dependent on the intratumoural milieu. In support of this, exercise prior to tumour implant causes significantly slower growth of B16-F10 tumours, and these tumours show higher mRNA expression of IL-2 and other NK-cell activating factors [30].
Macrophage phagocytosis and phenotype has also been reported to change with exercise in tumour-bearing rodents. Peritoneal macrophages from exercised mice produce more IFN-γ, IL-12, TNF-α and IL-4 than those from non-exercised mice and less of the immunosuppressive cytokines TGF-β and IL-10, suggesting a polarisation towards an antitumour M1 phenotype [97]. Furthermore, peritoneal macrophages from exercised, tumour-bearing rats are more phagocytic than those from non-exercising rats [98] and macrophages isolated from healthy, trained mice are able to induce higher cytolysis of tumour targets compared with those from non-exercised mice [99]. Finally, phagocytes isolated from subcutaneous breast tumours in moderately exercised mice have higher phagocytic activity against Staphylococcus aureus than those from non-exercised (or exhaustively exercised) mice [100].
In humans, it has been found that both acute [101, 102] and chronic [80, 103] exercise can improve cytotoxic activity of peripheral blood NK cells from healthy individuals against tumour targets, although one study found no change in NK cell cytotoxicity following exercise training [104] and another found decreased activity [105]. In cancer patients, chronic exercise has also been shown to increase NK cell cytotoxicity ex vivo [89, 106, 107]. In addition, ex vivo lymphocyte proliferation and phagocytic activity of monocytes is increased in post-exercise training, while neutrophil oxidative burst is unchanged [89].
Taken together, ex vivo immune functionality data from both human and animal studies suggest that exercise can improve antitumour cytotoxicity. In future, studies should aim to corroborate this in vivo if possible. In addition, only one study has utilised immune cells isolated from the tumour itself rather than from the spleen or peripheral blood [100]. As the phenotype of immune cells in the TME may differ from peripheral immune cells due to crosstalk with tumour cells, future studies should investigate the functionality of phagocytes and lymphocytes isolated directly from the tumour.
3.6.2.3 Intratumoural Immunity
Given the hypothesis that the transient lymphocytopenia following exercise is due to cytotoxic lymphocyte egress and surveillance of peripheral tissues, it follows that exercise may also redistribute these cells to the tumour. This is indirectly supported by work showing that NK cell and T cell numbers are increased in subcutaneous B16-F10 tumours following 6 weeks of exercise training [30] and that Cd8 gene expression is increased in mucosal scrapings from exercised compared with non-exercised ApcMin/+ mice [108]. In addition, Zielinski et al. found increased intratumoural lymphocyte density in subcutaneous EL-4 tumours following exhaustive exercise training compared with non-exercise mice [68]. Conversely, we observed no difference in T cell numbers in EO771 tumours between exercising and non-exercising ApoE−/− mice [37], and Bianco et al. found no change in numbers of tumour-infiltrating T cells into 4T1 tumours with post-implant exercise [109]. This may be due to the length of exercise, timing of exercise initiation or tumour model used. Pedersen et al. began exercise 4 weeks prior to tumour implant, whereas we and Bianco et al. started exercise at tumour implant [30, 37, 109]. Although Zielinski et al. also had a short exercise period of approximately 2 weeks, they used a tumour model which spontaneously regresses, indicating that this tumour cell line induces a strong antitumour immune response in vivo, which is enhanced by exercise [68]. Just one study has investigated B cell numbers and found that they were unchanged in the tumour with chronic exercise [30].
Whether or not absolute numbers of lymphocytes within the tumour change may be less important than the phenotype and cytotoxic functionality of those that are present. As described in the previous section, ex vivo data indicate that exercise may improve NK cell cytotoxicity. Data on T cells is much scarcer. Some studies suggest that exercise reduces Treg cell recruitment to the tumour (inferred from lower levels of the Treg cell recruiting cytokine CCL22 or lower mRNA expression of Foxp3 [108, 110]), but others have found no change in the proportion of intratumoural Treg cells [37, 109] or even an increase in intratumoural Foxp3 mRNA (alongside increased expression of inflammatory/cytotoxic cell markers) following exercise training [30]. Ex vivo functionality assays investigating the effect of exercise on intratumoural T cells have not yet been conducted.
The tumour microenvironment promotes an immunosuppressive phenotype of infiltrating immune cells, causing them to aid rather than inhibit tumour growth both by the inhibition of cytotoxic immune cells and by secreting factors that aid tumour growth such as VEGF [111]. Myeloid cells seem to be particularly susceptible to this reprogramming and often take on an immunosuppressive phenotype within the TME (e.g. M2 macrophages) [112]. Two studies have found reduced neutrophil infiltration into tumours with exercise [68, 113], and two have found reduced macrophage density [68, 114]. Additionally, gene expression of general macrophage markers (F4/80) and M2-specific markers (CD206, arginase) was reduced in mucosal scrapings from exercised compared with non-exercised mice [108]. Together with the above-described ex vivo data, this suggests that exercise reconditions the TME to reduce recruitment of and/or repolarise myeloid cells such as neutrophils and macrophages toward a more antitumour phenotype.
Comprehensive analysis of the types and subtypes of immune cells within the tumour microenvironment following exercise is still lacking. Current preliminary evidence suggests that exercise may repolarise immune cells to an antitumour phenotype and/or increase numbers of antitumour immune cells such as NK cells and CD8+ T cells, but this requires confirmation via flow cytometry, multiplex immunohistochemistry and functional assays, as well as investigation of possible differences between tumour type and exercise protocol.
3.6.2.4 Interplay of Immunity with Hypoxia and Angiogenesis
Hypoxia inhibits antitumour immunity by inhibiting lytic functions of cytotoxic T cells (CTL) and NK cells and promoting an immunosuppressive phenotype in both lymphoid and myeloid cells (reviewed in [115]). In addition, tumour vasculature is prohibitive to T cell entry in that it downregulates adhesion molecules required for extravasation and upregulates inhibitory and apoptotic ligands [116]. Conversely, Treg cells and M2 macrophages can promote angiogenesis, while Type 1 T helper (TH1) cells can promote intratumoural vessel normalisation [117,118,119]. Thus, the influence of hypoxia on immune cells and their influence on tumour vasculature (and vice versa) are integral to the overall tumour phenotype.
To our knowledge, just one study has directly investigated T cell subsets, hypoxia and perfusion in the same tumours, although this was in hyperlipidaemic ApoE−/− and not wild-type mice [37]. In this study, we found no change in the fraction of intratumoural CD8+ or Foxp3+ T cells, or perfusion, with post-implant exercise, but found a decrease in hypoxia in EO771 tumours from trained mice (using muscular COX-IV expression as a proxy for ‘fitness’). In order to better investigate how these aspects of the tumour microenvironment interact and change with exercise, an exercise protocol known to elicit tumour microenvironmental changes (such as a few weeks of pre-implant exercise continuing post-implant) could be used and the tumours analysed for hypoxia, perfusion, vascularity and immune cell composition.
3.6.3 Conclusions on the Immune Microenvironment
In cancer patients, acute exercise-induced lymphocytosis may be partially suppressed [83, 84] and chronic exercise may not be able to protect against chemotherapy-induced lymphopenia [90]. However, preclinical and clinical functional data indicate that exercise improves peripheral NK cell cytotoxicity (from both healthy individuals and cancer patients) [89, 91, 93] and possible repolarisation of macrophages towards an antitumour M1 phenotype [120]. It remains unclear whether these improvements in functionality of peripheral immune cells are translated to improved antitumour immunity within the TME, but they are a promising indication that exercise could improve immune responses in cancer patients.
3.7 Future Directions
As mentioned at the beginning of the chapter, the role of an individual’s ability to perform the physiological adaptations required for improvements in exercise capacity (exercise adaptability), and indeed inherent exercise capacity itself, has thus far been largely neglected in exercise oncology. Importantly, there is large inter-individual variation in these factors in both rodents and humans, determined by both inherited factors and activity levels [7,8,9]. In simple terms, this means that two individuals undergoing exactly the same exercise program will (a) not adapt to exercise to the same degree (chronic response) or (b) feel the same level of exertion (acute response). It is unclear what effect (if any) this might have on how exercise affects tumour characteristics, but is an important avenue of investigation if we are to fully understand how exercise effects physiological change in the TME—the key question being whether improvements in exercise capacity/muscular adaptations are required for beneficial effects of exercise on the TME. Parameters such as VO2 max, muscle protein synthesis/degradation and skeletal muscle mitochondrial content/function can be used to ascertain exercise capacity and exercise adaptability.
The vast majority of preclinical studies in exercise oncology to date have utilised exercise as a sole intervention (monotherapy). While these provide valuable insight into exercise-induced systemic and local changes affecting overall tumour growth characteristics, they do not accurately reflect the clinical situation in which a person diagnosed with cancer will almost always receive some form of treatment. A small number of studies have investigated the effect of exercise in combination with other cancer therapies (chemotherapy and hormone therapy) and found a reduction in tumour growth above and beyond that of the cancer treatment alone and sometimes in the complete absence of an exercise-only effect [28, 31, 67, 121, 122]. In addition to growth inhibitory effects, it is likely that the combination of exercise with other therapies will alter the TME distinct from the alterations caused by either therapy or exercise alone. Therefore, it is essential that future work combines exercise with cancer therapies such as chemotherapy, immunotherapy and surgical resection and measures not only the effect on tumour growth rate or survival but also the components of the TME outlined in this chapter.
It is well established that exercise can improve ex vivo antitumour toxicity of select immune cell types (particularly NK cells) isolated from both healthy and tumour-bearing mice [91,92,93,94,95, 98,99,100] or humans [80, 89, 101, 103, 106, 107]. However, with one exception, these studies have been conducted using immune cells isolated from either peripheral blood or the spleen. While these may give an indication of the individual’s general immune functioning, they do not account for effects that the TME might be having on immune cell phenotype. Future studies should aim to determine whether immune cells isolated from the tumour itself display the same enhanced cytotoxic capabilities as those isolated from the spleen or peripheral blood. Ideally, this would even be conducted in vivo, but this is limited by current technology.
With a few exceptions [31, 51, 63], investigation of the effects of exercise on the vascular TME have largely been limited to basic assessment of vessel number (overall, perfused or associated with pericytes) and hypoxic area. This gives a basic idea of functionality, but more comprehensive research into the effect of chronic exercise on tumour vessel haemodynamics would provide a more complete picture of whether exercise can induce similar vascular adaptations in tumour tissue as in skeletal muscle. In addition, how exercise affects the interaction between immune cells and tumour vessels has not yet been fully investigated.
3.8 Concluding Remarks
Exercise oncology is a hugely complex field and requires the collaboration of clinical oncologists, preclinical cancer researchers, immunologists and exercise physiologists (to name a few) for a thorough understanding of exercise and tumour physiology. The current state of knowledge supports a beneficial role of exercise in cancer prevention and survival in some cancer types, but comprehensive mechanistic data remain elusive and robust predictors of tumour response to exercise are non-existent. Delineating the effects of exercise on the TME (and of the tumour on the body, Fig. 3.3) may be the key to unravelling how and in which situations exercise exerts a tumour growth inhibitory effect.
Summary of exercise effects on the TME. Chronic exercise induces skeletal muscle remodelling to improve exercise capacity, including an increase in mitochondrial content and function, and improved vascularisation. However, this response may be blunted in cancer patients and varies strongly between individuals. It is unknown whether this variation in exercise adaptability affects the degree to which exercise can alter the TME. Myokines, dopamine, catecholamines and further unknown factors are released into the circulation with exercise and exert effects on the TME. Effects on vascularisation/hypoxia/perfusion and immunity are summarised in Figs. 3.1 and 3.2, respectively
References
Moore SC, Lee I-M, Weiderpass E, Campbell PT, Sampson JN, Kitahara CM, Keadle SK, Arem H, Berrington de Gonzalez A, Hartge P et al (2016) Association of leisure-time physical activity with risk of 26 types of cancer in 1.44 million adults. JAMA Intern Med 176:816
Li T, Wei S, Shi Y, Pang S, Qin Q, Yin J, Deng Y, Chen Q, Wei S, Nie S et al (2016) The dose–response effect of physical activity on cancer mortality: findings from 71 prospective cohort studies. Br J Sports Med 50:339–345
Christensen JF, Simonsen C, Hojman P (2019) Exercise training in cancer control and treatment. Compr Physiol 9:165–205
Glass OK, Bowie M, Fuller J, Darr D, Usary J, Boss K, Choudhury KR, Liu X, Zhang Z, Locasale JW et al (2017) Differential response to exercise in claudin-low breast cancer. Oncotarget 8:100989–101004
Lu M, Sanderson SM, Zessin A, Ashcraft KA, Jones LW, Dewhirst MW, Locasale JW, Hsu DS (2018) Exercise inhibits tumor growth and central carbon metabolism in patient-derived xenograft models of colorectal cancer. Cancer Metab 6:14
Goldstein RE (1990) Exercise capacity. Butterworths, Boston, MA. ISBN: 040990077X
Koch LG, Britton SL (2001) Artificial selection for intrinsic aerobic endurance running capacity in rats. Physiol Genomics 5:45–52
Avila JJ, Kim SK, Massett MP (2017) Differences in exercise capacity and responses to training in 24 inbred mouse strains. Front Physiol 8:974
Vellers HL, Kleeberger SR, Lightfoot JT (2018) Inter-individual variation in adaptations to endurance and resistance exercise training: genetic approaches towards understanding a complex phenotype. Mamm Genome 29:48–62
Thompson HJ, Jones LW, Koch LG, Britton SL, Neil ES, McGinley JN (2017) Inherent aerobic capacity-dependent differences in breast carcinogenesis. Carcinogenesis 38:920–928
Lundby C, Jacobs RA (2016) Adaptations of skeletal muscle mitochondria to exercise training. Exp Physiol 101:17–22
Bouchard C, An P, Rice T, Skinner JS, Wilmore JH, Gagnon J, Pérusse L, Leon AS, Rao DC (1999) Familial aggregation of VO2 max response to exercise training: results from the HERITAGE Family Study. J Appl Physiol 87:1003–1008
Jones LW, Courneya KS, Mackey JR, Muss HB, Pituskin EN, Scott JM, Hornsby WE, Coan AD, Herndon JE, Douglas PS et al (2012) Cardiopulmonary function and age-related decline across the breast cancer survivorship continuum. J Clin Oncol 30:2530–2537
Jones LW, Watson D, Herndon JE, Eves ND, Haithcock BE, Loewen G, Kohman L (2010) Peak oxygen consumption and long-term all-cause mortality in nonsmall cell lung cancer. Cancer 116:4825–4832
Christensen JF, Jones LW, Tolver A, Jørgensen LW, Andersen JL, Adamsen L, Højman P, Nielsen RH, Rørth M, Daugaard G (2014) Safety and efficacy of resistance training in germ cell cancer patients undergoing chemotherapy: a randomized controlled trial. Br J Cancer 111:8–16
Jones LW, Liang Y, Pituskin EN, Battaglini CL, Scott JM, Hornsby WE, Haykowsky M (2011) Effect of exercise training on peak oxygen consumption in patients with cancer: a meta-analysis. Oncologist 16:112–120
Brown JC, Winters-Stone K, Lee A, Schmitz KH (2012) Cancer, physical activity, and exercise. Compr Physiol 2:2775–2809
Mctiernan A, Friedenreich CM, Katzmarzyk PT, Powell KE, Macko R, Buchner D, Pescatello LS, Bloodgood B, Tennant B, Vaux-Bjerke A et al (2018) Physical activity in cancer prevention and survival: a systematic review. Med Sci Sport Exerc 51:1252–1261
Betof AS, Dewhirst MW, Jones LW (2013) Effects and potential mechanisms of exercise training on cancer progression: a translational perspective. Brain Behav Immun 30(Suppl):S75–S87
Eschke R-CK-R, Lampit A, Schenk A, Javelle F, Steindorf K, Diel P, Bloch W, Zimmer P (2019) Impact of physical exercise on growth and progression of cancer in rodents—a systematic review and meta-analysis. Front Oncol 9:35
Ashcraft KA, Peace RM, Betof AS, Dewhirst MW, Jones LW (2016) Efficacy and mechanisms of aerobic exercise on cancer initiation, progression, and metastasis: a critical systematic review of in vivo preclinical data. Cancer Res 76:4032–4050
Buss LA, Dachs GU (2018) The role of exercise and hyperlipidaemia in breast cancer progression. Exerc Immunol Rev 24:10–25
Newton RU, Kenfield SA, Hart NH, Chan JM, Courneya KS, Catto J, Finn SP, Greenwood R, Hughes DC, Mucci L et al (2018) Intense exercise for survival among men with metastatic castrate-resistant prostate cancer (INTERVAL-GAP4): a multicentre, randomised, controlled phase III study protocol. BMJ Open 8:e022899
Courneya KS, Booth CM, Gill S, O’Brien P, Vardy J, Friedenreich CM, Au HJ, Brundage MD, Tu D, Dhillon H et al (2008) The colon health and life-long exercise change trial: a randomized trial of the National Cancer Institute of Canada Clinical Trials Group. Curr Oncol 15:279–285
Zhang QB, Zhang BH, Zhang KZ, Meng XT, Jia QA, Zhang QB, Bu Y, Zhu XD, Ma DN, Ye BG et al (2016) Moderate swimming suppressed the growth and metastasis of the transplanted liver cancer in mice model: with reference to nervous system. Oncogene 35:4122–4131
Aveseh M, Nikooie R, Aminaie M (2015) Exercise-induced changes in tumour LDH-B and MCT1 expression are modulated by oestrogen-related receptor alpha in breast cancer-bearing BALB/c mice. J Physiol 593:2635–2648
Shalamzari SA, Agha-Alinejad H, Alizadeh S, Shahbazi S, Khatib ZK, Kazemi A, Saei MA, Minayi N (2014) The effect of exercise training on the level of tissue IL-6 and vascular endothelial growth factor in breast cancer bearing mice. Iran J Basic Med Sci 17:231–258
Betof AS, Lascola CD, Weitzel D, Landon C, Scarbrough PM, Devi GR, Palmer G, Jones LW, Dewhirst MW (2015) Modulation of murine breast tumor vascularity, hypoxia and chemotherapeutic response by exercise. J Natl Cancer Inst 107:djv040
Shewchuk LD, Baracos VE, Field CJ (1997) Dietary L-glutamine supplementation reduces the growth of the Morris Hepatoma 7777 in exercise-trained and sedentary rats. J Nutr 127:158–166
Pedersen L, Idorn M, Olofsson GH, Lauenborg B, Nookaew I, Hansen RH, Johannesen HH, Becker JC, Pedersen KS, Dethlefsen C et al (2016) Voluntary running suppresses tumor growth through epinephrine- and IL-6-dependent NK cell mobilization and redistribution. Cell Metab 23:554–562
Schadler KL, Thomas NJ, Galie PA, Bhang DH, Roby KC, Addai P, Till JE, Sturgeon K, Zaslavsky A, Chen CS et al (2016) Tumor vessel normalization after aerobic exercise enhances chemotherapeutic efficacy. Oncotarget 7:65429–65440
Jones LW, Antonelli J, Masko EM, Broadwater G, Lascola CD, Fels D, Dewhirst MW, Dyck JR, Nagendran J, Flores CT et al (2012) Exercise modulation of the host-tumor interaction in an orthotopic model of murine prostate cancer. J Appl Physiol 113:263–272
McCullough DJ, Nguyen LM, Siemann DW, Behnke BJ (2013) Effects of exercise training on tumor hypoxia and vascular function in the rodent preclinical orthotopic prostate cancer model. J Appl Physiol 115:1846–1854
Malicka I, Siewierska K, Pula B, Kobierzycki C, Haus D, Paslawska U, Cegielski M, Dziegiel P, Podhorska-Okolow M, Wozniewski M (2015) The effect of physical training on the N-methyl-N-nitrosourea-induced mammary carcinogenesis of Sprague-Dawley rats. Exp Biol Med 240:1408–1415
Regmi S, Fu A, Luo KQ, Mehlen P, Puisieux A, Fidler IJ, Gupta GP, Massagué J, Hart IR, Mitchell MJ et al (2017) High shear stresses under exercise condition destroy circulating tumor cells in a microfluidic system. Sci Rep 7:39975
Moreira VM, da Silva Franco CC, Prates KV, Gomes RM, de Moraes AMP, Ribeiro TA, Martins IP, Previate C, Pavanello A, Matiusso CCI et al (2018) Aerobic exercise training attenuates tumor growth and reduces insulin secretion in Walker 256 tumor-bearing rats. Front Physiol 9:465
Buss LA, Dachs GU (2018) Voluntary exercise slows breast tumor establishment and reduces tumor hypoxia in ApoE-/- mice. J Appl Physiol 124:938–949. https://doi.org/10.1152/japplphysiol.00738.2017
Zhang Q-B, Meng X-T, Jia Q-A, Bu Y, Ren Z-G, Zhang B-H, Tang Z-Y (2016) Herbal compound Songyou Yin and moderate swimming suppress growth and metastasis of liver cancer by enhancing immune function. Integr Cancer Ther 15:368–375
Davis JM, Kohut ML, Jackson DA, Colbert LH, Mayer EP, Ghaffar A (1998) Exercise effects on lung tumor metastases and in vitro alveolar macrophage antitumor cytotoxicity. Am J Physiol Integr Comp Physiol 274:R1454–R1459
Higgins KA, Park D, Lee GY, Curran WJ, Deng X (2014) Exercise-induced lung cancer regression: mechanistic findings from a mouse model. Cancer 120:3302–3310
Yan L, Demars LC (2011) Effects of non-motorized voluntary running on experimental and spontaneous metastasis in mice. Anticancer Res 31:3337–3344
Smeda M, Przyborowski K, Proniewski B, Zakrzewska A, Kaczor D, Stojak M, Buczek E, Nieckarz Z, Zoladz JA, Wietrzyk J et al (2017) Breast cancer pulmonary metastasis is increased in mice undertaking spontaneous physical training in the running wheel; a call for revising beneficial effects of exercise on cancer progression. Am J Cancer Res 7:1926–1936
Hoffmann C, Weigert C (2017) Skeletal muscle as an endocrine organ: the role of myokines in exercise adaptations. Cold Spring Harb Perspect Med 7:a029793
Kurgan N, Tsakiridis E, Kouvelioti R, Moore J, Klentrou P, Tsiani E (2017) Inhibition of human lung cancer cell proliferation and survival by post-exercise serum is associated with the inhibition of Akt, mTOR, p70 S6K, and Erk1/2. Cancers (Basel) 9:46
Dethlefsen C, Hansen LS, Lillelund C, Andersen C, Gehl J, Christensen JF, Pedersen BK, Hojman P (2017) Tumor and stem cell biology exercise-induced catecholamines activate the hippo tumor suppressor pathway to reduce risks of breast cancer development. Cancer Res 77:1–11
Dethlefsen C, Lillelund C, Midtgaard J, Andersen C, Pedersen BK, Christensen JF, Hojman P (2016) Exercise regulates breast cancer cell viability: systemic training adaptations versus acute exercise responses. Breast Cancer Res Treat 159:469–479
Hojman P, Dethlefsen C, Brandt C, Hansen J, Pedersen L, Pedersen BK (2011) Exercise-induced muscle-derived cytokines inhibit mammary cancer cell growth. Am J Physiol Endocrinol Metab 301:E504–E510
Rundqvist H, Augsten M, Strömberg A, Rullman E, Mijwel S, Kharaziha P, Panaretakis T, Gustafsson T, Östman A (2013) Effect of acute exercise on prostate cancer cell growth. PLoS One 8:e67579
Aoi W, Naito Y, Takagi T, Tanimura Y, Takanami Y, Kawai Y, Sakuma K, Hang LP, Mizushima K, Hirai Y et al (2013) A novel myokine, secreted protein acidic and rich in cysteine (SPARC), suppresses colon tumorigenesis via regular exercise. Gut 62:882–889
Gannon NP, Vaughan RA, Garcia-Smith R, Bisoffi M, Trujillo KA (2015) Effects of the exercise-inducible myokine irisin on malignant and non-malignant breast epithelial cell behavior in vitro. Int J Cancer 136:E197–E202
Garcia E, Becker VGC, McCullough DJ, Stabley JN, Gittemeier EM, Opoku-Acheampong AB, Siemann DW, Behnke BJ (2016) Blood flow responses to mild-intensity exercise in ectopic versus orthotopic prostate tumors; dependence upon host-tissue hemodynamics and vascular reactivity. J Appl Physiol 121:15–24
Prior BM, Lloyd PG, Yang HT, Terjung RL (2003) Exercise-induced vascular remodeling. Exerc Sport Sci Rev 31:26–33
Viboolvorakul S, Patumraj S (2014) Exercise training could improve age-related changes in cerebral blood flow and capillary vascularity through the upregulation of VEGF and eNOS. Biomed Res Int 2014:230791
Viboolvorakul S, Niimi H, Wongeak-in N, Eksakulkla S, Patumraj S (2009) Increased capillary vascularity in the femur of aged rats by exercise training. Microvasc Res 78:459–463
Iemitsu M, Maeda S, Jesmin S, Otsuki T, Miyauchi T (2006) Exercise training improves aging-induced downregulation of VEGF angiogenic signaling cascade in hearts. Am J Physiol Circ Physiol 291:H1290–H1298
Rossman MJ, Kaplon RE, Hill SD, McNamara MN, Santos-Parker JR, Pierce GL, Seals DR, Donato AJ (2017) Endothelial cell senescence with aging in healthy humans: prevention by habitual exercise and relation to vascular endothelial function. Am J Physiol Heart Circ Physiol 313:H890–H895
Jain RK (1988) Determinants of tumor blood flow: a review. Cancer Res 48:2641–2658
Schofield CJ, Ratcliffe PJ (2004) Oxygen sensing by HIF hydroxylases. Nat Rev Mol Cell Biol 5:343–354
Carmeliet P, Jain RK (2011) Principles and mechanisms of vessel normalization for cancer and other angiogenic diseases. Nat Rev Drug Discov 10:417–427
Tozer GM, Prise VE, Bell KM, Dennis MF, Chaplin DJ (1996) Reduced capacity of tumour blood vessels to produce endothelium-derived relaxing factor: significance for blood flow modification. Br J Cancer 74:1955–1960
McCullough DJ, Stabley JN, Siemann DW, Behnke BJ (2014) Modulation of blood flow, hypoxia, and vascular function in orthotopic prostate tumors during exercise. J Natl Cancer Inst 106:dju036
Jones LW, Viglianti BL, Tashjian JA, Kothadia SM, Keir ST, Freedland SJ, Potter MQ, Moon EJ, Schroeder T, Herndon JE 2nd et al (2010) Effect of aerobic exercise on tumor physiology in an animal model of human breast cancer. J Appl Physiol 108:343–348
Morrell MBG, Alvarez‐Florez C, Zhang A, Kleinerman ES, Savage H, Marmonti E, Park M, Shaw A, Schadler KL (2019) Vascular modulation through exercise improves chemotherapy efficacy in Ewing sarcoma. Pediatr Blood Cancer 66:e27835
Faustino-Rocha AI, Silva A, Gabriel J, Gil da Costa RM, Moutinho M, Oliveira PA, Gama A, Ferreira R, Ginja M (2016) Long-term exercise training as a modulator of mammary cancer vascularization. Biomed Pharmacother 81:273–280
Padilha CS, Testa MT, Marinello PC, Cella PS, Voltarelli FA, Frajacomo FT, Cechini R, Duarte JAR, Guarnier FA, Deminice R (2019) Resistance exercise counteracts tumor growth in two carcinoma rodent models. Med Sci Sport Exerc 51:2003–2011
Zhu Z, Jiang W, McGinley JN, Thompson HJ (2009) Energetics and mammary carcinogenesis: effects of moderate-intensity running and energy intake on cellular processes and molecular mechanisms in rats. J Appl Physiol 106:911–918
Isanejad A, Alizadeh AM, Amani Shalamzari S, Khodayari H, Khodayari S, Khori V, Khojastehnjad N (2016) MicroRNA-206, let-7a and microRNA-21 pathways involved in the anti-angiogenesis effects of the interval exercise training and hormone therapy in breast cancer. Life Sci 151:30–40
Zielinski MR, Muenchow M, Wallig MA, Horn PL, Woods JA (2004) Exercise delays allogeneic tumor growth and reduces intratumoral inflammation and vascularization. J Appl Physiol 96:2249–2256
Smith KA, Hill SA, Begg AC, Denekamp J (1988) Validation of the fluorescent dye Hoechst 33342 as a vascular space marker in tumours. Br J Cancer 57:247–253
Torii M, Fukui T, Inoue M, Kanao S, Umetani K, Shirai M, Inagaki T, Tsuchimochi H, Pearson JT, Toi M (2017) Analysis of the microvascular morphology and hemodynamics of breast cancer in mice using SPring-8 synchrotron radiation microangiography. J Synchrotron Radiat 24:1039–1047
Ostergaard L, Tietze A, Nielsen T, Drasbek KR, Mouridsen K, Jespersen SN, Horsman MR (2013) The relationship between tumor blood flow, angiogenesis, tumor hypoxia, and aerobic glycolysis. Cancer Res. 73:5618–5624
Graff RM, Kunz HE, Agha NH, Baker FL, Laughlin M, Bigley AB, Markofski MM, LaVoy EC, Katsanis E, Bond RA et al (2018) β2-Adrenergic receptor signaling mediates the preferential mobilization of differentiated subsets of CD8+ T-cells, NK-cells and non-classical monocytes in response to acute exercise in humans. Brain Behav Immun 74:143–153
Idorn M, Hojman P (2016) Exercise-dependent regulation of NK cells in cancer protection. Trends Mol Med 22:565–577
Peake JM, Neubauer O, Walsh NP, Simpson RJ (2017) Recovery of the immune system after exercise. J Appl Physiol 122:1077–1087
Bosch JA, Berntson GG, Cacioppo JT, Dhabhar FS, Marucha PT (2003) Acute stress evokes selective mobilization of T cells that differ in chemokine receptor expression: a potential pathway linking immunologic reactivity to cardiovascular disease. Brain Behav Immun 17:251–259
Anane LH, Edwards KM, Burns VE, Drayson MT, Riddell NE, van Zanten JJCSV, Wallace GR, Mills PJ, Bosch JA (2009) Mobilization of γδ T lymphocytes in response to psychological stress, exercise, and β-agonist infusion. Brain Behav Immun 23:823–829
Simpson RJ, Florida-James GD, Whyte GP, Guy K (2006) The effects of intensive, moderate and downhill treadmill running on human blood lymphocytes expressing the adhesion/activation molecules CD54 (ICAM-1), CD18 (β2 integrin) and CD53. Eur J Appl Physiol 97:109–121
Pascoe AR, Fiatarone Singh MA, Edwards KM (2014) The effects of exercise on vaccination responses: a review of chronic and acute exercise interventions in humans. Brain Behav Immun 39:33–41
Spielmann G, McFarlin BK, O’Connor DP, Smith PJW, Pircher H, Simpson RJ (2011) Aerobic fitness is associated with lower proportions of senescent blood T-cells in man. Brain Behav Immun 25:1521–1529
Nieman DC, Henson DA, Gusewitch G, Warren BJ, Dotson RC, Butterworth DE, Nehlsen-Cannarella SL (1993) Physical activity and immune function in elderly women. Med Sci Sports Exerc 25:823–831
Simpson RJ, Kunz H, Agha N, Graff R (2015) Exercise and the regulation of immune functions. Prog Mol Biol Transl Sci 135:355–380
Campbell JP, Turner JE (2018) Debunking the myth of exercise-induced immune suppression: redefining the impact of exercise on immunological health across the lifespan. Front Immunol 9:648
Evans ES, Hackney AC, McMurray RG, Randell SH, Muss HB, Deal AM, Battaglini CL (2015) Impact of acute intermittent exercise on natural killer cells in breast cancer survivors. Integr Cancer Ther 14:436–445
Galvão DA, Nosaka K, Taaffe DR, Peake J, Spry N, Suzuki K, Yamaya K, McGuigan MR, Kristjanson LJ, Newton RU (2008) Endocrine and immune responses to resistance training in prostate cancer patients. Prostate Cancer Prostatic Dis 11:160–165
Jönsson S, Olsson B, Jacobsson S, Palmqvist L, Ricksten A, Ekeland-Sjöberg K, Wadenvik H (2011) BCR-ABL1 transcript levels increase in peripheral blood but not in granulocytes after physical exercise in patients with chronic myeloid leukemia. Scand J Clin Lab Invest 71:7–11
Glass OK, Inman BA, Broadwater G, Courneya KS, Mackey JR, Goruk S, Nelson ER, Jasper J, Field CJ, Bain JR et al (2015) Effect of aerobic training on the host systemic milieu in patients with solid tumours: an exploratory correlative study. Br J Cancer 112:825–831
Hagstrom AD, Marshall PWM, Lonsdale C, Papalia S, Cheema BS, Toben C, Baune BT, Fiatarone Singh MA, Green S (2016) The effect of resistance training on markers of immune function and inflammation in previously sedentary women recovering from breast cancer: a randomized controlled trial. Breast Cancer Res Treat 155:471–482
Nieman D, Cook V, Henson D, Suttles J, Rejeski W, Ribisl P, Fagoaga O, Nehlsen-Cannarella S (1995) Moderate exercise training and natural killer cell cytotoxic activity in breast cancer patients. Int J Sports Med. 16:334–337
Kruijsen-Jaarsma M, Revesz D, Bierings MB, Buffart LM, Takken T (2013) Effects of exercise on immune function in patients with cancer: a systematic review. Exerc Immunol Rev 19:120–143
Schmidt T, Jonat W, Wesch D, Oberg H-H, Adam-Klages S, Keller L, Röcken C, Mundhenke C (2018) Influence of physical activity on the immune system in breast cancer patients during chemotherapy. J Cancer Res Clin Oncol 144:579–586
Hoffman-Goetz L, Arumugam Y, Sweeny L (1994) Lymphokine activated killer cell activity following voluntary physical activity in mice. J Sports Med Phys Fitness 34:83–90
Hoffman-Goetz L, MacNeil B, Arumugam Y, Simpson J (1992) Differential effects of exercise and housing condition on murine natural killer cell activity and tumor growth. Int J Sports Med 13:167–171
Hoffman-Goetz L, May KM, Arumugam Y (1994) Exercise training and mouse mammary tumour metastasis. Anticancer Res 14:2627–2631
MacNeil B, Hoffman-Goetz L (1993) Effect of exercise on natural cytotoxicity and pulmonary tumor metastases in mice. Med Sci Sports Exerc 25:922–928
MacNeil B, Hoffman-Goetz L (1993) Chronic exercise enhances in vivo and in vitro cytotoxic mechanisms of natural immunity in mice. J Appl Physiol 74:388–395
Lehmann C, Zeis M, Uharek L (2001) Activation of natural killer cells with interleukin 2 (IL-2) and IL-12 increases perforin binding and subsequent lysis of tumour cells. Br J Haematol 114:660–665
Abdalla DR, Aleixo AAR, Murta EFC, Michelin MA (2014) Innate immune response adaptation in mice subjected to administration of DMBA and physical activity. Oncol Lett 7:886–890
de Lima C, Alves LE, Iagher F, Machado AF, Bonatto SJ, Kuczera D, de Souza CF, Pequito DC, Muritiba AL, Nunes EA et al (2008) Anaerobic exercise reduces tumor growth, cancer cachexia and increases macrophage and lymphocyte response in Walker 256 tumor-bearing rats. Eur J Appl Physiol 104:957–964
Lu Q, Ceddia MA, Price EA, Ye S-M, Woods JA (1999) Chronic exercise increases macrophage-mediated tumor cytolysis in young and old mice. Am J Physiol Integr Comp Physiol 276:R482–R489
Woods JA, Davis JM, Kohut ML, Ghaffar A, Mayer EP, Pate RR (1994) Effects of exercise on the immune response to cancer. Med Sci Sports Exerc 26:1109–1115
Bigley AB, Rezvani K, Chew C, Sekine T, Pistillo M, Crucian B, Bollard CM, Simpson RJ (2014) Acute exercise preferentially redeploys NK-cells with a highly-differentiated phenotype and augments cytotoxicity against lymphoma and multiple myeloma target cells. Brain Behav Immun 39:160–171
Wang J-S, Weng T-P (2011) Hypoxic exercise training promotes antitumour cytotoxicity of natural killer cells in young men. Clin Sci (Lond) 121:343–353
Woods J, Ceddia M, Wolters B, Evans J, Lu Q, McAuley E (1999) Effects of 6 months of moderate aerobic exercise training on immune function in the elderly. Mech Ageing Dev 109:1–19
Campbell PT, Wener MH, Sorensen B, Wood B, Chen-Levy Z, Potter JD, McTiernan A, Ulrich CM (2008) Effect of exercise on in vitro immune function: a 12-month randomized, controlled trial among postmenopausal women. J Appl Physiol 104:1648–1655
Suzui M, Kawai T, Kimura H, Takeda K, Yagita H, Okumura K, Shek PN, Shephard RJ (2004) Natural killer cell lytic activity and CD56dim and CD56bright cell distributions during and after intensive training. J Appl Physiol 96:2167–2173
Na Y-M, Kim M-Y, Kim Y-K, Ha Y-R, Yoon DS (2000) Exercise therapy effect on natural killer cell cytotoxic activity in stomach cancer patients after curative surgery. Arch Phys Med Rehabil 81:777–779
Fairey AS, Courneya KS, Field CJ, Bell GJ, Jones LW, Mackey JR (2005) Randomized controlled trial of exercise and blood immune function in postmenopausal breast cancer survivors. J Appl Physiol 98:1534–1540
McClellan JL, Steiner JL, Day SD, Enos RT, Davis MJ, Singh UP, Murphy EA (2014) Exercise effects on polyp burden and immune markers in the Apc(Min)(/+) mouse model of intestinal tumorigenesis. Int J Oncol 45:861–868
Bianco TM, Abdalla DR, Desidério CS, Thys S, Simoens C, Bogers J-P, Murta EFC, Michelin MA (2017) The influence of physical activity in the anti-tumor immune response in experimental breast tumor. Immunol Lett 190:148–158
Goh J, Tsai J, Bammler TK, Farin FM, Endicott E, Ladiges WC (2013) Exercise training in transgenic mice is associated with attenuation of early breast cancer growth in a dose-dependent manner. PLoS One 8:e80123
Rabinovich GA, Gabrilovich D, Sotomayor EM (2007) Immunosuppressive strategies that are mediated by tumour cells. Annu Rev Immunol 25:267–296
Mantovani A, Marchesi F, Malesci A, Laghi L, Allavena P (2017) Tumour-associated macrophages as treatment targets in oncology. Nat Rev Clin Oncol 14:399–416
Almeida PWM, Gomes-Filho A, Ferreira AJ, Rodrigues CEM, Dias-Peixoto MF, Russo RC, Teixeira MM, Cassali GD, Ferreira E, Santos IC et al (2009) Swim training suppresses tumor growth in mice. J Appl Physiol 107:261–265
Baltgalvis KA, Berger FG, Peña MMO, Davis JM, Carson JA (2008) Effect of exercise on biological pathways in ApcMin/+ mouse intestinal polyps. J Appl Physiol 104:1137–1143
Aouali N, Bosseler M, Sauvage D, Van Moer K, Berchem G, Janji B (2017) The critical role of hypoxia in tumor-mediated immunosuppression. IntechOpen, London
Lanitis E, Irving M, Coukos G (2015) Targeting the tumor vasculature to enhance T cell activity. Curr Opin Immunol 33:55–63
Ren L, Yu Y, Wang L, Zhu Z, Lu R, Yao Z (2016) Hypoxia-induced CCL28 promotes recruitment of regulatory T cells and tumor growth in liver cancer. Oncotarget 7:75763–75773. ISBN: 1949-2553|escape
Riabov V, Gudima A, Wang N, Mickley A, Orekhov A, Kzhyshkowska J (2014) Role of tumor associated macrophages in tumor angiogenesis and lymphangiogenesis. Front Physiol 5:75
Tian L, Goldstein A, Wang H, Ching Lo H, Sun Kim I, Welte T, Sheng K, Dobrolecki LE, Zhang X, Putluri N et al (2017) Mutual regulation of tumour vessel normalization and immunostimulatory reprogramming. Nature 544:250–254
Goh J, Kirk EA, Lee SX, Ladiges WC (2012) Exercise, physical activity and breast cancer:the role of tumor-associated macrophages. Exerc Immunol Rev 18:158–176
Sturgeon K, Schadler K, Muthukumaran G, Ding D, Bajulaiye A, Thomas NJ, Ferrari V, Ryeom S, Libonati JR (2014) Concomitant low-dose doxorubicin treatment and exercise. Am J Physiol Regul Integr Comp Physiol 307:R685–R692
Zheng XI, Cui X-X, Gao ZHI, Zhao Y, Shi YI, Huang M-T, Liu YUE, Wagner GC, Lin Y, Shih WJ et al (2011) Inhibitory effect of dietary atorvastatin and celecoxib together with voluntary running wheel exercise on the progression of androgen-dependent LNCaP prostate tumors to androgen independence. Exp Ther Med 2:221–228
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Buss, L.A., Dachs, G.U. (2020). Effects of Exercise on the Tumour Microenvironment. In: Birbrair, A. (eds) Tumor Microenvironment. Advances in Experimental Medicine and Biology, vol 1225. Springer, Cham. https://doi.org/10.1007/978-3-030-35727-6_3
Download citation
DOI: https://doi.org/10.1007/978-3-030-35727-6_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-35726-9
Online ISBN: 978-3-030-35727-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)