Abstract
Just over a quarter of humanity is infected with the tubercle bacillus and risks developing active disease that routinely requires 6-month treatment. The impact of this scourge cannot be underestimated, and reducing the global burden of tuberculosis is the focus of much research. In addition to the need for improved chemotherapy regimens and monitoring thereof, understanding the risk and processes involved in transmission, a critical step in the life cycle of the organism, has even greater potential to impact the burden of disease. Our chance observation that lipid bodies (LBs) were present in Mycobacterium tuberculosis in sputum, but not in growing cultures of the lab strain in vitro, led us and others to examine this phenomenon further. Transcriptional analysis of the bacilli in sputum identified that upregulation of tgs1, a triacylglycerol synthase, was likely responsible for the presence of these LBs. Strikingly, in contrast to the then established view that tubercle bacilli in sputum arose directly from rapidly replicating populations, further transcriptional and cytological analyses led us to link the M. tuberculosis sputum phenotype to slow or non-growing persisters. As a result, we and others have directed research to further understanding the biological and clinical significance of LBs and neutral lipids in mycobacteria. There is now greater insight into the biosynthetic pathways and role of neutral lipids during infection, for both growing and dormant M. tuberculosis. Links have been made between tgs1-related triacylglycerol LB accumulation and growth arrest and with antibiotic tolerance potentially underpinning the need for protracted chemotherapy. The possible clinical significance of this is reflected in the finding that sustained high frequencies of LB-positive M. tuberculosis in sputum during treatment are associated with unsatisfactory outcomes. LB-positivity may also support transmission of the organism. Greater understanding of the significance of this “fat and lazy” population will open up new approaches to the combat of this long-standing foe.
Access provided by Autonomous University of Puebla. Download reference work entry PDF
Similar content being viewed by others
1 Introduction
1.1 Occurrence and Composition of Lipid Bodies
Lipid bodies (LBs) (also known as lipid droplets (LDs) or intracellular lipophilic inclusions (ILIs)) are cytoplasmic accumulations of lipids. LBs are widespread in eukaryotes, where they have generally been viewed as a carbon storage depot but are now recognized as dynamic organelles (Murphy 2012). In contrast, the occurrence of LBs in prokaryotes appears to be more restricted and includes the Actinobacteria Mycobacterium and Rhodococcus, some Streptomyces, and certain hydrocarbon-degrading species of Gram-negative genera including Acinetobacter, Marinobacter, Alcanivorax, and Thalassolituus (Alvarez 2016). Bacterial LBs are overwhelmingly viewed as a form of carbon storage, enabling cells to survive “feast or famine” and to colonize and thrive within different niches.
The core of LBs is composed of neutral lipid which is surrounded by a unit phospholipid membrane. Many proteins are associated with the phospholipid membrane and include enzymes involved in neutral lipid synthesis and degradation, but also proteins which stabilize the LB structure or regulate lipid homoeostasis and turnover (Murphy 2012). Eukaryotic LBs are composed of triacylglycerols (TAGs) and cholesterol esters. Bacterial LBs are predominantly composed of TAG, although in certain growth conditions, wax esters (WEs ) can form. Representative structures of these are shown in Fig. 1. The acyl composition and distribution of acyl chains on the glycerol backbone of bacterial TAG can vary with bacterial culture conditions and growth state. In general, the sn-1,2 positions are esterified with acyl chains of between 16 and 18 carbons which reflects the composition of phospholipids and the 3-position with longer or branched fatty acids (Walker et al. 1970; Alvarez and Steinbüchel 2002). The composition of bacterial LBs can affect their morphology, presumably a reflection of the melting temperature of the components and resulting fluidity. Compared to the relatively circular appearance of TAG-containing LBs, WEs accumulate as more disc-like structures (Alvarez and Steinbüchel 2002; Ishige et al. 2002; Sherratt 2008).
1.2 Mycobacterial Lipid Bodies
Mycobacterial LBs were first reported by Burdon who applied the lipophilic strain, Sudan Black B to smears of Mycobacterium tuberculosis (Mtb), Mycobacterium leprae, and saprophytic species (Burdon 1946). Since this time, mycobacterial LBs have been recognized by both light and electron microscopy (Garton et al. 2002). During a study of the native organization of envelope lipid domains, we identified LBs in live mycobacteria with application of select fluorescent lipophilic probes (Christensen et al. 1999). This observation was the impetus for our study of the natural history of these structures in the fast-growing saprophytic species Mycobacterium smegmatis (Garton et al. 2002). Consistent with a supposed carbon storage role, LBs accumulated in conditions of carbon excess and nutrient limitation. In nitrogen-limited medium LBs formed over a period of several days, whereas supplementation of growing cultures with long-chain fatty acids (LCFAs) resulted in rapid LB accumulation. Transfer to carbon-deficient medium resulted in assimilation of accumulated LBs. TAG was, for many years, considered to be a component of the mycobacterial cell envelope (Minnikin 1982; Ortalo-Magné et al. 1996). We found observation of M. smegmatis LBs to be coincident with the presence of TAG and the TAG acyl composition to reflect the culture conditions (Garton et al. 2002). A range of LCFA (C14–C24) were detected in TAG extracted from biomass cultured in Middlebrook broth, with palmitic (hexadecanoic C16:0) and oleic (octadecenoic C18:1) acyl substituents being most abundant. The chain length profile of acyl substituents of TAG from nitrogen-limited culture was similar, but was enriched in saturated, particularly stearic (octadecanoic C18:0) acyl chains. In contrast, the profile of TAG from oleic acid-supplemented culture was dominated by this acyl substituent (~80%) and also contained palmitoleoyl (hexadecenoic C16:1) and trace tuberculostearic (10-methyloctadecanoic brC19:10Me) acyl chains. TAG was not observed in cultures in which LBs had been assimilated. Acyl chain lengths of up to C28 have been reported in TAG of Mtb treated with nitric oxide (NO), with the most abundant chain length being C26 (Daniel et al. 2004).
Our particular interest in LBs of Mtb, the agent of tuberculosis (TB), was driven by the observation that LBs were more readily observed in acid-fast bacilli in TB patient sputum samples, compared with growing in vitro culture of the Mtb laboratory strain H37Rv (Garton et al. 2002, 2008). This was in contrast with the abundant presence of LBs in fast-growing M. smegmatis. It is interesting to note that Burdon described inconsistent detection of LBs in tubercle bacilli cultured on egg medium, compared with their ready detection in M. smegmatis (Burdon 1946). However, we did identify LBs in Mtb sampled from in an in vitro hypoxic model of Mtb dormancy and in response to NO, a treatment that results in growth arrest (Garton et al. 2008). Mtb LBs have since been reported in response to other growth arresting stimuli (Sherratt 2008) and in alternative in vitro models of dormancy (Deb et al. 2009), including a macrophage models of Mtb persistence (Daniel et al. 2011) and a granuloma model (Kapoor et al. 2013).
Significantly, Mtb growth arrest and dormancy result in a phenotypic state in which the bacilli become tolerant to the action of antibiotics, leading us to propose that Mtb bacilli in TB patient sputa may represent a similarly antibiotic tolerant population. The clinical significance of LBs was highlighted in a recent study linking sputum LB content of Mtb with increased probability of unsatisfactory treatment outcome (Sloan et al. 2015). Understanding the differences between Mtb in sputum and in vitro culture, the clinical significance of Mtb LB positivity in sputum, and how this may relate to bacilli in aerosols that transmit infection are major foci of our research.
2 TAG Synthesis, LB Assembly, and Assimilation
There is now much insight into the conditions and biochemical pathways involved in TAG and WE synthesis, organization as LBs, and their breakdown. Here we summarize what is known of these in members of the Mtb complex.
2.1 Synthesis of TAG and WE
Eukaryotic lipid droplets (LDs) contain TAG and cholesterol esters, which are synthesized by distinct acyl transferase enzymes. The biosynthetic (Kennedy) pathway of TAG, similar to that in bacteria shown in Fig. 2, branches from that of phospholipids at the key intermediate phosphatidic acid (diacylglycerol 3-phosphate). A specific phosphatidic acid phosphatase hydrolyzes phosphatidic acid to form diacylglycerol (DAG), the substrate for diacylglycerol acyl transferases (DGATs). DGATs catalyze the transfer of long-chain fatty acyl chains, activated as acyl CoA, onto the free hydroxyl of DAG to form TAG. Bacteria contain no homologues of eukaryotic DGATs. The first bacterial enzyme characterized as having DGAT activity was identified in an Acinetobacter (Acinetobacter sp. ADP1) (Kalscheuer and Steinbüchel 2003) with a screen of transposon mutants to identify those deficient in lipid accumulation. This Acinetobacter enzyme was found to possess both DGAT and wax ester synthase (WS) activity and was termed WS/DGAT. These authors identified homologues of this WS/DGAT in mycobacteria.
The biosynthetic pathway of TAG and wax esters in bacteria. Key: GPAT, glycerol 3-phosphate acyltransferase; AGPAT, 1-acylglycerol 3-phosphate acyltransferase; PAP, phosphatidic acid phosphatase; FACS, fatty acyl-CoA synthase; FCR, fatty acyl-CoA reductase; WS/DGAT, wax ester synthase/diacylglycerol acyltransferase; TGS, triacylglycerol synthase
Daniel and colleagues screened 15 putative Mtb WS/DGAT proteins for these activities with in vitro assays. All 15 recombinant proteins were shown to have greater DGAT activity than WS activity, leading to these investigators to term the mycobacterial enzymes TAG synthases (TGS) and those with the greatest TGS activity, as Tgs1 (Rv3130c), Tgs2 (Rv3734c), Tgs3 (Rv3234c), and Tgs4 (Rv3088) (Daniel et al. 2004). Overexpression of tgs1 in M. smegmatis resulted in enhanced TAG LB content (Garton et al. 2008) relating the activity of this enzyme with cytoplasmic LB accumulation. The accumulation in Mtb of TAG in response to hypoxia, or following exposure to NO, correlates with induced transcription of tgs1 (Daniel et al. 2004). An Mtb tgs1 mutant strain fails to accumulate TAG in response to these conditions (Sirakova et al. 2006) or in a multiple stress (low O2, high CO2, low nutrient, acidic pH) model (Deb et al. 2009).
Tgs1 is a member of the DosR-regulated dormancy-related regulon comprising ~48 genes (Park et al. 2003). DosR expression responds to hypoxia, NO, and carbon monoxide, with the signal transduced by two sensor proteins DosS and DosT (Roberts et al. 2004). It is noteworthy that although tgs1 shows the greatest induction in response to hypoxia or NO, expression of other tgs genes, e.g., tgs2, tgs3, and Rv3371, which are not members of the DosR regulon, is increased in these conditions also (Daniel et al. 2004).
TGS Rv3371 shows the greatest homology with Tgs1 (Daniel et al. 2004) although it was reported to possess weak TGS activity in vitro. Over-expression of Rv3371 in M. smegmatis resulted in enhanced TAG content (Rastogi et al. 2017). Colonies of this strain had a smooth appearance, and cells had altered cell surface properties, leading these authors to suggest that Rv3371 is involved in cell wall alterations. However, the intracellular LB content of strains was not examined. In addition to being induced in response to hypoxia and NO (Daniel et al. 2004), Rv3371 expression is upregulated in conditions of iron -limitation. Rv3371 expression is repressed by the putative transcriptional regulator Rv1404 (Golby et al. 2008). Rv1404 has an IdeR (iron-dependent repressor and activator) binding site upstream. Reduced expression of both ideR and Rv1404 in iron-limited conditions led Rastogi and colleagues to suggest that IdeR activates Rv1404, in turn relieving repression of Rv3371 expression (Rastogi et al. 2017).
In addition to the 15 TGSs, other proteins of the Mtb complex have been identified as having a role in TAG accumulation. Investigation of the protein content of isolated Mycobacterium bovis BCG LBs identified homologues of Tgs1 (BCG3153c), Tgs2 (BCG3794c), and two proteins, which, when deleted, reduced the TAG content of the mutants compared with the wild type, a putative 1-acyl glycerol 3-phosphate acyl transferase (BCG1489c) and an uncharacterized protein BCG1169c (Low et al. 2010). Furthermore, homologous over-expression of BCG1721, encoding a fifth LB-associated protein which was found to contain both long-chain acyl-CoA synthase (ACSL) and lipase domains, resulted in accumulation of LBs. This suggests that in those conditions, the ACSL activity of this protein was dominant. To date, the activity of the Mtb homologue of this novel bifunctional protein, Rv1109c, has not been investigated.
In 2011, Elamin and colleagues reported that Ag85a, one of three cell envelope mycolyl-transferases , also had TGS activity (Elamin et al. 2011). Over-expression of Ag85a in M. smegmatis resulted in the accumulation of TAG LBs and a thickening of the cell envelope. Recently, all three mycolyl-transferases Ag85a, Ag85b, and Ag85c have been reported to possess TGS activity in vitro, with Ag85c having the highest TGS activity (Viljoen et al. 2018). Recombinant WS/DGAT of Acinetobacter sp. ADP1 has been found to be rather promiscuous in the range of substrates accepted for acylation (Stöveken et al. 2005). It is not known if the Mtb Ag85 proteins have a physiological role in TAG synthesis in the mycobacterial cell, possibly contributing to TAG content of the outer layer of the cell envelope, or whether this activity simply reflects the ability of these proteins to accept DAG and shorter acyl chains as substrates in vitro.
In addition to TAG, WEs have been reported in lipid extracts of Mtb cultures under multiple stress (Deb et al. 2009) or iron -limitation (Bacon et al. 2007). Of the 15 TGS (WS/DGAT) assayed by Daniel and colleagues, TGS2 (Rv3734c) was shown to have the greatest WS activity (Daniel et al. 2004), although contribution of Tgs2 to WE accumulation in bacilli has not been demonstrated. Two long-chain fatty acyl-CoA reductases (FCRs) of Mtb, Fcr1 (Rv3391) and Fcr2 (Rv1543), have been identified as being important for WE synthesis (Sirakova et al. 2012). FCR catalyzes reduction of long-chain fatty acyl-CoA, via an aldehyde intermediate, to long-chain fatty alcohol, a substrate for acylation by WS/DGAT to form WE (Fig. 2). To date, the cellular location of mycobacterial WE is not known. A cell envelope location is implied by the study of Sirakova and colleagues; Mtb Fcr1 and Fcr2 mutants showed faster growth rates and increased 14C-glycerol uptake, suggesting increased cell envelope permeability. However, M. smegmatis cultured on hexadecanol accumulates LBs with disc-like morphology (Sherratt 2008) similar to those seen in Acinetobacter sp. cultured in conditions which induce WE formation (Singer et al. 1985; Ishige et al. 2002). Intracytoplasmic co-localization of WE and TAG may occur, with the relative concentration of each possibly impacting the LB morphology.
2.2 Proteins with Roles in the Organization of LBs
Eukaryotic LDs contain a high-protein content in the surrounding phospholipid unit membrane (Walther and Farese 2012). Some LD proteins have roles in the formation and stabilization of the LDs for long-term storage by restricting access of lipases; one such example is the oleosins associated with LBs in plant cells. Other LD proteins, such as mammalian perilipin, have roles in regulating lipolytic breakdown. The first protein identified to have a role in bacterial LB organization was a heparin-binding hemagglutinin homologue termed TadA (TAG accumulation deficient) of Rhodococcus opacus (MacEachran et al. 2010). A ΔtadA mutant showed reduced TAG content and LBs of reduced size and shape. Aggregation of purified LBs on addition of purified TadD led the authors to suggest that TadA has a role in the maturation of LBs, directing aggregation of smaller nascent LBs found in early lipid storage. To date, no similar role has been reported for the Mtb TadA homologue, the heparin-binding hemagglutinin, HbhA.
Two Mtb LB-associated proteins have recently been described which impact LB production or organization. Daniel and colleagues characterized an Mtb protein with weak homology to human perilipin-1 and termed this MPER1 (Daniel et al. 2016). An Mtb Δmper1 (Rv1039c) mutant did not accumulate LBs and showed reduced incorporation of radiolabeled fatty acid into TAG in multiple stress conditions (Daniel et al. 2016) known to result in TAG LB accumulation in wild-type Mtb (Deb et al. 2009). Armstrong and colleagues investigated PspA (Rv2744c), a phage shock protein which shows similar structural characteristics to Psp proteins in unrelated bacteria such as E. coli (Armstrong et al. 2016). Psp proteins are induced in response to cell envelope stress, whereupon they localize to the inner face of the plasma membrane, assembling to form a scaffold-like complex to maintain membrane integrity and prevent dissipation of the proton-motive force (Armstrong et al. 2016). Although Mtb pspA is induced by the cell envelope stressor, SDS, an Mtb pspA mutant strain did not show enhanced susceptibility to this stress. However, the authors identified a novel role for the Mtb Psp protein. Compared with wild type, cells of an M. smegmatis mutant of the Mtb pspA homologue MS_2695 were found to have a greater number of LBs with a size profile shifted to those of diameter < 50 nm. However, loss or overproduction of MS_2695, although influencing the number and the size profile of LBs, did not impact the amount of TAG detected in a purified LB fraction. These authors concluded that PspA is important in regulating LB homeostasis.
2.3 LB Turnover
Mycobacterial TAG LBs are assimilated rapidly in cells transferred to carbon-limited conditions (Garton et al. 2002; Dhouib et al. 2011) or on transfer to growth-permissive conditions following growth arrest, for example, during regrowth from an hypoxic in vitro model of Mtb dormancy (Low et al. 2009). LBs are also considered to be a store of carbon and energy to sustain mycobacteria during dormancy (Daniel et al. 2004). Mycobacterial LB turnover is susceptible to inhibition with the pancreatic lipase inhibitor, tetrahydrolipstatin (THL) (Low et al. 2009; Dhouib et al. 2011). Mtb has a Lip family of 24 lipid/ester hydrolases, with a GXSXG consensus motif and annotation as putative esterases or lipases. Assessment of the TAG hydrolytic (lipase) activity of these recombinant proteins revealed LipY (Rv3097c) to be the most active (Deb et al. 2006). Following culture in conditions which induce TAG accumulation, an Mtb ΔlipY mutant did not assimilate TAG on transfer to carbon-limited conditions (Deb et al. 2006). LipY is a member of the Mtb PE family of proteins named for the Pro-Glu (PE) motif at their N-terminal, and these proteins have roles in antigenic variation, immune evasion, and virulence (Mishra et al. 2008). Over-expression of lipY in mycobacteria reduces cellular TAG content. Interestingly, if the PE domain is lacking in the over-expressed protein, lipase activity is enhanced, suggesting a regulatory role for this domain.
3 Metabolic Significance of TAG LB Accumulation
3.1 TAG Formation During Growth
More so than any other bacteria, mycobacteria with their uniquely lipid-rich cell envelope have a greater requirement to synthesize and manipulate LCFAs to support growth. During infection Mtb utilizes host lipids as a carbon and energy source. Segal and Bloch (1956) first reported that Mtb bacilli recovered from experimentally infected murine lungs showed an enhanced respiratory rate when supplemented with LCFA, but not with simple sugars; in vitro grown bacilli utilized both carbon sources equally well. Subsequent studies have confirmed a requirement for catabolism of both host LCFAs and cholesterol for persistence (McKinney et al. 2000; Pandey and Sassetti 2008). Catabolism of host LCFA requires β-oxidation and the glyoxylate shunt in order to bypass steps of the TCA cycle in which carbon is lost as CO2 and to retain this for gluconeogenesis (McKinney et al. 2000). In addition to fuelling central metabolism, host LCFA could be incorporated directly into mycobacterial lipids required for cell envelope synthesis during growth.
In axenic culture, LCFAs can be toxic to mycobacteria, probably resulting from the detergent-like activity of these amphiphiles leading to disruption of membranes (Kondo and Kanai 1977). Growing mycobacteria which import LCFA need to balance this uptake with metabolic and biosynthetic requirements. Incorporation of LCFA into TAG would buffer this toxic activity, yet, with hydrolysis by lipases, the LCFA would be readily available for oxidation or incorporation into cell envelope lipid. In growing mycobacteria, LBs may therefore represent a more dynamic metabolic pool, rather than a store of carbon (Fig. 3).
A proposed dynamic role for the TAG lipid body system. Lipid bodies are formed depending on the environmental balance of availability of LCFA and conditions available for growth. Key: LCFA, long-chain fatty acids; FA, fatty acid; CoA, coenzyme A; FASI and FASII, fatty acid synthase systems 1 and 2; TAG, triacylglycerol. (Originally published in Barer and Garton 2010, published with kind permission of ©Springer Science+Business Media New York, 2003. All rights reserved)
3.2 TAG Accumulation in Slow/Non-growing Mtb
Bacterial accumulation of TAG, rather than alternative carbon storage compounds such as glycogen and polyhydroxybutyrate, provides greater energetic return on oxidation (Alvarez 2016). This is because TAG is the more reduced molecule. Synthesis of LCFA for TAG can act to balance metabolism, preventing accumulation of reduced pyridine nucleotide cofactors which otherwise could inhibit some enzymes of central metabolism. This is particularly the case in hypoxic conditions when Mtb switches to anaerobic respiration.
Baek and colleagues proposed a direct role of TAG accumulation in bringing about growth arrest (Baek et al. 2011). In their in vitro experiments, an Mtb H37Rv Δtgs1 mutant failed to respond to growth-limiting stresses including hypoxia, low pH, and iron -limitation, with a restriction of growth as observed in the wild-type strain (Baek et al. 2011); failure of the Δtgs1 mutant to accumulate TAG under these conditions was also confirmed. These authors proposed that induction of tgs1, which results in TAG accumulation, directs acetate (the precursor of fatty acid synthesis by FasI) away from the TCA cycle and into TAG, resulting in growth arrest. They supported this hypothesis by demonstrating that the Δtgs1 mutant phenotype could be replicated by increasing acetate flux through the TCA cycle with oxaloacetate supplementation or overexpression of citA. Acetate flows into the TCA cycle at citrate synthase (CitA) which catalyzes condensation of this with oxaloacetate to form citrate. Baek and colleagues proposed this would compete for acetyl-CoA that would otherwise be diverted into fatty acid synthesis and ultimately TAG by Tgs1. In growth-permissive conditions, TAG hydrolysis and β-oxidation would make acetyl-CoA available for central metabolism again. Interestingly, an Mtb Rv3371 mutant also fails to enter non-replicating persistence in response to hypoxia, NO, and iron limitation (Rastogi et al. 2017). This suggests that the activity of both Tgs1 and Rv3371 is required to bring about growth arrest in vitro.
Whether this hypothesis holds for Mtb which is supplemented with, or utilizing, LCFA as a carbon source has not been explored. It could be proposed that tgs1 or Rv3371 induction in these conditions would direct all imported LCFA into TAG at the expense of fuelling the TCA cycle. Would the likelihood of growth arrest be dependent on availability, or not, of this exogenous resource?
We originally proposed that synthesized TAG is transported to the cell envelope and that, when this became saturated, excess TAG would accumulate as cytoplasmic LBs (Barer and Garton 2010). Recently, Martinot and colleagues characterized two Mtb proteins, lipoprotein LprG (Rv1411c) and Rv1410c, which function in the export of TAG from the cytoplasm and which have a role in regulating intracellular TAG levels (Martinot et al. 2016). Mutation of the LprG-Rv1410c locus in Mtb was shown to result in TAG accumulation, and over-expression led to excess TAG identified in the culture medium. However, the Mtb mutant did not show a growth defect in standard culture medium, even though it is attenuated for growth during murine infection. Therefore, intracellular accumulation of TAG alone is not a sole requirement for growth arrest in vitro. Growth attenuation in vitro was observed in conditions which mimic infection, i.e., when cholesterol was used as a sole carbon source for culture. Furthermore, in these conditions, inhibiting TAG lipolysis through addition of THL enhanced this growth defect. Conversely, supplementation of the cultures with acetate partially relieved the growth arrest of the mutant. These findings lead Martinot and colleagues to propose a model in which during growth, TAG is either incorporated into the cell envelope by the action of Rv1410c and LprG or alternatively hydrolyzed to release LCFA for β-oxidation and anaplerosis of the TCA cycle. Loss of Rv1410c-LprG function resulted in no TAG transport to the cell envelope and accumulation of TAG, which they propose inhibits growth by a currently unknown mechanism.
3.3 Accumulation of TAG Is Not the Sole Factor Resulting in Growth Restriction
There is further evidence that TAG accumulation alone is not the sole contributing factor to growth arrest. TAG accumulation resulting from tgs1 or Rv3371 expression in M. smegmatis, M. bovis BCG, or Mtb is not sufficient to restrict growth (unpublished results). Beijing strains of Mtb accumulate TAG during growth (Reed et al. 2007), and we have confirmed that in contrast with H37Rv, Beijing strains contain high levels of cytoplasmic LBs during growth (unpublished results). The accumulation of TAG by Beijing strains is thought to be a consequence of having tgs1 constitutively upregulated; it has now been reported that the constitutive expression of the DosR regulon in Beijing strains is as a result of a SNP in the DosR promoter region (within Rv3134c), present in all Beijing strains (Domenech et al. 2017). Furthermore, we are now obtaining preliminary results which indicate that recent Mtb clinical isolates contain variable tgs1 expression and TAG LB content during growth, leading us to believe that H37Rv may be exceptional in showing very low levels (unpublished results). Our observations are supported by a recent report in which LBs were identified in electron micrographs of a small number of Mtb sputum isolates, and not in Mtb H37Rv, growing in liquid culture (Vijay et al. 2017). Therefore, the differing LB content between growth and stasis of some strains does not necessarily reflect the extremes of TAG LB content shown in the images in Fig. 3.
Taken together recent findings indicate that the balance between TAG synthesis, export to the cell envelope, accumulation as cytoplasmic LBs, and turnover by lipases is complex, and the balance between the different fates must be finely tuned in different conditions. If the rate of TAG synthesis does not impact acetyl-CoA flux through the TCA cycle, growth could continue. The tipping point which results in sufficient redirection of acetyl-CoA from the TCA cycle to TAG synthesis to impact growth is not known. Alternatively, factors in addition to TAG accumulation resulting from tgs1 or Rv3371 expression must be responsible for growth restriction in conditions which result in Mtb non-replicating persistence. Whether strains which express tgs1 during growth respond to growth-limiting stresses to the same extent and via similar mechanisms to those proposed for H37Rv remains unexplored. The importance of investigating these mechanisms in culture conditions which reflect those during infection and examining strains other than the laboratory-adapted H37Rv must be highlighted.
4 The Significance of LB Accumulation in the Pathogenesis of TB
4.1 Establishment of Infection
Mtb is transmitted via aerosol droplets which are inhaled and engulfed within alveolar macrophages of the lung. It is not known if Mtb bacilli containing LBs have a modified (TAG-laden) envelope which provides an advantage for macrophage uptake. Once within the macrophage, the bacilli resist macrophage bactericidal mechanisms and replicate. The macrophage environment is relatively nutrient limiting, and assimilation of LBs may provide Mtb with an early growth advantage. The potential role of LBs in early infection is a current focus of our research.
4.2 Persistence During Latent Infection
Despite initial bacillary replication, very few infected (~1 in 10) individuals go onto develop active TB. Recruitment of immune cells to the site of infection leads to the production of a granuloma, containing infected macrophages (Russell 2007). Conditions within the granuloma (low O2, low pH) are not favorable for Mtb growth, and the pathogen is believed to adopt a state of non-replicating persistence (via induction of dosR), with low metabolic activity, a switch to anaerobic respiration and accumulation of LBs.
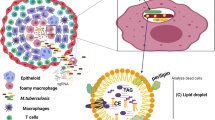
Lipid-laden foamy macrophages are a characteristic feature of the Mtb granuloma (Russell et al. 2009). Infection of macrophages with Mtb leads to remodelling of macrophage lipid metabolism (Lovewell et al. 2016). Transport of oxidized low-density lipoprotein in to the macrophages, and a downregulation of cholesterol efflux results in accumulation of macrophage LBs (hereafter referred to as LDs) and acquisition of a foamy macrophage phenotype. The lipids within the macrophage LDs can be accessed by the Mtb bacilli. In an in vitro human PBMC granuloma model in which foamy macrophages are induced following infection with Mtb, phagosomes containing bacilli were observed in close apposition with LDs (Peyron et al. 2008), and ultimately the bacilli were found within LDs. These foamy macrophages were not permissive for Mtb growth, and within the LD the bacilli were found to express tgs1 and accumulate LBs. Using a model in which foamy macrophages were induced by hypoxia, Daniel and colleagues demonstrated using both radioisotopic labeling and fluorescent LCFA derivatives, the uptake of host LCFA by intracellular Mtb and direct incorporation of this into Mtb TAG (Daniel et al. 2011). The bacilli, which did not grow within these hypoxic foamy macrophages, were also observed to contain LBs.
In addition to a role in bringing about growth arrest, LBs are thought to sustain Mtb throughout dormancy. Armstrong and colleagues reported PspA affects Mtb survival in non-replicating persistence (Armstrong et al. 2016). Both a ΔpspA Mtb mutant and an over-producing complemented strain showed reduced survival in a rapid anaerobic dormancy model, suggesting that tightly controlled concentrations of this protein are required. Interestingly, this phenomenon was specific to hypoxia-induced non-replicating persistence and was not reproduced when the same strains were examined for survival in phosphate-buffered saline. Perhaps this reflects requirement for interaction with different lipases.
4.3 Reactivation of Progressive Infection and Active Disease
Individuals with latent Mtb infection have a 10% lifetime chance of reactivation and development of active disease. The resource within TAG LBs may support bacillary regrowth in a newly permissive environment, for example, on aeration of a cavitating lesion. In vitro experiments with BCG adapted to non-replicating persistence following hypoxia have revealed a requirement for lipase activity and LB turnover for regrowth on reaeration (Low et al. 2009). An Mtb ΔlipY mutant fails to exit dormancy in an in vitro granuloma model (Kapoor et al. 2013).
With replication of Mtb within the open lesions, individuals with active disease become infectious and expectorate bacilli in sputum. We reported the presence of LB-positive acid-fast bacilli in TB patient sputa, at variable levels between patients (Garton et al. 2008), an observation supported by recent electron microscopy studies (Vijay et al. 2017). Strikingly, counter to the widely held belief that expectorated bacilli are replicating rapidly, findings of our Mtb sputum transcriptome study suggested quite the opposite (Garton et al. 2008). This transcriptome showed many signatures of slow or non-growth. Large clusters of down-regulated genes showed significant overlap with transcriptional signatures of Mtb in conditions in which the bacilli do not replicate, including during anaerobic non-replicating persistence (Muttucumaru et al. 2004; Voskuil et al. 2004; Garton et al. 2008), non-replicating persistence arising from nutrient deprivation (Betts et al. 2002) and during chronic murine infection (Shi et al. 2005). Groups of genes associated with ribosomal function, ATP synthesis, and aerobic respiration were also downregulated. The most highly expressed Mtb regulon in sputum was that induced by DosR. These findings led us to conclude that populations of non-replicating persister-like cells are present in sputum, a view consistent with the presence of LB-positive Mtb. The positive correlation between Mtb LB content and “time to positivity” in primary liquid culture of decontaminated sputum samples supported this view (Garton et al. 2008).
Bacterial replication is required for continuous population of sputum; therefore, environmental signals which result in this “persister” phenotype must be experienced by the bacilli as they exit the lung. The sputum environment would not be expected to be hypoxic and induction of the DosR regulon may result from exposure to NO in the airway. We have preliminary evidence that sputum Mtb LB content correlates with patient-expired NO (unpublished results).
Subsequent studies of the Mtb sputum transcriptome by others have supported our findings and provide further insight into how the environment of the host shapes the Mtb sputum transcriptome (Garcia et al. 2016; Honeyborne et al. 2016; Walter et al. 2016; Sharma et al. 2017). In addition to tgs1, the expression of TGS genes, Rv1425, Rv1760, Rv3087, and Rv3371, has been identified in Mtb in sputa of untreated patients (Honeyborne et al. 2016). This indicates that LB content of Mtb in sputum may not reflect activity of Tgs1 alone. Garcia and colleagues found that Mtb transcriptional profiles in sputum resembled those in cognate bronchoalveolar lavage samples, with the exception that genes of the DosR regulon showed slightly higher expression levels in lavages (Garcia et al. 2016). An assessment of Mtb transcription in sputum taken from HIV-negative and HIV-positive individuals with active TB revealed that Mtb adapts to the immune status of the host, with lower DosR regulon expression in samples from HIV-positive individuals (Walter et al. 2016). Concomitant host gene expression analysis led the authors to suggest this was a result of alternative pathway activation of macrophages, leading to lower NO production and poorer granuloma formation. A recently published study reporting on differential expression in sputum of Mtb lineage 4 (Euro-American lineage) and Mtb lineage 6 (Mycobacterium africanum (Maf) – the cause of ~40% of all TB cases in West Africa) noted that compared with Mtb lineage 4, the Maf lineage 6 shows significantly reduced expression of the DosR regulon (Ofori-Anyinam et al. 2017). Interestingly, preliminary results reveal a higher proportion of LB-positive bacilli of Maf in sputum compared with Mtb (Tientcheu et al. 2016), the converse of what might be expected as a consequence of reduced expression of dosR and may reflect activity of additional TGS enzymes.
Expectoration of Mtb is critical for the propagation of infection. The presence of LBs in Mtb in sputum will reflect the bacillary response to the lung environment immediately prior to, or during exit and may reflect adaptation for onward transmission.
5 The Clinical Significance of Mtb LBs
5.1 Implications for the Treatment of TB
At 6 months, chemotherapy for pulmonary TB is prolonged and patients show varying responses. In the majority, the burden of bacilli in sputum, as revealed by smear microscopy and culture, rapidly decreases in the first few days of treatment. The clinical trials underpinning the current standard regimen in the 1970s all showed that while most (~85%) were cured after 3–4 months, 6 months was required to bring the relapse rate below 5%. Despite multiple studies, as yet, no clinical assessments have enabled accurate identification of either those cured, or those requiring more time, and multiple trials to reduce treatment time have yet to identify a shorter regimen that can be given with confidence. Some patients, although infected with strains demonstrated to be drug sensitive in vitro, can still produce positive specimens after many months of treatment. Positive cultures after a 2-month therapy are associated with greater risk of treatment failure and relapse. Shortening the duration of treatment regimens and early identification of patients with high risk of treatment failure or relapse, are major goals of clinical research.
5.1.1 Antibiotic Tolerance Is a Feature of Dormant or Slow-Growing Mtb
In 1979, Mitchison proposed that it is physiological heterogeneity of Mtb bacilli in the tissues that results in persistence of bacilli in the face of chemotherapy (Mitchison 1979). Rapidly replicating bacilli are inactivated very quickly with antimicrobials, resulting in the initial rapid decline in bacilli detected by culture from patient samples. However, other bacilli persist, seemingly resistant to the action of the therapeutics. Mitchison proposed these were non-growing bacilli or those with low metabolic activity. Bacterial populations which show “phenotypic antibiotic tolerance” are characterized by having low metabolic activity, bacteriostasis, or slow growth (Kussell et al. 2005; Lewis 2007); in permissive growth conditions, such bacilli are fully drug sensitive.
The characteristic of “phenotypic antibiotic tolerance” has been observed in Mtb in conditions which lead to growth arrest and in many in vitro models of persistence (Wayne and Hayes 1996; Garton et al. 2008; Deb et al. 2009; Daniel et al. 2011; Baek et al. 2011; Kapoor et al. 2013). A common feature of these conditions is dosR induction, tgs1 expression, and LB accumulation. Mtb Δtgs1 mutant strains do not develop the antibiotic tolerance observed in the wild type. Furthermore, expression of MPER1 which associates with Mtb LBs is also required for development of antibiotic tolerance (Daniel et al. 2016), suggesting organization of the TAG into LB is required. Although TAG synthesis can bring about growth arrest, it is not known if LBs have a mechanistic role in antibiotic tolerance, or are solely a biomarker for cells with altered physiology. Hammond and colleagues utilized the differential buoyancy of LB-positive mycobacteria (which they refer to as lipid-rich) to physically separate these from lipid-poor bacilli at various points during culture (Hammond et al. 2015). Regardless of the age of the culture from which they were recovered, the subpopulation of “lipid-rich” cells showed greater tolerance of antibiotic action than lipid-poor cells.
The demonstration of differentially culturable (DC or resuscitation promoting factor-dependent Mtb) in sputum samples by Mukamolova and colleagues adds to the body of evidence consistent with a high proportion of bacilli therein being in a slow or non-replicating state (Mukamolova et al. 2010). More recently, multiple populations in sputum samples, including those in a DC state, were shown to be antibiotic tolerant and to lose this phenotype on subculture (Turapov et al. 2016). Work to determine the relationship between DC bacilli and LB positivity is in progress.
5.1.2 The Frequency of LB-Positive Mtb in Sputum Increases During Antibiotic Therapy and Has Relationship with Treatment Success
Mtb in sputum are a sample of those which must be eliminated by chemotherapy. Recognition that LB-positive persister-like bacilli are tolerant to the action of antibiotics has profound implications for the treatment of TB. Two studies now support the clinical significance of Mtb LB content in sputum (Kayigire et al. 2015; Sloan et al. 2015). Kayigire and colleagues determined Mtb LB content in serial sputum samples taken from TB patients in an early bactericidal activity clinical trial of a new compound SQ-109, assessed alone or in combination with rifampicin (RIF). They reported proportions of LB-containing Mtb bacilli patients’ sputa increased with antibiotic treatment over the 14 days of assessment (Kayigire et al. 2015). This increase in LB-positive bacilli was more prominent in samples from patients on regimens which contained RIF, reflecting the greater rate of decline in CFU count in those samples than from patients treated with SQ-109 alone. Monitoring sputum Mtb LB content may prove a valuable additional biomarker for assessment of novel antituberculous regimens developed with the aim of shortening treatment to less than 6 months.
Sloan and colleagues also assessed patient sputum Mtb LB content with treatment investigating the relationship between the LB content of patients’ sputa with treatment response (Sloan et al. 2015). High Mtb LB positivity in sputum taken after 3–4 weeks of treatment was found to correlate with poor treatment outcome (treatment failure or patient relapse), supporting the hypothesis that such bacilli are antibiotic tolerant persisters. Notably, this correlation was not apparent in sputum samples taken at baseline before the initiation therapy, suggesting that it is LB-positive persisters revealed by therapy that are predictive of treatment response and not the total LB-positive Mtb subpopulation in untreated patients’ sputa. This study demonstrates the potential value of monitoring LB-positive Mtb populations in patients’ sputa to inform management of patient treatment by providing an early indicator of those patients at risk of treatment failure.
5.2 LB-Positive Mtb and Patient Infectiousness
Transmission between hosts is critical for Mtb; that more than a quarter of humanity are believed to be infected is testament to its success in this regard. The bacilli are under strong selection pressure to maintain and display properties which facilitate transmission at every stage, from facilitating their exit from an infected host, through survival in transit, to establishing infection within a new susceptible host. TB patient infectiousness is currently based on sputum smear acid-fastness, informing infection control and screening of contacts. We proposed that LB-positive Mtb in sputum may reflect a population adapted for onward transmission (Barer and Garton 2010). Slowly replicating or non-replicating bacilli with low metabolic activity are more tolerant to stresses (Kolter et al. 1993; Smeulders et al. 1999). Furthermore, within TAG, LB-positive bacilli have a readily mobilized source of energy and lipid precursors with which to support growth in a new host. Supporting this hypothesis is a report that Mtb bacilli grown in reduced oxygen conditions, which we have shown to possess LBs (unpublished data), were tenfold more infectious for guinea pigs than aerobically grown controls (Bacon et al. 2004).
A relationship between Mtb of low metabolic activity in sputum and TB patient infectiousness has been reported (Datta et al. 2017). Quantitation of metabolically active Mtb bacilli in sputum was made following staining with fluorescein diacetate (FDA) which requires enzymatic hydrolysis within the bacillus to become fluorescent. FDA-negative Mtb in sputum was associated with greater transmission to household contacts. This supports the view that it is bacilli with low metabolic activity that are adapted for onward transmission. One study examining factors predictive of transmission risk including assessment of index case sputum Mtb LB positivity has been undertaken (Hector et al. 2017). Therein, univariate analysis showed that lower LB% was associated with greater probability of contact tuberculin skin test positivity. However, this was not the case when multivariate analysis was undertaken. Therefore, more extensive studies are required to test the significance of sputum Mtb LB-positivity as a measure of patient infectiousness.
It is important to note, however, that sputum is not the vehicle of transmission; Mtb is transmitted in aerosol droplets. We have preliminary TB patient transcriptional data which provides indication that aerosolized Mtb bacilli are a distinct population to those in sputa (unpublished results). To date, little is known of what promotes Mtb aerosolization. The high cell envelope lipid content and consequent cell surface hydrophobicity of mycobacteria has been linked to the efficacy with which mycobacteria aerosolize from aqueous suspensions (Falkinham 2003). Minnikin has proposed that changes in cell envelope lipid content and consequent increased cell surface hydrophobicity led to the evolution of Mtb strains that were able to enter and thereby via transmit via aerosol (Minnikin et al. 2015). LB-positive Mtb cells, which may have enhanced TAG envelope content and consequently enhanced surface hydrophobicity, may have a greater propensity for aerosolization. Furthermore, enhanced TAG LB content decreases the buoyant density of Mtb (Deb et al. 2009) and could contribute to a concentration of such bacilli at aqueous-air interfaces, i.e., at the surface of respiratory secretions in the alveolus, further increasing the likelihood for aerosolization. These proposals warrant further investigation.
6 Research Needs
Our initial finding that TAG LBs are particularly prominent in sputum samples provides major incentive for both basic and clinical research. Knowledge of the significance of TAG LBs has increased greatly in the last decade. Progress has been made toward understanding the Mtb pathways involved in the synthesis and regulation of neutral lipid accumulation and downstream utilization. Many protein players in these processes have been identified, but roles for all are not understood. Questions such as the functional significance of the 15 different Mtb TGS remain, particularly for those, which in addition to Tgs1, have transcripts identified in sputum. Neither do we appreciate the relationship between cell envelope composition and LB accumulation. Does LB accumulation reflect saturation of the cell envelope with TAG? There are obvious technical challenges which need to be overcome in order to perform specific and sensitive analyses of Mtb neutral lipid content ex vivo and in clinical samples, including patient aerosol samples.
We are now beginning to recognize that unlike Mtb H37Rv, some Mtb isolates exhibit significant LB content during aerobic growth. This fuels the need to understand the role of other TGS enzymes and how expression of tgs1 and others relates to growth deceleration in different strain backgrounds. Do strains with a high level of TGS activity and LBs during replication have the same capacity to respond to growth arresting stimuli and how does this impact development of antibiotic tolerance? Perhaps the composition of LBs which are present during bacterial replication differs from those which accumulate with growth deceleration; this may reflect the involvement of different TGS enzymes. Different proteins may stabilize and control access of lipases to the lipid reserve in different conditions. LBs which accumulate during growth may be turned over more rapidly than those which accumulate as a result of environmental changes which restrict growth. Different lipases may be involved in their degradation. Furthermore, how lipases are recruited to LBs to initiate TAG turnover remains to be investigated.
There is now support for the clinical significance of Mtb LBs and the value of monitoring these to inform management of patient treatment or assessment of new and potentially treatment-shortening regimens. Further clinical studies are required to validate the role of LBs as a useful biomarker for these assessments. These will be facilitated by developments for improved sputum LB analysis which is currently technically demanding or discovery of alternative biomarkers of the sputum LB population.
We are keen to understand the stimulus, or stimuli, responsible for inducing LBs in Mtb in sputum. This, in combination with transcriptional data, will allow us to develop in vitro conditions inducing Mtb cells into a comparable state. Mtb LB content, growth state, and antibiotic tolerance are anticipated to reflect variable characteristics of the both bacilli and host. Furthermore, we must understand the nature of Mtb in aerosol and how this relates to populations we measure in sputum. Being able to influence and study in vitro aspects of host-Mtb interplay and factors impacting aerosolization and aerosol survival, will provide new opportunities to explore the persister problem in treatment of TB and also transmission potential, which need to be understood to control dissemination.
Abbreviations
- ACSL:
-
Long-chain acyl-CoA synthase
- DAG:
-
Diacylglycerol
- DC:
-
Differentially culturable
- DGAT:
-
Diacylglycerol acyl transferase
- FACS:
-
Fatty acyl CoA synthase
- FCR:
-
Fatty acyl long-chain CoA reductase
- ILI:
-
Intracellular lipophilic inclusion
- LB:
-
Lipid body
- LCFA:
-
Long-chain fatty acid
- LD:
-
Lipid droplet
- NO:
-
Nitric oxide
- PBMC:
-
Peripheral blood mononuclear cell
- RIF:
-
Rifampicin
- TAG:
-
Triacylglycerol
- TB:
-
Tuberculosis
- TCA:
-
Tricarboxylic acid
- TGS:
-
Triacylglycerol synthase
- THL:
-
Tetrahydrolipstatin
- WE:
-
Wax ester
- WS:
-
Wax ester synthase
References
Alvarez HM (2016) Triacylglycerol and wax ester-accumulating machinery in prokaryotes. Biochimie 120:28–39
Alvarez HM, Steinbüchel A (2002) Triacylglycerols in prokaryotic microorganisms. Appl Microbiol Biotechnol 60:367–376
Armstrong RM, Adams KL, Zilisch JE, Bretl DJ, Sato H, Anderson DM, Zahrt TC (2016) Rv2744c is a PspA Ortholog that regulates lipid droplet homeostasis and nonreplicating persistence in Mycobacterium tuberculosis. J Bacteriol 198:1645–1661
Bacon J, James BW, Wernisch L, Williams A, Morley KA, Hatch GJ, Mangan JA, Hinds J, Stoker NG, Butcher PD, Marsh PD (2004) The influence of reduced oxygen availability on pathogenicity and gene expression in Mycobacterium tuberculosis. Tuberculosis (Edinb) 84:205–217
Bacon J, Dover LG, Hatch KA, Zhang Y, Gomes JM, Kendall S, Wernisch L, Stoker NG, Butcher PD, Besra GS, Marsh PD (2007) Lipid composition and transcriptional response of Mycobacterium tuberculosis grown under iron-limitation in continuous culture: identification of a novel wax ester. Microbiology 153:1435–1444
Baek SH, Li AH, Sassetti CM (2011) Metabolic regulation of mycobacterial growth and antibiotic sensitivity. PLoS Biol 9:e1001065
Barer MR, Garton NJ (2010) Mycobacterial lipid bodies and the chemosensitivity and transmission of tuberculosis. In: Timmis KN (ed) Handbook of hydrocarbon and lipid microbiology. Springer, Berlin/Heidelberg
Betts JC, Lukey PT , Robb LC, McAdam RA, Duncan K (2002) Evaluation of a nutrient starvation model of persistence by gene and protein expression profiling. Molecular Microbiology 43(3):717–731
Burdon KL (1946) Disparity in appearance of true Hansen’s bacilli and cultured “leprosy bacilli” when stained for fat. J Bacteriol 52:679–680
Christensen H, Garton NJ, Horobin RW, Minnikin DE, Barer MR (1999) Lipid domains of mycobacteria studied with fluorescent molecular probes. Mol Microbiol 31:1561–1572
Daniel J, Deb C, Dubey VS, Sirakova TD, Abomoelak B, Morbidoni HR, Kolattukudy PE (2004) Induction of a novel class of diacylglycerol acyltransferases and triacylglycerol accumulation in Mycobacterium tuberculosis as it goes into a dormancy-like state in culture. J Bacteriol 186:5017–5030
Daniel J, Maamar H, Deb C, Sirakova TD, Kolattukudy PE (2011) Mycobacterium tuberculosis uses host triacylglycerol to accumulate lipid droplets and acquires a dormancy-like phenotype in lipid-loaded macrophages. PLoS Pathog 7:e1002093
Daniel J, Kapoor N, Sirakova T, Sinha R, Kolattukudy P (2016) The perilipin-like PPE15 protein in Mycobacterium tuberculosis is required for triacylglycerol accumulation under dormancy-inducing conditions. Mol Microbiol 101:784–794
Datta S, Sherman JM, Tovar MA, Bravard MA, Valencia T, Montoya R, Quino W, D'Arcy N, Ramos ES, Gilman RH, Evans CE (2017) Sputum microscopy with fluorescein diacetate predicts tuberculosis infectiousness. J Infect Dis 216:514–524
Deb C, Daniel J, Sirakova TD, Abomoelak B, Dubey VS, Kolattukudy PE (2006) A novel lipase belonging to the hormone-sensitive lipase family induced under starvation to utilize stored triacylglycerol in Mycobacterium tuberculosis. J Biol Chem 281:3866–3875
Deb C, Lee CM, Dubey VS, Daniel J, Abomoelak B, Sirakova TD, Pawar S, Rogers L, Kolattukudy PE (2009) A novel in vitro multiple-stress dormancy model for Mycobacterium tuberculosis generates a lipid-loaded, drug-tolerant, dormant pathogen. PLoS One 4:e6077
Dhouib R, Ducret A, Hubert P, Carriere F, Dukan S, Canaan S (2011) Watching intracellular lipolysis in mycobacteria using time lapse fluorescence microscopy. Biochim Biophys Acta 1811:234–241
Domenech P, Zou J, Averback A, Syed N, Curtis D, Donato S, Reed MD (2017) Unique regulation of the DosR regulon in the Beijing lineage of Mycobacterium tuberculosis. J Bacteriol 199:e00696–e00716
Elamin AA, Stehr M, Spallek R, Rohde M, Singh M (2011) The Mycobacterium tuberculosis Ag85A is a novel diacylglycerol acyltransferase involved in lipid body formation. Mol Microbiol 81:1577–1592
Falkinham JO 3rd (2003) Mycobacterial aerosols and respiratory disease. Emerg Infect Dis 9:763–767
Garcia BJ, Loxton AG, Dolganov GM, Van TT, Davis JL, de Jong BC, Voskuil MI, Leach SM, Schoolnik GK, Walzl G, Strong M, Walter ND (2016) Sputum is a surrogate for bronchoalveolar lavage for monitoring Mycobacterium tuberculosis transcriptional profiles in TB patients. Tuberculosis (Edinb) 100:89–94
Garton NJ, Christensen H, Minnikin DE, Adegbola RA, Barer MR (2002) Intracellular lipophilic inclusions of mycobacteria in vitro and in sputum. Microbiology 148:2951–2958
Garton NJ, Waddell SJ, Sherratt AL, Lee SM, Smith RJ, Senner C, Hinds J, Rajakumar K, Adegbola RA, Besra GS, Butcher PD, Barer MR (2008) Cytological and transcript analyses reveal fat and lazy persister-like bacilli in tuberculous sputum. PLoS Med 5:e75
Golby P, Nunez J, Cockle PJ, Ewer K, Logan K, Hogarth P, Vordermeier HM, Hinds J, Hewinson RG, Gordon SV (2008) Characterization of two in vivo-expressed methyltransferases of the Mycobacterium tuberculosis complex: antigenicity and genetic regulation. Microbiology 154:1059–1067
Hammond RJ, Baron VO, Oravcova K, Lipworth SL, Gillespie SH (2015) Phenotypic resistance in mycobacteria: is it because I am old or fat that I resist you? J Antimicrob Chemother 70:2823–2827
Hector J, Anderson ST, Banda G, Kamdolozi M, Jefferys LF, Shani D, Garton NJ, Mwale A, Jobe A, Davies GR, Sloan DJ (2017) TST positivity in household contacts of tuberculosis patients: a case-contact study in Malawi. BMC Infect Dis 17:259
Honeyborne I, McHugh TD, Kuittinen I, Cichonska A, Evangelopoulos D, Ronacher K, van Helden PD, Gillespie SH, Fernandez-Reyes D, Walzl G, Rousu J, Butcher PD, Waddell SJ (2016) Profiling persistent tubercle bacilli from patient sputa during therapy predicts early drug efficacy. BMC Med 14:68
Ishige T, Tani A, Takabe K, Kawasaki K, Sakai Y, Kato N (2002) Wax ester production from n-alkanes by Acinetobacter sp. strain M-1: ultrastructure of cellular inclusions and role of acyl coenzyme A reductase. Appl Environ Microbiol 68:1192–1195
Kalscheuer R, Steinbüchel A (2003) A novel bifunctional wax ester synthase/acyl-CoA:diacylglycerol acyltransferase mediates wax ester and triacylglycerol biosynthesis in Acinetobacter calcoaceticus ADP1. J Biol Chem 278:8075–8082
Kapoor N, Pawar S, Sirakova TD, Deb C, Warren WL, Kolattukudy PE (2013) Human granuloma in vitro model, for TB dormancy and resuscitation. PLoS One 8:e53657
Kayigire XA, Friedrich SO, van der Merwe L, Donald PR, Diacon AH (2015) Simultaneous staining of sputum smears for acid-fast and lipid-containing Myobacterium tuberculosis can enhance the clinical evaluation of antituberculosis treatments. Tuberculosis (Edinb) 95:770–779
Kolter R, Siegele DA, Tormo A (1993) The stationary phase of the bacterial life cycle. Annu Rev Microbiol 47:855–874
Kondo E, Kanai K (1977) The relationship between the chemical structure of fatty acids and their mycobactericidal activity. Jpn J Med Sci Biol 30:171–178
Kussell E, Kishony R, Balaban NQ, Leibler S (2005) Bacterial persistence: a model of survival in changing environments. Genetics 169:1807–1814
Lewis K (2007) Persister cells, dormancy and infectious disease. Nat Rev Microbiol 5:48–56
Lovewell RR, Sassetti CM, VanderVen BC (2016) Chewing the fat: lipid metabolism and homeostasis during Mycobacterium tuberculosis infection. Curr Opin Microbiol 29:30–36
Low KL, Rao PS, Shui G, Bendt AK, Pethe K, Dick T, Wenk MR (2009) Triacylglycerol utilization is required for regrowth of in vitro hypoxic nonreplicating Mycobacterium bovis bacillus Calmette-Guerin. J Bacteriol 191:5037–5043
Low KL, Shui G, Natter K, Yeo WK, Kohlwein SD, Dick T, Rao SP, Wenk MR (2010) Lipid droplet-associated proteins are involved in the biosynthesis and hydrolysis of triacylglycerol in Mycobacterium bovis bacillus Calmette-Guerin. J Biol Chem 285:21662–21670
MacEachran DP, Prophete ME, Sinskey AJ (2010) The Rhodococcus opacus PD630 heparin-binding hemagglutinin homolog TadA mediates lipid body formation. Appl Environ Microbiol 76:7217–7225
Martinot AJ, Farrow M, Bai L, Layre E, Cheng TY, Tsai JH, Iqbal J, Annand JW, Sullivan ZA, Hussain MM, Sacchettini J, Moody DB, Seeliger JC, Rubin EJ (2016) Mycobacterial metabolic syndrome: LprG and Rv1410 regulate triacylglyceride levels, growth rate and virulence in Mycobacterium tuberculosis. PLoS Pathog 12:e1005351
McKinney JD, Honer zu Bentrup K, Munoz-Elias EJ, Miczak A, Chen B, Chan WT, Swenson D, Sacchettini JC, Jacobs WR Jr, Russell DG (2000) Persistence of Mycobacterium tuberculosis in macrophages and mice requires the glyoxylate shunt enzyme isocitrate lyase. Nature 406:735–738
Minnikin DE (1982) Lipids: complex lipids, their chemistry, biosynthesis and roles. In: Ratledge C, Stanford J (eds) The biology of the mycobacteria. Academic Press, London, pp. 95–185
Minnikin DE, Lee OY, Wu HH, Nataraj V, Donoghue HD, Ridell M, Watanabe M, Alderwick L, Bhatt A, Besra GS (2015) Pathophysiological implications of cell envelope structure in Mycobacterium tuberculosis and related taxa. In: Ribón W (eds) Tuberculosis – expanding knowledge. InTech Open Access Publisher, Rijeka, pp. 145–175
Mishra KC, de Chastellier C, Narayana Y, Bifani P, Brown AK, Besra GS, Katoch VM, Joshi B, Balaji KN, Kremer L (2008) Functional role of the PE domain and immunogenicity of the Mycobacterium tuberculosis triacylglycerol hydrolase LipY. Infect Immun 76:127–140
Mitchison DA (1979) Basic mechanisms of chemotherapy. Chest 76(6 Suppl):771–781
Mukamolova GV, Turapov O, Malkin J, Woltmann G, Barer MR (2010) Resuscitation-promoting factors reveal an occult population of tubercle bacilli in sputum. Am J Respir Crit Care Med 181:174–180
Murphy DJ (2012) The dynamic roles of intracellular lipid droplets: from archaea to mammals. Protoplasma 249:541–585
Muttucumaru DG, Roberts G, Hinds J, Stabler RA, Parish T (2004) Gene expression profile of Mycobacterium tuberculosis in a non-replicating state. Tuberculosis (Edinb) 84:239–246
Ofori-Anyinam B, Dolganov G, Van T, Davis JL, Walter ND, Garcia BJ, Voskuil M, Fissette K, Diels M, Driesen M, Meehan CJ, Yeboah-Manu D, Coscolla M, Gagneux S, Antonio M, Schoolnik G, Gehre F, de Jong BC (2017) Significant under expression of the DosR regulon in M. tuberculosis complex lineage 6 in sputum. Tuberculosis (Edinb) 104:58–64
Ortalo-Magné A, Lemassu A, Lanéelle MA, Bardou F, Silve G, Gounon P, Marchal G, Daffé M (1996) Identification of the surface-exposed lipids on the cell envelopes of Mycobacterium tuberculosis and other mycobacterial species. J Bacteriol 178:456–461
Pandey AK, Sassetti CM (2008) Mycobacterial persistence requires the utilization of host cholesterol. Proc Natl Acad Sci U S A 105:4376–4380
Park HD, Guinn KM, Harrell MI, Liao R, Voskuil MI, Tompa M, Schoolnik GK, Sherman DR (2003) Rv3133c/dosR is a transcription factor that mediates the hypoxic response of Mycobacterium tuberculosis. Mol Microbiol 48:833–843
Peyron P, Vaubourgeix J, Poquet Y, Levillain F, Botanch C, Bardou F, Daffe M, Emile JF, Marchou B, Cardona PJ, de Chastellier C, Altare F (2008) Foamy macrophages from tuberculous patients’ granulomas constitute a nutrient-rich reservoir for M. tuberculosis persistence. PLoS Pathog 4:e1000204
Rastogi S, Singh AK, Chandra G, Kushwaha P, Pant G, Singh K, Mitra K, Sashidhara KV, Krishnan MY (2017) The diacylglycerol acyltransferase Rv3371 of Mycobacterium tuberculosis is required for growth arrest and involved in stress-induced cell wall alterations. Tuberculosis (Edinb) 104:8–19
Reed MB, Gagneux S, Deriemer K, Small PM, Barry CM 3rd (2007) The W-Beijing lineage of Mycobacterium tuberculosis overproduces triglycerides and has the DosR dormancy regulon constitutively upregulated. J Bacteriol 189:2583–2589
Roberts DM, Liao RP, Wisedchaisri G, Hol WG, Sherman DR (2004) Two sensor kinases contribute to the hypoxic response of Mycobacterium tuberculosis. J Biol Chem 279:23082–23087
Russell DG (2007) Who puts the tubercle in tuberculosis? Nat Rev Microbiol 5:39–47
Russell DG, Cardona PJ, Kim MJ, Allain S, Altare F (2009) Foamy macrophages and the progression of the human tuberculosis granuloma. Nat Immunol 10:943–948
Segal W, Bloch H (1956) Biochemical differentiation of Mycobacterium tuberculosis grown in vivo and in vitro. J Bacteriol 72:132–141
Sharma S, Ryndak MB, Aggarwal AN, Yadav R, Sethi S, Masih S, Laal S, Verma I (2017) Transcriptome analysis of mycobacteria in sputum samples of pulmonary tuberculosis patients. PLoS One 12:e0173508
Sherratt AL (2008) Lipid bodies in mycobacteria. PhD, University of Leicester
Shi L, Sohaskey CD, Kana BD, Dawes S, North RJ, Mizrahi V, Gennaro ML (2005) Changes in energy metabolism of Mycobacterium tuberculosis in mouse lung and under in vitro conditions affecting aerobic respiration. Proc Natl Acad Sci U S A 102(43):15629–15634
Singer ME, Tyler SM, Finnerty WR (1985) Growth of Acinetobacter sp. strain HO1-N on n-hexadecanol: physiological and ultrastructural characteristics. J Bacteriol 162:162–169
Sirakova TD, Dubey VS, Deb C, Daniel J, Korotkova TA, Abomoelak B, Kolattukudy PE (2006) Identification of a diacylglycerol acyltransferase gene involved in accumulation of triacylglycerol in Mycobacterium tuberculosis under stress. Microbiology 152:2717–2725
Sirakova TD, Deb C, Daniel J, Singh HD, Maamar H, Dubey VS, Kolattukudy PE (2012) Wax ester synthesis is required for Mycobacterium tuberculosis to enter in vitro dormancy. PLoS One 7:e51641
Sloan DJ, Mwandumba HC, Garton NJ, Khoo SH, Butterworth AE, Allain TJ, Heyderman RS, Corbett EL, Barer MR, Davies GR (2015) Pharmacodynamic modeling of bacillary elimination rates and detection of bacterial lipid bodies in sputum to predict and understand outcomes in treatment of pulmonary tuberculosis. Clin Infect Dis 61:1–8
Smeulders MJ, Keer J, Speight RA, Williams HD (1999) Adaptation of Mycobacterium smegmatis to stationary phase. J Bacteriol 181:270–283
Stöveken T, Kalscheuer R, Malkus U, Reichelt R, Steinbüchel A (2005) The wax ester synthase/acyl coenzyme A:diacylglycerol acyltransferase from Acinetobacter sp. strain ADP1: characterization of a novel type of acyltransferase. J Bacteriol 187:1369–1376
Tientcheu LD, Bell A, Secka O, Ayorinde A, Otu J, Garton NJ, Sutherland JS, Ota MO, Antonio M, Dockrell HM, Kampmann B, Barer MR (2016) Association of slow recovery of Mycobacterium africanum-infected patients posttreatment with high content of persister-like bacilli in pretreatment sputum. Int J Mycobacteriol 5(Suppl 1):S99–S100
Turapov O, O'Connor BD, Sarybaeva AA, Williams C, Patel H, Kadyrov AS, Sarybaev AS, Woltmann G, Barer MR, Mukamolova GV (2016) Phenotypically adapted Mycobacterium tuberculosis populations from sputum are tolerant to first-line drugs. Antimicrob Agents Chemother 60:2476–2483
Vijay S, Hai HT, Thu DDA, Johnson E, Pielach A, Phu NH, Thwaites GE, Thuong NTT (2017) Ultrastructural analysis of cell envelope and accumulation of lipid inclusions in clinical Mycobacterium tuberculosis isolates from sputum, oxidative stress, and iron deficiency. Front Microbiol 8:2681
Viljoen A, Richard M, Nguyen PC, Fourquet P, Camoin L, Paudal RR, Gnawali GR, Spilling CD, Cavalier JF, Canaan S, Blaise M, Kremer L (2018) Cyclipostins and cyclophostin analogs inhibit the antigen 85C from Mycobacterium tuberculosis both in vitro and in vivo. J Biol Chem 293(8):2755–2769
Voskuil MI, Visconti KC, Schoolnik GK (2004) Mycobacterium tuberculosis gene expression during adaptation to stationary phase and low-oxygen dormancy. Tuberculosis (Edinb) 84:218–227
Walker RW, Barakat H, Hung JG (1970) The positional distribution of fatty acids in the phospholipids and triglycerides of Mycobacterium smegmatis and M. bovis BCG. Lipids 5:684–691
Walter ND, de Jong BC, Garcia BJ, Dolganov GM, Worodria W, Byanyima P, Musisi E, Huang L, Chan ED, Van TT, Antonio M, Ayorinde A, Kato-Maeda M, Nahid P, Leung AM, Yen A, Fingerlin TE, Kechris K, Strong M, Voskuil I, Davis JL, Schoolnik GK (2016) Adaptation of Mycobacterium tuberculosis to impaired host immunity in HIV-infected patients. J Infect Dis 214(8):1205–1211
Walther TC, Farese RV Jr (2012) Lipid droplets and cellular lipid metabolism. Annu Rev Biochem 81:687–714
Wayne LG, Hayes LG (1996) An in vitro model for sequential study of shiftdown of Mycobacterium tuberculosis through two stages of nonreplicating persistence. Infect Immun 64:2062–2069
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this entry
Cite this entry
Garton, N.J., Barer, M.R. (2020). Mycobacterial Lipid Bodies and the Chemosensitivity and Transmission of Tuberculosis. In: Goldfine, H. (eds) Health Consequences of Microbial Interactions with Hydrocarbons, Oils, and Lipids. Handbook of Hydrocarbon and Lipid Microbiology . Springer, Cham. https://doi.org/10.1007/978-3-030-15147-8_6
Download citation
DOI: https://doi.org/10.1007/978-3-030-15147-8_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-15146-1
Online ISBN: 978-3-030-15147-8
eBook Packages: Biomedical and Life SciencesReference Module Biomedical and Life Sciences