Abstract
Nearly 80% of global bacterial infections are associated with biofilm bacteria (Joo, Otto, Chem Biol 19:1503–1513, 2012). In contrast to planktonic bacteria, biofilms are a complex, organized bacterial community possessing a sophisticated protective armor, in the form of the extracellular polymeric substance (EPS), which acts as a robust defense mechanism against eradication. Chronic biofilm infections affect 17 million people annually, and approximately 550,000 people die as a result of their chronic infections (Wolcott et al J Wound Care 19:45–50, 2010). The challenge with biofilm-related infections is that they cannot be adequately confirmed via diagnostic tests in the clinical setting, and, more importantly, they are intrinsically resistant to host immunity, antibiotics, and biocides. This renders current therapeutic options inadequate to successfully eradicate the infection. Next Science™ has applied novel material science methods to combat biofilm through its innovative Xbio™ technology. Xbio technology, which includes the proprietary product, BlastX™, works by disrupting the biofilm matrix and creating an environment that compromises the biofilm’s structural integrity. In doing so, the EPS can be broken down and removed, thereby allowing the pathogens within the environment to be targeted and preventing the biofilm’s reformation.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Since BlastX is considered a combination product, a medical device with a drug component, there were some regulatory challenges in navigating the FDA clearance pathway. BlastX was first submitted to the FDA as an OTC device with limited and standard OTC claims. Once further data was obtained, Next Science submitted a second submission and received clearance for the use of BlastX on more chronic wounds by prescription only.
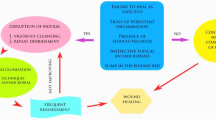
Normal wound physiology goes through four different steps [3]: hemostasis, inflammation, proliferation, and remodeling. Hemostasis takes place seconds to hours after the initial injury. Inflammation can include increased vasodilation and vasopermeability. This can lead to increased exudate, a release of cytokines and growth factors, immune cell recruitment, and, finally, bacterial clearance. The inflammation process typically occurs over a period of hours to days. Proliferation of the wound begins in days to weeks, provided the inflammation is controlled and no infection is present. However, if bacteria infiltrate the wound, a microbial infection can result and interrupt the healing process.
According to the US National Institutes of Health, biofilms account for over 80 percent of microbial infections in the human body [4]. Research has demonstrated that 80–90% of all chronic wounds contain microorganisms protected by biofilms (Fig. 1) [5]. Chronic infections are defined as wounds that take more than 12 weeks to heal, and research states that 70% of wounds worldwide fall under this definition [6]. Chronic biofilm infections can affect every organ system in the human body, including the skin [7]. Approximately 17 million people annually are affected by chronic biofilm infections, and approximately 550,000 people will die each year as a result [2].
The rising prevalence of antibiotic-resistant organisms, particularly within hospitals, is a contributing factor to the prevalence of chronic infections. Antibiotic-resistant organisms and their complications are responsible for more than two million hospital-acquired infections at a cost of $30.5 billion [8]. As discussed, healing for these infections can be routinely delayed by the introduction of microorganisms while the wound remains inflamed. Particularly at risk are those affected by diabetes and vascular disease, where explosive infected numbers have led to a rise in untreatable chronic wounds. This results in an increased burden that negatively impacts the patients’ quality of life [9].
Collectively, these chronic wounds significantly contribute to morbidity, mortality, and increased healthcare expenditures [10].
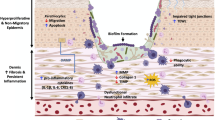
Bacteria exist in two essential forms: free floating (planktonic) and anchored/sessile (biofilms, spores). While planktonic bacteria are well understood and relatively easy to kill, biofilms pose a unique challenge. Biofilms are surface-adhering bacteria that are encased and defended by a glycocalyx, also known as an extracellular polymeric surface (EPS). This EPS begins to form after the bacteria secrete a sticky gel that protects them from initial eradication. Polymers inside the gel then become cross-linked by metallic bonds to strengthen the structure’s integrity and form the backbone of the extracellular polymeric surface. Once metallic bonds become established, the biofilm converts to an insoluble capsular environment that interacts with the host for bacterial growth, mutation, and proliferation. Ninety percent of the bacteria are enveloped within the structure, leaving less than 10% of free-floating bacteria in a wound. The resulting structure is mechanically resistant because metallically bonded polymers anchor the extracellular polymeric structure (EPS), preventing it from being washed off or eradicated by current treatment protocols (Fig. 2).
The EPS acts as a key mechanism in protecting the underlying pathogens by blocking large molecules such as antimicrobials, antibodies, and inflammatory cells from invading. Similarly, its biofilm matrixes act as diffusion barrier to small molecules like antibiotics, safeguarding it from extermination by conventional means [11]. Biofilm matrixes have also developed a mechanism for a subpopulation to become metabolically quiescent, i.e., to hibernate [12, 13]. Furthermore, the EPS exhibits cooperative protective effects. Some species of bacteria can assist others to attach and incorporate into the biofilm (quorum sensing) [14]. The overall effect of these mechanisms is to create a robust, well-defended bacterial community that thrives in spite of elimination efforts.
Antimicrobial drugs are the current mainstay treatment for the management of an acute bacterial infection. Antimicrobials target essential components of bacterial metabolism through the inhibition of cell wall synthesis, cell membrane function, protein synthesis, RNA synthesis, and DNA synthesis. Their primary action mechanism affects bacteria which are metabolically active during a synthesis process of active replication. If at any time bacterial cells become quiescent, or metabolically inactive, they become resistant to most antimicrobials [15]. Therefore, biofilms, with their ability to become metabolically quiescent, are intrinsically resistant to antimicrobials. The biofilms’ genetic mechanisms facilitate modification to the antimicrobial target in the form of decreased uptake, efflux pumps, modulation of metabolic pathways, and conferred resistance. Additional functional mechanisms involve modifications to the antimicrobial molecule, prevention of target access, bypass of target sites, or global cell adaption and resistance.
Biofilm bacteria exhibit up to 1000-fold more antimicrobial resistance when compared to planktonic bacteria. Various protective mechanisms render current therapeutic options inadequate to successfully eradicate the infection. Furthermore, the treating clinician often lacks definitive diagnostic data to confirm the presence of biofilm, making the decision to remove infected hardware and tissues, and to treat with antimicrobial agents even more difficult. The decision involves balancing the relative risks of treating or not treating the infection versus exposing a patient to the potential adverse effects of the available treatment strategies.
Current treatment strategies for chronic wound infections generally involve the use of topical antimicrobial dressings as well as local debridement. Debridement breaks biofilm into smaller colonies but does not entirely remove it and may spread the biofilm to other wound regions. Therefore, debridement can amplify the infection, spreading it more aggressively and causing it to undergo reformation faster than on its own. To mitigate these side effects, debridement is generally followed by a topical antimicrobial for highest effectiveness [16]. However, in the context of biofilm-based infections, dosages of antimicrobial drugs up to 500–1000 times the minimum inhibitory concentration are often required. Even if such concentrated dosages were to be administered, they would still be unsuccessful at completely eradicating the infection [22]. An optimal treatment for a biofilm infection should include the use of an antibiofilm agent in addition to the current strategies [17]. A targeted antibiofilm approach is necessary to disrupt and degrade the EPS matrix of the biofilm, target the bacteria for destruction, and prevent biofilm reformation in the wound [18, 19].
Next Science is leading a paradigm shift with a unique, unprecedented approach to eliminating both biofilm bacteria and planktonic bacteria with a proprietary, nontoxic technology that disrupts the biofilm’s extracellular polymeric substance (EPS) matrix and makes the bacteria within the biofilm more vulnerable to attack by antimicrobials, antibiotics, and the body’s natural immune defenses. This patented Xbio™ technology reduces the bacterial load which, in turn, helps to reduce the overall use of antibiotics (Fig. 3). More importantly, it has shown no known evidence of bacterial resistance [20].
Next Science’s Xbio uses proprietary composition-of-matter patents that contain technology to physically break down the biofilm’s protective structures (Fig. 4). The exposure and eradication of the formerly enveloped bacteria are achieved by the technology’s induced cell lysis.
Next Science has created BlastX™, an antimicrobial wound gel designed to facilitate natural wound healing. The use of the hydrogel on a wound creates a moist environment that reduces the buildup of necrotic tissue caused by apoptosis and enables the body’s natural wound healing process to take place. The moist environment created by the gel promotes granulation, epithelization, and autolytic debridement. The moist environment also prevents tissue dehydration and cell death, increases angiogenesis, and increases the breakdown of dead tissue and fibrin [21].
The gel additionally prevents bacterial growth and the formation of biofilms when applied to fresh wounds by preventing the bacteria from passing through the gel into the wound. BlastX is a topical polyethylene glycol-based hydrogel that disrupts and eliminates biofilms that become enveloped in the gel. This occurs largely by degrading the biofilm’s EPS matrix through removal of the metallic bonds in the EPS via chelation and hydrolysis. The hyperosmolar wound gel, and its contained surfactant, enables cell wall lysis, resulting in destruction of the microorganisms that were formerly protected by the biofilm’s EPS.
The citric acid in the gel binds to the biofilms’ metallic bonds , while the sodium citrate buffers the solution to a pH of 4. This allows the citric acid to attach and remove the metallic bonds that hold the EPS structure together and releases the polymers. Sodium molecules split off and cap the free polymer ends. The remaining sodium citrate molecules are then converted to citric acid. This conversion prevents the polymer from reattaching and replenishes the original citric acid that was depleted in breaking the metallic bonds, thereby sustaining the chelation process through buffering. The pathogens are destroyed when sodium citrate and citric acid in the gel mixture produce an osmotic pressure distending the bacterial cell wall. Aiding in cell lysis, the benzalkonium chloride surfactant then attaches to a protein in the cell wall and removes it. BlastX prevents the recolonization of the biofilms’ EPS structure by preventing the bacteria from passing through the gel for biofilm regrowth.
Specifically, once the biofilm enters the gel environment, the Next Science technology dissolves the slime layer permitting direct contact with individual bacteria. Typically, the RNA/proteins in the biofilm’s EPS deactivate treatment chemicals before they reach the bacteria. Next Science technology overwhelms these entities, ensuring that critical conditions for lysis are maintained throughout treatment (Fig. 5). Lysis is nondiscriminatory, effective against both gram-positive and gram-negative strains of bacteria, and active, downregulated, and persister cells. The bacteria have no resistance mechanism to cell lysis.
BlastX has been studied extensively to quantify its effectiveness on creating an ideal healing environment for chronic wounds and eliminating robust biofilms. Tests of Suspension Time Killing show that BlastX is effective against a broad range of bacteria and selective fungi, including C. albicans and A. brasiliensis. A study led by Montana State University demonstrated that BlastX has a nearly six times higher log reduction from control than leading wound gels SilvaSorb and Microcyn, based on a 24 -hour contact time and an 8-log control.
In addition, the applications of BlastX have also been evaluated in vivo. Research conducted at Texas Tech studied the infection reduction in 24-hour biofilm growth with LUX-modified S. aureus and P. aeruginosa bacteria. Twenty-four hours after the first BlastX application, rats were shown to have significant reduction in infection rates compared to the control. Similarly, WuXi modeled infected rats wound size over time (rats infected with S. aureus), modeling BlastX’s ability to provide a wound healing environment, which resulted in a decrease in wound size at faster rates than the control in the first 7 days of healing. At day 7, wounds covered with BlastX had reduced in size to below 20% of the original area compared to the control’s reduction to approximately 55% the original area. This coincided with a reduction in the CFU of bacteria recovered from the wounds, with the bacterial counts reduced from 4.3 log for the control animals to 0.7 log for the BlastX treated rats.
A clinical trial by the Mayo Clinic reviewed the efficacy of BlastX at creating a wound environment which enabled the natural reduction in the size of wounds in human patients. In a 12-week random trial with 43 participants, BlastX was shown to provide a wound environment that resulted in three times the area reduction of chronic wounds over a broad-spectrum antimicrobial ointment. In addition, patients saw a 205% relative increase in wound closure when the wound was covered with BlastX instead of a broad-spectrum antimicrobial ointment. Similarly, a study by Wolcott found that, over a period of 4 weeks, 45 subjects with chronic wounds saw 1.5 times more effective wound closure and 2 times more effective wound area reduction than the standard practice of care.
Next Science currently has four active generations of solution/gel technologies developed: BlastX, Bactisure™ for surgical lavage, Next Science Acne Gel (NAG), and TorrentX [22]. Within these generations, our Intellectual Property covers broad ranges of chemicals and solution properties. This allows Next Science to tailor formulations for specific use conditions, anatomical area, application time frame, and toxicity.
Because the Next Science technology is targeted to attack prokaryotic structures (bacteria and biofilms), they are nontoxic for use on eukaryotic tissues. The pH of Next Science solutions and gels are not hazardous to mammalian tissue. Cells are quite resistant to negative effects of osmolarity due to decreased permeability and the body’s ability to normalize the osmolarity from the non-exposed surfaces. There is broad evidence showing that cationic surfactants at low to moderate concentrations are safe for human use. Proteins on the surface of the bacteria are susceptible to binding with cationic surfactants. The solvents used in Next Science products are already used within patients and are used at low concentrations in these products. The enzymes used in Next Science products are commonly present in the human body and pose no toxicity concerns.
Since the Xbio technology is considered to be a combination product, a medical device with a drug component, it required a different path for FDA regulation than current drug-based treatments. For a drug to obtain FDA approval, it must undergo clinical testing and then be submitted to the FDA’s Center for Drug Evaluation and Research (CDER). This process can take years and be quite costly. A medical device is approved through the FDA’s Center for Devices and Radiological Health (CDRH). Depending on the classification, a device is either cleared or approved for sale. The BlastX device was considered a moderate risk device which required a submission to show substantial equivalence through the FDA’s 510(k) process. A 510(k) submission must demonstrate that the device is substantially equivalent to another device legally in commercial distribution in the United States: (1) before May 28, 1976 or (2) to a device that has been determined by FDA to be substantially equivalent [23].
According to the FDA, a combination product is defined as, “a product comprised of two or more regulated components (i.e., drug/device, biologic/device, drug/biologic, or drug/device/biologic) that are physically, chemically, or otherwise combined or mixed and produced as a single entity [often referred to as a “single-entity” combination product]“ [24] The Xbio technology is a combination product because BlastX is a wound dressing used to cover and protect the wound, giving it the properties of a device, but it also contains benzalkonium chloride , an antimicrobial agent, which constitutes the drug portion. BlastX was regulated by CDRH due to its primary mode of action being achieved by the device activities of the wound dressing.
BlastX was originally designed with the OTC monographs in mind. OTC monographs allow the marketing of drug products without the requirement for a New Drug Application (NDA), provided specific limitations are placed on the product. The OTC monographs currently allow for a 1:750 (0.13%) use concentration of benzalkonium chloride to be marketed under the category of “skin protectant.” The PEG and buffers in the BlastX gel are all accepted as inactive ingredients for US drug products. As such, BlastX could have been marketed as an OTC drug product. The FDA has been moving away from the use of OTC monographs for wound dressings and so Next Science took the next step to submit BlastX to the CDRH division of FDA as a combination wound dressing with an antimicrobial agent. The initial submission was to gain clearance for the same indications that were used with the OTC monographs. Once further data was obtained, Next Science submitted a second submission for the prescription only use of BlastX on more chronic wounds.
For future projects, Next Science will continue to evaluate the appropriate regulatory pathway for each of its new products. Some technologies will most likely be drugs, which will go to the FDA’s CDER, while others might be designated as new devices. These new devices would require either a premarket application (PMA) or a de novo application for the establishment of a new device type along with the classification, regulation, and necessary controls and product code. The de novo process is an option for lower-risk devices, and once approved, a de novo device can then serve as a predicate for new medical devices where appropriate to the 501(k) process [25].
Next Science has created a rapid-acting technology, providing options that have superior efficacy against both planktonic and biofilm bacterial forms. Xbio™ is gentle, with low toxicity and a favorable environmental impact. We are at the forefront of addressing the growing problem of biofilm-caused antimicrobial resistance.
References
Joo, H. S., & Otto, M. (2012). Molecular basis of in-vivo biofilm formation by bacterial. Chemistry & Biology, 19(12), 1503–1513.
Wolcott, R., Rhoads, D., Bennett, M., Wolcott, B., Gogokhia, L., Costerton, J., & Dowd, S. (2010). Chronic wounds and the medical biofilm paradigm. Journal of Wound Care, 19(2), p45–p50.
Landén, N. X., & Li, D. (2016). Mona Ståhle.Transition from inflammation to proliferation: a critical step during wound healing. Cellular and Molecular Life Sciences, 73(20), 3861–3885.
Attinger, C., & Wolcott, R. (2012). Clinically addressing biofilm in chronic wounds. Advances in Wound Care, 1(3), 127–132.
Aziz-Abdel, M. S. (2014). Bacterial biofilm: Dispersal and inhibition strategies. SAJ Biotechnology, 1, 105. https://doi.org/10.18875/2375-6713.1.105.
Frykberg, R. G., & Banks, J. (2015). Challenges in the Treatment of Chronic Wounds. Advances in Wound Care, 4(9), 560–582. https://doi.org/10.1089/wound.2015.0635.
Flemming, H. C., & Wingender, J. (2010). The Biofilm Matrix. Nature Reviews Microbiology, 8, 623–633.
Performance of the Massachusetts Health Care System Series: A focus on provider quality. CHIA Center for Health Information and Analysis, chiamass.gov.
Bjarnsholt, T., Kirketerp-Møller, K., Jensen, P. Ø., et al. (2008). Why chronic wounds will not heal: a novel hypothesis. Wound Repair and Regeneration, 16(10), 2–10.
Sen, C., Gordillo, G., Roy, S., Kirsner, R., Lambert, L., Hunt, T., Gottrup, F., Gurtner, G., & Longaker, M. (2009). Human skin wounds: A major and snowballing threat to public health and economy. Wound Repair and Regeneration, 17(6), 763–771.
Hall-Stoodley, L., & Stoodley, P. (2009). Evolving concepts in biofilm infections. Cell Microbiol, 11(7), 1034–1043.
Brooun, A., Liu, S., & Lewis, K. (2000). A dose response study of antibiotic resistance in Pseudomonas aeruginosa biofilms. Antimicrobial Agents and Chemotherapy, 44(3), 640–646.
Lewis, K. (2007). Persister cells, dormancy and infectious disease. Nature Reviews. Microbiology, 5(1), 48–56.31.
Davies, D. G., Parsek, M. R., Pearson, J. P., et al. (1998). The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science, 280(5361), 295–298.
Carroll, K., Hobden, J., Miller, S., Morse, S., Mietzner, T., Detrick, B., Mitchell, T., McKerrow, J., & Sakanari, J. (2016). Jawetz, Melnick, & Adelbergs medical microbiology (27th ed.). Shenzen: McGraw-Hill.
Kim et al. (2018). Wounds, 30(5), 114–119. Epub 2018 February 23 (Biofilms Made Easy (wounds international) – Phillips; Skin and Wound Care 2013 – Kevin Wu; Wound Education Update Feb 2014 – Wounds UK Best Practice Statement)
Snyder, R. J., Bohn, G., Hanft, J., Harkless, L., Kim, P., Lavery, L., Schultz, G., & Wolcott, R. (2017). Wound bioflm: current perspectives and strategies on biofilm disruption and treatments. Wounds, 29(6), S1–S17.
Snyder, R. J., Bohn, G., Hanft, J., Harkless, L., Kim, P., Lavery, L., Schultz, G., & Wolcott, R. (2017). Wound Biofilm: Current Perspectives and Strategies on Biofilm Disruption and Treatments. Wounds, 29(6), S1–S17.
Ryder, M. (2005). Catheter related infections – It’s all about biofilms. Topics in Advanced Practice Nursing eJournal, 5(3), 1–6.
Data on file. Center for Biofilm Engineering at Montana State University. Next Science Report TR-10-12-004.
Mallefet, P., & Dweck, A. C. (2008). Mechanisms involved in wound healing. Biomedical Scientist, 52(7), 609.
Next Science website. http://www.nextscience.com
Code of Federal Regulations. https://www.fda.gov/medicaldevices/deviceregulationandguidance/overview/default.htm
FDA.gov https://www.fda.gov/combinationproducts/aboutcombinationproducts/ucm101496.htm#CP
Evaluation of Automatic Class III Designation (De Novo). FDA.gov https://www.fda.gov/medicaldevices/deviceregulationandguidance/howtomarketyourdevice/premarketsubmissions/ucm462775.htm
Disclaimer
Dr. Myntti has financial interest in Next Science and the technologies discussed.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Myntti, M. (2019). Translation of Antibiofilm Technologies to Wounds and Other Clinical Care. In: Williams, D. (eds) Targeting Biofilms in Translational Research, Device Development, and Industrial Sectors. Springer, Cham. https://doi.org/10.1007/978-3-030-30667-0_6
Download citation
DOI: https://doi.org/10.1007/978-3-030-30667-0_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-30666-3
Online ISBN: 978-3-030-30667-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)