Abstract
Hemophagocytic lymphohistiocytosis (HLH) is a severe cytokine storm syndrome (CSS) which until the turn of the century was barely known but is now receiving increased attention. The history of HLH dates back to 1939 when it was first described in adults, followed by the first description of its primary, familial form in children in 1952. Secondary forms of HLH are far more frequent and occur with infections, malignancies, metabolic diseases, iatrogenic immune suppression, and autoinflammatory/autoimmune diseases. Identification of genetic defects leading to defective function of natural killer cells and cytotoxic T cells, as well as corresponding mouse models, have revolutionized our understanding of HLH and of immune function. Diagnosis relies on clinical and laboratory criteria; functional and genetic tests can help separate primary forms from secondary forms. Treatment with immunochemotherapy and hematopoietic stem cell transplantation has considerably improved survival in children with primary HLH, a formerly uniformly fatal disease.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Hemophagocytic lymphohistiocytosis
- Hemophagocytic syndrome
- Cytokines
- Genetic defect
- Immune deficiency
- Natural killer cells
- Cytotoxic T cell
- Degranulation
- Etoposide
- Hematopoietic stem cell transplantation
First Descriptions
Hemophagocytic lymphohistiocytosis (HLH) is a severe cytokine storm syndrome (CSS ) which was described in its primary, familial form (pHLH) in 1952 by Farquhar and Claireaux as “haemophagocytic reticulosis” [1]. Interestingly, hemophagocytosis, which gave HLH its name was not present during life in the two siblings but was found at autopsy only. Nevertheless, until not long ago hemophagocytosis was considered a sine qua non for the diagnosis of HLH.
Thirteen years earlier a clinical picture typical for HLH (fever, hepatosplenomegaly, cytopenias, and widespread histiocytic infiltration on autopsy) had been described in adults by Scott and Robb-Smith under the term “histiocytic medullary reticulosis” (HMR ) [2]. Eight of these cases were later reviewed with more refined histological techniques. Five patients were found to have had malignant lymphomas; four were of T cell origin [3]. Lymphomas are now a well-known cause for secondary HLH (sHLH).
Another form of sHLH associated with viral infections (virus-associated hemophagocytic syndrome [VAHS ]) was described in 1979 by Risdall and coworkers [4]. Interestingly, their cohort not only included adults under immunosuppression but also some children who may have had primary (familial) HLH (pHLH). Subsequent reports by other investigators linked sHLH to various infectious agents, including bacteria, protozoa, and fungi, though viruses, especially herpes viruses remain the most common trigger. The majority of Epstein–Barr virus-associated cases of HLH have been reported from Asia. The reason for this susceptibility remains mysterious to date.
Risdall et al. emphasized that the histological picture in sHLH is distinct from malignant histiocytosis. Not all authors agreed, and the first case of sHLH due to Leishmania was published under the title “Systemic Leishmaniasis Mimicking Malignant Histiocytosis” [5]. Until the 1980s, malignant histiocytosis was considered a true histiocytic malignancy. Nearly all cases could later be reclassified as neoplasm of lymphoid origin, now called large-cell anaplastic lymphoma.
Since then, the list of conditions predisposing to the development of HLH has expanded, including metabolic diseases, other rare inborn immune defects, acquired immune deficiencies such as AIDS, and iatrogenic immunosuppression. The HLH-like picture in autoinflammatory/autoimmune diseases is usually called macrophage activation syndrome (MAS ), a special subset of sHLH. pHLH is a rare disease with an estimated incidence of 1:50,000 live births [6]. Secondary forms are far more frequent. Between the first description of pHLH in 1952 and the turn of the century, PubMed lists only about 200 publications on HLH. Since then this syndrome has rapidly gained increased attention resulting in about 4600 additional publications of which more than 75% appeared in the last 10 years.
First Personal Experience
My first encounter with HLH was in the 1970s with four children of one family, three of whom died in early infancy [7]. The parents and an older sibling were healthy. The babies had unexplained high fever, hepatosplenomegaly, and cytopenias, the characteristic triad of HLH. The first child went from a cellular marrow to bone marrow aplasia after weeks of uncontrolled fever; lacking a better alternative at that time the final diagnosis was aplastic anemia. When the second child developed the same symptoms it became evident that this must be an inherited disease. Abundant histiocytes in the bone marrow and a liver biopsy prompted a search in the huge medical index called Index Medicus—a painstaking effort at a time when Internet research was far away. The search led to the publication by Farquhar and Claireaux. Since in their second patient a temporary remission was obtained with adrenocorticotropic hormone, our patient was treated with prednisolone. However, only some minor and transient improvement was achieved. When the third baby showed the same symptoms the family refused treatment but had the generosity to allow diagnostic procedures including a bone marrow aspirate and spinal tap, the latter showing a lymphocytic pleocytosis and increased protein level—my first encounter with central nervous system (CNS)-HLH. Unfortunately, no material was stored; thus, one can only speculate as to the type of genetic defect in this family.
Terminology
Various terms for pHLH were initially used such as familial reticulosis, lymphohistiocytic reticulosis, familial erythrophagocytic lymphohistiocytosis, familial histiocytosis, generalized lymphohistiocytic infiltration and others. After the first review on pHLH in 1983, which included 121 cases from the literature and 6 of my own cases [8], the term hemophagocytic lymphohistiocytosis was adopted by most authors. There has been some recent discussion whether to use a different name for HLH which takes into account that HLH is a severe hyperinflammatory syndrome and that lymphocytes (T cells) are not an absolute necessity for the syndrome to develop. However, as yet no consensus has been reached. sHLH is often called macrophage activation syndrome (MAS ), especially in internal medicine. Pediatricians agree to reserve the term MAS for HLH in autoinflammatory/autoimmune diseases.
First HLH Symposium and Foundation of the FHL Study Group
Initially, there were only few pediatricians and pathologists who were interested in HLH. Through a colleague who had worked in Paris, I gained access to a doctoral thesis on pHLH (“histiocytose familiale”) from Alain Fischer’s group in which eight cases were described. Parts of it were later published [9]. The pathologist’s description was presented by Christian Nezelof from Paris and Julio Goldberg, a visiting pathologist from Argentina. [10]. Another group led by Jan-Inge Henter in Stockholm had started to thoroughly investigate all Swedish cases. A third group, represented by Maurizio Aricó and Roberto Burgio, who also had a general interest in histiocytosis, finally organized a first international workshop on HLH in Pavia in 1988. One year later, the FLH (pHLH) Study Group was founded as a part of the international Histiocyte Society which had existed since 1985. FHL Study Group founding members were Maurizio Aricó, Göran Elinder, Blaise Favara, Jan-Inge Henter, Gritta Janka, Diane Komp, Christian Nezelof, and Jon Pritchard; at the HLH protocol meeting in 1994, several additional members joined.
In 1991, the FHL Study Group presented the first diagnostic guidelines for HLH [11]. The suggested diagnostic criteria consisted of five clinical and laboratory items which were easy to ascertain and which gained wide acceptance.
State of Knowledge Before Study HLH-94
When the first international HLH study started in 1994, the knowledge about HLH was still quite limited. It was evident that there were familial (genetic) and secondary forms; the latter due to infections or malignancies. However, the distinction between pHLH and infection-associated HLH was not possible when the family history was blank, since children with pHLH were found to have viral infections as well [8, 12]. Involvement of the central nervous system (CNS) was recognized as a serious complication; the common appearance on autopsy was that of “leptomeningitis,” but parenchymal lesions were described as well [13]. With long-term survival, cognitive and psychosocial sequelae due to CNS involvement have now become a major concern in children with HLH [14]. CNS-HLH is still poorly understood, and treatment options are limited [15].
Although high levels of cytokines and soluble interleukin-2 receptor alpha chain [16,17,18] suggested uncontrolled activity of macrophages and lymphocytes, the etiology and pathogenesis of HLH remained elusive for a long time. Various, but inconsistent, immunological abnormalities were reported initially. The general assumption that HLH must be an immunodeficiency was finally confirmed by a profound deficit in natural killer (NK) cell activity [19]. Impaired NK cell activity had already previously been described in two immune deficiencies with partial albinism, namely, Griscelli syndrome (GS-2 ) and Chédiak–Higashi syndrome (CHS ). GS-2 and CHS are frequently complicated by HLH and are now counted among the primary forms of HLH.
Study HLH-1994 could fall back only on limited experience regarding therapy of HLH. Various measures had been tried, such as splenectomy, exchange transfusions, corticosteroids, and cytotoxic drugs, but the prognosis of pHLH was dismal; only four children with prolonged survival had been reported in the first review [8]. A promising agent seemed to be the epipodophyllotoxin derivate, etoposide (VP-16), which produced longer remissions but could not prevent reactivations, including CNS relapses [20]. The efficacy of VP-16 combined with steroids and CNS directed therapy could be confirmed by other groups [7, 21]. Treatment with chemotherapy or immunotherapy, however, was only able to reverse disease activity for some period but was not curative in familial cases. Thus, it was a big step forward when the first patient receiving a hematopoietic stem cell transplant (HSCT ) from his HLA-identical sibling remained free of disease without therapy [22].
There was another promising approach from a single institution with anti-thymocyte globulin (ATG ), corticosteroids, and cyclosporin A [23] which, however, was not considered for the large international study due to less general experience with ATG and inferior availability.
HLH-94 Study
Between July 1994 and December 2003, study HLH-1994 recruited 249 patients fulfilling inclusion criteria from 25 countries. The protocol included an initial intensive therapy with dexamethasone and etoposide for 8 weeks. Dexamethasone was chosen due to its better penetration into the cerebrospinal fluid (CSF ). Intrathecal methotrexate therapy was recommended for patients with progressive neurological symptoms and/or persisting CSF abnormalities. For patients with familial, persistent, or relapsing disease, continuation therapy, with cyclosporin A and periodic etoposide and dexamethasone pulses until HSCT, was recommended. Interim results were published in 2002 [24]. In the final report at 6.2-years median follow-up, probability of survival was 54% [25]. This was a very gratifying result, although at least about 20% of the patients must have had non familial disease, as evident from reactivation-free survival in 49 children without HSCT. Death was due to poor response to treatment within the first 8 weeks, reactivations before HSCT (median time to HSCT 6.1 months), and complications after HSCT, mostly due to the toxicity of myeloablative conditioning.
The second international study, HLH-2004, was based on the HLH-94 protocol with only few changes: Cyclosporin A was added up front, and intrathecal therapy was supplemented by corticosteroids. The revised diagnostic criteria also included impaired natural killer cell activity, a hallmark of the disease, hyperferritinemia, and increased levels of soluble interleukin-2 receptor alpha chain [26]. The results of study HLH-2004 have recently been published, reporting a 62% 5-year probability of survival [27].
Advances in Understanding HLH
Genetics
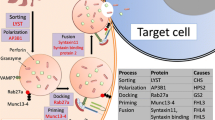
The description of the first genetic defect in pHLH [28] revolutionized our understanding of the pathogenesis of HLH. Previously, linkage analysis in a Pakistani family had revealed a putative disease gene on chromosome 9 [29]; thus, pHLH due to mutations in the Perforin (PRF1) gene was called FHL-2. Perforin, a component of cytolytic granules in cytotoxic cells is critical for the access of proteolytic enzymes to the target cell to initiate apoptotic death. Perforin is released into the immunological synapse between effector and target cell by cytolytic granules which traffic to the contact site, dock, and fuse with the plasma membrane. Notably, the cytotoxic effector response not only targets infected cells but also antigen-presenting cells (APCs ). Elimination of APCs is an important negative feedback for the immune response. The identification of perforin deficiency as cause of pHLH showed the importance of this protein for controlling and terminating the immune response. The failure to contract the immune response in patients with pHLH explains the persistently high cytokine levels which are responsible for the symptomatology. Later, another critical role for perforin, which is immune surveillance, became evident from temperature-sensitive missense mutations where residual protein activity was present. These mutations were associated with late-onset pHLH and the occurrence of lymphomas or leukemia [30].
Within the next 10 years, three additional genetic defects for pHLH were described; all were found to also impair granule-dependent cytotoxic function. The genes are UNC13D (FHL-3) [31], Syntaxin11 (FHL-4) [32], and STXBP2 (FHL-5) [33, 34]. In addition, mutations in Griscelli syndrome (RAB27A) and Chédiak–Higashi syndrome (LYST) were found to also affect the cytolytic granule pathway of cytotoxic cells, and present as pHLH. Another rare disorder of lysosomal trafficking, Hermansky–Pudlak syndrome type 2 (HPS2), has only a low risk of developing HLH. Additionally, X-linked lymphoproliferative (XLP) disease types 1 and 2 were noted to have a high risk for developing HLH, particularly in the setting of EBV infection. Despite the fact that the XLP mutations do not affect the cytolytic pathway, they are now counted among the primary forms of HLH.
Disease onset and severity in primary forms of HLH are highly variable, depending on the gene involved and the type of mutation, determining complete or only partial loss of protein structure and function. When age at onset was taken as a surrogate marker for disease severity in patients with predicted severe protein impairment, FHL-2 patients had the earliest onset followed by GS-2, FHL-4, and CHS [35]. In FHL-5, patients with missense or splice-site mutations differ markedly in age of onset and presence of diarrhea, an atypical newly described symptom [36]. There are still a small number of patients with absent degranulation, indicative for biallelic mutations in the cytolytic pathway, but no mutation in the known genes.
Classical cases of pHLH are either autosomal recessive with biallelic mutations (FHL2–5, GS, CHS, and HPS) or hemizygous as in XLP-1 and XLP-2. Digenic inheritance with mutations within PRF1 and a degranulation gene or within two genes of the degranulation pathway has also been described [37]. Reports on heterozygous mutations in patients with HLH have appeared with increasing frequency. They were found to have only reduced but not completely absent perforin expression or degranulation [38]. This suggests that these patients either have other unknown genetic factors outside the cytolytic pathway or environmental factors contributing to the development of HLH. It has to be emphasized that parents or heterozygous siblings of patients with pHLH typically do not show signs of HLH. Heterozygous carriers are accepted as donors for HSCT.
Mouse Models
Mouse models, created for pHLH and sHLH, have largely contributed to our understanding of how the genetic defects could be responsible for the aberrant immune response in HLH. Studies in perforin-deficient mice, infected with LCMV, have shown that activated CD8 T cells and interferon-γ (IFNγ) production play a central role in the pathogenesis of HLH. HLH could be prevented by neutralization of IFNγ [39]. This was confirmed also for Rab27a deficient mice [40]. In the PRF1 deficiency mouse model, the therapeutic effect of etoposide involved deletion of activated T cells [41]. Polyclonal T cells are activated in both pHLH and sHLH [42], and INFγ is increased [16, 17]. However, the pathogenesis of HLH does not always involve T cells; patients with severe combined immunodeficiency and T cells <1000/μL can develop HLH, as well [43]. Activation of T cells as a prerequisite for HLH was also not required in a mouse model with a normal genetic background where repeated toll-like receptor 9 stimulation produced an HLH-like picture [44]. Innate immune stimulation may be an important pathway to develop HLH in patients without a cytotoxic defect.
A recent study in mouse and human cytotoxic cells demonstrated that failed target cell killing, leading to a prolonged synapse time, greatly amplifies cytokine secretion by NK cells and cytotoxic lymphocytes. Of note, blocking caspase in the target cell, important for the extrinsic pathway of apoptosis, phenocopied perforin deficiency regarding prolonged synapse time. This could be an explanation for sHLH in viral infections and malignancies since virus-infected or transformed cells often have defects in their apoptotic machinery [45].
The clinical and laboratory symptoms of HLH can all be explained by hypercytokinemia and organ infiltration by activated lymphocytes and histiocytes [46]. Macrophages secrete plasminogen, which is cleaved into plasmin, mediating fibrinolysis. A recent investigation in a murine model showed plasmin to be also an important regulator for the influx of inflammatory cells and the production of inflammatory cytokines leading to HLH [47].
Advances in HLH in Adults
Although the first cases of HLH had been described in adults [2], it took a long time until HLH received adequate interest in internal medicine. HLH was commonly regarded as are rare pediatric syndrome, and HLH patients outside of pediatric centers have been at high risk of not being diagnosed. Only recently, case reports and larger case series [48] of HLH in adults have been published with greater frequency. The recognition that mutations in HLH-relevant genes are also found in adults in a substantial number of patients [49] has further increased interest. Nevertheless, in most countries, a network of experts, as is present in pediatrics, is missing, although there is even a registry in one country (www.hlh-registry.org). Thus, HLH in adults is still very likely underdiagnosed.
The majority of HLH cases in adults are secondary to infections and malignancies; a smaller number is due to autoimmune diseases [48]. The true incidence of genetic cases is not known; mutations in these patients allow for residual protein expression and hence partially preserved cytotoxic function. There are no separate diagnostic criteria for HLH in adults. Usually the HLH-2004 criteria [26] or a recently developed score, the HScore [50], is used. Treatment of HLH in adults is challenging since older patients have a diminished hematopoietic reserve and may have comorbidities which limit treatment intensity. Lymphomas, often occult, have to be ruled out vigorously before treatment. Recommendations for the management of HLH in adults have been published [51].
Advances in Diagnostics
Diagnosis of HLH is based on a set of diagnostic and laboratory parameters; no single parameter, including hemophagocytosis, is sufficiently specific for HLH. pHLH and sHLH cannot be distinguished by these parameters, including NK cell activity. Differentiation between genetic and secondary forms, however, is crucial for early organization of a stem cell transplant. In 2012, a joint collaboration between five European countries showed that degranulation assays, a measure quantifying lytic granule exocytosis, were a reliable tool to identify patients with mutations in the cytolytic pathway [52]. In combination with intracellular measurement of perforin, SAP (XLP-1), and XIAP (XLP-2), also obtained by flow cytometry, this approach can give guidance whether a genetic analysis should be performed and a transplant should be prepared. A diagnostic algorithm how to proceed in patients with HLH has been proposed [53]. Recently, perforin and degranulation testing were shown to be superior to measurement of NK cell function for screening patients for genetic HLH [54]. Unfortunately, these assays have been established in larger HLH centers only and require rapid transport of material. Additionally, repeated testing may be necessary with equivocal results. In the future, with increasingly more rapid and less costly genetic testing, these functional assays may eventually be replaced by mutation analysis although functional testing will remain important as our knowledge of the entire genetic landscape of HLH remains incomplete. Moreover, a blurring of the distinction between pHLH and sHLH has been noted for patients with complete [55] or partial dominant-negative [56] heterozygous mutations in known HLH-associated genes.
Developments in Therapy
Stem Cell Transplantation
Like in other immunodeficiencies, HSCT is the only curative treatment for pHLH. In the absence of a suitable related or unrelated donor, haploidentical transplantation or cord blood HSCT constitute alternatives [57]. Post-transplant mortality after myeloablative conditioning (MAC) was high with a survival probability of only 49–64% in larger studies [58]. Liver (mainly veno-occlusive disease) and lung problems were leading causes of death. It was important to learn that sustained remissions could be achieved in all patients with a donor chimerism ≥20% [59]. In line with this, in the PRF1 mouse model either a mixed hematopoietic or CD8 T cell chimerism of ~10–20% was sufficient for reestablishment of immune regulation [60]. The introduction of reduced-intensity conditioning was a big step forward; more than 80% of patients with pHLH can now be expected to survive HSCT [58, 61]. An increased incidence of mixed chimerism can be seen after RIC conditioning. In a large retrospective study from 23 bone marrow transplant centers, the protective effect of >20% donor cells against late reactivations could be confirmed. Interestingly, there were five patients living reactivation-free for 1.1–10 years (median 5.1) with ≤10% donor cells [62].
Whereas in XLP-2, HSCT should be reserved for patients with severe disease, there is a general indication for HSCT in patients with XLP-1, even before first exposure to EBV [57]. These patients not only have life-threatening EBV-associated HLH but also progressive hypogammaglobulinemia, and the risk of developing lymphomas.
Although HSCT should be reserved for patients with pHLH, there are some patients with EBV-associated HLH who may need a transplant. In a survey from Japan, fourteen pediatric patients with sHLH due to EBV were collected who failed HLH-2004 therapy and underwent HSCT [63]. Since such transplants were also successful with autologous stem cells, cells from an identical twin, and with graft failure, resetting of the adaptive immune response was suggested as mechanism for success, rather than replacement of a genetically defective immune system [63].
New Therapies
Etoposide-based treatment can currently be regarded as the standard of care for HLH. Two studies conducted in North America and Europe evaluated the combination of ATG, dexamethasone, and etoposide. The studies have been closed; results are not published yet. A French study is currently evaluating the role of the monoclonal anti-CD52-antibody alemtuzumab as first line treatment. Another promising approach is therapy with an anti-IFNγ antibody, which is currently tested in a phase II/III study. Recently, two groups used several mouse models to show that ruxolitinib, a Janus kinase inhibitor was not only successful in preventing [64] but also in treating manifest HLH [65]. As yet there are only two case reports in humans [66, 67]. A review on salvage therapy of HLH identified alemtuzumab as the only drug with data in a larger number of patients. A partial response was achieved in 14 of 22 patients [68]. Plasma exchange, a very old method, has received renewed interest [69]. It may still be of value, especially when therapy with etoposide is not possible due to renal failure. An interesting approach is cytokine absorption which has been successfully applied in some adult patients with HLH [70]. Specific targeting of pro-inflammatory cytokines, including IL-1, IL-6, and tumor necrosis factor is also being explored as therapy for CSS [71, 72].
Open Questions And Outlook
Our knowledge of HLH has increased rapidly within the last 20 years. However, our understanding is still incomplete, and many questions remain. Just to name a few: What other genes in the degranulation pathway are involved? What activates the T cells in children with pHLH where no readily identifiable infectious organism is found? What are the pathogenetic mechanisms of sHLH? Why do some genetic defects have a higher likelihood of CNS-HLH? Is there a place for drugs with good penetration into brain tissue such as thiotepa in refractory CNS disease? Why do some patients respond so poorly to frontline chemoimmunotherapy? Can efficacy and toxicity of frontline therapy be improved with the newer drugs? What compensatory mechanisms prevent recurring HLH in some patients with graft failure? Which patients in the ICU with severe hyperinflammation, fulfilling HLH criteria, could profit from immunosuppressive therapy?
HLH is no longer a disease of marginal existence but is increasingly being recognized as an important and dangerous syndrome. New drugs as supplement or substitution in initial therapy will hopefully improve response rates and long-term results. Gene therapy is presently being explored in animal models of HLH but will take time until introduction into the human setting.
References
Farquhar, J. W., & Claireaux, A. E. (1952). Familial haemophagocytic reticulosis. Archives of Disease in Childhood, 27, 519–525.
Scott, R. B., & Robb-Smith, A. H. T. (1939). Histiocytic medullary reticulosis. Lancet, 2, 194–198.
Falini, B., Pileri, S., De Solas, I., Martelli, M. F., Mason, D. Y., Delsol, G., et al. (1990). Peripheral T-cell lymphoma associated with hemophagocytic syndrome. Blood, 75, 434–444.
Risdall, R. J., McKenna, R. W., Nesbit, M. E., Krivit, W., Balfour Jr., H. H., Simmons, R. L., et al. (1979). Virus-associated hemophagocytic syndrome: A benign histiocytic proliferation distinct from malignant histiocytosis. Cancer, 44, 993–1002.
Matzner, Y., Behar, A., Beeri, E., Gunders, A. E., & Hershko, C. (1979). Systemic leishmaniasis mimicking malignant histiocytosis. Cancer, 43, 398–402.
Henter, J. I., Elinder, G., Söder, O., & Ost, A. (1991b). Incidence in Sweden and clinical features of hemophagocytic lymphohistiocytosis. Acta Paediatrica Scandinavica, 80, 428–435.
Janka, G. E. (1989). Familial hemophagocytic lymphohistiocyosis: Therapy in the German experience. Pediatric Hematology and Oncology, 6, 227–231.
Janka, G. E. (1983). Familial hemophagocytic lymphohistiocytosis. European Journal of Pediatrics, 140, 221–230.
Devictor, E., Fischer, A., Mamas, S., de Saint Basile, G., Durandy, A., Buriot, D., et al. (1982). Etude immunologique de la lymphohistiocytose familiale. Archives Francaises de Pediatrie, 39, 135–140.
Goldberg, J., & Nezelof, C. (1986). Lymphohistiocytosis: A multi-factorial syndrome of macrophagic activation clinico-pathological study of 38 cases. Hematological Oncology, 4, 275–289.
Henter, J. I., & Elinder, G. (1991). Diagnostic guidelines for hemophagoytic lymhohistiocytosis. The FHL study group of the histiocyte society. Seminars in Oncology, 18, 29–33.
Henter, J. I., Ehrnst, A., Anderson, J., & Elinder, G. (1993). Familial hemophagocytic lymphohistiocytosis and viral infections. Acta Paediatrica, 82, 369–372.
Akima, M., & Sumi, S. M. (1984). Neuropathology of familial erythrophagocytic lymphohistiocytosis. Human Pathology, 15, 161–168.
Jackson, J., Titman, P., Butler, S., Bond, K., Rao, A., Veys, P., et al. (2013). Cognitive and psychosocial function in post hematopoietic stem cell transplantation in children with hemophagocytic lymphohistiocytosis. Journal of Allergy and Clinical Immunology, 132, 889–895.
Horne, A. C., Wickström, R., Jordan, M. B., Yeh, E. A., Naqvi, A., Henter, J. I., et al. (2017). How to treat involvement of the central nervous system in hemophagocytic lymphohistiocytosis. Current Treatment Options in Neurology, 19, 3.
Henter, J. I., Elinder, G., Söder, O., Hansson, M., Andersson, B., & Andersson, U. (1991). Hypercytokinemia in familial hemophagocytic lymphohistiocytosis. Blood, 78, 2918–2922.
Imashuku, S., Ikushima, S., Esumi, N., Todo, S., & Saito, M. (1991). Serum levels of interferon-gamma, cytotoxic factor and soluble interleukin-2 receptor in childhood hemophagocytic syndromes. Leukemia & Lymphoma, 3, 287–292.
Komp, D. M., McNamara, J., & Buckley, P. (1989). Elevated soluble interleukin-2 receptor in childhood hemophagocyctic histiocytic syndromes. Blood, 73, 2128–2132.
Perez, N., Virelizier, J. L., Arenzana-Seisdedos, S., Fischer, A., & Griscelli, C. (1984). Impaired natural killer cell activity in lymphohistiocytosis syndrome. The Journal of Pediatrics, 104, 569–573.
Ambruso, D. R., Hays, T., Zwartjes, W. J., Tubergen, D. G., & Favara, B. E. (1980). Successful treatment of lymphohistiocytic reticulosis with phagocytosis with epipodophyllotoxin VP 16-213. Cancer, 45, 2516–2520.
Fischer, A., Virelizier, J. L., Arenzana-Seisdedos, F., Perez, N., Nezelof, C., & Griscelli, C. (1985). Treatment of four patients with erythrophagocytic lymphohistiocytosis by a combination of epipodophyllotoxin, steroids, intrathecal methotrexate, and cranial irradiation. Pediatrics, 76, 263–268.
Fischer, A., Cerf-Bensussan, N., Blanche, S., Le Deist, F., Bremard-Oury, C., Leverger, G., et al. (1986). Allogeneic bone marrow transplantation for erythrophagocytic lymphohistiocytosis. The Journal of Pediatrics, 108, 267–270.
Stéphan, J. L., Donadieu, J., Ledeist, F., Blanche, S., Griscelli, C., & Fischer, A. (1993). Treatment of familial hemophagocytic lymphohistiocytosis with antithymocyte globulins, steroids, and cyclosporin A. Blood, 82, 2319–2323.
Henter, I. J., Samuelsson-Horne, A. C., Aricò, M., Egeler, R. M., Elinder, G., Filopovich, A. H., et al. (2002). Treatment of hemophagocytic lymphohistiocytosis with HLH94 immunochemotherapy and bone marrow transplantation. Blood, 100, 2367–2373.
Trottestam, H., Horne, A. C., Aricò, M., Egeler, R. M., Filipovich, A. H., Gadner, H., et al. (2011). Chemoimmunotherapy for hemophagocytic lymphohistiocytosis: Long-term results of the HLH-94 treatment protocol. Blood, 118, 4577–4584.
Henter, J. I., Horne, A. C., Aricó, M., Egeler, R. M., Filopovich, A. H., Imashuku, S., et al. (2007). HLH-2004: Diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatric Blood & Cancer, 48, 124–131.
Bergsten, E., Horne, A., Astigarraga, I., Egeler, R. M., Filipoivich, A. H., Ishii, E., et al. (2017). Confirmed efficacy of etoposide and dexamethasone in HLH treatment: Long-term results of the cooperative HLH-2004 study. Blood, 130, 2728–2738.
Stepp, S. E., Dufourcq-Lagelouse, R., Le Deist, F., Bhawan, S., Certain, S., Mathew, P. A., et al. (1999). Perforin gene defects in familial hemophagocytic lymphohistiocytosis. Science, 286, 1957–1959.
Ohadi, M., Lalloz, M. R., Sham, P., Zhao, J., Dearlove, A. M., Shiach, C., et al. (1999). Localization of a gene for familial hemophagocytic lymphohistiocytosis at chromosome 9q21.3-22 by homozygosity mapping. American Journal of Human Genetics, 64, 165–171.
Chia, J., Yeo, K. P., Whisstock, J. C., Dunstone, M. A., Trapani, J. A., & Voskoboinik, I. (2009). Temperature sensitivity of human perforin mutants unmasks subtotal loss of cytotoxicity, delayed FHL, and a predisposition to cancer. Proceedings of the National Academy of Sciences of the United States of America, 106, 9809–9814.
Feldmann, J., Callebaut, I., Raposo, G., Certain, S., Bacq, D., Dumont, C., et al. (2003). Munc13-4 is essential for cytolytic granules fusion and is mutated in a form of familial hemophagocytic lymphohistiocytosis (FHL3). Cell, 115, 461–473.
Zur Stadt, U., Schmidt, S., Kasper, B., Beutel, K., Diler, A. S., Henter, J. I., et al. (2005). Linkage of familial hemophagocytic lymphohistiocytosis (FHL) type-4 to chromosome 6q24 and identification of mutations in syntaxin 11. Human Molecular Genetics, 14, 827–834.
Côte, M., Ménager, M. M., Burgess, A., Mahlaoui, N., Picard, C., Schaffner, C., et al. (2009). Munc18-2 deficiency causes familial hemophagocytic lymphohistiocytosis type 5, and impairs cytotoxic granule exocytosis in patient NK cells. The Journal of Clinical Investigation, 119, 3765–3773.
Zur Stadt, U., Rohr, J., Seifert, W., Koch, F., Grieve, S., Pagel, J., et al. (2009). Familial hemophagocytic lymphohistiocytosis type 5 (FHL-5) is caused by mutations in Munc18-2 and impaired binding to syntaxin 11. American Journal of Human Genetics, 85, 482–492.
Jessen, B., Kögl, T., Sepulveda, F. E., de Saint Basile, G., Aichele, P., & Ehl, S. (2013). Graded defects in cytotoxicity determine severity of hemophagocytic lymphohistiocytosis in humans and mice. Frontiers in Immunology, 4, 448.
Pagel, J., Beutel, K., Lehmberg, K., Koch, F., Maul-Pavicic, A., Rohlfs, A. K., et al. (2012). Distinct mutations in STXBP2 are associated with variable clinical presentations in patients with familial hemophagocytic lymphohistiocytosis type 5 (FHL5). Blood, 119, 6016–6024.
Zhang, K., Chandrakasan, S., Chapman, H., Valencia, C. A., Husami, A., Kissell, D., et al. (2014). Synergistic defects of different molecules in the cytotoxic pathway lead to clinical familial hemophagocytic lymphohistiocytosis. Blood, 124, 1331–1334.
Cetica, V., Sieni, E., Pende, D., Danesino, C., De Fusco, C., Locatelli, F., et al. (2016). Genetic predisposition to hemophagocytic lymphohistiocytosis: Report on 500 patients from the Italian registry. The Journal of Allergy and Clinical Immunology, 137, 188–196.
Jordan, M. B., Hildeman, D., Kappler, J., & Marrack, P. (2004). An animal model of hemophagocytic lymphohistiocyosis (HLH): CD8+ T cells and interferon gamma are essential for the disorder. Blood, 104, 735–743.
Pachlopnik-Schmidt, J., Ho, C. H., Chrétien, F., Lefebvre, J. M., Pivert, G., Kosco-Vilbois, M., et al. (2009). Neutralization of IFNgamma defeats haemophagocytosis in LCMV-infected perforin- and Rab27a-deficient mice. EMBO Molecular Medicine, 1, 112–124.
Johnson, T. S., Terrell, C. E., Millen, S. H., Katz, J. D., Hildemann, D. A., & Jordan, M. B. (2014). Etoposide selectively ablates activated T cells to control the immunoregulatory disorder hemophagocytic lymphohistiocytosis. Journal of Immunology, 192, 84–91.
Amman, S., Lehmberg, K., zur Stadt, U., Janka, G., Rensing-Ehl, A., Klemann, C., et al. (2017). Primary and secondary hemophagocytic lymphohistiocytosis have different patterns of T-cell activation, differentiation and repertoire. European Journal of Immunology, 47, 364–373.
Bode, S. F. N., Ammann, S., Al-Herz, W., Bataneant, M., Dvorak, C. C., Gehring, S., et al. (2015). The syndrome of hemophagocytic lymophohistiocytosis in primary immunodeficiencies: Implications for differential diagnosis and pathogenesis. Haematologica, 100, 978–988.
Behrens, E. M., Canna, S. W., Slade, K., Rao, S., Kreiger, P. A., Paessler, M., et al. (2011). Repeated TLR9 stimulation results in macrophage activation syndrome-like disease in mice. The Journal of Clinical Investigation, 121, 2264–2277.
Jenkins, M. R., Rudd-Schmidt, J. A., Lopez, J. A., Ramsbottom, K. M., Mannering, S. I., Andrews, D. M., et al. (2015). Failed CTL/NK cell killing and cytokine hypersecretion are directly linked through prolonged synapse time. The Journal of Experimental Medicine, 212, 307–317.
Janka, G. E. (2012). Familial and acquired hemophagocytic lymphohistiocytosis. Annual Review of Medicine, 63, 233–246.
Shimazu, H., Munakata, S., Tashiro, Y., Salama, Y., Dhahri, D., Eiamboonsert, S., et al. (2017). Pharmacological targeting of plasmin prevents lethality in a murine model of macrophage activation syndrome. Blood, 130, 59–72.
Ramos-Casals, M., Brito-Zerón, P., López-Guillermo, A., Khamashta, M. A., & Bosch, X. (2014). Adult haemophagocytic syndrome. Lancet, 383, 1503–1516.
Zhang, K., Jordan, M. B., Marsh, R. A., Johnson, J. A., Kissell, D., Meller, J., et al. (2011). Hypomorphic mutations in PRF1, MUNC13-4, and STXBP2 are associated with adult-onset familial HLH. Blood, 118, 5794–5798.
Fardet, L., Galicier, L., Lambotte, O., Marzac, C., Aumont, C., Chahwan, D., et al. (2014). Development and validation of the HScore, a score for the diagnosis of reactive hemophagocytic syndrome. Arthritis & Rhematology, 66, 2613–2620.
La Rosée, P., Horne A. C., Hines M., von Bahr Greenwood T., Machowicz, R., Berliner, N., et al. (2019). Recommendations for the management of hemophagocytic lymphohistiocytosis in adults. Blood, 133, 2465–2477.
Bryceson, A. T., Pende, D., Maul-Pavicic, A., Gilmour, K. C., Ufheil, H., Vraetz, T., et al. (2012). A prospective evaluation of degranulation assays in the rapid diagnosis of familial hemophagocytic syndromes. Blood, 119, 2754–2763.
Lehmberg, K., & Ehl, S. (2013). Diagnostic evaluation of patients with suspected haemophagocytic lymphohistiocytosis. British Journal of Haematology, 160, 275–287.
Rubin, T. S., Zhang, K., Gifford, C., Lane, A., Choo, S., Bleesing, J. J., et al. (2017). Perforin and CD107a testing is superior to NK cell function testing for screening patients for genetic HLH. Blood, 129, 2993–2999.
Spessott, W. A., Sanmillan, M. L., McCormick, M. E., Patel, N., Villanueva, J., Zhang, K., et al. (2015). Hemophagocytic lymphohistiocytosis caused by dominant-negative mutations in STXBP2 that inhibit SNARE-mediated membrane fusion. Blood, 125, 1566–1577.
Zhang, M., Bracaglia, C., Prencipe, G., Bemrich-Stolz, C. J., Beukelman, T., Dimmitt, R. A., et al. (2016). A heterozygous RAB27A mutation associated with delayed cytolytic granule polarization and hemophagocytic lymphohistiocytosis. Journal of Immunology, 196, 2492–2503.
Janka, G. E., & Lehmberg, K. (2014). Hemophagocytic syndromes—an update. Blood Reviews, 28, 135–142.
Marsh, R. A., Jordan, M. B., & Filipovich, A. H. (2011). Reduced-intensity conditioning haematopoietic cell transplantation for haemophagocytic lymphohistiocytosis: An important step forward. British Journal of Haematology, 154, 556–563.
Ouachée-Chardin, M., Elie, C., de Saint Basile, G., Le Deist, F., Mahlaoui, N., Picard, C., et al. (2006). Hematopoietic stem cell transplantation in hemophagocytic lymphohistiocytosis: A single-center report of 48 patients. Pediatrics, 117, e743–e750.
Terrell, C. E., & Jordan, M. B. (2013). Mixed hematopoietic or T cell chimerism above a minimal threshold restores perforin-dependent immune regulation in perforin-deficient mice. Blood, 122, 2618–2621.
Lehmberg, K., Albert, M. H., Beier, R., Beutel, K., Gruhn, B., Kröger, N., et al. (2013). Treosulfan-based conditioning regimen for children and adolescents with hemophagocytic lymphohistiocytosis. Haematologica, 99, 180–184.
Hartz, B., Marsh, R., Rao, K., Henter, J. I., Jordan, M., Filipovich, L., et al. (2016). The minimum required level of donor chimerism in hereditary hemophagocytic lymphohistiocytosis. Blood, 127, 3281–3290.
Ohga, S., Kudo, K., Ishii, E., Honjo, S., Morimoto, A., Osugi, Y., et al. (2010). Hematopoietic stem cell transplantation for familial hemophagocytic lymphohistiocytosis and Epstein-Barr virus-associated hemophagocytic lymphohistiocytosis in Japan. Pediatric Blood & Cancer, 54, 299–306.
Das, R., Guan, P., Sprague, L., Verbist, K., Tedrick, P., An, Q. A., et al. (2016). Janus kinase inhibition lessens inflammation and ameliorates disease in murine models of hemophagocytic lymphohistiocytosis. Blood, 127, 1666–1675.
Maschalidi, S., Sepulveda, F. E., Garrigue, A., Fischer, A., & de Saint Basile, G. (2016). Therapeutic effect of JAK1/2 blockade on the manifestations of hemophagocytic lymphohistiocytosis in mice. 2016. Blood, 128, 60–71.
Broglie, L., Pommert, L., Rao, S., Thakar, M., Phelan, R., Margolis, D., et al. (2017). Ruxolitinib for treatment of refractory hemophagocytic lymphohistiocytosis. Blood Advances, 1, 1533–1536.
Sin, J. H., & Zangardi, M. L. (2017). Ruxolitinib for secondary hemophagocytic lymphohistiocytosis: First case report. Hematology/Oncology and Stem Cell Therapy.
Marsh, R. A., Jordan, M. B., Talano, J. A., Nichols, K. E., Kumar, A., Naqvi, S. R., et al. (2016). Salvage therapy for refractory hemophagocytic lymphohistiocytosis: A review of the published experience. Pediatr. Blood Cancer, 64, e26308.
Bosnak, M., Erdogan, S., Aktekin, E. H., & Bay, A. (2016). Therapeutic plasma exchange in primary hemophagocytic lymphohistiocytosis: Reports of two cases and a review of the literature. Transfusion and Apheresis Science, 55, 353–356.
Greil, C., Roether, F., La Rosée, P., Grimbacher, B., Duerschmied, D., & Warnatz, K. (2016). Rescue of cytokine storm due to HLH by hemoadsorption in a CTLA4-deficient patient. Journal of Clinical Immunology, 37, 273–276.
Maude, S. L., Barrett, D., Teachey, D. T., & Grupp, S. A. (2014). Managing cytokine release syndrome associated with novel T cell-engaging therapies. Cancer Journal, 20, 119–122.
Miettunen, P. M., Narendran, A., Jayanthan, A., Behrens, E. M., & Cron, R. Q. (2011). Successful treatment of severe paediatric rheumatic disease-associated macrophage activation syndrome with interleukin-1 inhibition following conventional immunosuppressive therapy: Case series with 12 patients. Rheumatology (Oxford), 50, 417–419.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Janka, G.E. (2019). History of Hemophagocytic Lymphohistiocytosis. In: Cron, R., Behrens, E. (eds) Cytokine Storm Syndrome. Springer, Cham. https://doi.org/10.1007/978-3-030-22094-5_1
Download citation
DOI: https://doi.org/10.1007/978-3-030-22094-5_1
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-22093-8
Online ISBN: 978-3-030-22094-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)